Abstract
Background
In response to the ongoing overdose crisis, some clinicians in Canada have started prescribing immediate release hydromorphone (IRH) as an alternative to the toxic unregulated drug supply. This practice is often referred to as safer supply. We aimed to identify and characterize patients receiving safer supply IRH and their prescribers in Ontario.
Methods
Using provincial administrative health data, we identified individuals with opioid use disorder prescribed safer supply IRH from January 2016 to March 2020 and reported the number of initiations over time. We summarized demographic, health, and medication use characteristics among patients who received safer supply IRH, and examined select clinical outcomes including retention and death. Finally, we characterized prescribers of safer supply IRH and compared frequent and infrequent prescribers.
Results
We identified 534 initiations of safer supply IRH (447 distinct individuals) from 155 prescribers. Initiations increased over time with a peak in the third quarter of 2019 (103 initiations). Patients’ median age was 42 (interquartile range [IQR] 34–50), and most were male (60.2%), urban residents, (96.2%), and in the lowest neighborhood income quintile (55.7%), with 13.9% having overdosed in the previous one year. The prevalence of HIV was 13.9%. The median duration on IRH was 272 days (IQR 30–1,244) and OAT was co-prescribed in 62.9% of courses. Death while receiving IRH or within 7 days of discontinuation was rare (≤5 courses; ≤0.94 per person-year for each).
Conclusions
Clinicians are increasingly prescribing safer supply IRH in Ontario. Patients prescribed safer supply IRH had demographic and clinical characteristics associated with high risk of death from opioid-related overdose. Short-term deaths among people receiving safer supply IRH were rare.
Keywords: Opioid-related disorders, Opioid agonist therapy, Hydromorphone, Harm reduction
INTRODUCTION
The opioid-related overdose crisis is one of the most pressing public health concerns in Canada and the United States, and the number of overdose-related deaths continues to increase (Ahmad, Rossen, & Sutton, 2020; Special Advisory Committee on The Epidemic of Opioid Overdoses, 2020). In Canada, more than 6200 opioid toxicity deaths were recorded in 2020, with Ontario, Canada’s most populous province, having the country’s largest absolute number of overdose deaths (Ontario Drug Policy Research Network, Office of the Chief Coroner for Ontario/Ontario Forensic Pathology Service, Ontario Agency for Health Protection and Promotion (Public Health Ontario), & Evaluation, 2020; Special Advisory Committee on the Epidemic of Opioid Overdoses, 2021). Throughout Canada and the United States, 73% to 85% of overdose deaths are related to fentanyl in the unregulated drug supply (BC Coroners Service, 2021; Centers for Disease Control and Prevention, 2021; Gomes et al., 2021).
Opioid agonist therapy (OAT), including methadone and buprenorphine-based medications, reduces all-cause and overdose-related mortality among patients with opioid use disorder (OUD) (Larochelle et al., 2018; Sordo et al., 2017). However, traditional oral OAT fails to benefit some people or may not be in line with their goals or preferences (British Columbia Centre on Substance Use, 2017; Canadian Association of People Who Use Drugs, 2019). For such individuals who continue to use non-prescribed opioids, the toxicity of the current unregulated drug supply and criminalization of drug use places them at ongoing risk of death, overdose, infectious diseases, violence, and incarceration (Degenhardt et al., 2011; Haber, Demirkol, Lange, & Murnion, 2009). In recognition of these factors, there have been increasing calls for a “safer opioid supply” as a harm reduction measure. Some physicians and nurse-practitioners (hereafter IRH prescribers) in Ontario have begun prescribing pharmaceutical-grade opioids – most commonly daily dispensed immediate release hydromorphone (IRH) tablets – as an off-label indication with the aim of minimizing the harms associated with the unregulated drug market (British Columbia Centre on Substance Use, 2020a; Hales et al., 2019b). A safer supply guidance document has been published in Ontario outlining recommended practice based on consensus opinion of several IRH prescribers, although it is not known how closely most prescribers adhere to these recommendations (Hales et al., 2019b). According to the guidance document, patients on IRH intended as a safer supply are prescribed several tablets of IRH each day, which is typically daily dispensed at community pharmacies for unwitnessed use. The IRH is often prescribed in combination with a daily dispensed long-acting opioid or OAT, such as methadone or slow release oral morphine, with ingestion recommended to be witnessed by pharmacy staff (Hales et al., 2019b). Implicit within this prescribing practice is the understanding that some patients will choose to inject IRH formulated as oral tablets; therefore, it is recommended that education is provided on injecting techniques and sterile injection supplies are given in order to reduce harms the risk of injection-related harms (British Columbia Centre on Substance Use, 2020b; Hales et al., 2019b). Critics have raised concerns about IRH, including diversion, infection, and opioid-related overdose (Bromley, 2020).
A few physicians and nurse practitioners in Ontario have spoken publicly about prescribing IRH for people who use unregulated opioids. These are often prescribers who work within the small number of established safer supply programs in the province, the first of which was established in 2016 and are now located in a few major Ontario cities (CBC News, 2020). In 2019, the Canadian federal government announced an increase in funding for safer alternatives to the unregulated drug supply which helped expand some of these programs (Health Canada, 2019). Safer supply prescribing – while not officially recognized by provincial opioid prescribing guidelines - has been cautiously recognized by provincial regulators as an emerging area of clinical practice (College of Physicians and Surgeons of Ontario, 2021), yet remains controversial amongst many in the field of addiction medicine. From anecdotal reports, we hypothesized that other clinicians have adopted this practice but have not publicly declared this practice because of this perceived controversy ((Hales et al., 2019a); Rai, Sereda, Hales, & Kolla, 2019). In contrast to witnessed injectable opioid agonist therapy (iOAT) with diacetylmorphine or hydromorphone, which has evidence to support its use for reduction in non-prescribed opioid use, overdose risk, and medical consequences of unsafe injection practices but is largely unavailable in Ontario due to regulatory and resource barriers, (Ferri, Davoli, & Perucci, 2011; Oviedo-Joekes et al., 2016; Strang et al., 2015) there is extremely limited evidence on the impact of and outcomes associated with unwitnessed IRH. It is currently not known how widespread the prescribing practice of daily dispensed IRH is across Ontario. It is also possible that prescribing practices differ between practitioners who prescribe IRH to a large number of patients (such as within safer supply programs) relative to those who do so less frequently. As a first step toward understanding this new approach to harm reduction, we sought to explore the implementation of daily dispensed IRH as safer supply by physicians and nurse practitioners in Ontario and to describe the characteristics of patients receiving IRH, duration of use, and associated prescriber attributes.
METHODS
Setting and design
We conducted a retrospective cohort study of patients with OUD receiving safer supply IRH between January 1, 2016 and March 31, 2020. In Ontario, all residents have access to fully publicly-funded physician and hospital services. Medication coverage is publicly-funded for Ontario residents below a certain income cut-off or over the age of 65 but is not universal.
