Abstract
Objective:
To investigate the influence of contextual factors on self-reports of cognitive abilities, this study investigated whether the frequency of self-reported memory failures was affected by level of daily busyness (Busyness) and daily routines (Routine) and whether age moderated these relations. The influence of the COVID-19 pandemic on self-reported memory failures also was explored.
Method:
902 community-dwelling participants (mean age= 56.98 years; sd= 18.96; range: 22–97 years) completed self-report questionnaires. Multiple linear regressions examined predictors of the frequency of retrospective (RM) and prospective memory (PM) failures and interactions with age. A pilot measure of the Influence of the Pandemic was added in secondary analyses.
Results:
Frequency of PM failures was significantly predicted by Age, Busyness, and Routine, such that people who were younger and those with busier and less routine activities reported more frequent PM failures. Frequency of RM failures was significantly predicted by Busyness, and the Age x Busyness and Age x Routine interactions. Busyness was associated with more frequent RM failures for people of all ages, but the effect was stronger for younger people. By contrast, more routine daily schedules were associated with fewer RM failures only for older people. PM/RM failures were predicted by the Influence of the Pandemic in exploratory analyses.
Conclusions:
Self-reports of cognitive abilities are influenced by contextual factors in adults of all ages. Contextual factors, including everyday task demands, daily routines, and acute stressors that disrupt daily activities, should be considered when interpreting self-reports of cognitive abilities in research and clinical practice settings.
Keywords: Subjective Cognitive Complaints, Retrospective Memory, Prospective Memory, Contextual Factors, COVID-19
Introduction
Self-report of cognitive abilities has long been the topic of research within clinical science. Studies from the late twentieth century suggested that physicians tended to underestimate patients’ reports of their functional difficulties, including emotional and physical complaints, leading to patient dissatisfaction and lower quality of life (Calkins et al., 1991; Nelson et al., 1983). There has since been a shift to focus on patient-reported outcomes in the development of medical interventions (FDA, 2009) to aid in prediction of future cognitive decline in the case of impending neurodegeneration, and as sensitive measures of the cognitive and functional consequences of various neurological disorders (e.g., HIV, ADHD, MS). At the same time, numerous studies have shown that self-reports of cognitive abilities may be biased by non-cognitive, person-level factors, such as personality and mood, raising caution in interpretation of self-report methods as measures of raw cognitive capacity (Edmonds, Delano-Wood, Galasko, Salmon, & Bondi, 2014; Smit et al., 2021; Studer, Donati, Popp, & von Gunten, 2014). Contextual factors, such as a busy lifestyle and the complexity of daily activities, also have been shown to influence self-report measures of cognitive abilities. Although not as extensively investigated in studies of self-report (Gondo et al., 2010), increased task demands are known to affect performance and increase errors on objective tests, particularly in people with lower cognitive ability levels (Suchy, Ziemnik, Niermeyer, & Brothers, 2020). Thus, self-report may differ markedly between people with similar cognitive ability levels, depending on their daily task demands.
Understanding what self-report of memory failures may encompass is important because it can be weighted heavily in a clinical context. For example, subjective cognitive decline (SCD; Jessen et al., 2020, 2014), a condition in which cognitive difficulties are observed on self-report but not objective tests, has been identified as a significant risk factor for later cognitive decline in older people (Dufouil, Fuhrer, & Alpérovitch, 2005; Reisberg, Shulman, Torossian, Leng, & Zhu, 2014; Slot et al., 2018). In a meta-analysis reported by Mitchell and colleagues (2014), older adults who self-reported poor cognitive abilities but who did not demonstrate objective impairment showed a higher annual conversion rate to mild cognitive impairment (MCI; 6.6%) and dementia (2.3%) compared to older people without any complaint of self-reported cognitive abilities (1%; see also Mendonca et al., 2016). Additionally, among people aged 65 and older self-reported cognitive abilities worsen across the older adult lifespan (Larrabee & Cook III, 1994; Reid & MacLullich, 2006), a pattern that is consistent with data from objective cognitive tests (Burmester, Leathem, & Merrick, 2016; Jonker, Jonker, & Schmand, 2000). Larrabee and Cook (1994) found that 88% of people over 85-years-old reported complaints about their cognitive abilities as compared to 43% of those aged 65–74 years. Further, subjective cognitive reports correlate with performance on objective cognitive tests in people with HIV (Sheppard, Woods, Massman, & Gilbert, 2019) and lowered functional productivity in people with MS (Kobelt, Langdon, & Jönsson, 2019). As such, subjective reports of cognitive abilities have demonstrated utility in several clinical and research contexts.
A different pattern of subjective cognitive reports is observed in studies that include participants across the entire adult lifespan. For example, in a study of community-dwelling people aged 16 – 75 years old, subjective cognitive reports reflected worsening abilities up to age 55 followed by improvement between the ages of 55 and 75 and then a subsequent decline in people older than 75 (Begum et al., 2014). Investigators have used the term the “age-paradox” and the “age-PM paradox” to describe findings showing no differences between younger and older people on subjective reports of cognitive abilities, despite worse performance by older people on objective tests with standardized task demands (de Winter et al., 2015; Rendell & Craik, 2000). Further concern over using solely subjective reports as a proxy for cognitive capacity is raised by the fact that even in studies focused on older adults, subjective reports do not consistently correlate with objective cognitive measures. Edmonds and colleagues found that healthy older adults tend to overestimate their cognitive problems whereas those with dementia tend to underestimate cognitive difficulties (Edmonds, Delano-Wood, Galasko, Salmon, & Bondi, 2015). Even the association between subjective cognitive decline and risk for future impairment has not been consistently reported. Jonker and colleagues (2000) and Reid and MacLullich (2006) have suggested that the validity of subjective report of cognitive difficulties for predicting neurodegenerative disease might be dependent on whether cognitive impairment is present on objective tests at baseline.
The equivocal findings regarding self-reported cognitive abilities could be explained by a variety of factors. Person-level factors, other than objective cognitive ability level, have been shown to significantly influence self-reports. People with more symptoms of depression (Balash et al., 2013; Jessen et al., 2020; Lee, Sung, & Choi, 2020) and women compared to men (Brucki & Nitrini, 2009; Tomita et al., 2014) are more likely to report cognitive difficulties, although this sex difference is inconsistently found (Markova et al., 2017). Montejo and colleagues (2014) demonstrated that depression was a stronger predictor of self-reported cognitive difficulties than objective memory performance, but both depressive symptoms and objective memory performance made significant independent contributions to self-reported cognitive abilities. There also has been evidence of anxiety symptoms (Cooper et al., 2011; Dux et al., 2008), social functioning (Kuiper et al., 2017), and personality traits, such as neuroticism and self-directedness, influencing self-reported cognitive abilities (Pearman, Hertzog, & Gerstorf, 2014; Rönnlund, Vestergren, Mäntylä, & Nilsson, 2011).
In addition to person-level factors, external or contextual factors also may play an important role in self-reported cognition. Decades of research have shown that cognitive performance suffers under conditions of increased task demands and that age-related cognitive difficulties are exacerbated under conditions of high cognitive load on most tests, including measures of prospective memory (Kidder, Park, Hertzog, & Morrell, 1997; Park, Hertzog, Kidder, Morrell, & et al, 1997) and everyday function (Seligman, Giovannetti, Sestito, & Libon, 2014). Thus, people with higher demands (i.e., busier) in daily life will likely experience/report more cognitive difficulties than people with lower demands (i.e., less busy). Because older adults are more susceptible to cognitive failures under conditions that tax their cognitive resources (Kidder et al., 1997; Maujean et al., 2003; Park et al., 1997), the self-reports of older adults might be most strongly influenced by busyness. In fact, busyness predicted medication adherence errors in older adults with rheumatoid arthritis (Martin & Park, 2003). Also, in a large study conducted in people age 18 – 90 in Japan, Gondo and colleagues (2010) showed that people who reported higher rates of busyness also reported more frequent prospective memory (PM) failures; however, the relation between busyness and retrospective memory (RM) failures was observed only in older participants (i.e., people age 50 and older). Further, the effect of busyness on PM/RM failures was significant even after controlling for person-level variables, such as personality traits.
Other contextual factors might have a different effect on cognitive performance and self-report. For example, Martin and Park (2003) posited that the predictability of events in one’s schedule (i.e., routine) as a contextual factor that might preclude memory failures in daily life but found no relation between a measure of routine and errors in medication adherence in older people. Additionally, contrary to expectation, Gondo and colleagues (2010) showed that individuals aged 55 and over with more routine daily schedules reported more frequent memory failures. The authors raised the possibility that older people with cognitive difficulties might lead a more routinized life because of their functional limitations.
The Current Study
The current study examined the influence of contextual factors (e.g., busyness and routine) on the frequency of self-reported memory failures in a sample of adults aged 22 and older. We hypothesized that the frequency of self-reported memory failures would be significantly related to contextual factors, such that older and younger people who are busier and follow a less routine schedule would also report more frequent memory failures. We examined whether age had a moderating role on the association between contextual factors and frequency of memory failures and hypothesized that age would moderate the relation between contextual factors and the frequency of memory failures, such that older adults would be especially vulnerable to the increased task demands and cognitive load/burden associated with a busy and less routine schedule.
