Abstract
Widespread public vaccination is one of the effective mechanisms to ensure the health and prevent deaths in societies. The coronavirus disease 2019 (COVID-19) vaccine is a stark instance in this regard. Vaccine development is a complex process requiring firm-level capabilities, various infrastructures, long-term planning, and stable and efficient policies. Due to the global demand for vaccines during the pandemic, the national capability to produce vaccines is critical. To this end, the current paper investigates influential factors, at the firm- and policy-level, in the COVID-19 vaccine development process in Iran. By adopting a qualitative research method and conducting 17 semi-structured interviews and analyzing policy documents, news, and reports, we extracted internal and external factors affecting the success and failure of a vaccine development project. We also discuss the characteristics of the vaccine ecosystem and the gradual maturity of policies. This paper draws lessons for vaccine development in developing countries at both firm and policy levels.
Keywords: COVID-19 vaccines, Vaccine, COVID-19, Ecosystem, Health policy, Iran
Introduction
Amid the outbreak of the coronavirus disease 2019 (COVID-19) pandemic, vaccine research centers and companies across the world set out to develop vaccines through different platforms to control pandemics [1,2,3]. Iran—a country with 85 million people in the Middle East with a history of nearly 100 years in the pharmaceutical and vaccine industry—was no exception, and several vaccine development projects were launched. Such activities were reinforced and accelerated by creating a discourse on vaccine production. In 2021, Iran was among the top 10 countries in the world regarding the number of vaccine production projects in the pre-clinical and clinical studies phase [4]. Under normal circumstances, a vaccine takes 10 to 15 years to develop [5], but in the COVID-19 pandemic, the first approved vaccine by the World Health Organization (WHO) launched in the market in less than 2 years, in December 2020 [6]. The most important factors accelerating the development of the COVID-19 vaccine include coopetition (simultaneous cooperation and competition) in research centers and vaccine companies [7] and government financial and regulatory support.
The COVID-19 pandemic underscored the strategic importance of the vaccine industry in controlling disease and collective health. This pandemic created a vast global demand for vaccination while only a limited number of countries were able to produce the COVID-19 vaccine in a timely manner which highlights the importance of producing vaccines as a strategic asset.
By the end of January 2022, 33 vaccines in at least one country had received emergency licenses and entered the market. Five vaccines produced in Iran, COVIran Barekat, SpikoGen, PastoCovac, FAKHRAVAC, and Razi Cov Pars, are also among these vaccines [8]. In addition, the Noora vaccine was recently licensed for emergency use. Iran, along with the United States, Germany, China, the United Kingdom, Russia, Cuba, Taiwan, India, and Japan, was among the first countries to launch the COVID-19 vaccine in the market [9,10]. The other three vaccines with a non-replicating viral vector, Inactivated, and messenger RNA (mRNA) platforms in are undergoing pre-clinical or clinical studies in Iran (Table 1). Accordingly, in terms of the number of authorized domestic vaccines for emergency use, Iran’s experience could be useful for other developing countries.
Table 1. Overview of coronavirus disease 2019 vaccines development in Iran as of July 2022 (author’s conclusion from interviews, news, and reports).
| Product name | Developer | Manufacturer | Platform | Stage |
|---|---|---|---|---|
| COVIran Barekat | Shifa Pharmed Industrial Co. | Shifa Pharmed Industrial Co. | Inactivated | EUA by Iranian FDO |
| SpikoGen | CinnaGen Co. | CinnaGen Co. | Protein subunit | EUA by FDO |
| PastoCovac | Pasteur Institute and Instituto Finlay de Vacunas Cuba | Pasteur Institute | Protein subunit | EUA by FDO |
| Razi Cov Pars | Razi Vaccine and Serum Research Institute | Razi Vaccine and Serum Research Institute | Protein subunit | EUA by FDO |
| Noora vaccine | Bagheiat-allah University of Medical Sciences | Noargen Co. | Protein subunit | EUA by FDO |
| FakhraVac | Milad Group, Organization of Defensive Innovation and Research | Milad Group, Organization of Defensive Innovation and Research | Inactivated | EUA by FDO |
| OSVID-19 | Paya FanYakhte Co. | Not manufactured yet; Osve Pharmaceutical Co. will manufacture the vaccine | Inactivated | Clinical trial–phase 1 is in progress |
| Corenapcin | ReNAP Group Co. | Not manufactured yet | mRNA | Awaiting for clinical trial permission |
| HUM Immune Biotechnology Vaccine | HUM Immune Biotechnology Co. | Not manufactured yet | Viral vector (non-replicating) | Pre-clinical phase |
EUA, Emergency Use Authorization; FDO, Food and Drug Organization.
Iran has a relatively long experience in vaccine production. Iranian companies and institutions introduced their COVID-19 vaccine to the market just a few months after world-leading vaccines. While all these vaccines were developed by domestic companies, their developers differ greatly in terms of motivation, capabilities, and strategies. The experience of vaccine development projects in terms of effective management and policy factors has been less studied, and in this paper, we will look into that through the lens of Iran. Analyzing this experience reveals the reasons for the success and failure of vaccine development and has practical and policy implications for other countries, especially developing ones. This article reviews Iran’s experience in developing COVID-19 vaccines and explains the influential factors in this process. The following sections relate to vaccine background in Iran, research methods, and findings, which include giving an account of major COVID-19 vaccine development projects and supportive policies. In the subsequent section, we discuss the success factors in each vaccine project as well as the vaccine ecosystem in Iran and the challenges faced by vaccine manufacturers. The last section concludes our findings and provides some practical implications for future vaccine development efforts in developing countries.