Data sources
We used Ontario’s administrative health databases, which are held securely in linkable files without any direct personal identifiers at ICES. We used the Narcotics Monitoring System (NMS) to capture all outpatient opioid dispensing of hydromorphone and medications for OAT (methadone, buprenorphine, or slow release oral morphine products). The NMS captures all opioid prescriptions dispensed from community pharmacies, regardless of payer (i.e. public drug program, private insurance or out-of-pocket). We used the Canadian Institute for Health Information’s (CIHI) Discharge Abstract Database, the National Ambulatory Care Reporting System, and the Ontario Mental Health Reporting System to identify diagnoses and procedures during inpatient hospital admissions, emergency department visits, and mental health-related hospitalizations, respectively. We used the Ontario Health Insurance Plan (OHIP) database to identify claims for outpatient services, and the Registered Persons Database, a registry of all individuals eligible for OHIP, to identify demographic characteristics and dates of death. We used two validated databases at ICES to define patients with diagnoses of HIV and chronic obstructive pulmonary disease (COPD), which both have high sensitivity and specificity (Antoniou, Zagorski, Loutfy, Strike, & Glazier, 2011; Gershon et al., 2009). We used the Ontario Cancer Registry and the Activity Level Reporting datasets for cancer diagnoses and treatment. We obtained information regarding patient enrollment with family physicians and physician characteristics (e.g., specialty, year of graduation) using the Client Agency Program Enrolment dataset and the ICES Physician Database, respectively. Nurse practitioners are permitted to prescribe IRH in Ontario but are not captured in the ICES Physician Database. These datasets were linked using unique encoded identifiers and analyzed at ICES. ICES is an independent, non-profit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement. The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board.
Cohort definition
Our definition of a course of daily dispensed IRH was based on a safer opioid supply guidance document from Ontario and expert consultation with prescribers who provide IRH as safer supply (Hales et al., 2019b). We included hydromorphone dispensed at a total daily dose of at least 32 mg on at least two of the first three consecutive days of prescribing during the study period; the index date was the first date of a course of IRH during the study period. We allowed for one day without dispensation during the first three days based on feedback from prescribers that missed doses may occur during treatment initiation. We included patients who had previously been prescribed IRH but excluded patients initiating IRH in formulations other than 4 mg or 8 mg tablets in the first three days, as these are not commonly used as safer opioid supply. We restricted the cohort to individuals who had either a hospital or outpatient visit with a diagnostic code of OUD in the two years prior to and including the index date (Supplementary Table 1), were prescribed OAT in the four years prior to and including the index date, or had an opioid-related overdose in the two years prior to and including the index date (Supplementary Table 1). We excluded individuals who received a cancer diagnosis or treatment (Supplementary Table 1) within the 365 days prior to and including the index date to avoid including individuals prescribed IRH to treat cancer-related pain. However, in consultation with prescribers at safer opioid supply programs, we did not exclude individuals who recently accessed palliative care services because safer opioid supply has been used as a tool for linkage to care for patients that been given a palliative prognosis for conditions such as infective endocarditis or untreated HIV. Finally, we excluded non-residents of Ontario.
We defined discontinuation of a course of IRH as a gap in dispensing of 8 mg or 4 mg IRH hydromorphone tablets extending for 14 day or longer, incorporating the duration of each IRH dispense (i.e., 14 or more days between end of supply of previous IRH prescription and subsequent dispense). In this case, the end date was defined as the day on which the last dispensed IRH prescription would have ended (i.e., dispense date + days supply). Individuals could re-enter the cohort if they restarted IRH according to our definition. Thus, one individual could contribute multiple courses of therapy.
Prescriber characteristics
We characterized physicians or nurse practitioners who prescribed the index prescription of IRH by age, sex, primary medical speciality (for physicians), number of years in practice (less than 10 years versus 10 or more years), and whether they prescribed OAT during the study period. We also reported the number of index safer supply IRH prescriptions they prescribed during the study period. We categorized clinicians as infrequent (prescribed one to two safer supply IRH courses on the index date) or frequent prescribers (>2 courses).
Patient characteristics
We reported characteristics for each participant at their first index date, including sociodemographic characteristics (age, sex, rurality, neighbourhood income quintile) and whether patients were diagnosed with COPD or HIV before the index date. We described recent receipt of palliative care services (Supplementary Table 1) in the 180 days prior to the index date. We identified health service use related to substance use, including infective complications consistent with injection drug use (Supplementary Table 2), alcohol use disorder (Supplementary Table 1), and opioid-related overdose (Supplementary Table 1), in the 365 days prior to the index date. We also reported prior treatment for OUD or prescribing of either long acting or immediate release hydromorphone. Specifically, we captured dispensing of OAT in the 30, 180, or 365 days prior to the index date, and evidence of receipt of any long acting or immediate release hydromorphone (of any dose) in the 180 days prior to the index date. Additionally, we reported whether patients had been dispensed a prescription for benzodiazepines in the 30 days prior to the index date.
We described patterns of safer supply IRH prescribing (i.e. dose, duration, take-home doses, and co-prescription with long-acting opioids and benzodiazepines) and selected clinical outcomes during the observation period. For each patient, we recorded the number and rate (per person-year) of hospitalizations and deaths while receiving IRH and within 7 days of discontinuation. We also determined the number of people who had a hospitalization lasting 14 or more days while receiving IRH to assess the frequency with which an inpatient hospital stay could lead to safer supply discontinuation. We also determined the geographic location of the index prescription based on the 34 Ontario Public Health Units, which are official health agencies that provide health promotion and disease prevention to their local municipalities.
Analysis
All baseline characteristics were summarized using percentages for binary variables and medians with interquartile ranges for continuous variables. In alignment with privacy requirements, we suppressed any results with counts ≤5. We used chi-squared tests to compare proportion for binary variables and the Kruskal-Wallis test to compare medians; we used a type 1 error rate of 0.05 to define statistical significance. Finally, we used Kaplan Meier curves to examine time to discontinuation (censored on hospitalization ≥ 14 days and death) stratified by frequency of prescriber, first courses of IRH and any subsequent courses, and by calendar time (2016–2017 vs. 2018–2020). We used the log-rank test to determine any differences between strata. Analyses were performed at ICES (www.ices.on.ca) using SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, North Carolina).
RESULTS
We identified 1133 courses of IRH among 577 individuals. After applying exclusion criteria, a final sample of 534 treatment courses among 447 individuals met the cohort definition (Fig. 1). Apart from the first quarter of the study period, the quarterly number of initiations of daily dispensed IRH was fairly stable between 2016 and the third quarter of 2018 (20 initiations or less), increasing in the final quarter of 2018 (Fig. 2). This increase is seen primarily among courses prescribed by frequent prescribers as the number of courses prescribed by infrequent prescribers was fairly stable at less than 20 after the first quarter of the study. The largest number of initiations of daily dispensed IRH occurred in the third quarter of 2019, during which 103 new initiations occurred.
Figure 1.
