Abstract
Early-life iron deficiency is a common nutrient condition worldwide and can result in cognitive impairment in adulthood despite iron treatment. In rodents, prenatal choline supplementation can diminish long-term hippocampal gene dysregulation and neurocognitive deficits caused by iron deficiency. Since fetal iron status is generally unknown in humans, we determined whether postnatal choline supplementation exerts similar beneficial effects. Male rat pups were made iron deficient (ID) by providing pregnant and nursing dams an ID diet (3-6 ppm Fe) from gestational day (G) 3 through postnatal day (P) 7, and an iron-sufficient (IS) diet (200 ppm Fe) thereafter. Control pups were provided IS diet throughout. Choline (5 ppm) was given to half the nursing dams and weanlings in each group from P11-P30. P65 rat cognitive performance was assessed by novel object recognition (NOR). Real-time PCR was performed to validate expression levels of synaptic plasticity genes known to be dysregulated by early-life iron deficiency. Postnatal choline supplementation prevented impairment of NOR memory in formerly iron-deficient (FID) adult rats but impaired NOR memory in IS controls. Gene expression analysis revealed a recovery of 4 out of 10 dysregulated genes compared to 8 of the same 10 genes that we previously demonstrated to recover following prenatal choline supplementation. Recognition memory deficits induced by early-life iron deficiency can be prevented by postnatal choline supplementation and disrupted expression of a subset of synaptic plasticity genes can be ameliorated. The positive response to postnatal choline represents a potential adjunctive therapeutic supplement to treat iron-deficient anemic children in order to spare long-term neurodevelopmental deficits.
Keywords: Iron deficiency, novel object recognition, memory, hippocampus, choline supplementation, gene expression
Introduction:
Iron deficiency is a common early-life nutrient deficiency in humans that has harmful effects on the developing brain, leading to a risk of cognitive deficits that persist even after re-establishment of normal iron status [1, 2]. Deleterious effects of fetal neonatal iron deficiency are also found in rodent models. These include impairments in recognition [3, 4] and spatial [5, 6] memory as well as disruption of normal hippocampal gene expression and development [7, 8] that last into adulthood in spite of iron repletion [9]. Thus, there is a strong rationale for adjunct treatments in order to ameliorate the long-term effects on cognitive function of iron deficiency that is diagnosed at or after birth. Choline is a widely available and developmentally important nutrient [10] that has previously been shown to reduce the detrimental effects of iron deficiency in rodents when given prenatally [3]. Specifically, choline supplementation during embryonic days 11 - 18 improved recognition memory and normalized hippocampal expression of a number of synaptic plasticity genes in a rat model early life iron deficiency [3, 11].
Developmental studies have revealed two sensitive periods during which choline supplementation can improve learning and memory in rats exposed to developmental insults, a prenatal period [3, 12, 13] and an early postnatal period starting around P11 [13] or P16 [12] and ending at P30. The beneficial effect of postnatal choline supplementation was further underscored by a recent trial in young children suggesting that postnatally administered choline reduces the cognitive memory deficits associated with fetal alcohol exposure [14]. An early postnatal supplementation approach is ideal for an adjunct treatment of iron deficiency due to the challenge of diagnosis and intervention of iron deficiency during the prenatal period. Although maternal iron deficiency is a risk factor for fetal and early postnatal iron deficiency, fetal and maternal iron stores are not necessarily correlated [15]. For instance, some risk factors for fetal iron deficiency such as hypertension during pregnancy or maternal diabetes mellitus can restrict maternal-fetal iron transfer, altering fetal and neonatal iron stores without affecting maternal iron status [16, 17]. Such cases of neonatal iron deficiency may go undetected until anemia screening at 12 months of age, a point when iron alone is not sufficient to restore normal cognitive function [2, 18], and thus could potentially benefit from an adjunctive treatment such as choline supplementation.
