Abstract
Ubiquitin C-terminal hydrolase-L1 (UCH-L1), a member of the lesser-known deubiquitinating enzyme family, has deubiquitinase and ubiquitin (Ub) ligase activity and the role of stabilizing Ub. UCH-L1 was first discovered in the brain and is associated with regulating cell differentiation, proliferation, transcriptional regulation and numerous other biological processes. UCH-L1 is predominantly expressed in the brain and serves a role in tumor promotion or inhibition. There is still controversy about the effect of UCH-L1 dysregulation in cancer and its mechanisms are unknown. Extensive research to investigate the mechanism of UCH-L1 in different types of cancer is key for the future treatment of UCH-L1-associated cancer. The present review details the molecular structure and function of UCH-L1. The role of UCH-L1 in different types of cancer is also summarized and how novel treatment targets provide a theoretical foundation in cancer research is discussed.
Keywords: ubiquitin C-terminal hydrolase-L1, cancer, deubiquitylation, invasion, metastasis, inhibitor
1. Introduction
As with phosphorylation, ubiquitination is a highly reversible post-translational modification that binds ubiquitin (Ub) proteins to target proteins, modifying their activity and stability. This is also essential for the editing and recycling of Ub (1). The critical members involved in the ubiquitination pathway are Ub-activating (E1s) and -conjugating enzymes (E2s) and Ub ligases (E3s), which use the energy supplied by ATP hydrolysis to establish a multi-step cascade, resulting in Ub binding to the substrate. By contrast, as anti-ubiquitinase enzymes, deubiquitinases (DUBs) regulate the target protein degradation and cleave its attached polyubiquitination chain, thereby regulating target protein stability (2). The ubiquitin-proteasome system consists of ubiquitinase enzymes, DUBs and the 26S proteasome complex, which has a key role in regulating protein degradation (3). To date, ~100 DUBs have been classified into five families: Ubiquitin C-terminal hydrolases (UCHs), ovarian tumor-related, ubiquitin-specific and Machado-Josephin domain proteases and Jab1/MPN domain-associated metallopeptidases. The UCH family consists of four members, UCH-L1, UCH-L3, UCH37 and BAP1 (4).
UCH-L1, a protein predominantly expressed in the brain, has been demonstrated to serve a critical role in neurodegenerative diseases, such as Parkinson's and Alzheimer's disease (5–8). There have been some reviews that discuss UCH-L1 in the brain (9–12), but to the best of our knowledge, there are few that discuss UCH-L1 in cancer (13–15). New studies suggest that UCH-L1 has a role in cancer (16–20). However, the effect of UCH-L1 on cancer is debatable. Certain research suggests that decreased UCH-L1 expression is associated with malignancies (21–28). Due to promoter methylation, UCH-L1 expression is silenced or decreased, resulting in the progression of numerous types of cancer, including esophageal (21), gastric (22), ovarian and (23), renal cancer (24), head and neck squamous cell (20) and hepatocellular carcinoma (HCC) (25), breast cancer (26), pancreatic neuroendocrine tumor (PNET) (27) and nasopharyngeal carcinoma (NPC) (28). By contrast, UCH-L1 is considered to be an oncogenic factor promoting the occurrence, invasion and metastasis of breast (29) and non-small cell lung cancer (NSCLC) (30), lymphoma (31), parathyroid carcinoma (32,33), cutaneous squamous cell cancer (34), osteosarcoma (35), uterine serous carcinoma (36) and neuroblastoma (37). However, the specific mechanism of UCH-L1 in these types of cancer remains unclear.
The molecular structure and function of UCH-L1 are discussed and summarized in the present review, focusing on its role in carcinogenesis. Although more evidence is needed to support UCH-L1 as a marker and therapeutic target for various types of cancer, the present review details necessary future research to understand the role of UCH-L1 in cancer. UCH-L1 substrate analysis will significantly help the research and development of associated drugs (38).
