Abstract
Information regarding patients who were treated for breast cancer in 2018 was extracted from the National Clinical Database (NCD), which is run by Japanese physicians. This database continues from 1975, created by the Japanese Breast Cancer Society (JBCS). A total of 95,620 breast cancer cases were registered. The demographics, clinical characteristics, pathology, surgical treatment, adjuvant chemotherapy, adjuvant endocrine therapy, and radiation therapy of Japanese breast cancer patients were summarized. We made comparisons with other reports to reveal the characteristics of our database. We also described some features in Japanese breast cancer that changed over time. The unique characteristics of breast cancer patients in Japan may provide guidance for future research and improvement in healthcare services.
Keywords: Japanese Breast Cancer Society, Breast neoplasms, Registry, National Clinical Database in Japan, Annual report
Introduction
There are three breast cancer registries in Japan. The first is a national registry for which data are gathered through local governments [1]. These data are provided by all Japanese hospitals and some clinics. The second is a registry for which data are collected by cancer hospitals designated by Japanese authorities [2]. The third is a registry run by the Japanese Breast Cancer Society (JBCS), which consists of experts specializing in breast cancer. Therefore, the last registry contains a wide range of breast cancer information. This registry was integrated into the National Clinical Database (NCD) in 2012. The NCD, a Japanese online database run by medical experts, mainly contains data regarding patients who underwent surgery. In total, 1423 hospitals contribute to this database. After the integration of the JBCS database into the NCD, the number of registered breast cancer cases increased significantly. The data are used for planning healthcare services, evaluating the activity of individual surgeons, and for breast cancer research.
Several reports concerning breast cancer in Japan based on data from the JBCS or NCD databases have been published [3–5]. Herein, we provide a summary of the NCD registry data in 2018.
Patients and methods
The study participants were extracted from NCD data. The inclusion criteria were as follows: patients whose date of surgery was in 2018, and for non-surgery patients, those who began treatment in 2018.
From this database, the demographics, pathological information, and information on surgery, chemotherapy, endocrine therapy, and radiation therapy were extracted. Pathological information was treated according to Japanese and international guidelines [6, 7]. For the purpose of calculating breast cancer cases per 100,000 population, the report “Current Population Estimate as of October 1, 2018” issued by the Statistics Bureau of Japan was used [8]. Estrogen receptor (ER) and progesterone receptor (PgR) were defined as positive when they were expressed at ≥ 1% in the tumor tissue. Human epidermal growth factor receptor type 2 (HER2) positivity was defined according to the 2013 ASCO/CAP guidelines [9].
Key findings
Demographics and clinical characteristics of the patients
In 2018, 95,620 breast cancer cases were registered. According to the national registry gathered through local government, 94,519 breast cancer cases were registered [1]. It is not too much to say that almost all of the Japanese breast cancer patients were registered in the NCD database.
Among 95,620 patients with breast cancer, 94,999 patients (99.4%) were women and 621 patients (0.7%) were men. Herein, we studied 94,999 female breast cancer patients (Table 1). Male breast cancer patients were not analyzed in this report.
Table 1.
Demographics and clinical characteristics of the patients
| Number of patients | Percentage of patients (%) | ||
|---|---|---|---|
| Number of female patients | 94,999 | ||
| Laterality | Unilateral | 84,964 | 89.4 |
| Synchronous bilateral | 6265 | 6.6 | |
| Metachronous bilateral | 3770 | 4.0 | |
| Family historya | Yes | 14,776 | 15.6 |
| No | 73,235 | 77.1 | |
| Missing | 6988 | 7.4 | |
| Menopausal status | Premenopausal | 29,365 | 30.9 |
| Postmenopausal | 62,833 | 66.1 | |
| Unknownb | 2801 | 3.0 | |
| Body mass index | < 18.5 | 9131 | 9.6 |
| 18.5–24.9 | 58,609 | 61.7 | |
| 25.0–29.9 | 19,371 | 20.4 | |
| 30–34.9 | 4791 | 5.0 | |
| 35–39.9 | 1005 | 1.1 | |
| ≥ 40 | 217 | 0.2 | |
| Missing | 1875 | 2.0 | |
| Detection method | Symptoms | 48,189 | 50.7 |
| Screening with symptoms | 5688 | 6.0 | |
| Screening without symptoms | 26,793 | 28.2 | |
| Other | 13,544 | 14.3 | |
| Missing | 785 | 0.8 |
aIf a patient had first- or second-degree relatives with breast cancer, she was considered to have a family history of breast cancer
bPatients with hysterectomy were included in this category
The median age of the female patients was 65 years. It has been reported that the age distribution of Japanese breast cancer patients is biphasic [3–5]. We confirmed this pattern in the cases registered in 2018, which showed peaks at ages 45–49 years and 65–69 years (Fig. 1A). In addition to this, we created a diagram of the age distribution of the patients per 100,000 population, because Japan had birth surges after World War II (Fig. 1B). Although the distribution curve was still biphasic after adjusting for population, this adjustment reduced its biphasic character.
Fig. 1.
