Abstract
Small RNA (sRNA) mediated gene regulation during Sri Lankan Cassava Mosaic Virus (SLCMV) infection was studied from the Indian Cassava Cultivar H226. Our study generated high throughput sRNA dataset of 23.64 million reads from the control and SLCMV infected H226 leaf libraries. mes-miR9386 was detected as the most prominent miRNA expressed in control and infected leaf. Among the differentially expressed miRNAs, mes-miR156, mes- miR395 and mes-miR535a/b showed significant down regulation in the infected leaf. Genome-wide analysis of the three small RNA profiles revealed critical role of virus-derived small RNAs (vsRNAs) from the infected leaf tissues of H226. The vsRNAs were mapped to the bipartite SLCMV genome and high expression of siRNAs generated from the virus genomic region encoding AV1/AV2 genes in the infected leaf pointed towards the susceptibility of H226 cultivars to SLCMV. Furthermore, the sRNA reads mapped to the antisense strand of the SLCMV ORFs was higher than the sense strand. These vsRNAs were potential to target key host genes involved in virus interaction such as aldehyde dehydrogenase, ADP-ribosylation factor1 and ARF1-like GTP-binding proteins. The sRNAome-assisted analysis also revealed the origin of virus-encoded miRNAs from the SLCMV genome in the infected leaf. These virus-derived miRNAs were predicted to have hair-pin like secondary structures, and have different isoforms. Moreover, our study revealed that the pathogen sRNAs play a critical role in the infection process in H226 plants.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-023-03494-2.
Keywords: Cassava, H226, Sri Lankan Cassava Mosaic virus, Virus-derived small RNAs, miRNAs
Introduction
Cassava (Manihot esculenta Crantz) serves as major source of food for millions of people worldwide and it contributes the third largest source of carbohydrates in the tropics (Jose et al. 2011). Cassava is not only a staple food in Africa but also a prominent industrial crop in Latin America and Asia (Legg et al. 2015). Even though cassava is an excellent crop to resist the negative impacts of climate change, the occurrence of cassava mosaic disease (CMD) is a major threat that affects the food security in the tropics. Cassava mosaic disease belong to the group of most economically important crop viral diseases in the world. Despite the CMD resistance in cassava landraces, most farmer-preferred varieties are susceptible to the disease (Vanderschuren et al. 2012). As cassava plant is a good candidate for genetic engineering, RNA interference (RNAi) technology has immense potential to improve the tolerance to virus diseases. RNAi technology that deploys the expression of hairpin RNA homologous to virus gene sequences such as coat proteins has revealed virus-resistance in cassava (Vanderschuren et al. 2009, 2007; Vanitharani et al. 2003) and recently AC1, AC2, AC4 from DNA-A and BC1 from DNA-B of African Cassava Mosaic Virus (ACMV) were reported as effective targets for virus resistance (Patil et al. 2016).
Sri Lankan Cassava Mosaic Virus (SLCMV) infection is the most prominent in Indian Cassava cultivars and their molecular response mediated by the front-line players of defence mechanism such as sRNAs and miRNAs are not yet fully understood. Cassava Mosaic Virus genome is bipartite as it comprises of two circular, single-stranded DNA components which are DNA A and DNA B. The common region is a segment of high sequence identity and harbors viral promoter, origin of replication, sequences involved in binding Replication associated protein (Ntui et al. 2015). DNA A encodes two overlapping ORFs in sense orientation and four overlapping complementary sense ORFs. AV1 and AV2 are sense ORFs of DNA A. The DNA-A complementary ORFs such as AC1, AC2, AC3 and AC4 encodes respectively replication associated protein (Rep), transcriptional activator protein (TrAP), Replication enhancer protein (REn) and Suppressor of RNA silencing (Ntui et al. 2015; Vanitharani et al. 2004). BV1 and BC1 that respectively encodes the nuclear shuttle protein and movement protein are the gene products from DNA B (Vanitharani et al. 2004).
Plants and other eukaryotes counteract virus infections through conserved defence mechanism called RNA silencing. During RNA silencing, short RNAs of 20–30 nucleotides are produced and it recognize and manipulate complementary nucleic acids. A pathogen that could evade this line of defence will be successful in infecting the host. Through anti-viral RNA silencing, plant miRNAs counteract virus infections (Maghuly et al. 2014). microRNAs are a group of small RNAs of 21–24 nucleotide that target messenger RNAs in a sequence dependent manner and regulate gene expression at the post-transcriptional level through cleavage or translational repression (Jones-Rhoades et al. 2006). During plant: pathogen interaction miRNAs are expressed not only in the host plant but also in viruses. A combined computational-experimental analysis identified several conserved miRNAs involved in normal seedling development and stress responses in cassava (Zeng et al. 2009). Subsequent to this, genome-wide computational-experimental analysis identified several conserved miRNAs that have significant role in normal seedling development and stress responses in cassava (Patanun et al. 2013; Zeng et al. 2009). While the deep sequencing further revealed conserved as well as cassava-specific miRNAs (Rogers 2016, Chen et al. 2015a, b), and their role in heat, drought (Ballén-Taborda et al. 2013) and chilling responses (Xia et al. 2014; Zeng et al. 2017). The role of microRNAs in starch metabolism and root development was also revealed by miRNA transcriptome profiling of cassava cultivars and their wild progenitor. Further co-expression analysis revealed the negative relationship of certain miRNAs and their corresponding targets such as MYB33, ARF10, GRF1, RD19, APL2, NF-YA3 and SPL2 in the storage roots and leaves (Chen et al. 2015a, b). Later, small RNA deep sequencing of leaf, stem, callus, male and female flower tissues revealed novel 38 miRNAs in cassava (Khatabi et al. 2016). Other classes of small RNAs involved in post-transcriptional gene silencing such as trans-acting small interfering RNAs (ta-siRNAs) and natural cis-antisense siRNAs (cis-nat-siRNAs) were also identified in cassava through detailed bioinformatic (Perez-Quintero et al. 2012) and NGS analysis (Quintero et al. 2013; Xia et al. 2014). Among the 54 ta-siRNA loci identified in cassava, 39 candidates were found to repress the bacterial infection of Xanthomonas axonopodis pv. manihotis (Xam). Meanwhile cis-nat -siRNAs identified from the overlapping regions in the genome were found as differentially expressed upon the bacterial infection (Perez-Quintero et al. 2012). Phased and half-phased miRNA-like sRNAs were also reported in cassava during chilling responses, and a half-phased miR171d.3 was found to cleave the target gene P-glycoprotein (Zeng et al. 2017).
The role of small RNAs has been elucidated in different cassava genotype upon SACMV (South African Cassava Mosaic Virus) interaction and virus derived small RNAs were found lower in tolerant plant compared to the non-recovery plants (Rogans et al. 2016). Meanwhile, small RNA deep sequencing of cassava genotypes NASE 3, TME 204 and 60,444 infected with Cassava brown streak virus (CBSV) and Ugandan cassava brown streak virus (UCBSV) revealed abundance of 21-24nt vsRNAs (Ogwok et al. 2016). Along with the virus-derived small RNAs that mapped to entire CBSV and UCBSV genomes, large sRNA populations mapped with the cytoplasmic inclusion, P1 and P3 protein-encoding regions were also detected. CBSV-derived sRNAs were found in greater occurrence than UCBSV-derived sRNAs in susceptible genotypes (TME 204 and 60,444). Deep sequencing of small RNAs were also attempted from CMD susceptible and resistant cassava genotypes after inoculation with infectious clones of ACMV Cameroon strain (ACMV-CM) and East African cassava mosaic virus Kenyan strain (EACMV KE2 K201). Although the total vsRNAs were abundant at 20dpi and 35dpi of CMD, they were reduced significantly upon recovery. While in susceptible varieties the vsRNAs were found abundant throughout the experimental stages (Kuria et al. 2017).