2. Molecular structure of UCH-L1
The gene encoding UCH-L1, also known as the PARK5 gene, is located on chromosome 4P14 (39). The peptide encoded by UCH-L1 consists of 223 amino acids and has a molecular weight of ~24.8 kDa. Although UCH-L1 is widely present in neurons and neuroendocrine cells of the brain, trace expression of UCH-L1 has also been found in the placenta, kidney, retina, testis and ovaries (6,40–44). UCH-L1 structure is comparable to UCH-L3 (45). The basic structure of UCH-L1 monomers consists of two lobes: One lobe comprises five α-helices and the other comprises two α-helices and six β-sheets. These α-helices and β-sheets constitute the α-β-α structure of UCH-L1. The cleft between these two lobes is the site of the catalytic cysteine C90 and consists of three secondary structures: An α-helix (α3), a β-strand (β3) and an L9-loop (Fig. 1). However, ring L8, which is located above C90, frequently obscures the fissure at the active site location (5). Notably, the L8 ring also forms a small tunnel in the fissure. This arrangement may allow the substrate to pass through while preventing the larger Ub chains from binding to UCH-L1 (4).
Figure 1.
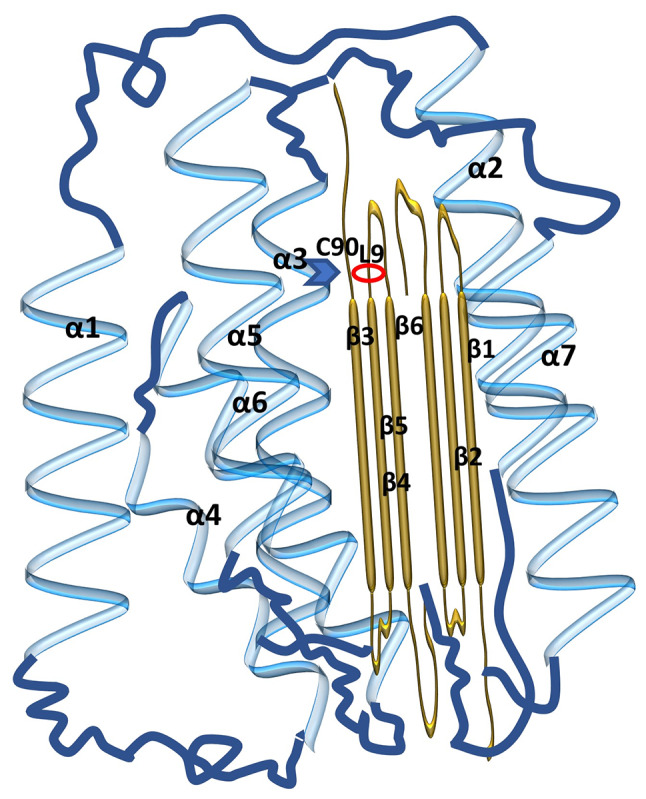
Molecular structure of UCH-L1. The secondary structure in blue represents α-helices and the secondary structure in orange represents the β-sheets. The unlabeled blue lines are unstructured regions. The five blue structures, α1, α3, α4, α5, α6, constitute one lobe of the UCH-L1 monomer, and the other lobe consists of two helices (α2 and α7) and six β-sheets (β1-β6). The cleft constitutes the catalytic cysteine, C90, which consists of three secondary structures: 3-helix, 3-strand and loop L9, between the two lobes. UCH-L1, ubiquitin C-terminal hydrolase-L1.
3. Molecular function of UCH-L1
Although specific molecular functions of UCH-L1 are not well understood, a number of studies have found that UCH-L1 participates in regulating free Ub pools, lysosomal activity, signaling molecules and cytoskeletal dynamics (46,47). UCH-L1 dysregulation contributes to various diseases, including cancer (48).