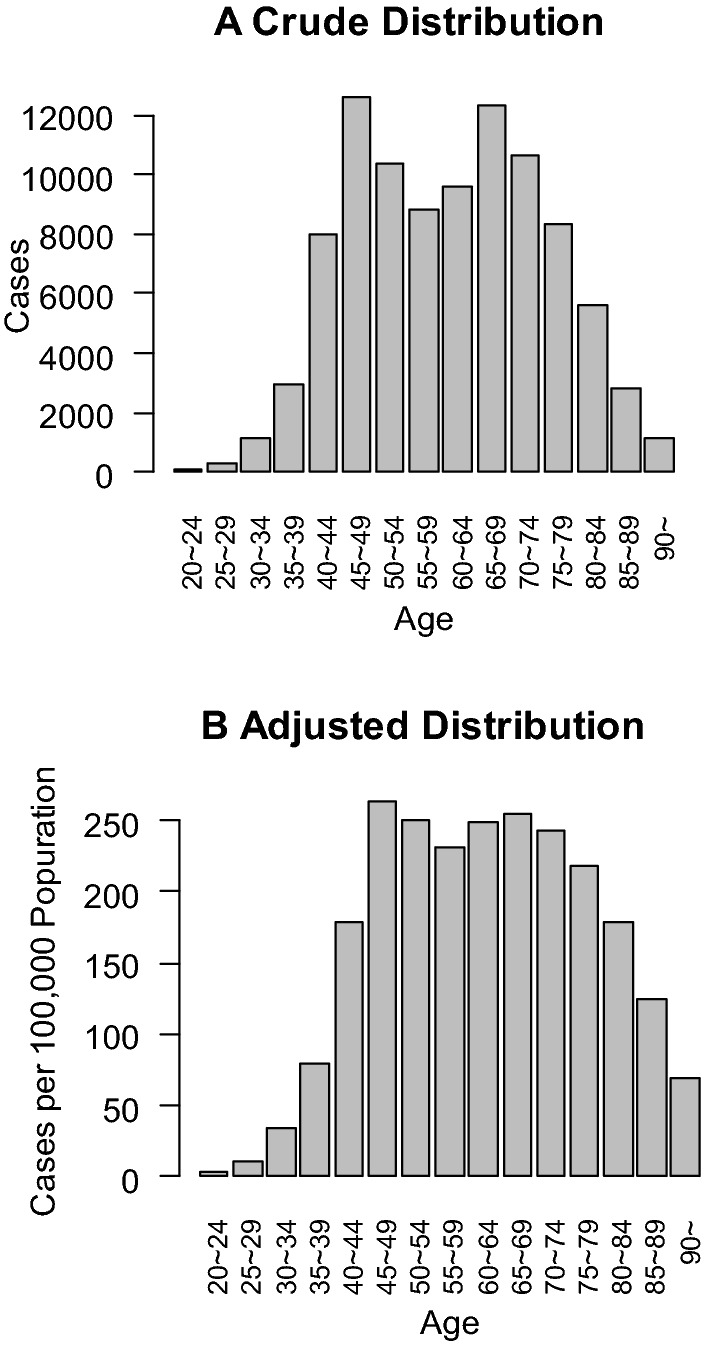
The crude age distribution and the age distribution adjusted per 100,000 population are shown in A and B, respectively. The age in this database was defined as follows. In patients with preoperative treatment, the age at which they received preoperative treatment was adopted. Those who had no surgical treatment conform with this rule. When patients underwent up-front surgery, the age at which they had surgical treatment was adopted
This biphasic distribution is not specific to Japanese breast cancer. This characteristic has also been reported in the USA and other countries [10]. When cancer develops according to the multistep theory, a log–log graph of age-specific cancer frequency versus age is linear [11]. However, the log–log graph for breast cancer shows a bend around the age of 50 years. This bend is called “Clemmesen’s hook” [12], and was reproduced in our analysis (Fig. 2). Several hypotheses have been suggested to explain Clemmesen’s hook; one is that estrogen plays a role in carcinogenesis [12]. Another is that breast cancer consists of early-onset and late-onset subtypes [13]. However, a definitive explanation remains to be found.
Fig. 2.
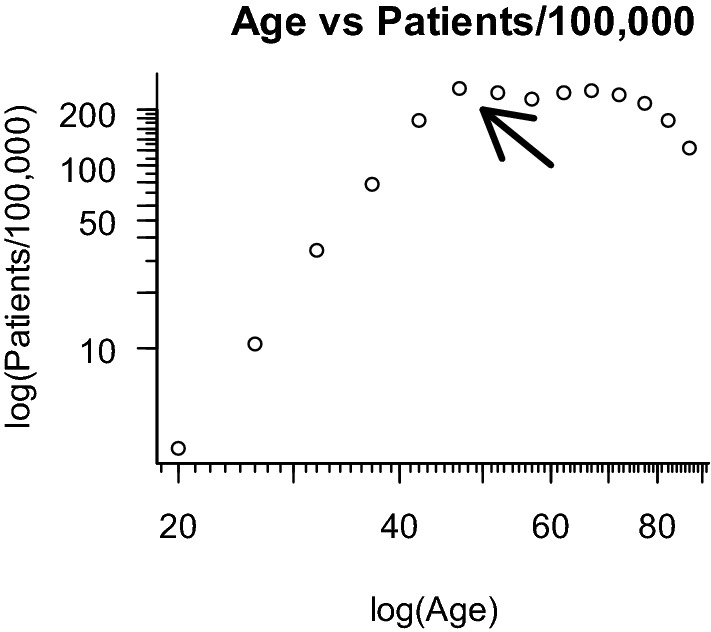
The log–log graph of age versus frequency. The arrow indicates the bend around 50 years of age, which is termed “Clemmesen’s hook”
Laterality, family history of breast cancer and menopausal status are shown in Table 1. Synchronous and metachronous bilateral breast cancer accounted for 6.6 and 4.0% of all Japanese breast cancer cases in 2018, respectively. On the other hand, synchronous bilateral breast cancer accounted for only 2.9% of breast cancer patients registered in the Surveillance Epidemiology and End Result (SEER) database in 2014 [14], and other studies report values from 1.0 to 1.5% [15–17]. Although synchronous bilateral breast cancer in Japan looks more frequent, we consider that this frequency is comparable with that in other regions. That is because our annual report defines one unilateral breast cancer as one case. Therefore, one synchronous breast cancer patient counts as two cases.
Our data showed that patients who had a first- or second-degree relative with breast cancer accounted for 15.6% of all female breast cancer patients. The same figures from other countries ranged from 10.0 to 26.7% [18–22]. Our figure was relatively low among these studies.
Breast cancer patients with BMI values of less than 18.5 accounted for 9.6% of all patients. According to the National Health and Nutrition Survey, 12.7% of Japanese women aged 20 years or older had a BMI of less than 18.5 [8]. On the other hand, 26.7% of breast cancer patients had a BMI of 25 or greater, whereas 21.9% of Japanese women in the general population had a BMI of 25 or greater. According to these data, breast cancer patients tended to weigh more than the general population. This finding does not suggest that obesity is associated with breast cancer development, because the age distribution of breast cancer patients is different from that of the general population.
