Abstract
Doxorubicin is a widely used anticancer drug whose efficacy is limited due to its cardiotoxicity. There is no ideal cardioprotection available against doxorubicin-induced cardiotoxicity. This study aimed to investigate the anticipated cardioprotective potential of metformin and dapagliflozin against doxorubicin-induced acute cardiotoxicity in Wistar rats. At the beginning of the experiment, cardiac screening of experimental animals was done by recording an electrocardiogram (ECG) before allocating them into the groups. Thereafter, a total of thirty healthy adult Wistar rats (150–200 g) were randomly divided into five groups (n = 6) and treated for eight days as follows: group I (normal control), group II (doxorubicin control), group III (metformin 250 mg/kg/day), group IV (metformin 180 mg/kg/day), and group V (dapagliflozin 0.9 mg/kg/day). On the 7th day of the treatment phase, doxorubicin 20 mg/kg was administered intraperitoneal to groups II, III, IV, and V. On the 9th day (immediately after 48 h of doxorubicin administration), blood was collected from anesthetized animals for glucose, lipid profile, CK-MB & AST estimation, and ECG was recorded. Later, animals were sacrificed, and the heart was dissected for histopathological examination. We found that compared to normal control rats, CK-MB, AST, and glucose were significantly increased in doxorubicin control rats. There was a significant reversal of doxorubicin-induced hyperglycemia in the rats treated with metformin 250 mg/kg compared to doxorubicin control rats. Both metformin (180 mg/kg and 250 mg/kg) and dapagliflozin (0.9 mg/kg) significantly altered doxorubicin-induced ECG changes and reduced the levels of cardiac injury biomarkers CK-MB and AST compared to doxorubicin control rats. Metformin and dapagliflozin protected the cellular architecture of the myocardium from doxorubicin-induced myocardial injury. Current study revealed that both metformin and dapagliflozin at the FDA-recommended antidiabetic doses mitigated doxorubicin-induced acute cardiotoxicity in Wistar rats. The obtained data have opened the perspective to perform chronic studies and then to clinical studies to precisely consider metformin and dapagliflozin as potential chemoprotection in the combination of chemotherapy with doxorubicin to limit its cardiotoxicity, especially in patients with comorbid conditions like type II diabetes mellitus.
Keywords: Cardiotoxicity, Antidiabetic drugs, Chemotherapy, Chemoprotectant, Electrocardiograph, Cardio-oncology
Introduction
Cancer survival rates are rising exponentially, owing to advances in cancer screening, diagnostic imaging, and therapy methods. However, treatment-related side effects are associated with a higher cancer survival rate, which can significantly influence a patient's health and quality of life [1]. Doxorubicin is an anthracycline anticancer antibiotic used to treat many cancers, including breast cancer and lymphoma [2–4]. It is more likely to produce cardiotoxicity, which is a major cause of morbidity and mortality in cancer patients [5]. Doxorubicin’s therapeutic potential is limited by its cardiotoxicity [6]. Dose-dependent cardiotoxicity caused by doxorubicin can occur at any point throughout treatment and can linger for years after treatment is completed [7]. Because of the various definitions of cardiotoxicity and the wide range of disorders caused by this chemotherapeutic agent, the incidence of doxorubicin-induced cardiotoxicity varies significantly between studies [8–10].
The mechanisms of doxorubicin's therapeutic advantages on tumor cells are distinct from those of its cardiotoxicity. Because of their significant reliance on oxidative substrate metabolism, cardiomyocytes are substantially more vulnerable to the oxidative stress generated by this agent. According to many studies, the primary mechanism for doxorubicin-induced cardiotoxicity is oxidative stress [11–13]. Reactive oxygen species are produced as doxorubicin is oxidized to semiquinone, an unstable metabolite that is then transformed back into doxorubicin [14]. This agent creates an overabundance of reactive oxygen species (ROS) in the mitochondria, resulting in oxidative damage to biological macromolecules such as lipids, proteins, and DNA and affecting the structure and function of cardiac cell membranes [15]. As cardiomyocytes have lower defenses from antioxidant enzymes, doxorubicin decreases endogenous antioxidants and increases lipid peroxidation, altering cardiac function and other toxicities, according to Singal et al. [16]. It has been shown to cause hyperglycemia by increasing insulin resistance. [17–19]. The risk of cardiovascular death in doxorubicin-treated individuals is higher than the chance of tumor recurrence [19]. Doxorubicin has been shown to impair AMPK-alpha (adenosine monophosphate-activated protein kinase-alpha) signaling in the heart, resulting in cellular energy shortages [20]. Doxorubicin can be converted to doxorubicinol, a metabolite that affects the control of iron and calcium by the sarcoplasmic reticulum's calcium pump (ATP2A2) and the sarcolemma's Na+/K+ pump (RYR2) as well as the mitochondria's F0F1 proton pump [21]. AKR1C3, AKR1A1, CBR1, and CBR3 are potential candidate genes for the synthesis of doxorubicinol [14]. Nitric oxide synthases as well as the NADPH oxidase complex genes NCF4, CYBA, and RAC2 are candidate genes for the production of reactive oxygen species or reactive nitrogen species from the metabolism of doxorubicin [22, 23]. Doxorubicin metabolism within the mitochondria can impair respiration and cause the release of cytochrome-C, which starts apoptosis [24]. Doxorubicin has been reported to interact negatively with phenytoin and cyclosporine. Drug interactions causing cardiotoxicity from co-treatment with doxorubicin and trastuzumab or taxanes such as paclitaxel and docetaxel are more clinically significant [25, 26].
Dexrazoxane is occasionally used in most cancer patients to suppress cardiotoxicity induced by anthracycline.
It may protect against cardiotoxicity by sequestering iron and reducing the production of free radicals [27]. The usage was limited based on concerns that dexrazoxane might increase the risk of second primary malignancies, myelosuppression, and infection, particularly in children. The review of the recent evidence leads to reassessing the European label for dexrazoxane found that it is an effective cardioprotectant in children and adolescents and is not associated with an increased risk of second primary malignancies. It may also be may be associated with specific but reversible toxicities, including myelosuppression [28]. Dexrazoxane side effects frequently include dose-limiting myelotoxicity, which is quite similar to the anthracycline side-effect profile. Therefore, it can be difficult to discern between the side effects of anthracycline treatment and those of dexrazoxane usage. This could also cause testicular atrophy which leads to infertility in males based on its effects on a repeated dose [29]. Due to these serious adverse effects of dexrazoxane, a novel cardioprotective agent is necessary to mitigate doxorubicin-induced cardiotoxicity. An important area of anthracycline research has been figuring out how to keep effectiveness while lowering toxicity.