Vaccine Development Background in Iran
The vaccine industry in Iran has a long history (from 1920) and two key players including Razi and Pasteur institutes. So, it had a good performance in eradicating infectious diseases and improving the level of community health. The Razi Serum and Vaccine Research Institute began producing veterinary vaccines in 1929 by producing the Rinderpest vaccine. Razi also produces human vaccines such as measles, polio, measles-mumps-rubella (MMR), measles-rubella, trivalent vaccines, and divalent. Presently, Razi Institute is affiliated with the Ministry of Agriculture and works closely with the Ministry of Health and Veterinary Organization [11]. The Institute Pasteur of Iran was founded in 1920 with the cooperation of the Institute Pasteur of France. It produced many vaccines such as smallpox, cholera, Bacillus Calmette–Guérin (BCG), and recombinant hepatitis B and played a key role in controlling infectious diseases in Iran. Now Pasteur plays an instrumental national role in infectious disease control programs and making diagnostic kits for laboratories in Iran [12].
Vaccine production and general vaccination were effective in preventing and eradicating infectious diseases from 1920 to 1970. After this period, there was a relative stagnation in the Iranian vaccine industry for several decades, which might be associated with the closure of government institutions producing vaccines in old technologies, and low investment in research and development (R&D) to develop new products and technologies. The capital-intensive and time-taking process of vaccine’s R&D, the low-profit margin of vaccine production compared to other drugs, and the government’s inclination to import vaccines, instead of supporting domestic vaccine production, created a long-lasting reluctance in the companies until the mid-2010s. In the last decade, with the support of the government, private companies have been involved in the vaccine ecosystem. Noyan Pajouhan Biopharma made its first serious effort to develop advanced vaccines in 2013 with the support of the government completed the R&D of cervical cancer vaccine—human papillomavirus (HPV) in 2020 and obtained a license to enter the Iranian market (IRCT20090526001952N9). Following these efforts, the seasonal influenza vaccine was developed by Nivad Pharmed Salamat, a spin-off from Noyan Pajouhan Biopharma, in 2020 (IRCT20200318046812N4).
Two specialized accelerators, PersisGene and Paya FanYakhte, provided technical and business services in vaccines and biotechnology sectors which were also supported by the government. They provide a variety of services, including R&D infrastructure, training and mentoring, financing vaccine’s development, production, marketing, and sales. Government agencies such as the Biotechnology Development Headquarters (BDH) and the Iran National Innovation Fund (INIF) offer low-interest facilities, grants, and venture capital (VC) investments to support vaccine production projects. Owing to this support, the vaccine production ecosystem in Iran has been revived and many companies are currently researching, developing, and producing vaccines or providing concerning services.
Before the advent of COVID-19, Haemophilus influenzae type b, bivalent oral poliovirus, diphtheria/tetanus (DT), hepatitis B, BCG, and MMR vaccines were produced by Pasteur and Razi institutes. Other critical vaccines in public vaccination, such as pneumococcal, rotavirus, pentavalent, rabies, influenza, and meningitis, are imported.
With the outbreak of the COVID-19 pandemic, several public and private institutions and companies in Iran developed vaccines with different motives. Out of 10 major corona vaccine projects in Iran, nine vaccines have successfully passed the pre-clinical study phase and six vaccines had an emergency use license from the FDO. Among the approved vaccines, three vaccines SpikoGen, Razi Cov Pars, and PastoCovac have been produced with international cooperation and transfer of technical knowledge. In contrast, COVIran Barekat FAKHRAVAC (MIVAC) and Noora vaccine vaccines have been developed internally. The remaining three COVID-19 vaccine projects failed due to insufficient research and production infrastructure, financial and human resources, and production experience (Table 1). Clinical studies and industrial production of these vaccines have not yet accomplished. One of the notable ones is the COReNAPCIN vaccine, developed by the ReNAP Group Company, using mRNA technology. The vaccine has already completed its development and evaluation in animal models and is awaiting approval to begin phase 1 clinical trial in humans. COReNAPCIN produces the same antigen as the vaccine from Pfizer/BioNTech and Moderna. If the vaccine is successfully developed, the new strains can be quickly customized in the production line.
COVIran Barekat, PastoCovac, and SpikoGen are the first Iranian-produced COVID-19 vaccines, respectively introduced to the market in June, July, and October of 2021. However, domestic vaccine production did not meet Iran’s need for vaccines until the first few months of production. Due to the availability of foreign vaccines, the Iran government imported vaccines such as AstraZeneca, Sinopharm, and Sputnik V to increase coverage and accelerate vaccination.
As of February 2022, approximately 22% of the 193.6 million doses of vaccines purchased by the government for general vaccination were domestically produced vaccines [13]. Until 29 May 2022, 149.85 million doses were injected in Iran, of which 64.52, 57.82, and 27.5 million doses were respectively first, second, and booster shots (https://behdasht.gov.ir).