Early detection of breast cancer is crucial to decrease breast cancer death. However, most breast cancers (56.7%) were detected because the patients experienced symptoms or underwent screening after experiencing symptoms. Only 28.2% of breast cancer patients were detected by screening without symptoms. Although the positive impact of breast screening is established [23], education about self-examination of the breast is also important to reduce breast cancer mortality.
The prevalence of comorbidities is shown in Table 2. No comorbidity was reported in 65.7% of patients. The most frequent comorbidity was hypertension (23.4%). Diabetes mellitus, ischemic heart disease, and heart failure were reported in 7.6, 1.9 and 0.9% of patients respectively. These findings are important when anthracyclines and trastuzumab are administered in adjuvant therapy.
Table 2.
Prevalence of comorbidities
| Number of patients | Percentage of patients (%) | |
|---|---|---|
| Hypertensiona | 22,187 | 23.4 |
| Diabetes mellitusb | 7261 | 7.6 |
| Malignant neoplastic disease other than breast cancer | 5152 | 5.4 |
| Cerebral or peripheral vascular disease | 3037 | 3.2 |
| Ischemic heart disease | 1780 | 1.9 |
| Renal dysfunctionc | 1463 | 1.5 |
| Chronic hepatitis | 1361 | 1.4 |
| Collagen disease | 1012 | 1.1 |
| Heart failure | 816 | 0.9 |
| Chronic obstructive pulmonary disease | 413 | 0.4 |
| No comorbidity | 62,406 | 65.7% |
aPatients were defined as having hypertension if they received anti-hypertension agents
bPatients were defined as having diabetes if they received insulin treatment
cPatients were defined as having renal dysfunction if their serum creatinine level was over 1.0 mg/dl or if their estimated glomerular filtration rate (eGFR) was less than 60 ml/min/1.73m2
Pathology
The pathological characteristics based on the tumor, node, metastasis (TNM) classification are summarized in Table 3. Breast cancers classified as Tis or T1 in the tumor factor, N0 in the nodal factor and stage 0 to IIB accounted for 60.6, 81.1 and 88.0%, respectively. Many newly diagnosed breast cancers were at the early stage. The distribution of estrogen receptor (ER) expression and human epidermal growth factor receptor 2 (HER2) expression is presented in Tables 4, 5 and Fig. 3. ER and HER2 were determined based on surgical material. The characterization of ER or HER2 was missing in 24.3% of patients. It is regrettable that this percentage was higher than the value of 7% observed in SEER [24]. Distribution of histological data is shown in Table 6. Histological classification is defined by the committee belonging to Japanese Breast Cancer Society [7].
Table 3.
Pathological Characteristics
| Number of patients | Percentage of patients (%) | |
|---|---|---|
| T factor | ||
| Tis | 13,600 | 14.3 |
| T1 | 43,970 | 46.3 |
| T2 | 27,484 | 28.9 |
| T3 | 2949 | 3.1 |
| T4 | 4648 | 4.9 |
| Missing | 2348 | 2.5 |
| N factor | ||
| N0 | 77,021 | 81.1 |
| N1 | 12,137 | 12.8 |
| N2 | 1972 | 2.1 |
| N3 | 1905 | 2.0 |
| Missing | 1964 | 2.1 |
| M factor | ||
| M0 | 90,699 | 95.5 |
| M1 | 2021 | 2.1 |
| Missing | 2279 | 2.4 |
| Stage | ||
| 0 | 13,515 | 14.2 |
| I | 40,661 | 42.8 |
| IIA | 22,050 | 23.2 |
| IIB | 7374 | 7.8 |
| IIIA | 2177 | 2.3 |
| IIIB | 3051 | 3.2 |
| IIIC | 1326 | 1.4 |
| IV | 2021 | 2.1 |
| Missing | 2824 | 3.0 |
Table 4.
Immunohistochemical Characteristics
| Number of patients | Percentage of patients (%) | |
|---|---|---|
| ER | ||
| Negative | 12,902 | 13.6 |
| 1–9% | 2751 | 2.9 |
| 10% or more | 65247 | 68.7 |
| Missing | 14,099 | 14.8 |
| PgR | ||
| Negative | 21,254 | 22.4 |
| 1–9% | 6456 | 67.8 |
| ≥ 10% | 53,043 | 55.8 |
| Missing | 14,246 | 15.0 |
| HER2 | ||
| Negative | 61,433 | 64.7 |
| Positive | 10,849 | 11.4 |
| Missing | 22,717 | 23.9 |
ER estrogen receptor, PgR progesterone receptor, HER2 human epidermal growth factor receptor type 2
Table 5.
HER2 Evaluation According to Immunochemistry and FISH test
| Number of patients | Percentage of patients (%) | |
|---|---|---|
| HER2 Immunochemistry | ||
| 0 | 25,462 | 26.8 |
| 1 + | 27,051 | 28.5 |
| 2 + | 14,384 | 15.1 |
| 3 + | 8770 | 9.2 |
| Missing | 19,332 | 20.3 |
| HER2 FISH in HER2 = 2 + | ||
| Positive | 2079 | 14.5 |
| Negative | 8920 | 62.0 |
| Missing | 3385 | 23.5 |
FISH Fluorescence in situ hybridization
Fig. 3.