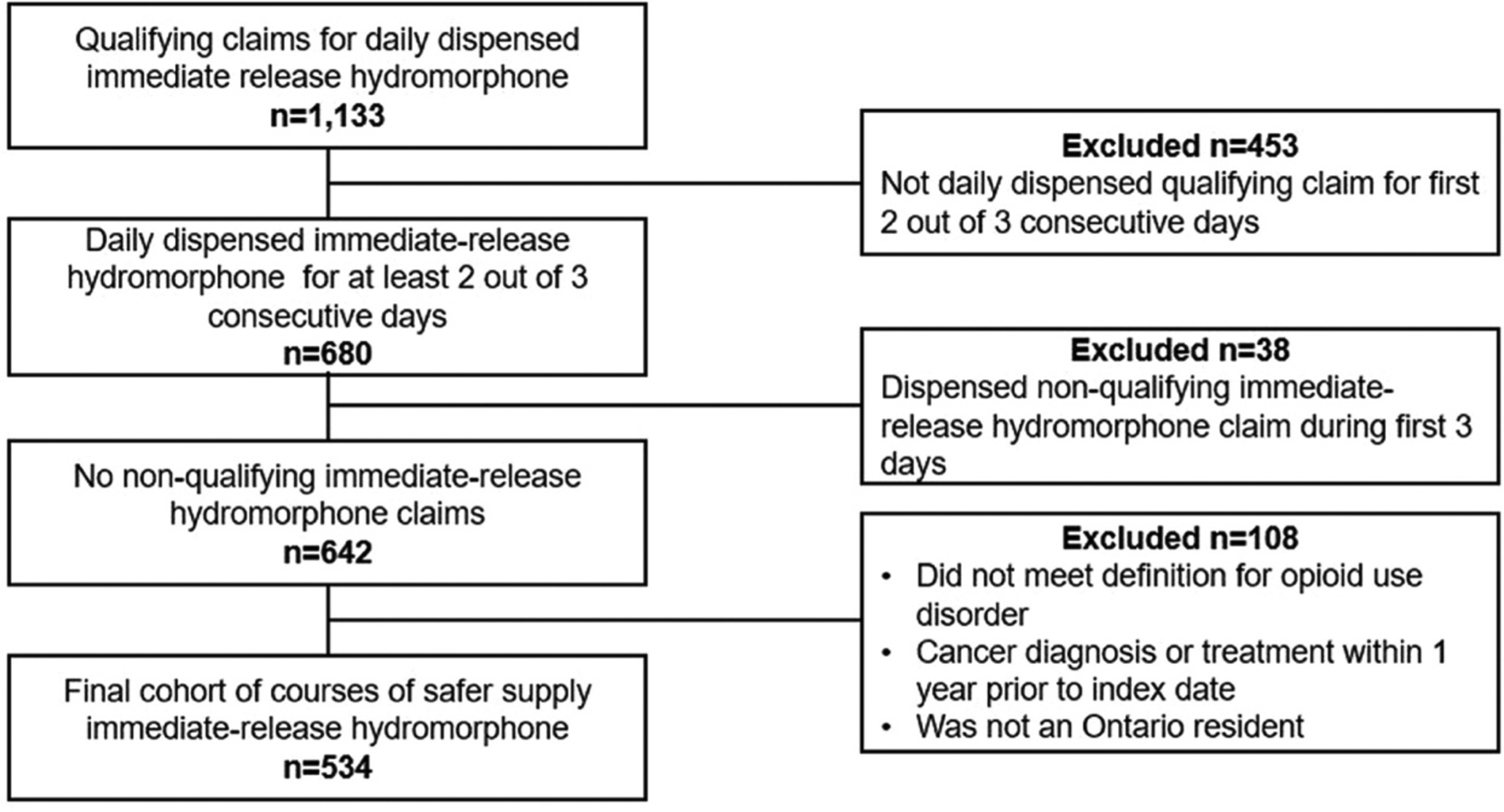
Flowchart showing the derivation of our final study cohort of 534 courses of safer supply (447 individual patients) based on inclusion and exclusion criteria.
Qualifying claim defined as 4 × 8mg or 8 8 × 4mg immediate-release hydromorphone. Non-qualifying claims include immediate-release hydromorphone other than 4mg or 8mg tablets.
Figure 2.
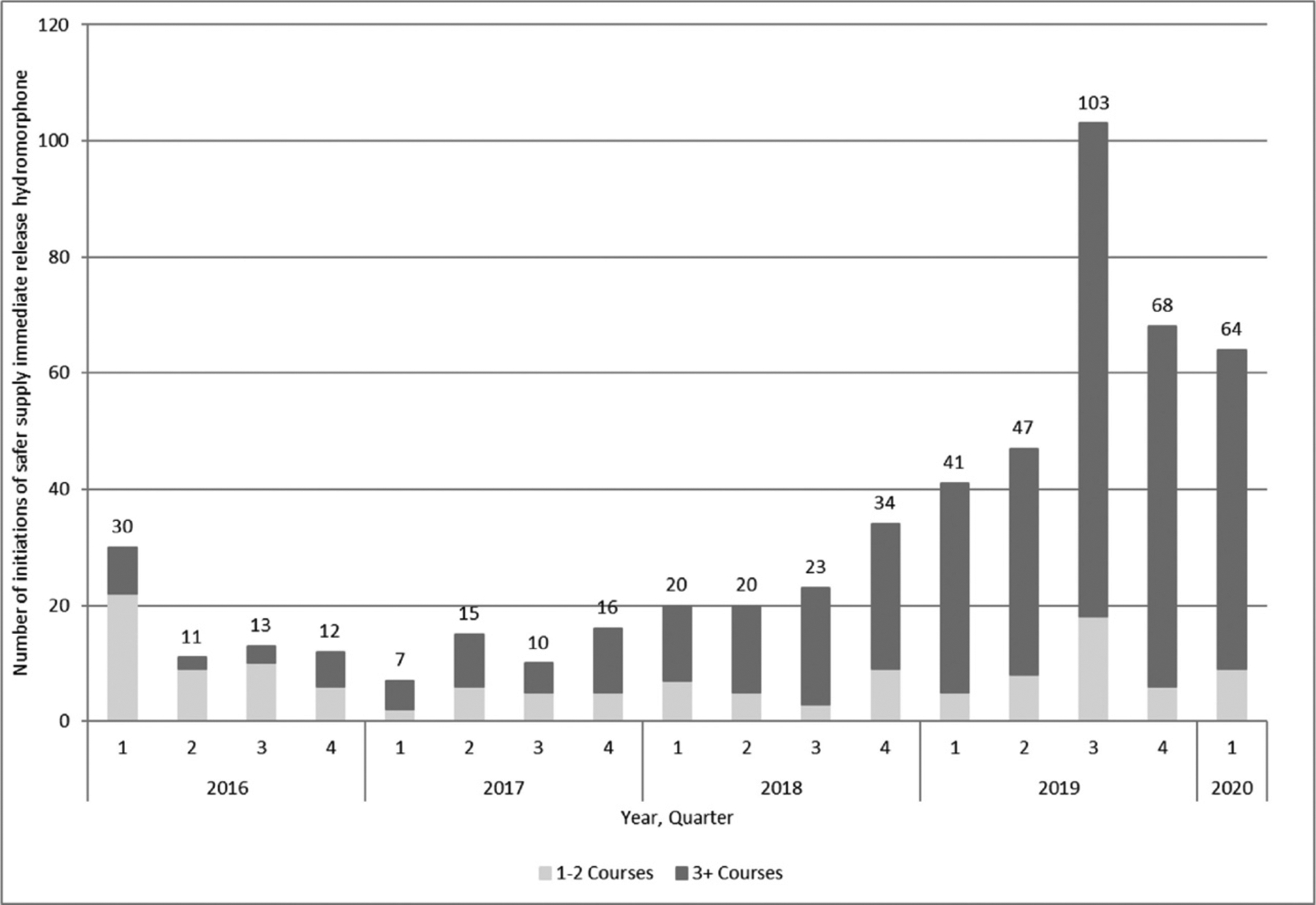
Number of initiations of safer supply immediate release hydromorphone per quarter in Ontario (n=534): January 2016 to March 2020.
A total of 155 clinicians prescribed at least one course of safer supply IRH, of which 132 could be linked to the ICES Physician Database and included in the analysis. Of those that could not be linked, 10 were nurse practitioners. A minority of prescribers (n=26; 19.7%) prescribed 3 or more index prescriptions during the study period, but accounted for 74.7% (n=399) of all index prescriptions. Overall, the median prescriber age was 50 (IQR 39–58) and 64.4% were male (Table 1). Family medicine was the most common specialty of prescribers (81.8%) and the majority (79.5%) had been in practice 10 years or longer at the time of their first index prescription during the study period. Frequent prescribers were more likely to have also prescribed OAT during the study period (n=25, 96.2%) compared to infrequent prescribers (n=77, 72.6%, p=0.01).
Table 1.