Research Method and Data Gathering
This article aims at reviewing Iran’s experience in developing COVID-19 vaccines and explaining the positive and negative influencing factors in this process. The research method is qualitative and for collecting data, semi-structured interviews, as well as data available in news, websites, and reports of governmental organizations, and companies involved in the vaccine development process have been utilized. For this purpose, the chief executive officer (CEO), project manager, R&D manager, or production manager of 11 vaccine companies/institutes were interviewed (Table 2). Additionally, we interviewed the CEO of two accelerators specialized in vaccine products and technologies as well as two policymakers with expertise on vaccine development. Accelerators are intermediary organizations that provide technical, lab, manufacturing, and business (including financial, marketing, legal, and strategy) services to startups, firms, and manufacturers. Accelerators are usually specialized in an area or sector. In this paper, accelerator refers to two specialized accelerators on vaccines in Iran, i.e., PersisGene and Paya FanYakhte. Interviews were conducted in person from March to May 2022. Depending on the interviewee (including vaccine developer, accelerator, and policymaker), the questions were asked in several main areas. We categorized interview questions into four main sections: (1) interviewee’s involvement and background in vaccine development; (2) vaccine development processes and strategies; (3) motivation, experience, financial and human resources, and existing infrastructure; and (4) business environment, competition, politics, and regulation.
Table 2. List of conducted interviews.
| Interview code | Organizations | No. of interviews | Interviewee’s position | |
|---|---|---|---|---|
| Vaccine developers | ||||
| 1 | Shifa Pharmed Industrial Co. | 1 | Project manager | |
| 2 | CinnaGen | 1 | CEO | |
| 3 | Pasteur Institute of Iran | 1 | Production manager | |
| 4 | Razi Vaccine and Serum Research Institute | 1 | Project manager | |
| 5 | Baqiyatallah University of Medical Sciences | 1 | Project manager | |
| 6 | Milad Group of Organization of Defensive Innovation and Research | 1 | Project manager | |
| 7 | Osve Pharmaceutical Co. | 1 | CEO | |
| 8 | ReNAP Group Co. | 1 | CEO | |
| 9 | HUM Immune Biotechnology Co. | 1 | CEO | |
| 10, 11 | Noyan Pajouhan Biopharma | 2 | CEO, R&D manger | |
| 12, 13 | Nivad Pharmed Salamat | 2 | CEO, R&D manger | |
| Specialized accelerators | ||||
| 14 | PersisGene | 1 | CEO | |
| 15 | Paya FanYakhte | 1 | CEO | |
| Policy makers | ||||
| 16, 17 | BDH | 2 | Senior expert, vaccine evaluator expert | |
CEO, chief executive officer; R&D, research and development.
In interviews with the accelerators, we asked two types of questions—i.e., the services provided by the accelerator and the vaccine ecosystem. In interviews with policymakers, we inquired regarding the role of the government, the support provided, and the supportive vaccine policies. To collect secondary data, we did content analysis of reports published by WHO and Iran’s governmental organizations and several reputable international websites.
Findings
By collecting and analyzing the data obtained from the interviews, reports, and documents pertinent to the COVID-19 vaccine development in Iran, the research findings are presented in two parts. The first part addresses the development process of Iranian COVID-19 vaccines as well as the influential factors in this process (Fig. 1). The second part of the findings is dedicated to explaining the government’s support policies for the COVID-19 vaccine.
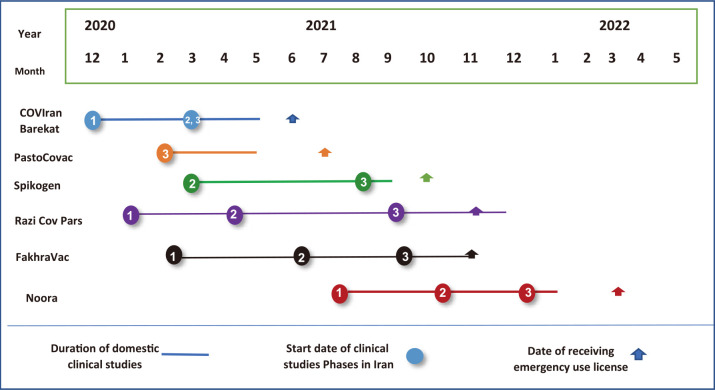
Fig. 1. Timeline of development and launch of the coronavirus disease 2019 (COVID-19) vaccines in Iran.
COVIran Barekat (Shifa Pharmed Industrial Company)
Shifa Pharmed Industrial Company developed COVIran Barekat—the first introduced COVID-19 vaccine in Iran. Shifa Pharmed’s main field of activity is the pharmacy and is one of the 25 companies affiliated with Barkat Pharmaceutical Group.
Barkat Pharmaceutical Group is also a public joint-stock company, under the Execution of Imam Khomeini’s Order (EIKO)—a major public organization in Iran. Pre-clinical studies of this vaccine started in March 2020 with the beginning of a pandemic and on December 21, 2020, the development of the vaccine entered the phase of clinical studies. Phases 2 and 3 of the mentioned studies were carried out simultaneously in six cities of Iran and ended on May 21, 2021. The protocol of these studies has been reported in the Iranian Registry of Clinical trials (IRCT20201202049567N1, IRCT20201202049567N3). COVIran Barekat received an emergency use license from the FDO in June 2021. This vaccine is used in the national vaccination basket and is on the WHO review and approval waiting list. According to studies, the use of such a vaccine is effective in preventing infection, hospitalization, and disease mortality [14] and the use of two 5-µg doses of Kovayran Barakat vaccine 28 days apart is safe and causes provides immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus [15]. At present, the production capacity of this vaccine is 30 million doses per month, 60 million doses have been produced, and 15 million doses have been purchased by the Iran government. Initial plans and agreements are underway to export the vaccine to ten African countries and Nicaragua in Central America. Given the fact that the vaccination rate in 40 countries is below 25% and the first and second dose vaccinations have not been performed in many African countries, there is a good export opportunity for COVIran Barekat and Iranian vaccines in general.