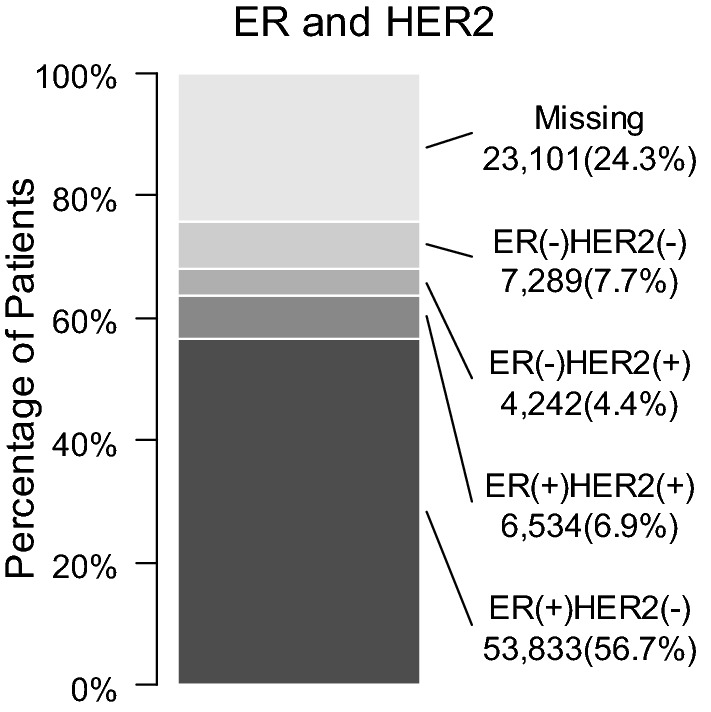
Distribution of ER and HER2. ER and HER2 denote the estrogen receptor and the human epidermal growth factor receptor type 2, respectively
Table 6.
Histology
| Number of patients | Percentage of patients (%) | |
|---|---|---|
| Epithelial tumors | ||
| Ductal carcinoma in situ | 12,903 | 14.1 |
| Lobular carcinoma in situ | 382 | 0.4 |
| IDC-Papillotubular carcinoma | 15,623 | 17.1 |
| IDC-Solid-tubular carcinoma | 12,365 | 13.5 |
| IDC-Scirrhous carcinoma | 27,985 | 30.6 |
| IDC (not sub-classified) | 6472 | 7.1 |
| Mucinous carcinoma | 3228 | 3.5 |
| Medullary carcinoma | 247 | 0.3 |
| Invasive lobular carcinoma | 3938 | 4.3 |
| Adenoid cystic carcinoma | 65 | 0.1 |
| Squamous cell carcinoma | 152 | 0.2 |
| Spindle cell carcinoma | 103 | 0.1 |
| Apocrine carcinoma | 1105 | 1.2 |
| Tubular carcinoma | 362 | 0.4 |
| Invasive micro-papillary carcinoma | 806 | 0.9 |
| Matrix-producing carcinoma | 50 | 0.1 |
| Other special subtypes | 602 | 0.7 |
| Paget’s disease | 250 | 0.3 |
| Mixed connective tissue and epithelial tumors | ||
| Malignant phyllodes tumor | 142 | 0.2 |
| Carcinosarcoma | 7 | 0.0 |
| Non-epithelial tumors | ||
| Stromal sarcoma | 26 | 0.0 |
| Other non-epithelial tumors | 45 | 0.0 |
| Unclassified tumors | 521 | 0.6 |
| Unknown | 3987 | 4.4 |
Classification was performed according to the general rules for clinical and pathological recording of breast cancer [7]
IDC Invasive ductal carcinoma
Surgery
The pattern of surgical treatment in patients without distant metastasis is presented in Table 7. Fewer patients underwent partial mastectomy compared to mastectomy, including skin-sparing mastectomy and nipple-sparing mastectomy. Furthermore, the percentage of patients who underwent partial mastectomy decreased over the period 2014–2018 (Fig. 4A). On the other hand, the rate of mastectomy increased over time (Fig. 4A).
Table 7.
Surgical treatment in patients without distant metastasis
| Number of patients | Percentage of patients (%) | ||
|---|---|---|---|
| M0 patients with surgery | 87,852 | ||
| Breast surgery | Partial mastectomy | 39,054 | 44.5 |
| Mastectomy | 46,699 | 53.1 | |
| Total mastectomy | 42,208 | 48.0 | |
| Nipple-sparing mastectomy | 2334 | 2.7 | |
| Skin-sparing mastectomy | 1843 | 2.1 | |
| Radical mastectomya | 314 | 0.4 | |
| No breast surgery | 396 | 0.5 | |
| Other | 1703 | 1.9 | |
| Axillary surgery | No axillary surgery | 6804 | 7.7 |
| Sentinel node biopsy | 57,816 | 65.8 | |
| Sentinel node biopsy followed by axillary clearance | 6915 | 7.9 | |
| Axillary clearance | 13,307 | 15.1 | |
| Sampling | 1192 | 1.4 | |
| Other | 1818 | 2.1 |
aThis procedure means mastectomy with removal of the pectoral muscles
Fig. 4.

A Trends in breast surgery between 2014 and 2018. Mastectomy includes total mastectomy, nipple-sparing mastectomy, skin-sparing mastectomy and radical mastectomy. B Trends in axillary surgery between 2014 and 2018. The cases of intraoperative conversion from sentinel node biopsy to axillary dissection are included in the axillary dissection group
73.7% of patients with surgery had a sentinel node biopsy alone. The number of cases treated with a sentinel node biopsy alone increased slightly over the period 2014–2018 (Fig. 4B). On the other hand, axillary clearance was performed in 23.0% of total patients, including those whose sentinel node biopsy was converted into axillary dissection during the operation.
Adjuvant systemic treatment
A total of 12,846 patients (14.2%) had preoperative treatment (Table 8). Chemotherapy was delivered to 9,551 women (10.5%) and endocrine therapy to 3454 patients (3.8%). Considering the regimens containing anthracyclines, epirubicin was used more frequently than doxorubicin. In the taxane therapy category, docetaxel was given with higher frequency than paclitaxel. Figure 5A demonstrates the preoperative use of major chemotherapy regimens stratified by subtypes. Because there are many ER-positive HER2-negative breast cancer patients, every regimen except for trastuzumab was likely to be used for these patients.
Table 8.