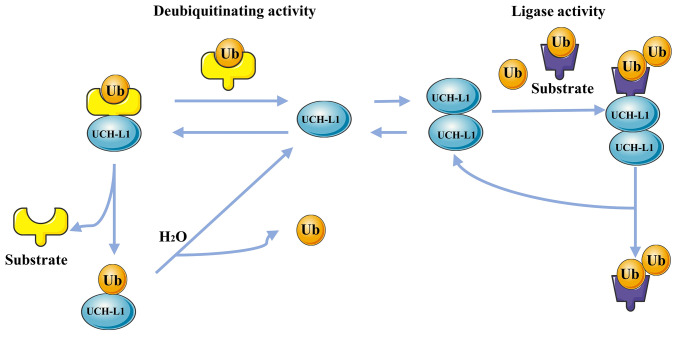
As a member of the UCH family of deubiquitinating enzymes, UCH-L1 exhibits low-activity hydrolase action that primarily hydrolyzes Ub chains of small polymeric or unfolded proteins (42,49). For example, its deubiquitinating activity controls expression of β-catenin (Fig. 2), which activates the β-catenin/T cell factor (TCF) pathway and enhances expression of related genes, including c-myc and c-jun (50). The β-catenin/TCF signaling pathway promotes UCH-L1 expression (51). Furthermore, UCH-L1 maintains IκB-α in vascular cells while decreasing TNF-α-induced NF-κB activation (Fig. 2) (52,53). Thus, UCH-L1 may decrease expression of NF-κB-driven cytokines, such as inflammatory cytokines or adhesion molecules. Recently, UCH-L1 has been reported to be involved in activating the TGF-β/SMAD signaling pathway (54). UCH-L1 has Ub ligase activity in addition to deubiquitinase activity (55). A recent study suggested that UCH-L1 serves as a Ub ligase enzyme, mediating cortactin (CCTN) degradation by increasing ubiquitination of K48-linked CCTN (28). The upregulation of CCTN is associated with the progression of tumors (56). UCH-L1 can also stabilize the expression of Ub monomers in vivo (42). UCH-L1, depending on its ubiquitination enzyme activity, acts on the target protein and produces free Ub monomers, which participate in the Ub metabolism of target proteins. These Ub monomers also prevent degradation by binding to nearby UCH-L1. Furthermore, UCH-L1 stabilization of Ub monomers is unaffected by its deubiquitination activity (57).
Figure 2.
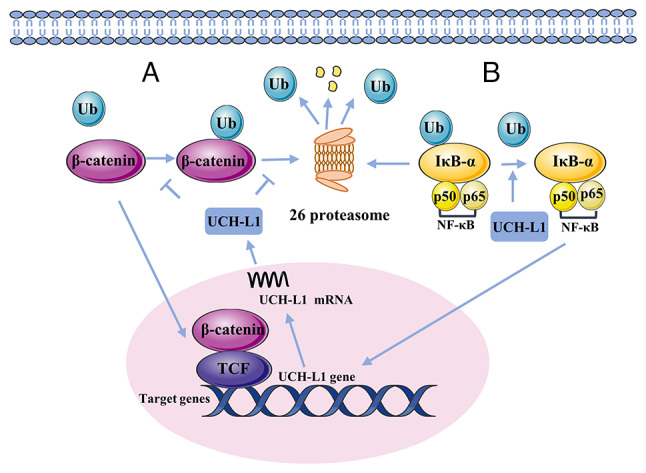
Deubiquitinating activity of UCH-L1 in cell signaling. (A) Mutual regulation between UCH-L1 and β-catenin. By downregulating the polyubiquitination of β-catenin, overexpressed UCH-L1 stabilizes β-catenin and upregulates transcription of β-catenin/TCF, further inducing the expression of oncogenes, such as c-myc, cyclin D, c-jun and survivin. TCF binds to the promoter of UCH-L1 and upregulates the translation of UCH-L1 mRNA. (B) UCH-L1 deubiquitinates IκB-α (an endogenous inhibitor of the NF-κB signaling pathway) and upregulates its expression, leading to decreased expression of NF-κB. NF-κB activation upregulates UCH-L1 expression. TCF, T cell factor; Ub, ubiquitin; UCH-L1, Ub C-terminal hydrolase-L1.
UCH-L1 is associated with skeletal muscle cell proliferation, sperm formation, angiogenesis and numerous other biological processes (13). For example, cell mitosis is inhibited by overexpression of UCH-L1, resulting in decreased cell proliferation (58). In addition, the C-terminus of UCH-L1 promotes phosphorylation of Akt, which facilitates survival and metabolic activity of malignant B cells (59). τ protein is the most abundant microtubule-associated protein in neurons, participating in regulation of synaptic plasticity, axonal transport and neuronal survival by promoting microtubule assembly and stabilizing microtubule networks (60). However, UCH-L1 inhibition reduces the enzyme activity of histone deacetylase 6 by reducing the production of K63-linked ubiquitin chain, leading to abnormal accumulation of τ protein, and finally affecting the brain (61,62). Furthermore, UCH-L1 may strengthen the ubiquitination and degradation of tubulin, arrest proliferation of cells and further inhibit microtubule formation (63). In addition, UCH-L1 increases the ubiquitination and degradation of microphthalmia-related transcription factor (MITF) by binding to ubiquitinated MITF (64).