According to an interview with the project development manager of this vaccine, one of the main reasons behind its success is availability of financial resources and equipment and infrastructure of EIKO. EIKO’s Civic Center, Pars Oil Company, Biosun Pharmed Company, KBC Company, and 4030 System, companies affiliated to EIKO, respectively, contributed to creating physical infrastructure, developing vaccine production processes, large-scale production, preparation of raw materials, and clinical studies. Forming small and agile problem-oriented R&D teams is another factor involved in the success of such a project. By contrast, the lack of a Shifa Pharmed Industrial Company experience in the development of inactivated vaccines and the lack of timely provision of industrial production infrastructure owing to increasing global demand for this equipment are among the challenges of producing this vaccine (interviewee 1).
SpikoGen (CinnaGen Company)
The SpikoGen (Covax) protein subunit vaccine was developed by CinnaGen Company. CinnaGen is a privately owned biopharmaceutical company, founded in 1994, and is currently the largest biopharmaceutical companies in West Asia. It manufactures a variety of biological drugs for the treatment of multiple sclerosis, infertility medicine, cancer, etc. and exports them to Middle Eastern, Eastern European, and Latin American countries [16]. It decided to develop the COVID-19 vaccine, because of its social responsibility, availability of infrastructure, and the ability to produce biopharmaceuticals. CinnaGen produced SpikoGen in collaboration with an external partner, the Australian company Vaccine, and purchased the effective protein subunit cell technology from the Vaccine. Pre-clinical studies and the first clinical studies phase of this vaccine were performed in Australia, and the second and third phases of clinical studies were conducted in Tehran and Iran (IRCT20150303021315N24, IRCT20150303021315N23). These studies were finished in September 2021 and in October 2021, and the SpikoGen emergency use license was issued in Iran. This vaccine was placed in the general vaccination of Iran, has proper safety with the use of non-aluminum adjuvants, and has fewer side effects than alum adjuvants [4]. According to the SpikoGen scientific findings, it has acceptable safety and humoral and cellular immune responses to the SARS-CoV-2 virus have been promising in this vaccine [17]. According to CinnaGen CEO, the company has no plans to develop vaccines in the future and by use of its existing capacity in producing biopharmaceutical products it could produce 4 million doses of SpikoGen per month.
Adopting purchasing technical knowledge strategy, experience in developing biopharmaceuticals, accumulation of R&D know-how, production of drugs and bioproducts similar to protein recombinant vaccines, a renowned brand in biopharmaceuticals, and higher speed in clinical studies compared to other competitors led to the rapid and efficient development of SpikoGen. From a managerial point of view, the agile private structure in decision-making and resource allocation, providing the required funding, and the efficient international network for supplying raw materials under COVID-19 conditions proved effective in the success of this vaccine (interviewee 2).
PastoCovac (Pasteur Institute of Iran)
The Pasteur Institute of Iran produced the PastoCovac vaccine in Iran by transferring technology from the Instituto Finlay de Vacunas Cuba. Due to previous experiences in international technological collaboration, national responsibility to combat infectious diseases, and the Possession of infrastructure for the industrial production of vaccines, this institute decided to acquire the technology of production of Soberna, a conjugated protein subunit vaccine, by technology transfer. In this process, in addition to producing an effective COVID-19 vaccine, Pasteur also acquired conjugation technology. Pre-clinical and phase 1 and 2 of clinical studies of this vaccine were performed in Cuba and phase 3 studies were conducted jointly in Cuba and eight cities in Iran (IRCT20210303050558N1). The vaccine underwent clinical studies in May 2021, and in July 2021 received an emergency use license in Iran and is in the general vaccination basket of Iran. According to studies, its effectiveness is 71% after two injections and 92.4% after a booster injection [18]. By February 2022, about 10 million doses of this vaccine have been purchased by the Iran government [13].
More than 20 years of collaboration between the Pasteur Institute of Iran and Cuba in the transfer of technical knowledge and production of hepatitis B, rotavirus, and pneumococcal vaccines, networking and international collaboration, access to vaccine infrastructure and equipment development, Pasteur national brand in the field of vaccines are the key factors involved in the successful production of PastoCovac. In contrast, the government management structure and the small number of human resources and specialists in vaccine development are also among the pastor’s challenges in this project (interviewee 3).
Razi Cov Pars (Razi Serum and Vaccine Research Institute)
Razi Cov Pars is a protein subunit vaccine developed by the Razi Serum and Vaccine Research Institute. Razi Institute is a government agency with a long history of developing animal and human vaccines. Considering the mission of this institute in preventing the spread of the disease, previous experiences in making human vaccines, and the existence of infrastructure and resources required for vaccine development, Razi Institute decided to develop the COVID-19 vaccine in Iran. The vaccine consists of two main components, including pure coronavirus spike antigen and an advanced adjuvant. Razi Cov Pars vaccine is given in three doses, two doses for intramuscular injection 21 days apart and an inhalable dose being sprayed into the nasal cavity on day 51. This is because the primary route of entry and proliferation of the respiratory virus is the inhaled dose of the vaccine to create local immunity in the upper respiratory tract (interviewee 4).
Razi Institute collaborated technologically with a French and a Sino-American company to achieve an effective cell against the virus. Razi conducted pre-clinical and clinical studies in cooperation with the Iran University of Medical Sciences. Clinical trials of this vaccine began on 29 January 2021 till 1 December 2021 (IRCT20201214049709N1, IRCT20201214049709N2, IRCT20201214049709N3). The vaccine received a voluntary emergency use permit in Iran in November 2021 during the implementation of phase 3 clinical trials. So far, 4,777,970 doses have been bought by the Iran government [13].
