Abstract
Introduction:
Reductions in fetal growth are associated with adverse outcomes at birth and later in life. However, identifying fetuses with pathologically small growth remains challenging. Definitions of small-for-gestational age (SGA) are often used as a proxy to identify those experiencing pathological growth (i.e., fetal growth restriction). However, this approach is subject to limitation as most babies labeled SGA are constitutionally, not pathologically small. Incorporating repeated ultrasound measures to examine fetal growth trajectories may help distinguish pathological deviations in growth from normal variability, beyond a simple definition of SGA.
Objective:
Characterize phenotypes of growth using ultrasound trajectories of fetal growth among SGA births.
Study Design:
We identified and described trajectories of fetal growth among SGA births (< 10th percentile weight-for-gestational age; n = 245) in the LIFECODES Fetal Growth Study using univariate and multivariate trajectory modeling approaches. We abstracted available ultrasound measures of fetal growth (estimated fetal weight [EFW], head circumference [HC], abdominal circumference [AC], and femur length [FL]) from health records. First, we applied univariate group-based trajectory modeling to define trajectories of EFW z-scores during gestation. Second, we applied group-based multi-trajectory modeling to identify trajectories based on concurrent measures of HC, AC, and FL z-scores. Last, we described how these trajectories were related to patient demographics, pregnancy characteristics, and birth outcomes compared to those observed among AGA controls.
Results:
We identified three univariate trajectories of EFW and four multivariate trajectories of fetal growth among SGA births. In our univariate approach, infants with the smallest EFW trajectory throughout pregnancy had poorer outcomes, including the highest risk of NICU admission. The remaining univariate trajectory groups did not have elevated risk of adverse birth outcomes relative to AGA controls. In our multivariate approach, we identified two groups at increased or moderately increased risk of NICU admission, including infants that remained extremely small for all parameters throughout pregnancy as well as those who had disproportionately smaller FL and AC compared to HC. Again, the remaining multivariate trajectory groups did not have elevated risk of adverse birth outcome relative to AGA controls.
Conclusion:
Latent class group-based trajectory modeling applied to ultrasound measures of fetal growth may help distinguish pathologic vs. constitutional growth profiles among babies born SGA. While trajectories cannot be fully characterized until delivery, limiting direct clinical application of these methods, they may still contribute to the development of approaches for separating growth restriction from constitutional smallness.
Keywords: Fetal growth restriction, ultrasound measures of fetal growth, group-based multi-trajectory modeling, latent class trajectory analysis
INTRODUCTION
Aberrations in normal fetal growth have long been associated with adverse outcomes at birth and later in life. For example, babies born small-for-gestational age (SGA) have been shown to be at an increased risk of infant mortality and neurodevelopmental disorders.1,2 However, SGA infants comprise two distinct subpopulations of individuals: those that are pathologically small (e.g., growth restricted) and those that are constitutionally small (e.g., small but healthy).3 Despite this knowledge, research on the effects of growth restriction is hindered by the difficulty in distinguishing these groups from one another. Acknowledging this limitation, several recent analyses have tried to refine definitions of growth restriction through the incorporation of various clinical measures or other pregnancy characteristics.4–7
One refinement, which remains minimally explored, could be to account for the overall trajectory of growth during gestation. For example, latent class trajectory modeling approaches can identify subgroups of fetuses with homogenous growth trajectories. These methodologies posit that individuals with shared trajectories may reflect a shared etiology or mechanism underlying them.8,9 Thus, these trajectories may be more useful when identifying risk factors or adverse outcomes that are believed to have specific mechanisms relating to fetal growth better than a blanket definition of SGA. One example of this approach is a recent study conducted in collaboration between our research group and the Generation R cohort, which identified different latent class trajectories of estimated fetal weight (EFW) among SGA births.7 The identified trajectories had different associations with childhood neurodevelopmental outcomes. These findings suggested that trajectory modeling to characterize fetal growth phenotypes may help to appropriately isolate groups of growth restricted infants better than the SGA definition.
In this study, we used two latent class trajectory modeling approaches to identify phenotypes of fetal growth among SGA births using ultrasound records from mid- through late pregnancy in the LIFECODES Fetal Growth Study. We examined 1) univariate trajectories of EFW and 2) multivariate trajectories of head circumference (HC), abdominal circumference (AC), and femur length (FL), which have not been examined previously and may better account for fetal body composition. We hypothesize that multivariate trajectory modeling approaches may provide a more nuanced analysis of fetal growth relative to approaches relying on weight as a summary measure of growth.
METHODS
Study population
The LIFECODES cohort, which began in 2006, is an ongoing prospective pregnancy cohort that has been described in detail elsewhere.10 Briefly, participants are recruited from antenatal clinics in Brigham and Women’s Hospital (BWH) in Boston, MA. Participants are eligible for enrollment if they are at least 18 years of age, initiate prenatal care before 15 weeks gestation, and intend on delivering at BWH. All participants provide written and informed consent at the time of enrollment. Participants attend three study visits (i.e., median 10, 26, and 35 weeks gestation). At the first study visit, participants complete detailed questionnaires about sociodemographics and medical history. The LIFECODES study was approved by the Institutional Review Board of BWH, and the current analysis was deemed exempt by the NIEHS.
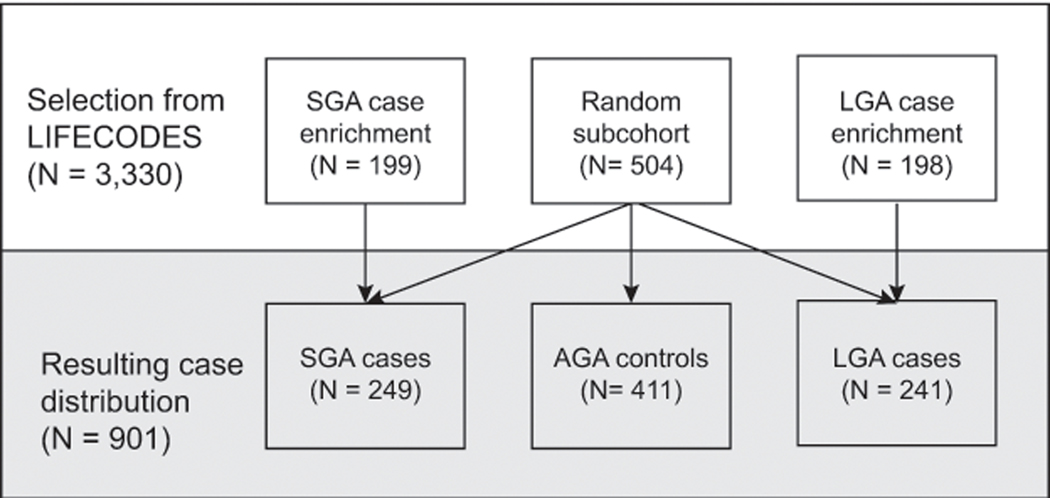
This analysis utilizes the LIFECODES Fetal Growth Study, a case-cohort of 901 participants sampled from those participating in LIFECODES with a singleton delivery between 2008 – 2018 (N = 3,330). Prior to sampling, we abstracted birthweight and gestational age at delivery from medical records. Gestational age was calculated according to recommendations by the American College of Obstetrics and Gynecologists.11 We constructed birthweight-for-gestational age z-scores using an internal standard previously created from the hospital clinic population by our research group.12 Births were defined as SGA if their birthweights were < 10th percentile, appropriate-for-gestational age (AGA) if they were between the 10th and 90th percentile, and large-for-gestational age (LGA) if they were > 90th percentile. Sampling into the case-cohort took place in two steps. First, we randomly selected a subcohort (i.e., sample; N = 504) from the LIFECODES study population. Second, two additional groups of participants with either an SGA (N = 199) or LGA birth (N = 198) were randomly sampled from identified cases as part of an enrichment subset. This sampling scheme resulted in a total of 249 SGA, 411 AGA, and 241 LGA births (Figure 1). For the purposes of this analysis, we present our findings according to case status and focus primarily on SGA cases and AGA controls.