Additionally, because the current study was conducted from August 2020 through February 2021, which was in the first year of the global COVID-19 pandemic and prior to the widespread distribution of vaccines, we explored participants’ perception of the effect of the pandemic on the frequency of their self-reported memory failures. Due to the disruptive and burdensome effect that the pandemic had on individuals of all ages, we felt it important to explore its relation to self-reported cognitive abilities in secondary analyses.
Finally, we explored whether contextual factors differentially influenced prospective PM and RM. People differ in their RM and PM abilities, and caregivers report that PM impairments may be more disruptive to everyday functioning than RM impairments (Martin & Park, 2003). Thus, understanding how contextual factors influence PM versus RM could have implications for future work on environmental adaptations (e.g., decreasing task demands, increasing routine) to improve memory abilities.
Methods
Participants
This study was conducted in accordance with the Helsinki Declaration and was approved by the Temple University Institutional Review Board. Participants were community dwelling adults (ages 18+) from North America who were recruited to complete an online questionnaire via Temple University’s undergraduate psychology research pool, social media, word of mouth, and Qualtrics participant recruitment services. We sought an even distribution of participants across 10-year age bands. Qualtrics relies on an actively managed, double-opt-in market research panel to recruit participants based on designated inclusion/exclusion parameters. Regarding compensation, participants who were recruited via Temple University’s undergraduate pool were given course credit for their participation. Qualtrics determines compensation for the participants they recruit based on survey length and other factors and the types of rewards varies (e.g., cash, gift cards, airline miles, redeemable points, etc.).
As shown in Figure 1, 1908 participants responded to the survey. After eliminating participants that were flagged for robot detection, duplicates, suspiciously fast responses (survey completion times of less than 200 seconds), unusual responses to open-ended questions (e.g., responses that were either nonsensical and/or unrelated, such as “very nice” for all free response options), and unreasonable entries for year of birth (e.g., 207, 4444, 999, 1–11), 1514 participants remained. Because of the different lifestyle and demands on college students compared to adults outside of college, we chose to exclude participants younger than 22, leaving 1218 participants aged 22 and older. Finally, participants who did not respond to any of the items on either the PRMQ or the MPED questionnaires, which were the predictors/outcomes for our primary analyses, were excluded; the final sample included 902 participants, 204 of whom were recruited via word of mouth and 698 of whom were recruited via Qualtrics.
Figure 1.
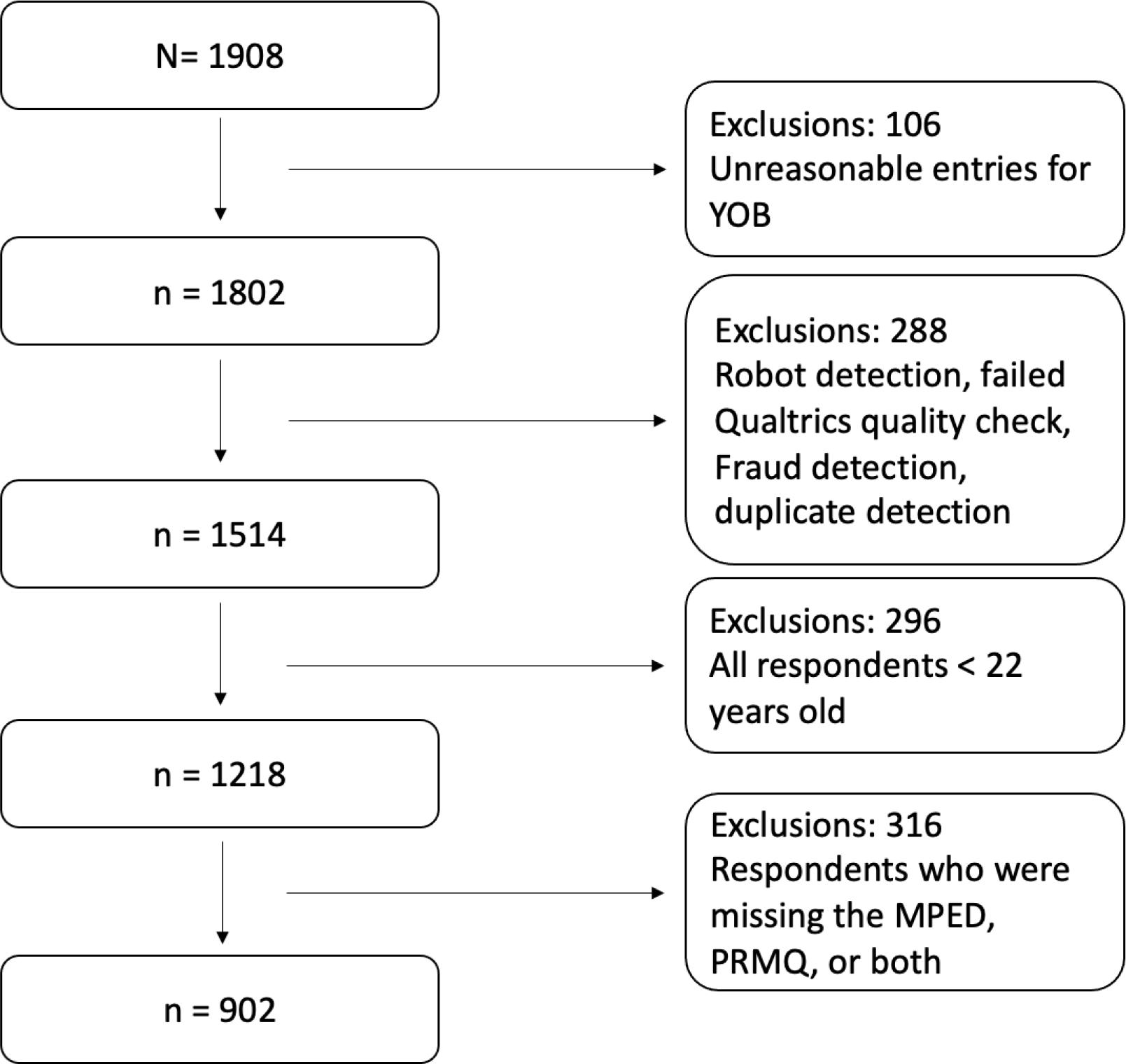
Participant Exclusion/Inclusion Flow Chart
Measures
Measures of demographic characteristics, frequency of memory failures (Prospective Retrospective Memory Questionnaire; Smith, Della Sala, Logie, & Maylor, 2000), contextual factors (Martin and Park Environmental Demands Questionnaire; Martin & Park, 2003), compensatory strategies (Memory Compensatory Questionnaire; De Frìas & Dixon, 2005), and everyday cognition (Everyday Cognition Questionnaire; Farias et al., 2008) were administered. A novel questionnaire on the performance of everyday activities (Temple Activities Engagement Questionnaire, TAEQ) also was administered. The order of the questionnaires was randomized and counterbalanced across participants. Participants completed all measures via the internet using Qualtrics software. The entire study took about 20–30 minutes. The PRMQ, MPED, and questions regarding demographic characteristics were the primary measures for the current study.
Frequency of Self-reported Memory Failures
Prospective and Retrospective Memory Failures.
The PRMQ (Smith, Della Sala, Logie, & Maylor, 2000) is a 16-item questionnaire with response options on a 5-point Likert scale (never [1]; very often [5]), asking participants to rate the frequency of specific memory failures over the past month. The PRMQ contains eight questions regarding the frequency of PM failures (e.g., “Do you forget to buy something you planned to buy [e.g., a birthday card], even when you see it in the store?”) and eight questions about the frequency of RM failures (e.g., “Do you fail to recall things that have happened to you in the last few days?”) in daily life. Separate scores were calculated for each type of memory failure (PM range = 8 – 40; RM range = 8 – 40), with higher scores representing more frequent memory failures. The PRMQ has strong psychometric properties, with good internal consistency for both the PRMQ total score (Cronbach’s alpha = .89), the PRMQ PM subscale (Cronbach’s alpha = 0.84), and the PRMQ RM subscale (Cronbach’s alpha = 0.80; Crawford et al., 2003). Previous research has also supported the construct validity of the PRMQ through factor analysis and comparisons across clinical populations with known memory impairment (Kliegel & Jäger, 2006; Smith et al., 2000; Woods et al., 2007; Zimprich, Kliegel, & Rast, 2011).
Contextual Factors
Busyness and Routine.
The MPED Questionnaire (Martin & Park, 2003) was administered to investigate the extent to which participants engaged in daily activities that made them busy (i.e., busyness) and how predictable and routinized their daily schedules typically are (i.e., routine). The MPED includes seven questions inquiring about participants’ level of daily busyness in the last month (e.g., “How often do you have too many things to do each day to actually get them done?”) and four questions about the degree to which participants maintain a routine schedule (e.g., “how often do your days follow a basic routine?”). Each question contains a response set on a 5-point Likert scale rating how frequently the item occurs (never [1]; very often [5]). Higher scores indicate higher self-reported busyness and higher self-reported routine. The MPED Questionnaire shows good internal consistency for the busyness subscale and fair for routine (Cronbach’s alpha for busyness = 0.88; 0.74 for routine). Martin and Park also demonstrated the external validity of the MPED in a sample of 121 community-dwelling adults between the ages of 34 and 84, as people who reported more busyness were younger, employed, lived in larger households, and reported more errors in taking their medications (Martin & Park, 2003).
Influence of COVID-19 Pandemic.
