Abstract
Transcription from the Pseudomonas CF600-derived ς54-dependent promoter Po is controlled by the aromatic-responsive activator DmpR. Here we examine the mechanism(s) by which integration host factor (IHF) stimulates DmpR-activated transcriptional output of the Po promoter both in vivo and in vitro. In vivo, the Po promoter exhibits characteristics that typify many ς54-dependent promoters, namely, a phasing-dependent tolerance with respect to the distance from the regulator binding sites to the distally located RNA polymerase binding site, and a strong dependence on IHF for optimal promoter output. IHF is shown to affect transcription via structural repercussions mediated through binding to a single DNA signature located between the regulator and RNA polymerase binding sites. In vitro, using DNA templates that lack the regulator binding sites and thus bypass a role of IHF in facilitating physical interaction between the regulator and the transcriptional apparatus, IHF still mediates a DNA binding-dependent stimulation of Po transcription. This stimulatory effect is shown to be independent of previously described mechanisms for the effects of IHF at ς54 promoters such as aiding binding of the regulator or recruitment of ς54-RNA polymerase via UP element-like DNA. The effect of IHF could be traced to promotion and/or stabilization of open complexes within the nucleoprotein complex that may involve an A+T-rich region of the IHF binding site and promoter-upstream DNA. Mechanistic implications are discussed in the context of a model in which IHF binding results in transduction of DNA instability from an A+T-rich region to the melt region of the promoter.
DmpR is the specific regulator of the dmp operon that encodes the enzymes for the sequential catabolism of (methyl)phenols to pyruvate and acetyl coenzyme A by Pseudomonas sp. strain CF600 (41, 44). Transcription of the dmp operon from the −24,−12 ς54-dependent Po promoter is very tightly controlled, with detectable transcription only in the presence of pathway substrates or some structural analogues (42, 43). As is typical for members of the ς54-dependent family, DmpR has a distinctive domain structure, with the amino-terminal A domain involved in signal reception, a central C domain mediating essential transcriptional activation functions, and a carboxy-terminal DNA binding D domain (see Fig. 1A and reference 27). The activities of ς54-dependent regulators are controlled by different mechanisms, including phosphorylation cascades and signal-responsive protein-protein interactions, and/or in response to small ligand effectors (40). The activity of DmpR is directly controlled by binding aromatic effectors through a single site on its amino-terminal regulatory A domain (28, 29).
FIG. 1.
Schematic illustrations. (A) Domain structure of DmpR and ΔA2*-His-DmpR. The functions of the different domains are described in the text. The hatched boxes represent the extent of the nucleoside triphosphate motif discussed by Walker et al. (49) and found in this class of regulators (G--G-GKE--A---H--S [27]). (B) Po promoter region with the locations of motifs discussed in the text shown. The nucleotide sequences in key derivatives used in this study are shown. The large inverted repeat comprising UAS1 and UAS2 is underlined and the −24,−12 promoter sequence and the transcriptional initiation start point are shown in bold italics, while the ATG initiation codon is bold and underlined. Residues altered to generate unique restriction sites are shown in bold type above the sequence. Potential IHF binding sites are compared with two different consensus signature sequences. WATCANNNNTTR (W, A or T; R, A or G) is the consensus IHF sequence from Friedman (16). The consensus sequence at-aatt--attaaAATCAA-aagTTA------a-a (with hyphens representing less conserved bases and lowercase letters representing any base) is the IHF signature identified in a comparison of 37 known IHF binding sites that can be searched using the IHF search program (http://www.bmb.psu.edu/seqscan). The arrows indicate the point defined as the center of the IHF binding motifs. In panels A and B, the numbers are residue and base pair positions, respectively.
Transcriptional activation in prokaryotes can be mediated by at least two different mechanisms: (i) direct contact between the activator and the holoenzyme RNA polymerase (Eς) and (ii) alteration of the geometry of the promoter region to modulate binding of one or more of the regulatory components involved (reviewed in references 10, 35, and 47). In the case of ς54-dependent regulators that bind DNA via sites located unusually far upstream (100 to 200 bp) of the cognate −24,−12 promoters they control, the regulators engage Eς54 via DNA looping (25). Upon ATP hydrolysis by the regulator, Eς54 closed promoter complexes are stimulated to isomerize the DNA and form open complexes that are competent to initiate transcription (1, 51). The formation of a DNA loop that assists physical proximity and thus interaction between the regulator and Eς54 can be facilitated by either a static DNA bend or binding of DNA-bending proteins (5, 7, 21). Transcriptional activity from the DmpR-Po regulatory circuit (45) and that of a number of other ς54-dependent systems (17, 21, 33) are greatly stimulated by integration host factor (IHF). The small heterodimeric IHF protein binds to specific DNA signatures and bends DNA more than 160° (16, 36). In addition to localizing the regulator and Eς54 in close proximity, the IHF-mediated DNA topology has been proposed to exert a number of other influences that contribute to both the specificity and magnitude of transcription from ς54 promoters. These include the following: (i) restricting the transcriptional activation specifically either to a single regulator bound to specific upstream sequences (9, 11, 12, 31) or to a single signal transduction pathway (50), (ii) aiding binding of the regulator (23), and (iii) recruiting Eς54 to the promoter (2).
Here we examine activation of the ς54 Po promoter by identifying the binding sites for DmpR and IHF and assessing the importance of correct phasing of these sites relative to the binding site for Eς54. To determine the role of IHF in Po transcriptional activity, we performed in vitro transcription and complex formation assays with purified components. In particular, we were interested in resolving which of the two potential IHF binding sites within the Po regulatory sequence (see Fig. 1B) was of physiological significance and, further, to test if IHF binding mediates regulation mechanisms through its effect on DNA topology in addition to playing a role in assisting the close physical proximity of DmpR and ς54-RNA polymerase at the Po promoter.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Escherichia coli strains used were DH5 (19) for construction and maintenance of plasmids, BL21(DE3)/plysS (37) for expression of ΔA2*-His-DmpR, and S90C (as well as its IHF mutant derivative DPB101) (3). Pseudomonas putida strain KT2440::dmpR (41) was constructed by insertion of a minitransposon carrying dmpR transcribed from its native promoter. An equivalent IHF mutant derivative, KT2440-ΔihfA::dmpR, harboring a deletion of ihfA (4), was constructed in an analogous manner using KT2440-ΔihfA (from Silvia Marquéz). Luria broth (38) was used for culturing E. coli strains at 37°C and P. putida strains at 30°C. Plasmids were introduced into E. coli strains by transformation (24) and into P. putida strains by either conjugation or electroporation using a Bio-Rad Gene Pulser. For selection of resident plasmids, carbenicillin was added to a concentration of 1 mg/ml for P. putida and to a concentration of 100 μg/ml for E. coli.
Plasmids and DNA manipulations.
Plasmids were constructed by using standard recombinant techniques, and the fidelity of all PCR-amplified DNA was confirmed by DNA sequence analysis. For quantification of in vivo transcription, the broad-host-range luciferase reporter plasmids pVI466 (45), pVI360 (42), and derivatives thereof were used. Both pVI466 and pVI360 carry the luxAB genes under the control of the Po promoter. Plasmid pVI466 also carries dmpR in its native configuration with respect to Po. To analyze the importance of the phasing of binding sites in the Po regulatory region, insertion derivatives of pVI360 (see Fig. 1) were constructed as follows. To introduce bases between the upstream activating sequences UAS1 and UAS2, an EcoRI site at position −152 of the Po region of pVI360 was generated, using site-directed mutagenesis, to yield pVI580. An EcoRI digest of pVI580 was then filled using Klenow fragment DNA polymerase, and the blunt ends were ligated to generate pVI581, which had a 4-bp insertion. For pVI582 (with a 10-bp insertion), pVI583 (with a 15-bp insertion), and pVI584 (with a 20-bp insertion), synthetic oligonucleotide linkers of various lengths with EcoRI-compatible ends were inserted into the EcoRI site of pVI580. Similarly, to introduce bases between UAS2 and the −24,−12 Po promoter, an NdeI site at position −90 of the Po region of pVI360 was generated to yield pVI585. Insertions of oligonucleotide linkers with NdeI-compatible ends into this site of pVI585 gave rise to pVI586 (with a 6-bp insertion), pVI587 (with a 10-bp insertion), pVI588 (with a 15-bp insertion) and pVI589 (with a 20-bp insertion). The noninserted parents bearing the unique restriction site for each series possess in vivo activities indistinguishable from those of wild-type Po reporter plasmid pVI360 (data not shown).