UCH-L1 has hydrolase and ligase activity and stabilizes the Ub monomer effect (Fig. 3). In addition, it also has an important effect on the regulation of cell metabolic kinetics and the morphological structure of cellular proteins (13,33,65). For example, by reducing polyubiquitination and degradation of functional proteins such as p27Kip1 in podocytes of membranous nephropathy, UCH-L1 increases the accumulation of p27Kip1 protein, eventually leading to podocyte hypertrophy (66). Abnormal enzymatic activity of UCH-L1 may also be associated with the development and progression of disease, particularly cancer (55). For example, UCH-L1, according to its deubiquitination activity, inhibits the progression of malignant tumors such as NPC (67), breast cancer (26) and HCC (25) by activating p53 signaling, but promotes metastasis of gastric cancer cells by upregulating Akt and Erk1/2 signaling, and metastasis of breast and lung cancer cells by upregulating HIF-1α activity (68,69). While there are few reports on the role of the ligase activity of UCH-L1 in cancer (28,64), research is necessary to explore the association between them.
Figure 3.
Deubiquitinating and ligase activity of UCH-L1. When UCH-L1 exists as a monomer, its deubiquitinating enzyme activity specifically cleaves the heteropeptide bond between Ub and the protein substrate, which produces Ub monomers. When UCH-L1 is present as a dimer, it has an ATPase-independent Ub ligase activity, which forms K63-linked polyubiquitin chains that protect Ub from proteasomal degradation. Ub, ubiquitin; UCH-L1, Ub C-terminal hydrolase-L1.
4. UCH-L1 suppresses cancer
UCH-L1 in cancer
Studies have shown that UCH-L1, which also possesses an antitumor effect, is often deleted or silenced due to promoter methylation in various types of tumor tissue, such as esophageal (21) and gastric cancer (22,70), renal cell carcinoma (24,71), prostate cancer (72), primary head and neck squamous cell carcinoma (20) and ovarian (73) and colorectal cancer (74), resulting in adverse clinical outcomes. However, the tumor suppressor mechanism of UCH-L1 remains unclear.
UCH-L1 in HCC
The loss of UCH-L1 expression caused by promoter methylation occurs in most HCC tissue (25,75). However, adding UCH-L1 or demethylating drugs in HCC cell lines can inhibit cancer cell proliferation (25). On the one hand, restoring UCH-L1 expression in silenced cells leads to cell cycle disruption at G2/M and induces programmed cell death (25). On the other hand, UCH-L1 decreases degradation of p53 by interfering with ubiquitination of p53 and further stabilizes p53 expression, and ultimately inhibits proliferation of cancer cells (Fig. 4) (25). As a tumor inhibitor and biomarker, UCH-L1 may provide a new treatment strategy for HCC. Chemotherapy is one of the treatment measures for most patients with cancer, and chemotherapy resistance is a known side effect. In many cancers, such as breast cancer, lung cancer and esophageal squamous cell carcinoma, this chemoresistance can be reversed by verapamil (VER), a calcium channel-blocking drug (76–78). In treating HCC, the overexpression of UCH-L1 enhances the effect of VER in the reversal of adriamycin resistance and promotion of cancer cell apoptosis (79), however the underlying mechanism is unclear.
Figure 4.