As the present study was conducted during a time of a global pandemic, we were interested in the extent to which the pandemic influenced participants’ responses to the questionnaires. Without a validated, existing measure to estimate the influence of the pandemic, we generated three novel questions for the current study. At the end of three study questionnaires (PRMQ, MPED, and the TAEQ), participants were asked to estimate the extent to which they believed the pandemic influenced their responses. Participants used a 5-point Likert scale, with lower response options indicating that COVID-19 had no influence (1) and higher options indicating that COVID-19 had a significant influence (5). Responses from the three questions were summed to create a measure of the Influence of the Pandemic (range = 3 to 15).
Statistical Analysis Plan
Prior to conducting the primary analyses, we obtained measures of internal consistency and descriptive statistics and then conducted bivariate correlations to determine relations among all study variables. Variables were transformed to z-scores (based on the mean and standard deviation of the full sample; M = 0, SD = 1 for the transformed variables) before inclusion in the regression equation to reduce multicollinearity, and calculation of interaction terms for tests of moderation involved creating cross-product terms using these standardized scores.
Multiple linear regression models were used to examine the main effects of contextual factors on self-reported memory failures and to determine whether age moderated the relations between contextual factors and self-reported memory failures. Separate models were run using PM failures and RM failures as the two outcome variables. Each regression equation included age and the contextual variables (i.e., Busyness and Routine) and the interactions between age and each contextual factor (e.g., Age × Busyness; Age × Routine). Standardized beta coefficients were examined to estimate effect sizes for each predictor in the regression models (<|0.2| = weak; | 0.2| < | 0.5| = moderate; > | 0.5| = strong (Acock, 2014; p. 272).
Post-hoc probing was conducted for significant interactions based on methods described in Holmbeck (2002). Specifically, we created conditional moderator variables (+/− 1 SD from the z-scored values for age) to reflect younger and older adults. We then created new interaction terms that included these conditional moderator variables. Post-hoc regressions were then performed, involving simultaneous entry of the contextual factor, the conditional moderator variable, and the interaction of these two predictors. From these regressions we derived unstandardized betas as the slopes and the intercepts in regression equations that were +/− 1 SD from the mean of the contextual factor.
Finally, to explore the effect that the COVID-19 pandemic has had on the frequency of self-reported memory failures additional, exploratory analyses were conducted that included the measure of the Influence of the Pandemic as well as the Age x Influence of the Pandemic interaction term.
Results
Missing Data
As stated earlier, of the 1218 participants aged 22 and older who participated in the study and provided valid responses, 316 did not respond to any items on the PRMQ and/or the MPED and were excluded from the study. We compared the final sample of 902 participants to the 316 who did not complete the primary study measures. Results showed that those who were missing PRMQ/MPED data were significantly older (t [1216] =5.38, p < 0.001; mean = 63.25 years old, sd = 14.05 vs. mean = 56.98, sd = 18.96) and included fewer people who self-reported Asian race and American Indian/Alaska Native race (X2 = 12.16, p = .02). There were no other demographic differences between those who completed the study and those who did not. Notably, the Influence of the Pandemic variable had several missing responses (n=894; <1% of our total sample), and analyses including this variable were conducted by excluding missing values pairwise.
Participant Demographic Characteristics
The distribution of the 902 participants across 10-year age bands and demographic data of each age band are shown in Table 1. Overall, 59.1% reported female sex and gender identity. Most of the sample self-reported non-Hispanic ethnicity (94.7%) and White (87.6%) race. Other self-reported racial identities included Black (4.7%), Asian (2.7%), or not listed (2.8%). Overall, 4.1% of the sample reported a current cognitive disorder (e.g., MCI, AD); 7.1% neurological disorder (e.g., stroke, seizures), 12.5% a history of concussion or traumatic brain injury (TBI), and 5.2% a past or current diagnosis of COVID-19.
Table 1.
Distribution of Participants and Demographic Data across Different Age Groups in the Final Study Sample (N = 902)
| 20’s | 30’s | 40’s | 50’s | 60’s | 70’s | 80’s | 90’s | Overall | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| n (% of total cohort) | 64 (7.10) | 153 (17.00) | 124 (13.70) | 127 (14.10) | 147 (16.30) | 152 (16.90) | 123 (13.60) | 12 (1.30) | 902 |
| Sex (F) | 50 | 93 | 66 | 80 | 81 | 85 | 74 | 8 | 537 |
| Gender (F) | 49 | 92 | 65 | 80 | 81 | 84 | 74 | 8 | 533 |
| Race (%)* | |||||||||
| White | 70.3 | 81.7 | 86.3 | 87.4 | 93.7 | 95.3 | 91.9 | 100 | 87.6 |
| Black | 7.8 | 8.5 | 6.5 | 6.3 | 2.7 | 0.7 | 2.4 | 0 | 4.7 |
| Asian | 12.5 | 4.6 | 0.8 | 0.8 | 2.0 | 1.3 | 1.6 | 0 | 2.7 |
| Not listed | 1.6 | 1.3 | 3.2 | 4.7 | 1.4 | 2.6 | 4.0 | 0 | 2.8 |
| Ethnicity (%)* | |||||||||
| Hispanic | 12.7 | 9.8 | 6.5 | 7.9 | 0.7 | 2.6 | 0.8 | 8.3 | 5.3 |
| Non-Hispanic | 85.9 | 90.2 | 93.5 | 92.1 | 98.0 | 97.4 | 99.2 | 91.7 | 94.7 |
| Mean Years of Education (SD) | 16.39 (1.82) | 15.17 (3.80) | 15.04 (4.07) | 14.43 (3.15) | 15.24 (3.02) | 15.70 (3.67) | 15.38 (3.15) | 14.20 (2.44) | 15.25 (3.42) |
| Cognitive Disorder Diagnosis (% yes) | 0 | 6.5 | 10.5 | 4.7 | 1.4 | 1.3 | 3.3 | 0 | 4.1 |
| Other Neurological Diagnosis (% yes) | 7.8 | 10.5 | 11.3 | 8.7 | 4.1 | 5.3 | 3.3 | 0 | 7.1 |
| History of TBI or Concussion (% yes) | 17.2 | 14.4 | 21 | 15.7 | 11.6 | 6.6 | 5.7 | 0 | 12.5 |
| Past or current COVID-19 diagnosis (% yes) | 1.6 | 7.8 | 10.5 | 8.7 | 1.4 | 1.3 | 4.1 | 8.3 | 5.2 |
Note: N=902
Several responses missing.
Internal Consistency of the PRMQ, MPED, and Questions about the Influence of the Pandemic
Cronbach’s alphas for the Total PRMQ (0.95) and separate PM (0.92) and RM (0.91) measures were excellent (Cicchetti, 1994). Internal consistency was excellent for the MPED Busyness items (Cronbach’s alpha = 0.92) and good for the Routine items (Cronbach’s alpha = 0.81). For the three questions concerning the Influence of the Pandemic, internal consistency was fair (Cronbach’s alpha of 0.73).
Frequency of Self-Reported Memory Failures
The mean of the frequency of reported PM and RM failures and descriptive statistics for all variables are reported in Table 2. PM and RM scores were comparable (i.e., within one sd) to published normative data obtained from a community-dwelling (non-clinical) sample ages 17–94 by Crawford and colleagues (2003), providing support for the validity of our participants’ responses.
Table 2.
Descriptive Statistics for the Study Variables
| Skewness | Kurtosis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Min | Max | Mean | SD | Statistic | SE | Statistic | SE | |
|
| |||||||||
| Prospective Memory Failures | 902 | 8 | 40 | 17.93 | 6.72 | 0.89 | 0.08 | 0.68 | 0.16 |
| Retrospective Memory Failures | 902 | 8 | 40 | 16.11 | 6.43 | 1.21 | 0.08 | 1.57 | 0.16 |
| Busyness | 902 | 7 | 35 | 16.58 | 6.60 | 0.65 | 0.08 | −0.22 | 0.16 |
| Routine | 902 | 4 | 20 | 13.71 | 3.51 | −0.42 | 0.08 | −0.12 | 0.16 |
| Influence of the Pandemic | 894 | 3 | 15 | 6.61 | 3.07 | 0.72 | 0.08 | −0.17 | 0.16 |
Note: <1% of participants were missing some of the items used to calculate Influence of the Pandemic were missing.
Bivariate correlations among the study variables are reported in Table 3 and showed a significant positive relation between RM and PM scores, such that individuals who reported more frequent PM failures also reported more frequent RM failures.
Table 3.
Bivariate Correlations among the Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
|
| ||||||
| 1. Age | - | |||||
| 2. Prospective Memory Failures | −.30** | - | ||||
| 3. Retrospective Memory Failures | −.27** | .89** | - | |||
| 4. Busyness | −.51** | .44** | .43** | - | ||
| 5. Routine | .17** | −.11** | −.07* | −.10** | - | |
| 6. Influence of the Pandemic | −.25** | .41** | .43** | .36** | 0.01 | - |
Note: N=902 for all variables except the influence of the pandemic. <1% of items were missing for the influence of the pandemic questions (n = 894).
p < 0.05.
p < 0.01.
Relations between PRMQ and Demographic Variables
Contrary to our expectation, there was a significant negative relation between age and the frequency of both types of memory failures (see Table 3), such that older people reported fewer PM and RM failures. Correlation coefficients also showed significant relations between the PRMQ and several demographic variables (not included in Table 3), such as education (PM: r = −.09, p < 0.01; RM: r = −.13, p < .001), reported history of concussion or TBI (PM: r = .16, p < .001; RM: r = .17, p < .001), report of cognitive impairment (PM: r = .29, p < .001; RM: r = .31, p < .001), report of other neurologic disease (e.g., epilepsy, Parkinson’s disease, etc.; PM: r = .18, p < .001; RM: r = .22, p < .001), and report of past or current COVID-19 infection (PM: r = .14, p < .001; RM: r = .16, p < .001). There was no relation between ethnicity and PRMQ measures and no difference between the different race groups on the PRMQ (ANOVA).