In the present study high throughput sRNA sequencing was carried out from the control and SLCMV infected leaves of cassava cultivar H226. A detailed analysis of three libraries further revealed the regulatory role of host and pathogen derived sRNAs on infection process in cassava. Major hotspots of vsRNA origin and cleavage were mapped from the SLCMV genome. Moreover, prospective candidates of virus-encoded miRNAs from the SLCMV genome and their potential gene regulatory function by regulating the host mRNAs were analysed. This study is the first to utilize high throughput small RNA sequencing to dissect the regulatory role of plant and virus derived sRNAs upon SLCMV infection in cassava plants.
Materials and methods
Plant material and pathogen screening
Three weeks old field grown plants that were screened for SLCMV infection were used for the study. In vitro grown disease-free plants of cassava variety H226 were used as the control. The plants were PCR screened using SLCMV coat-protein specific primers (FP 5' GGATCCATGTCGAAGCGCCA3' and RP 5'AAGCTTTTAATTGCTGACCGA3') (Makeshkumar et al. 2005). Triple Antibody Sandwich ELISA (TAS-ELISA) was further used for detection of virus in cassava leaf samples showing different symptoms of infection. A healthy non host sample collected from the field was used as the negative control for the test. Wells of ELISA micro titre plates were coated with 200 μL of purified IgG (ACMV Polyclonal antibody) diluted to 1:1000 in coating buffer (carbonate buffer) and incubated at 37 °C for 3 h. The plates were washed three times with PBS-Tween, soaked for three minutes during each wash and dried by tapping upside down on a tissue paper spread on the bench. When the plates became completely dried, 200 μl of blocking solution was added to each well (blocking) and incubated at 37 °C for 30 min. After the incubation period, the blocking solution was removed, tap dried the plates and again washed three times using PBS-Tween solution. After the plates become dried, 200 μl of test samples was loaded in duplicate wells and incubated overnight at 4 °C. The test samples were prepared by grinding 100 mg test leaves in sample extraction buffer (PBS-T + 2% PVP) and centrifuged at 8000 rpm for 10 min and the supernatant was taken. After the incubation period, plates were washed thrice using PBT-Tween solution and tap dried. Then the plates were coated with 200 μl of monoclonal antibody (ICMV/SLCMV SCR 60) diluted to 1:500 in conjugate buffer and plates were incubated at 37 °C for 3 h. After the incubation period, the plates were washed thrice using PBS-Tween solution and tap dried. Then the plates were coated with 200 μl of conjugate antibody [Alkaline Phosphatase (ALP) conjugated anti-mouse IgG] in appropriate conjugate buffer incubated at 37 °C for 2 h. Then 200 μl aliquots of freshly prepared substrate dissolved in 10 ml of substrate buffer was added to each well and incubated at room temperature in dark condition. The A405 for sample in each well was measured in a BIO-RAD iMark Microplate Reader (USA).
Small RNA sequencing and analysis
Total RNA was isolated from the control uninfected leaves and the SLCMV infected leaves using trizol method (Invitrogen) as per the manufacture’s protocol. The quality of the purified total RNA was checked on Nanodrop Spectrophotometer (Thermo Scientific) and the Agilent Technologies 2100 Bioanalyzer. Total RNA with RNA Integrity Number (RIN) value greater than or equal to 8 was used for the small RNA library preparation with Illumina TruSeq miRNA Sample Preparation protocol.
In brief, RNA 5' adapter (5' GTTCAGAGTTCTACAGTCCGACGATC) and RNA 3' adapter (3' AGATCGGAAGAGCACACGTCTGAACTC) were specifically ligated to each end of miRNAs and other small RNAs. The cDNAs synthesized from the RT reaction was then PCR amplified using a common primer and a primer containing one of 48 index sequences. The overall work flow for the small RNA library preparation is shown in Fig.S1. The libraries constructed were sequenced on HiSeq 2500 with 1 × 50 bp reads. The illumina small RNA-Seq data after the sequencing run were processed to generate FASTQ files. The basic data QC metrics were analyzed from the fastq files of each library. The raw reads were further processed to remove adapter sequences with the adapter removal tool cutadapt (v-1.3). The adapter removed reads were aligned against different noncoding RNA database such as GtRNAdb (ttp://gtrnadb.ucsc.edu/), Rfam (http://rfam.xfam.org/), piRNABank (http://pirnabank.ibab.ac.in/index.shtml), siRNAdb (http://sirna.sbc.su.se/sirnadb_050915.txt) NCBI Genbank (http://www.ncbi.nlm.nih.gov/genbank/) and deepBase (http://deepbase.sysu.edu.cn/download.php) to filter out tRNA, rRNA, piRNA, siRNA, snRNA and snoRNA respectively, using Bowtie2 program (version 2.1.0). The adapter processed small RNAs were further mapped to the host genome (Manihot esculenta). The small RNA analysis pipeline followed in the study is represented in Fig.S2. The sRNA datasets were deposited to the NCBI GEO Database under the accession number GSM 5,175,960- 5,175,962.
Mapping of virus-derived small RNAs
The unaligned reads from contamination/host/miRNA removal steps were aligned to the Sri Lankan cassava mosaic virus Genome A (KP455486.1) and B (KP455487.1.) using http://bowtie-bio.sourceforge.net/index.shtml Bowtie program version 0.12.9). The mapped small RNAs were further analyzed for their length distribution, abundance, genome origin, and potential targets.
Prediction of virus-encoded miRNAs and their targets
The vsRNAs were further analyzed for their ability to derive from miRNA precursors. The secondary structures of sRNA flanking region were in silico predicted for potential miRNA hair-pin like precursor structure. The potential targets of the conserved and novel miRNAs and virus derived small RNAs were predicted from the Manihot esculenta unigene DFCI gene index (MAESGI) using psRNATargetserver (Dai and Zhao 2011).
Results and discussion
Screening of plants for SLCMV infection
The cassava variety H226 at three weeks after planting in the field were visually screened for the symptoms of SLCMV infection such as mosaic, deformation and leaf curl (Fig. 1a). PCR analysis with SLCMV coat protein gene specific primers confirmed positive infection in symptomatic plants (Fig. 1b). Further the viral load in the selected plants were confirmed through ELISA. The in-vitro grown disease free plants of H226 were used as the control.
Fig. 1.