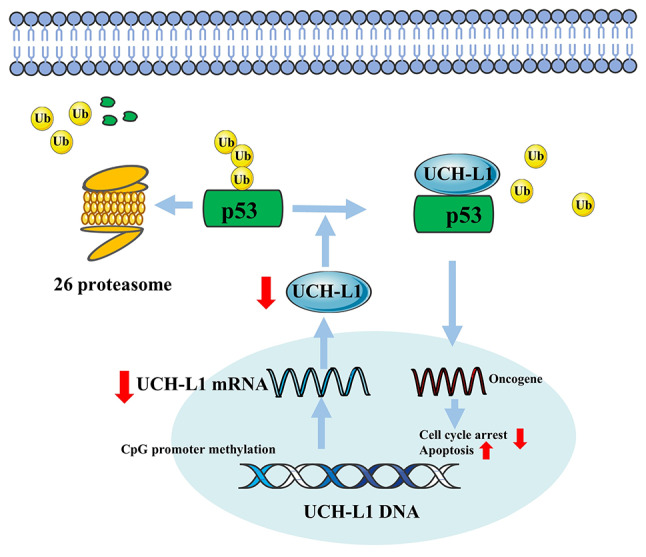
UCH-L1 methylation downregulates expression of UCH-L1. UCH-L1 promoter hypermethylation inhibits its transcription, causing decreased p53 stability and further decreasing or even eliminating the effects of UCH-L1 in promoting apoptosis and inhibiting cell proliferation. Ub, ubiquitin; UCH-L1, Ub C-terminal hydrolase-L1.
UCH-L1 in NPC
As with HCC, restoring UCH-L1's expression in NPC can significantly promote tumor cell apoptosis. UCH-L1 prolongs the half-life of p53 and p14ARF proteins through its deubiquitinase activity and shortens the half-life of MDM2 proteins through ubiquitination, resulting in the inhibition of NPC cell proliferation (67). In addition, the methylation of UCH-L1 leads to its deletion in NPC, which is conducive to the metastasis of NPC (67). By contrast, demethylated UCH-L1, which acts as a Ub ligase, prevents invasion of NPC by reducing CCTN stability (28).
UCH-L1 in breast cancer
UCH-L1 also blocks the proliferation and induces apoptosis in breast cancer cells (26,80). When the expression level of UCH-L1 increases, UCH-L1 further activates and stabilizes p53 signaling by inhibiting the degradation of p53, leading to cell cycle arrest at G0/G1 and apoptosis (Fig. 4) (26). UCH-L1 also induced apoptosis in breast cancer cells via the PI3K/Akt signaling pathway (80). As a tumor suppressor, UCH-L1 restrains the proliferation of tumor cells, but the silencing or deletion of UCH-L1 expression caused by promoter methylation reverses this tumor inhibition. A recent study proposed that for cancer caused by abnormal methylation of UCH-L1, the construction of CRISPR-Cas9-based vectors and targeted methylation of UCH-L1 may regulate expression of UCH-L1 to a basal level, but this needs to be proven in future research (81).
UCH-L1 in PNET
Low expression of UCH-L1 is an independent predictor of the invasiveness of PNET (82). UCH-L1 silencing or downregulation caused by promoter methylation participates in the metastasis of PNET (27). Pharmacological demethylation reactivates expression of UCH-L1 in the PNET, BON and QGP-1 cell lines, and UCH-L1 upregulates the expression levels of cyclins checkpoint kinase 2 and p21, leading to cell cycle arrest (27).
5. UCH-L1 promotes cancer
UCH-L1 promotes breast cancer
Breast cancer cell metastasis is the primary cause of mortality in most patients with breast cancer (83). The high invasiveness of breast cancer is regulated by abnormal expression of UCH-L1 (84,85). Wang et al (86) transfected MCF7 cells (a highly invasive breast cancer cell line) with a vector carrying UCH-L1; UCH-L1 enhanced the invasive ability of breast cancer by activating the MAPK/Erk signaling pathway. However, the opposite effect emerged when a vector specific for UCH-L1 small interfering RNA was transfected into MCF7/Adr cells (a multidrug resistance breast cancer cell line) (86). In addition, Luo et al (17) showed that highly expressed UCH-L1 directly binds to Akt2 to activate the Akt signaling pathway, resulting in a significant increase in MCF7 cell invasion. Epithelial-mesenchymal transition (EMT) facilitates cancer cell invasion and metastasis, which are enhanced by the cytokine TGF-β (87). In the most aggressive triple-negative breast cancer [loss of estrogen receptor (ER), progesterone receptor and HER2 expression], overexpression of UCH-L1 promotes TGF-β signaling-induced metastasis by inhibiting degradation of the TGF-β type I receptor and its downstream effector molecule SMAD2 (29). Therefore, UCH-L1 may be a therapeutic target for malignant breast cancer.