The prolongation of phase one clinical trials of this vaccine made it challenging to conduct phase 3 clinical trials because a significant portion of the Iranian population had received the first or second dose of the COVID-19 vaccine.
Razi Institute with a long history and active production line of MMR vaccines and DT vaccines for children and adults has a team of vaccine development specialists (from R&D, animal studies, and clinical phase). International collaboration and efficient import of raw materials are the strengths of the vaccine development process (interviewee 4).
FAKHRAVAC (Milad Daroo Company)
The Fakhra vaccine is an inactivated vaccine produced by Milad Daroo Company affiliated with the Organization of Defensive Innovation and Research. Considering the existence of vaccine R&D infrastructure in the Ministry of Defense (MOD) and the conditions of need for COVID-19 vaccine, persuaded the MOD to produce a vaccine in a low dose in response to its mission to maintain national security and prevent the spread of disease in the armed forces (interviewee 5).
Clinical trials of this vaccine began in March 2021 and ended in November 2021 (IRCT20210206050259N1, IRCT20210206050259N2, IRCT20210206050259N3). Studies show that this vaccine has made it well concerning animal testing and sustained immunity [19]. The emergency use permit for this vaccine was also issued in November 2021. The production capacity of this vaccine is 0.5 million doses per month and so far, 465,595 doses have been delivered by the government [13].
As with the Razi Cov Pars vaccine, the third phase of FakhraVac’s clinical studies was conducted simultaneously with the general vaccination in the country, which caused delays and difficulties in attracting volunteers for clinical trials.
Noora vaccine (Baqiyatallah University of Medical Sciences)
The Noora vaccine is a type of protein subunit developed by researchers at Baqiyatallah University of Medical Sciences (BUMS). The researchers of this university, who had the vaccine R&D infrastructure, produced the Noora vaccine according to the country’s need, using internal networking and the use of existing infrastructure in Iran. After R&D and obtaining effective vaccine protein on a laboratory scale, BUMS cooperated with PersisGen accelerator and various domestic companies to perform standardization operations, clinical studies, preparation of common technical document, filling, industrial production, and so forth (interviewee 6). The third phase of clinical studies of this vaccine began in July 2021 in four cities till January 21, 2022 (IRCT20210620051639N1, IRCT202106-20051639N2, IRCT20210620051639N3). The emergency use permit for this vaccine was also issued in March 2022. Official information about the effectiveness of the vaccine has not been published yet.
The key to Noora’s successful production is the high motivation and perseverance of the vaccine research team and efficient networking to use the internal infrastructure. So far, 3 million doses of the vaccine have been delivered by the government and the production capacity of this vaccine is about 5 million doses per month (interviewee 6).
Government support aimed at developing COVID-19 vaccines
Government support for COVID-19 vaccine production could be divided into three categories, i.e., financial support, domestic production discourse, and faster accreditations as described below.
Financial support
Government financial support was given to three different types of organizations engaged in developing COVID-19 vaccines proportionate to their technological maturity [20]. The first type was pioneer companies in the vaccine and biotech industry in Iran. Manufacturers of Razi Cov Pars, COVIran Barekat, PastoCovac, and Spikogen received subsidies for importing raw materials and vaccine production equipment [21] (interviewee 1, 2, 3, 4, and 16). The second type includes companies that passed the research phase successfully. INIF provided low-interest rates loans to two companies to scale up and mass-produce their research results. Small ambitious companies as well as talented teams engaged in the early stages of vaccine development were the third type of financial support receivers. BDH provided risk-taking direct financial support to performers of research in the early stages of development. Financial support for BDH, including VCs and grants, aimed at vaccine research, was given to numerous companies and teams based at universities (interviewee 16). Although the amount of awarded financial support was not significant compared to the government vaccine supports in countries such as China and the United States, it created motivation and momentum for vaccine development by the Iranian companies.
Domestic production discourse and the promise of banning the import of vaccines in case of supply internal needs by domestic producers
The persistent emphasis of the Supreme Leader of Iran and other key policymakers on the domestic production of the COVID-19 vaccine contributed to forming a strong national discourse. In addition, the promise of pre-purchase of vaccines, guaranteed purchase of domestic vaccines, and restriction of imports if the domestic vaccine is produced by the Ministry of Health as much as the country needs encouraged companies and research institutes to work on vaccine development. As a result of such a discourse, out of the 10 major vaccine production projects by April 2022, six companies and institutions have made it possible to obtain licenses for emergency consumption and industrial production.
Accelerating the administrative process of obtaining licenses
The FDO, responsible for setting standards and issuing licenses for human vaccines, cooperated more actively with vaccine companies and institutions during the pandemic, and the administrative bureaucracy in terms of time and process was reduced in the case of COVID-19 vaccines. For instance, the organization promptly processed correspondence and requests for vaccines and authorized the COVIran Barekat vaccine, which entered the clinical study phase prior to the other vaccines, to conduct phases 2 and 3 of the clinical trial simultaneously.
Discussion
With the start of COVID-19 worldwide, including Iran, vaccine development by various platforms was pursued. Vaccine types produced in Iran until January 2022, like most vaccines produced in Asian countries (India [Covaxin, Corbevax], China [Convidecia, CoronaVac, ZF2001, BBIBP-CorV], Kazakhstan [QazCovidin], and Taiwan [Medigen]), were either inactivated or protein subunit [18].