Contextual Factors
There was a weak but statistically significant negative relation between Busyness and Routine, such that people who reported being busier also reported less routine in their daily life (see Table 3). Age was significantly and negatively associated with Busyness and positively, though weakly, associated with Routine (see Table 3). Similarly, there were significant, positive relations between the frequency of PM and RM failures and Busyness. Relations between the frequency of PM and RM failures and Routine were significant, although weak, and negative, indicating that people who reported more memory failures also reported less routine in in their daily life.
Multivariate Effects of Contextual Factors on Reported Memory Failures and the Role of Age as a Moderating Factor
Hypotheses were tested with multivariate models that examined the effect of contextual factors on self-reported memory failures and the moderating role of age. Results are presented in Tables 4–5 and described below.
Table 4.
Regression Predicting the Frequency of Self-Reported Memory Failures
| Outcome | Predictor Variable | B | SE B | β |
|---|---|---|---|---|
|
| ||||
| Prospective Memory Failures | ||||
| Education | −0.17 | 0.06 | −0.09** | |
| TBI | 0.19 | 0.65 | 0.01 | |
| Cognitive Impairment | 6.56 | 1.06 | 0.19*** | |
| Neurologic Disorder | 1.48 | 0.82 | 0.06 | |
| COVID History | 1.24 | 0.95 | 0.04 | |
| Age | −0.56 | 0.22 | −0.09* | |
| Busyness | 2.18 | 0.25 | 0.32*** | |
| Routine | −0.44 | 0.21 | −0.06* | |
| Age × Busyness | −0.42 | 0.23 | −0.06 | |
| Age × Routine | −0.12 | 0.21 | −0.02 | |
| Retrospective Memory Failures | ||||
| Education | −0.25 | 0.06 | −0.13*** | |
| TBI | 0.30 | 0.61 | 0.02 | |
| Cognitive Impairment | 6.48 | 1.00 | 0.20*** | |
| Neurologic Disorder | 2.35 | 0.77 | 0.09** | |
| COVID History | 1.39 | 0.90 | 0.05 | |
| Age | −0.36 | 0.21 | −0.06 | |
| Busyness | 2.00 | 0.24 | 0.31*** | |
| Routine | −0.30 | 0.20 | −0.05 | |
| Age × Busyness | −0.59 | 0.21 | −0.09** | |
| Age × Routine | −0.39 | 0.20 | −0.06* | |
Note: N=902 for all variables.
Model predicting prospective memory failures: F(10, 860) = 30.71, p < .001.
Model predicting retrospective memory failures: F(10, 860) = 33.57, p < .001.
p < 0.05
p < 0.01
p < 0.001
Table 5.
Regressions Predicting the Frequency of Self-Reported Memory Failures with the Influence of the Pandemic Added to the Model
| Outcome | Predictor Variable | B | SE B | β |
|---|---|---|---|---|
|
| ||||
| Prospective Memory Failures | ||||
| Education | −0.19 | 0.06 | −0.10** | |
| TBI | 0.14 | 0.62 | 0.01 | |
| Cognitive Impairment | 5.78 | 1.02 | 0.17*** | |
| Neurologic Disorder | 1.39 | 0.79 | 0.05 | |
| COVID History | 0.68 | 0.93 | 0.02 | |
| Age | −0.37 | 0.21 | −0.06 | |
| Busyness | 1.72 | 0.25 | 0.26*** | |
| Routine | −0.49 | 0.20 | −0.07* | |
| Influence of the Pandemic | 1.68 | 0.22 | 0.25*** | |
| Age × Busyness | −0.23 | 0.22 | −0.03 | |
| Age × Routine | −0.07 | 0.20 | −0.01 | |
| Age × Influence of the Pandemic | −0.31 | 0.20 | −0.05 | |
| Retrospective Memory Failures | ||||
| Education | −0.28 | 0.05 | −0.14*** | |
| TBI | 0.25 | 0.58 | 0.01 | |
| Cognitive Impairment | 5.62 | 0.96 | 0.17*** | |
| Neurologic Disorder | 2.24 | 0.74 | 0.09** | |
| COVID History | 0.74 | 0.87 | 0.02 | |
| Age | −0.16 | 0.20 | −0.03 | |
| Busyness | 1.53 | 0.23 | 0.24*** | |
| Routine | −0.36 | 0.19 | −0.06 | |
| Influence of the Pandemic | 1.72 | 0.20 | 0.27*** | |
| Age × Busyness | −0.37 | 0.21 | −0.05 | |
| Age × Routine | −0.32 | 0.19 | −0.05 | |
| Age × Influence of the Pandemic | −0.49 | 0.19 | −0.08** | |
Note: N = 902 for all variables except the influence of the pandemic. <1% of items were missing for the influence of the pandemic questions (n = 894).
Model predicting prospective memory failures: F(12, 853) = 33.47, p < .001.
Model predicting retrospective memory failures: F(12, 853) = 39.10, p < .001.
p < 0.05
p < 0.01
p < 0.001
PM.
As shown in Table 4, after controlling for demographic factors, Age, Busyness, and Routine were significant predictors of PM failures. All of the statistically significant standardized beta coefficients reflected weak effect sizes, with the exception of the coefficient for Busyness, which indicated a moderate effect.
RM.
As shown in Table 4, after controlling for demographic variables, Busyness, the Age × Busyness interaction, and the Age × Routine interaction significantly predicted RM failures. All of the statistically significant standardized beta coefficients reflected weak effect sizes, with the exception of the coefficient for Busyness, which indicated a moderate effect.
Post-hoc probing of the significant Age × Busyness interaction revealed that the slope for both older and younger ages were significantly different from zero, suggesting that adults of all ages who reported greater busyness also reported more frequent RM failures. As shown in Figure 2, the significant interaction indicates that this relation was particularly strong for younger participants.
Figure 2.
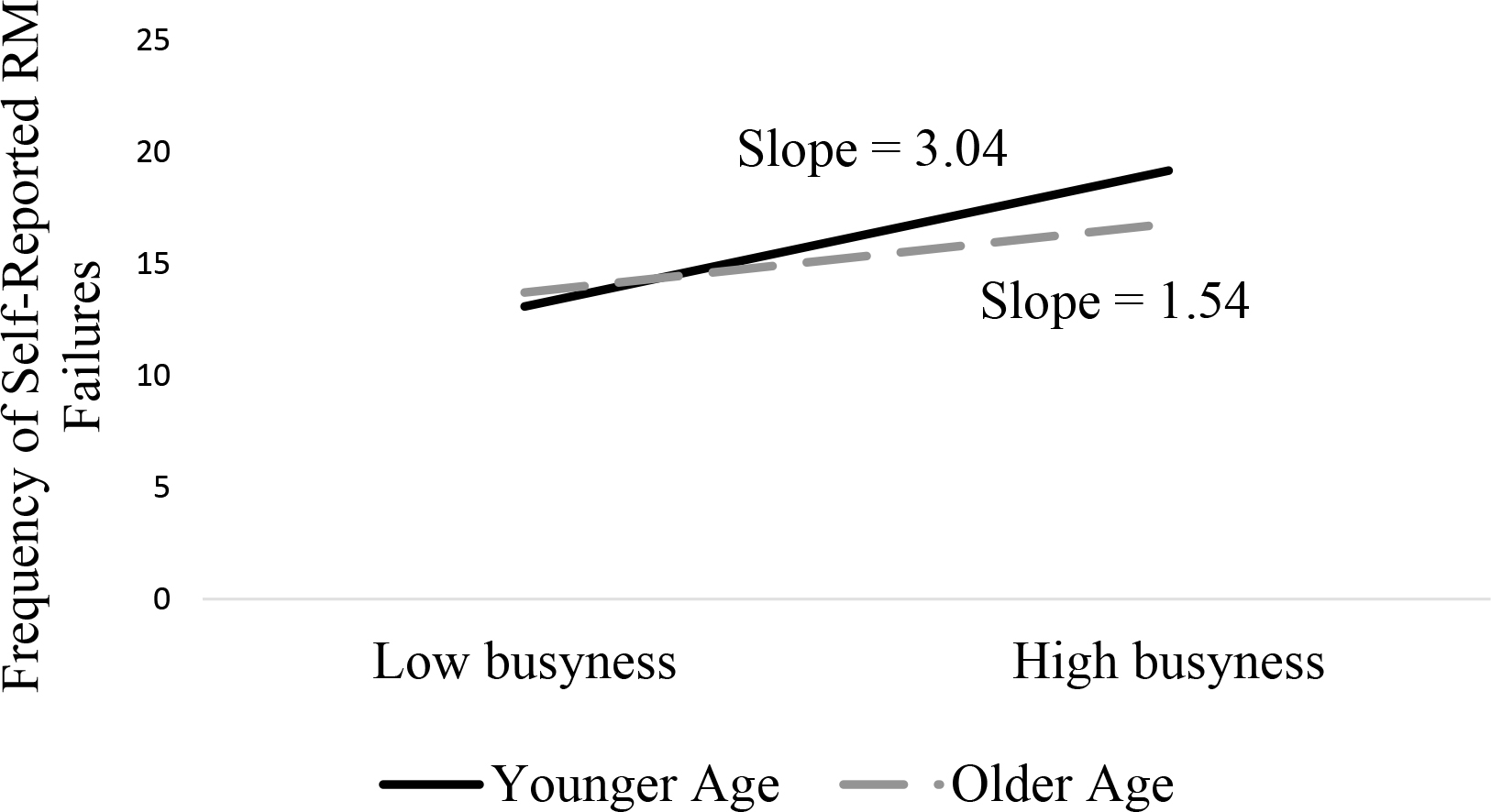
Moderating Effect of Age on the Relation between Busyness and Frequency of Self-Reported Retrospective Memory Failures
Note: N=902 for all variables.