Symptomatology and molecular detection of SLCMV infection. a Symptoms of SLCMV infection in H226 cassava plants. b Molecular screening of SLCMV infection using SLCMV coat protein-specific primers
Summary of small RNA sequencing
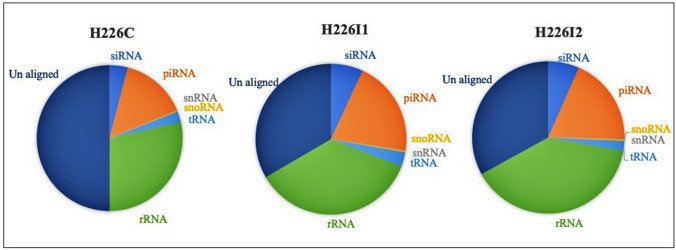
Three small RNA libraries were sequenced from the leaves of control uninfected and SLCMV infected plants. After removing the low-quality reads, a total of 13,660,874 raw reads were obtained in the control leaf library. While the infected leaf library replicate 1 and replicate 2 constituted 13,488,952 and 10,169,846 total raw reads. The data summary of fastq file of samples is given in the supplementary Table 1 and the unique small RNAs read statistics were listed in Table 1. The unique reads were further annotated into different RNA classes such as siRNA, piRNA, snRNA, snoRNA, tRNA and rRNA (Fig. 2). The small RNA read counts among the different functional categories of are listed in Supplementary Table 2. The clean reads after removal of other non-coding RNAs constituted 4,388,762 total reads, contributing 1,950,971 unique small RNAs. Among these, 96.53 percent of the unique small RNAs had length range from 17 to 35 bp. The clean reads after removal of other non-coding RNAs constitute 373,065 unique small RNAs with 946,630 reads in H226I1 library, and 322,535 unique small RNAs, with total read count of 902,510 in H226I2 library.
Table 1.
Overall summary of raw reads of samples after adapter trimming
| Library | Total reads | Unique reads |
|---|---|---|
| H226-C | 13,660,874 | 2,627,476 |
| H226-I1 | 13,488,952 | 1,116,532 |
| H226-I2 | 10,169,846 | 977,318 |
Fig. 2.
Functional categorization of small RNAs into different RNAs
Identification of host-derived miRNAs
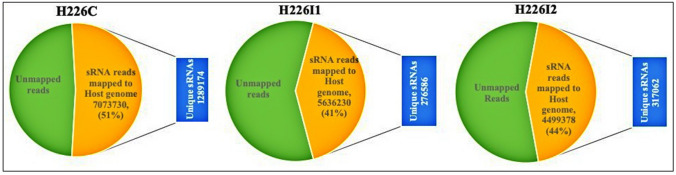
From the control leaf library (H226C), 51% of the total reads were mapped to the host genome (Manihot esculenta L.) (Fig. 3). While 41.78% and 44.24% of the reads, that constitute about 276,586 and 317,062 unique small RNAs were respectively mapped from H226I1 and H226I2. Among the control leaf library, 3744 sRNA candidates, constituting a total read of 671,719 mapped to the miRbase miRNA sequences. Meanwhile the frequency of miRNA reads was low in both the infected leaf sRNAome. 836 conserved mature miRNAs previously reported from cassava were identified with read number ≥ 10. Mature miRNAs were mapped to the previously identified 150 miRNA precursors from the M. esculenta. The length of the precursors ranged from 68 to 255 nt, with 55% average A + T.
Fig. 3.
Statistics of small RNAs mapped to the host genome Manihot esculenta
The most abundant miRNA detected from the H226C was mes-miR9386, a 21nt cassava-specific miRNA with a read count of 113,209, followed by mes-miR166e with 96,905 reads (Table 2). Most plant miRNAs are expected to possess a size range of 20 to 25nt, and are conserved across widely diverged plant species. Multiple miRNA members were also detected from a particular MIRNA family. The family-wise distribution of miRNA reads showed MIR159 as the most prominent family, constituting 23% reads followed by MIR9386 and MIR166 each comprising of 21% of total miRNA reads (Fig.S3). From the H226I1, 402 small RNAs constituting about 6368 total reads were mapped as miRNAs, while 331 small RNAs with total read count of 3343 were identified as miRNAs from H226I2. The average read counts of miRNAs among H226I1 and H226I2 revealed mes-miR9386 as the most prominent miRNA in the infected leaf tissues. miR9386 was previously predicted to target a gene encoding a phosphatidylglycerol-specific phospholipase C, that have critical role in cell growth, cell survival and signal transduction (Khatabi et al. 2016). Meanwhile a low expression of MIR395, MIR156 and MIR482 family were evident in both infected leaf libraries. Four novel miRNAs were predicted from the library data. The size of the predicted miRNAs ranged from 18 to 25nt. The precursor characteristics of the novel miRNAs are listed in Table 3.
Table 2.
The most prominent miRNAs detected from the cassava leaf library (H226C)
| miRNA | mature miRNA sequence | miRNA read frequency | Length |
|---|---|---|---|
| mes-MIR9386 | TTTGCAGTTCGAAAGTGGAAG | 113,209 | 21 |
| mes-MIR166e | TCGGACCAGGCTTCATTCCC | 96,905 | 20 |
| mes-MIR159b | TTTGGATTGAAGGGAGCTC | 86,929 | 19 |
| mes-MIR159b | TTTGGATTGAAGGGAGCTCT | 42,550 | 20 |
| mes-MIR395c | CTGAAGTGTTTGGGGGAACT | 24,891 | 20 |
| mes-MIR156i | TTGACAGAAGATAGAGAG | 21,124 | 18 |
| mes-MIR395b | CTGAAGTGTTTGGGGGAAC | 16,186 | 19 |
| mes-MIR167a | TGAAGCTGCCAGCATGATCT | 14,234 | 20 |
| mes-MIR319e | TTGGACTGAAGGGAGCTCC | 10,853 | 19 |
| mes-MIR156h | GCTCTCTATGCTTCTGTCAT | 10,783 | 20 |
| mes-MIR396a | TTCCACAGCTTTCTTGAACT | 10,026 | 20 |
Table 3.