Preoperative treatment
| Number of patients | Percentage of patients (%) | |
|---|---|---|
| Preoperative therapy | ||
| Yes | 12,846 | 14.2 |
| Chemotherapy | 9551 | 10.5 |
| Endocrine therapy | 3454 | 3.8 |
| Molecular targeted therapy | 3782 | 4.2 |
| Radiation therapy | 82 | 0.1 |
| Others | 160 | 0.2 |
| No | 77,672 | 85.6 |
| Missing | 181 | 0.2 |
| Total | 90,699 | |
| Chemotherapy | ||
| AC or CAF | 1711 | 17.9 |
| EC or CEF | 6288 | 65.8 |
| TC | 375 | 3.9 |
| DTX | 4853 | 50.8 |
| PTX | 2782 | 29.1 |
| nab-PTX | 695 | 7.3 |
| Carboplatin | 169 | 1.8 |
| Others | 846 | 8.9 |
| Total | 9551 | |
| Molecular targeted Tx | ||
| Trastuzumab | 3523 | 3.9 |
| Pertuzumab | 479 | 0.5 |
| Bevacizumab | 138 | 0.2 |
| Others | 113 | 0.1 |
| Total | 3782 | |
AC doxorubicin cyclophosphamide, CAF doxorubicin cyclophosphamide and 5-fluorouracil, EC epirubicin cyclophosphamide, CEF epirubicin cyclophosphamide and 5-fluorouracil, TC docetaxel and cyclophosphamide, DTX docetaxel, PTX paclitaxel, nab-PTX nab-paclitaxel, Tx therapy
Fig. 5.
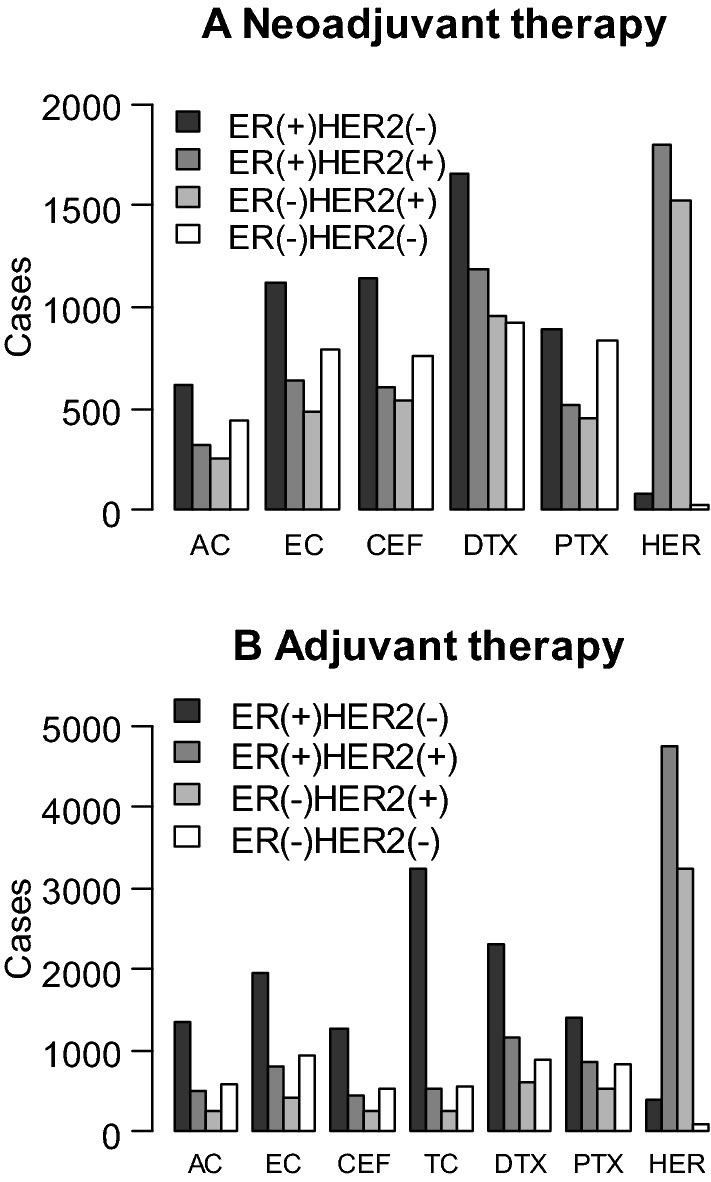
The use of major preoperative (A) and postoperative (B) chemotherapy regimens stratified by immuno-histological subtype. AC Adriamycin and Cyclophosphamide, EC Epirubicin and Cyclophosphamide, CEF Cyclophosphamide, Epirubicin and Fluorouracil, DTX Docetaxel, PTX Paclitaxel, HER Trastuzumab, TC Docetaxel and Cyclophosphamide
A total of 71,278 patients had local or systemic postoperative therapy. As shown in Table 9, chemotherapy was administered to 18,989 women (21.6%), endocrine therapy was given to 54,124 women (61.6%), and radiation therapy was delivered to 35,278 patients (40.2%). In this setting, epirubicin and docetaxel were used more frequently than doxorubicin and paclitaxel, respectively. Figure 5B demonstrates the postoperative use of major chemotherapy regimens stratified by subtypes. TC regimen was likely to be used in ER-positive HER2-negative patients compared with the patients of other subtypes. A total of 33,127 patients received an aromatase inhibitor, 20,426 women received tamoxifen, and 4128 women received gonadotropin-releasing hormone agonist. In this gonadotropin-releasing hormone agonist cases, 3660 cases (88.7%) and 203 cases (5.5%) also received tamoxifen and aromatase inhibitor, respectively. In 157 cases (3.8%), gonadotropin-releasing hormone agonist was used as a single agent. Figure 6 shows the use of major hormonal agents stratified by menopausal status. The ratio of tamoxifen to gonadotropin-releasing hormone was approximately 4 to 1 in premenopausal patients. On the other hand, the proportion of aromatase inhibitor to tamoxifen was around 8 to 1 in postmenopausal patients.
Table 9.