Multidrug resistance (MDR) is one of the leading causes of poor treatment outcomes for patients with breast cancer and a severe challenge to managing breast cancer (16). UCH-L1 is associated with the regulation of chemotherapy resistance in breast cancer. Immunohistochemistry by Jin et al (88) found that UCH-L1 can inhibit degradation of EGFR, resulting in high expression of P-glycoprotein (P-gp), CD147 and matrix metalloproteinase (MMP) in MDR breast cancer cells. In addition, a study demonstrated that upregulation of MDR1, CD147 and MMP can also be achieved via activation of the MAPK/Erk signaling pathway by UCH-L1 (Fig. 5) (86). Notably, high expression of P-gp, CD147 and MMP has also been proven to be the primary molecular mechanism mediating MDR (89). Activation of the UCH-L1/EGFR signaling pathway also inhibits ERα expression in breast cancer and downregulation of the deubiquitinase activity of UCH-L1 has the opposite effect, leading to Erα-breast cancer cells being more sensitive to treatment with tamoxifen and fulvestrant (90). Furthermore, ER− breast cancer has a worse prognosis than ER+ breast cancer (91,92). A new target for the treatment of EGFR-associated MDR breast cancer is expected to be identified in further studies of UCH-L1.
Figure 5.
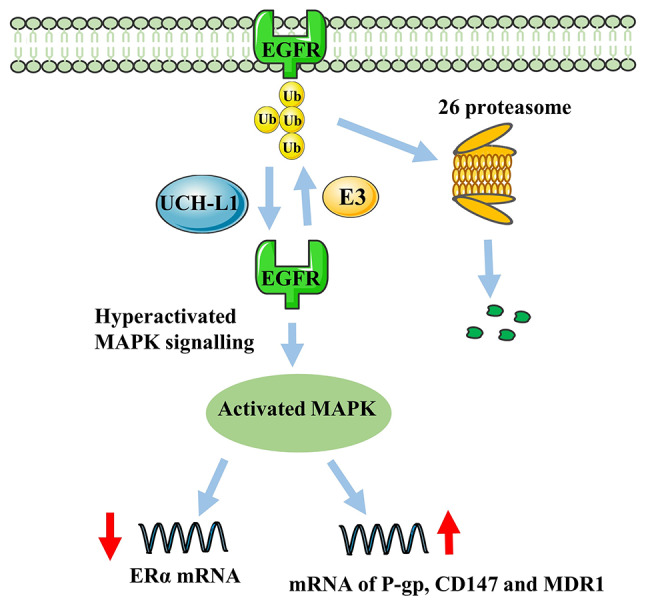
UCH-L1 balances EGFR expression. UCH-L1 deubiquitinates and regulates levels of EGFR, further activating MAPK signaling. Over-activated MAPK signaling downregulates ER or induces expression of P-gp, CD147 and MDR1. ER, estrogen receptor; MDR1, multidrug resistance protein 1; P-gp, P-glycoprotein; Ub, ubiquitin; UCH-L1, Ub C-terminal hydrolase-L1.
UCH-L1 promotes lung cancer
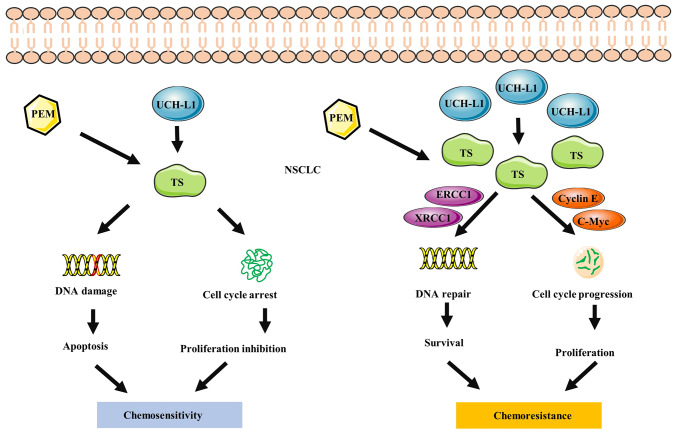
Poor prognosis in NSCLC is associated with high expression of UCH-L1 (93). The invasion of NSCLC cells is enhanced due to activation of the Akt signaling pathway by UCH-L1 (Fig. 6) (33). It has been reported that UCH-L1 is associated with chemoresistance in patients with NSCLC (19). Overexpression of UCH-L1 upregulates thymidylate synthase, decreasing pemetrexed-induced DNA degradation and cell cycle disturbance in NSCLC cells (Fig. 7) (19). Other findings have demonstrated that UCH-L1 overexpression is associated with high-grade neuroendocrine lung cancer (30,68), demonstrating that targeting UCH-L1 may be a novel strategy for treating drug resistance in lung cancer. As aforementioned, UCH-L1 is primarily found in the brain, reproductive organs and placenta. These normal tissues are primarily immune-privileged organs, which may imply that UCH-L1 is associated with tumor immune evasion. Programmed cell death ligand 1 (PD-L1) with antitumor effects is expressed in numerous cancer cells, including NSCLC cells, and is involved in the immune escape from cancer (94). Mao et al (95) hypothesized that inhibition of UCH-L1 may prevent immune escape development in NSCLC. Their findings suggested that UCH-L1 leads to upregulation of PD-L1 expression in NSCLC cell lines by activating the Akt/p65 signaling pathway.