Novel miRNAs predicted from cassava
| Total read count | Mature sequence | Star sequence | Genome Location | Strand | Length of mature miRNA | Length of precursor | AU(%) of precursor | MFE FOR PRECURSOR | |
|---|---|---|---|---|---|---|---|---|---|
| Novel miR1 | 1695 | UUGGACCAGUCUUCAUUCCC | GAAUGUUGGCUGGCUCUAAGC | Chromosome07:1,953,068–1,953,150 | + | 20 | 82 | 53 | -27.79 |
| Novel miR2 | 631 | CGGCGAUGAUGAUGAAUAAGACCUU | GGUUUCAAGCACUUUUGAGCCGCA | Chromosome05:26,503,411–26,503,490 | - | 25 | 79 | 57 | -20.28 |
| Novel miR3 | 208 | CACGGUUGCCUGACAGAC | AGGUCCGUGAGCUGUAGAG | Chromosome09:1,853,122–1,853,193 | + | 18 | 71 | 37 | -32.29 |
| Novel miR4 | 145 | UUAACGGCUAAGGAUUCU | GGUCCGUGAGCUUGUAGAGG | Chromosome09:1,873,855–1,873,896 | + | 18 | 41 | 52 | -10.94 |
Differential expression of miRNAs
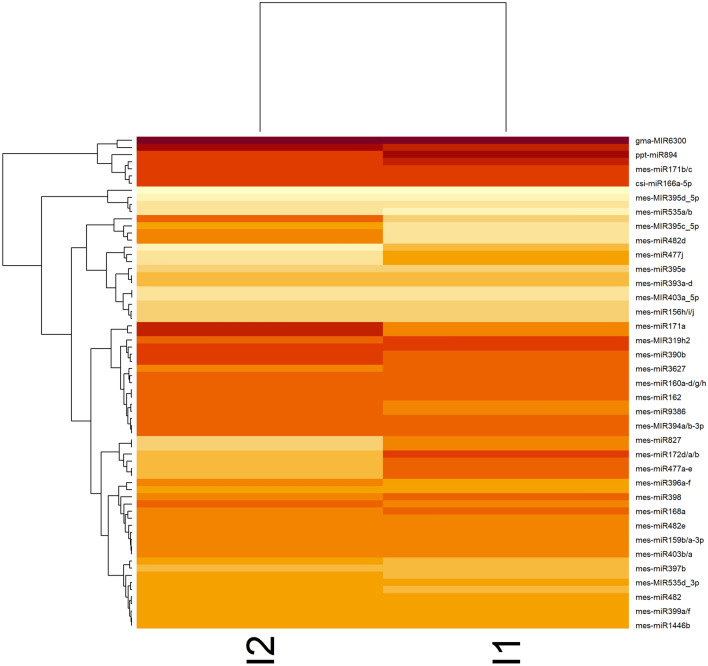
The comparative miRNA expression analysis revealed downregulation of most of the conserved miRNAs in the SLCMV infected H226 leaves. The expression of miRNAs analyzed from their corresponding read numbers in each library revealed differentially expressed sixty-nine miRNAs (Supplementary Table 3). A significant reduction in expression was detected for the miRNA candidates such as mes-miR156a-h, mes-miR395a-d, mes-MIR477j, mes-miR535a/b, mes-miR482b, mes-MIR403a_5p, mes-miR408 as these candidates were not detected in the infected leaf library. Meanwhile mes-miR159b/a-3p, mes-miR9386, mes-miR166a-h, mes-miR395a-d, mes-miR156h/i/j, mes-miR167a-h, mes-miR319a-h, mes-miR396a-f mes-miR535d/c, mes-miR482e were downregulated during infection. Only very few miRNAs were detected upregulated during infection. It is suggested that viral suppressors of RNA silencing can interfere with miRNA mediated regulation of host genes (Chapman et al. 2004; Kasschau et al. 2003). The vvi-miR3630 and gma-miR6300 showed significant up-regulation (p ≤ 0.05) during infection (Fig. 4). Consistent with previous reports on ACMV infection on N. benthamiana (Amin et al. 2011), a decreased accumulation of miR156, miR160 and miR169 were detected upon SLCMV infection in cassava. miR156 targets squamosa promoter like protein, a critical component of vegetative phase transition. In addition to this, a common pattern of upregulation of host derived miRNAs upon infection of begomoviruses such as African cassava mosaic virus (ACMV), Cabbage leaf curl virus (CbLCuV), Tomato yellow leaf curl virus (TYLCV) and Cotton leaf curl Multan virus/Cotton leaf curl betasatellite (CLCuV/CLCuMB) was detected in N. benthamiana plants (Amin et al. 2011). Virus infection generally alters the accumulation of sRNAs in host plants (Zhang et al. 2019). Plant miRNA can mediate antiviral defense through targeting the viral genomes and the efficiency of host miRNAs to target viral RNAs depend on the nature and accessibility to the target mRNAs. Plant miRNAs counteract viral attack via antiviral RNA silencing and viruses in turn have anti-host defense mechanisms blocking these RNA silencing pathways and establish a counter-defense. Thus, the miRNA mediated silencing also function as a selective force for shaping viral genomes (Liu et al. 2017).
Fig. 4.
Differentially expressed miRNAs among control and SLCMV infected leaf tissues of H226
Mapping of small RNAs to SLCMV genome
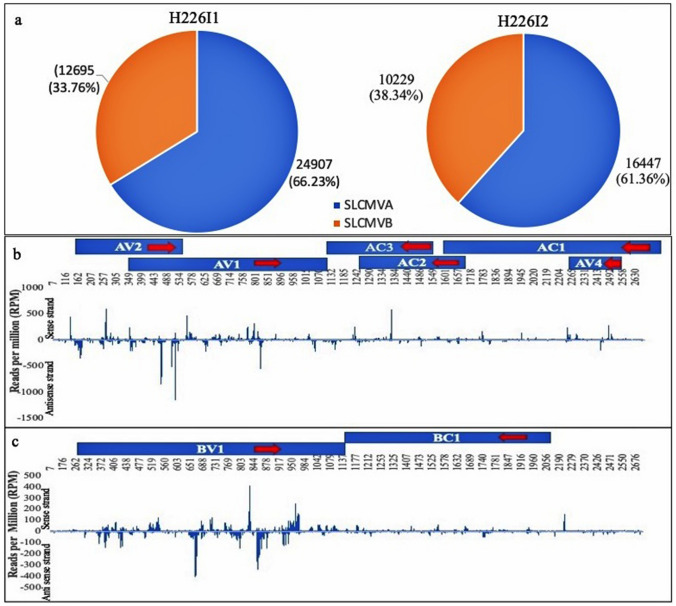
Small RNAs mapped to the virus genome were detected in the cassava leaves on SLCMV infection. The virus-derived small RNA reads constituted respectively of total read frequency of 37,602 and 26,676 from H226I1 and H226I2 (Fig. 5a). The mapping of small RNA reads from the control RNA library was negligible. Altogether, an average of 3.46% of the total reads from the infected leaf library reads mapped to SLCMV genome. Among the total reads mapped, the number of reads mapped to the SLCMVA genome was detected to be high in both the infected library, and it constituted respectively of 66.23% and 61.36% in H226I1 and H226I2. Small RNAs were mapped at the sense and antisense polarity of the bipartite SLCMV DNA-A and DNA-B genome (Fig. 5b, c).
Fig. 5.
a Small RNA reads mapped to the SLCMV bipartite genome A and B. b Virus- derived sRNAs mapped to the sense and antisense polarity of SLCMV DNA- A genome. c Virus- derived sRNAs mapped to the sense and antisense polarity of SLCMV DNA- B genome
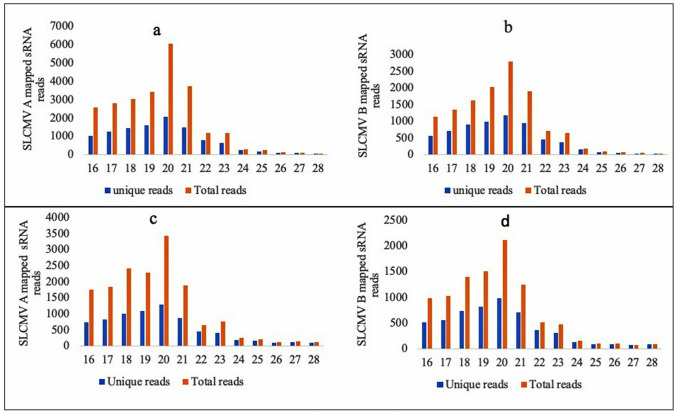
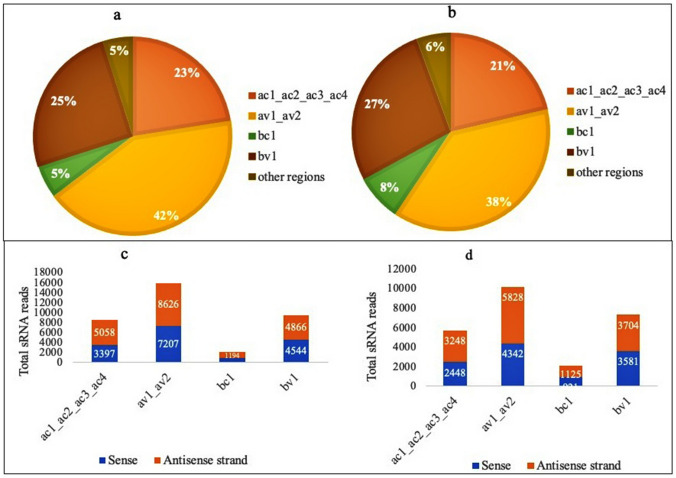
The length distribution of the SLCMV genome A and B mapped reads further revealed highest percentage of sRNAs with 20nt length derived from both genome (Fig. 6a-d). The most prominent sRNAs mapped to the SLCMV genome is listed in the Table 4. Even though vsRNAs were mapped in the sense and complementary strands of DNA-B, higher reads were detected for vsRNAs derived from the complementary strand of DNA-A, particularly from AV1/AV2. Both the infected libraries showed similar trend of small RNA distribution across the SLCMV genome and virus-derived small RNAs mapped at the av1/av2 protein encoding region was high (38–42%), followed by that of bv1 region (25–27%) and ac1/ac2/ac3/ac4 (21–23%) (Fig. 7a, b). Meanwhile, total sRNA reads mapped to the antisense strand of the SLCMV ORFs was observed to be higher than the sense strand (Fig. 7c, d). Gemini virus derived small RNAs were characterized from different plant species during infection (Akbergenov et al. 2006; Aregger et al. 2012). SiRNAs of both polarities derived from the coding and intergenic regions were reported from Cabbage leaf curl begomovirus in Arabidopsis and African cassava mosaic begomovirus in Nicotiana benthamiana and cassava (Akbergenov et al. 2006). Furthermore, the abundance of virus derived small RNAs generated from the coding regions were reported from the susceptible cultivars during the infection of SACMV (Rogans et al. 2016), EACMV and ACMV (Kuria et al. 2017), and CBSV (Ogwok et al. 2016). Begomovirus siRNAs are suggested to be generated by more than two RNA silencing pathways as there are distinct size classes of small RNAs generated by DCL1 proteins (Akbergenov et al. 2006).