In contrast, the major vaccines produced in the United States and Europe (BNT162b2, Moderna mRNA-1273, AstraZeneca AZD1222, Ad26.COV2.S) are made with new mRNA and non-replicating viral vector technologies, which are a new generation of vaccine [21]. Such vaccines have been developed and entered the market faster than other ones [6]. Researches demonstrate that COVID-19 vaccines using these technologies are more effective than inactivated and protein subunit vaccines [18]. Corenapcin and HUM Immune Biotechnology Vaccine, two vaccines under research in Iran, also use mRNA and non-replicating viral vector technologies. Both of these vaccines have not yet entered clinical studies. The reason for the failure of new vaccine generation in Iran and most Asian countries can be related to the lack of accumulation of knowledge, previous experience, and sufficient investment to develop such technologies in the years before the epidemic.
Factors involved in the success of COVID-19 vaccine development efforts in Iran can be divided into two categories including internal and external factors. Internal factors are related to the capabilities, strategies, and capacities of vaccine firms/institutions and external ones are related to the country’s environmental and policy conditions. Each factor belongs to one or more vaccines, concluded in Table 3. We will subsequently discuss these factors and their effects on vaccine development in Iran.
Table 3. Success factors in Iranian coronavirus disease 2019 vaccines (author’s conclusion based-on interviews and policy reports).
| Success factor | Product | |||||
|---|---|---|---|---|---|---|
| COVIran Barekat | SPikoGen | PastoCovac | Razi Cov Pars | Noora | FakhraVac | |
| Internal factors | ||||||
| Internal networking within Iran | ○ | ○ | ||||
| International technological collaboration | ○ | ○ | ○ | |||
| Private ownership of the developer | ○ | |||||
| Access to finance mainly from inside resources | ○ | ○ | ○ | ○ | ○ | ○ |
| Accumulation of experience and tacit knowledge related to vaccine development | ○ | ○ | ○ | |||
| External factors | ||||||
| Well-known brand and popularity | ○ | ○ | ○ | |||
| Speed in issuing permits and administrative processes | ○ | |||||
| Large unvaccinated population of Iran during the clinical trials of vaccines | ○ | ○ | ○ | |||
| Receive subsidies for importing raw materials and vaccine production equipment | ○ | ○ | ○ | ○ | ||
Access to financial resources is an important internal factor. There were no critical financial restrictions in the process of successful Iranian COVID-19 vaccines. In contrast, the three ongoing projects (as stated in Table 1) face financial constraints leading to delay and failure, despite receiving support from the government.
Another internal factor is the type of ownership. CinnaGen is the only private company that successfully developed a vaccine. Although the company did not have similar experience in vaccine development in the past, it was able to use its knowledge and infrastructure in the production of biopharmaceuticals, and in collaboration with the Vaccine, it developed the recombinant Spikogen vaccine in a short time. The flexibility and high speed of CinnaGen can be attributed to its private ownership. By providing its existing infrastructure to produce several million doses of vaccine per month, the company could produce vaccines faster than other vaccines that needed to import equipment or use the infrastructure of other companies.
Networking and technological collaboration have also contributed to the success of vaccine development projects. Internal networking was more considerable in the vaccine development process at COVIran Barekat and Noora. This accelerated the COVIran Barekat development process and in Noora helped to overcome the lack of infrastructure. International technological collaboration in Spikogen accelerated vaccine development and in PastoCovac led to learning and technology transfer.
Another internal factor is the accumulation of experience and know-how regarding vaccine development. The process of developing a new vaccine (achieving an effective antigen-producing cell against the virus, quality control tests, stabilization, standardization, purification, cloning, etc.), unlike drugs, is new and less routine [22]. However, having a workforce familiar with the process increases the likelihood of success and speed of vaccine development. In SpikoGen and Razi Cov Pars vaccines, tacit knowledge and experience play a positive and pivotal role. Shafa Pharmed also used the tacit knowledge in line with its goal in COVIran Barekat by forming R&D teams and networking with experienced researchers. A well-known brand in the field of vaccines and drugs is the last internal factor contributing to the success of vaccine development and its acceptance. The vaccine, unlike the medicine prescribed for people with the disease, is injected into healthy people in the community and may have side effects. In such a situation, some people avoid injecting it due to a lack of trust [23]. The brand of the vaccine firm/institute can be effective in accepting the vaccine and building public trust in it. In the case of produced vaccines, the well-known brand and popularity of CinnaGen and Pasteur helped people popular with Spikogen and PastoCovac vaccines and accelerated the clinical studies of these vaccines. Additionally, the well-known brand of such companies led the government to provide them with foreign exchange subsidies for importing raw materials and equipment for the production of vaccines.
The increase in the speed and flexibility of administrative processes and issuing of the necessary licenses by IFDA at various stages of the vaccine development process as an external factor had a great impact on the success and introduction of vaccines to the market. For instance, COVIran Barekat was licensed to conduct phase 2 and 3 clinical trials simultaneously, which led to an increase in the issuance of emergency licenses for this vaccine.
The number of unvaccinated people in Iran during the clinical study phase of the three vaccines COVIran Barekat, SpikoGen, and PastoCovac was another external factor that increased the ease and speed of their clinical studies. Clinical trials of the remaining vaccines, Razi Cov Pars, Noora, and FakhraVac, became challenging due to the temporal concurrency with widespread public vaccination. The allocation of a total of 111 million Euros in foreign exchange subsidies also had a positive and effective effect on the preparation of raw materials and equipment for the four vaccines COVIran Barekat, Razi Cov Pars, SpikoGen, and PastoCovac.