In the present study, we assessed whether postnatal choline supplementation (P11-30) of rats born iron-deficient could improve the long-term deficit in recognition memory and normalize alterations of hippocampal gene expression in adulthood. In order to more directly compare the effects of pre- versus postnatal choline supplementation, we evaluated memory in the formerly iron-deficient adult animals using the same measure of novel object recognition (NOR) as in our previous report on prenatal choline supplementation [3]. Likewise, we assessed ten genes related to synaptic plasticity in the adult hippocampus that have previously been shown to be altered by fetal-neonatal iron deficiency and partially restored by prenatal choline supplementation [19].
Materials and Methods:
Animals and diet manipulations:
All experiments were approved by the University of Minnesota Institutional Animal Care and Use Committee. Timed-pregnant Sprague-Dawley dams (Charles River, Wilmington, MA) were randomly assigned to either iron-deficient (ID, 2-6 ppm iron, Harlan-Teklad TD# 110137, see reference [3] for diet composition) or iron-sufficient (IS, 200 ppm iron, TD# 110138) diet (Harlan-Teklad, Madison, WI) ad libitum from G3 through P7. Shortly after birth, litters were culled to 8 pups containing at least 2 females. Blood was collected from culled pups for hematocrits. On P7, all dams were placed on the IS diet until their pups were weaned at P21. Pups were maintained on the IS diet for the duration of the experiment. This ID diet induced a lower hematocrit in newborn pups born to dams receiving the ID diet (data not shown, Χ2 <0.009) consistent with previous reports using this model [20, 21]. These reductions in brain iron and hematocrits were normalized by P65 [3, 20, 21]. From P11-30, half of the litters in each diet group were further randomized to an IS diet with fortified choline (5 ppm choline chloride, TD# 110140), or remained on the IS diet with standard choline content (1.1 ppm). The final randomization strategy resulted in 4 groups in adulthood (Figure 1): formerly iron-deficient with supplemental choline (FID-Ch), formerly iron-deficient without supplemental choline (FID), always iron-sufficient with supplemental choline (IS-Ch), and always iron-sufficient without supplemental choline (IS). Only male animals were used for behavioral and gene analyses.
Figure 1:
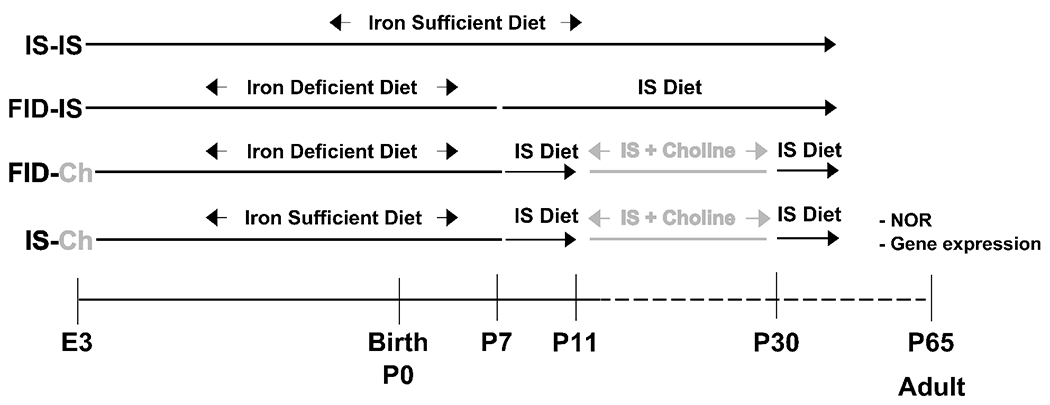
Experimental paradigm of dietary-induced early-life ID and early postnatal choline supplementation.