Fig. 6.
Length distribution of sRNAs mapped at the SLCMV A and SLCMV B genome. a SLCMV A mapped sRNA reads from H226I1, b SLCMV B mapped sRNA reads from H226I1. c SLCMV A mapped sRNA reads from H226I2, d SLCMV B mapped sRNA reads from H226I2
Table 4.
The most prominent sRNAs mapped to the SLCMV genome
| sRNAs mapped | Mapped region | Start | End | Strand | Length | |
|---|---|---|---|---|---|---|
| SLCMV A (Acc. No KP455486.1) | CATCTGGGCTTTTGAACATC | AV1/AV2: Antisense | 467 | 487 | - | 20 |
| TCTGGACTCAAACGATTGAAC |
AV1/AV2: Antisense |
516 | 537 | - | 21 | |
| GATTTGATCTCTGTCATCAG |
AV1/AV2: sense |
266 | 286 | + | 20 | |
| AAGGTCATGTGCATCTCTGATGT |
AV1/AV2: sense |
558 | 581 | + | 23 | |
| CTGGACTCAAACGATTGAAC |
AV1/AV2: Antisense |
516 | 536 | - | 20 | |
| TGGGCTGTCGAAGTTCAGACG |
AV1/AV2: Antisense |
354 | 375 | - | 21 | |
| AGATTTGATCTCTGTCATCAG |
AV1/AV2: sense |
265 | 286 | + | 21 | |
| TGGATGATCCTGAAATAG | AC1/AC2/AC3:Antisense | 1371 | 1389 | + | 18 | |
| SLCMV B (KP455487.1) | TACCCTATCTGGACTAGTTT | BV1 | 959 | 979 | + | 20 |
Fig. 7.
Virus derived small RNAs reads mapped to different ORFs encoded in SLCMV genome A and B. a The read distribution of H226I1sRNAs mapped among the SLCMV ORFs. b The read distribution of H226I2 sRNAs mapped among the SLCMV ORFs. c Total sRNA reads of H226I1 distributed among the sense and antisense strands of SLCMV ORFs. d Total sRNA reads of H226I2 distributed among the sense and antisense strands of SLCMV ORFs
Identification of virus-encoded miRNAs
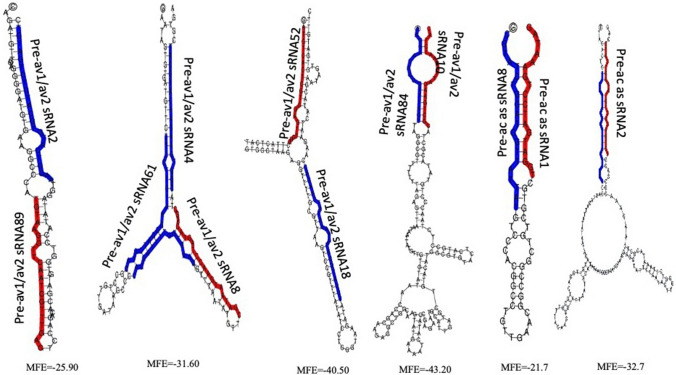
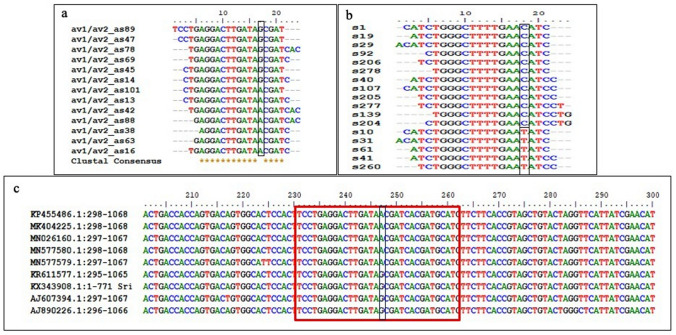
Consistent with previous reports of EACMV and ACMV infection in cassava (Maghuly et al. 2014), potential virus-derived miRNAs were detected from the SLCMV genome during the interaction with susceptible host plant H226 (Supplementary Table 4). Hair-pin like secondary structures were predicted for 13 miRNAs from the AV1/AV2 and AC1/AC2/AC3/AC4 genes encoded in the virus genome (Fig. 8). The combined approach of sequencing of cloned sRNA sequences and computational analysis of miRNA-like hairpin structure validates virus-derived miRNAs (Kincaid and Sullivan 2012; Mishra et al. 2020). DNA virus can utilize the cellular machinery of the host and express miRNAs to modulate host and viral gene expression (Mishra et al. 2020). Thus, the viral miRNAs mimic host miRNAs and participate in the conserved network of host miRNA targeting (Kincaid and Sullivan 2012). Virus-encoded miRNAs also regulate both viral life cycle and the interaction with their hosts. These miRNAs evolve rapidly and are critical mode of gene regulation in the virus itself (Nair and Zavolan 2006). Although virus-encoded miRNAs were identified as critical regulators of gene expression in animal-virus interactions, there are very few reports on existence of plant virus-derived miRNAs. The capacity of virus encoded miRNAs generated from the 5' and 3' hairpin arms of African cassava mosaic virus (ACMV) and East African cassava mosaic virus-Uganda (EACMV-UG) to bind the genomic sequences of the host plants revealed their potential role in virus stress response (Maghuly et al. 2014). Virus derived miRNAs were also reported from Sugarcane streak mosaic virus (SCSMV) (Gao et al. 2012), Hibiscus chlorotic ringspot virus (HCRSV) (Viswanathan et al. 2014), and more recently from the Banana bract mosaic virus (BBMV) (Sankaranarayanan et al. 2020). Meanwhile, two potential miRNAs such as pre-av1/av2 as sRNA1 and pre-av1/av2 as sRNA13 that mapped at the antisense AV1/AV2 revealed different isoforms in the dataset (Fig. 9a, b). The sequence alignment of the predicted precursors to other isolates of SLCMV genome revealed the possibility of different isolates in the infected leaf tissues of H226 plants (Fig. 9c). Furthermore, virus derived sRNA reads also revealed the possibility of novel SLCMV strains in the Indian cultivar H226.