Postoperative adjuvant therapy
| Number of patients | Percentage of patients (%) | |
|---|---|---|
| Postoperative therapy | ||
| Yes | 71,278 | 81.1 |
| Chemotherapy | 18,989 | 21.6 |
| Endocrine therapy | 54,124 | 61.6 |
| Molecular targeted therapy | 8999 | 10.2 |
| Radiation therapy | 35,278 | 40.2 |
| Others | 1697 | 1.8 |
| No | 12,936 | 14.7 |
| Missing | 3638 | 4.1 |
| Total | 87,852 | |
| Chemotherapy | ||
| AC or CAF | 2799 | 14.7 |
| EC or CEF | 7038 | 37.1 |
| TC | 4813 | 25.3 |
| DTX | 5267 | 27.7 |
| PTX | 3740 | 19.7 |
| nab-PTX | 356 | 1.9 |
| CMF | 218 | 1.1 |
| Carboplatin | 892 | 0.6 |
| Others | 1725 | 9.1 |
| Total | 18,989 | |
| Endocrine therapy | ||
| Tamoxifen | 20,426 | 37.7 |
| Gonadotropin-releasing Hormone Agonist | 4128 | 7.6 |
| Aromatase Inhibitor | 33,127 | 61.2 |
| Others | 1099 | 2.0 |
| Total | 54,124 | |
| Molecular targeted Tx | ||
| Trastuzumab | 8826 | 98.1 |
| Pertuzumab | 1202 | 13.4 |
| Trastuzumab Emtansine | 11 | 0.1 |
| Others | 179 | 2.0 |
| Total | 8999 | |
With regard to the abbreviated names, refer to the footnote of Table 6
Fig. 6.
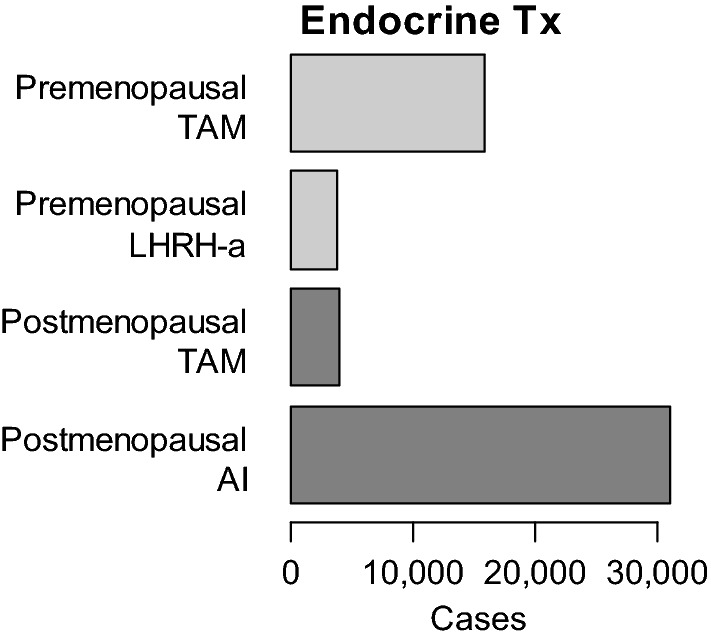
The use of major hormonal agents stratified by menopausal status. TAM tamoxifen, LHRH-a Gonadotropin-releasing Hormone Agonist, AI aromatase inhibitor, Tx therapy
The rate of neoadjuvant and adjuvant chemotherapy administration stratified by stage and receptor expression profile is shown in Fig. 7A and Fig. 7B, respectively. Patients with a higher stage were more likely to receive neoadjuvant chemotherapy. On the other hand, patients with a lower stage are likely to receive adjuvant chemotherapy. Those with Stage I or IIa whose subtype was ER (+) and HER2 (−) tended to do without chemotherapy.
Fig. 7.
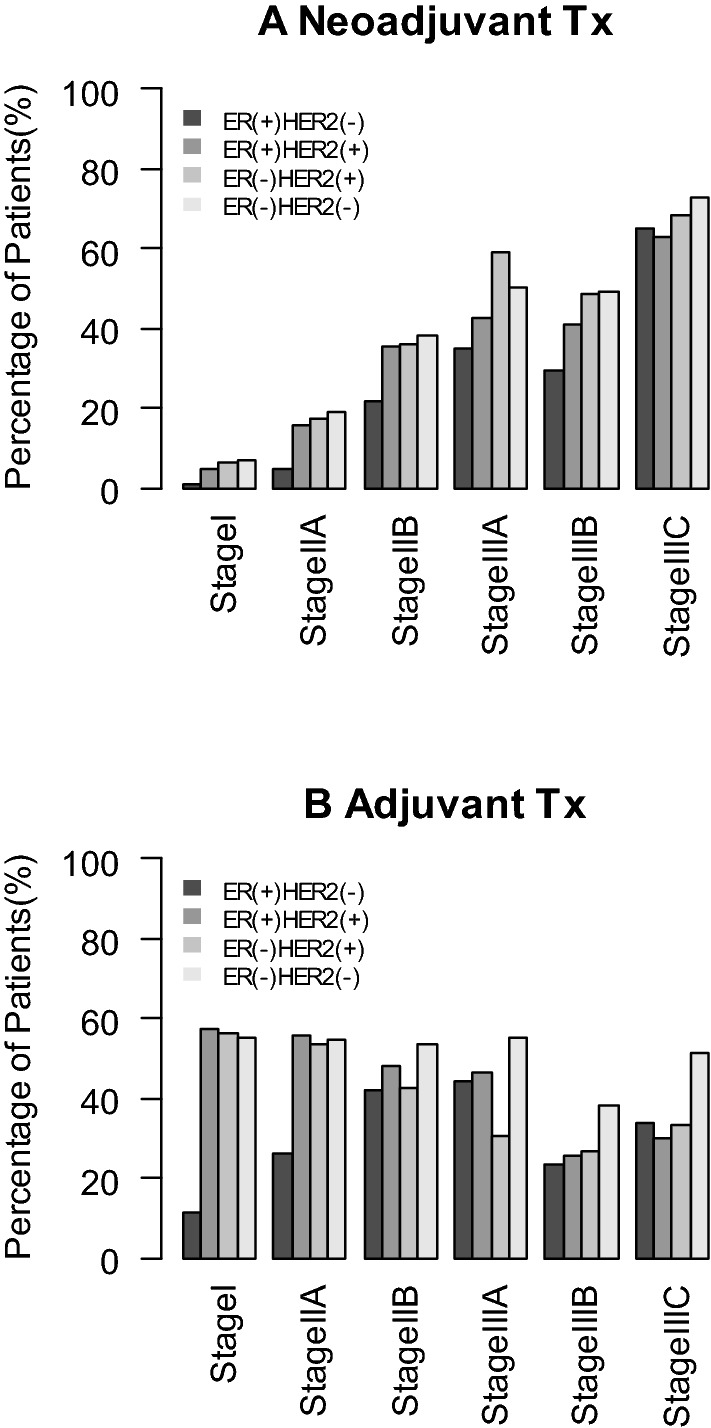
Distribution of patients who received preoperative (A) and postoperative (B) chemotherapy stratified by stage and immuno-biological subtype. Tx therapy
Radiation therapy
Postoperative radiation therapy among M0 patients is summarized in Table 10. In the partial mastectomy patients, 29,012 patients (74.3%) received radiation therapy. Among these patients with radiation therapy, 27,797 patients (95.8%) and 6,301 patients (21.7%) had radiation at the total breast and boost, respectively. In the total mastectomy patients, 6,166 patients (17.1%) received radiation therapy. Among these patients with radiation therapy, 4969 patients (80.6%) and 4,708 patients (76.4%) had radiation at the chest wall and supra-clavicular region, respectively. Furthermore, 6068 patients had radiation therapy for the supra-clavicular lymph nodes regardless of the radiation to the breast. Considering that at least 4794 patients had 4 or more positive axillary lymph nodes (data not shown), radiation therapy for this region is considered to have been delivered properly.