Novel Object Recognition Memory (NOR) Assessment:
The effects of diet on memory were assessed using the NOR task in P65 rats as previously described [3]. Following handling and habituation to the test chamber (10 min per day over two days), rats were assessed for NOR at either a 1- or 6-h delay between acquisition and testing. During the acquisition phase, two identical objects were placed in the chamber and exploration of each object was video-recorded for 3 minutes. After the appropriate delay, animals were returned to the chamber which now contained a copy of the object from the acquisition phase (familiar object) and a novel object for a 2-minute test. Object pairs, the novel object, and novel object locations were counterbalanced between treatment groups. Blind observers rated acquisition and test videos for NOR, which was defined as percent of total exploration directed toward the location of novel object. Our measure of object investigation is commonly used in NOR studies and is defined as orientation to the object and either proximity (within a few cm) or contact with the object.
Real-time PCR analysis:
A subset of animals from each treatment group were euthanized for hippocampal dissections at P65. Hippocampal extraction, RNA isolation, cDNA synthesis, and quantitative Real-Time PCR (RT-qPCR) were performed using methods previously described [8]. Briefly, RNA from the hippocampus was isolated using the RNAqueous® total RNA isolation kit (Ambion®, Austin, TX) following the manufacturer’s instructions and samples were assessed for purity and concentration using a NanoDrop ND-1000 (Invitrogen). 1 μg of total RNA was used to generate cDNA (High Capacity RNA-to-cDNA™ kit, Applied Biosystems, Carlsbad, CA) following the manufacturer’s instructions. The cDNA was diluted 10-fold with ddH2O prior to RT-qPCR analysis. Each sample was run in duplicates, where each RT-qPCR reaction consisted of 5.0 μl qPCR FastStart Universal Probe Mastermix (Roche, Indianapolis, IN), 4.5 μl diluted cDNA, and 0.5 μl of Taqman probes and primers mix (20X). Reactions were run in a 96-well plate in a MX3000P thermocycler (Stratagene, La Jolla, CA).
Statistical Analysis:
Performance on the NOR task was assessed using mixed model 2x2x2 ANOVAs for iron and choline diet treatment (between-subjects) and test phase (within-subjects) at each delay time. Novelty preference was directly tested using planned contrasts between test and averaged acquisition preferences for each dietary treatment group at both delay times. Gene expression data were analyzed by ANOVA with post hoc Tukey-corrected t-test for group comparisons.
Results:
Object preferences during the acquisition phase of the NOR task were equivalent for each diet treatment (F[3,91] = 0.489, P=0.72) and at both test delays, although there was a trend for higher preferences at the 6 hr delay (F[1,93] = 3.3, P=0.07). Regardless of this borderline effect, acquisition preferences from all groups and delays did not differ from the chance preference of 50% (Ps>0.05). In order to reduce variability, acquisition preferences for each animal were averaged across tests conducted at both delay times.
At the 1-h delay, rats generally exhibited a higher preference for the novel object at the test vs acquisition phase (Figure 2; F[1,91] = 51.9, P<0.001) indicating overall novelty preference. Furthermore, this effect was dependent on choline supplementation (Choline x phase interaction, F[1,91] = 6.87, P<0.05) such that choline improved NOR at the 1-h delay (novelty preferences, Choline = 78.4%, No Choline = 66.4%). However, performance at the 1-h delay was not affected by fetal-neonatal iron status (F[1,91] = 0.02, P=0.884). Planned contrasts between the acquisition and test preferences at the 1-h delay were conducted to further assess effects of diet on object memory. Relative to acquisition, IS, IS-Ch, and FID-Ch rats exhibited a significant preference for the novel object (P<0.05), whereas this preference was on the threshold of significance for FID rats (P = 0.05).