Fig. 8.
Virus-derived miRNAs predicted from SLCMV genome
Fig. 9.
Different isoforms and variants of v-miRNAs detected in the cassava small RNA library of SLCMV infected H226 leaves. a miRNA isoforms and variants detected from av1/av2 as sRNA13 and b av1/av2 as sRNA1. c The sequence variation detected at the av1/av2 as sRNA13 mapped regions of the different SLCMV isolates from cassava
Targets of host and virus-derived sRNAs
The targets of the differentially expressed miRNAs and novel miRNAs were predicted from the Manihot esculenta unigene DFCI gene index (MAESGI, version1/2010) from the psRNATargetserver. The Type IIIa membrane protein cp-wap13, 60S ribosomal protein L23a and Cytochrome c oxidase subunit I were predicted as targets of novel miRNA1. Branched-chain alpha keto-acid dehydrogenase E1-alpha subunit, Methylcrotonoyl-CoA carboxylase subunit alpha, Protein kinase and RAP2-like protein were predicted as targets of novel miRNA 2. Tyrosine recombinase, Proton-translocating NADH-quinone oxidoreductase were predicted as targets of novel miR3. Anthranilate N-hydroxycinnamoyl/benzoyltransferase were predicted as potential target for the novel miR4. The targets of novel miRNAs were listed in the Table 5. Targets were also predicted for the SLCMV derived small RNAs from the host genome (Table 6). Transcripts encoding Aldehyde dehydrogenase and Nitrite oxidoreductase beta-subunit were predicted as targets of SLCMV sRNA1. ADP-ribosylation factor, ARF1-like GTP-binding protein, Zinc finger (C3HC4-type RING finger) protein, Eukaryotic translation initiation factor, 60 s acidic ribosomal protein-like protein, glutamine synthase precursor etc. were potential host mRNA targets predicted for SLCMV sRNA3. Meanwhile SLCMV sRNA4 was predicted to target mRNAs of phosphate transporter, cytosine transporter, zinc finger protein and senescence associated protein.
Table 5.
Targets predicted for the novel miRNAs of cassava

Table 6.
Targets predicted for SLCMV derived small RNAs/microRNAs from the transcriptome of the host plant M. esculenta
| Virus sRNAs | Target Accession No | Alignment Score | Target inhibition | Target Function |
|---|---|---|---|---|
| SLCMV sRNA1 | DB933423 | 3.5 | Cleavage | Aldehyde dehydrogenase |
| SLCMV sRNA1 | DB941541 | 3.5 | Cleavage | Nitrite oxidoreductase beta-subunit |
| SLCMV sRNA3 | TC1305 | 2 | Cleavage | ADP-ribosylation factor 1 |
| SLCMV sRNA3 | FG806228 | 2.5 | Cleavage | ARF1-like GTP-binding protein |
| SLCMV sRNA3 | TC11819 | 3.5 | Cleavage | Zinc finger (C3HC4-type RING finger) protein-like |
| SLCMV sRNA3 | DV456692 | 3.5 | Cleavage | Eukaryotic translation initiation factor |
| SLCMV sRNA3 | TC1732 | 3.5 | Cleavage | 60 s acidic ribosomal protein-like protein |
| SLCMV sRNA3 | TC3840 | 3.5 | Cleavage | 60 s acidic ribosomal protein-like protein |
| SLCMV sRNA3 | TC2154 | 3.5 | Cleavage | Glutamine synthetase precursor |
| SLCMV sRNA3 | DV454971 | 3.5 | Cleavage | NADH-ubiquinone oxidoreductase chain 1 |
| SLCMV sRNA4 | CK643326 | 2.5 | Cleavage | Phosphate transporter |
| SLCMV sRNA4 | TC2216 | 3 | Cleavage | Cytosine transporter |
| SLCMV sRNA4 | DV455651 | 3 | Cleavage | Phosphate transporter |
| SLCMV sRNA4 | DB933778 | 3.5 | Cleavage | Zinc finger protein 780B |
| SLCMV sRNA4 | TC351 | 3.5 | Cleavage | Senescence-associated protein |
| SLCMV sRNA4 | DB923868 | 3.5 | Cleavage | Phosphate transporter |
| SLCMV sRNA4 | DV447769 | 3.5 | Cleavage | GA10363-PA |
| SLCMV sRNA5 | TC3915 | 3.5 | Cleavage | Phosphoglycerate kinase |
| SLCMV sRNA5 | TC11748 | 3.5 | Cleavage | Phosphoglycerate kinase |
| SLCMV sRNA6 | DB953267 | 3 | Cleavage | Amino acid permease family protein |
| SLCMV sRNA6 | DB952321 | 3.5 | Cleavage | Peptide synthetase |
| SLCMV sRNA6 | TC9642 | 3.5 | Cleavage | E3 ubiquitin-protein ligase CHIP |
| SLCMV sRNA6 | FF535522 | 3.5 | Cleavage | Cytochrome b5 isoform Cb5-A |
| SLCMV sRNA6 | TC380 | 3.5 | Cleavage | Cytochrome b5 isoform Cb5-A |
| SLCMV sRNA7 | TC1305 | 3 | Cleavage | ADP-ribosylation factor 1 |
| SLCMV sRNA7 | DB950702 | 3 | Translation | Predicted protein |
| SLCMV sRNA7 | TC1732 | 3 | Cleavage | 60 s acidic ribosomal protein-like protein |
| SLCMV sRNA7 | TC3840 | 3 | Cleavage | 60 s acidic ribosomal protein-like protein |
| SLCMV sRNA7 | DV456692 | 3.5 | Cleavage | Eukaryotic translation initiation factor |
| SLCMV sRNA7 | FG806228 | 3.5 | Cleavage | ARF1-like GTP-binding protein |
| SLCMV sRNA7 | TC2154 | 3.5 | Cleavage | Glutamine synthetase precursor |
| SLCMV sRNA7 | DV454971 | 3.5 | Cleavage | NADH-ubiquinone oxidoreductase chain 1 |
| SLCMV sRNA7 | TC9497 | 3.5 | Cleavage | Starch branching enzyme I precursor |
Conclusion
Geminiviruses are not only the largest group of plant viruses but also the major plant pathogens that threaten food security globally (Hesketh et al. 2018). In the present study, the high throughput small RNA sequencing was carried out from the susceptible cassava plant H226 on SLCMV infection. Sequence analysis of the infected leaf libraries revealed virus derived small RNAs mapped to the bipartite SLCMV genome with the abundance of siRNAs generated from the AV1/AV2 genes. Furthermore, potential virus-encoded miRNAs were also identified along with host derived miRNAs. Several host genes involved in virus interaction were predicted as possible targets of the vsRNAs/miRNAs, indicating their key role in infection process in cassava plants. The biogenesis, origin, cleavage hotspots and the distribution of vsRNAs provide the insights of precise processing events from the virus genome. The current understanding of host and virus sRNAs laid a foundation for developing new molecular strategies for crop improvement in the Indian cassava cultivars against SLCMV infections. Future efforts will be directed to the elucidation of overall mechanisms of biogenesis and function of these small RNAs and development of tools for effective resistance to the pathogen.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
SA greatly acknowledge the DST-SERB NPDF (PDF/2017/000914) and KBC KSCSTE PDF (No. 049/PDF/KBC/2017/KSCSTE). SM acknowledge the Research Fellowship from KSCSTE. TM acknowledge the financial support from ICAR-New Delhi.