Characteristics of prescribers of at least one index prescription of safer supply immediate release hydromorphone from January 2016 to March 2020, stratified by infrequent versus frequent prescribers.
| Characteristics | All Prescribers | Infrequent Prescribers | Frequent Prescribers | P Value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| n=132 | n=106 | n=26 | ||
| Age (years) | ||||
| Median (IQR) | 50 (39–58) | 51 (40–58) | 46 (37–59) | 0.488 |
| Male Sex | 85 (64.4) | 68 (64.2) | 17 (65.4) | 0.914 |
| Main practice specialty | ||||
| Family medicine | 108 (81.8) | 86 (81.1) | 22 (84.6) | 0.455 |
| Emergency medicine | 6 (4.5) | * | * | - |
| Other | 18 (13.6) | * | * | - |
| Duration in practice | ||||
| 10 or more years | 105 (79.5) | 84 (79.2) | 21 (80.8) | 0.863 |
| Prescribed OAT during the study period | ||||
| Any | 102 (77.3) | 77 (72.6) | 25 (96.2) | 0.01 |
| Methadone | 59 (44.7) | 45 (42.5) | 14 (53.8) | 0.295 |
| Buprenorphine/naloxone | 91 (68.9) | 69 (65.1) | 22 (84.6) | 0.054 |
| Daily dispensed slow release oral morphine | 58 (43.9) | 37 (34.9) | 21 (80.8) | <0.001 |
OAT=opioid agonist therapy.
Suppressed to prevent disclosure of counts of 5 or less due to privacy requirements.
The median age of patients prescribed safer supply IRH was 42 (interquartile range [IQR] 34–50), and patients were predominantly male (60.2%), resided in urban areas (96.2%) and in neighbourhoods with the lowest income quintile (55.7%) (Table 2). Patients of frequent prescribers had a slightly higher prevalence of HIV although not statistically significant (n=50, 15.5%, p=0.11) and were more likely to have received palliative care within 180 days prior to the index date (n=49, 15.2%, p=0.03) than patients of infrequent prescribers. The prevalence of any infective complications potentially related to injection drug use in the one year preceding the index date was 41.6%, with 34.2%, 13.0%, and 4.9% of individuals having had a diagnosis of a skin and soft tissue infection, osteomyelitis or discitis, or infective endocarditis, respectively. Most patients (n=309, 69.1%) had been dispensed OAT within the year prior to cohort entry, and this was more common amongst patients of frequent prescribers (n=244, 75.5%, p<0.001). Of these, methadone was the most common form of OAT (56.2% in prior year). Recent dispensation of hydromorphone was more common among patients of infrequent prescribers, with 81.5% (n=101) having received any immediate or controlled release hydromorphone within the prior 180 days compared to 48.9% (n=158) of patients of frequent prescribers (p<0.001).
Table 2.
Baseline characteristics of patients initiating their first course of daily dispensed immediate release hydromorphone between January 2016 and March 2020, stratified by infrequent versus frequent prescribers.
| Characteristic | All Individuals | People Initiated by Infrequent Prescribers | People Initiated by Frequent Prescribers | P Value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| n=447 | n=124 | n=323 | ||
| Sociodemographic Characteristics | ||||
| Age (in years) | ||||
| Median (IQR) | 42 (34–50) | 46 (38–54) | 41 (33–49) | <0.001 |
| Male Sex | 269 (60.2) | 76 (61.3) | 193 (59.8) | 0.766 |
| Urban Residence | 430 (96.2) | 117 (94.4) | 313 (96.9) | 0.053 |
| Income Quintile | ||||
| 1 (lowest) | 249 (55.7) | 63 (50.8) | 186 (57.6) | 0.567 |
| 2 | 85 (19.0) | 27 (21.8) | 58 (18.0) | |
| 3 | 62 (13.9) | 17 (13.7) | 45 (13.9) | |
| 4 | 23 (5.1) | 9 (7.3) | 14 (4.3) | |
| 5 (highest) | 21 (4.7) | 7 (5.6) | 14 (4.3) | |
| Missing | 7 (1.6) | 1 (0.8) | 6 (1.9) | - |
| Health-related characteristics | ||||
| Has a family physician | 157 (35.1) | 66 (53.2) | 91 (28.2) | <0.001 |
| HIV seropositive prior to index date | 62 (13.9) | 12 (9.7) | 50 (15.5) | 0.112 |
| COPD diagnosis prior to index date | 91 (20.4) | 43 (34.7) | 48 (14.9) | <0.001 |
| Infective complication in prior 1 year | ||||
| Any | 186 (41.6) | 44 (35.5) | 142 (44.0) | 0.103 |
| Infective endocarditis | 22 (4.9) | 1–5 (0.8–4.0)* | 17–21 (5.3–6.5)* | 0.304 |
| Osteomyelitis or discitis | 58 (13.0) | 24 (19.4) | 34 (10.5) | 0.013 |
| SSTI | 153 (34.6) | 38 (30.6) | 115 (35.6) | 0.323 |
| Received palliative care services in prior 180 days | 58 (13.0) | 9 (7.3) | 49 (15.2) | 0.026 |
| Utilized health services for alcohol use disorder in prior 1 year | 100 (22.4) | 29 (23.4) | 71 (22.0) | 0.75 |
| Opioid-related overdose in prior 1 year | 62 (13.9) | 14 (11.3) | 48 (14.9) | 0.328 |
| ED visit or hospitalization in prior 3 years for: | ||||
| Substance-related disorder | 192 (43.0) | 47 (37.9) | 145 (44.9) | 0.181 |
| Deliberate self-harm | 87 (19.5) | 21 (16.9) | 66 (20.4) | 0.403 |
| Schizophrenia | 25 (5.6) | 6 (4.8) | 19 (5.9) | 0.667 |
| Mood disorder | 34 (7.6) | 10 (8.1) | 24 (7.4) | 0.821 |
| ED visit within 2 days prior to baseline | 28 (6.3) | 9 (7.3) | 19 (5.9) | 0.591 |
| Inpatient hospital discharge within 2 days prior to baseline | 56 (12.5) | 20 (16.1) | 36 (11.1) | 0.154 |
| Medication characteristics | ||||
| Benzodiazepines in prior 30 days | 77 (17.2) | 41 (33.1) | 36 (11.1) | <0.001 |
| Methadone | ||||
| In prior 30 days | 160 (35.8) | 44 (35.5) | 116 (35.9) | 0.932 |
| In prior 180 days | 206 (46.1) | 46 (37.1) | 160 (49.5) | 0.018 |
| In prior 1 year | 251 (56.2) | 52 (41.9) | 199 (61.6) | <0.001 |
| Buprenorphine/naloxone | ||||
| In prior 30 days | 27 (6.0) | 1–5 (0.8–4.0)* | 22–26 (6.8–8.0)* | 0.122 |
| In prior 180 days | 80 (17.6) | 13 (10.5) | 67 (20.7) | 0.011 |
| In prior 1 year | 108 (24.2) | 19 (15.3) | 89 (27.6) | 0.007 |
| Daily dispensed slow release oral morphine | ||||
| In prior 30 days | 35 (7.8) | 6 (4.8) | 29 (9.0) | 0.0145 |
| In prior 180 days | 46 (10.3) | 7 (5.6) | 39 (12.1) | 0.045 |
| In prior 1 year | 47 (10.5) | 7 (5.6) | 40 (12.4) | 0.038 |
| Any opioid agonist therapy in prior 1 year | 309 (69.1) | 65 (52.4) | 244 (75.5) | <0.001 |
| Oral hydromorphone in prior 180 days | ||||
| Any | 259 (57.9) | 101 (81.5) | 158 (48.9) | <0.001 |
| mmediate release | 251 (56.2) | 100 (80.6) | 151 (46.7) | <0.001 |
| Daily dispensed long acting | 61 (13.6) | 31 (25.0) | 30 (9.3) | <0.001 |
Infrequent prescribers were defined as prescribers of 1–2 index prescriptions of immediate release hydromorphone during the study period. Frequent prescribers were defined as prescribers of 3 or more index prescriptions of immediate release hydromorphone during the study period. IQR=interquartile range; COPD=chronic obstructive pulmonary disease; SSTI=skin or soft tissue infection. ED=emergency department. Opioid agonist therapy includes methadone, buprenorphine/naloxone, and daily dispensed slow release oral morphine.