Post-hoc probing of the significant Age × Routine interaction revealed that the slope for older adults was significantly different from zero, but the slope for the younger adults was not. Thus, older adults with more routinized daily activities are less likely to report RM failures as compared to older adults with less routine schedules (See Figure 3).
Figure 3.
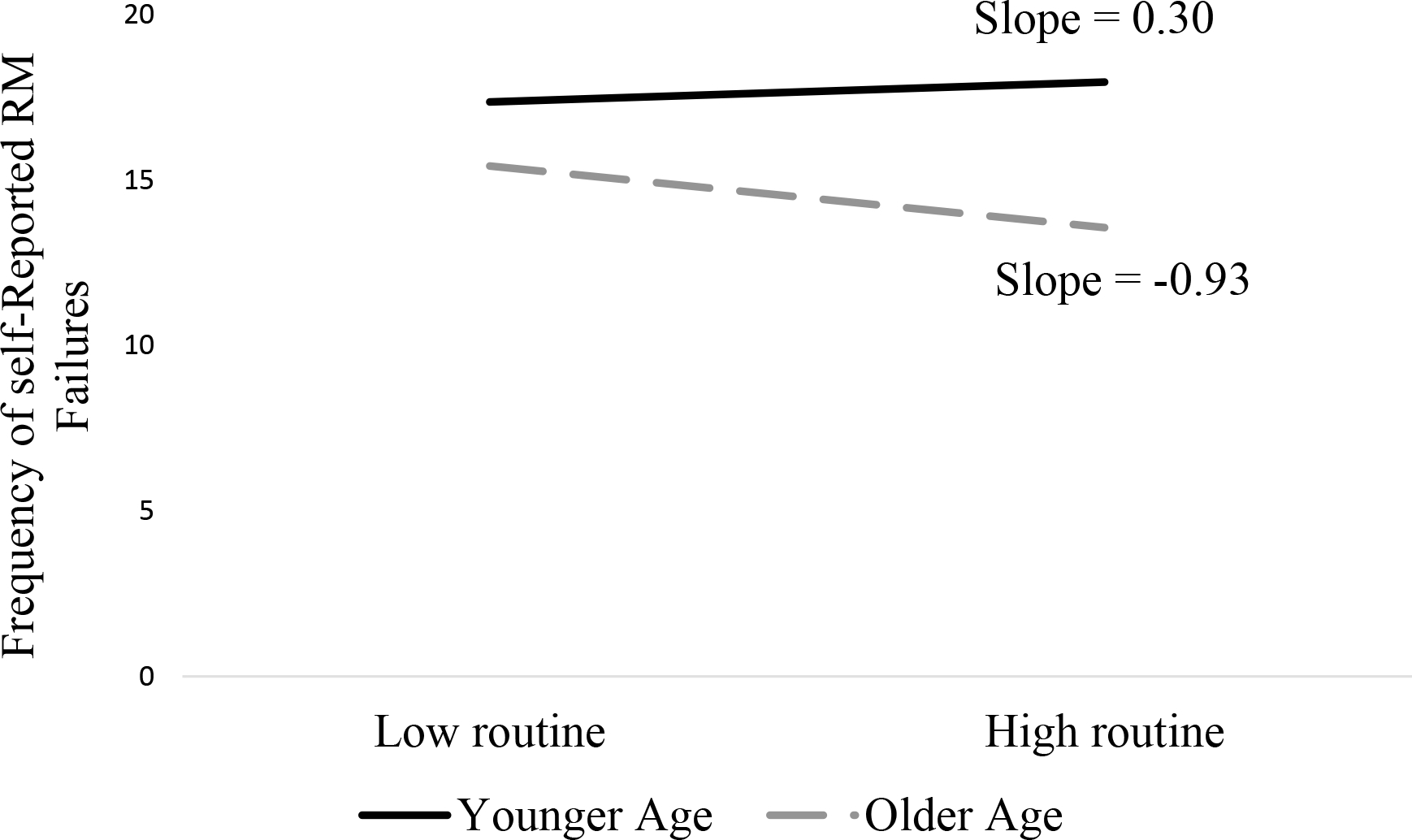
Moderating Effect of Age on the Relation between Routine and Frequency of Self-Reported Retrospective Memory Failures.
Note: N=902 for all variables.
Exploratory Analyses Including the Influence of the COVID-19 Pandemic
PM.
When a measure estimating the extent to which participants were influenced by the COVID-19 pandemic was included in the multivariate model predicting the frequency of self-reported PM failures, the significant effect of Busyness and Routine remained statistically significant, and the Influence of the Pandemic was also a significant predictor. None of the interactions between contextual factors and age were significant. All of the statistically significant standardized beta coefficients reflected weak effect sizes, with the exception of the coefficient for Busyness and the Influence of the Pandemic, which indicated a moderate effect. See Table 5.
RM.
A model predicting the frequency of RM failures that included the Influence of the Pandemic demonstrated that Busyness remained a statistically significant predictor, and the Influence of the Pandemic and the Age × Influence of the Pandemic interaction also were significant predictors. All of the statistically significant standardized beta coefficients reflected weak effect sizes, with the exception of the coefficient for Busyness and the Influence of the Pandemic, which indicated a moderate effect. See Table 5.
Post hoc probing of the significant Age × Influence of the Pandemic interaction showed that the slopes for both older and younger adults were significantly different from zero, suggesting that adults of all ages who reported greater influence of the pandemic also reported more frequent RM failures. As shown in Figure 4, the significant interaction indicates that this relation was particularly strong for younger adults.
Figure 4.
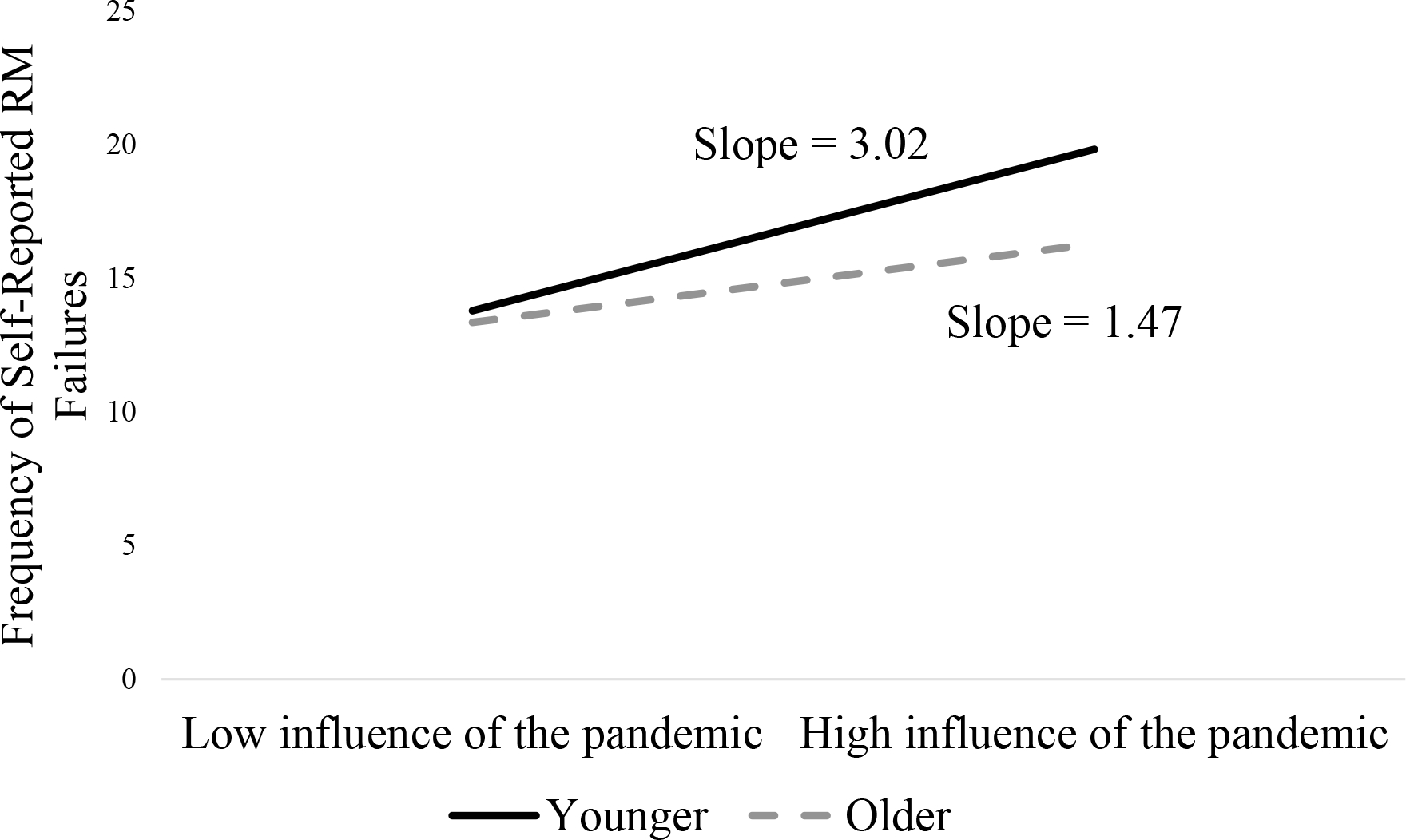
Moderating Effect of Age on the Relation between the Influence of the Pandemic and the Frequency of Self-Reported Retrospective Memory Failures.