Table 10.
Postoperative radiation therapy in M0 breast cancer
| Number of patients | Percentage of patients (%) | Number of patients | Percentage of patients (%) | |
|---|---|---|---|---|
| Partial mastectomy | 39,054 | 100 | ||
| Partial mastectomy with radiation | 29,012 | 74.3 | ||
| Partial mastectomy with radiation | 29,012 | (100) | ||
| Total breast | 27,797 | (95.8) | ||
| Boost | 6,301 | (21.7) | ||
| Partial breast | 967 | (3.3) | ||
| Supra-clavicular | 1,360 | (4.7) | ||
| Axilla | 803 | (2.8) | ||
| Total mastectomya | 42,208 | 100 | ||
| Total mastectomy with radiation | 6166 | 17.1 | ||
| Total mastectomy with radiation | 6,166 | (100) | ||
| Chest wall | 4,969 | (80.6) | ||
| Supra-clavicular | 4,708 | (76.4) | ||
| Para-sternal | 911 | (14.8) | ||
| Axilla | 848 | (13.8) |
aTotal mastectomy includes nipple-sparing mastectomy, skin-sparing mastectomy and radical mastectomy
Postscript
A total of 94,999 female breast cancer cases were studied. The distribution of patients’ ages was biphasic, which was also observed in other countries. The breast cancer cases detected by screening without symptoms accounted for only 28.2%. Mastectomy was performed more often than partial mastectomy. Epirubicin-containing regimens and docetaxel were used more than doxorubucin-containing regimens and paclitaxel. A total of 74.3% of women with partial mastectomy received radiation therapy, whereas 17.1% of patients with total mastectomy had radiation therapy.
The primary aim of this article is the global announcement of the latest statistics of breast cancer in Japan. Compared with previous reports of NCD breast cancer database, this article addresses some special issues: interpretation of the age distribution, the distribution of cases according to histopathological classification, comorbidities in patients, and practical use of anticancer agents and radiation therapy [3–5].
We defined one unilateral breast cancer as one case. Therefore, one synchronous breast cancer patient counts as two cases. Because synchronous breast cancer cases account for only 6.6% in the NCD database, we believe these cases will not overturn our interpretations of the findings. However, we propose to reinvent format of the future annual reports.
ER and HER2 were determined based on surgical material. Therefore, it is possible that ER and HER2 in neoadjuvant chemotherapy or non-surgery cases were not properly presented. The high rate of missing data in ER and HER2 profile may be due to this procedure. We need to improve this in future report.
Our report has some limitations. The NCD database was originally established based on surgical cases. Therefore, breast cancer patients who undergo surgery are likely to be enrolled in this database. On the other hand, those without surgery are more likely to be missed. That is why the percentage of stage IV breast cancer patients in the NCD was 2.1%, which is lower than another database based on a nationwide survey [1]. Furthermore, the reliability of this database needs to be increased. This concern arises because the data are entered by many busy physicians, and there are no systematic measures to confirm the accuracy.
Acknowledgements
The authors thank all the affiliated institutes participating in the Breast Cancer Registry of the Japanese Breast Cancer Society for their efforts to register the patients’ data. This work was funded by the Registration Committee of the Japanese Breast Cancer Society.
Author contributions
Assembly of data: All authors belong to or supervise The Registration Committee of Japanese Breast Cancer Association. The members of this committee play key roles in database management, including maintenance, modification, promotion, publication, and utilization. Study concept and design in demographic part: K. Tada, MK, and NH Study concept and design in pathology part: MY Study concept and design in surgery part: TK, K. Tanakura, MN, and MM Study concept and design in systemic treatment part: SA, KI, K. Tamura, and NN. Study concept and design in radiation part: EO. Analysis of data: HK and HM. Manuscript writing: K. Tada. Critical revision of the manuscript for important intellectual content: H.J., Y. K., and S. I. Approval of manuscript: All authors.
Declarations
Conflict of interest
K. Tada, H. K., N. H., M. K., M. M., Y. K., N. N., and S. I. have received honorariums as speakers or consultant/advisory roles from Chugai Pharmaceutical Co. K. Tada, Y. K. and N. N. have received honorariums as speakers or consultant/advisory roles from AstraZeneca.Y.K. Have received honorariums as speakers or consultant/advisory roles from Eisai, Eli Lilly, Daiichi-Sankyo, Kyowa Hakko Kirin and Pfizer. N. N. have received honorariums as speakers or consultant/advisory roles from Novartis, Eisai, Eli Lilly, Daiichi-Sankyo, and Pfizer. H. K. Have received honorariums as speakers or consultant/advisory roles from Mitsubishi-Tanabe Pharma Corporation, and EP Croit Co., Ltd. S.I. have received honorariums as speakers or consultant/advisory roles from Eizai and Taiho. N.N. received research funding to Tokai University from Novartis, Chugai Pharmaceutical Co, Daiichi-Sankyo, Pfizer, Eisai, Eli Lilly and Mochida. HK and HM are affiliated with the Department of Health Quality Assessment at the University of Tokyo, a social collaboration department supported by the National Clinical Database, Johnson & Johnson K.K., Nipro Corporation, and Intuitive Surgical Sarl. S.O., S.I. and H.J. are board members of the Japanese Breast Cancer Society.