Counts of 5 or less are censored due to privacy requirements and ranges are provided elsewhere to prevent residual disclosure of these suppressed data.
The median time to discontinuation was 272 days; however this differed when stratified by infrequent prescribers (147 days) and frequent prescribers (289 days; log-rank test p=0.011) and by calendar time (median time to discontinuation 179 days 2016–2017 vs. 309 days in 2018–2020; p=0.024) (Supplementary Figures 1 and 2). In contrast, there was no significant difference in time to discontinuation between first and subsequent courses (log-rank test p=0.21; Supplementary Figure 3). Hospitalizations for 14 or more days were uncommonly associated with treatment discontinuation, occurring among 3.2% of all courses. Deaths while receiving treatment (≤ 5 courses; ≤0.016 per person-year) or within 1 to 7 days of treatment discontinuation (≤ 5 courses; ≤ 0.94 per person-year) were rare, as were hospitalizations (0.53 per person-year while receiving treatment; 3.01 per person-year within 1–7 days of discontinuation). The median maximum dose of IRH dispensed during follow-up was 88 mg per day (IQR 48–144), with frequent prescribers having a higher median maximum of 96 mg per day (IQR 64–160) compared to infrequent prescribers at 48 mg per day (IQR 32–72, p<0.001). In 57.3% of courses, there was at least one occurrence of multi-day dispensing of IRH. Slow release oral morphine was the most common co-prescribed OAT while receiving IRH (32.8%) followed by methadone (30.3%) and buprenorphine (14.0%). In terms of geographic location, London, Ontario had the highest number of safer supply IRH initiations – almost all of which were by frequent prescribers – followed by Toronto, Ottawa, and Hamilton, (Table 3).
Table 3.
Patient outcomes while receiving safer supply immediate release hydromorphone, stratified by infrequent versus frequent prescribers.
| Outcomes | All Individuals | People Initiated by Infrequent Prescribers | People Initiated by Frequent Prescribers | P Value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| n=534 | n=135 | n=399 | ||
| Location of index prescription * | ||||
| London | 208 (39.0) | 19 (14.1) | 189 (47.4) | <0.001 |
| oronto | 93 (17.4) | 31 (23.0) | 62 (15.5) | |
| Ottawa | 76 (14.2) | 11 (8.2) | 65 (16.3) | |
| Hamilton | 24 (4.5) | 7 (5.2) | 17 (4.3) | |
| Other (total of 31 health units) | 125 (23.4) | 66 (48.8) | 59 (14.8) | |
| Missing | 8 (1.5) | 1 (0.7) | 7 (1.8) | |
| Median time to discontinuation (in days) | 272 | 147 | 289 | 0.011† |
| Maximum dose (in mg/day) of IRH | ||||
| Median (IQR) | 88 (48–144) | 48 (32–72) | 96 (64–160) | <0.001 |
| Multi-day dispensing | ||||
| Received any dispensation ≥ 1 day | 306 (57.3) | 86 (63.7) | 220 (55.1) | 0.082 |
| Maximum consecutive days dispensed, median (IQR) | 2 (1–7) | 3 (1–8) | 2 (1–4) | <0.001 |
| Co-prescribed medications ‡ | ||||
| Any opioid agonist therapy | 336 (62.9) | 59 (43.7) | 277 (69.4) | <0.001 |
| Methadone | 162 (30.3) | 49 (36.3) | 113 (28.3) | 0.081 |
| Buprenorphine | 75 (14.0) | 10 (7.4) | 65 (16.3) | 0.01 |
| Slow release oral morphine (any) | 175 (32.8) | 7 (5.2) | 168 (42.1) | <0.001 |
| Slow release oral morphine (daily dispensed) | 174 (32.6) | 6 (4.4) | 168 (42.1) | <0.001 |
| Benzodiazepine | 122 (22.8) | 55 (40.7) | 67 (16.8) | <0.001 |
| Long acting hydromorphone | 100 (18.7) | 48 (35.6) | 52 (13.0) | <0.001 |
| Hospitalized for less than 14 days | 98 (18.4) | 27 (20.0) | 71 (17.8) | 0.567 |
| Number of emergency department visits | ||||
| 0 | 268 (50.2) | 66 (48.9) | 202 (50.6) | 0.366 |
| 1 | 87 (16.3) | 18 (13.3) | 69 (17.3) | |
| 2 or more | 179 (33.5) | 51 (37.8) | 128 (32.1) | |
| Death within 7 days of discontinuation § | ≤5 (<1.8) | ≤5 (<5.4) | ≤5 (<2.7) | - |
Infrequent prescribers were defined as prescribers of 1–2 index prescriptions of immediate release hydromorphone during the study period. Frequent prescribers were defined as prescribers of 3 or more index prescriptions of immediate release hydromorphone during the study period. Opioid agonist therapy includes methadone, buprenorphine/naloxone, and daily dispensed slow release oral morphine. IRH=immediate release hydromorphone. IQR=interquartile range.
Location was determined based on Ontario Public Health Unit.
P-value for log-rank test.
At any point during safer supply immediate release hydromorphone continuous use period.
Denominator is only courses that ended in discontinuation (n=280).
DISCUSSION
In this population-based study, we found increasing use of daily dispensed IRH prescribing in Ontario, presumed to reflect safer supply, particularly after mid-2018. The largest increase in new initiations of safer supply IRH was seen in mid to late 2019, which may reflect the Government of Canada’s funding call announcement for proposals related to the provision of safer drug supplies and a call to action by safer opioid supply prescribers in the summer of that year (Health Canada, 2019; Rai et al., 2019). This increase appears to be driven primarily by a small number of frequent prescribers, which may indicate expansion of existing safer supply programs rather than adoption of safer supply prescribing by prescribers outside of established programs during the time period under study. Despite the increased prescribing, the number of individuals receiving IRH is small compared with those receiving OAT. The number of patients prescribed methadone or buprenorphine/naloxone in Ontario has risen to 66,348 in 2020, with over 11,000 new yearly users (Ontario Drug Policy Research Network, 2020), compared to an average of 125 courses of safer opioid supply annually in this study.
Three quarters of courses of safer supply IRH in our cohort were initiated by a small number of prescribers, likely practicing within established safer opioid supply programs. However, our study indicates that many more prescribers have initiated one or two courses of safer supply, often in health regions without established programs, although the number of initiations by infrequent prescribers did not increase notably over the time period studied. We suspect this reflects a cautious interest in implementing this practice in the context of the escalating overdose crisis. Policy changes such as a letter from the federal Minister of Health on safer supply (Government of Canada, 2020b) and the publication of a statement on opioid prescribing that addressed safer supply from the College of Physicians and Surgeons of Ontario occurred in 2020 (College of Physicians and Surgeons of Ontario, 2021) following the end of the period under study here. Continued monitoring of scale-up of safer supply following policy shifts is warranted, and offering education and support to prescribers may be helpful to help standardize care for patients receiving safer supply.