There is no ideal cardioprotectant readily available against doxorubicin-induced cardiotoxicity and no ideal drug which can be used for both malignancies and diabetes. In this attempt, we did a literature review on the pleiotropic effects of metformin and dapagliflozin. Metformin is a well-established oral antihyperglycemic drug used to treat type II diabetes mellitus. Many in vitro and in vivo studies have reported that metformin exerts a cardioprotective role by activating the AMPK pathway [30–35]. Metformin enhances vascular functioning, which reduces cardiovascular events and death as a result, according to one study [36]. Whittington et al. reported that metformin improves myocardial function by reducing oxidative stress and cardiac fibrosis [37]. Dapagliflozin is a sodium-glucose cotransporter 2 inhibitor that is used to treat type 2 diabetes. Dapagliflozin appears to reduce cardiac fibrosis and improve cardiac function in diabetic rats and reduce glucose reabsorption and promote blood glucose excretion to the urine [38, 39]. Dapagliflozin was found to reduce mortality and the risk of heart failure in type 2 diabetes individuals in the dapagliflozin-Heart Failure (DAPA-HF) trial [40]. The key mechanisms underpinning their cardioprotective effect are improvements in cardiac cell metabolism, ventricular loading circumstances, blockage of Na+/H+ exchange in myocardial cells, an increase in cytokine generation, and a decrease in cardiac cell necrosis and fibrosis [41].
The cardioprotective potential of metformin and dapagliflozin has been reported at a quite higher dose than the US FDA-recommended maximum dose for the treatment of type II diabetes mellitus. Considering the already defined adverse effects associated with the US FDA-recommended dose of metformin and dapagliflozin, it is not advisable to use these drugs beyond the recommended dose to achieve cardioprotection. At the pre-clinical stage, the cardioprotective potential of these drugs needs to be investigated following the conversion of the recommended human dose to rat dose as per the body surface area ratio given by Paget and Barnes [42]. The combined impact of metformin and dapagliflozin at human equivalent doses against doxorubicin-induced cardiotoxicity has yet to be explored. So, we aimed to investigate the anticipated cardioprotective potential of metformin and dapagliflozin at their normal antidiabetic doses against doxorubicin-induced acute cardiotoxicity in Wistar rats.
Materials and Methods
Animals
In this experiment, female Wistar rats weighing 150–200 g were employed. They were kept in separate polypropylene cages under conventional circumstances, which included a temperature of 22–24 °C, a 12-h light/12-h dark cycle, and relative air humidity of 40–60%. The rats received constant access to a regular calorie standard rat pellet diet and tap water (Hindustan Lever Ltd., Mumbai, India). The rats were acclimatized to the laboratory conditions for one week before the experiment began after being randomly assigned to different groups. Fasted animals were deprived of food for 10 h but given unlimited access to water. The Institutional Animal Ethics Committee (IAEC/KMC/33/2021) accepted the experimental protocol, and the studies were carried out by the Government of India's Committee for Control and Supervision of Experiments on Animals (CPCSEA) guidelines.
Drugs and Reagents
Sigma-Aldrich-Merck Ltd., Bangalore, provided active pharmaceutical ingredient versions of doxorubicin, metformin, and dapagliflozin (India). ASPEN Laboratories Ltd., New Delhi, provided assay kits for lipid profile, creatine kinase-MB (CK-MB), and aminotransferase (AST) quantification (India). This research employed only analytical-grade compounds.
The Rationale for a Dose Selection of Doxorubicin
Doxorubicin is commonly used to treat a variety of malignancies at a therapeutic dose of 60–75 mg/m2 IV once every 21 days. In rats, this dose is similar to 20–25 mg/kg [43].
The Rationale for a Dose Selection of Metformin
The maximum daily dose of metformin recommended by the US Food and Drug Administration to treat type 2 diabetes patients is 2000 mg per day. According to Paget and Barne's dose conversion table for surface area ratios of certain common laboratory species and man, this dose is equivalent to 180 mg/kg/day in rats [42].
The Rationale for a Dose Selection of Dapagliflozin
According to the US Food and Drug Administration (US FDA guideline), the dose of dapagliflozin for type 2 diabetes patients is 10 mg per day. According to Paget and Barne's dose conversion table for surface area ratios of certain common laboratory species and man, this dose is equivalent to 0.9 mg/kg/day in rats [42].
The Rationale for Use of Gum Acacia and Normal Saline (0.9% NaCl)
Gum acacia is a pharmacologically inert substance used widely as emulsifying and tablet binding agent. We dissolved the powder of our test drugs in 2% gum acacia so that while administering these drugs to rats orally, the drug particles remain intact in the feeding needle. Doxorubicin powder is soluble in normal saline (0.9% NaCl). Therefore, we have used gum acacia and normal saline (0.9% NaCl) in control groups to rule out their therapeutic effects.
Experimental Design
Animals having depressed ST segment/absence of P-wave/inverted P-wave/nonspecific ST-segment/ST-segment elevation were removed from the experiment because of pre-existing cardiac problems and diabetes mellitus. Following that, 30 healthy adult female Wistar rats were randomly divided into five groups and treated for eight days as follows:
Group I (Normal Healthy Control)
2% gum acacia; 1 mL/kg/day orally for 8 days + 0.9% NaCl; 1 mL/kg (single dose); i.p. on 7th day.
Group II (Doxorubicin Control)
2% gum acacia; 1 mL/kg/day orally for 8 days + doxorubicin; 20 mg/kg (single dose); i.p. on 7th day.
Group III (Test-Doxorubicin + Metformin 250 mg/kg)
Metformin; 250 mg/kg/day orally for 8 days + doxorubicin; 20 mg/kg (single dose); i.p. on 7th day.
Group IV (Test-Doxorubicin + Metformin 180 mg/kg)
Metformin; 180 mg/kg/day orally for 8 days + doxorubicin; 20 mg/kg (single dose); i.p. on 7th day.
Group V (Test-Doxorubicin + Dapagliflozin 0.9 mg/kg)
Dapagliflozin; 0.9 mg/kg/day orally for 8 days + doxorubicin; 20 mg/kg (single dose); i.p. on 7th day.
On the ninth day (immediately after 48 h of doxorubicin administration), all the experimental animals were anesthetized by administering intraperitoneal ketamine (60 mg/kg) and xylazine (10 mg/kg).
Estimation of Fasting Blood Glucose
Fasting blood samples were taken from the tail vein (tail tip) of rats for blood glucose determination using glucose oxidase–peroxidase reactive strips and a glucometer (Accu-chek, Roche Diagnostics, USA) [44].
ECG (Electrocardiogram) Recording
In this study, ECG was recorded at two stages. At the beginning of the experiment, cardiac screening of experimental animals was done by recording an ECG before allocating them into the groups. Later, it was recorded on the 9th day (immediately after 48 h of doxorubicin administration). Each rat was anesthetized and placed on an animal surgery table for ECG recording. Electrodes were attached to the palmer surface of rats' shaved limbs. A conductive ECG gel was carefully put over each electrode to avoid the formation of a gel bridge between them. The ECG was recorded for one minute for each animal, and the analysis only used the average of data from 11 consecutive ECG signals. Each experimental animal's ECG was examined qualitatively and quantitatively, and an interventional cardiologist double-checked the results. The PR interval, QT interval, QTc interval, and QRS complex amplitude were all measured in the ECG. The P-wave and ST segment were qualitatively evaluated [43]. The interventional cardiologist validated the results of ECG recording in rats.
Blood Collection and Serum Preparation
Following ECG recording, capillary tubes were used to collect 2 mL of blood from each sedated rat's retro-orbital venous plexus. Blood was preserved in microcentrifuge tubes, and serum was obtained by centrifuging the entire blood at 3000 rpm for 20 min at 4 °C using a Remi C-24 refrigerated centrifuge following clot formation. The serum was kept at 80 °C for additional biochemical analysis.