Figure 2:
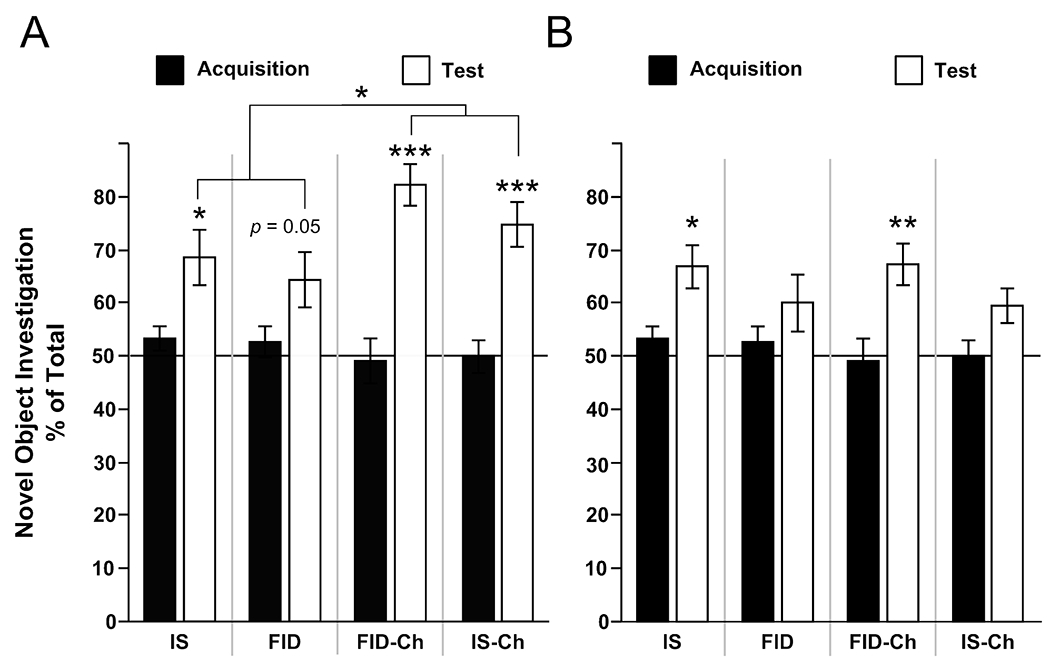
Effects of postnatal choline supplementation on NOR performance in FID P65 male rats. (A) At 1-h delay, postnatal choline supplementation enhanced preference for the novel object regardless of prenatal iron status. (B) At the 6-h delay IS, but not FID, rats exhibited intact object memory. Choline supplementation improved preference for the novel object in the FID-Ch, but impaired NOR in IS-Ch animals. Values are mean ± SEM, n = 10-12/group, different from averaged acquisition preferences (planned contrasts), *P<0.05, **P<0.01, ***P<0.001.
NOR was also assessed in a more difficult memory task by increasing the delay to 6-h (effect of phase, F[1,91] = 17.28, P<0.0005), but performance was not dependent on dietary choline (F[1,91] = 0.64, P=0.43) or iron status (F[1,91] = 0.01, P=0.92). Planned contrasts between acquisition and test preferences revealed a different pattern of performance compared to the 1-h delay. At the 6-h delay, NOR was maintained in the IS group (P<0.05) but was absent in the FID animals (P=0.2). By contrast, choline-supplemented FID animals exhibited normal NOR performance (FID-Ch, P<0.01), but choline-supplemented IS control animals showed impaired novelty recognition memory (P>0.05) compared to the IS group.
We analyzed ten long-term dysregulated genes induced by fetal-neonatal iron deficiency (FID vs. IS, P<0.05, Table), 8 of which were recovered by prenatal choline supplementation. Postnatal choline supplementation normalized 4 of these 10 genes (Table, FID-Ch vs. IS, P>0.05). Conversely, choline supplementation dysregulated 5 of these genes in the IS-Ch group (Table, IS-Ch vs. IS, P<0.05) in the same direction as the changes induced by fetal-neonatal iron deficiency.