Additionally, local and provincial government policies in Canada may have an influence on the acceptability and feasibility of safer opioid supply prescribing across different cities and provinces (Nowell, 2021), and federal funding for these services has been largely concentrated in Ontario and British Columbia (Government of Canada, 2020a; Health Canada, 2019). In British Columbia, IRH prescribing was recently scaled up as a form of “risk mitigation prescribing” in response to the COVID-19 pandemic, and a policy directive has been released in support of such prescribing (British Columbia Centre on Substance Use, 2020a; British Columbia Ministry of Mental Health and Addictions & British Columbia Ministry of Health, 2021). While our study predates the pandemic, the number of patients we identified who were prescribed IRH in Ontario is much lower than British Columbia, where 1317 individuals were prescribed hydromorphone as an alternative to the unregulated drug supply between March 27th and August 31st, 2020 (Slaunwhite et al., 2021). This difference may relate to the lack of an official provincial guideline or directive for safer opioid prescribing in Ontario, although further research examining the effect of the pandemic on safer supply IRH prescribing in Ontario is needed.
Individuals prescribed safer supply IRH tended to be young adult males and urban residents, which parallels the characteristics of those at highest risk for overdose death in Ontario (Ontario Drug Policy Research Network et al., 2020). The baseline prevalence of HIV in our cohort was high, particularly among patients in the frequent prescribers’ group, compared with a prevalence of 0.7% among Ontario residents prescribed OAT between 2011 and 2015. (Morin et al., 2020). This may reflect the use of IRH as a tool for engagement in antiretroviral treatment for patients with untreated HIV in some safer supply programs such as the London-based program (Bonn, Felicella, Johnson, & Sereda, 2020; Nowell, 2021). In addition, most safer supply IRH recipients had been prescribed OAT in the previous year, with rates of prior year OAT exceeding 75% for patients of frequent prescribers. Taken together, our findings suggest that safer supply with IRH is being prescribed for individuals with severe OUD, at high risk of complications from unregulated opioid use, and who may have failed to adequately benefit from traditional treatment.
Frequent, compared with infrequent prescribers, prescribed higher doses of IRH, concomitant OAT more frequently (nearly 70%), and multi-day dispensing less often. Together, these findings suggest that an association between prescribing frequency and adherence to safer supply guidance, with important implications for assessing the quality of prescribing of safer supply IRH (Hales et al., 2019b). For example, long-acting opioid such as slow release oral morphine or methadone are recommended as part of safer opioid supply prescribing to prevent withdrawal and help manage cravings (Hales et al., 2019b). As well, almost all co-prescribed slow release oral morphine was dispensed daily, which is another important quality indicator given evidence of increased risk of infective endocarditis with long-acting opioid formulations (Silverman et al., 2020; Wiese et al., 2019). In our study, the median maximum daily dose of IRH dispensed (88 mg) was well within the recommended maximum of 192 mg (24 × 8 mg tablets) per day in the Ontario safer opioid supply guidance document, although frequent prescribers appear more comfortable prescribing higher doses than infrequent prescribers with a median maximum dose of 96 mg compared to 48 mg, respectively.
Retention in safer supply IRH at one year was similar to retention in methadone in Ontario (range 39.3–48.9%) (Eibl et al., 2015), although individuals prescribed safer supply may be more likely to have previously tried traditional OAT without benefit. Retention rates for OAT are highly variable and range from 37–91% at 12 months based on a systematic review of randomized controlled trials (Timko, Schultz, Cucciare, Vittorio, & Garrison-Diehn, 2016). An important area of future study is whether safer supply IRH offers an advantage for retention compared with traditional OAT alone among certain individuals, as has been shown for iOAT (Ferri et al., 2011; Strang et al., 2015).
Five or less people died with a rate of less than one death per person-year of follow up. Similar findings were noted in British Columbia, where fewer than 0.4% of patients died while receiving safer supply prescribing (including 1317 individuals prescribed IRH) (Slaunwhite et al., 2021). A recent study in British Columbia found the crude mortality rate while on OAT was 0.0109 per person-year compared to 0.0243 per person-year among those not on OAT (Pearce et al., 2020), and a previous international systematic review found a mortality rate of 0.0235 per person-year for people who inject drugs. (Mathers et al., 2013) The small number of deaths in our study requiring suppression of results and relatively short follow-up period make comparison to this and other published mortality literature difficult but is an important area for future research. The rate of hospitalizations while receiving safer supply was just over 0.5 per person-year, which is higher than the rate seen in a previously published retrospective cohort study of people who use drugs in Ontario, where the rate of hospitalization per person-year was 0.182 for men and 0.309 for women (Kendall et al., 2017). However, comparisons to earlier data should be approached with caution; the saturation of fentanyl in the unregulated drug supply has been identified as a major factor that is likely responsible for increasing prevalence of health conditions among people who use drugs, including high rates of hospitalization due to overdose, increases in serious sequalae from non-fatal overdose and increasing rates of infectious complications (; (Gomes et al., 2021) Kitchen et al., 2021). Given high rates of HIV and risk factors for overdose in our cohort, this rate of hospitalization may reflect elevated baseline acuity leading to increased risk of hospitalization for individuals prescribed safer supply overall. Further study comparing individuals prescribed safer supply to similar individuals not on safer supply or in the period prior to initiation would be helpful to contextualize these results.
Safer supply prescribing has generated much interest as a novel option for addressing the overdose crisis in Canada, with potential applications in other international jurisdictions grappling with increasing overdose rates or seeking to improve treatment options for people who use drugs who have not been retained with traditional OAT programs (Bonn et al., 2020; Chang, Agliata, & Guarinieri, 2020). Given the newness of the practice, data on prescribers, prescribing patterns, and characteristics of individuals receiving safer supply are lacking in the literature and necessary for informing policy in this area. While preliminary, our results provide important information on the scale of prescribing in an area of Canada with a high burden of overdose-related morbidity and mortality. Our data also suggests that safer supply prescribing is reaching a group of people who have high rates of concomitant medical conditions, as well as high rates of previous OAT treatment attempts, suggesting prescribers are reserving this intervention for individuals with more severe or longstanding opioid use disorder. IRH prescribers frequently also prescribed traditional OAT during the study period, suggesting that prescribers have experience and familiarity with the range of options available for the treatment of opioid use disorder. Our data also provides important baseline information on rates of safer supply prescribing prior to the onset of the COVID-19 pandemic and the publication of COVID-19 related guidance documents for safer supply/risk mitigation prescribing in other provinces; the impacts of the pandemic and policy shifts on prescribing patterns is an important area for future research.
Strengths and limitations
Strengths of our study include the use of population-based data to characterize safer supply IRH. However, our study has some limitations. Importantly, while our study aimed to identify individuals with OUD prescribed IRH as a safer opioid supply, it is possible that some patients were receiving IRH for another indication, such as post-operative pain, and we cannot confirm that it was intended to be used as an alternative to the unregulated drug supply. However, our requirements of daily dispensation and a starting dose of at least 32 mg of hydromorphone (using only 4 or 8 mg tablet formulations) to define IRH as safer supply prescribing are aligned with the safer opioid supply guidance. Moreover, such an approach is not typically used when prescribing IRH for pain, even in non-opioid naïve individuals. Furthermore, fewer than 2% of patients received a prescription for 14 or more take-home doses and none received 28 or more take-home doses within 30 days of the index date, providing reassurance that our definition did not misclassify patients receiving IRH for reasons other than safer supply. The percentage of individuals who recently accessed palliative care in our cohort is consistent with patient characteristics in the London, Ontario safer opioid supply program, the site of the first and largest program in Ontario (Sereda, 2021). Additionally, London had the highest number of new initiations in our cohort (N=208), followed by Toronto, Ottawa, and Hamilton, which are the other locations with known safer opioid supply programs, which supports the validity of our definition. In our effort to maximize the specificity of our definition, we may have excluded some individuals who received safer supply prescribing resulting in an underestimate the true number of courses. We could not ascertain safety of IRH as safer supply, including risk of overdose and infectious complications. However, the intent of our descriptive study was to characterize IRH use for safer supply in Ontario and explore the feasibility of using administrative data for defining this practice. Future research examining safety of IRH as safer supply is an important next step.