Figure 1.
Sampling diagram for the LIFECODES Fetal Growth Study. AGA = appropriate-for-gestational age; LGA = large-for-gestational age, SGA = small-for-gestational age.
Fetal growth measures
Using medical records, we abstracted ultrasound measures of fetal growth, including HC, AC, and FL. Using these measures, we calculated EFW using Hadlock Formula #3 (i.e., based on HC, AC, and FL).13 For a subset of participants (n = 49), HC was not recorded. For these participants, we abstracted their biparietal diameters (BPD) and calculated EFW using Hadlock Formula #2 (i.e., based on BPD, AC, and FL).13 Ultrasound measures from 16 weeks gestation until delivery were converted into gestational age-specific z-scores using ultrasound standards constructed from the underlying clinic population.12 The standards did not account for fetal sex, as this factor is not commonly used in the clinic or in epidemiologic studies to assess growth during pregnancy.6,14–16 For participants without measured HC, their BPD z-score was substituted for their HC z-score. Measures collected before 16 weeks gestation were not utilized due unclear clinical relevance.14,17
Participant characteristics
The questionnaire administered at the first study visit assessed basic participant characteristics, including sociodemographic characteristics, behavioral factors, and medical history. For the purposes of this analysis, we summarized race and ethnicity as “non-Hispanic white”, “non-Hispanic Black”, “Hispanic” and “Other”. We expect that race does not have a biological effect on fetal growth, but instead, that observed differences across racial categories are the result of unmeasured cultural or social factors, including structural racism.18 Basic clinical information was collected at each study visit and medical diagnoses that occurred during pregnancy were abstracted from medical records and validated by two maternal fetal medicine physicians.
Birth outcomes
Birth outcomes were abstracted from medical records after delivery and included: gestational age at the time of delivery, birthweight z-scores, delivery via c-section, neonatal intensive care unit (NICU) admission, and length of NICU stay. Birthweight z-scores, like ultrasound measure z-scores, were derived from an internal growth standard that did not account for sex.12 While the inclusion of measures such as placental pathologies or uterine artery doppler would be informative in the context of fetal growth, this information was not collected uniformly on all pregnancies in this study population. Therefore, we have not included them due to selection bias.
Statistical methods
All statistical analyses were performed using SAS v 9.4 (SAS Institute, Cary, NC). We summarized demographic characteristics of participants according to case or control status by calculating the median (interquartile range [IQR]) or n (%). In addition, we visualized the distribution of ultrasound fetal growth measures through scatter plots.
We first used group-based trajectory modeling to identify the optimal number of EFW trajectories within the subset of SGA births.19 We used EFW z-scores to account for both the relative size of participants within the cohort and the velocity of growth across gestation. We explored solutions ranging from one to six groups. The optimal number of groups was identified using the following statistical criteria: a log Bayes Factor > 2.0, average posterior probability of class membership ≥ 0.70, the smallest group had at least 5% of the sample, and that the odds of correct classification ≥ 5.0.20,21 Subject-matter knowledge was also used to make decisions about the optimal number of groups. Participants were assigned to the trajectory group that corresponded to their highest posterior inclusion probability. Trajectories were visualized graphically by displaying the predicted mean EFW z-score and 95% confidence interval (CI) for each trajectory group from 16 to 40 weeks gestation.
Second, we applied group-based multi-trajectory modeling to identify subgroups of participants that share trajectories on multiple indicators of fetal growth, namely, HC, AC, and FL z-scores.22 To conduct group-based multi-trajectory modeling, we identified the number of univariate trajectories needed to describe each growth parameter individually, and this number was used to determine a range of possible solutions for the multivariate trajectory model. Groups were optimized using the same statistical criteria described above.22 After optimization, group membership was assigned, and trajectories were visualized as previously described.
We examined the distribution of demographics, pregnancy characteristics, and birth outcomes by summarizing the median (IQR) or n (%) within each trajectory group compared to those observed in AGA births as a reference. Because this is a descriptive analysis, we highlight the magnitude of difference in these variables between groups rather than presenting p-values from statistical tests.23 We also examined how the identified trajectory groups compare to other ways of characterizing pathologically small fetal growth: suspected fetal growth restriction (i.e., EFW < 10th percentile on ultrasound after 20 weeks gestation) and low birth weight (i.e., birthweight < 2500 g). As a sensitivity analysis to account for uncertainty in group membership, we replicated these descriptive statistics using a case-weight approach, where the posterior inclusion probabilities of group membership were used as inverse probability weights in our analysis instead of assigning fixed trajectory groups.24 Additionally, we determined that our main conclusions were consistent when we relaxed statistical criteria and arbitrarily chose a higher number of group solutions for our latent class trajectory models (data not shown).
RESULTS
Participant characteristics and birth outcomes
This study comprises 249 SGA cases and 411 AGA controls (Table 1). Participants who delivered SGA were more likely to be primiparas, have lower pre-pregnancy BMI, and less likely to have access to private health insurance compared to AGA controls. They were also more likely to self-identify as a race or ethnicity other than non-Hispanic white and to give birth to a baby assigned female at birth. The distribution of birth outcomes, aside from birthweight z-scores, was similar between SGA and AGA births.
Table 1.
Maternal demographics, pregnancy characteristics, and birth outcomes among SGA cases and AGA controls in the LIFECODES Fetal Growth Study
| Variable | Median (25th, 75th percentile) or N (%) |
|
|---|---|---|
| SGA cases (N = 249) | AGA births (N = 411) | |
|
| ||
| Age, years | 32.0 (26.9, 35.5) | 32.4 (28.8, 36.2) |
| Education a | ||
| High school or less | 41 (17) | 49 (12) |
| Some college or associate degree | 54 (22) | 89 (22) |
| Bachelor’s degree or greater | 149 (61) | 268 (66) |
| Race and ethnicity | ||
| Non-Hispanic white | 113 (45) | 243 (59) |
| Non-Hispanic Black | 53 (21) | 55 (13) |
| Hispanic | 46 (l8) | 63 (15) |
| Other | 37 (15) | 50 (12) |
| Health insurance b | ||
| Private | 164 (66) | 298 (74) |
| Public | 83 (34) | 107 (26) |
| Pre-pregnancy BMIc, kg/m2 | 22.9 (20.7, 27.3) | 24.8 (21.5, 29.2) |
| Height, inches | 64 (62, 65) | 64 (63, 66) |
| Alcohol consumption in pregnancy d | ||
| No | 226(91) | 377 (93) |
| Yes | 21 (9) | 29 (7) |
| Smoking in pregnancy | ||
| No | 229 (92) | 379 (92) |
| Yes | 20 (8) | 32 (8) |
| Primiparous | ||
| No | 131(53) | 254 (62) |
| Yes | 118 (47) | 157 (38) |
| ART | ||
| No | 219 (88) | 364 (89) |
| Yes | 30 (12) | 47 (11) |
| Preeclampsia | ||
| No | 230 (92) | 397 (97) |
| Yes | 19 (8) | 14 (3) |
| Gestational diabetes | ||
| No | 239 (96) | 387 (94) |
| Yes | 10 (4) | 24 (6) |
| Infant sex e | ||
| Female | 142 (57) | 199 (48) |
| Male | 106(43) | 212 (52) |
| Gestational age at delivery, weeks | 39.0 (37.7, 39.6) | 39.0 (37.9, 39.7) |
| Birthweight z-score | −1.59 (−1.84, −1.42) | −0.15 (−0.64, 0.33) |
| Delivery via C-Section d | ||
| No | 151 (61) | 249 (61) |
| Yes | 95 (39) | 158 (39) |
| NICU Admission c | ||
| No | 212 (86) | 363 (89) |
| Yes | 34 (14) | 45 (11) |
| Length of NICU Stay, days | 7 (4, 18) | 5 (3, 17) |
Abbreviations: AGA = appropriate-for-gestational age, ART = assisted reproductive technologies, BMI = body mass index, NICU = neonatal intensive care unit, SGA = small-for-gestational age
n = 10 missing;
n = 8 missing;
n = 6 missing;
n = 7 missing;
n = 1 missing.