To analyze the physiological significance of the IHF binding motif overlapping UAS2, the UAS2 of pVI580 was modified, using PCR mutagenesis, to simultaneously destroy the IHF motif and alter the sequence to match that of the UAS2 of the Pu promoter, generating pVI590. Construction of a Po-luxAB luciferase reporter plasmid, pVI363, containing just the UAS2 of Po has been described previously (45). An equivalent plasmid, pVI591, having the UAS2 of Pu derived from pVI590, was generated in an analogous manner.
To provide templates suitable for DNA footprinting and complex formation assays, three regions of the Po promoter were PCR amplified and inserted into pBluescript SK (Stratagene). Plasmid pVI592 harbors bp −183 to +2 of the Po promoter region as an EcoRI-to-BamHI fragment, pVI593 carries the bp −224 to −85 region as an EcoRI fragment, while pVI594 carries the bp −206 to +67 region as a NotI fragment.
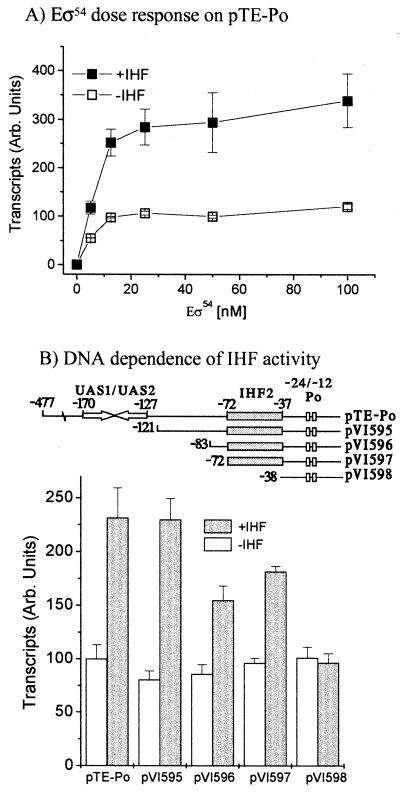
Templates for in vitro transcription assays are all based on the plasmid pTE103 (13), which carries a strong T7 transcriptional terminator downstream from a multicloning site. Plasmid pTE-Po (8) carries the bp −471 to +2 region of Po as an EcoRI-to-BamHI fragment in pTE103. Plasmids pVI595 (bp −121 to +2), pVI596 (bp −83 to +2), pVI597 (bp −72 to +2), and pVI598 (bp −38 to +2), were constructed in an analogous manner by insertion of the indicated PCR-amplified regions of Po as EcoRI-to-BamHI fragments in pTE103.
Construction of plasmid pVI453, carrying ΔA2-dmpR as an NdeI-to-BamHI fragment, with the NdeI site overlapping the ATG initiation codon, has previously been described (43). A poly(His) tag was placed in frame with the 5′ end of ΔA2-dmpR by insertions of an oligonucleotide linker that has NdeI-compatible ends but which regenerates the NdeI site only at the 5′ end when inserted in the correct orientation. The resulting derivative, pVI599, encodes a protein, ΔA2*-His-DmpR, with the amino acid sequence MRGHHHHHHVGM linked to residue L-219 and the remainder of DmpR. For purification of ΔA2*-His-DmpR, the NdeI-to-BamHI fragment of pVI599 was cloned between these sites of the T7 promoter expression plasmid pET3a (37) to generate pVI621.
In vivo luciferase assays.
To ensure balanced growth, cells were grown overnight, diluted 1:1,000, grown to mid-exponential phase, and then diluted again before initiation of the experiment by the addition of the effector 2-methylphenol to a final concentration of 2 mM. Aliquots of the culture were taken at the time points indicated in the figures, and luciferase assays were performed with 1:2,000 diluted decanal, as described previously (45).
Purified proteins.
Core RNA polymerase was purchased from Epicentre Technology and E. coli ς54 and IHF were from V. de Lorenzo and S. Goodman or purified essentially as previously described (22, 48), while P. putida IHF was provided by Frank Bartels. For purification of ΔA2*-His-DmpR, fresh transformants of BL21(DE3)/plysS harboring the T7 promoter expression plasmid pVI621 were inoculated into Luria broth supplemented with antibiotics for retention of the resident plasmids. Cultures were grown at 30°C to an optical density at 650 nm (OD650) of 0.3 and then shifted to 19°C (to aid solubility of the protein) and grown to an OD650 of 0.7. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a concentration of 0.4 mM, and growth continued for another 2 h. Cells were harvested, and the resulting pellets were stored at −80°C until used. Cells (2.5 g [wet weight]) were resuspended in 10 ml of buffer B (20 mM Na phosphate buffer [pH 7.2], 0.5 M NaCl, 0.1% ultrapure Triton X-100, 10% glycerol) containing protease inhibitor Complete-EDTA (Boehringer Mannheim) and sonicated. After centrifugation, the crude extract was filtered through a 0.45-μm-pore-size filter and loaded on a Ni-chelated column (Pierce) equilibrated with buffer B containing 5 mM imidazole. The column was then extensively washed with buffer B containing 10 to 50 mM imidazole. Column-bound proteins were eluted in 1-ml fractions using a stepwise imidazole gradient (100 mM to 1 M). Peak fractions containing ΔA2*-His-DmpR were identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis adjusted with ultra- pure Triton X-100 to 0.25% and then clarified through Micro Bio-Spin P30 Columns (Bio-Rad) equilibrated with storage buffer (20 mM Tris-HCl [pH 7.5], 0.5 M NaCl, 30% glycerol, 0.25% ultrapure Triton X-100, 1 mM EDTA, 2 mM β-mercaptoethanol). The resulting protein preparation was judged to be more than 90% pure and was stored as a 1-mg/ml solution at −80°C.
DNase I footprinting.
DNase I footprinting assays were performed on end-labeled restriction fragments of pBluescript-based plasmids pVI592 and pVI593. The restriction enzymes used for generating the fragments are as stated in the figure legends. Purified fragments were labeled by Klenow fragment DNA polymerase fill-in of 5′ overhangs with [α-32P]dCTP (for NotI sites) or [α-32P]dATP–[α-32P]dCTP (for HindIII sites) and the appropriate cold deoxynucleoside triphosphates. Unincorporated radioisotopes were removed using Micro Bio-Spin P30 columns (Bio-Rad). The labeled fragments were then diluted to a final concentration of 0.5 nM in 100 μl of a solution containing 20 mM Tris-HCl (pH 7.5), 2 mM MgCl2, 0.2 mM CaCl2, 0.1 mM EDTA, 40 mM KCl, 100 μg of bovine serum albumin (BSA) per ml, and 20 μg of salmon sperm DNA per ml. Mixtures were preincubated for 3 min at 30°C with the indicated amount of purified P. putida IHF or ΔA2*-His-DmpR and then subjected to digestion by 3 ng of DNase I for 2 min. Reactions were stopped by addition of 50 μl of a solution containing 0.1 M EDTA, 0.1% sodium dodecyl sulfate, 1.6 M ammonium acetate, and 0.2 mg of salmon sperm DNA per ml. After ethanol precipitation, nucleic acids were resuspended in 20 mM Tris-HCl (pH 7.5)–7 M urea with tracking dyes, heat denatured, and separated on a 7 M urea–7% polyacrylamide sequencing gel. A+G reactions (26) were carried out with the labeled fragments and loaded beside the corresponding reaction samples.