Table:
Pre- vs. postnatal choline supplementation effects on hippocampal gene expression.
| Gene | Prenatal choline1 (Fold of IS) | Postnatal choline (Fold of IS) | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| IS-C | FID | FID-C | IS-C | FID | FID-C | ||
|
|
|
||||||
| Cartpt 2 | 0.94 | 0.58 | 1.15 | 1.21 | 0.51* | 1.26 | |
| Drd1a 2 | 1.18 | 1.39** | 1.07 | 1.25** | 1.28*** | 1.36*** | |
| Folr1 3 | 1.07 | 2.15*** | 1.37 | 1.32* | 1.39* | 1.29* | |
| Gas5 2 | 0.35*** | 0.44** | 0.66*** | 1.06 | 0.73** | 0.90 | |
| Hcrt 2 | 0.75 | 0.44** | 1.07 | 1 69*** | 1.54* | 0.92 | |
| Kl 3 | 1.28 | 9 21*** | 1.31 | 0.89 | 1.76** | 2.32*** | |
| Mbp 2 | 0.64** | 0.61*** | 0.79 | 1.19 | 1.47*** | 1.48*** | |
| Pld5 2 | 1.39** | 2.35*** | 1.11 | 2.06*** | 2.02*** | 0.98 | |
| Pmf1 2 | 0.61*** | 0.62*** | 0.74** | 1.45*** | 1.34* | 1.52** | |
| Ttr 3 | 1.09 | 60.1*** | 0.78 | 0.71 | 2.43** | 3.65** | |
Prenatal choline data adapted with permission from Tran et al., 2016. Values are averages, n=5 for Real-time PCR. Note that for most genes, the lack of a significant effect in the FID-C group indicates recovery due to choline supplementation.
Different from IS, P<0.05;
Different from IS, P<0.01,
Different from IS, P<0.001. Abbreviations: Formerly iron-deficient (FID), FID-choline (FID-C), iron sufficient (IS), IS choline (IS-C).
Prenatal choline expression data generated using
Real-time PCR or
RNA-seq.
Discussion:
Postnatal choline supplementation restored recognition memory and partially normalized hippocampal gene expression following early-life iron deficiency. These data suggest that like prenatal choline supplementation [3], postnatal choline has the potential to reduce the long-term cognitive deficits caused by fetal-neonatal iron deficiency evidenced by the improved NOR performance at the 6-h delay. Consistent with previous findings [3], FID rats performed on par with the IS control group at a short delay time (i.e. 1-h delay) but showed a deficit at a longer delay time (6-h delay). These results parallel the recognition memory impairments observed in human adult subjects who were iron-deficient in early life [2]. The data also support mounting evidence that cognitive deficits observed in FID animals are task-dependent and may only be evident in more difficult memory tasks [6]. The beneficial effects of postnatal choline on cognitive function in FID rats are consistent with the effects of the same treatment, administered during a similar treatment window, on cognitive function following other early-life insults in the same species [12, 13].
Concomitant with memory impairment, the long-term dysregulated hippocampal genes induced by fetal-neonatal iron deficiency [7, 9, 19] can be partly restored by choline supplementation. Postnatal choline supplementation normalized 4, whereas prenatal choline supplementation normalized 8 of the 10 analyzed genes (Table) [19]. The greater extent of recovery following prenatal choline suggests that although postnatal choline can be beneficial in restoring normal hippocampal gene expression, supplementation during the prenatal sensitive period is likely more effective. The findings are consistent with previous studies that demonstrate a more robust and long-lasting improvement on a memory task in rats supplemented with choline during prenatal compared to postnatal periods [12, 13]. The molecular basis for different genomic outcomes between pre- and postnatal choline supplementation may depend on how much of the supplementation period overlaps with the critical window(s) of hippocampal development, when choline can exert its beneficial effects on neural differentiation and maturation [22]. Clearly, additional studies are needed to investigate the relative strength of pre- versus postnatal choline supplementation, as well as the underlying molecular mechanism by which choline restores normal cognition and hippocampal gene expression in FID animals.