Note: Other race and ethnicity includes Asian (n = 50), More than one race (n = 19) and Other (n = 18). Public insurance category includes those using Medicaid/Mass Health/SSI (n = 180), Self pay (n = 7) and no health insurance (n = 3). Length of NICU stay only calculated among individuals with a known NICU admission (n = 79).
Ultrasound measures of fetal growth
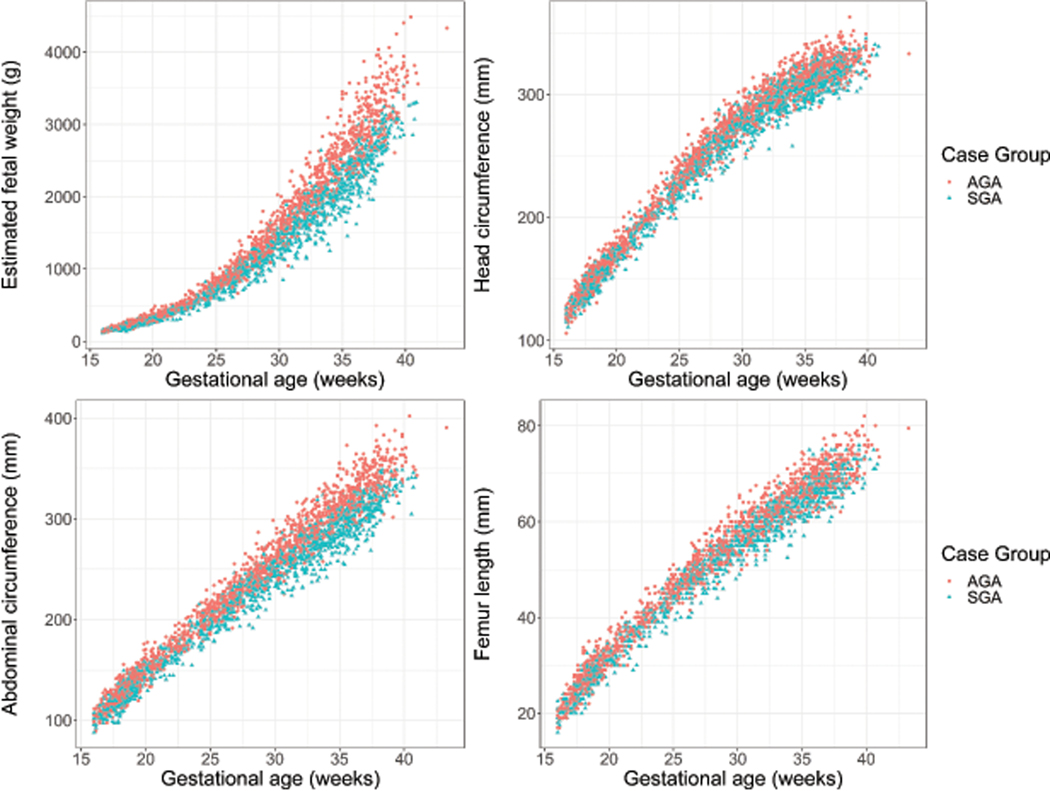
Participants in this study had an average of 4.5 ultrasound visits. The distribution of ultrasound measures of fetal growth according to case status are shown in Figure 2. Among SGA cases in this study, 245 (98%) had at least one recorded measure of each growth parameter and were used in subsequent growth trajectory modeling.
Figure 2.
Distribution of ultrasound measures of fetal growth by case status in the LIFECODES Fetal Growth Study. Measures of fetal growth abstracted from ultrasounds (i.e., estimated fetal weight, head circumference, abdominal circumference, and femur length) are shown according to case status. AGA = appropriate-for-gestational age, SGA = small-for-gestational age.
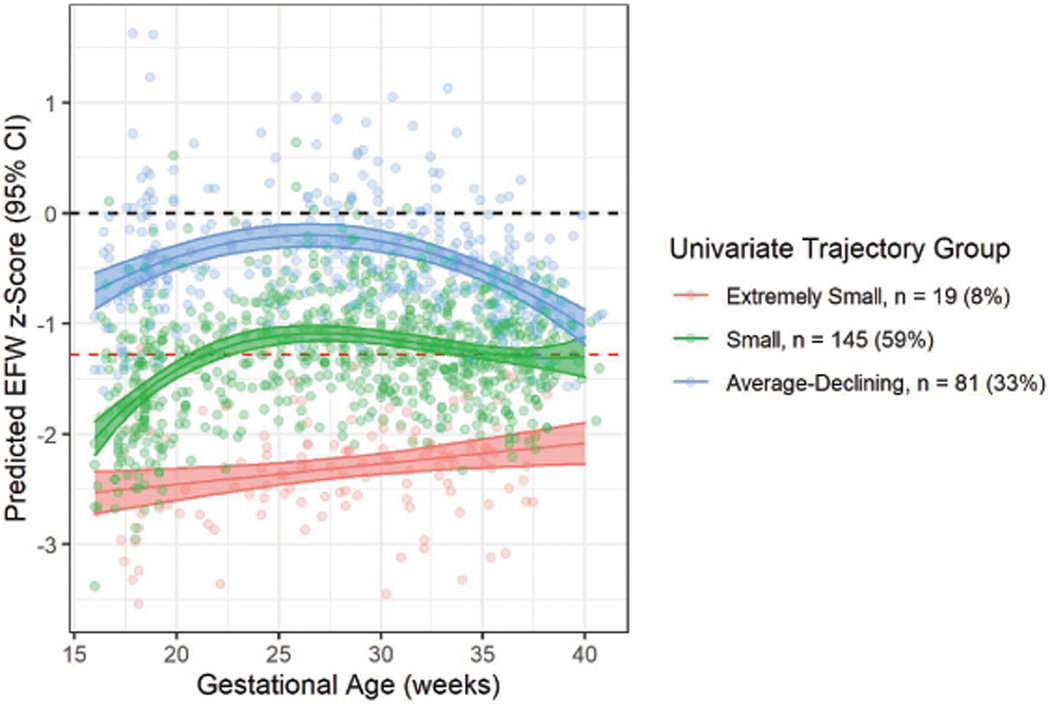
Univariate trajectories of fetal growth among SGA births
For EFW trajectories, a 3-group solution was the best fit (Figure 3; Supplemental Table 1). The groups identified are described as “Extremely Small” (n = 19 [8%]), where fetuses had the smallest EFW z-scores across gestation; “Small” (n = 145 [59%]), where fetuses had z-scores close to the 10th percentile across most of gestation; and “Average-Declining” (n = 81 [33%]) in which fetuses had approximately average size in mid-pregnancy but whose relative size was small during late pregnancy (Table 2). Participants assigned to the “Extremely Small” group were more likely to self-identify as non-Hispanic Black, use public health insurance, be multiparous, and less likely to have received a bachelor’s degree compared to other SGA and AGA births (Table 3). In addition, participants in the “Extremely Small” group were also more likely to have used assisted reproductive technologies to conceive and moderately more likely to be diagnosed with preeclampsia compared to other SGA and AGA births, though numbers were small.
Figure 3.
Predicted mean EFW z-scores (95% CI) in univariate EFW trajectory groups identified among SGA births in the LIFECODES Fetal Growth Study. Red dashed line indicates reference line for defining SGA (z-score = −1.28). Black dashed line indicates average growth (z-score = 0). EFW = estimated fetal weight.