Note: N=902 for all variables except the influence of the pandemic. <1% of items were missing for the influence of the pandemic questions (n = 894).
Discussion
The overarching aim of this study was to understand the relation between self-reported memory abilities and contextual factors across adulthood. Specifically, we predicted that the frequency of self-reported RM and PM failures would be associated with the quantity and type of activities that a person performs in daily life (i.e., Busyness, Routine) and that this association would be stronger in older adults. Consistent with our predictions, people who were busier reported more frequent PM and RM failures, and people with more routine schedules reported less frequent PM failures. Also consistent with predictions, age moderated the effect of Routine on the frequency of RM failures, such that only older participants with routine schedules reported less frequent RM failures; routine did not influence the frequency of RM failures in younger adults. Exploratory analyses of a third contextual factor, the COVID-19 pandemic, suggested that the pandemic also significantly influenced the frequency of RM and PM failures, such that people who reported a greater Influence of the Pandemic on their daily life also reported more frequent PM/RM failures.
Unexpected associations between age and the frequency of self-reported RM and PM failures also were observed across several analyses. For example, bivariate correlations showed that older age was associated with less frequent self-reported PM and RM failures (Table 3). Despite decades of research showing worse memory abilities in older versus younger people, prior studies have reported similar findings using self-report measures (Begum et al., 2014; de Winter, Dodou, & Hancock, 2015) and others have demonstrated discrepancies between self-report ratings of cognitive abilities and objective cognitive test scores (Edmonds et al., 2018; Graves et al., 2022; Lenehan, Klekociuk, & Summers, 2012). The “paradoxical decrease of self-reported cognitive failures” across adulthood has been speculated to be due to a variety of factors, including older adults’ difficulties in recalling memory failures, stronger fluid intelligence, greater compensatory abilities, as well older adults’ more “lenient lifestyle” (de Winter et al., 2015). In support of the lenient lifestyle account, our results showed that younger participants were more busy and less routine than older participants (Table 3), suggesting the possibility that younger people may have experienced more frequent memory failures because their lifestyles are more cognitively demanding (busier, less routine). Thus, the associations between age and frequency of self-reported memory failures are more interpretable when contextual factors are considered. We suspect that if older people had daily life demands that were comparable to younger people, then they might have reported more frequent memory failures and the results of self-reports would be more in line with the literature on cognitive abilities based on objective, standardized tests, after controlling for other person-level variables that are known to influence subjective cognitive concerns (e.g., psychological distress). Future research should use longitudinal designs to evaluate whether contextual factors, such as Busyness, may be best conceptualized as mediators of the relation between age and self-reported memory failures.
As this study was conducted within the first year of the COVID-19 pandemic, when people’s everyday lives were suddenly disrupted due to the quarantine and stay-at-home orders, we were interested in knowing the effect of the pandemic (i.e., an acute contextual factor) on the frequency of self-reported memory failures. Without any available standardized measures at the time, we generated novel, pilot questions to explore the effects of the disruption due to the pandemic on our data. Exploratory analyses showed that those who reported being more greatly influenced by the pandemic (i.e., the pandemic markedly disrupted their day-to-day lives) also reported more frequent PM and RM failures (Table 3). Furthermore, younger adults reported a greater influence of the pandemic on their daily lives (Table 3), and multivariate analyses revealed that the influence of the pandemic on the frequency of RM failures was greatest for younger people (Figure 4). These findings suggest that the unusual work and childcare demands, distress due to potential unemployment and financial uncertainty and hardships during the pandemic, increased the burden of daily activities among younger people, which may have contributed to downstream cognitive difficulties and/or greater perception of memory failures in younger adults during the pandemic (Cunningham, Fields, Garcia, & Kensinger, 2021; Varma, Junge, Meaklim, & Jackson, 2021). Our results suggest the possibility that a variety of acute stressors that disrupt daily life, such as the death of a spouse, divorce, employment transitions, and so on, could have similar effects on memory abilities and/or perceptions of memory failures and should be considered when interpreting self-reported cognitive abilities in clinical practice and research studies.
Another exploratory aim of our study was to determine whether different contextual factors influenced the frequency of PM versus the frequency of RM. Results showed that greater Busyness and the Influence of the Pandemic were associated with both more frequent PM and RM failures in people of all ages (Tables 4 & 5). For RM, but not PM, the effect of contextual factors was significantly moderated by age. As predicted and noted earlier, more routine in daily activities (Routine) was associated with less frequent RM failures only in older participants (Figure 3). By contrast, and contrary to expectation, the effect of Busyness and the Pandemic on the frequency of RM failures was the greatest on younger people (Figure 2 & 4). Thus, the interaction between Age and contextual factors appears to be especially important for the frequency of self-reported RM. The interaction between Age and Routine in predicting the frequency of RM failures is consistent with accounts that posit contextual factors are most impactful for people with cognitive vulnerabilities (Kidder et al., 1997; Maujean et al., 2003; Suchy, Ziemnik, Niermeyer, & Brothers, 2020) and suggest future research should evaluate the potential for routinization of daily schedules as a compensatory strategy to decrease the frequency of RM errors in older people. Furthermore, Age was a significant predictor for the frequency of PM but not RM, such that younger age was associated with more frequent PM memory compared to older adults. This counterintuitive finding is generally consistent with the “Age-PM paradox,” which describes differences between age groups in PM performance across different settings. Older adults typically perform better than younger adults on PM tasks in naturalistic settings but worse than younger adults when task demands are standardized (Rendell & Craik, 2000).
Our results have important implications for clinical neuropsychological assessment and research. Self-reported cognitive abilities are often used to inform diagnoses (e.g., attention deficit hyperactivity disorder, MCI vs. dementia), generate recommendations, and identify primary care patients who might need more comprehensive assessment. For example, as the demands for neuropsychological assessment increase due to the growing population of people over age 65, the utility of cognitive complaints to triage patients for neuropsychological assessment (in both the primary care and neurology/neuropsychology settings) will likely increase (Isaacson & Saif, 2020; Sabbagh et al., 2020). Also, in geriatric neuropsychology, research on subjective cognitive complaints has increased dramatically, with growing interest in identifying pre-clinical neurodegenerative disease. Our study results indicate that self-report of cognitive abilities is influenced strongly by contextual factors, which may vary widely across people. Caution should be taken when using self-reports for diagnosis or between-group comparisons, though the use of self-report data may be improved by considering contextual factors, particularly Busyness and acute stressors that might disrupt daily activities.
Self-report measures of cognitive abilities are useful for understanding how individuals are managing their daily lives, how well they are compensating for cognitive impairments, and their clinical concerns. However, it is important to understand that self-reported memory failures will be reported more frequently by people who are very active and who may be struggling with transient external factors that may increase busyness and therefore cognitive burden (e.g., changes in job duties, divorce, illness of a spouse). Thus, the accurate evaluation of memory abilities via self-report questionnaires will require adjustments for contextual factors such as busyness and stressful events. New measures that enable statistical adjustments for contextual factors, in the same way age adjustments are applied to objective tests of cognitive abilities, should be explored. Another approach suggested by Groen et al. (2019) is to evaluate cognitive abilities by asking individuals to predict how well they would perform in a specific contextual scenario (e.g., ability to read text over a span of 2 hours) to standardize context across raters. Future research is needed to determine whether consideration of contextual factors minimizes differences between self-report and objective test scores.
It is important to mention several limitations of our study. First, the participants in the current study were homogeneous regarding race and ethnicity (i.e., majority White, non-Hispanic), and the participants who completed the primary study measures and were included in the analyses were significantly younger and included fewer people who self-reported Asian race and American Indian/Alaska Native race, thereby limiting the generalizability of our study findings. Second, our study was limited in scope and did not include objective tests of cognitive abilities, symptom and performance validity measures, self-reports of cognitive abilities other than memory, information on person-factors, such as depression (Mendonça, Alves, & Bugalho, 2016), or personal beliefs about self-efficacy of memory (West et al., 2005), which are known to influence self-report questionnaires. This limitation is specifically salient in the context of the COVID-19 pandemic, which increased psychological distress, particularly in younger adults (Varma et al., 2021). As mentioned earlier, future studies should assess psychological distress to determine whether external factors are stronger or weaker predictors or interact with person-level factors in explaining memory complaints. Third, that our measure of the Influence of the Pandemic was a rudimentary, pilot assessment of the day-to-day life disruption associated with the pandemic that has not been validated. Our measure of the Influence of the Pandemic referenced the self-report measures of cognition, which introduces criterion contamination. Further work should look at the Influence of the Pandemic more thoroughly, to glean a clearer picture of the relation with self-reported memory failures. Fourth, our sample included community dwelling participants who were not seeking a clinical evaluation. People seeking a clinical evaluation typically report more frequent memory failures (Ryu, Lee, Kim, & Lee, 2016), and the ConVExA model (Suchy et al., 2020), which posits that contextual factors have stronger effects on people with cognitive vulnerabilities, would predict clinic samples might experience greater effects of contextual factors. However, we acknowledge that this point remains unknown and suggest that it is pursued in future research.