Development and maturity of vaccine ecosystem in Iran before the onset of COVID-19 epidemic
As mentioned earlier, after decades of stagnation in the vaccine industry, in the 2010s government support for the development of biopharmaceuticals and vaccines intensified systematically. In particular, this support from the BDH (responsible for policy-making, planning, coordination, and oversight in the field of biotechnology), with the approach of developing capable companies in the field of biopharmaceuticals and vaccines, contributed significantly to the development of the ecosystem. BDH increased the motivation of the companies to develop advanced vaccines by providing low-interest facilities and loans, indirect financing through tax exemptions, direct financing, and government purchases of domestic vaccine products. BDH also encouraged and supported the establishment of specialized vaccine and biopharmaceutical accelerators, i.e., PersisGene and Paya FanYakhte (interviewee 14, 15, and 16). The development of the vaccine ecosystem matured with the formation of mediating entities, like these accelerators. They helped develop the ecosystem by providing technical and business services in the field of vaccines, including R&D infrastructure, training and mentoring, investing in vaccine development, industrial-scale vaccine production, and vaccine marketing and sales.
The establishment of specialized financial institutions such as INIF and technology research funds and the provision of the low-interest, grant, and VC facilities to vaccine projects led to the formation and maturation of financing the vaccine ecosystem in the 2010s.
This support in the past decade led many companies to conduct R&D activities with the aim of vaccine production or providing vaccine-related services. The capability of Iran to simultaneously run several COVID-19 vaccine development projects with a high rate of success has to do with government support, particularly BDH direct and indirect support that shaped the vaccine ecosystem intensified from the 2010s.
Collaboration and networking between universities, R&D experts, biotechnological companies, vaccine institutions, intermediaries, and the government is a sign of vaccine ecosystem maturity in Iran which greatly contributed to COVID-19 vaccine development. Diversification of large pharmaceutical companies such as Osveh Pharmaceutical Company and Shifa Pharmed Industrial Company into vaccine development is expected to bring fruitful progress in the near future.
Common challenges faced by vaccine manufacturers
In addition to the factors affecting the success of vaccine development in Iran, there were also challenges in the development process. The lack of pre-purchase and lack of transparency in demand for vaccines by the government, delays in payment of vaccines to domestic companies were the most important. Furthermore, the timeliness and rising costs of supplying devices and raw materials owing to increasing global demand and international payment difficulties in Iran is a common challenge in all vaccine projects. Finally, the lack of transparency in the licensing process due to the IFDA’s lack of experience in licensing new vaccines was also a common challenge for vaccine development projects.
Conclusion
In the present paper, with the aim of examining Iran’s experience in the development of COVID-19 vaccines and the factors influencing this process, after collecting field evidence and interviewing vaccine development stakeholders in government and companies, this experience was analyzed. In addition, the effective internal and external success factors and challenges involved in each of the vaccine development projects were described.
According to our findings, a successful vaccine development—particularly in a pandemic situation where the timing of large-scale vaccine production is important—depends on factors including prior experience and knowledge accompanied by production infrastructure are crucial. Due to the time consumption and uncertainty of the vaccine development process, access to financial resources is another factor that should be considered by vaccine developers from the beginning.
Adopting a pragmatic and sound strategy toward licensing, managing costs, and pace of development is also instrumental in the success of vaccine development. Vaccine development takes place in both laboratory and industrial stages, but due to the different scales of such stages, the processes, considerations, and equipment required are fundamentally different. Undertaking pre-clinical and clinical stages also requires various specializations and infrastructures. Hence, networking, outsourcing, and technological collaboration for joint development or technology transfer contribute to the success of vaccine development.
The maturity of the vaccine ecosystem in a country is of high importance in networking and technological collaboration, and active government support is needed in this regard. The vaccine industry requires high investment with low-profit margins [22], so without government support, companies are not motivated enough to enter. The establishment and maturation of vaccine-specific intermediaries created an efficient ecosystem in Iran in the late 2010s, and during the COVID-19 epidemic, it played an empowering and facilitating role in all vaccine development projects. This ecosystem provided the momentum and perquisite conditions for the development of influenza and HPV vaccines from 2015 to 2020.