Table 2.
Descriptions of fetal growth phenotypes identified by univariate and multivariate latent class trajectory models in the LIFECODES Fetal Growth Study.
| Univariate trajectory groups | ||
|---|---|---|
|
| ||
| Group | Name | Description |
|
| ||
| 1 | Extremely Small | Has modest consistent increase in growth rate, but overall, the smallest relative EFW from mid- to late pregnancy. |
| 2 | Small | Exhibits an increase in relative EFW during mid-pregnancy. Growth rate stabilizes in late pregnancy and relative size remains small (e.g., close to 10th percentile). |
| 3 | Average-Declining | EFW within normal range throughout, but growth rate declines after approximately 30 weeks gestation. |
|
| ||
| Multivariate trajectory groups | ||
|
| ||
| Group | Name | Description |
|
| ||
| 1 | Extremely Small | Smallest relative size of HC, AC, FL from mid- to late pregnancy with constant growth rate. |
| 2 | Small-Asymmetric HC:AC | Growth within normal range in mid-pregnancy. Relative HC normal through late pregnancy, but AC and FL growth rate slows across pregnancy. |
| 3 | Small-Symmetric HC:AC | All growth parameters within small or normal range from mid- to late-pregnancy. Stable growth rate from mid- to late pregnancy. |
| 4 | Average-Declining | Normal HC, AC, and FL size throughout, but growth rate slows across pregnancy for all measures of growth. |
Table 3.
Maternal demographics, pregnancy characteristics, and birth outcomes for univariate EFW trajectory groups in SGA cases and AGA controls in the LIFECODES Fetal Growth Study
| Median (25th, 75th percentile) or n (%) |
||||
|---|---|---|---|---|
| Variable | AGA Controls (N = 411) | Group 1: Extremely Small (N = 19) | Group 2: Small (N = 145) | Group 3: Average-Declining (N = 81) |
|
| ||||
| Age, years | 32.4 (28.8, 36.2) | 30.5 (24.7, 39.7) | 31.7 (26.9, 35.5) | 32.4 (28.1, 35.5) |
| Education a | ||||
| High school or less | 49 (12) | 4 (22) | 26 (18) | 11(14) |
| Some college or associate degree | 89 (22) | 5 (28) | 31 (22) | 17 (21) |
| Bachelor’s degree or greater | 268 (66) | 9 (50) | 84 (60) | 53 (65) |
| Race and ethnicity | ||||
| Non-Hispanic white | 243 (59) | 7 (37) | 66 (46) | 38 (47) |
| Non-Hispanic Black | 55 (13) | 7 (37) | 33 (23) | 13 (l6) |
| Hispanic | 63 (15) | 3 (16) | 24 (17) | 19(23) |
| Other | 50 (12) | 2 (11) | 22 (15) | 11(14) |
| Health insurance b | ||||
| Private | 298 (74) | 9 (47) | 92 (64) | 60 (74) |
| Public | 107 (26) | 10 (53) | 51 (36) | 21 (26) |
| Pre-pregnancy BMIc, kg/m2 | 24.8 (21.5, 29.2) | 23.2 (20.9, 27.9) | 22.7 (20.4, 27.4) | 23.2 (21.3, 26.5) |
| Height, inches | 64 (63, 66) | 64 (62, 65) | 63 (62, 65) | 64 (62, 65) |
| Alcohol consumption in pregnancy d | ||||
| No | 377(93) | 19(100) | 130 (91) | 73 (90) |
| Yes | 29 (7) | 0 (0) | 13 (9) | 8 (10) |
| Smoking in pregnancy | ||||
| No | 379 (92) | 18 (95) | 130 (90) | 77 (95) |
| Yes | 32 (8) | 1 (5) | 15 (10) | 4 (5) |
| Primiparous | ||||
| No | 254 (62) | 14 (74) | 80 (55) | 36 (44) |
| Yes | 157 (38) | 5 (26) | 65 (45) | 45 (56) |
| ART | ||||
| No | 364 (89) | 15 (79) | 131 (90) | 69 (85) |
| Yes | 47 (11) | 4 (21) | 14 (10) | 12 (15) |
| Preeclampsia | ||||
| No | 397 (97) | 16 (84) | 136 (94) | 74 (91) |
| Yes | 14 (3) | 3 (16) | 9 (6) | 7 (9) |
| Gestational diabetes | ||||
| No | 387 (94) | 19(100) | 138 (95) | 78 (96) |
| Yes | 24 (6) | 0 (0) | 7 (5) | 3 (4) |
| Infant sex e | ||||
| Female | 199 (48) | 14 (74) | 84 (58) | 42 (52) |
| Male | 212 (52) | 5 (26) | 60 (42) | 39 (48) |
| Gestational age at delivery, weeks | 39.0 (37.9, 39.7) | 37.6 (36.0, 38.7) | 38.6 (37.6, 39.3) | 39.3 (38.7, 40.1) |
| Birthweight z-score | −0.15 (−0.64, 0.33) | −2.31 (−2.70, −1.63) | −1.62 (−1.84, −1.44) | −1.50 (−1.69, −1.38) |
| Delivery via C-Section d | ||||
| No | 249 (61) | 7 (37) | 90 (63) | 51 (64) |
| Yes | 158 (39) | 12 (63) | 53 (37) | 29 (36) |
| NICU Admission c | ||||
| No | 363 (89) | 7 (39) | 127 (89) | 74 (91) |
| Yes | 45 (11) | 11 (61) | 16 (11) | 7 (9) |
| Length of NICU Stay, days | 5 (3, 17) | 9 (5, 31) | 7 (3.5, 16.5) | 4 (4, 12) |
Abbreviations: AGA = appropriate-for-gestational age, ART = assisted reproductive technologies, BMI = body mass index, NICU = neonatal intensive care unit, SGA = small-for-gestational age
n = 10 missing;
n = 8 missing;
n = 6 missing;
n = 7 missing;
n = 1 missing.
Note: Other race and ethnicity includes Asian (n = 50), More than one race (n = 19) and Other (n = 18). Public insurance category includes those using Medicaid/Mass Health/SSI (n = 180), Self pay (n = 7) and no health insurance (n = 3). Length of NICU stay only calculated among individuals with a known NICU admission (n = 79).
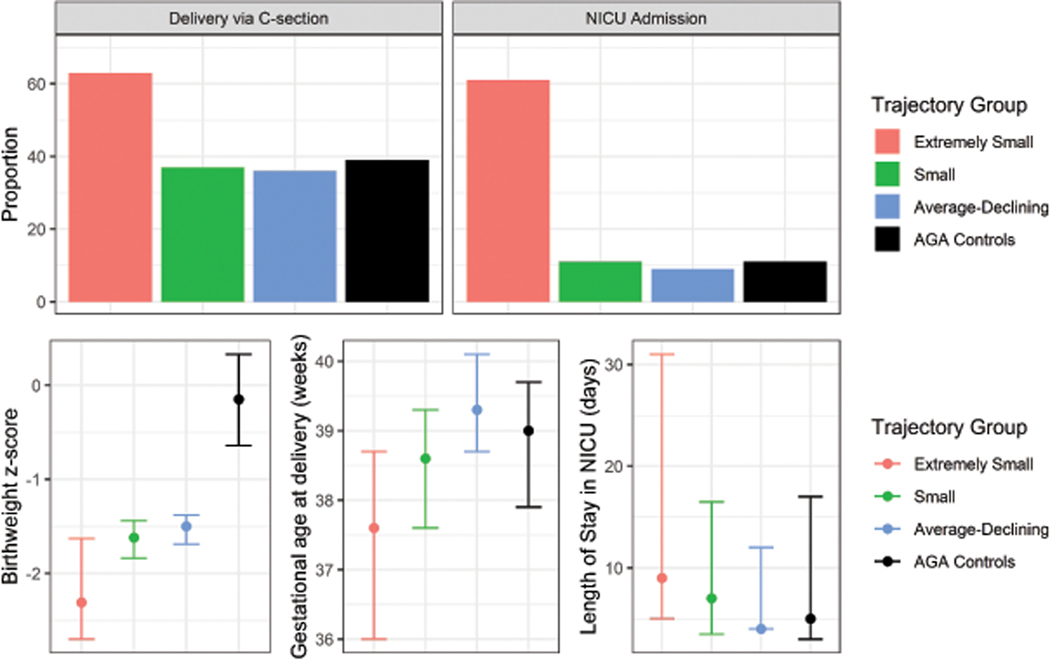
With respect to birth outcomes, participants in the “Extremely Small” group had earlier gestational ages at the time of delivery, smaller birthweight z-scores, the greatest proportion of babies admitted to the NICU, and longer lengths of stay in the NICU compared to other SGA and AGA births (Figure 4; Table 3). Findings were similar when our case-weight approach was used as an alternative to fixed group assignments (Supplemental Table 2).
