Abstract
Jumonji domain-containing 6 (JMJD6) protein has been reported to be upregulated in different cancer cells; however, to the best of our knowledge, no report has analyzed serum anti-JMJD6 antibodies (s-JMJD6-Abs) in patients with cancer. Therefore, the present study evaluated the clinical significance of s-JMJD6-Abs in patients with colorectal cancer. Preoperative serum samples were analyzed from 167 patients with colorectal cancer who underwent radical surgery between April 2007 and May 2012. The pathological stages were as follows Stage I (n=47), stage II (n=56), stage III (n=49) and stage IV (n=15). In addition, 96 healthy participants were analyzed as controls. s-JMJD6-Abs were analyzed by amplified luminescent proximity homology assay-linked immunosorbent assay. The cutoff value of s-JMJD6-Abs for detecting colorectal cancer was calculated to be 5,720 using the receiver operating characteristic curve. The positive rate of s-JMJD6-Abs was 37% in patients with colorectal cancer (61 of 167), independent of carcinoembryonic antigen or carbohydrate antigen 19-9 and p53-Abs. Clinicopathological factors and prognosis were compared between the s-JMJD6-Abs-positive group and the s-JMJD6-Abs-negative group. The s-JMJD6-Ab-positive status was significantly associated with older age (P=0.03), but was not associated with other clinicopathological variables. Regarding recurrence-free survival, the s-JMJD6-positive status was a significant poor prognostic factor in both univariate (P=0.02) and multivariate (P<0.01) analyses. Similarly, regarding overall survival, the s-JMJD6-Abs-positive status was a significant poor prognostic factor in both univariate (P=0.03) and multivariate (P=0.01) analyses. In conclusion, preoperative s-JMJD6-Abs was positive in 37% of patients with colorectal cancer and may be considered an independent poor prognostic biomarker.
Keywords: Jumonji domain-containing 6, autoantibody, colorectal cancer, prognosis, tumor marker
Introduction
The jumonji domain-containing protein 6 (JMJD6) plays a vital role in epigenetic regulation and demonstrates the tyrosine kinase activity (1). The JMJD6 has been reported in cellular proliferation and migration (2). Abnormal over expression of JMJD6 may contribute to the development of different types of cancer (breast cancer, malignant melanoma, oral cancer, lung adenocarcinoma, hepatocellular carcinoma, ovarian cancer, colorectal cancer, glioblastoma, glioma) (3–11). Overexpression of the JMJD6 gene promotes cell proliferation and migration and enhances tumor growth in vivo (2). JMJD6 expression has been linked to a poor prognosis in lung adenocarcinoma, hepatocellular carcinoma, ovarian cancer, and colorectal cancer.
JMJD6 was reported as a regulatory gene that works with Myc to promote tumorigenesis (12). Cancer cells in which EMT (epithelial-mesenchymal transition) is induced acquire invasive and metastatic potential. JMJD6 overexpression increases tumor volume, cause EMT, and enhances invasion in breast cancer (12). JMJD6 protein was found in intestinal glands where the intestinal epithelium is constantly regenerating, according to Wang et al (9). These reports suggested that JMJD6 may be involved in intestinal cell proliferation and may be a new biomarker for colorectal cancer development.
Generally, an immune response to aberrant tumor antigens produces autoantibodies early in carcinogenesis and the autoantibodies are frequently elevated in patients even at early disease stages due to an antigen expression within the tumor (13). Serum p53 autoantibodies have been reported as the most common autoantibody and are used as a standard biomarker in patients with colorectal cancer (14). Since the JMJD6 protein is specifically exhibited in cancer cells, it may cause autoantibodies. Nevertheless, there are no reports of the analysis of autoantibodies against JMJD6 in solid cancer patients. Therefore, this study aimed to analyze the anti-JMJD6 antibody in patients with colorectal cancer and to evaluate the clinicopathological and prognostic significance of the anti-JMJD6 antibody.
Materials and methods
Patients and sera
Overall, 167 patients with colorectal cancer who underwent radical surgery at Toho University Omori Medical Center were evaluated between April 2007 and May 2012. The control group comprised 96 healthy subjects provided by a health screening clinic, Port Square Kashiwado Clinic. The patients comprised 97 male and 70 female patients (mean age, 64.9 years; range, 33–90 years). The pathological stages were as follows; stage I (n=47), stage II (n=56), stage III (n=49), and stage IV (n=15). The control group comprised 51 healthy male and 45 healthy female (mean age, 58 years; range, 50–76 years).
The study was conducted following the guidelines Ethical statement of the Declaration of Helsinki and approved by the Ethics Committee of Faculty of Medicine, Toho University (approval no. A18103_A17052_A16035_A16001_26095_25024_24038_22047), Chiba University Graduate School of Medicine (approval no. 2018-320) (Japan), and Port Square Kashiwado Clinic, Kashiwado Memorial Foundation (approval no. 2012-001). Before surgery, serum samples were obtained and frozen at −80°C until analysis. Written, informed consent was obtained from all subjects. The patient's medical records were retrospectively reviewed according to the ethics committee of Toho University Omori Medical Center (approval nos. M21038_20197_19213 and M21320_21039_20200_30196_19056_18002).