A key lesson to draw from Iran’s experience in vaccine development is the need for integrated management and unity of command in the manufacture and supply of vaccines in pandemic conditions. In a pandemic, governments are committed to providing effective vaccines and general vaccinations, as well as managing the supply and distribution of vaccines. As such, government policies and support have a direct impact on the development of domestic vaccines and vaccine ecosystems. Due to the uncertainty in the success of vaccine projects, the parallel vaccine projects by different platforms were and still is a common sense and justified. However, integrated management should be considered by policymakers to encourage collaboration and synergy between domestic developers. Iran’s experience shows that although the government has been very effective in facilitating and accelerating standards and licensing, the lack of unity of command has led to vaccine development projects being carried out separately without interconnection synergies. However, most of the major vaccine developers in Iran were state-owned, and their cooperation with each other made it possible for synergy and faster access to large-scale production. In addition, the issue of demand and guaranteed purchase transparency has influenced the development of the COVID-19 vaccine and the vaccine ecosystem in general. Decentralized management of vaccine development and lack of transparency in demand has led to the production of six different vaccines in Iran, and these vaccines face challenges in production volume and reduced export opportunities. Despite some vaccine-related management deficiencies at the national level amid the COVID-19 pandemic, collective efforts of vaccine ecosystem actors led to the overall enhancement of knowledge and infrastructure required for vaccine development in Iran which may become a precious asset in the years to come.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Excler JL, Saville M, Berkley S, Kim JH. Vaccine development for emerging infectious diseases. Nat Med. 2021;27:591–600. doi: 10.1038/s41591-021-01301-0. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global vaccine market report. Geneva: World Health Organization; 2020. [Google Scholar]
- 3.Patel SP, Patel GS, Suthar JV. Inside the story about the research and development of COVID-19 vaccines. Clin Exp Vaccine Res. 2021;10:154–170. doi: 10.7774/cevr.2021.10.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li L, Honda-Okubo Y, Huang Y, et al. Immunisation of ferrets and mice with recombinant SARS-CoV-2 spike protein formulated with Advax-SM adjuvant protects against COVID-19 infection. Vaccine. 2021;39:5940–5953. doi: 10.1016/j.vaccine.2021.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han S. Clinical vaccine development. Clin Exp Vaccine Res. 2015;4:46–53. doi: 10.7774/cevr.2015.4.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang M, Liang Y, Yu D, et al. A systematic review of vaccine breakthrough infections by SARS-CoV-2 delta variant. Int J Biol Sci. 2022;18:889–900. doi: 10.7150/ijbs.68973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crick JM, Crick D. Coopetition and COVID-19: collaborative business-to-business marketing strategies in a pandemic crisis. Ind Mark Manag. 2020;88:206–213. [Google Scholar]
- 8.Krasilnikova A. Brief overview of the currently available COVID-19 vaccines. J Clin Health Sci. 2022;7:5–24. [Google Scholar]
- 9.Kandimalla R, Chakraborty P, Vallamkondu J, et al. Counting on COVID-19 vaccine: insights into the current strategies, progress and future challenges. Biomedicines. 2021;9:1740. doi: 10.3390/biomedicines9111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudlay D, Svistunov A. COVID-19 vaccines: an overview of different platforms. Bioengineering (Basel) 2022;9:72. doi: 10.3390/bioengineering9020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Razi Vaccine and Serum Research Institute. Razi Vaccine and Serum Research Institute website [Internet] Karaj: Razi Vaccine and Serum Research Institute; 2022. [cited 2022 Jul 10]. Available from: https://english.rvsri.ac.ir/portal/home/?239735/Home . [Google Scholar]
- 12.Maslehat S, Esmaeili Rastaghi AR, Siavashi MR, Mostafavi E. In honor of Dr. Mehdi Assmar, a distinguished researcher at the Pasteur Institute of Iran. J Res Hist Med. 2021;10:81–94. [Google Scholar]
- 13.Iranbio. Iranbio website [Internet] Tehran: Iranbio; 2022. [cited 2022 Jul 10]. Available: https://iranbio.info/en. [Google Scholar]
- 14.Heidarzadeh A, Moridani MA, Khoshmanesh S, Kazemi S, Hajiaghabozorgi M, Karami M. Effectiveness of COVID-19 vaccines on hospitalization and death in Guilan, Iran: a test negative case-control study. Int J Infect Dis. 2022 Dec 23; doi: 10.1016/j.ijid.2022.12.024. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohraz M, Salehi M, Tabarsi P, et al. Safety and immunogenicity of an inactivated virus particle vaccine for SARS-CoV-2, BIV1-CovIran: findings from double-blind, randomised, placebo-controlled, phase I and II clinical trials among healthy adults. BMJ Open. 2022;12:e056872. doi: 10.1136/bmjopen-2021-056872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majidpour M, Saber A, Elahi S, Shayan A, Khorasani SM. Technological catch-up in the biopharmaceutical sector: evidence from Iran. Technol Soc. 2021;67:101695 [Google Scholar]
- 17.Tabarsi P, Anjidani N, Shahpari R, et al. Safety and immunogenicity of SpikoGen, an Advax-CpG55.2-adjuvanted SARS-CoV-2 spike protein vaccine: a phase 2 randomized placebo-controlled trial in both seropositive and seronegative populations. Clin Microbiol Infect. 2022;28:1263–1271. doi: 10.1016/j.cmi.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eroglu B, Nuwarda RF, Ramzan I, Kayser V. A narrative review of COVID-19 vaccines. Vaccines (Basel) 2021;10:62. doi: 10.3390/vaccines10010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghasemi S, Naderi Saffar K, Ebrahimi F, et al. Development of inactivated FAKHRAVAC vaccine against SARS-CoV-2 virus: preclinical study in animal models. Vaccines (Basel) 2021;9:1271. doi: 10.3390/vaccines9111271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez C. Technological revolutions and financial capital. Cheltenham: Edward Elgar Publishing; 2003. [Google Scholar]
- 21.Tasnim News Agency. Medical news, financial support of Iranian COVID-19 vaccine development [Internet] Tehran: Tasnim News Agency; 2022. [cited 2022 Jul 10]. Available from: https://tn.ai/2599659 . [Google Scholar]
- 22.World Health Organization. Increasing access to vaccines through technology transfer and local production. Geneva: World Health Organization; 2011. [Google Scholar]
- 23.Al-Mistarehi AH, Kheirallah KA, Yassin A, et al. Determinants of the willingness of the general population to get vaccinated against COVID-19 in a developing country. Clin Exp Vaccine Res. 2021;10:171–182. doi: 10.7774/cevr.2021.10.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]