Estimation of Lipid Profile, CK-MB, and AST
Lipid profile, CK-MB, and AST were calculated using a semi-automated analyzer (Star 21 Plus, Mumbai, India) according to the standard technique included with the commercially available kits.
Heart Isolation, Gross Examination, and Histopathological Analysis
Animals were sacrificed according to the Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA) standards for Laboratory Animal Facility, annexure-6 of euthanasia of laboratory animals. A surgical scalpel was used to make an incision in the thoracic cavity, and the heart was collected from the mediastinum by dissecting it out from the major blood vessels. Following gross examination, it was preserved in 10% formalin for further histopathological evaluation. Following a 24-h fixation in 10% formalin, tissue samples were dehydrated in 50% ethanol for 48 h, 70% ethanol for 24 h, 90% ethanol for 24 h, and 100% ethanol for 24 h. Following that, heart tissues were maintained in xylene until they became translucent. Using embedding rings, tissues were embedded in paraffin wax to make the block, and tissue blocks were stored at 18 °C for 24 h. Then, using a rotary microtome, 5-μm-thick histological sections were taken. The sectioned tissues were stored in a water bath until they were ready to be mounted on lysine-coated slides. After that, the slides with tissues were dried on a hot plate and stained with Haematoxylin and Eosin (H & E). All the slides were observed for the presence of intermuscular edema, cardiomyocyte degeneration, infiltration with inflammatory cells, and myofibrillar loss. From each slide, 10 different fields were observed at ×400 magnification under the light microscope, and histopathological scoring was done as follows—absent (0), mild (1), moderate (2), and severe (3) [45]
Statistical Analysis
Normally distributed data were reported in terms of mean and standard deviation and analyzed using one-way analysis of variance (ANOVA) followed by post hoc Tukey's test using the Statistical Package for the Social Sciences (SPSS version 16.0). A significance level of p ≤ 0.05 was judged statistically significant.
Results
Effect on Fasting Blood Glucose Level and Lipid Profile
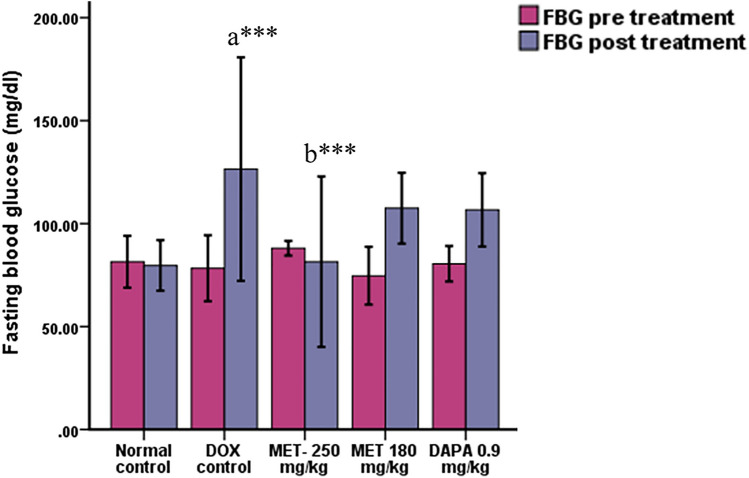
Fasting blood glucose was significantly increased in the doxorubicin control group (p < 0.001) in comparison with the normal control group. Metformin at the dose of 250 mg/kg significantly (p = 0.001) prevented the increase in glucose levels compared to the doxorubicin control group (Fig. 1). We did not find any significant changes in the lipid profile of doxorubicin-administered animals compared to the normal control.
Fig. 1.
Effect on fasting blood glucose level (Mean ± SD). a Compared to normal control, b compared to doxorubicin control; *** p ≤ 0.001
Effect on Cardiac Injury Biomarkers (CK-MB and AST)
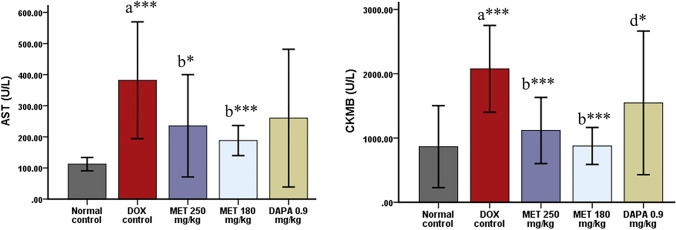
There was a significant upsurge in CK-MB (p < 0.001) and AST (p < 0.001) levels among doxorubicin control rats compared to the normal control group. CK-MB was significantly reduced by both the doses of metformin (p < 0.001) and dapagliflozin 0.9 mg/kg (p = 0.022) in comparison with the doxorubicin control rats. AST was significantly decreased among metformin 250 mg/kg (p = 0.019) and 180 mg/kg (p = 0.001) treated rats compared to doxorubicin control rats (Fig. 2).
Fig. 2.
Effect on cardiac injury biomarkers-CK-MB and AST (Mean ± SD). a Compared to normal control, b compared to doxorubicin control, d compared to doxorubicin + metformin 180 mg/kg; ***p ≤ 0.001 and *p ≤ 0.05
Effect on ECG
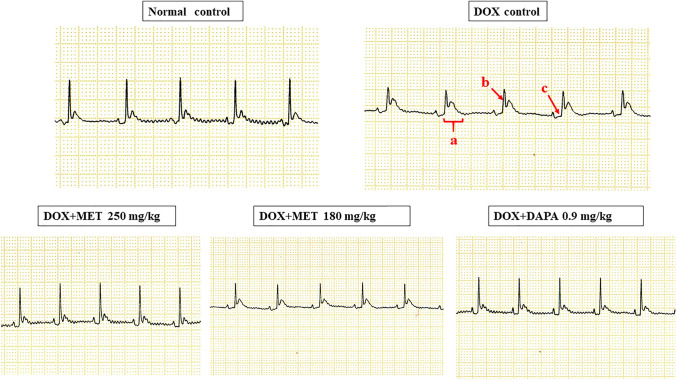
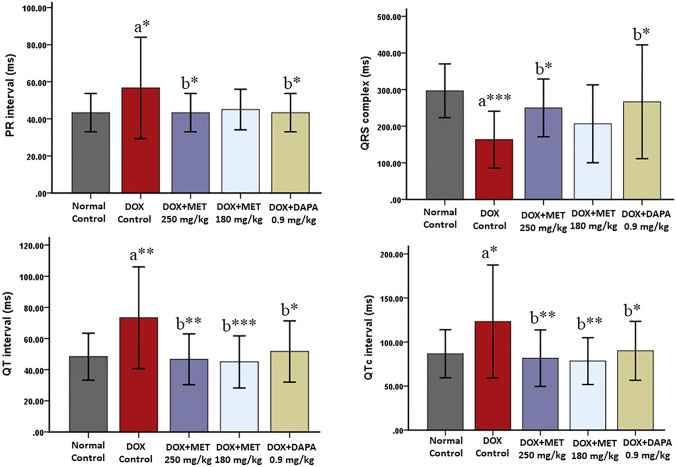
The doxorubicin control group exhibited significant prolongation of QT (p = 0.003), QTc (p = 0.026), and PR (p = 0.044) interval and reduction in QRS complex amplitude (p = 0.001) compared to normal control rats. QT interval was significantly decreased among the metformin 250 mg/kg (p = 0.002), metformin 180 mg/kg (p = 0.001), and dapagliflozin 0.9 mg/kg (p = 0.012) treated rats in comparison with doxorubicin control rats. Metformin 250 mg/kg (p = 0.009), metformin 180 mg/kg (p = 0.004), and dapagliflozin 0.9 mg/kg (p = 0.050) treated rats exhibited a significant reduction in QTc interval compared to the doxorubicin control group. There was a significant decrease in PR interval for metformin 250 mg/kg (p = 0.044) and dapagliflozin 0.9 mg/kg (p = 0.044) groups compared to doxorubicin control rats. QRS complex amplitude was significantly improved in metformin 250 mg/kg (p = 0.050) and dapagliflozin 0.9 mg/kg (p = 0.015) groups compared to the doxorubicin control group (Figs. 3, 4).