A disparity between pre- and postnatal choline supplementation was also evident for their effects on always-IS individuals. Postnatal choline supplementation in the IS control group improved NOR memory at the 1-h delay, but impaired NOR performance at a more challenging 6-h delay, suggesting differential effects on memory encoding and retrieval processes. Both pre- and postnatal choline supplementation also dysregulated approximately half of the genes analyzed in IS-Ch group. The pattern of gene dysregulation in IS-Ch group was remarkably similar to that induced by fetal/neonatal ID and might underlie the poorer NOR performance at the 6-h delay memory task. Thus, it is of high interest to determine whether choline and iron employ similar epigenetic mechanisms to establish the pattern of long-term gene (dys)regulation.
The negative effects associated with choline supplementation in the IS control group observed in a previous study [19] and the present study support the possibility that choline supplementation has the potential to be both beneficial or harmful depending on iron status. A similar bidirectional effect of choline on cognitive function was observed in human patients given choline acutely. While the cognitive abilities of poorer performers were enhanced by acute choline supplementation, these abilities were diminished in high performers [23]. Although choline supplementation seems broadly beneficial in rats following early-life iron deficiency or other developmental insults [13], the lack of consensus regarding the effects of choline in normal controls highlights a need for additional studies.
In summary, the present study provides evidence for a postnatal sensitive period during which choline supplementation can reduce the harmful effects of early-life iron deficiency on hippocampal function and memory. Thus, postnatal choline supplementation has the potential to be a more practical approach than prenatal supplementation as an adjunctive treatment to address the widespread and pervasive effects of early-life iron deficiency. The mixed outcomes for choline supplementation of healthy individuals suggest that the development of a therapeutic strategy involving choline can be tailored to specific conditions. For example, prenatal choline supplementation may be beneficial for pregnancies at-risk for severe maternal-fetal ID, whereas postnatal supplementation may be useful for infants diagnosed with iron deficiency anemia through routine screening. Given the wide range of foods containing high choline content and multiple therapeutic windows, choline supplementation holds promise as an adjunct treatment to mitigate the long-term effects of early-life iron deficiency.
Acknowledgements:
This work was supported by the National Institute of Health [HD29421-20]
Abbreviations:
- G
Gestational day
- ID
Iron-deficient
- IS
Iron-sufficient
- NOR
Novel Object Recognition
- P
Postnatal day
- RT-qPCR
Real-time quantitative PCR
Footnotes
Author disclosures: All authors declare no conflict of interest.
References:
- [1].Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T, Long-lasting neural and behavioral effects of iron deficiency in infancy, Nutr Rev 64(5 Pt 2) (2006) S34–43; discussion S72-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lukowski AF, Koss M, Burden MJ, Jonides J, Nelson CA, Kaciroti N, Jimenez E, Lozoff B, Iron deficiency in infancy and neurocognitive functioning at 19 years: evidence of long-term deficits in executive function and recognition memory, Nutr Neurosci 13(2) (2010) 54–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kennedy BC, Dimova JG, Siddappa AJ, Tran PV, Gewirtz JC, Georgieff MK, Prenatal choline supplementation ameliorates the long-term neurobehavioral effects of fetal-neonatal iron deficiency in rats, J Nutr 144(11) (2014) 1858–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schmidt AT, Waldow KJ, Grove WM, Salinas JA, Georgieff MK, Dissociating the long-term effects of fetal/neonatal iron deficiency on three types of learning in the rat, Behavioral neuroscience 121(3) (2007) 475–82. [DOI] [PubMed] [Google Scholar]