Purification of recombinant JMJD6 and detection of s-JMJD6-antibody by amplified luminescent proximity homology assay-linked immunosorbent assay (Alpha-LISA)
Serum samples were obtained before surgery and stored frozen at −80°C until use. Glutathione S-transferase (GST) and GST-fused JMJD6 proteins were purified as described previously (15–17). s-JMJD6 Ab levels were assessed using an amplified luminescent proximity homology assay-linked immunosorbent assay (Alpha-LISA), as described previously (15–17). Briefly, Alpha-LISA was performed in 384-well microtiter plates (white opaque OptiPlate, PerkinElmer) containing either 2.5 µl of 1:100 diluted serum and 2.5 µl of 10 µg/ml of GST or GST-JMJD6 protein in AlphaLISA buffer (25-mM HEPES, pH 7.4, 0.1% casein, 0.5% Triton X-100, 1 mg/ml dextran-500, and 0.05% Proclin-300). The reaction mixture was incubated at room temperature for 6–8 h, following which anti-human IgG-conjugated acceptor beads (2.5 µl at 40 µg/ml) and glutathione-conjugated donor beads (2.5 µl at 40 µg/ml) were added and incubated further at room temperature in the dark for 1–21 days. Chemical emissions were read on an EnSpire Alpha microplate reader (PerkinElmer). Specific reactions were estimated by subtracting the emission photon counts of the GST controls from the counts of GST-JMJD6 proteins.
Statistical analysis
The cutoff value for detecting colorectal cancer was calculated using the receiver operating characteristic curve. Patients with a cutoff value greater than 5,720 were categorized as serum anti-JMJD6-Ab positive. Using 5,720 as the cutoff value, patients with colorectal cancer were categorized into the s-JMJD6-Abs-positive group (n=61) and s-JMJD6-Abs-negative group (n=106), and the following analyses were performed.
Clinicopathologic factors and prognosis were compared between the Ab-positive and Ab-negative groups using the Mann-Whitney U test or Fisher's exact probability test. Clinicopathological parameters associated with survival were assessed by univariate analysis with a log-rank test based on Kaplan-Meier survival curves. Multivariate analysis was conducted using the Cox proportional hazards model. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University; Saitama, Japan) (18), a graphical user interface of R (The R Foundation for Statistical Computing; version 2.13.0). P<0.05 was considered to indicate a statistically significant difference.
Results
Comparison of s-JMJD6-Abs positivity rates
A comparison of anti-JMJD6 antibody levels in serum from healthy controls and colorectal cancer patients is shown in Fig. 1. The serum antibody (s-JMJD6-Ab) levels against JMJD6 examined using Alpha-LISA. The cutoff value for detecting colorectal cancer was calculated using the receiver operating characteristic curve (ROC) and determined to be 5,720 (Fig. S1). The positive rate of s-JMJD6-Abs in colorectal cancer was significantly higher than that of healthy controls (37 vs. 14%, P<0.05; Fig. 1). The associations of positivity of s-JMJD6-Abs, CEA, CA19-9, and p53-Abs are shown in Fig. 2. Overall, 20 patients (12%) were solely positive for s-JMJD6-Abs. Therefore, entirely negative patients for tumor markers were reduced from 88 (53%) by combinatory use of CEA, CA19-9 to 49 (29%) by combinatory use of CEA, CA19-9, p53-Abs and s-JMJD6-Abs (Fig. 2).
Figure 1.
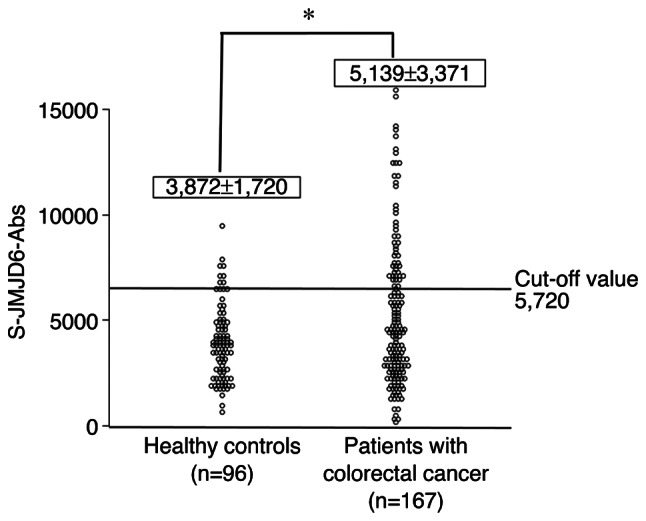
Comparing the serum anti-JMJD6 antibody levels between healthy controls and patients with colorectal cancer. This figure shows the levels of s-JMJD6-Abs examined using amplified luminescence proximity homogeneous assay-linked immunosorbent assay (AlphaLISA). A scatter dot plot of s-JMJD6-Abs (Alpha photon counts) is shown. Data are presented as the mean ± standard deviation. P-values were calculated using the Mann-Whitney U test. *P<0.05. The total sample numbers, a cutoff value are shown. The positive rate for patients with colorectal cancer was 37%, compared to 14% for healthy controls. s-JMJD6-Abs, serum antibodies against JMJD6.