Figure 4.
Proportion or median (25th, 75th percentiles) of observed birth outcomes in pregnancies within univariate EFW trajectory groups identified among SGA births and AGA controls in the LIFECODES Fetal Growth Study. AGA = appropriate-for-gestational age, NICU = neonatal intensive unit. Data underlying this figure can be found in Table 2.
When compared to other definitions of disordered fetal growth, most participants assigned to the “Extremely Small” group were also classified as low birthweight or having fetal growth restriction based on ultrasound measures of growth in the clinic (Supplemental Table 3). Likewise, a substantial proportion of participants in the “Small” trajectory group were deemed to have fetal growth restriction or low birthweight.
Multivariate trajectories of fetal growth among SGA births
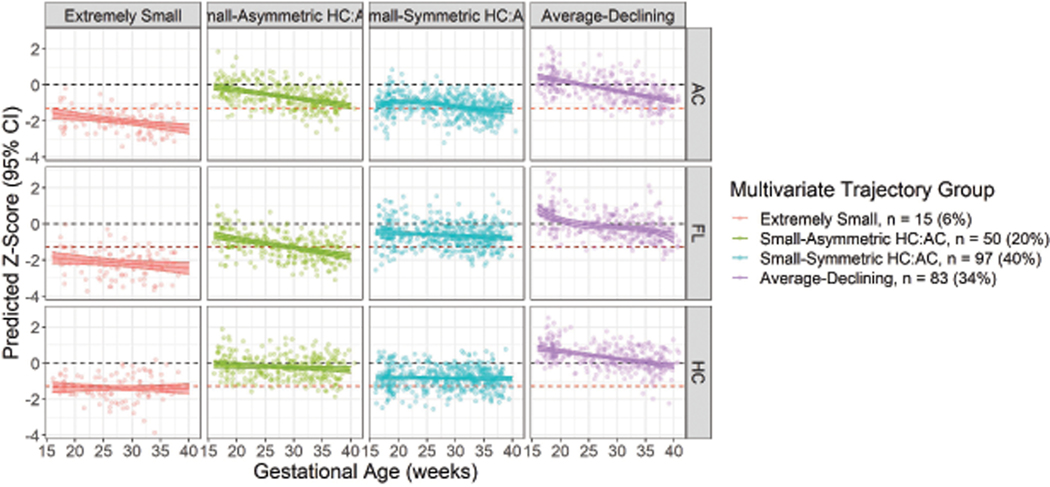
A 4-group multi-trajectory solution was deemed optimal (Table 2; Figure 5; Supplemental Table 4). Again, we identified an “Extremely Small” (n = 15 [6%]) group and an “Average-Declining” (n = 83 [34%]) group. We additionally identified two groups distinguished by the symmetry of their HC to AC and FL, named “Small-Asymmetric HC:AC” (n = 50 [20%]) and “Small-Symmetric HC:AC” (n = 97 [40%]). These groups had similar patterns of demographic characteristics as the groups identified above (Table 4).
Figure 5.
Predicted HC, AC, and FL z-scores (95% CI) in multivariate trajectory groups identified among SGA births in the LIFECODES Fetal Growth Study. Red dashed line indicates reference line for defining SGA (z-score = −1.28). Black dashed line indicates average growth (z-score = 0). AC = abdominal circumference, HC = head circumference, FL = femur length.
Table 4.
Maternal demographics, pregnancy characteristics, and birth outcomes of multivariate fetal growth trajectories identified among SGA births and AGA controls in the LIFECODES Fetal Growth Study
| Variable | Median (25th, 75th percentile) or n (%) |
||||
|---|---|---|---|---|---|
| AGA Controls (N = 411) | Group 1: Extremely Small (N = 15) | Group 2: Small-Asymmetric HC:AC (N = 50) | Group 3: Small-Symmetric HC:AC (N = 97) | Group 4: Average-Declining (N = 83) | |
|
| |||||
| Age, years | 32.4 (28.8, 36.2) | 30.5 (24, 39.7) | 33.3 (29.4, 36.8) | 31.53 (26.9, 34.8) | 32.0 (26.7, 35.1) |
| Education a | |||||
| High school or less | 49 (12) | 3 (21) | 7 (14) | 17 ( 18) | 14(17) |
| Some college or associate degree | 89 (22) | 3 (21) | 9 (18) | 24 (25) | 17 (21) |
| Bachelor’s degree or greater | 268 (66) | 8 (57) | 34 (68) | 54 (57) | 50 (62) |
| Race and ethnicity | |||||
| Non-Hispanic white | 243 (59) | 5 (33) | 24 (48) | 43 (44) | 39 ( 47) |
| Non-Hispanic Black | 55 (13) | 6 (40) | 8 (16) | 27 (28) | 12 (14) |
| Hispanic | 63 (15) | 2 (13) | 8 (16) | 17 (18) | 19(23) |
| Other | 50 (12) | 2 (13) | 10 (20) | 10 (10) | 13 (l6) |
| Health insurance b | |||||
| Private | 298 (74) | 7 (47) | 33 ( 66) | 61 ( 63) | 60 ( 74) |
| Public | 107(26) | 8 (53) | 17 (34) | 36 (37) | 21 (26) |
| Pre-pregnancy BMIc, kg/m2 | 24.8 (21.5, 29.2) | 23.0 (20.1, 27.6) | 22.1 (20.2, 27.5) | 23.2 (20.8, 27.4) | 23.1 (21.1, 26.5) |
| Height, inches | 64 (63, 66) | 64 (61, 64) | 63 (62, 65) | 64 (62, 65) | 63 (62, 65) |
| Alcohol consumption in pregnancy d | |||||
| No | 377 (93) | 15(100) | 44 ( 88) | 89 ( 92) | 74 ( 91) |
| Yes | 29 (7) | 0 (0) | 6 (12) | 8 (8) | 7 (9) |
| Smoking in pregnancy | |||||
| No | 379 (92) | 14 (93) | 43 ( 86) | 90 ( 93) | 78 ( 94) |
| Yes | 32 (8) | 1 (7) | 7 (14) | 7 (7) | 5 (6) |
| Primiparous | |||||
| No | 254 (62) | 11 ( 73) | 27 ( 54) | 58 ( 60) | 34 (41) |
| Yes | 157 (38) | 4 (27) | 23 (46) | 39 (40) | 49 (59) |
| ART | |||||
| No | 364 (89) | 12 (80) | 42 ( 84) | 90 ( 93) | 71 ( 86) |
| Yes | 47 (11) | 3 (20) | 8 (16) | 7 (7) | 12 (14) |
| Preeclampsia | |||||
| No | 397 (97) | 14 (93) | 45 (90) | 91 (94) | 76 (92) |
| Yes | 14 (3) | 1 (7) | 5 (10) | 6 (6) | 7 (8) |
| Gestational diabetes | |||||
| No | 387 (94) | 15(100) | 47 (94) | 93 (96) | 80 (96) |
| Yes | 24 (6) | 0 (0) | 3 (6) | 4 (4) | 3 (4) |
| Infant sex e | |||||
| Female | 199(48) | 10 (67) | 27 (54) | 63 (66) | 40 (48) |
| Male | 212 (52) | 5 (33) | 23 (46) | 33 (34) | 43 (52) |
| Gestational age at delivery, weeks | 39.0 (37.9, 39.7) | 37.1 (36.0, 38.6) | 38.2 (37.4, 39.4) | 38.7 (37.6, 39.3) | 39.3 (38.7, 40.1) |
| Birthweight z-score | −0.15 (−0.64, 0.33) | −2.51 (−2.70, − 1.63) | −1.59 (−1.84, −1.41) | −1.66 (−1.87, − 1.43) | −1.51 (−1.69, − 1.38) |
| Delivery via C-Section d | |||||
| No | 249(61) | 6 (40) | 31 (63) | 58 (60) | 53 (65) |
| Yes | 158 (39) | 9 (60) | 18 (37) | 38 (40) | 29 (35) |
| NICU Admission c | |||||
| No | 363 (89) | 5 (33) | 39 (80) | 86 (91) | 7S (94) |
| Yes | 45 (11) | 10 (67) | 10 (20) | 9 (9) | 5 (6) |
| Length of NICU Stay, days | 5 (3, 17) | 9 (5, 22) | 8 (4, 20) | 7 (2, 18) | 4 (4, 6) |
Abbreviations: AGA = appropriate-for-gestational age, ART = assisted reproductive technologies, BMI = body mass index, NICU = neonatal intensive care unit, SGA = small-for-gestational age
n = 10 missing;
n = 8 missing;
n = 6 missing;
n = 7 missing;
n = 1 missing.