- [5].Felt BT, Beard JL, Schallert T, Shao J, Aldridge JW, Connor JR, Georgieff MK, Lozoff B, Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats, Behav Brain Res 171(2) (2006) 261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Carlson ES, Tkac I, Magid R, O’Connor MB, Andrews NC, Schallert T, Gunshin H, Georgieff MK, Petryk A, Iron is essential for neuron development and memory function in mouse hippocampus, J Nutr 139(4) (2009) 672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Carlson ES, Stead JD, Neal CR, Petryk A, Georgieff MK, Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus, Hippocampus 17(8) (2007) 679–91. [DOI] [PubMed] [Google Scholar]
- [8].Tran PV, Carlson ES, Fretham SJ, Georgieff MK, Early-life iron deficiency anemia alters neurotrophic factor expression and hippocampal neuron differentiation in male rats, J Nutr 138(12) (2008)2495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tran PV, Fretham SJ, Carlson ES, Georgieff MK, Long-term reduction of hippocampal brain-derived neurotrophic factor activity after fetal-neonatal iron deficiency in adult rats, Pediatr Res 65(5) (2009) 493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zeisel SH, Nutritional importance of choline for brain development, J Am Coll Nutr 23(6 Suppl) (2004) 621S–626S. [DOI] [PubMed] [Google Scholar]
- [11].Tran PV, Kennedy BC, Pisansky MT, Won K, Gewirtz JC, Simmons RA, Georgieff MK, Prenatal choline supplementation diminishes early-life iron deficiency induced reprogramming of molecular networks associated with behavioral abnormalities in the adult rat hippocampus, J Nutr 146(3) (2016) 484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Meck WH, Williams CL, Cermak JM, Blusztajn JK, Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia, Front Integr Neurosci 1 (2007) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ryan SH, Williams JK, Thomas JD, Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: effects of varying the timing of choline administration, Brain research 1237 (2008) 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wozniak JR, Fuglestad AJ, Eckerle JK, Fink BA, Hoecker HL, Boys CJ, Radke JP, Kroupina MG, Miller NC, Brearley AM, Zeisel SH, Georgieff MK, Choline supplementation in children with fetal alcohol spectrum disorders: a randomized, double-blind, placebo-controlled trial, Am J Clin Nutr 102(5) (2015) 1113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kilbride J, Baker TG, Parapia LA, Khoury SA, Shuqaidef SW, Jerwood D, Anaemia during pregnancy as a risk factor for iron-deficiency anaemia in infancy: a case-control study in Jordan, International journal of epidemiology 28(3) (1999) 461–8. [DOI] [PubMed] [Google Scholar]
- [16].Rao R, Georgieff MK, Iron in fetal and neonatal nutrition, Semin Fetal Neonatal Med 12(1) (2007) 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK, The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations, Neonatology 92(2) (2007) 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Riggins T, Miller NC, Bauer PJ, Georgieff MK, Nelson CA, Consequences of low neonatal iron status due to maternal diabetes mellitus on explicit memory performance in childhood, Developmental neuropsychology 34(6) (2009) 762–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tran PV, Kennedy BC, Pisansky MT, Won KJ, Gewirtz JC, Simmons RA, Georgieff MK, Prenatal Choline Supplementation Diminishes Early-Life Iron Deficiency-Induced Reprogramming of Molecular Networks Associated with Behavioral Abnormalities in the Adult Rat Hippocampus, J Nutr 146(3) (2016) 484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jorgenson LA, Sun M, O’Connor M, Georgieff MK, Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus, Hippocampus 15(8) (2005) 1094–102. [DOI] [PubMed] [Google Scholar]
- [21].Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK, Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus, J Nutr 133(10) (2003) 3215–21. [DOI] [PubMed] [Google Scholar]
- [22].Fretham SJ, Carlson ES, Wobken J, Tran PV, Petryk A, Georgieff MK, Temporal manipulation of transferrin-receptor-1-dependent iron uptake identifies a sensitive period in mouse hippocampal neurodevelopment, Hippocampus 22(8) (2012) 1691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Knott V, de la Salle S, Choueiry J, Impey D, Smith D, Smith M, Beaudry E, Saghir S, Ilivitsky V, Labelle A, Neurocognitive effects of acute choline supplementation in low, medium and high performer healthy volunteers, Pharmacol Biochem Behav 131 (2015) 119–29. [DOI] [PubMed] [Google Scholar]