Figure 2.
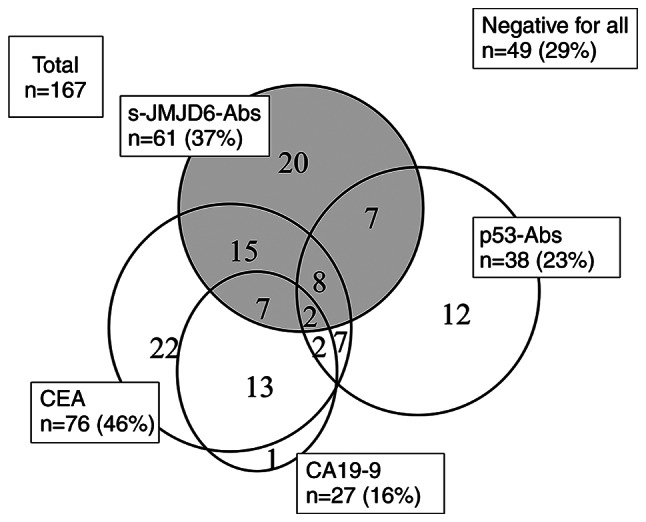
Relationship between positive serum tumor marker findings in patients with colorectal cancer. A total of 20 patients (12%) were solely positive for s-JMJD6-Abs. Therefore, entirely negative patients for tumor markers were reduced from 88 (53%) by combinatory use of CEA, CA19-9, p53-Abs to 49 (29%) by combinatory use of CEA, CA19-9, p53-Abs and s-JMJD6-Abs. CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; s-JMJD6-Abs, serum antibodies against JMJD6.
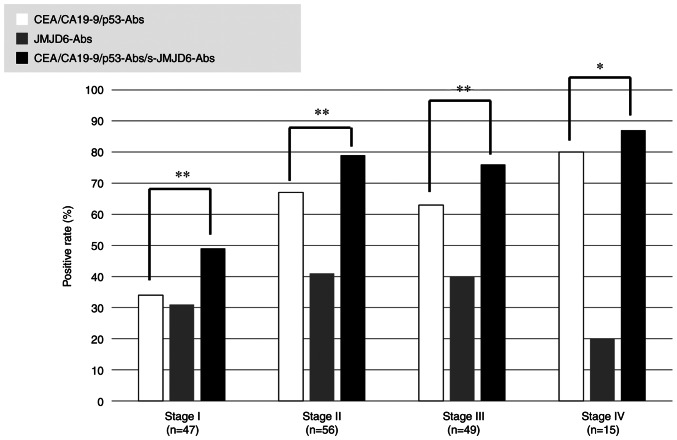
Comparisons of the positive rates according to tumor stages are shown in Fig. 3. The positive combinatory rates of CEA, CA19-9, p53-Abs were related to tumor progression (stage I: 34%, stage II: 68%, stage III: 63%, stage IV: 80%). Nevertheless, the positive rates of s-JMJD6-Abs were not associated with tumor progression (stage I: 32%, stage II: 41%, stage III: 41%, stage IV: 17%). Combinatory use of all these four markers significantly increased the positive rate than the combinatory use of CEA, CA19-9, and p53-Abs (Fig. 3).
Figure 3.
Comparison of positivity rates for CEA + CA19-9 + p53-Ab combination with that for CEA + CA19-9 + p53-Ab + s-JMJD6-Abs among patients with colorectal cancer according to the tumor stage. The P-values were calculated by Fisher's exact probability test. Combinatory use of all four markers significantly increased the positive rate than the combinatory use of CEA, CA19-9, and p53-Ab. **P<0.01, *P<0.05. CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; s-JMJD6-Abs, serum antibodies against JMJD6.
Comparison of clinicopathological factors between s-JMJD6-Ab-positive and s-JMJD6-Ab-negative groups
s-JMJD6-Ab positivity was significantly higher in the elderly group (P=0.03). In contrast, no other clinicopathological backgrounds demonstrated a significant association with s-JMJD6-Ab positivity. Additionally, no correlation was noticed between s-JMJD6-Abs and other tumor markers (Table I).
Table I.