Note: Other race and ethnicity includes Asian (n = 50), More than one race (n = 19) and Other (n = 18). Public insurance category includes those using Medicaid/Mass Health/SSI (n = 180), Self pay (n = 7) and no health insurance (n = 3). Length of NICU stay only calculated among individuals with a known NICU admission (n = 79).
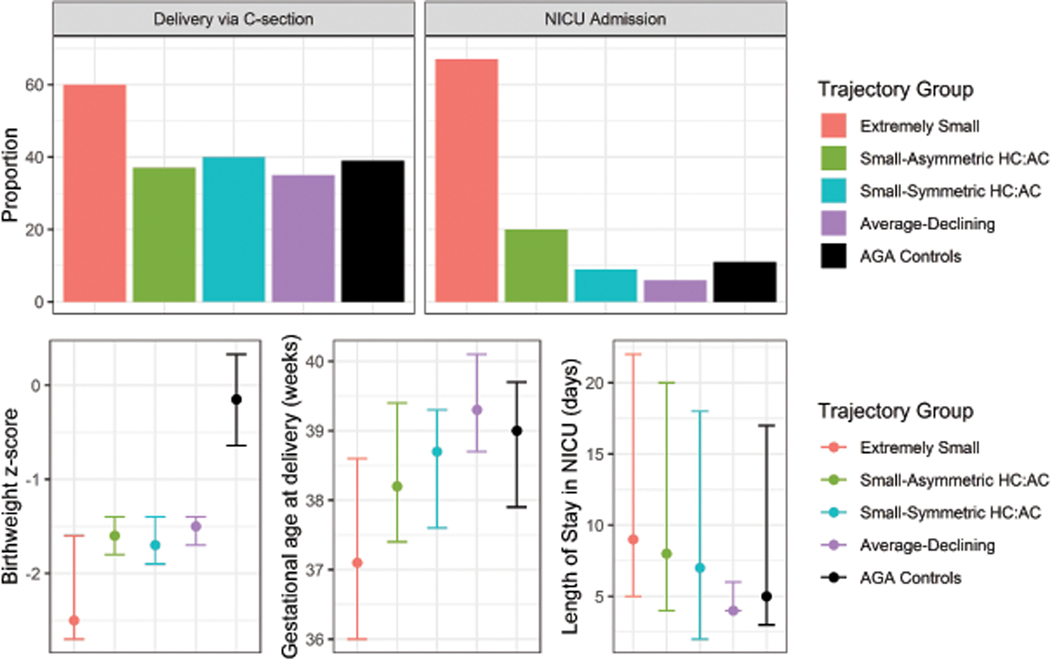
With respect to birth outcomes, the “Extremely Small” group had the earliest gestational age at delivery, lowest birthweight z-scores, highest proportion of babies admitted to the NICU with longer NICU stays relative to the other SGA births and AGA controls. Interestingly, the “Small-Asymmetric HC:AC” group also had a moderately elevated risk of NICU admission relative to both the “Small-Symmetric HC:AC” and “Average-Declining” trajectories and AGA controls (Figure 6; Table 4). In our sensitivity analysis using a case-weight approach, findings were largely consistent (Supplemental Table 5).
Figure 6.
Proportion or median (25th, 75th percentiles) of observed birth outcomes in pregnancies within multivariate trajectory groups identified among SGA births and AGA controls in the LIFECODES Fetal Growth Study. AGA = appropriate-for-gestational age, NICU = neonatal intensive unit. Data underlying this figure can be found in Table 3.
Most participants grouped as “Extremely Small” were also classified as low birthweight or having suspected fetal growth restriction based on ultrasound measures of growth in the clinic. Similar proportions of participants in either the “Small-Asymmetric HC:AC” and “Small-Symmetric HC:AC” groups were also identified as having fetal growth restriction or low birthweights (Supplemental Table 3).
Most of the participants in the univariate “Extremely Small” trajectory group were also assigned to the “Extremely Small” multivariate trajectory group (Table 5). Similarly, most participants in the univariate “Average-Declining” trajectory group were grouped in the “Average-Declining” multivariate trajectory group. Participants in the univariate “Small” trajectory group were primarily assigned to either the “Small-Asymmetric HC:AC” or the “Small-Symmetric HC:AC” multivariate trajectory groups.
Table 5.
N (%) comparing univariate and multivariate trajectory group membership among SGA births.
| Univariate Trajectory Group |
||||
|---|---|---|---|---|
| Group 1: Extremely Small | Group 2: Small | Group 3: Average-Declining | ||
| Multivariate Trajectory | Group 1: Extremely Small | 15 (79) | 0 (0) | 0 (0) |
| Group 2: Small-Asymmetric HC:AC | 0 (0) | 41 (28) | 9 (11) | |
| Group 3: Small-Symmetric HC:AC | 4 (21) | 91 (63) | 2 (2) | |
| Group 4: Average-Declining | 0 (0) | 13 (9) | 70 (86) | |
COMMENT
Principal findings
In this study, we characterized phenotypes of fetal growth based on longitudinal ultrasound measurements among SGA births in the LIFECODES Fetal Growth Study. Pregnancies within these groups had unique characteristics, which could reflect unique causes as well as consequences that are not captured by the SGA aggregate. Both our univariate and multivariate approaches identified a group of fetuses with the smallest EFW across pregnancy that were more likely to have adverse outcomes, such as NICU admission. However, the multivariate approach additionally distinguished phenotypes of asymmetric and symmetric growth.