Ethical approval
This article does not contain any studies with animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.https://www.mhlw.go.jp/content/10900000/000553552.pdf. 2022. Accessed 13 2022.
- 2.https://ganjoho.jp/public/qa_links/report/hosp_c/hosp_c_registry.html. 2022. Accessed 13 May 2022.
- 3.Hayashi N, Kumamaru H, Isozumi U, Aogi K, Asaga S, Iijima K, et al. Annual report of the Japanese breast cancer registry for 2017. Breast Cancer. 2020;27:803–809. doi: 10.1007/s12282-020-01139-3. [DOI] [PubMed] [Google Scholar]
- 4.Kubo M, Kumamaru H, Isozumi U, Miyashita M, Nagahashi M, Kadoya T, et al. Annual report of the japanese breast cancer society registry for 2016. Breast Cancer. 2020;27:511–518. doi: 10.1007/s12282-020-01081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurebayashi J, Miyoshi Y, Ishikawa T, Saji S, Sugie T, Suzuki T, et al. Clinicopathological characteristics of breast cancer and trends in the management of breast cancer patients in Japan: based on the breast cancer registry of the Japanese breast cancer society between 2004 and 2011. Breast Cancer. 2015;22:235–244. doi: 10.1007/s12282-015-0599-6. [DOI] [PubMed] [Google Scholar]
- 6.Brierley JD, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. 8. John Wiley & Sons; 2017. [Google Scholar]
- 7.Society JBC . General rules for clinical and pathological recording of breast cancer. 17. Tokyo: Kanehara Shuppan; 2012. [Google Scholar]
- 8.https://www.e-stat.go.jp/dbview?sid=0003224179. 2022. Accessed Apr 26 2022.
- 9.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 10.Anderson WF, Reiner AS, Matsuno RK, Pfeiffer RM. Shifting breast cancer trends in the United States. J Clin Oncol. 2007;25:3923–3929. doi: 10.1200/JCO.2007.11.6079. [DOI] [PubMed] [Google Scholar]
- 11.Knudson AG. Two genetic hits (more or less) to cancer. Nat Rev Cancer. 2001;1:157–162. doi: 10.1038/35101031. [DOI] [PubMed] [Google Scholar]
- 12.Lilienfeld AM, Johnson EA. The age distribution in female breast and genital cancers. Cancer. 1955;8:875–882. doi: 10.1002/1097-0142(1955)8:5<875::AID-CNCR2820080504>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 13.Allott EH, Shan Y, Chen M, Sun X, Garcia-Recio S, Kirk EL, et al. Bimodal age distribution at diagnosis in breast cancer persists across molecular and genomic classifications. Breast Cancer Res Treat. 2020;179:185–195. doi: 10.1007/s10549-019-05442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakai T, Ozkurt E, DeSantis S, Wong SM, Rosenbaum L, Zheng H, et al. National trends of synchronous bilateral breast cancer incidence in the United States. Breast Cancer Res Treat. 2019;178:161–167. doi: 10.1007/s10549-019-05363-0. [DOI] [PubMed] [Google Scholar]
- 15.Hartman M, Czene K, Reilly M, Adolfsson J, Bergh J, Adami HO, et al. Incidence and prognosis of synchronous and metachronous bilateral breast cancer. J Clin Oncol. 2007;25:4210–4216. doi: 10.1200/JCO.2006.10.5056. [DOI] [PubMed] [Google Scholar]
- 16.Mruthyunjayappa S, Zhang K, Zhang L, Eltoum IA, Siegal GP, Wei S. Synchronous and metachronous bilateral breast cancer: clinicopathologic characteristics and prognostic outcomes. Hum Pathol. 2019;92:1–9. doi: 10.1016/j.humpath.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Jobsen JJ, van der Palen J, Ong F, Riemersma S, Struikmans H. Bilateral breast cancer, synchronous and metachronous; differences and outcome. Breast Cancer Res Treat. 2015;153:277–283. doi: 10.1007/s10549-015-3538-5. [DOI] [PubMed] [Google Scholar]
- 18.Roseman DL, Straus AK, Shorey W. A positive family history of breast cancer. Does it effect diminish with age? Arch Intern Med. 1990;150:191–194. doi: 10.1001/archinte.1990.00390130157025. [DOI] [PubMed] [Google Scholar]
- 19.Sattin RW, Rubin GL, Webster LA, Huezo CM, Wingo PA, Ory HW, et al. Family history and the risk of breast cancer. JAMA. 1985;253:1908–1913. doi: 10.1001/jama.1985.03350370104033. [DOI] [PubMed] [Google Scholar]
- 20.Paul C, Skegg DC, Spears GF. Oral contraceptives and risk of breast cancer. Int J Cancer. 1990;46:366–373. doi: 10.1002/ijc.2910460305. [DOI] [PubMed] [Google Scholar]
- 21.Adami HO, Hansen J, Jung B, Rimsten A. Familiality in breast cancer: a case-control study in a Sweden population. Br J Cancer. 1980;42:71–77. doi: 10.1038/bjc.1980.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rookus MA, van Leeuwen FE. Oral contraceptives and risk of breast cancer in women aged 20–54 years. Netherlands oral contraceptives and breast cancer study group. Lancet. 1994;344:844–851. doi: 10.1016/S0140-6736(94)92826-6. [DOI] [PubMed] [Google Scholar]
- 23.Independent UKPoBCS The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380:1778–1786. doi: 10.1016/S0140-6736(12)61611-0. [DOI] [PubMed] [Google Scholar]
- 24.https://seer.cancer.gov/statfacts/html/breast-subtypes.html The last Access date May 23th 2022