Fig. 3.
Qualitative analysis of ECG. a prolongation of the QT interval, b reduced QRS complex amplitude, c increased PR interval
Fig. 4.
Quantitative analysis of ECG (Mean ± SD). a compared to normal control, b compared to doxorubicin control; ***p ≤ 0.001, **p ≤ 0.01, and *p ≤ 0.05
Gross Examination of Isolated Hearts
Ischemic changes were observed in the hearts of doxorubicin control rats as pale/yellow with hyperemic or hemorrhagic borders/white–gray (scar), but these appearances were missing in normal control and the metformin/dapagliflozin-treated groups.
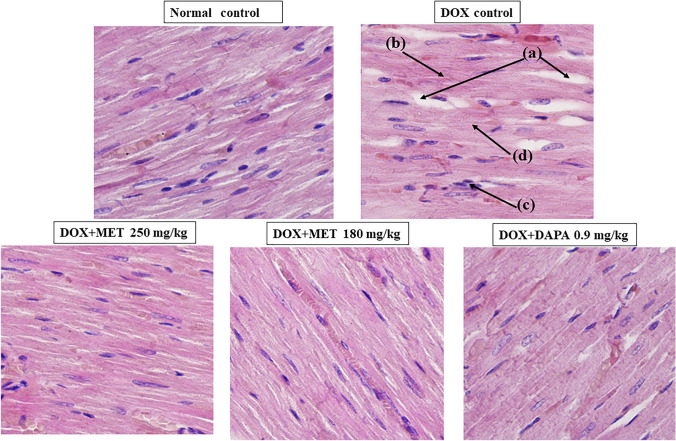
Histopathological Examination of the Heart
Cardiomyocytes from doxorubicin control rats showed significant cardiomyocyte degeneration, intermuscular edema, and mild inflammatory cell infiltration, myofibrillar loss under light microscopy (400 X). These pathological alterations were reduced in metformin and dapagliflozin-treated cardiomyocytes, and their architecture was nearly identical to that of the normal control group (Fig. 5).
Fig. 5.
Histopathological examination of the heart (longitudinal Sect. 400X). a intermuscular edema, b- cardiomyocytes degeneration, c- infiltration with inflammatory cells, d- myofibrillar loss
Effect of Metformin and Dapagliflozin on Cardiomyocyte Architecture Seen Under the Light Microscope Following H & E Staining
There was a significant increase in intermuscular edema (p < 0.001), and cardiomyocyte generation (p < 0.001) among the DOX-intoxicated control rats compared to normal healthy control rats (Table 1). These pathological alterations were reduced in metformin and dapagliflozin-treated cardiomyocytes, and their architecture was nearly identical to that of the normal control group (Fig. 5).
Table 1.
Quantitative scoring of histopathological examination of heart tissues using H & E staining
| Groups | Scoring for intermuscular edema (Mean ± SD) | Scoring for cardiomyocytes degeneration (Mean ± SD) |
|---|---|---|
| Normal control | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Doxorubicin control | 2.33 ± 0.51***a | 2.16 ± 0.40***a |
| Doxorubicin + MET 250 mg/kg | 1.50 ± 0.54*b | 1.16 ± 0.40***b |
| Doxorubicin + MET 180 mg/kg | 1.33 ± 0.51**b | 1.33 ± 0.51**b |
| Doxorubicin + DAPA 0.9 mg/kg | 1.16 ± 0.40***b | 1.16 ± 0.40***b |
Discussion
The present study has demonstrated acute cardiotoxicity with a single dose of doxorubicin administration to the Wistar rats. Doxorubicin binds to the negatively charged phospholipid cardiolipin, which is prevalent in the inner mitochondrial membrane and accumulates in cardiomyocytes’ mitochondria [43]. Doxorubicin causes an overproduction of reactive oxygen species (ROS) in the mitochondria, causing oxidative damage to biological macromolecules such as lipids, proteins, and DNA and disrupting cardiac cell membrane structure and function [46]. Doxorubicin causes acute cardiotoxicity that manifests during and within 2–3 days after a single-dose administration [46, 47].
We observed that doxorubicin has significantly altered ECG waves in the form of an inverted p-wave/increased PR interval/prolonged QT and QTc interval/reduced QRS complex amplitude/nonspecific ST segment. The majority of the ECG changes could be related to a change in membrane function caused by doxorubicin-induced lipid peroxidation. The absence or changed form of the P-wave occurs in a variety of cardiac arrhythmias, the most prevalent of which is atrial fibrillation. [48–50]. The distribution of depolarization from the atria to the ventricles is reflected by the PR interval [51, 52]. To diagnose atrioventricular blockages, the length of the PR interval must be measured. Doxorubicin has been shown to increase the PR interval [53, 54]. Supraventricular arrhythmias are linked to complex QRS narrowing. In cardiac insufficiency, myocardial ischemia, and right and left bundle branch blockages, the big QRS complex shows ventricular rhythms as well as abnormalities in intraventricular conduction. In doxorubicin-treated rats, wide QRS complexes were seen [55]. Due to cancelations and decreased electromotive force generation, several myocardial infarctions have been associated with a reduction in the amplitude of QRS complexes [56–58]. The ST segment is the time between the end of the QRS complex and the start of the T wave, and it reflects the moment when the ventricles depolarize. After myocardial infarction and myocardial ischemia, considerable alterations in the ST segment were identified, but specific criteria for major ST-segment abnormalities were unclear. In reaction to doxorubicin, nonspecific ST alterations have been described [59, 60]. Detecting the ST segment in rat ECG is difficult due to the T wave's continual increase in continuity with the S wave [61]. This parameter's pathological duration reflects heart electrical activity abnormalities produced by intrinsic heart disease or exogenous chemical harmful effects. An extended QT interval [62, 63] is thought to be a good predictor of drug-induced cardiotoxicity. Doxorubicin has been shown to cause QT prolongation in numerous investigations [53, 54, 63, 64]. The length of the QT interval is known to be dependent on heart rate (HR). As the ratio of systole and diastole duration increases, a rise in the HR usually shortens QT. As a result, the corrected QT interval (QTc), which accounts for variations in HR, is frequently utilized as a more objective ventricular depolarization and repolarization metric [66, 67]. Metformin and dapagliflozin were found to significantly counteract DOX-induced PR, QT, and QTc prolongation, as well as a reduction in QRS complex amplitude, in the current investigation. ECG alterations in patients using metformin and dapagliflozin have been reported to be similar. [55, 68–72]. This could be due to their potential to reduce doxorubicin-induced lipid peroxidation and so stabilize membranes.