Results in the context of what is known
Our findings for univariate trajectories of EFW among SGA births are consistent with results from the Generation R study, which also identified three growth trajectories.7 Similar to our analysis, the group of pregnancies where fetuses had the smallest relative growth from mid-to late-pregnancy were at highest risk of adverse neurodevelopmental outcomes at age six.7 In our analysis, babies that consistently had the smallest EFW (i.e., “Extremely Small”) were at the highest risk of NICU admission, had earlier gestational ages at delivery, and smaller birthweight z-scores relative to other SGA births and AGA controls in this cohort. Compared with existing approaches of classifying babies as growth restricted (any ultrasound with EFW < 10th percentile after 20 weeks gestation) or low birthweight (< 2500 g), most births classified as “Extremely Small” also met these definitions. However, most births that met a definition of fetal growth restriction or low birthweight were assigned to the “Small” group. This may indicate that our approach was better able to isolate a small group of high-risk infants compared to previous approaches based on either a single ultrasound measure or birthweight alone. However, further research is needed to interrogate the improvement this approach could offer over existing methods. Taken together, these findings suggest that babies with persistently small EFW throughout pregnancy may be at the highest risk of adverse outcomes and, therefore, could represent a growth restricted phenotype. On the other hand, participants in our “Average-Declining” group most closely resembled AGA controls with respect to demographics and birth outcomes and this phenotype may represent fetuses that are constitutionally small.
Despite similarities to previous analyses, there are some limitations to relying on EFW as a summary measure of growth, including: high error relative to birthweight, use of outdated equations based on non-generalizable populations, and that fetuses with different body compositions can obtain the same EFW despite different growth profiles.16,25,26 Thus, we applied multi-trajectory modeling to consider profiles of multiple growth indicators as a more comprehensive approach to assessing fetal growth. Using this approach, we identified similar “Extremely Small” and “Average-Declining” trajectory groups as our univariate approach. However, we also distinguished between phenotypes of “Small-Symmetric HC:AC” and “Small-Asymmetric HC:AC” growth, which primarily comprised fetuses that had been previously classified “Small” in our univariate analysis. Notably, these groups not only differed in body composition, but also in risk of adverse health outcomes. Overall, both the “Extremely Small” and “Small-Asymmetric HC:AC” groups had increased risk of NICU admission compared to other births. The growth profiles of these babies were also similar to previous descriptions of growth restricted phenotypes. Specifically, “Extremely Small” babies presented growth trajectories consistent with a description of symmetric growth restriction.27 The group of “Small-Asymmetric HC:AC” babies appeared consistent with definitions of asymmetric growth restriction.27 While the symmetry of fetal growth and its relationship to birth outcomes is debated27–30 and not included in current recommendations31,32 or consensus statements5,6 for assessing fetal growth restriction, our ability to recapitulate these previously described phenotypes demonstrates the utility of this method in describing fetal growth.
Clinical Implications
Numerous attempts have been made to utilize ultrasound measures of fetal growth, to improve the prediction of adverse birth outcomes beyond traditional approaches of classifying fetal growth restriction. These approaches include the use of repeated ultrasound measures (including across multiple dimensions of fetal size),33 growth velocity,34,35 and conditional growth measures.36,37 However, these approaches have yet to show consistent improvement over the use of a single measure of growth or size at birth. More recently, our group and others have explored the use of latent class methods, including trajectory modeling, to identify clinically meaningful phenotypes based on either longitudinal ultrasound measures of growth or the combination of clinical measures and size at birth.4,38,39 As noted by others, these methods are not well-suited for clinical applications as group memberships (e.g., growth trajectories) cannot be known until after delivery and instead are best suited to research applications. However, this work may ultimately have clinical relevance as our ability to evaluate clinical biomarkers for growth restriction will be improved when the outcome is better defined.
Research Implications
Our use of trajectory modeling identified subgroups of SGA births which may comprise fetuses with similar underlying mechanisms or causes of their relative smaller size at delivery.8 While these trajectory groups should not be thought of as rigid or distinct entities, they serve to approximate a more complicated reality by clustering individuals with similar growth patterns for downstream use.9 Given the differences in demographics and risk of birth outcomes observed across the identified groupings, these phenotypes may be more informative and preferable for epidemiologic studies when examining risk factors or outcomes that are hypothesized to relate to fetal growth.
Strengths and Limitations
This study was limited by restricting our analysis of growth trajectories to SGA births. While we expect that most babies experiencing fetal growth restriction would be considered SGA, our approach was not able to consider babies born AGA but who may still have not attained their full growth potential. Second, there were small sample sizes within groups, which may limit the number of groups identified and the degree to which the trajectories can be characterized. Yet, this study population still comprises a large number of SGA cases with rich clinical information and stored biospecimens not available in other studies, which will foster continued examination of these phenotypes. Third, given differences in the timing of delivery between trajectory groups (e.g., smallest babies deliver earlier), we may be limited in our ability to characterize growth profiles at later gestational ages. Fourth, infant and childhood follow-up is not a component of the LIFECODES study. Thus, the number of outcomes that can be examined in relation to trajectory groupings was limited. Last, the LIFECODES cohort recruits participants from several clinics at BWH, including a Maternal Fetal Medicine clinic, which may represent higher-risk pregnancies. Thus, growth trajectories may not be generalizable to all populations, although enrichment for high-risk pregnancies may have improved our ability to identify disordered growth trajectories.
A major strength of this study was the large number of ultrasounds (mean 4.5 per participant) relative to some previous studies taking a similar approach.7 Aided by this wealth of data, we identified trajectory groupings that were well separated and appear to be minimally impacted by uncertainty, as demonstrated by our case-weight sensitivity analysis. In addition, these findings validate previously identified EFW trajectory groups derived in a geographically distinct population using a different birthweight standard.7 Notably, rather than using only EFW measures to summarize fetal growth, we also applied group-based multi-trajectory modeling as a novel approach to characterize fetal growth across multiple dimensions. This allowed us to consider the overall body composition of fetuses rather than relying on a single measure, which we expect provides a more nuanced analysis of fetal growth.
Conclusions
We applied group-based trajectory modeling to identify univariate and multivariate trajectories of fetal growth among SGA births in the LIFECODES Fetal Growth Study. We identified several growth trajectories that either replicate findings from previous studies or that are cohesive with past attempts at phenotyping fetal growth. These trajectories present different associations with birth outcomes, including NICU admission, suggesting their possible utility in distinguishing pathological from constitutional fetal growth profiles.
Supplementary Material
CONDENSATION:
This study applies latent class trajectory modeling approaches to characterize phenotypes of fetal growth among small-for-gestational age births using repeated ultrasound measures.
AJOG AT A GLANCE:
Why was this study conducted?
This study aimed to contribute to the characterization of fetal growth by applying two related trajectory modeling approaches to repeated ultrasound measures of fetal growth.
What are the key findings?
Across both approaches utilized in this study, we observed that fetuses with the smallest relative growth across mid- to late pregnancy were at higher risk of experiencing adverse health outcomes at the time of delivery, suggesting that these births may represent a fetal growth restricted phenotype. We additionally identified a group with asymmetric growth that was also at moderately elevated risk of neonatal intensive care unit admission.
What does this add to what is known?
This study partially replicates and expands upon findings from studies taking a similar approach to characterizing fetal growth trajectories.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the LIFECODES study participants and the many BWH faculty involved in collecting and validating medical record information for making this work possible.
FUNDING:
This research was funded by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health (ZIA E103321). The funder had no role in the conduct of this research or preparation of this article.