Comparison of pretreatment JMJD6-Ab level with clinicopathological factors.
| Variable | Low JMJD6-Ab group <5,720, n=106 (%) | High JMJD6-Ab group ≧5,720, n=61 (%) | P-valuea |
|---|---|---|---|
| Sex | 0.15 | ||
| Female | 49 (70) | 21 (30) | |
| Male | 57 (59) | 40 (41) | |
| Age, years | 0.03 | ||
| <65 | 52 (73) | 19 (27) | |
| ≥65 | 54 (56) | 42 (44) | |
| Tumor depth | 0.61 | ||
| pT1pT2 | 34 (67) | 17 (33) | |
| pT3pT4 | 72 (62) | 44 (38) | |
| Nodal status | 1 | ||
| Negative | 68 (64) | 39 (36) | |
| Positive | 38 (63) | 22 (37) | |
| Stage | 0.17 | ||
| I/II/III | 93 (62) | 58 (38) | |
| IV | 13 (81) | 3 (19) | |
| Distant metastasis | 0.26 | ||
| Negative | 94 (62) | 58 (38) | |
| Positive | 12 (80) | 3 (20) | |
| Histology | 0.65 | ||
| Muc, Poor | 4 (80.0) | 1 (20) | |
| Tub | 101 (63) | 60 (37) | |
| CEA, 5 ng/ml | 0.20 | ||
| Negative | 62 (68) | 29 (32) | |
| Positive | 44 (58) | 32 (42) | |
| CA19-9, 37 U/ml | 0.67 | ||
| Negative | 90 (64) | 50 (36) | |
| Positive | 16 (59) | 11 (41) | |
| p53-Abb | 0.34 | ||
| Negative | 79 (65) | 42 (35) | |
| Positive | 21 (55) | 17 (45) |
Fisher's exact probability test;
excluding eight untested cases. Muc, mucinous adenocarcinoma; Poor, poorly differentiated adenocarcinoma; Tub, tubular adenocarcinoma.
Prognostic impact of s-JMJD6-Abs
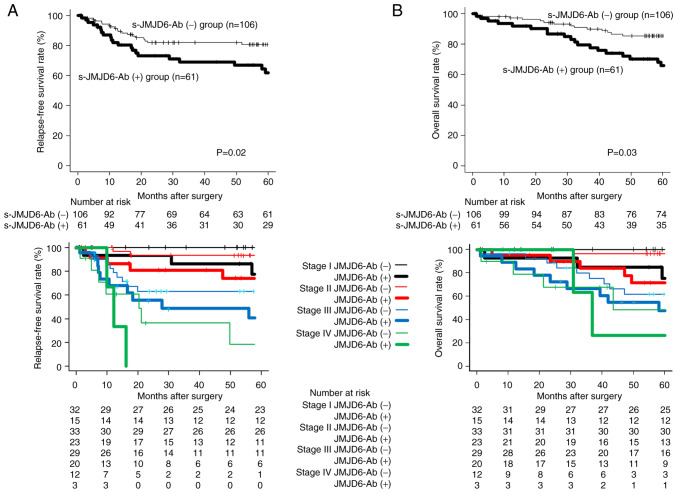
Comparisons of prognosis between the s-JMJD6-Ab-positive group and s-JMJD6-Ab-negative groups are shown in Fig. 4. Recurrence-free survival was significantly worse in the high s-JMJD6-Ab group than in the low s-JMJD6-Ab group (P=0.02; Fig. 4A). Overall survival was also significantly worse in the high s-JMJD6-Ab group than in the low s-JMJD6-Ab group (P=0.03; Fig. 4B). When the prognosis of the antibody-positive and antibody-negative groups was compared by stage, the antibody-positive group had a significantly poorer prognosis in stages I, II, and III than the antibody-negative groups. The antibody-positive group had a worse prognosis in stage IV, albeit this difference was not statistically significant.
Figure 4.
(A) Comparison of recurrence-free survival between the s-JMJD6-Ab-positive group and s-JMJD6-Ab-negative groups. (B) Comparison of overall survival between the s-JMJD6-Ab positive group and s-JMJD6-Ab negative groups. The bottom row is a comparison by stage. The P-values were calculated by log-rank test based on Kaplan-Meier. Relapse-free survival and overall survival were significantly worse in the high s-JMJD6-Ab group than in the low s-JMJD6-Ab group (P=0.02, P=0.03). The antibody-positive group had a significantly poorer prognosis in stages I, II, and III than the antibody-negative groups. s-JMJD6-Ab, serum antibody against JMJD6.