CONCLUSION
Safer supply IRH prescribing for patients with OUD has increased considerably since 2016 in Ontario, particularly since 2018. Individuals prescribed IRH commonly reside in urban, low-income neighbourhoods that are concentrated in health regions with known safer opioid supply programs, and the majority have previous experience with OAT. Overall, the prevalence of safer supply IRH prescribing in Ontario remains very low compared to traditional OAT, which likely reflects slow uptake in the absence of provincial guidelines and a desire for additional evidence on safety and efficacy. Although mortality in our study was reassuringly low, future research examining the effect of safer opioid supply on overdose risk, infection, and mortality are needed as calls for alternatives to the increasingly toxic unregulated drug supply continue to grow.
Supplementary Material
Highlights.
Safer supply immediate release hydromorphone prescribing is increasing in Ontario.
Patients prescribed safer supply appear to be those at high risk of overdose.
Deaths of people prescribed safer supply immediate release hydromorphone was rare.
Acknowledgments
We thank IQVIA Solutions Canada Inc. for use of their Drug Information File. This study and its data are drawn from the traditional territory and home of many diverse Indigenous people from across Turtle Island. We thank Andrea Sereda, Nanky Rai, Jess Hales, and Emmet O’Reilly for their consultation.
Funding sources
This project was supported by grant funding from the CIHR (grant # 153070). Samantha Young is supported by the University of British Columbia Clinician Investigator Program, the Canadian Institutes of Health Research Vanier Canada Graduate Scholarships and the International Collaborative Addiction Medicine Research Fellowship (NIDA grant R25-DA037756). Gillian Kolla is supported by a Canadian Network on Hepatitis C (CanHepC) Postdoctoral Fellowship and a Canadian Institutes of Health Research Banting Postdoctoral Researcher Award. Tara Gomes is supported by a Tier 2 Canada Research Chair. Ahmed Bayoumi is supported by the Fondation Baxter and Alma Ricard Chair in Inner City Health at St. Michael’s Hospital and the University of Toronto.
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). Parts of this material are based on data and information compiled and provided by CIHI, CCO, and the MOH. The analyses, conclusions, opinions, and statements reported in this article are those of the authors and do not necessarily reflect those of the funders, Cancer Care Ontario (CCO) or Canadian Institute for Health Information (CIHI). No endorsement by ICES, Ontario Health, CCO, MOHLTC, or CIHI is intended or should be inferred.
Declarations of Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Tara Gomes has received grant funding from the Ontario Ministry of Health. No other authors report conflicts of interest.
Footnotes
Ethics approval
The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board.
REFERENCES
- Ahmad FB, Rossen LM, & Sutton P (2020). Provisional drug overdose death counts. Retrieved from https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm?source=email#nature_sources_of_data.
- Antoniou T, Zagorski B, Loutfy MR, Strike C, & Glazier RH (2011). Validation of case-finding algorithms derived from administrative data for identifying adults living with human immunodeficiency virus infection. Plos One, 6 (6), e21748. 10.1371/journal.pone.0021748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BC Coroners Service. (2021). Illicit drug toxicity deaths in BC: January 1, 2011 to June 30, 2021 . Retrieved from https://www2.gov.bc.ca/assets/gov/birth-adoption-death-marriage-and-divorce/deaths/coroners-service/statistical/illicit-drug.pdf
- Bonn M, Felicella G, Johnson C, & Sereda A (2020). COVID-19, substance use, and safer supply: Clinical guidance to reduce risk of infection and overdose. Retrieved from https://www.bccsu.ca/blog/event/webinars-covid-19-substance-use-and-safer-supply/
- Bonn M, Palayew A, Bartlett S, Brothers TD, Touesnard N, & Tyndall M (2020). Addressing the syndemic of HIV, hepatitis C, overdose, and COVID-19 among people who use drugs: The potential roles for decriminalization and safe supply. Journal of Studies on Alcohol and Drugs, 81 (5), 556–560. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33028465. [PubMed] [Google Scholar]
- British Columbia Centre on Substance Use. (2017). A guideline for the clinical management of opioid use disorder. Retrieved from https://www.bccsu.ca/wp-content/uploads/2017/06/BC-OUD-Guidelines_June2017.pdf
- British Columbia Centre on Substance Use. (2020a). Risk mitigation in the context of dual public health emergencies. Retrieved from https://www.bccsu.ca/wp-content/uploads/2020/04/Risk-Mitigation-in-the-Context-of-Dual-Public-Health-Emergencies-v1.4.pdf
- British Columbia Centre on Substance Use. (2020b). Safer tablet injection. A resource for clinicians providing care to patients who may inject oral formulations Retrieved from https://www.bccsu.ca/wp-content/uploads/2020/09/Resource-Safer-Tablet-Injection.pdf
- British Columbia Ministry of Mental Health and Addictions, & British Columbia Ministry of Health. (2021). Access to prescribed safer supply in British Columbia: Policy direction. Retrieved from https://www2.gov.bc.ca/assets/gov/overdose-awareness/prescribed_safer_supply_in_bc.pdf
- Bromley LA (2020). Problems with hydromorphone prescribing as a response to the opioid crisis. Canadian Medical Association Journal, 192 (9), E219–E220. 10.1503/cmaj.74065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Association of People Who Use Drugs. (2019). SAFE SUPPLY: Concept document. Retrieved from https://static1.squarespace.com/static/5ef3cdaf47af2060a1cc594e/t/608c29e8d9137244ec7da81f/1619798507472/CAPUD+safe+supply+English+March+3+2019.pdf
- CBC News. (2020,. 21 September 2021). London-based safe opioid supply program gets $6.5M boost from the feds. Centers for Disease Control and Prevention. (2021). Drug overdose deaths remain high. Retrieved from https://www.cdc.gov/drugoverdose/deaths/index.html [Google Scholar]
- Chang J, Agliata J, & Guarinieri M (2020). COVID-19 - Enacting a “new normal” for people who use drugs. International Journal of Drug Policy, 83, Article 102832. 10.1016/j.drugpo.2020.102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- College of Physicians and Surgeons of Ontario. (2021). Advice to the profession: Prescribing drugs. Retrieved from https://www.cpso.on.ca/Physicians/Policies-Guidance/Policies/Prescribing-Drugs/Advice-to-the-Profession-Prescribing-Drugs [Google Scholar]
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, et al. (2011). Mortality among regular or dependent users of heroin and other opioids: A systematic review and meta-analysis of cohort studies. Addiction, 106 (1), 32–51. 10.1111/j.1360-0443.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- Eibl JK, Gomes T, Martins D, Camacho X, Juurlink DN, Mamdani MM, et al. (2015). Evaluating the effectiveness of first-time methadone maintenance therapy across northern, rural, and urban regions of Ontario, Canada. Journal of Addiction Medicine, 9 (6), 440–446. 10.1097/ADM.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri M, Davoli M, & Perucci CA (2011). Heroin maintenance for chronic heroin-dependent individuals. Cochrane Database of Systematic Reviews (Online), (12), Article CD003410. 10.1002/14651858.CD003410.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, et al. (2009). Identifying individuals with physician diagnosed COPD in health administrative databases. Journal of Chronic Obstructive Pulmonary Disease, 6 (5), 388–394. 10.1080/15412550903140865. [DOI] [PubMed] [Google Scholar]