In vitro transcription assays.
All templates used were prepared by CsCl gradients, extensively dialyzed, and clarified through Micro Bio-Spin P30 columns (Bio-Rad) equilibrated with sterile H2O to remove trace CsCl. Single-round transcription assays were performed at 37°C essentially as described previously (9). Assays of a final volume of 50 μl were in a transcriptional buffer (50 mM Tris-HCl [pH 7.5], 50 mM KCl, 10 mM MgCl2, 1 mM dithiothreitol, 0.1 mM EDTA, 0.275 mg of BSA per ml). Different amounts of core RNA polymerase and ς54 were premixed for 5 min in buffer with ATP (final concentration, 4.25 mM) to allow holoenzyme formation. Templates, ΔA2*-His-DmpR and IHF were then added, and the incubation was continued for 20 min to allow open complex formation. In standard assays, after 10 min on ice, a single cycle of transcription was initiated by adding a mixture of ATP, GTP, and CTP (final concentration, 0.4 mM [each]), as well as UTP (final concentration, 0.06 mM), [α-32P]UTP (5 μCi at >3,000 Ci/mmol), and heparin (0.1 mg/ml, to prevent reinitiation). After an additional 10 min at 37°C, the reactions were terminated by adding an equal volume of stop mix (350 mM NaCl, 50 mM EDTA, 0.1 mg of carrier tRNA per ml, 20 μl of seeDNA [Amersham Pharmacia Biotech] per ml), and the products were precipitated with ethanol. Samples were analyzed on a 7 M urea–4% polyacrylamide sequencing gel and quantified using a Molecular Dynamics Phosphorimager.
Eς54 nucleoprotein complex assays.
Assays were performed using a linear template spanning bp −206 to +67 of Po (NotI fragment derived from pVI594) and the constitutively active DmpR derivative ΔA2*-His-DmpR by a method modified from one given in reference 14. The DNA template, radiolabeled as described under “DNase I footprinting,” was added to a final concentration of 5 nM in 15 μl of transcription buffer (see above) in the presence of 3 μg of nonspecific denatured salmon sperm DNA per ml and 0.1 mM (each) GTP and CTP required for detection of open complexes on this linear Po template. Reaction mixtures were supplemented with core RNA polymerase (20 nM), ς54 (80 nM), E. coli IHF (20 nM), ΔA2*-His-DmpR (100 nM), and the regulator nucleotide dATP (4 mM). ATP and dATP are equally efficient in serving as the regulator nucleotide in in vitro transcription (P. Wikström, E. O'Neill, L. C. Ng, and V. Shingler, unpublished data), and both generate similar complexes as shown here for dATP (data not shown). Reaction mixtures were incubated at 37°C for 15 min (or as indicated) and reactions were stopped by the addition of 3 μl of load (50% glycerol, 0.1% xylene cyanol, 0.05% bromophenol blue) for detection of all complexes or 3 μl of load containing 330 μg of heparin per ml to detect heparin-stable complexes. Samples were analyzed on a 4% polyacrylamide-bis (80:1)–25 mM Tris (pH 8.6) gel containing 2% glycerol and 0.4 M glycine, as described previously (14), and quantified using a Molecular Dynamics PhosphorImager.
RESULTS
IHF2 is the in vivo relevant site in Pseudomonas.
The transcriptional start from the Po promoter in Pseudomonas CF600 upon induction with aromatic compounds has previously been mapped by primer extension (41). As illustrated in Fig. 1B, two potential binding sites for IHF (IHF1 and IHF2) are located upstream of the start site. IHF1 was the only potential site initially identified on the basis of the consensus sequence reported by Friedman (WATCANNNNTTR, where W is A or T and R is A or G) (16). More recent searches using the consensus based on comparison of 37 known IHF binding sites (at-aatt--attaaAATCAA-aagTTA------a-a where lowercase letters represent less conserved bases and hyphens represent any base [http://www.bmb.psu.edu/seqscan]) also identified IHF2. The IHF1 site is located overlapping the promoter-proximal half of a large inverted repeat, designated UAS1-UAS2, that is essential for transcriptional activation from Po in vivo and has been presumed to be the binding site for DmpR (45). The IHF2 site is positioned between the inverted repeat and the RNA polymerase binding site and is thus in a more conventional location for IHF-stimulated ς54-dependent promoters.
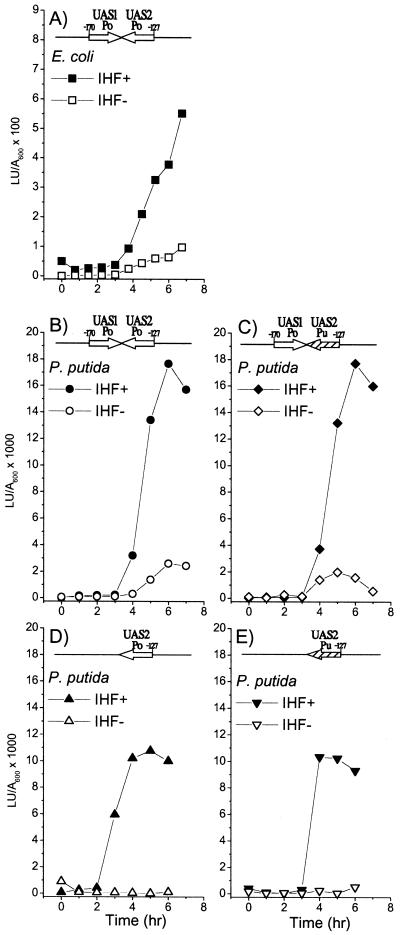
To determine the contribution of the IHF1 site to transcriptional activity from Po, we utilized the fact that DmpR and an analogous aromatic-responsive regulator, XylR, can each efficiently promote transcription from the other's cognate promoter through binding to similar UAS sequences (15, 32). Since the UAS2 equivalent of the XylR-regulated Pu promoter does not have an IHF binding signature, we could mutate UAS2 of Po to the sequence of UAS2 of Pu and thereby eliminate potential IHF recognition without abolishing DmpR-regulated transcription (pVI590 structure shown in Fig. 1B). A series of luciferase reporter plasmids in which the Po and Pu UAS2 sequences are present either in isolation or together with Po UAS1 in the native configuration relative to Po were generated. These plasmids were then introduced into isogenic IHF+ and IHF− E. coli and P. putida hosts to determine transcriptional levels from Po in vivo. Global regulation via the alarmone (p)ppGpp restricts DmpR-mediated transcription of Po to postexponential growth in rich media (46). Therefore, in the in vivo transcription experiments, the results of which are shown in Fig. 2, the peak activity observed across the growth curve is taken as the measure of Po transcriptional activity. The presence of IHF stimulates transcription from Po by four- to fivefold in both E. coli and P. putida (Fig. 2, compare panels A and B), and elimination of IHF1 by using the Pu UAS2 sequence does not affect the level of transcription (Fig. 2, compare panels B and C). As previously shown, Po UAS2 alone is sufficient to mediate trancriptional activation up to 60 to 70% of the level achieved in the presence of both UAS1 and UAS2 (Fig. 2D) (45). Pu UAS2 alone appears to be as efficient or slightly less efficient, mediating approximately 55 to 60% of the wild-type Po transcriptional activity (Fig. 2E). In both cases, the derivatives with only a UAS2 appear to be more dependent on IHF (compare IHF− levels shown in Fig. 2B to D).