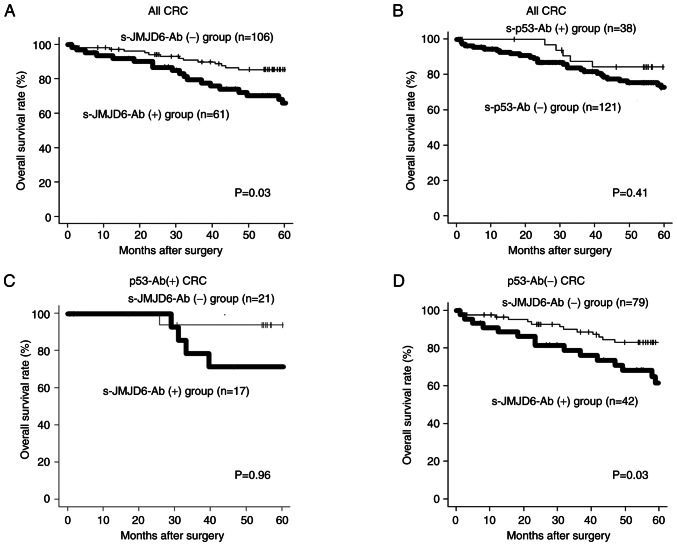
For all colorectal cancers, overall survival was significantly worse in the high s-JMJD6-Ab group than in the low s-JMJD6-Ab group (P=0.03; Fig. 5A). On the other hand, there was no significant difference in overall survival with or without p53 antibody (P=0.41; Fig. 5B). In p53 antibody-positive cases, no prognostic difference was observed between the presence or absence of s-JMJD6-Ab group (Fig. 5C). On the other hand, among p53-Abs-negative cases, s-JMJD6-Ab-positive cases had a worse prognosis than p53-Abs-negative cases (P=0.03, Fig. 5D).
Figure 5.
(A) Overall survival was significantly worse in the high s-JMJD6-Ab group than in the low s-JMJD6-Ab group in patients with colorectal cancer (P=0.03). (B) No significant difference in overall survival between the presence or absence of p53 antibody in patients with colorectal cancer (P=0.41). (C) In p53 antibody-positive cases, no prognostic difference was observed between the presence or absence of s-JMJD6-Ab group (P=0.96). (D) Among p53-Abs-negative cases, s-JMJD6-Ab-positive cases had a worse prognosis than p53-Abs-negative cases (P=0.03). CRC, colorectal cancer; s-JMJD6-Ab, serum antibody against JMJD6.
Univariate and multivariate analyzes of relapse-free survival
In the univariate analysis of recurrence-free survival, tumor depth (P<0.01), lymph node metastasis (P<0.01), distant metastasis (P<0.01), CA19-9 positivity (P<0.01), and high JMJD6-Abs (P=0.02) were significant poor prognostic factors regarding Relapse-free survival (Table II). In multivariate analysis, tumor depth (P=0.01), lymph node metastasis (P<0.01), distant metastasis (P<0.01), CA19-9 positivity (P=0.04), and high JMJD6-Abs (P<0.01) were independent poor prognostic factors.
Table II.
Univariate and multivariate analysis of clinicopathological factors to predict relapse-free survival.
| Multivariate analysis | ||||
|---|---|---|---|---|
|
|
||||
| Variable | Univariate P-valuea | HRb | 95% CIc | P-valued |
| Sex | ||||
| Male | 0.42 | |||
| Female | ||||
| Age, years | ||||
| <65 | 0.27 | |||
| ≧65 | ||||
| Tumor depth | ||||
| pT3pT4 | <0.01 | 4.59 | 1.36-15.57 | 0.01 |
| pT1pT2 | ||||
| Nodal status | ||||
| Positive | <0.01 | 3.30 | 1.67-6.52 | <0.01 |
| Negative | ||||
| JMJD6-Abs | ||||
| ≥5,720 | 0.02 | 2.39 | 1.28-4.46 | <0.01 |
| <5,720 | ||||
| Distant metastasis | ||||
| Positive | <0.01 | 3.29 | 1.52-7.14 | <0.01 |
| Negative | ||||
| Histology | ||||
| Muc, Poor | 0.36 | |||
| Tub | ||||
| CEA | ||||
| Positive | 0.32 | 0.71 | 0.36-1.39 | 0.32 |
| Negative | ||||
| CA19-9 | ||||
| Positive | <0.01 | 2.26 | 1.06-4.81 | 0.04 |
| Negative | ||||
| p53-Ab | ||||
| Positive | 0.41 | |||
| Negative | ||||
Log-rank test analysis;
adjusted HR;
adjusted 95% CI;
Cox proportional hazards regression analysis. CI, confidence interval; HR, hazards ratio. Muc, mucinous adenocarcinoma; Poor, poorly differentiated adenocarcinoma; Tub, tubular adenocarcinoma.
Univariate and multivariate analyzes of overall survival
Similarly, in a univariate analysis of overall survival (Table III), tumor invasiveness (P<0.01), lymph node metastasis (P<0.01), distant metastasis (P<0.01), CA19-9 (P<0.01) and high JMJD6-Abs (P=0.03) were significant poor prognostic factors. In multivariate analysis, tumor depth (P=0.03), lymph node metastasis (P<0.01), distant metastasis (P<0.01), and high JMJD6-Abs (P=0.01) were independent poor prognostic factors.
Table III.