In conclusion, our results suggest that contextual factors should be considered when interpreting self-report questionnaires of cognitive abilities. Understanding the various factors that contribute to an increase in subjective cognitive concerns will improve the ability to identify clinically significant problems and advance our knowledge of awareness and insight into cognitive difficulties. Because cognitive performance is strongly influenced by contextual factors and because task demands and task complexity differ markedly across people, it critical to consider contextual factors in interpretation of self-report measures of cognitive abilities.
Acknowledgements
This project was supported by the National Institute on Aging (Grants R21AG060422, R01AG062503, R21AG066771 to TG). A portion of the study was presented at the 2022 North American meeting of the International Neuropsychological Society. Authors report no conflicts of interest.
References
- Acock AC (2008). A gentle introduction to Stata. Stata press. [Google Scholar]
- Balash Y, Mordechovich M, Shabtai H, Giladi N, Gurevich T, & Korczyn AD (2013). Subjective memory complaints in elders: Depression, anxiety, or cognitive decline? Acta Neurologica Scandinavica, 127(5), 344–350. 10.1111/ane.12038 [DOI] [PubMed] [Google Scholar]
- Begum A, Dewey M, Hassiotis A, Prince M, Wessely S, & Stewart R (2014). Subjective cognitive complaints across the adult life span: A 14-year analysis of trends and associations using the 1993, 2000 and 2007 english psychiatric morbidity surveys. Psychological Medicine, 44(9), 1977–1987. 10.1017/S0033291713002444 [DOI] [PubMed] [Google Scholar]
- Brucki SMD, & Nitrini R (2009). Subjective memory impairment in a rural population with low education in the Amazon rainforest: An exploratory study. International Psychogeriatrics, 21(1), 164–171. 10.1017/S1041610208008065 [DOI] [PubMed] [Google Scholar]
- Burmester B, Leathem J, & Merrick P (2016). Subjective Cognitive Complaints and Objective Cognitive Function in Aging: A Systematic Review and Meta-Analysis of Recent Cross-Sectional Findings. Neuropsychology Review, 26(4), 376–393. 10.1007/s11065-016-9332-2 [DOI] [PubMed] [Google Scholar]
- Calkins DR, Rubenstein LV, Cleary PD, Davies AR, Jette AM, Fink A, … Delbanco TL (1991). Failure of physicians to recognize functional disability in ambulatory patients. Annals of Internal Medicine, 114(6), 451–454. 10.7326/0003-4819-114-6-451 [DOI] [PubMed] [Google Scholar]
- Cicchetti DV (1994). Guidelines, Criteria, and Rules of Thumb for Evaluating Normed and. Psychological Assessment, 6(4), 284–290. [Google Scholar]
- Cooper C, Bebbington P, Lindesay J, Meltzer H, McManus S, Jenkins R, & Livingston G (2011). The meaning of reporting forgetfulness: A cross-sectional study of adults in the English 2007 Adult Psychiatric Morbidity Survey. Age and Ageing, 40(6), 711–717. 10.1093/ageing/afr121 [DOI] [PubMed] [Google Scholar]
- Crawford JR, Smith G, Maylor EA, Della Sala S, & Logie RH (2003). The Prospective and Retrospective Memory Questionnaire (PRMQ): Normative data and latent structure in a large non-clinical sample. Memory, 11(3), 261–275. 10.1080/09658210244000027 [DOI] [PubMed] [Google Scholar]
- Cunningham TJ, Fields EC, Garcia SM, & Kensinger EA (2021). The Relation Between Age and Experienced Stress, Worry, Affect, and Depression During the Spring 2020 Phase of the COVID-19 Pandemic in the United States. Emotion, 21(8), 1660–1670. 10.1037/emo0000982 [DOI] [PubMed] [Google Scholar]
- De Frìas CM, & Dixon RA (2005). Confirmatory factor structure and measurement invariance of the memory compensation questionnaire. Psychological Assessment, 17(2), 168–178. 10.1037/1040-3590.17.2.168 [DOI] [PubMed] [Google Scholar]
- de Winter JCF, Dodou D, & Hancock PA (2015). On the paradoxical decrease of self-reported cognitive failures with age. Ergonomics, 58(9), 1471–1486. 10.1080/00140139.2015.1019937 [DOI] [PubMed] [Google Scholar]
- Dufouil C, Fuhrer R, & Alpérovitch A (2005). Subjective cognitive complaints and cognitive decline: Consequence or predictor? The epidemiology of vascular aging study. Journal of the American Geriatrics Society, 53(4), 616–621. 10.1111/j.1532-5415.2005.53209.x [DOI] [PubMed] [Google Scholar]
- Dux MC, Woodard JL, Calamari JE, Messina M, Arora S, Chik H, & Pontarelli N (2008). The moderating role of negative affect on objective verbal memory performance and subjective memory complaints in healthy older adults. Journal of the International Neuropsychological Society, 14(2), 327–336. [DOI] [PubMed] [Google Scholar]
- Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, & Bondi MW (2014). Subjective cognitive complaints contribute to misdiagnosis of mild cognitive impairment. Journal of the International Neuropsychological Society, 20(8), 836–847. 10.1017/S135561771400068X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, & Bondi MW (2015). Subtle Cognitive Decline and Biomarker Staging in Preclinical Alzheimer’s Disease. Journal of Alzheimer’s Disease, 47(1), 231–242. 10.3233/JAD-150128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds EC, Weigand AJ, Thomas KR, Eppig J, Delano-Wood L, Galasko DR, … Bondi MW (2018). Increasing Inaccuracy of Self-Reported Subjective Cognitive Complaints over 24 Months in Empirically Derived Subtypes of Mild Cognitive Impairment. Journal of the International Neuropsychological Society, 24(8), 842–853. 10.1017/S1355617718000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Reed BR, Cahn-Weiner D, Jagust WJ, Baynes K, & DeCarli C (2008). The measurement of everyday cognition (ECog): Scale development and psychometric properties. 22(4), 531–544. 10.1037/0894-4105.22.4.531.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. (2009). Guidance for Industry Use in Medical Product Development to Support Labeling Claims Guidance for Industry. Clinical/Medical Federal Register, (December), 1–39. [Google Scholar]
- Gondo Y, Renge N, Ishioka Y, Kurokawa I, Ueno D, & Rendell P (2010). Reliability and validity of the Prospective and Retrospective Memory Questionnaire (PRMQ) in young and old people: A Japanese study. Japanese Psychological Research, 52(3), 175–185. 10.1111/j.1468-5884.2010.00433.x [DOI] [Google Scholar]
- Graves LV, Edmonds EC, Thomas KR, Weigand AJ, Cooper S, Stickel AM, … Bondi MW (2022). Diagnostic accuracy and differential associations between ratings of functioning and neuropsychological performance in non-Hispanic Black and White older adults. Clinical Neuropsychologist, 36(2), 287–310. 10.1080/13854046.2021.1971766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen Y, Fuermaier ABM, Tucha L, Weisbrod M, Aschenbrenner S, & Tucha O (2019). A situation-specific approach to measure attention in adults with ADHD: The everyday life attention scale (ELAS). Applied Neuropsychology:Adult, 26(5), 411–440. 10.1080/23279095.2018.1437730 [DOI] [PubMed] [Google Scholar]
- Holmbeck GN (2002). Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. Journal of Pediatric Psychology, 27(1), 87–96. 10.1093/jpepsy/27.1.87 [DOI] [PubMed] [Google Scholar]
- Isaacson R, & Saif N (2020). A Missed Opportunity for Dementia Prevention? Current Challenges for Early Detection and Modern-Day Solutions. (7), 7–9. [DOI] [PubMed] [Google Scholar]
- Jessen F, Amariglio RE, Buckley RF, van der Flier WM, Han Y, Molinuevo JL, … Wagner M (2020). The characterisation of subjective cognitive decline. The Lancet Neurology, 19(3), 271–278. 10.1016/S1474-4422(19)30368-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Amariglio RE, Van Boxtel M, Breteler M, Ceccaldi M, Chételat G, … Wagner M (2014). A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s and Dementia, 10(6), 844–852. 10.1016/j.jalz.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker C, Jonker MI, & Schmand B (2000). Are memory complaints predictive for dementia? A review of clinical and population-based studies. International Journal of Geriatric Psychiatry, 15(11), 983–991. [DOI] [PubMed] [Google Scholar]
- Kidder DP, Park DC, Hertzog C, & Morrell RW (1997). Prospective memory and aging: The effects of working memory and prospective memory task load. Aging, Neuropsychology, and Cognition, 4(2), 93–112. 10.1080/13825589708256639 [DOI] [Google Scholar]
- Kliegel M, & Jäger T (2006). Can the Prospective and Retrospective Memory Questionnaire (PRMQ) predict actual prospective memory performance? Current Psychology, 25(3), 182–191. 10.1007/s12144-006-1002-8 [DOI] [Google Scholar]