FIG. 2.
In vivo effects of IHF on transcription from the Po promoter. (A) Luciferase activity of E. coli S90C (closed squares) and its IHF− counterpart, DPB101 (open squares), harboring the reporter plasmid pVI466 (dmpR-Po-luxAB). (B, C, D and E) Luciferase activity of P. putida KT2440::dmpR (closed symbols) and its IHF counterpart, KT2440-ΔihfA::dmpR (open symbols), harboring the Po-luxAB reporter plasmids pVI360 (circles), pVI590 (diamonds), pVI363 (up triangles), and pVI591 (down triangles), respectively. Schematic inserts indicate the number and derivation of the UASs on the different reporter plasmids. The results are representative of duplicate independent experiments.
IHF and DmpR footprinting.
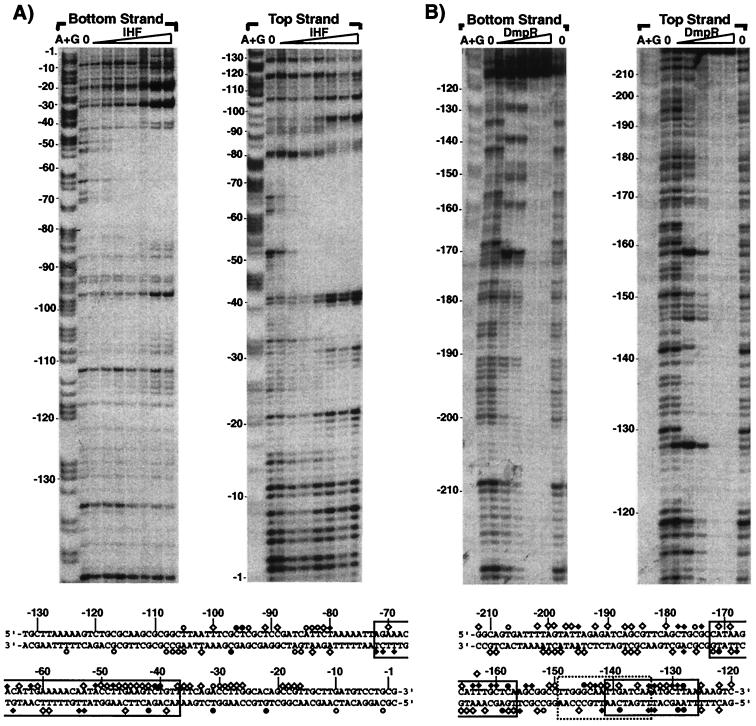
The experiments described above suggest that IHF2 functions as a binding site for IHF in vivo and that IHF1 contributes little, if any, to the IHF-mediated stimulation of transcription from Po. To address IHF binding directly, we performed in vitro DNase I footprinting with increasing concentrations of IHF. Figure 3A shows IHF binding to IHF2 which could be detected with as little as 2.5 nM IHF and was saturated by 30 nM IHF under the conditions used. The footprints totally span the IHF2 site from bp −37 to −72 of Po. The extent of IHF-mediated protection and the concentration range with which it is observed are consistent with other IHF in vitro footprints (see, e.g., references 21 and 50). In similar experiments with DNA spanning the IHF1 site, no footprint was discernible until IHF was added to concentrations that far exceed physiological levels (>150 nM [data not shown]). The results confirm the conclusion from the in vivo experiments that IHF2 is the physiologically significant site through which IHF mediates its stimulatory activity.
FIG. 3.
Interactions of IHF (A) and ΔA2*-His-DmpR (B) with the Po promoter region. The gel in panel A shows footprints made on labeled EcoRV-NotI (top strand) and HindIII-SacI (bottom strand) fragments containing the bp −183 to +2 region of the Po promoter. Lanes: A+G, Maxam and Gilbert sequencing reactions of the labeled fragments; 0, no addition of purified P. putida IHF; IHF (gradient lanes), IHF added to a final concentration of 2.5, 5, 10, 20, 30, 40 or 50 nM. Sequence coordinates are indicated at the sides. At the bottom of panel A, the sequence of the Po promoter region and positions that are in contact with IHF are shown. Diamonds indicate strong (⧫) or weak (◊) protection from DNase I digestion, while circles indicate strong (●) or weak (○) enhancement of sensitivity to DNase I. The region corresponding to the IHF binding consensus of 5′-at-aatt--attaaAATCAA-aagTTA-3′ (Fig. 1) is boxed. The gel in panel B shows footprints made on labeled HindIII-SmaI (top strand) and NotI-EcoRV (bottom strand) fragments containing the bp −224 to −85 region of the Po promoter. The lanes are as described for panel A, except that it was ΔA2*-His-DmpR, that was not added (lanes 0) or was added to a final concentration of 0.1, 0.2, 0.4, 0.6 or 0.8 μM. At the bottom of panel B, the sequence of the Po promoter region and positions in contact with ΔA2*-His-DmpR are shown, with the symbols as described for panel A. The three regions corresponding to the proposed UAS consensus sequence 5′-TTGATCAA TTGATCAA-3′ (32) are boxed, with that with highest homology boxed with a dashed line.
Figure 3 also shows the DNA footprint generated using an amino-terminal His-tagged variant of DmpR lacking its regulatory A domain, ΔA2*-His-DmpR. The truncation to remove the repressive A domain renders this derivative effector independent and constitutively active and thus bypasses the requirement for phenolic effectors in the reaction mixes (28, 43). With low concentrations of ΔA2*-His-DmpR (0.2 μM), a specific footprint from bp −127 to −172, spanning the inverted repeat region of UAS1 and UAS2 is clearly observed, confirming that the large inverted repeat is the DNA target for DmpR binding. No preferential occupancy of one UAS over the other has been observed using this assay (Fig. 3B and data not shown). At concentrations of 0.4 μM and higher, ΔA2*-His-DmpR protects DNA outside of the UAS1-UAS2 region, with concentrations of 0.6 and 0.8 μM ΔA2*-His-DmpR resulting in protection extending over the whole DNA fragment used. These findings suggest that at high concentrations in vitro, ΔA2*-His-DmpR might bind DNA nonspecifically and/or form high-order oligomers.
Relative phasing of DmpR, IHF, and Eς54 binding sites.
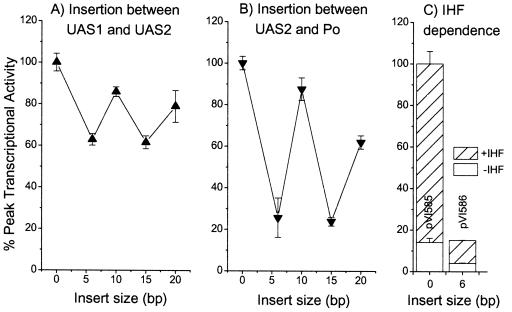
To assess the importance of the relative phasing of the identified DNA motifs for transcription from Po, we generated a series of derivatives of the Po luciferase reporter plasmid pVI360 that harbored insertions between the two UAS sequences or between UAS2 and Po. Insertions between UAS1 and UAS2 affected only the distance and helical phasing of UAS1 relative to the Po promoter, with UAS2 retaining its native location. As shown in Fig. 4A, moving UAS1 one or two helical turns further upstream from Po (10- and 20-bp insertions) had only a comparatively minor effect, and these derivatives retained more than 80% of their promoter activity. Insertion of a half helical turn or one-and-a-half helical turns (4- or 15-bp insertions) had a greater effect, resulting in about 60% of Po promoter activity (Fig. 4B). Thus, the net effect of moving UAS1 further away and altering its helical phasing relative to Po had the same outcome as a complete deletion of UAS1, i.e., reduction to approximately 60% activity. Insertions between UAS2 and the Po promoter simultaneously modulated the distance and helical phasing of both UAS1 and UAS2 relative to Po. In these cases, offsetting the relative phasing had a marked effect, reducing transcription fourfold to 25% activity, while insertion of a whole helical turn reduced promoter activity to only approximately 85% (Fig. 4B). Irrespective of the relative phasing, the activities of these derivatives were still dependent on IHF to the same degree (four- to fivefold [Fig. 4C]). These results demonstrate that the helical phasing of both UAS1 and UAS2 relative to Po are important for promoter activity. However, as with other ς54-dependent systems, there is a degree of flexibility in the location of the regulator binding sites so long as the correct phasing relative to the promoter is maintained.