Univariate and multivariate analysis of clinicopathological factors to predict overall survival.
| Multivariate analysis | ||||
|---|---|---|---|---|
|
|
||||
| Variable | Univariate P valuea | HRb | 95% CIc | P-valued |
| Sex | ||||
| Male | 0.30 | |||
| Female | ||||
| Age, years | ||||
| <65 | 0.24 | |||
| ≥65 | ||||
| Tumor depth | ||||
| pT3pT4 | <0.01 | 3.85 | 1.14-12.99 | 0.03 |
| pT1pT2 | ||||
| Nodal status | ||||
| Positive | <0.01 | 3.70 | 1.87-7.34 | <0.01 |
| Negative | ||||
| JMJD6-Abs | ||||
| ≥5,720 | 0.03 | 2.14 | 1.16-3.94 | 0.01 |
| <5,720 | ||||
| Distant metastasis | ||||
| Positive | <0.01 | 3.05 | 1.43-6.48 | 0.01 |
| Negative | ||||
| Histology | ||||
| Muc, Poor | 0.28 | |||
| Tub | ||||
| CEA | ||||
| Positive | 0.23 | 0.73 | 0.37-1.48 | 0.39 |
| Negative | ||||
| CA19-9 | ||||
| Positive | <0.01 | 2.13 | 0.98-4.63 | 0.06 |
| Negative | ||||
| p53-Ab | ||||
| Positive | 0.41 | |||
| Negative | ||||
Log-rank test analysis;
adjusted HR;
adjusted 95% CI;
Cox proportional hazards regression analysis. CI, confidence interval; HR, hazards ratio. Muc, mucinous adenocarcinoma; Poor, poorly differentiated adenocarcinoma; Tub, tubular adenocarcinoma.
Discussion
This study analyzed the anti-JMJD6 antibody in colorectal cancer patients and evaluated the clinicopathological and prognostic significance of the anti-JMJD6 antibody. The positive rate of s-JMJD6-Abs in patients with colorectal cancer was 37%. s-JMJD6-Abs along with CEA/CA19-9/s-p53-Abs was 71%. s-JMJD6-Abs showed no correlation with TNM factors. However, the presence of s-JMJD6-Abs was an independent poor prognostic factor.
The mechanism by which the JMJD6 protein generates autoantibodies is thought to be the induction of autoantibodies due to the leakage of antigens into the blood due to overexpression in the cancer cells and subsequent cancer cell destruction. Taking p53 as an example, in cancers with p53 mutations, Mdm2, one of p53′s target genes, is not induced, resulting in increased intracellular p53 protein levels. Therefore, when cancer cells are destroyed, many antigens leak into the blood, and it is speculated that p53 autoantibodies also increase. It has been reported that the JMJD6 protein is overexpressed in many cancer cells, including colon cancer, suggesting a mechanism similar to that of the p53 autoantibody production.
Because of the high frequency of advanced disease, the prognosis of JMJD6 immunostaining-positive patients with cancer was poor (2). We analyzed recurrence patterns in our current research population between antibody-positive and antibody-negative groups and found no significant difference in recurrence patterns (data not shown). Interestingly, such prognostic impact of JMJD6-Abs was evident in p53-Ab-negative cases. Because JMJD6 has been reported to suppress the activity of the tumor suppressor gene p53 via p53 hydroxylation (9), JMJD6 may suppress the activity of wild-type p53 and promote carcinogenesis in p53-Ab-negative cases. On the other hand, in the case of p53-Ab-positive cases, since p53 is mutated and does not function, it is thought that the presence or absence of JMJD6 has little prognostic effect. s-JMJD6-Abs did not affect intracellular JMJD6, suggesting that tissue destruction of cells with high levels of JMJD6 expression increases autoantibodies. In other words, we believe that the elevation of JMJD6-Abs is a result of its high expression in cancer cells. Although we could not directly compare the results with immunostaining, the antibody-positive cases probably fared worse than the immunostaining-positive ones. It has been reported that increased JMJD6 expression is associated with poor prognosis in colorectal cancer (9), and our findings imply that the s-JMJD6-Ab positivity may reflect increased JMJD6 exhibition in cancerous cells.
Since JMJD6 works as a transcriptional and splicing regulator for histone and non-histone proteins via arginine demethylation or lysine hydroxylation, reducing JMJD6 enzyme activity might be a promising new cancer therapy. For instance, inhibitors of lysine demethylase activity are entering the clinical trials (19). A small molecule inhibitor that can hinder the enzymatic activity of JMJD6 has been found (20). Such molecules suppress JMJD6-dependent cancer cell growth, including cervical cancer cells and hepatocellular carcinoma. Therefore, JMJD6 inhibitors may be a future option in cancer therapy. Clinical trials of molecularly-targeted agents should also assess whether to target immunostaining-positive tissue or antibody-positive patients. An autoantibody monitoring-based treatment technique may be effective in circumstances when monitoring the progress of therapy or tissue biopsy is challenging.
The positive rates of s-JMJD6-Abs slightly increased in stage II. However, no elevations were observed in stages III or IV. This tendency is frequent in autoantibody markers in colorectal cancer (21,22). The weakness of immune reactions against tumor antigens in the liver metastases may be why antibody titers decrease in stage IV patients. Notably, a tendency for the positive rate to decrease in stage IV compared with stage III has also been noticed in the other autoantibodies in the different types of cancer (23). The decrease in the autoantibody levels may be attributable to the immune system breakdown or the absorption of autoantibodies by antigens leaked due to excessive tissue destruction.