- Kobelt G, Langdon D, & Jönsson L (2019). The effect of self-assessed fatigue and subjective cognitive impairment on work capacity: The case of multiple sclerosis. Multiple Sclerosis Journal, 25(5), 740–749. 10.1177/1352458518769837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper JS, Oude Voshaar RC, Zuidema SU, Stolk RP, Zuidersma M, & Smidt N (2017). The relationship between social functioning and subjective memory complaints in older persons: a population-based longitudinal cohort study. International Journal of Geriatric Psychiatry, 32(10), 1059–1071. 10.1002/gps.4567 [DOI] [PubMed] [Google Scholar]
- Larrabee GJ, & Crook III TH (1994). Estimated Prevalence of Age-Associated Memory Impairment Derived From Standardized Tests of Memory Function. International Psychogeriatrics, 6(1), 95–104. 10.1017/S1041610294001663 [DOI] [PubMed] [Google Scholar]
- Lee JH, Sung J, & Choi MK (2020). The factors associated with subjective cognitive decline and cognitive function among older adults. Journal of Advanced Nursing, 76(2), 555–565. 10.1111/jan.14261 [DOI] [PubMed] [Google Scholar]
- Lenehan ME, Klekociuk SZ, & Summers MJ (2012). Absence of a relationship between subjective memory complaint and objective memory impairment in mild cognitive impairment (MCI): Is it time to abandon subjective memory complaint as an MCI diagnostic criterion? International Psychogeriatrics, 24(9), 1505–1514. 10.1017/S1041610212000695 [DOI] [PubMed] [Google Scholar]
- Markova H, Andel R, Stepankova H, Kopecek M, Nikolai T, Hort J, … Vyhnalek M (2017). Subjective Cognitive Complaints in Cognitively Healthy Older Adults and Their Relationship to Cognitive Performance and Depressive Symptoms. Journal of Alzheimer’s Disease, 59(3), 871–881. 10.3233/JAD-160970 [DOI] [PubMed] [Google Scholar]
- Martin M, & Park DC (2003). The Martin and Park Environmental Demands (MPED) Questionnaire: Psychometric properties of a brief instrument to measure self-reported environmental demands. Aging Clinical and Experimental Research, 15(1), 77–82. 10.1007/BF03324483 [DOI] [PubMed] [Google Scholar]
- Maujean A, Shum D, & McQueen R (2003). Effect of Cognitive Demand on Prospective Memory in Individuals with Traumatic Brain Injury. Brain Impairment, 4(2), 135–145. 10.1375/brim.4.2.135.27024 [DOI] [Google Scholar]
- Mendonça MD, Alves L, & Bugalho P (2016). From Subjective Cognitive Complaints to Dementia. American Journal of Alzheimer’s Disease and Other Dementias, 31(2), 105–114. 10.1177/1533317515592331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, & Stubbs B (2014). Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatrica Scandinavica, 130(6), 439–451. 10.1111/acps.12336 [DOI] [PubMed] [Google Scholar]
- Montejo P, Montenegro M, Fernández-Blázquez MA, Turrero-Nogués A, Yubero R, Huertas E, & Maestú F (2014). Association of perceived health and depression with older adults’ subjective memory complaints: contrasting a specific questionnaire with general complaints questions. European Journal of Ageing, 11(1), 77–87. 10.1007/s10433-013-0286-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E, Conger B, Douglass R, Gephart D, Kirk J, Page R, … Zubkoff M (1983). Functional Health Status Levels of Primary Care Patients. JAMA: The Journal of the American Medical Association, 249(24), 3331–3338. 10.1001/jama.1983.03330480037027 [DOI] [PubMed] [Google Scholar]
- Park DC, Hertzog C, Kidder DP, Morrell RW, & et al. (1997). Effect of age on event-based and time-based prospective memory. Psychology and Aging, 12(2), 314–327. 10.1037//0882-7974.12.2.314 [DOI] [PubMed] [Google Scholar]
- Pearman A, Hertzog C, & Gerstorf D (2014). Little evidence for links between memory complaints and memory performance in very old age: Longitudinal analyses from the Berlin Aging Study. Psychology and Aging, 29(4), 828. [DOI] [PubMed] [Google Scholar]
- Reid LM, & MacLullich AMJ (2006). Subjective memory complaints and cognitive impairment in older people. Dementia and Geriatric Cognitive Disorders, 22(5–6), 471–485. 10.1159/000096295 [DOI] [PubMed] [Google Scholar]
- Reisberg B, Shulman MB, Torossian C, Leng L, & Zhu W (2014). Outcome over seven years of healthy adults with and without subjective cognitive impairment Barry. 71(11), 3831–3840. 10.1016/j.jalz.2009.10.002.Outcome [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell PG, & Craik FIM (2000). Virtual Week and Actual Week: Age-related Differences in Prospective Memory. Applied Cognitive Psychology, 14(SPEC. ISS.). 10.1002/acp.770 [DOI] [Google Scholar]
- Rönnlund M, Vestergren P, Mäntylä T, & Nilsson LG (2011). Predictors of self-reported prospective and retrospective memory in a population-based sample of older adults. Journal of Genetic Psychology, 172(3), 266–284. 10.1080/00221325.2010.538450 [DOI] [PubMed] [Google Scholar]
- Ryu SY, Lee SB, Kim TW, & Lee TJ (2016). Memory complaints in subjective cognitive impairment, amnestic mild cognitive impairment and mild Alzheimer’s disease. Acta Neurologica Belgica, 116(4), 535–541. 10.1007/s13760-016-0604-7 [DOI] [PubMed] [Google Scholar]
- Sabbagh MN, Boada M, Borson S, Chilukuri M, Dubois B, Ingram J, … Hampel H (2020). Early Detection of Mild Cognitive Impairment (MCI) in Primary Care. Journal of Prevention of Alzheimer’s Disease, 7(3), 165–170. 10.14283/jpad.2020.21 [DOI] [PubMed] [Google Scholar]
- Seligman SC, Giovannetti T, Sestito J, & Libon DJ (2014). A new approach to the characterization of subtle errors in everyday action: Implications for mild cognitive impairment. Clinical Neuropsychologist, 28(1), 97–115. 10.1080/13854046.2013.852624 [DOI] [PubMed] [Google Scholar]
- Sheppard DP, Woods SP, Massman PJ, & Gilbert PE (2019). Frequency and Correlates of Subjective Cognitive Impairment in HIV Disease. AIDS and Behavior, 23(3), 617–626. 10.1007/s10461-018-2297-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot RER, Sikkes SAM, Berkhof J, Brodaty H, Buckley R, Cavedo E, … van der Flier WM (2018). Subjective cognitive decline and rates of incident Alzheimer’s disease and non–Alzheimer’s disease dementia. Alzheimer’s and Dementia, 15, 465–476. 10.1016/j.jalz.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit D, Koerts J, Bangma DF, Fuermaier ABM, Tucha L, & Tucha O (2021). Look who is complaining: Psychological factors predicting subjective cognitive complaints in a large community sample of older adults. Applied Neuropsychology:Adult. 10.1080/23279095.2021.2007387 [DOI] [PubMed] [Google Scholar]
- Smith G, Della Sala S, Logie RH, & Maylor EA (2000). Prospective and retrospective memory in normal ageing and dementia: A questionnaire study. Memory, 8(5), 311–321. 10.1080/09658210050117735 [DOI] [PubMed] [Google Scholar]
- Studer J, Donati A, Popp J, & von Gunten A (2014). Subjective cognitive decline in patients with mild cognitive impairment and healthy older adults: Association with personality traits. Geriatrics and Gerontology International, 14(3), 589–595. 10.1111/ggi.12139 [DOI] [PubMed] [Google Scholar]
- Suchy Y, Ziemnik RE, Niermeyer MA, & Brothers SL (2020). Executive functioning interacts with complexity of daily life in predicting daily medication management among older adults. Clinical Neuropsychologist, 34(4), 797–825. 10.1080/13854046.2019.1694702 [DOI] [PubMed] [Google Scholar]
- Tomita T, Sugawara N, Kaneda A, Okubo N, Iwane K, Takahashi I, … Yasui-Furukori N (2014). Sex-specific effects of subjective memory complaints with respect to cognitive impairment or depressive symptoms. Psychiatry and Clinical Neurosciences, 68(3), 176–181. 10.1111/pcn.12102 [DOI] [PubMed] [Google Scholar]
- Varma P, Junge M, Meaklim H, & Jackson ML (2021). Younger people are more vulnerable to stress, anxiety and depression during COVID-19 pandemic: A global cross-sectional survey. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 109(December 2020), 110236. 10.1016/j.pnpbp.2020.110236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RL, Bagwell DK, & Dark-Freudeman A (2005). Memory and goal setting: The response of older and younger adults to positive and objective feedback. Psychology and Aging, 20(2), 195–201. 10.1037/0882-7974.20.2.195 [DOI] [PubMed] [Google Scholar]
- Woods SP, Carey CL, Moran LM, Dawson MS, Letendre SL, & Grant I (2007). Frequency and predictors of self-reported prospective memory complaints in individuals infected with HIV. Archives of Clinical Neuropsychology, 22(2), 187–195. 10.1016/j.acn.2006.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich D, Kliegel M, & Rast P (2011). The factorial structure and external validity of the prospective and retrospective memory questionnaire in older adults. European Journal of Ageing, 8(1), 39–48. 10.1007/s10433-011-0174-8 [DOI] [PMC free article] [PubMed] [Google Scholar]