Footnotes
CONFLICTS OF INTEREST:
The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mullis PE, Tonella P. Regulation of fetal growth: consequences and impact of being born small. Best Pract Res Clin Endocrinol Metab. 2008;22(1):173–190. [DOI] [PubMed] [Google Scholar]
- 2.van Wassenaer A.Neurodevelopmental consequences of being born SGA. Pediatr Endocrinol Rev. 2005;2(3):372–377. [PubMed] [Google Scholar]
- 3.Ananth CV, Vintzileos AM. Distinguishing pathological from constitutional small for gestational age births in population-based studies. Early Hum Dev. 2009;85(10):653–658. [DOI] [PubMed] [Google Scholar]
- 4.Hutcheon JA, Riddell CA, Himes KP. A New Approach for Classifying Fetal Growth Restriction. Epidemiology. 2021;32(6):860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beune IM, Bloomfield FH, Ganzevoort W, et al. Consensus Based Definition of Growth Restriction in the Newborn. J Pediatr. 2018;196:71–76.e71. [DOI] [PubMed] [Google Scholar]
- 6.Gordijn SJ, Beune IM, Thilaganathan B, et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol. 2016;48(3):333–339. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson KK, Sammallahti S, Rosen E, et al. Fetal Growth Trajectories Among Small for Gestational Age Babies and Child Neurodevelopment. Epidemiology. 2021;32(5):664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagin D.Group-based modeling of development. Harvard University Press; 2009. [Google Scholar]
- 9.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. [DOI] [PubMed] [Google Scholar]
- 10.McElrath TF, Lim K-H, Pare E, et al. Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy. American journal of obstetrics and gynecology. 2012;207(5):407. e401–407. e407. [DOI] [PubMed] [Google Scholar]
- 11.American College of Obstetrics and Gynecologists. Committee Opinion No 700: Methods for Estimating the Due Date. Obstet Gynecol. 2017;129(5):e150–e154. [DOI] [PubMed] [Google Scholar]
- 12.Cantonwine DE, Ferguson KK, Mukherjee B, et al. Utilizing Longitudinal Measures of Fetal Growth to Create a Standard Method to Assess the Impacts of Maternal Disease and Environmental Exposure. PLOS ONE. 2016;11(1):e0146532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements—A prospective study. American Journal of Obstetrics and Gynecology. 1985;151(3):333–337. [DOI] [PubMed] [Google Scholar]
- 14.Buck Louis GM, Eunice Kennedy Shriver National Institute of Child H, Human Development Fetal Growth Studies’ Research T, Grewal J. Clarification of estimating fetal weight between 10–14 weeks gestation, NICHD fetal growth studies. American journal of obstetrics and gynecology. 2017;217(1):96–101. [DOI] [PubMed] [Google Scholar]
- 15.Grantz KL, Grewal J, Kim S, et al. Unified standard for fetal growth: the Eunice Kennedy Shriver National Institute of Child Health and Human Development Fetal Growth Studies. American Journal of Obstetrics and Gynecology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papageorghiou AT, Kennedy SH, Salomon LJ, et al. The INTERGROWTH-21st fetal growth standards: toward the global integration of pregnancy and pediatric care. American Journal of Obstetrics & Gynecology. 2018;218(2):S630–S640. [DOI] [PubMed] [Google Scholar]
- 17.Grantz KL, Hediger ML, Liu D, Buck Louis GM. Fetal growth standards: the NICHD fetal growth study approach in context with INTERGROWTH-21st and the World Health Organization Multicentre Growth Reference Study. American Journal of Obstetrics and Gynecology. 2018;218(2, Supplement):S641S655.e628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: Patterns and prospects. Health Psychol. 2016;35(4):407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological methods & research. 2001;29(3):374–393. [Google Scholar]
- 20.Andruff H, Carraro N, Thompson A, Gaudreau P, Louvet B. Latent class growth modelling: a tutorial. Tutorials in Quantitative Methods for Psychology. 2009;5(1):11–24. [Google Scholar]
- 21.Klijn SL, Weijenberg MP, Lemmens P, van den Brandt PA, Lima Passos V. Introducing the fit-criteria assessment plot - A visualisation tool to assist class enumeration in group-based trajectory modelling. Stat Methods Med Res. 2017;26(5):2424–2436. [DOI] [PubMed] [Google Scholar]
- 22.Nagin DS, Jones BL, Passos VL, Tremblay RE. Group-based multi-trajectory modeling. Stat Methods Med Res. 2018;27(7):2015–2023. [DOI] [PubMed] [Google Scholar]
- 23.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. Annals of Internal Medicine. 2007;147(8):W-163-W-194. [DOI] [PubMed] [Google Scholar]
- 24.Kamata A, Kara Y, Patarapichayatham C, Lan P. Evaluation of Analysis Approaches for Latent Class Analysis with Auxiliary Linear Growth Model. Frontiers in Psychology. 2018;9(130). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dudley NJ. A systematic review of the ultrasound estimation of fetal weight. Ultrasound Obstet Gynecol. 2005;25(1):80–89. [DOI] [PubMed] [Google Scholar]
- 26.Nesbitt-Hawes EM, Tetstall E, Gee K, Welsh AW. Ultrasound (in)accuracy: it’s in the formulae not in the technique - assessment of accuracy of abdominal circumference measurement in term pregnancies. Australas J Ultrasound Med. 2014;17(1):38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dashe JS, McIntire DD, Lucas MJ, Leveno KJ. Effects of symmetric and asymmetric fetal growth on pregnancy outcomes. Obstetrics & Gynecology. 2000;96(3):321–327. [DOI] [PubMed] [Google Scholar]
- 28.Lin C-C, Su S-J, River LP. Comparison of associated high-risk factors and perinatal outcome between symmetric and asymmetric fetal intrauterine growth retardation. American Journal of Obstetrics and Gynecology. 1991;164(6, Part 1):1535–1542. [DOI] [PubMed] [Google Scholar]
- 29.Suhag A, Berghella V. Intrauterine Growth Restriction (IUGR): Etiology and Diagnosis. Current Obstetrics and Gynecology Reports. 2013;2(2):102–111. [Google Scholar]
- 30.Hiersch L, Melamed N. Fetal growth velocity and body proportion in the assessment of growth. American Journal of Obstetrics and Gynecology. 2018;218(2, Supplement):S700–S711.e701. [DOI] [PubMed] [Google Scholar]
- 31.Melamed N, Baschat A, Yinon Y, et al. FIGO (International Federation of Gynecology and Obstetrics) initiative on fetal growth: Best practice advice for screening, diagnosis, and management of fetal growth restriction. International Journal of Gynecology & Obstetrics. 2021;152(S1):3–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gynecologists ACoOa. Fetal Growth Restriction: ACOG Practice Bulletin, Number 227. Obstetrics & Gynecology. 2021;137(2):e16–e28. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Kim S, Grewal J, Albert PS. Predicting large fetuses at birth: do multiple ultrasound examinations and longitudinal statistical modelling improve prediction? Paediatr Perinat Epidemiol. 2012;26(3):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grantz KL, Kim S, Grobman WA, et al. Fetal growth velocity: the NICHD fetal growth studies. Am J Obstet Gynecol. 2018;219(3):285.e281–285.e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavallaro A, Veglia M, Svirko E, Vannuccini S, Volpe G, Impey L. Using fetal abdominal circumference growth velocity in the prediction of adverse outcome in near-term small-for-gestational-age fetuses. Ultrasound Obstet Gynecol. 2018;52(4):494–500. [DOI] [PubMed] [Google Scholar]
- 36.Hutcheon JA, Egeland GM, Morin L, Meltzer SJ, Jacobsen G, Platt RW. The predictive ability of conditional fetal growth percentiles. Paediatr Perinat Epidemiol. 2010;24(2):131–139. [DOI] [PubMed] [Google Scholar]
- 37.Hutcheon JA, Jacobsen GW, Kramer MS, Martinussen M, Platt RW. Small Size at Birth or Abnormal Intrauterine Growth Trajectory: Which Matters More for Child Growth? Am J Epidemiol. 2016;183(12):1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barker ED, McAuliffe FM, Alderdice F, et al. The Role of Growth Trajectories in Classifying Fetal Growth Restriction. Obstetrics & Gynecology. 2013;122(2 PART 1):248–254. [DOI] [PubMed] [Google Scholar]
- 39.Slaughter JC, Herring AH, Thorp JM. A Bayesian latent variable mixture model for longitudinal fetal growth. Biometrics. 2009;65(4):1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.