FIG. 4.
In vivo effects of the relative phasing of binding motifs on transcription from the Po promoter. The luciferase activity of P. putida KT2440::dmpR harboring reporter plasmid pVI580 and derivatives pVI581 to pVI584 harboring inserts between UAS1 and UAS2 (A) or derivatives pVI585 to pVI589 harboring inserts between UASs and Po (B) and the levels obtained with the indicated derivatives in isogenic IHF+ and IHF− P. putida strains as described in the legend to Fig. 2 (C). Values are the averages of peak activities from two independent experiments where the luciferase activity was followed over the entire growth curve. Error bars, standard errors.
IHF stimulation of transcription in vitro.
The data described above clearly demonstrate that IHF has a stimulatory effect on transcription from Po that is independent of the relative phasing of the regulator and Eς54 binding sites. To dissect the mechanism underlying this IHF stimulation of Po promoter activity, we performed a series of single-round in vitro transcription assays using the constitutively active DmpR derivative ΔA2*-His-DmpR. The different supercoiled DNA templates used all originate a transcript of 311 nucleotides from Po and differ only in the extent of the Po upstream region.
In both E. coli and P. putida, IHF stimulates transcription from Po to the same extent (Fig. 2). Likewise, titration of IHF purified from E. coli or P. putida into reaction mixes that contained all the other necessary components (template pTE-Po, ΔA2*-His-DmpR, and ATP for hydrolysis by the regulator, as well as Eς54) showed similar activity curves, with IHF stimulating transcription in vitro by two- to threefold over the 10 to 30 nM range. The Po promoter is very dependent on the supercoiled nature of the DNA template, with linear templates giving >20-fold-lower transcript levels compared to a supercoiled counterpart; however, IHF still stimulates transcription two- to threefold from linear templates (data not shown).
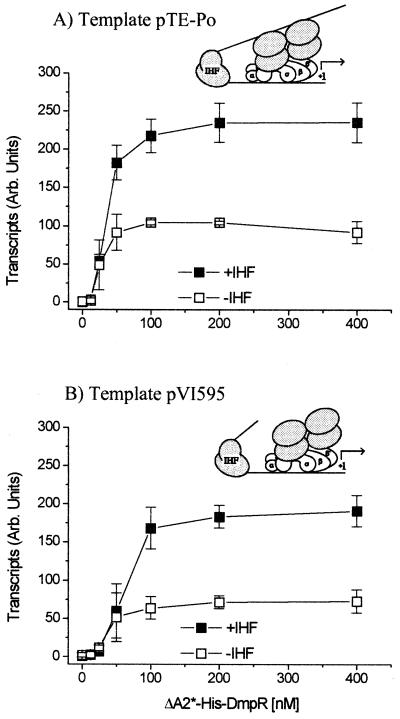
To elucidate if IHF influenced Po output by modulating binding of the regulator, as has been observed for PspF (23), we used two supercoiled DNA templates that differ in possession of the UAS1-UAS2 DmpR binding sites. A common feature of ς54-dependent regulators is that while they normally act in cis (i.e., bound to DNA), at higher concentrations they can also function in trans (i.e., from solution). As shown in Fig. 5, IHF stimulates transcription by approximately two- to three-fold irrespective of the presence or absence of binding sites for the regulator and maintains its stimulatory effect when the regulator is present at saturating concentrations. Thus, we conclude that IHF does not cause its stimulatory effect via recruitment of the regulator.
FIG. 5.
In vitro transcription from Po in response to increasing concentrations of ΔA2*-His-DmpR in the presence and absence of IHF in single-round transcription assays. (A) pTE-Po; (B) pVI595. Shown are transcript levels in the presence of 20 nM core, 80 nM ς54, 0 to 400 nM ΔA2*-His-DmpR, and either no IHF (open symbols) or 20 nM IHF (closed symbols). The inserts schematically represent the extent of the DNA in the templates used and the consequent forced activation from solution using the pVI595 template. The results are the weighted averages of triplicate independent experiments, in which the average of the plateau values for pTE-Po in the absence of IHF was set at 100. Error bars, standard errors; Arb., arbitrary.
The other major player in the regulatory circuit is the ς54 RNA polymerase itself. For XylR regulation of Pu, IHF has recently been shown to recruit Eς54 to the promoter by providing a promoter architecture that allows interaction of the α-subunit with a distally located UP-like DNA element (upstream of the IHF binding site) that is otherwise out of reach (2, 6). To test if such a mechanism could underlie the IHF stimulation of Po transcription, we determined the effect of IHF with increasing concentrations of Eς54 and on templates with different portions of the Po regulatory region upstream of the IHF2 site. A two- to threefold stimulation of transcription in the presence of IHF was observed even at saturating concentrations of Eς54 (Fig. 6A). IHF stimulation was still observed on templates (pVI596 and pVI597) that were designed to remove potential UP elements and possess only the IHF2 site and the Po promoter (Fig. 6B). The stimulatory effect of IHF on transcription from these templates is clearly mediated by binding of IHF to the IHF2 site since no effect of IHF was observed with the template that lacks this site, pVI598 (Fig. 6B). Hence, recruitment of Eς54 via an UP element does not appear to be the mechanism by which IHF exerts its effect on transcription from Po. However, the nature of the DNA upstream of the IHF2 site does appear to play some role, since IHF stimulation on the templates lacking all or part of this region (pVI596 and pVI597) is clearly less than that seen with pTE-Po or pVI595 (Fig. 6B). As expanded upon in the Discussion, both pVI596 and pVI597 lack part of an unusually A+T-rich region of the promoter upstream region.
FIG. 6.
In vitro transcription. (A) In vitro transcription from Po in the presence and absence of IHF in response to increasing concentrations of Eς54. Standard single-round transcription assays included 5 nM supercoiled template pVI595, 20 nM IHF, 100 nM ΔA2*-His-DmpR, 0 to 100 nM core with a threefold excess of ς54, and either no IHF (open symbols) or 20 nM IHF (closed symbols). The results are the weighted averages of duplicate independent experiments, in which the average of the plateau values in the absence of IHF was set at 100. (B) In vitro transcription from templates with modified Po regulatory regions as schematically indicated. Standard single-round transcription assays included a 5 nM concentration of the indicated supercoiled template, 200 nM ΔA2*-His-DmpR, 20 nM core, 80 nM ς54, and either no IHF (open bars) or 20 nM IHF (shaded bars). The results are the averages of duplicate experiments in which the average of pTE-Po in the absence of IHF is set at 100. Error bars, standard errors; Arb., arbitrary.
IHF-stimulated nucleoprotein complex formation.