The limitations of this study are as follows. First, we did not evaluate the association between protein expression and the s-JMJD6-Ab reaction using the resected specimens although the antibody reactions may reflect protein expression. Second, the relationship between changes in antibody titer and recurrence could not be analyzed because of the lack of postoperative antibody titer changes and postoperative monitoring data. Third, regarding the grade of malignancy, it is implied that it is related to resistance to therapeutic drugs. Forth, relatively high false positive rate in heathy controls. It was found that the s-JMJD6-Ab-positive rate tended to be high in elderly people aged 65 and over and men.
Antibody markers are highly sensitive and have the potential to detect early-stage cancers. Thus far, precise data on the recurrence and/or treatment time are limited and unusable. In conclusion, although the presence of s-JMJD6-Abs was not substantially connected with TNM variables or stage in patients with colorectal cancer, it was an independent poor prognostic factor, suggesting that it is a valuable biomarker for predicting the malignant potential of colorectal cancer.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Xiao-Meng Zhang (Department of Biochemistry and Genetics, Graduate School of Medicine, Chiba University) for identification and purification of the antigen.
Funding Statement
This study was partly supported by Grant-in-Aid for Scientific Research (grant nos. 15K10117, 16K10520, 19K09451 and 21K08695) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Availability of data and materials
The datasets used and/or analyzed in the present study are available from the corresponding author on reasonable request.
Authors contributions
KY, HS, TH, HT and YI conceived and designed the current study. KY, MU, KF and HT acquired patient samples. KY, MU and KF contributed to the acquisition of the patient's clinicopathological data. KY, MI, MU, KF, SYL, BSZ and TH analyzed patient data. KY and HS drafted the manuscript. HS and TH confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was conducted following the Ethical statement of the Declaration of Helsinki guidelines. The collection of serum samples was approved by the Ethics Committee of Faculty of Medicine, Toho University (approval no. A18103_A17052_A16035_A16001 _26095_25024_24038_22047), Chiba University Graduate School of Medicine (approval no. 2018-320), and Port Square Kashiwado Clinic, Kashiwado Memorial Foundation (approval no. 2012-001). Written informed consent was obtained from all subjects. Retrospective analysis of patients' medical records was approved by the ethics committee of Toho University Omori Medical Center (approval nos. M21038_20197_19213 and M21320_21039_20200_30196_19056_18002).
Patient consent for publication
No applicable.
Competing interests
Professor Hideaki Shimada received research grants from the Medical & Biological Laboratories Co., Ltd. (Nagoya, Japan). All other authors have no competing interests.
References
- 1.Yang J, Chen S, Yang Y, Ma X, Shao B, Yang S, Wei Y, Wei X. Jumonji domain-containing protein 6 protein and its role in cancer. Cell Prolif. 2020;53:e12747. doi: 10.1111/cpr.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poulard C, Rambaud J, Lavergne E, Jacquemetton J, Renoir JM, Tredan O, Chabaud S, Treilleux I, Corbo L, Le Romancer M. Role of JMJD6 in breast tumourigenesis. PLoS One. 2015;10:e0126181. doi: 10.1371/journal.pone.0126181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee YF, Miller LD, Chan XB, Black MA, Pang B, Ong CW, Salto-Tellez M, Liu ET, Desai KV. JMJD6 is a driver of cellular proliferation and motility and a marker of poor prognosis in breast cancer. Breast Cancer Res. 2012;14:R85. doi: 10.1186/bcr3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X, Si W, Liu X, He L, Ren J, Yang Z, Yang J, Li W, Liu S, Pei F, et al. JMJD6 promotes melanoma carcinogenesis through regulation of the alternative splicing of PAK1, a key MAPK signaling component. Mol Cancer. 2017;16:175. doi: 10.1186/s12943-017-0744-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CR, Lee SH, Rigas NK, Kim RH, Kang MK, Park NH, Shin KH. Elevated expression of JMJD6 is associated with oral carcinogenesis and maintains cancer stemness properties. Carcinogenesis. 2016;37:119–128. doi: 10.1093/carcin/bgv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Ni SS, Zhao WL, Dong XC, Wang JL. High expression of JMJD6 predicts unfavorable survival in lung adenocarcinoma. Tumour Biol. 2013;34:2397–2401. doi: 10.1007/s13277-013-0789-9. [DOI] [PubMed] [Google Scholar]