Myocardial damage is indicated by elevated blood levels of CK-MB and AST [10]. Doxorubicin increased the levels of myocardial damage indicators such as CK-MB and AST in our findings. This is consistent with earlier research that suggests DOX-induced oxidative stress can result in cardiac lipid peroxidation and the release of these enzymes into the bloodstream [20, 73, 74]. Cardiomyocytes from doxorubicin control rats exhibited significant cardiomyocyte degeneration, intermuscular edema but mild inflammatory cell infiltration, and myofibrillar loss. Out of the four prominent histopathological features perceived following myocardial infarction, two features i.e., intermuscular edema and cardiomyocyte degeneration are usually seen during the first 48 h of myocardial infarction, and the other two features i.e., inflammatory cell infiltration and myofibrillar loss are seen gradually in a time-dependent fashion later on. Myocardial infarction is evident in the current study from ECG and biochemical perspectives. It has been commonly observed that structural changes are seen following biochemical alterations.
Both metformin and dapagliflozin significantly reduced the levels of cardiac damage indicators CK-MB, AST, and histological changes in doxorubicin-treated rats, according to our findings. Doxorubicin-induced cardiotoxicity has been linked to a variety of pathways [55, 75]. Its inhibitory effects on AMPK, on the other hand, have been thoroughly described [55, 76].
Metformin has been shown to activate AMPK, resulting in increased glucose absorption and stimulation of glycolysis in cardiomyocytes. AMPK activation also inhibits cell growth and proliferation, which aids in the prevention of cardiac remodeling after a heart attack [77]. Metformin has also been shown to inhibit reactive oxygen species production and oxidative stress caused by doxorubicin [51, 55]. SGLT-2 inhibitors have been found to reduce myocardial oxidative stress, fibrosis, and vascular remodeling which play important roles in the pathogenesis of cardiovascular diseases [71].
In diabetic cardiomyopathy models in mice and rats, heart failure models in zebrafish embryos, and a myocardial ischemia model in rats, SGLT-2 inhibitors have been found to ameliorate cardiac histopathologic alterations [78]. Dapagliflozin has been shown to reduce the augmentation of mitochondrial reactive oxygen species generation, depolarization, and edema in a cardiac ischemia–reperfusion injury model [79–81]. PGC1- and CPT1 are mitochondrial metabolism-related proteins in the heart that play important roles in fatty acid oxidation [30]. Dapagliflozin therapy enhanced CPT1 protein expression and boosted complex I of the ETC expression, indicating that it prevents myocardial energy metabolism depletion when I/R damage occurs [71]. When combined with existing guideline-directed medical therapy, sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin has been shown to minimize hospitalization for heart failure or mortality linked with cardiovascular causes in recent randomized controlled trials, including DAPA-HF [82–84]. According to one study, dapagliflozin treatment for patients with ST-segment elevation myocardial infarction (STEMI) and type 2 diabetes mellitus after percutaneous coronary intervention (PCI) can improve cardiac function, reduce inflammation, and lower the risk of adverse cardiovascular outcomes [85]. Metformin and dapagliflozin both decreased doxorubicin-induced myocardial damage in our investigation, possibly through the mechanisms outlined above.
Conclusion
Our findings revealed that both metformin and dapagliflozin at the FDA-recommended antidiabetic doses exert cardioprotection against doxorubicin-induced acute cardiotoxicity in Wistar rats. This research can be elaborated further to investigate potential molecular mechanisms underlying the anticipated optimal cardioprotective action of metformin and dapagliflozin against doxorubicin-induced acute and chronic cardiotoxicity at single and repeated doses.
Acknowledgements
The authors are grateful to RAK Medical & Health Sciences University, UAE, and Manipal Academy of Higher Education, India for their research facility support toward the accomplishment of this work. We would also like to thank Dr. Abdul Rehman, Assistant Professor of Pathology, RAK College of Medical Sciences, RAK Medical & Health Science University, Ras Al Khaimah (UAE) for his valuable histopathological inputs during the revision of the manuscript.
Author Contribution
SMS conceptualized, designed, and supervised this study. SMS, SP, PS, and AZA performed the experiments. LKB, VKS, SB, and AAJ assisted with the experiments. SMS, LKB, SP, and PS analyzed the data. SMS and SP wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors have no relevant financial or non-financial interests to disclose. The authors have no financial or proprietary interests in any material discussed in this article.
Declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saleh Y, Abdelkarim O, Herzallah K, Abela GS. Anthracycline-induced cardiotoxicity: Mechanisms of action, incidence, risk factors, prevention, and treatment. Heart Failure Reviews. 2021;26:1159–1173. doi: 10.1007/s10741-020-09968-2. [DOI] [PubMed] [Google Scholar]
- 2.Cho H, Lee S, Sim SH, Park IH, Lee KS, Kwak MH, Kim HJ. Cumulative incidence of chemotherapy-induced cardiotoxicity during a 2-year follow-up period in breast cancer patients. Breast Cancer Research and Treatment. 2020;182:333–343. doi: 10.1007/s10549-020-05703-5. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Chen Z, Chua CC, Ma YS, Youngberg GA, Hamdy R, Chua BH. Melatonin as an effective protector against doxorubicin-induced cardiotoxicity. American Journal of Physiology. Heart and Circulatory Physiology. 2002;283:254–263. doi: 10.1152/ajpheart.01023.2001. [DOI] [PubMed] [Google Scholar]
- 4.Aygun H, Gul SS. Cardioprotective effect of melatonin and agomelatine on doxorubicin-induced cardiotoxicity in a rat model: An electrocardiographic, scintigraphic and biochemical study. Bratislavske Lekarske Listy. 2019;120:249–255. doi: 10.4149/BLL_2019_045. [DOI] [PubMed] [Google Scholar]
- 5.Outomuro D, Grana DR, Azzato F, Milei J. Adriamycin-induced myocardial toxicity: New solutions for an old problem. International Journal of Cardiology. 2007;117:6–15. doi: 10.1016/j.ijcard.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Indu R, Azhar TS, Nair A, Nair CK. Amelioration of doxorubicin-induced cardio-and hepatotoxicity by carotenoids. Journal of Cancer Research and Therapeutics. 2014;10:62–67. doi: 10.4103/0973-1482.131370. [DOI] [PubMed] [Google Scholar]
- 7.Bristow MR, Thompson PD, Martin RP, Mason JW, Billingham ME, Harrison DC. Early anthracycline cardiotoxicity. American Journal of Medicine. 1978;65:823–832. doi: 10.1016/0002-9343(78)90802-1. [DOI] [PubMed] [Google Scholar]