The in vitro transcription experiments described above demonstrate a two- to threefold stimulation of transcription from Po through binding of IHF to the IHF2 site. This level of stimulation is lower than that seen in vivo (four- to fivefold). This observation prompted us to test if the incubation times and/or temperatures in the in vitro assay influenced the level of stimulation. In all the experiments described above, open complexes were generated at 37°C for 20 min and then placed on ice for 10 min prior to initiation of a single round of transcription. Prolonged incubation on ice (>20 min) or at 37°C (>60 min) resulted in a general decrease in transcriptional proficiency in vitro over time; however, the presence of IHF results in a three- to fourfold comparative stimulation of transcriptional output (data not shown). Since the single-round transcription assay measures the number of productive open complexes present when transcription is initiated, these findings suggest that binding of IHF at the Po promoter promotes or stabilizes open complexes.
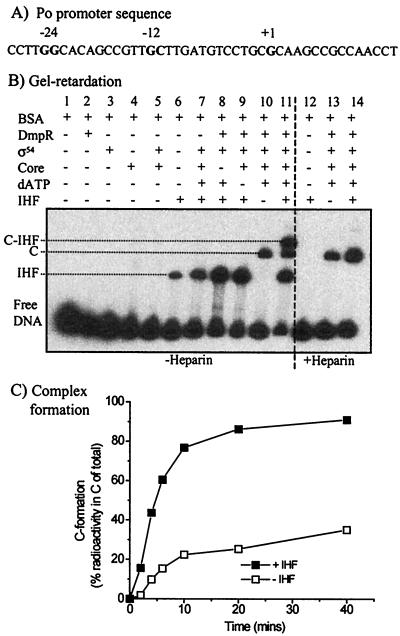
To directly assess the role of IHF on the formation of Eς54 nucleoprotein complexes, we used a radiolabeled linear DNA fragment spanning bp −206 to +67 of the Po promoter region and quantified heparin-resistant complexes by gel retardation analysis. Reactions were performed under the same buffer conditions and protein concentrations used in the in vitro transcription assays. It should be noted that open complexes could not be detected using this linear template without the stabilizing effect of the addition of the two initiating nucleotides (GTP and CTP), as has been observed with unstable Eς70 open complexes (18, 20). Since dATP used as the regulator nucleotide for hydrolysis (see Materials and Methods) can be incorporated inefficiently into transcripts, a nascent transcript of up to 14 nucleotides could be formed (see Fig. 7A). Thus, the complexes detected under the conditions used are short initiation complexes.
FIG. 7.
Complex formation. (A) Sequence of the Po promoter region, with the −24, −12, and +1 positions shown in bold. The sequence up to and including the first T(U) nucleotide downstream of the initiation site is shown; this sequence defines the maximum length of the nascent transcript under the conditions used to obtain the experimental results shown in panels B and C. (B) Complexes formed using the NotI fragment from pVI594 (bp −206 to +67) in the presence of the indicated supplements, as well as in the absence (left side) or presence (right side) of heparin. The labeled complexes are discussed in the text. (C) Effect of IHF on C formation over time. The complexes were generated under the conditions shown for panel B, lanes 13 (no IHF) and 14 (20 nM IHF). C, nucleoprotein complex; C-IHF, supershifted IHF-bound nucleoprotein complex; IHF, IHF-bound complex.
Complexes formed with the bp −206 to +67 linear template in the presence of various combinations of ΔA2*-His-DmpR, dATP, IHF, core, and ς54 are shown in Fig. 7B. The nucleoprotein complex (C) is heparin resistant (Fig. 7B, compare lanes 10 and 13), and its formation is dependent on the additions of the regulator, its cognate nucleotide for hydrolysis, and Eς54, i.e., conditions required for open complex formation and transcription (Fig. 7B, lanes 7, 8, and 9). Complexes formed with IHF alone and the supershifted IHF-bound C are heparin sensitive (Fig. 7B, compare lanes 6 and 12 to lanes 11 and 14). To determine the effect of IHF on C formation, we quantified the amount of this complex formed over time in the presence and absence of IHF. It is shown in Fig. 7C that IHF results in 3.5-fold-higher levels of C at all time points from 4 to 40 min, i.e., it promotes and/or stabilizes open initiation complex formation to the same degree as it stabilizes in vitro transcription. Taken together, the results above provide evidence for IHF-mediated effects on open transcriptional complexes that are transduced through binding at the IHF2 site.
DISCUSSION
The current dissection of the effects of IHF in vitro at the Po promoter demonstrates a new, distinct role for IHF at a ς54 promoter. In addition to having a conventional role in facilitating correctly phased close physical contact between DmpR and Eς54 (Fig. 2 and 4), IHF stimulated transcription and nucleoprotein complex formation in vitro by a mechanism that is clearly independent of UP element recruitment of Eς54 or recruitment of DmpR to the Po promoter (Fig. 5 and 6). In this respect it is interesting to note that the DmpR-regulated Po promoter appears to have a much higher affinity for Eς54 than the XylR-regulated Pu promoter in which IHF-mediated Eς54 recruitment is required for efficient promoter output. In the case of Pu, in vitro titration of Eς54 in the absence of IHF showed that this promoter was not saturated even at 400 nM (6), while Po under similar conditions was saturated by 12.5 to 25 nM Eς54 (Fig. 6). Direct comparison by gel retardation of Eς54 binding to Po and Pu in the presence and absence of IHF showed that this is indeed the case, with Eς54 binding Po with substantially higher affinity than Pu in the absence of IHF but binding both promoters with similar affinities in the presence of IHF (M. Carmona and V. de Lorenzo, personal communication).
Some, but not all, ς54-dependent promoters are greatly dependent on the supercoiled status of the template. Supercoiling dependency and torsional constraints have been proposed to provide an additional physiological checkpoint for certain ς54-dependent promoters, increasing the thermodynamic barrier to the formation of the initial open complex and contributing to the activator dependence of Eς54 transcription (see reference 34 and references therein). Binding by IHF between the regulator and Eς54, in addition to facilitating protein-protein interactions, could thus function to transduce or constrain torsional stress. In this study, for the supercoiled ς54-dependent promoter Po, the effect of IHF via provision of DNA architecture to facilitate DmpR-Eς54 interaction was dissected away from any additional role(s) in vitro by using DNA templates that lack the regulator binding sites. Under these conditions, IHF still mediates an approximately two- to threefold enhancement of transcription that is dependent on its binding to the IHF2 site (Fig. 3, 5, and 6). The results from in vitro transcription and complex formation assays (Fig. 7) provide evidence for a structural role of IHF that is dependent on IHF binding and is transduced to effectively promote and/or stabilize open complexes at Po. The properties of Po and the functional outcome of IHF binding are similar to those reported for the Eς70 promoter ilvPG (30, 39). At the ilvPG promoter, IHF activation of transcription is mediated by a supercoil-dependent DNA structural transmission mechanism involving an A+T-rich upstream region (bp −153 to −67), designated SIDD (for supercoiling-induced duplex destabilized), that overlaps the IHF binding site. IHF binding to its site between bp −95 and −80 stabilizes the SIDD, with consequent destabilization of base pairing at the −11 and −10 positions and enhanced isomerization of closed to open complexes. Conceptually, so long as transmission of the destabilizing effect is to the region of DNA unwinding, such a mechanism need not be restricted to ς70 promoters (39). The Po promoter is derived from Pseudomonas CF600, which has intrinsically G+C-rich DNA, with the structural genes it controls having a G+C content of 57.6 to 66.7% (44). Consistently, the DNA spanning the Po promoter to the transcription start (bp −27 to +1) is likewise G+C rich (61% [Fig. 1]). However, the region from bp −105 to −28, immediately upstream of Po and including the IHF2 site, is markedly A+T rich (67% A+T, 33% G+C [Fig. 1]). Thus, although more-detailed studies of the DNA structure of the Po region are required to determine the duplex status under various conditions, the structural analogies between the Po and ilvPG promoters in combination with the similar functional output of IHF binding suggest that a structural transmission mechanism may also underlie the activity of IHF at the Po promoter. However, in the case of Po, the effects of IHF are not limited to supercoiled templates and are also observed on linear templates. Thus, such a transmission mechanism would likely involve a microdomain structure (47) that on linear templates is determined and constrained by binding of DmpR to its UAS and Eς54 to the promoter. Transmission of duplex destabilization to the melt region of Po could effectively reduce the thermodynamic barrier to the formation of the initial open complex and/or could be utilized to stabilize polymerase binding within the open complex. This being the case, reduced propensity to destabilization by partial deletion of this A+T-rich region and replacement by E. coli vector DNA might underlie the mild reduction in the IHF stimulation observed with templates lacking various portions of the Po promoter upstream region (Fig. 6B).