Abbreviations
- SLCMV
Sri Lankan Cassava Mosaic Virus
- miRNA
MicroRNA
- vsRNAs
Virus-derived small RNAs
- siRNAs
Small interfering RNAs
- CMD
Cassava Mosaic Disease
- ACMV
African Cassava Mosaic Virus
- ORF
Open reading frame
Author contributions
SA and MK contributed to the study conception and design. Material preparation, data collection and analysis were performed by SA, SM and TM. The first draft of the manuscript was written by SA and all authors read and approved the final manuscript.
Funding
The research leading to these results received funding from National Post- Doctoral Fellowship – Science and Engineering Research Board—Department of Science and Technology, India (SERB -DST) (PDF/2017/000914) and Kerala Biotechnology Commission (KBC)—Kerala State Council for Science, Technology and Environment (KSCSTE) Post-Doctoral Fellowship (No.049/PDF/KBC/2017/KSCSTE).
Data availability statement
The sRNA datasets generated from the study was deposited to the NCBI GEO Database under the accession number GSM 5175960- 5175962.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Research involving human participants and/or animals
Not applicable to the current research as we have not used any human or animal subject.
Informed consent
Not applicable.
References
- Akbergenov R, Si-Ammour A, Blevins T, Amin I, Kutter C, Vanderschuren H, Zhang P, Gruissem W, Meins FJ, Hohn T, Pooggin MM. Molecular characterization of geminivirus-derived small RNAs in different plant species. Nucl Acids Res. 2006;34(2):462–471. doi: 10.1093/nar/gkj447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin I, Patil BL, Briddon RW, Mansoor S, Fauquet CMA. Common set of developmental miRNAs are upregulated in Nicotiana benthamiana by diverse begomoviruses. Virol J. 2011;8:143. doi: 10.1186/1743-422X-8-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aregger M, Borah BK, Seguin J, Rajeswaran R, Gubaeva EG, Zvereva AS, Windels D, Vazquez F, Blevins T, Farinelli L, Pooggin MM. Primary and secondary siRNAs in geminivirus-induced gene silencing. PLOS Pathog. 2012;8(9):1–19. doi: 10.1371/journal.ppat.1002941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell MJ, Meyers BC. Revisiting criteria for plant microRNA annotation in the era of big data. Plant Cell. 2018;30:272–284. doi: 10.1105/tpc.17.00851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballén-Taborda C, Plata G, Ayling S, Rodríguez-Zapata F, Becerra Lopez-Lavalle LA, Duitama J, Tohme J (2013) Identification of cassava microRNAs under abiotic stress. Int J Genom 857986. 10.1155/2013/857986. [DOI] [PMC free article] [PubMed]
- Bredeson JV, Lyons JB, Prochnik SE, Wu GA, Ha CM, Edsinger-Gonzales E, Grimwood J, Schmutz J, Rabbi IY, Egesi C, Nauluvula P, Lebot V, Ndunguru J, Mkamilo G, Bart RS, Setter TL, Gleadow RM, Kulakow P, Ferguson ME, Rounsley S, Rokhsar DS. Sequencing wild and cultivated cassava and related species reveals extensive interspecific hybridization and genetic diversity. Nat Biotechnol. 2016;34(5):562–570. doi: 10.1038/nbt.3535. [DOI] [PubMed] [Google Scholar]
- Chapman EJ, Prokhnevsky AI, Gopinath K, Dolja VV, Carrington JC. Viral RNA silencing suppressors inhibit the microRNA pathway at an intermediate step. Genes Dev. 2004;18(10):1179–1186. doi: 10.1101/gad.1201204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chen X, Chai X, Qiu Y, Gong C, Zhang Z, Wang T, Zhang Y, Li J, Wang A. Effects of low temperature on mRNA and Small RNA transcriptomes in Solanum Lycopersicoides leaf revealed by RNA-Seq. Biochem Biophys Res Commun. 2015;464(3):768–773. doi: 10.1016/j.bbrc.2015.07.029. [DOI] [PubMed] [Google Scholar]
- Chen X, Xia J, Xia Z, Zhang H, Zeng C, Lu C, Zhang W, Wang W. Potential functions of microRNAs in starch metabolism and development revealed by miRNA transcriptome profiling of cassava cultivars and their wild progenitor. BMC Plant Biol. 2015;15:33. doi: 10.1186/s12870-014-0355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Zhao PX. PsRNATarget: a plant small RNA target analysis server. Nucl Acids Res. 2011;39(SUPPL. 2):1–5. doi: 10.1093/nar/gkr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Liu P, Wong SM (2012) Identification of a plant viral RNA genome in the nucleus. PLoS One 7(11):e48736. 10.1371/journal.pone.0048736. [DOI] [PMC free article] [PubMed]
- Hefferon KL. DNA Virus vectors for vaccine production in plants: spotlight on geminiviruses. Vaccines. 2014;2(3):642–653. doi: 10.3390/vaccines2030642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh EL, Saunders K, Fisher C, Potze J, Stanley J, Lomonossoff GP, Ranson NA (2018) The 3.3 Å structure of a plant geminivirus using Cryo-EM. Nat Commun 9(1):1–10. [DOI] [PMC free article] [PubMed]
- Jones-Rhoades MW, Bartel DP, Bartel B. MicroRNAs and their regulatory roles in plants. Annu Rev Plant Biol. 2006;57:19–53. doi: 10.1146/annurev.arplant.57.032905.105218. [DOI] [PubMed] [Google Scholar]
- Jose A, Makeshkumar T, Edison S. Survey of cassava mosaic disease in Kerala. J Root Crop. 2011;37:41–47. [Google Scholar]
- Kasschau KD, Xie Z, Allen E, Llave C, Chapman EJ, Krizan KA, Carrington JC. P1/HC-pro a viral suppressor of RNA silencing interferes with arabidopsis development and miRNA function. Dev Cell. 2003;4(2):205–217. doi: 10.1016/s1534-5807(03)00025-x. [DOI] [PubMed] [Google Scholar]
- Khatabi B, Arikit S, Xia R, Winter S, Oumar D, Mongomake K, Meyers BC, Fondong VN. High-Resolution identification and abundance profiling of cassava (Manihot esculenta Crantz) MicroRNAs. BMC Genomics. 2016;17:85. doi: 10.1186/s12864-016-2391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid RP, Sullivan CS. Virus-encoded microRNAs: an overview and a look to the future. PLOS Pathog. 2012;8(12):1–11. doi: 10.1371/journal.ppat.1003018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuria P, Ilyas M, Ateka E, Miano D, Onguso J, Carrington JC, Taylor NJ. Differential response of cassava genotypes to infection by Cassava Mosaic Geminiviruses. Virus Res. 2017;227:69–81. doi: 10.1016/j.virusres.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legg JP, Kumar PL, Makeshkumar T, Tripathi L, Ferguson M, Kanju E, Ntawuruhunga P, Cuellar W (2015) Cassava Virus Diseases: biology epidemiology and management. In: Advances in virus research, Elsevier, pp 85–142 [DOI] [PubMed]