- 8.McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline chemotherapy, and cardiotoxicity. Cardiovascular Drugs and Therapy. 2017;31:63–75. doi: 10.1007/s10557-016-6711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 10.Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 11.Sangomla S, Saifi MA, Khurana A, Godugu C. Nanoceria ameliorates doxorubicin-induced cardiotoxicity: Possible mitigation via reduction of oxidative stress and inflammation. Journal of Trace Elements in Medicine and Biology. 2018;47:53–62. doi: 10.1016/j.jtemb.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Qi W, Boliang W, Xiaoxi T, Guoqiang F, Jianbo X, Gang W. Cardamonin protects against doxorubicin-induced cardiotoxicity in mice by restraining oxidative stress and inflammation associated with Nrf2 signaling. Biomedicine & Pharmacotherapy. 2020 doi: 10.1016/j.biopha.2019.109547. [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Daim MM, Khalifa HA, Ahmed AA. Allicin ameliorates doxorubicin-induced cardiotoxicity in rats via suppression of oxidative stress, inflammation, and apoptosis. Cancer Chemotherapy and Pharmacology. 2017;80:745–753. doi: 10.1007/s00280-017-3413-7. [DOI] [PubMed] [Google Scholar]
- 14.Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, Altman RB. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenetics and Genomics. 2011;21(7):440. doi: 10.1097/2FFPC.0b013e32833ffb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuznetsov AV, Margreiter R, Amberger A, Saks V, Grimm M. Changes in mitochondrial redox state, membrane potential, and calcium precede mitochondrial dysfunction in doxorubicin-induced cell death. Biochimica et Biophysica Acta. 2011;1813:1144–1152. doi: 10.1016/j.bbamcr.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Singal P, Li T, Kumar D, Danelisen I, Iliskovic N. Adriamycin-induced heart failure: Mechanisms and modulation. Molecular and Cellular Biochemistry. 2000;207:77–86. doi: 10.1023/a:1007094214460. [DOI] [PubMed] [Google Scholar]
- 17.Dos-Santos DS, dos Santos Goldenberg RC (2018) Doxorubicin-induced cardiotoxicity from mechanisms to the development of efficient therapy. In: Cardiotoxicity, Intechopen, UK, pp 3–24. 10.5772/intechopen.79588
- 18.Morrow GR, Andrews PL, Hickok JT, Roscoe JA, Matteson S. Fatigue associated with cancer and its treatment. Supportive Care in Cancer. 2002;10:389–398. doi: 10.1007/s005200100293. [DOI] [PubMed] [Google Scholar]
- 19.Gorselink M, Vaessen SF, van der Flier LG, Leenders I, Kegler D, Caldenhoven E, van der Beek E, van Helvoort A. Mass-dependent decline of skeletal muscle function in cancer cachexia. Muscle and Nerve. 2006;33:691–693. doi: 10.1002/mus.20467. [DOI] [PubMed] [Google Scholar]
- 20.Kobashigawa LC, Xu YC, Padbury JF, Tseng YT, Yano N. Metformin protects cardiomyocytes from doxorubicin-induced cytotoxicity through an AMP-activated protein kinase-dependent signaling pathway: An in vitro study. PLoS ONE. 2014 doi: 10.1371/journal.pone.0104888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson RD, Mushlin PS, Brenner DE, Fleischer S, Cusack BJ, Chang BK, Boucek RJ., Jr Doxorubicin cardiotoxicity may be caused by its metabolite, doxorubicinol. Proceedings of the National Academy of Sciences. 1988;85(10):3585–3589. doi: 10.1073/pnas.85.10.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinstein DM, Mihm MJ, Bauer JA. Cardiac peroxynitrite formation and left ventricular dysfunction following doxorubicin treatment in mice. Journal of Pharmacology and Experimental Therapeutics. 2000;294(1):396–401. [PubMed] [Google Scholar]
- 23.Wojnowski L, Kulle B, Schirmer M, Schlüter G, Schmidt A, Rosenberger A, Vonhof S, Bickeböller H, Toliat MR, Suk EK, Tzvetkov M. NAD (P) H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation. 2005;112(24):3754–3762. doi: 10.1161/CIRCULATIONAHA.105.576850. [DOI] [PubMed] [Google Scholar]
- 24.Clementi ME, Giardina B, Di Stasio E, Mordente A, Misiti F. Doxorubicin-derived metabolites induce release of cytochrome C and inhibition of respiration on cardiac isolated mitochondria. Anticancer Research. 2003;23(3B):2445–2450. [PubMed] [Google Scholar]
- 25.Cusack BJ, Tesnohlidek DA, Loseke VL, Vestal RE, Brenner DE, Olson RD. Effect of phenytoin on the pharmacokinetics of doxorubicin and doxorubicinol in the rabbit. Cancer Chemotherapy and Pharmacology. 1988;22(4):294–298. doi: 10.1007/bf00254234. [DOI] [PubMed] [Google Scholar]
- 26.Gianni L, Salvatorelli E, Minotti G. Anthracycline cardiotoxicity in breast cancer patients: Synergism with trastuzumab and taxanes. Cardiovascular Toxicology. 2007;7(2):67–71. doi: 10.1007/s12012-007-0013-5. [DOI] [PubMed] [Google Scholar]
- 27.Doroshow JH. Dexrazoxane for the prevention of cardiac toxicity and treatment of extravasation injury from the anthracycline antibiotics. Current Pharmaceutical Biotechnology. 2012;13(10):1949–1956. doi: 10.2174/138920112802273245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reichardt P, Tabone MD, Mora J, Morland B, Jones RL. Risk-benefit of dexrazoxane for preventing anthracycline-related cardiotoxicity: Re-evaluating the European labeling. Future Oncology. 2018;14(25):2663–2676. doi: 10.2217/fon-2018-0210. [DOI] [PubMed] [Google Scholar]
- 29.Eneh, C., & Lekkala, M. R. (2022). Dexrazoxane. In StatPearls 2022. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK560559/ [PubMed]
- 30.Oidor-Chan VH, Hong E, Pérez-Severiano F, Montes S, Torres-Narváez JC, del Valle-Mondragón L, Pastelín-Hernández G, Sánchez-Mendoza A. Fenofibrate plus metformin produces cardioprotection in a type 2 diabetes and acute myocardial infarction model. PPAR Research. 2016;2016:1–14. doi: 10.1155/2016/8237264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Legtenberg RJ, Houston RJ, Oeseburg B, Smits P. Metformin improves cardiac functional recovery after ischemia in rats. Hormone and Metabolic Research. 2002;34:182–185. doi: 10.1055/s-2002-26705. [DOI] [PubMed] [Google Scholar]
- 32.Solskov L, Løfgren B, Kristiansen SB, Jessen N, Pold R, Nielsen TT, Bøtker HE, Schmitz O, Lund S. Metformin induces cardioprotection against ischemia/reperfusion injury in the rat heart 24 hours after administration. Basic & Clinical Pharmacology & Toxicology. 2008;103:82–87. doi: 10.1111/j.1742-7843.2008.00234.x. [DOI] [PubMed] [Google Scholar]
- 33.Kravchuk E, Grineva E, Bairamov A, Galagudza M, Vlasov T. The effect of metformin on the myocardial tolerance to ischemia-reperfusion injury in the rat model of diabetes mellitus type II. Experimental Diabetes Research. 2011;2011:1–5. doi: 10.1155/2011/907496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amara VR, Surapaneni SK, Tikoo K. Metformin attenuates cardiovascular and renal injury in uninephrectomized rats on DOCA-salt: Involvement of AMPK and miRNAs in cardioprotection. Toxicology and Applied Pharmacology. 2019;362:95–104. doi: 10.1016/j.taap.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Ramachandran R, Saraswathi M. Postconditioning with metformin attenuates apoptotic events in cardiomyoblasts associated with ischemic reperfusion injury. Cardiovascular Therapeutics. 2017;35:1–10. doi: 10.1111/1755-5922.12279. [DOI] [PubMed] [Google Scholar]