The in vitro identification of the additional role of IHF at the Po promoter relied on circumventing the role of IHF in facilitating close physical contact between the players in the system by forcing DmpR to act from solution. We attempted to force DmpR to act from solution in intact cells either by using reporter gene constructs lacking the UASs or by overexpressing a derivative of DmpR lacking its DNA binding domain. However, in both cases transcriptional activation was too poor (less than 10% that of its wild-type counterpart) to be able to assess the role of IHF under these conditions (data not shown). Hence, the data reported here do not allow us to put a quantitative value on (i) the relative contribution of IHF in facilitating close physical contact between the players or (ii) its effect on open complex formation and stability, with regard to the overall four- to fivefold stimulatory outcome of IHF in vivo. Nevertheless, the in vitro identification of a mechanism for IHF involving open complex formation and/or stability provides an additional regulatory mechanism to the growing list of the different ways IHF can exert regulatory effects via the DNA topology at ς54 promoters.
ACKNOWLEDGMENTS
Thanks are due to many colleagues for generously providing reagents: V. de Lorenzo for ς54, S. Goodman for E. coli IHF, F. Bartels for P. putida IHF, S. Marquéz for P. putida KT2440-ΔihfA, and M. Carmona for pTE-Po. We are indebted to M. Carmona and V. de Lorenzo for sharing unpublished results.
This research was supported by grants from the Swedish Research Councils for Natural and Engineering Sciences, the Swedish Foundation for Strategic Research, and the J. C. Kempe Foundation.
REFERENCES
- 1.Austin S, Dixon R. The prokaryotic enhancer binding protein NTRC has an ATPase activity which is phosphorylation and DNA dependent. EMBO J. 1992;11:2219–2228. doi: 10.1002/j.1460-2075.1992.tb05281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertoni G, Fujita N, Ishihama A, de Lorenzo V. Active recruitment of ς54-RNA polymerase to the Pu promoter of Pseudomonas putida: role of IHF and αCTD. EMBO J. 1998;17:5120–5128. doi: 10.1093/emboj/17.17.5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biek D P, Cohen S N. Involvement of integration host factor (IHF) in maintenance of plasmid pSC101 in Escherichia coli: characterization of pSC101 mutants that replicate in the absence of IHF. J Bacteriol. 1989;171:2056–2065. doi: 10.1128/jb.171.4.2056-2065.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calb R, Davidovitch A, Koby S, Giladi H, Goldenberg D, Margalit H, Holtel A, Timmis K N, Sanchez-Romero J M, de Lorenzo V, Oppenheim A B. Structure and function of the Pseudomonas putida integration host factor. J Bacteriol. 1996;178:6319–6326. doi: 10.1128/jb.178.21.6319-6326.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmona M, Claverie-Martin F, Magasanik B. DNA bending and the initiation of transcription at ς54-dependent bacterial promoters. Proc Natl Acad Sci USA. 1997;94:9568–9572. doi: 10.1073/pnas.94.18.9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmona M, de Lorenzo V, Bertoni G. Recruitment of RNA polymerase is a rate-limiting step for the activation of the ς54 promoter Pu of Pseudomonas putida. J Biol Chem. 1999;274:33790–33794. doi: 10.1074/jbc.274.47.33790. [DOI] [PubMed] [Google Scholar]
- 7.Carmona M, Magasanik B. Activation of transcription at ς54-dependent promoters on linear templates requires intrinsic or induced bending of the DNA. J Mol Biol. 1996;261:348–356. doi: 10.1006/jmbi.1996.0468. [DOI] [PubMed] [Google Scholar]
- 8.Carmona M, Rodriguez M J, Martinez-Costa O, de Lorenzo V. In vivo and in vitro effects of (p)ppGpp on the ς54 promoter Pu of the TOL plasmid of Pseudomonas putida. J Bacteriol. 2000;182:4711–4718. doi: 10.1128/jb.182.17.4711-4718.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claverie-Martin F, Magasanik B. Positive and negative effects of DNA bending on activation of transcription from a distant site. J Mol Biol. 1992;227:996–1008. doi: 10.1016/0022-2836(92)90516-m. [DOI] [PubMed] [Google Scholar]
- 10.Dai X, Rothman-Denes L B. DNA structure and transcription. Curr Opin Microbiol. 1999;2:126–130. doi: 10.1016/S1369-5274(99)80022-8. [DOI] [PubMed] [Google Scholar]
- 11.Dworkin J, Jovanovic G, Model P. Role of upstream activation sequences and integration host factor in transcriptional activation by the constitutively active prokaryotic enhancer-binding protein PspF. J Mol Biol. 1997;273:377–388. doi: 10.1006/jmbi.1997.1317. [DOI] [PubMed] [Google Scholar]
- 12.Dworkin J, Ninfa A J, Model P. A protein-induced DNA bend increases the specificity of a prokaryotic enhancer-binding protein. Genes Dev. 1998;12:894–900. doi: 10.1101/gad.12.6.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott T, Geiduschek E P. Defining a bacteriophage T4 late promoter: absence of a “−35” region. Cell. 1984;36:211–219. doi: 10.1016/0092-8674(84)90091-6. [DOI] [PubMed] [Google Scholar]
- 14.Eydmann T, Söderbäck E, Jones T, Hill S, Austin S, Dixon R. Transcriptional activation of the nitrogenase promoter in vitro: adenosine nucleotides are required for inhibition of NIFA activity by NIFL. J Bacteriol. 1995;177:1186–1195. doi: 10.1128/jb.177.5.1186-1195.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez S, Shingler V, de Lorenzo V. Cross-regulation by XylR and DmpR activators of Pseudomonas putida suggests that transcriptional control of biodegradative operons evolves independently of catabolic genes. J Bacteriol. 1994;176:5052–5058. doi: 10.1128/jb.176.16.5052-5058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman D I. Integration host factor: a protein for all reasons. Cell. 1988;55:545–554. doi: 10.1016/0092-8674(88)90213-9. [DOI] [PubMed] [Google Scholar]
- 17.Goosen N, van de Putte P. The regulation of transcription initiation by integration host factor. Mol Microbiol. 1995;16:1–7. doi: 10.1111/j.1365-2958.1995.tb02386.x. [DOI] [PubMed] [Google Scholar]
- 18.Gourse R L. Visualization and quantitative analysis of complex formation between E. coli RNA polymerase and an rRNA promoter in vitro. Nucleic Acids Res. 1988;16:9789–9809. doi: 10.1093/nar/16.20.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanahan D. Techniques for transformation of E. coli. In: Glover D M, editor. DNA cloning. 1. A practical approach. Oxford, United Kingdom: IRL Press Ltd.; 1985. pp. 109–136. [Google Scholar]
- 20.Heinemann M, Wagner R. Guanosine 3′,5′-bis(diphosphate) (ppGpp)-dependent inhibition of transcription from stringently controlled Escherichia coli promoters can be explained by an altered initiation pathway that traps RNA polymerase. Eur J Biochem. 1997;247:990–999. doi: 10.1111/j.1432-1033.1997.00990.x. [DOI] [PubMed] [Google Scholar]
- 21.Hoover T R, Santero E, Porter S, Kustu S. The integration host factor stimulates interaction of RNA polymerase with NIFA, the transcriptional activator for nitrogen fixation operons. Cell. 1990;63:11–22. doi: 10.1016/0092-8674(90)90284-l. [DOI] [PubMed] [Google Scholar]