- 7.Wan J, Liu H, Yang L, Ma L, Liu J, Ming L. JMJD6 promotes hepatocellular carcinoma carcinogenesis by targeting CDK4. Int J Cancer. 2019;144:2489–2500. doi: 10.1002/ijc.31816. [DOI] [PubMed] [Google Scholar]
- 8.Zheng H, Tie Y, Fang Z, Wu X, Yi T, Huang S, Liang X, Qian Y, Wang Xi, Pi R, et al. Jumonji domain-containing 6 (JMJD6) identified as a potential therapeutic target in ovarian cancer. Signal Transduct Target Ther. 2019;4:24. doi: 10.1038/s41392-019-0055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang F, He L, Huangyang P, Liang J, Si W, Yan R, Han X, Liu S, Gui B, Li W, et al. JMJD6 promotes colon carcinogenesis through negative regulation of p53 by hydroxylation. PLoS Biol. 2014;12:e1001819. doi: 10.1371/journal.pbio.1001819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller TE, Liau BB, Wallace LC, Morton AR, Xie Q, Dixit D, Factor DC, Kim LJY, Morrow JJ, Wu Q, et al. Transcription elongation factors represent in vivo cancer dependencies in glioblastoma. Nature. 2017;547:355–359. doi: 10.1038/nature23000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou DX, Zhou D, Zhan SQ, Wang P, Qin K, Gan W, Lin XF. Inhibition of JMJD6 expression reduces the proliferation, migration and invasion of neuroglioma stem cells. Neoplasma. 2017;64:700–708. doi: 10.4149/neo_2017_507. [DOI] [PubMed] [Google Scholar]
- 12.Aprelikova O, Chen K, El Touny LH, Brignatz-Guittard C, Han J, Qiu T, Yang HH, Lee MP, Zhu M, Green JE. The epigenetic modifier JMJD6 is amplified in mammary tumors and cooperates with c-Myc to enhance cellular transformation, tumor progression, and metastasis. Clin Epigenetics. 2016;8:38. doi: 10.1186/s13148-016-0205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaneker P, Gray ES, Ziman MR. Autoantibody production in cancer-the humoral immune response toword autologous antigens in cancer patients. Autoimmun Rev. 2016;15:477–483. doi: 10.1016/j.autrev.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Ushigome M, Shimada H, Miura Y, Yoshida K, Kaneko T, Koda T, Nagashima Y, Suzuki T, Kagami S, Funahashi K. Changing pattern of tumor markers in recurrent colorectal cancer patients before surgery to recurrence: Serum p53 antibodies, CA19-9 and CEA. Int J Clin Oncol. 2020;25:622–632. doi: 10.1007/s10147-019-01597-6. [DOI] [PubMed] [Google Scholar]
- 15.Sumazaki M, Shimada H, Ito M, Shiratori F, Kobayashi E, Yoshida Y, Adachi A, Matsutani T, Iwadate Y, Mine S, et al. Serum anti-LRPAP1 is a common biomarker for digestive organ cancers and atherosclerotic diseases. Cancer Sci. 2020;111:4453–4464. doi: 10.1111/cas.14652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiwasa T, Wang H, Goto K, Mine S, Machida T, Kobayashi E, Yoshida Y, Adachi A, Matsutani T, Sata M, et al. Serum anti-DIDO1, anti-CPSF2, and anti-FOXJ2 antibodies as predictive risk markers for acute ischemic stroke. BMC Med. 2021;19:131. doi: 10.1186/s12916-021-02001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li SY, Yoshida Y, Kobayashi E, Kubota M, Matsutani T, Mine S, Machida T, Maezawa Y, Takemoto M, Yokote K, et al. Serum anti-AP3D1 antibodies are risk factors for acute ischemic stroke related with atherosclerosis. Sci Rep. 2021;11:13450. doi: 10.1038/s41598-021-92786-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maes T, Carceller E, Salas J, Ortega A, Buesa C. Advances in the development of histone lysine demethylase inhibitors. Curr Opin Pharmacol. 2015;23:52–60. doi: 10.1016/j.coph.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Ran T, Xiao R, Huang Q, Yuan H, Lu T, Liu W. In silico discovery of JMJD6 inhibitors for cancer treatment. ACS Med Chem Lett. 2019;10:1609–1613. doi: 10.1021/acsmedchemlett.9b00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ushigome M, Shimada H, Nabeya Y, Shiratori F, Soda H, Takiguchi N, Hoshino I, Kuwajima A, Kaneko T, Funahashi K. Possible predictive significance of serum RalA autoantibodies on relapse-free survival in patients with colorectal cancer. Mol Clin Oncol. 2021;14:18. doi: 10.3892/mco.2020.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ushigome M, Nabeya Y, Soda H, Takiguchi N, Kuwajima A, Tagawa M, Matsushita K, Koike J, Funahashi K, Shimada H. Multi-panel assay of serum autoantibodies in colorectal cancer. Int J Clin Oncol. 2018;23:917–923. doi: 10.1007/s10147-018-1278-3. [DOI] [PubMed] [Google Scholar]
- 23.Takashi S, Satoshi Y, Akihiko O, Naoya Y, Yusuke T, Kentaro M, Yu O, Yasuaki N, Koichi Y, Takashi F, et al. Clinical impact of preoperative serum p53 antibody titers in 1487 patients with surgically treated esophageal squamous cell carcinoma: A multi-institutional study. Esophagus. 2021;18:65–71. doi: 10.1007/s10388-020-00761-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed in the present study are available from the corresponding author on reasonable request.