- 36.Abbasi F, Chu JW, McLaughlin T, Lamendola C, Leary ET, Reaven GM. Effect of metformin treatment on multiple cardiovascular disease risk factors in patients with type 2 diabetes mellitus. Metabolism. 2004;53:159–164. doi: 10.1016/j.metabol.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 37.Whittington HJ, Hall AR, McLaughlin CP, Hausenloy DJ, Yellon DM, Mocanu MM. Chronic metformin-associated cardioprotection against infarction: Not just a glucose-lowering phenomenon. Cardiovascular Drugs and Therapy. 2013;27:5–16. doi: 10.1007/s10557-012-6425-x. [DOI] [PubMed] [Google Scholar]
- 38.Fang CX, Dong F, Thomas DP, Ma H, He L, Ren J. Hypertrophic cardiomyopathy in high-fat diet-induced obesity: Role of suppression of forkhead transcription factor and atrophy gene transcription. American Journal of Physiology Heart and Circulatory Physiology. 2008;295:1206–1215. doi: 10.1152/ajpheart.00319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang WT, Lin YW, Ho CH, Chen ZC, Liu PY, Shih JY. Dapagliflozin protects doxorubicin-induced cardiotoxicity in breast cancer patients with diabetes via suppressing ER stress. Archives of Toxicology. 2021;95:659–671. doi: 10.1007/s00204-020-02951-8. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt AM. Diabetes mellitus and cardiovascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2019;39:558–568. doi: 10.1161/ATVBAHA.119.310961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vattam KK, Raghavendran HR, Murali MR, Savatey H, Kamarul T. Co-administration of alloxan and nicotinamide in rats produces biochemical changes in blood and pathological alterations comparable to the changes in type II diabetes mellitus. Human and Experimental Toxicology. 2016;35:893–901. doi: 10.1177/0960327115608246. [DOI] [PubMed] [Google Scholar]
- 42.Paget, G. E., & Barnes, J. M. (1964). Toxicity tests in the evaluation of drug activities. In: Laurence, D. R., & Bacharach, A. L. (Eds.) Pharmacometrics (1st edn). Massachusetts, pp 135–166. 10.1016/B978-1-4832-2845-7.50012-8
- 43.Ahmed AZ, Satyam SM, Shetty P, D’Souza MR. Methyl gallate attenuates doxorubicin-induced cardiotoxicity in rats by suppressing oxidative stress. Scientifica. 2021 doi: 10.1155/2021/6694340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Satyam SM, Bairy LK, Pirasanthan R, Vaishnav RL. Grape seed extract and Zinc containing nutritional food supplement delays onset and progression of Streptozocin-induced diabetic cataract in Wistar rats. Journal of Food Science and Technology. 2015;52:2824–2832. doi: 10.1007/s13197-014-1305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdulkareem Aljumaily SA, Demir M, Elbe H, Yigitturk G, Bicer Y, Altinoz E. Antioxidant, anti-inflammatory, and anti-apoptotic effects of crocin against doxorubicin-induced myocardial toxicity in rats. Environmental Science and Pollution Research. 2021;46:65802–65813. doi: 10.1007/s11356-021-15409-w. [DOI] [PubMed] [Google Scholar]
- 46.Parker MA, King V, Howard KP. Nuclear magnetic resonance study of doxorubicin binding to cardiolipin containing magnetically oriented phospholipid bilayers. Biochimica et Biophysica Acta. 2001;1514:206–216. doi: 10.1016/s0005-2736(01)00371-6. [DOI] [PubMed] [Google Scholar]
- 47.Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology. 2010;115:155–162. doi: 10.1159/000265166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ammar ES, Said SA, El-Damarawy SL, Suddek GM. Cardioprotective effect of grape-seed proanthocyanidins on doxorubicin-induced cardiac toxicity in rats. Pharmaceutical Biology. 2013;51:339–344. doi: 10.3109/13880209.2012.729065. [DOI] [PubMed] [Google Scholar]
- 49.Konopelski P, Ufnal M. Electrocardiography in rats: a comparison to a human. Physiological Research. 2016;65:717–725. doi: 10.33549/physiolres.933270. [DOI] [PubMed] [Google Scholar]
- 50.Haugan K, Lam HR, Knudsen CB, Petersen JS. Atrial fibrillation in rats induced by rapid transesophageal atrial pacing during brief episodes of asphyxia: A new in vivo model. Journal of Cardiovascular Pharmacology. 2004;44:125–135. doi: 10.1097/00005344-200407000-00017. [DOI] [PubMed] [Google Scholar]
- 51.Nattel S, Shiroshita-Takeshita A, Brundel BJ, Rivard L. Mechanisms of atrial fibrillation: Lessons from animal models. Progress in Cardiovascular Diseases. 2005;48:9–28. doi: 10.1016/j.pcad.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Hoffman BF, Cranefield PF, Stuckey JH, Bagdonas AA, Piera J. Electrical activity during the PR interval. Circulation Research. 1960;8:1200–1211. doi: 10.1161/01.res.8.6.1200. [DOI] [PubMed] [Google Scholar]
- 53.Beinfield WH, Lehr DA. PR interval of the rat electrocardiogram. American Journal of Physiology. 1968;214:205–211. doi: 10.1152/ajplegacy.1968.214.1.205. [DOI] [PubMed] [Google Scholar]
- 54.Hazari MS, Haykal-Coates N, Winsett DW, Costa DL, Farraj AK. Continuous electrocardiogram reveals differences in the short-term cardiotoxic response of Wistar-Kyoto and spontaneously hypertensive rats to doxorubicin. Toxicological Sciences. 2009;110:224–234. doi: 10.1093/toxsci/kfp092. [DOI] [PubMed] [Google Scholar]
- 55.Emeka PM, Al-Ahmed A. Effect of metformin on ECG, HR, and BP of rats administered with cardiotoxic agent doxorubicin. International Journal of Basic & Clinical Pharmacology. 2017;6:1054–1059. doi: 10.18203/2319-2003.ijbcp20171656. [DOI] [Google Scholar]
- 56.Kelishomi RB, Ejtemaeemehr S, Tavangar SM, Rahimian R, Mobarakeh JI, Dehpour AR. Morphine is protective against doxorubicin-induced cardiotoxicity in the rat. Toxicology. 2008;243:96–104. doi: 10.1016/j.tox.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 57.Madias JE. Low QRS voltage and its causes. Journal of Electrocardiology. 2008;41:498–500. doi: 10.1016/j.jelectrocard.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 58.Kilickap S, Akgul E, Aksoy S, Aytemir K, Barista I. Doxorubicin-induced second degree and complete atrioventricular block. Europace. 2005;7:227–230. doi: 10.1016/j.eupc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 59.Chrastina A, Pokreisz P, Schnitzer JE. Experimental model of transthoracic, vascular-targeted, photo dynamically induced myocardial infarction. American Journal of Physiology. Heart and Circulatory Physiology. 2014;306:270–278. doi: 10.1152/ajpheart.00818.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Speechly-Dick ME, Mocanu MM, Yellon DM. Protein kinase C: Its role in ischemic preconditioning in the rat. Circulation Research. 1994;75:586–590. doi: 10.1161/01.res.75.3.586. [DOI] [PubMed] [Google Scholar]