- Liu SR, Zhou JJ, Hu CG, Wei CL, Zhang JZ. MicroRNA-mediated gene silencing in plant defense and viral counter-defense. Front Microbiol. 2017;8:1801. doi: 10.3389/fmicb.2017.01801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghuly F, Ramkat RC, Laimer M. Virus versus host plant MicroRNAs: who determines the outcome of the interaction? PLoS ONE. 2014;9(6):e98263. doi: 10.1371/journal.pone.0098263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeshkumar T, Nair RRA, Edison S. Detection of Indian cassava mosaic virus through polymerase chain reaction and nucleic acid hybridization techniques. J Root Crops. 2005;31(1):1–6. [Google Scholar]
- Meyers BC, Axtell MJ, Bartel B, Bartel DP, Baulcombe D, Bowman JL, Cao X, Carrington JC, Chen X, Green PJ, Griffiths-Jones S, Jacobsen SE, Mallory AC, Martienssen RA, Poethig RS, Qi Y, Vaucheret H, Voinnet O, Watanabe Y, Weigel D, Zhu JK. Criteria for annotation of plant microRNAs. Plant Cell. 2008;20(12):3186–3190. doi: 10.1105/tpc.108.064311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R, Kumar A, Ingle H, Kumar H. The interplay between viral-derived miRNAs and host immunity during infection. Front Immunol. 2020;10:3079. doi: 10.3389/fimmu.2019.03079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair V, Zavolan M. Virus-encoded microRNAs: novel regulators of gene expression. Trends Microbiol. 2006;14(4):169–175. doi: 10.1016/j.tim.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Ntui VO, Kong K, Khan RS, Igawa T, Janavi GJ, Rabindran R, Nakamura I, Mii M (2015) Resistance to Sri Lankan Cassava Mosaic Virus (SLCMV) in genetically engineered cassava cv. ku50 through rna silencing. PLoS One 10(4): e0120551. 10.1371/journal.pone.0120551 [DOI] [PMC free article] [PubMed]
- Ogwok E, Ilyas M, Alicai T, Rey MEC, Taylor NJ. Comparative analysis of virus-derived small RNAs within cassava (Manihot Esculenta Crantz) infected with Cassava Brown Streak Viruses. Virus Res. 2016;215:1–11. doi: 10.1016/j.virusres.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patanun O, Lertpanyasampatha M, Sojikul P, Viboonjun U, Narangajavana J. Computational identification of microRNAs and their targets in cassava (Manihot Esculenta Crantz.) Mol Biotechnol. 2013;53(3):257–269. doi: 10.1007/s12033-012-9521-z. [DOI] [PubMed] [Google Scholar]
- Patil BL, Bagewadi B, Yadav JS, Fauquet CM. Mapping and identification of Cassava Mosaic Geminivirus DNA-A and DNA-B genome sequences for efficient siRNA expression and RNAi based virus resistance by transient agro-infiltration studies. Virus Res. 2016;213:109–115. doi: 10.1016/j.virusres.2015.11.011. [DOI] [PubMed] [Google Scholar]
- Perez-Quintero AL, Quintero A, Urrego O, Vanegas P, Lopez C. Bioinformatic identification of cassava miRNAs differentially expressed in response to infection by Xanthomonas Axonopodis Pv. Manihotis BMC Plant Biol. 2012;12:29. doi: 10.1186/1471-2229-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero A, Perez-Quintero AL, Lopez C. Identification of ta-SiRNAs and Cis-Nat-SiRNAs in cassava and their roles in response to cassava bacterial blight. Genom Proteom Bioinf. 2013;11(3):172–181. doi: 10.1016/j.gpb.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogans SJ, Allie F, Tirant JE, Rey MEC. Small RNA and methylation responses in susceptible and tolerant landraces of cassava infected with South African Cassava Mosaic Virus. Virus Res. 2016;225:10–22. doi: 10.1016/j.virusres.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R, Palani SN, Kumar A, Selvakumar ASP, Tennyson J. Prediction and experimental confirmation of banana bract mosaic virus encoding miRNAs and their targets. ExRNA. 2020;2(1):5. doi: 10.1186/s41544-019-0044-7. [DOI] [Google Scholar]
- Vanderschuren H, Akbergenov R, Pooggin MM, Hohn T, Gruissem W, Zhang P. Transgenic cassava resistance to african cassava mosaic virus is enhanced by viral DNA-A bidirectional promoter-derived siRNAs. Plant Mol Biol. 2007;64(5):549–557. doi: 10.1007/s11103-007-9175-6. [DOI] [PubMed] [Google Scholar]
- Vanderschuren H, Alder A, Zhang P, Gruissem W. Dose-dependent RNAi-mediated geminivirus resistance in the tropical root crop cassava. Plant Mol Biol. 2009;70(3):265–272. doi: 10.1007/s11103-009-9472-3. [DOI] [PubMed] [Google Scholar]
- Vanderschuren H, Moreno I, Anjanappa R. B, Zainuddin I. M, Gruissem W (2012) Exploiting the combination of natural and genetically engineered resistance to cassava mosaic and cassava brown streak viruses impacting cassava production in Africa. PLoS One 9: e45277. 10.1371/journal.pone.0045277 [DOI] [PMC free article] [PubMed]
- Vanitharani R, Chellappan P, Fauquet CM. Short interfering RNA-mediated interference of gene expression and viral DNA accumulation in cultured plant cells. Proc Natl Acad Sci USA. 2003;100(16):9632–9636. doi: 10.1073/pnas.1733874100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanitharani R, Chellappan P, Pita JS, Fauquet CM. Differential roles of AC2 and AC4 of cassava geminiviruses in mediating synergism and suppression of posttranscriptional gene silencing. J Virol. 2004;78(17):9487–9498. doi: 10.1128/JVI.78.17.9487-9498.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan C, Anburaj J, Prabu G. Identification and validation of Sugarcane Streak Mosaic Virus-encoded microRNAs and their targets in sugarcane. Plant Cell Rep. 2014;33(2):265–276. doi: 10.1007/s00299-013-1527-x. [DOI] [PubMed] [Google Scholar]
- Xia J, Zeng C, Chen Z, Zhang K, Chen X, Zhou Y, Song S, Lu C, Yang R, Yang Z, Zhou J, Peng H, Wang W, Peng M, Zhang W. Endogenous small-noncoding RNAs and their roles in chilling response and stress acclimation in cassava. BMC Genom. 2014;15:634. doi: 10.1186/1471-2164-15-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Wang W, Zheng Y, Chen X, Bo W, Song S, Zhang W, Peng M. Conservation and divergence of micrornas and their functions in Euphorbiaceous plants. Nucl Acids Res. 2009;38(3):981–995. doi: 10.1093/nar/gkp1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Xia J, Chen X, Zhou Y, Peng M, Zhang W. MicroRNA-like RNAs from the same miRNA precursors play a role in cassava chilling responses. Sci Rep. 2017;7(1):17135. doi: 10.1038/s41598-017-16861-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Li W, Zhang J, Wang L, Wu J (2019) Roles of small RNAs in virus-plant interactions. Viruses 11(9). 10.3390/v11090827. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sRNA datasets generated from the study was deposited to the NCBI GEO Database under the accession number GSM 5175960- 5175962.