- Gomes T, Kitchen SA, Tailor L, Men S, Murray R, Bayoumi AM, et al. (2021). Trends in hospitalizations for serious infections among people with opioid use disorder in Ontario, Canada. Journal of Addiction Medicine. 10.1097/ADM.0000000000000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes T, Murray R, Kolla G, Leece P, Bansal S, Besharah J, et al. (2021). Changing circumstances surrounding opioid-related deaths in Ontario during the COVID-19 pandemic. Retrieved from Toronto, Ontario: [Google Scholar]
- Government of Canada. (2020a). Government of Canada highlights support for safer drug supply projects in Ontario [Press release]. Retrieved from https://www.canada.ca/en/health-canada/news/2020/09/government-of-canada-highlights-support-for-safer-drug-supply-projects-in-ontario.html
- Government of Canada. (2020b). Letter from the Minister of Health regarding treatment and safer supply. Retrieved from https://www.canada.ca/en/health-canada/services/substance-use/minister-letter-treatment-safer-supply.html
- Haber PS, Demirkol A, Lange K, & Murnion B (2009). Management of injecting drug users admitted to hospital. The Lancet, 374 (9697), 1284–1293. 10.1016/S0140-6736(09)61036-9. [DOI] [PubMed] [Google Scholar]
- Hales J, Kolla G, Man T, O’Reilly E, Rai N, & Sereda A (2019a). Safer opioid supply programs (SOS): A harm reduction informed guiding document for primary care teams. Retrieved from https://docs.google.com/document/d/e/2PACX-1vTMQEhchBfmTjeBxpDRi6w7pXE5EDuInMiKARuxBcxvFUtjPmqk8l7AFPGYvWn3hOHWkTMo8-m5QPI0/pub
- Hales J, Kolla G, Man T, O’Reilly E, Rai N, & Sereda A (2019b). Safer opioid supply programs (SOS): A harm reduction informed guiding document for primary care teams - April 2020 update. Retrieved from https://bit.ly/3dR3b8m
- Health Canada. (2019). Backgrounder: New measures to address the opioid crisis and emerging drug threats. Retrieved from https://www.canada.ca/en/health-canada/news/2019/07/backgrounder-new-measures-to-address-the-opioid-crisis-and-emerging-drug-threats.html
- Kendall CE, Boucher LM, Mark AE, Martin A, Marshall Z, Boyd R, et al. (2017). A cohort study examining emergency department visits and hospital admissions among people who use drugs in Ottawa, Canada. Harm Reduction Journal, 14 (1), 16. 10.1186/s12954-017-0143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen SA, McCormack D, Werb D, Caudarella A, Martins D, Matheson FI, et al. (2021). Trends and outcomes of serious complications associated with non-fatal opioid overdoses in Ontario, Canada. Drug and Alcohol Dependence, 225, Article 108830. 10.1016/j.drugalcdep.2021.108830. [DOI] [PubMed] [Google Scholar]
- Larochelle MR, Bernson D, Land T, Stopka TJ, Wang N, Xuan Z, et al. (2018). Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: A cohort study. Annals of Internal Medicine, 169 (3), 137–145. 10.7326/M17-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, & Hickman M (2013). Mortality among people who inject drugs: A systematic review and meta-analysis. Bulletin of the World Health Organization, 91 (2), 102–123. 10.2471/BLT.12.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin KA, Prevost CR, Eibl JK, Franklyn MT, Moise AR, & Marsh DC (2020). A retrospective cohort study evaluating correlates of deep tissue infections among patients enrolled in opioid agonist treatment using administrative data in Ontario, Canada. Plos One, 15 (4), Article e0232191. 10.1371/journal.pone.0232191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell M (2021). Safe supply: What is it and what is happening in Canada? Retrieved from https://www.catie.ca/en/pif/spring-2021/safe-supply-what-it-and-what-happening-canada
- Ontario Drug Policy Research Network, Office of the Chief Coroner for Ontario/Ontario Forensic Pathology Service, Ontario Agency for Health Protection and Promotion (Public Health Ontario), & Evaluation, C. o. D. P. (2020). Preliminary patterns in circumstances surrounding opioid-related deaths in Ontario during the COVID-19 pan-demic. Retrieved from Toronto, Ontario: [Google Scholar]
- Ontario Drug Policy Research Network. (2020). Ontario prescription opioid tool. Retrieved from https://odprn.ca/ontario-opioid-drug-observatory/ontario-prescription-opioid-tool/ [Google Scholar]
- Oviedo-Joekes E, Guh D, Brissette S, Marchand K, MacDonald S, Lock K, et al. (2016). Hydromorphone compared with diacetylmorphine for long-term opioid dependence: A randomized clinical trial. JAMA Psychiatry, 73 (5), 447–455. 10.1001/jamapsychiatry.2016.0109. [DOI] [PubMed] [Google Scholar]
- Pearce LA, Min JE, Piske M, Zhou H, Homayra F, Slaunwhite A, et al. (2020). Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: Population based retrospective cohort study. British Medical Association, 368, m772. 10.1136/bmj.m772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai N, Sereda A, Hales J, & Kolla G (2019). Urgent call on clinicians: Prescribe alternatives to poisoned drug supply. Retrieved from https://healthydebate.ca/opinions/safer-supply-opioids [Google Scholar]
- Sereda A (2021). [Personal communication].
- Silverman M, Slater J, Jandoc R, Koivu S, Garg AX, & Weir MA (2020). Hydromorphone and the risk of infective endocarditis among people who inject drugs: A population-based, retrospective cohort study. The Lancet Infectious Diseases, 20 (4), 487–497. 10.1016/S1473-3099(19)30705-4. [DOI] [PubMed] [Google Scholar]
- Slaunwhite A, Palis H, Zhao B, Nosyk B, Pauly B, Urbanoski K, et al. (2021). Evaluation of the risk mitigation guidance in British Columbia - Interim Findings (Knowledge update) Retrieved from Vancouver, BC. [Google Scholar]
- Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, et al. (2017). Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. British Medical Association, 357, j1550. 10.1136/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Special Advisory Committee on The Epidemic of Opioid Overdoses. (2020). Opioids and stimulant related harms in Canada. Retrieved from https://health-infobase.canada.ca/substance-related-harms/opioids-stimulants
- Special Advisory Committee on the Epidemic of Opioid Overdoses. (2021). Opioid and stimulant-related harms in Canada. Retrieved from https://health-infobase.canada.ca/substance-related-harms/opioids-stimulants [Google Scholar]
- Strang J, Groshkova T, Uchtenhagen A, van den Brink W, Haasen C, Schechter MT, et al. (2015). Heroin on trial: Systematic review and meta-analysis of randomised trials of diamorphine-prescribing as treatment for refractory heroin addiction dagger. British Journal of Psychiatry, 207(1), 5–14. 10.1192/bjp.bp.114.149195. [DOI] [PubMed] [Google Scholar]
- Timko C, Schultz NR, Cucciare MA, Vittorio L, & Garrison-Diehn C (2016). Retention in medication-assisted treatment for opiate dependence: A systematic review. Journal of Addictive Diseases, 35(1), 22–35. 10.1080/10550887.2016.1100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese AD, Griffin MR, Schaffner W, Stein CM, Greevy RA, Mitchel EF, et al. (2019). Long-acting opioid use and the risk of serious infections: A retrospective cohort study. Clinical Infectious Diseases, 68(11), 1862–1869. 10.1093/cid/ciy809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