- 61.Sambhi MP, White FN. The electrocardiogram of the normal and hypertensive rat. Circulation Research. 1960;8:129–134. doi: 10.1161/01.res.8.1.129. [DOI] [PubMed] [Google Scholar]
- 62.Hanada E, Ohtani H, Kotaki H, Sawada Y, Sato H, Iga T. Pharmacodynamic analysis of the electrocardiographic interaction between disopyramide and erythromycin in rats. Journal of Pharmaceutical Sciences. 1999;88:234–240. doi: 10.1021/js980256r. [DOI] [PubMed] [Google Scholar]
- 63.Roden DM. Drug-induced prolongation of the QT interval. New England Journal of Medicine. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 64.Porta-Sanchez A, Gilbert C, Spears D, Amir E, Chan J, Nanthakumar K, Thavendiranathan P. Incidence, diagnosis, and management of QT prolongation induced by cancer therapies: A systematic review. Journal of the American Heart Association. 2017;6:1–18. doi: 10.1161/JAHA.117.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Venugopal A, Rajasree O, Koshy RC. Effect of anthracyclines and isoflurane on QTc interval. Egypt J Anaesth. 2014;30:83–87. doi: 10.1016/j.egja.2013.08.003. [DOI] [Google Scholar]
- 66.Funck-Brentano C, Jaillon P. Rate-corrected QT interval: Techniques and limitations. American Journal of Cardiology. 1993;72:17–22. doi: 10.1016/0002-9149(93)90035-b. [DOI] [PubMed] [Google Scholar]
- 67.Ahnve S. Correction of the QT interval for heart rate: A review of different formulas and the use of Bazett's formula in myocardial infarction. American Heart Journal. 1985;109:568–574. doi: 10.1016/0002-8703(85)90564-2. [DOI] [PubMed] [Google Scholar]
- 68.Soraya H, Khorrami A, Garjani A, Maleki-Dizaji N, Garjani A. Acute treatment with metformin improves cardiac function following isoproterenol-induced myocardial infarction in rats. Pharmacological Reports. 2012;64:1476–1484. doi: 10.1016/s1734-1140(12)70945-3. [DOI] [PubMed] [Google Scholar]
- 69.Costa EC, Gonçalves AA, Areas MA, Morgabel RG. Effects of metformin on QT and QTc interval dispersion of diabetic rats. Arquivos Brasileiros de Cardiologia. 2008;90:232–238. doi: 10.1590/s0066-782x2008000400004. [DOI] [PubMed] [Google Scholar]
- 70.Carlson GF, Tou CK, Parikh S, Birmingham BK, Butler K. Evaluation of the effect of dapagliflozin on cardiac repolarization: A thorough QT/QTc study. Diabetes Ther. 2011;2:123–132. doi: 10.1007/s13300-011-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lahnwong S, Palee S, Apaijai N, Sriwichaiin S, Kerdphoo S, Jaiwongkam T, Chattipakorn SC, Chattipakorn N. Acute dapagliflozin administration exerts cardioprotective effects in rats with cardiac ischemia/reperfusion injury. Cardiovascular Diabetology. 2020;19:1–3. doi: 10.1186/s12933-020-01066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Akhan O, Sahin EŞ, Ramazan RA, Aktas AA. Comparison of the effects of SGLT 2 inhibitors and sulfonylurea on electrocardiographic parameters. Cumhuriyet Medical Journal. 2021;43:49–54. doi: 10.7197/cmj.879083. [DOI] [Google Scholar]
- 73.Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE, Andreyev A, Quach T, Ly S, Shekhtman G, Nguyen W. AMPK dysregulation promotes the diabetes-related reduction of superoxide and mitochondrial function. The Journal of Clinical Investigation. 2013;123:4888–4899. doi: 10.1172/JCI66218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shah SL, Mali VR, Zambare GN, Bodhankar SL. Cardioprotective activity of methanol extract of the fruit of Trichosanthes cucumerina on doxorubicin-induced cardiotoxicity in Wistar rats. Toxicology International. 2012;19:167–172. doi: 10.4103/0971-6580.97218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saeed NM, El-Naga RN, El-Bakly WM, Abdel-Rahman HM, El-Din RAS, El-Demerdash E. Epigallocatechin-3-gallate pretreatment attenuates doxorubicin-induced cardiotoxicity in rats: A mechanistic study. Biochemical Pharmacology. 2015;95:145–155. doi: 10.1016/j.bcp.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 76.Wang S, Song P, Zou MH. Inhibition of AMP-activated protein kinase α (AMPKα) by doxorubicin accentuates genotoxic stress and cell death in mouse embryonic fibroblasts and cardiomyocytes: Role of p53 and SIRT1. Journal of Biological Chemistry. 2012;287:8001–8012. doi: 10.1074/jbc.M111.315812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circulation Research. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 78.Lahnwong S, Chattipakorn SC, Chattipakorn N. Potential mechanisms responsible for cardioprotective effects of sodium-glucose co-transporter 2 inhibitors. Cardiovascular Diabetology. 2018;17:1–7. doi: 10.1186/s12933-018-0745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tanajak P, Sa-Nguanmoo P, Sivasinprasasn S, Thummasorn S, Siri-Angkul N, Chattipakorn SC, Chattipakorn N. Cardioprotection of dapagliflozin and vildagliptin in rats with cardiac ischemia-reperfusion injury. Journal of Endocrinology. 2018;236:69–84. doi: 10.1530/JOE-17-0457. [DOI] [PubMed] [Google Scholar]
- 80.Lucas E, Vila-Bedmar R, Arcones AC, Cruces-Sande M, Cachofeiro V, Mayor F, Murga C. Obesity-induced cardiac lipid accumulation in adult mice is modulated by G protein-coupled receptor kinase 2 levels. Cardiovascular Diabetology. 2016 doi: 10.1186/s12933-016-0474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duncan JG. Peroxisome proliferator-activated receptor-alpha (PPARα) and PPAR gamma coactivator-1alpha (PGC-1α) regulation of cardiac metabolism in diabetes. Pediatric Cardiology. 2011;32:323–328. doi: 10.1007/s00246-011-9889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gupta M, Rao S, Manek G, Fonarow GC, Ghosh RK. The role of dapagliflozin in the management of heart failure: An update on the emerging evidence. Therapeutics and Clinical Risk Management. 2021;17:823–830. doi: 10.2147/TCRM.S275076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Belohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett J, Katova T, Kitakaze M, Ljungman CEA, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020;323:1353–1368. doi: 10.1001/jama.2020.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miaobo Z, Changmei L, Xin D, Huipu X. The effects of Dapagliflozin in patients with heart failure complicated with type II diabetes mellitus: A meta-analysis of placebo-controlled randomized trials. Front. Clin. Diabetes Healthc. 2021;2:1–10. doi: 10.3389/fcdhc.2021.703937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xue L, Yuan X, Zhang S, Zhao X. Investigating the effects of dapagliflozin on cardiac function, inflammatory response and cardiovascular outcome in patients with STEMI complicated with T2DM after PCI. Evid Based Complement Alternat Med. 2021;2021:1–6. doi: 10.1155/2021/9388562. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]