- 22.Hunt T P, Magasanik B. Transcription of glnA by purified Escherichia coli components: core RNA polymerase and the products of glnF, glnG, and glnL. Proc Natl Acad Sci USA. 1985;82:8453–8457. doi: 10.1073/pnas.82.24.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jovanovic G, Model P. PspF and IHF bind co-operatively in the psp promoter-regulatory region of Escherichia coli. Mol Microbiol. 1997;25:473–481. doi: 10.1046/j.1365-2958.1997.4791844.x. [DOI] [PubMed] [Google Scholar]
- 24.Kushner S R. An improved method for transformation of Escherichia coli with ColE1 derived plasmids. In: Boyer H W, Nicosia S, editors. Genetic engineering. Amsterdam, The Netherlands: Elsevier/North Holland Publishing Co.; 1978. pp. 17–23. [Google Scholar]
- 25.Kustu S, Santero E, Keener J, Popham D, Weiss D. Expression of ς54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol Rev. 1989;53:367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maxam A M, Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65:499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- 27.Morett E, Segovia L. The ς54 bacterial enhancer-binding protein family: mechanism of action and phylogenetic relationship of their functional domains. J Bacteriol. 1993;178:6067–6074. doi: 10.1128/jb.175.19.6067-6074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Neill E, Ng L C, Sze C C, Shingler V. Aromatic ligand binding and intramolecular signaling of the phenol-responsive ς54-dependent regulator DmpR. Mol Microbiol. 1998;28:131–141. doi: 10.1046/j.1365-2958.1998.00780.x. [DOI] [PubMed] [Google Scholar]
- 29.O'Neill E, Sze C C, Shingler V. Novel effector control through modulation of a preexisting binding site of the aromatic-responsive ς54-dependent regulator DmpR. J Biol Chem. 1999;274:32425–32432. doi: 10.1074/jbc.274.45.32425. [DOI] [PubMed] [Google Scholar]
- 30.Parekh B S, Sheridan S D, Hatfield G W. Effects of integration host factor and DNA supercoiling on transcription from the ilvPG promoter of Escherichia coli. J Biol Chem. 1996;271:20258–20264. doi: 10.1074/jbc.271.34.20258. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Martin J, de Lorenzo V. Integration host factor suppresses promiscuous activation of the ς54-dependent promoter Pu of Pseudomonas putida. Proc Natl Acad Sci USA. 1995;92:7277–7281. doi: 10.1073/pnas.92.16.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Martin J, de Lorenzo V. Physical and functional analysis of the prokaryotic enhancer of the ς54-promoters of the TOL plasmid of Pseudomonas putida. J Mol Biol. 1996;258:562–574. doi: 10.1006/jmbi.1996.0269. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Martin J, de Lorenzo V. Clues and consequences of DNA bending in transcription. Annu Rev Microbiol. 1997;51:593–628. doi: 10.1146/annurev.micro.51.1.593. [DOI] [PubMed] [Google Scholar]
- 34.Qureshi M, Eydmann T, Austin S, Dixon R. Torsional constraints on the formation of open promoter complexes on DNA minicircles carrying ς54-dependent promoters. Biochemistry. 1997;36:12303–12316. doi: 10.1021/bi9701179. [DOI] [PubMed] [Google Scholar]
- 35.Rhodius V A, Busby S J. Positive activation of gene expression. Curr Opin Microbiol. 1998;1:152–159. doi: 10.1016/s1369-5274(98)80005-2. [DOI] [PubMed] [Google Scholar]
- 36.Rice P A, Yang S, Mizuuchi K, Nash H A. Crystal structure of an IHF-DNA complex: a protein-induced DNA U-turn. Cell. 1996;87:1295–1306. doi: 10.1016/s0092-8674(00)81824-3. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg A H, Lade B N, Chui D-S, Lin S-W, Dunn J J, Studier F W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56:125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Sheridan S D, Benham C J, Hatfield G W. Activation of gene expression by a novel DNA structural transmission mechanism that requires supercoiling-induced DNA duplex destabilization in an upstream activating sequence. J Biol Chem. 1998;273:21298–21308. doi: 10.1074/jbc.273.33.21298. [DOI] [PubMed] [Google Scholar]
- 40.Shingler V. Signal sensing by ς54-dependent regulators: derepression as a control mechanism. Mol Microbiol. 1996;19:409–416. doi: 10.1046/j.1365-2958.1996.388920.x. [DOI] [PubMed] [Google Scholar]
- 41.Shingler V, Bartilson M, Moore T. Cloning and nucleotide sequence of the positive regulator, DmpR, of the phenol catabolic pathway of pVI150: a member of the NtrC family of transcriptional activators. J Bacteriol. 1993;175:1596–1604. doi: 10.1128/jb.175.6.1596-1604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shingler V, Moore T. Sensing of aromatic compounds by the DmpR transcriptional activator of phenol-catabolizing Pseudomonas sp. strain CF600. J Bacteriol. 1994;176:1555–1560. doi: 10.1128/jb.176.6.1555-1560.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shingler V, Pavel H. Direct activation of the ATPase activity of the transcriptional activator DmpR by aromatic compounds. Mol Microbiol. 1995;17:505–513. doi: 10.1111/j.1365-2958.1995.mmi_17030505.x. [DOI] [PubMed] [Google Scholar]
- 44.Shingler V, Powlowski J, Marklund U. Nucleotide sequence and functional analysis of the complete phenol/3,4-dimethylphenol catabolic pathway of Pseudomonas sp. strain CF600. J Bacteriol. 1992;174:711–724. doi: 10.1128/jb.174.3.711-724.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sze C C, Moore T, Shingler V. Growth-phase-dependent transcription of the ς54-dependent Po promoter controlling the Pseudomonas-derived (methyl)phenol dmp operon of pVI150. J Bacteriol. 1996;178:3727–3735. doi: 10.1128/jb.178.13.3727-3735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sze C C, Shingler V. The alarmone (p)ppGpp mediates physiological-responsive control at the ς54-dependent Po promoter. Mol Microbiol. 1999;31:1217–1228. doi: 10.1046/j.1365-2958.1999.01264.x. [DOI] [PubMed] [Google Scholar]
- 47.Travers A, Muskhelishvili G. DNA microloops and microdomains: a general mechanism for transcription activation by torsional transmission. J Mol Biol. 1998;279:1027–1043. doi: 10.1006/jmbi.1998.1834. [DOI] [PubMed] [Google Scholar]
- 48.Vorgias C E, Wilson K S. A rapid purification procedure of recombinant integration host factor from Escherichia coli. Prot Expr Purif. 1991;2:317–320. doi: 10.1016/1046-5928(91)90089-2. [DOI] [PubMed] [Google Scholar]
- 49.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wassem R, de Souza E M, Yates M G, Pedrosa F D, Buck M. Two roles for integration host factor at an enhancer-dependent nifA promoter. Mol Microbiol. 2000;35:756–764. doi: 10.1046/j.1365-2958.2000.01746.x. [DOI] [PubMed] [Google Scholar]
- 51.Weiss D S, Batut J, Klose K E, Keener J, Kustu S. The phosphorylated form of the enhancer-binding protein NTRC has an ATPase activity that is essential for activation of transcription. Cell. 1991;67:155–167. doi: 10.1016/0092-8674(91)90579-n. [DOI] [PubMed] [Google Scholar]