Figure 6.
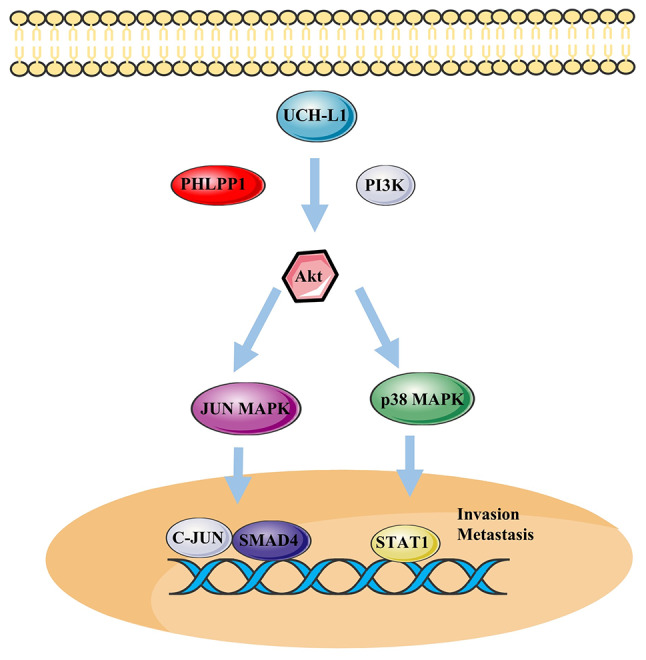
UCH-L1 expression boosts signaling through the Akt. UCH-L1 overexpression may regulate Akt signaling by inhibiting PHLLP1 or PI3K, resulting in increased MAPK signaling, which participates in progression of lymphoma and prostate and gastric cancer. SMAD4, recombinant mothers against decapentaplegic homolog 4; PHLPP1, PH domain and leucine-rich repeat protein phosphatase 1; UCH-L1, ubiquitin C-terminal hydrolase-L1.
Figure 7.
UCH-L1 induces PEM resistance in NSCLC. Overexpressed UCH-L1 regulates Cyclin E and c-myc by upregulating TS in NSCLC cells. High levels of Cyclin E and c-myc promote DNA repair and inhibit apoptosis, thereby alleviating PEM-induced DNA destruction and cell cycle disorder in NSCLC cells. NSCLC, non-small cell lung cancer; PEM, pemetrexed; TS, thymidylate synthase; UCH-L1, ubiquitin C-terminal hydrolase-L1.
UCH-L1 promotes osteosarcoma
UCH-L1 overexpression affects the prognosis of patients with osteosarcoma and UCH-L1 overexpression corresponds to high tumor metastasis rate (35). UCH-L1 may be regulated through Akt and MAPK/Erk signaling pathways, leading to proliferation, apoptosis and metastasis of osteosarcoma cells (35).
UCH-L1 promotes lymphoma
Overexpression of UCH-L1 participates in the development of lymphoma and high levels of UCH-L1 downregulate expression of PH domain and leucine-rich repeat protein phosphatase 1 (PHLPP1), which activates the Akt pathway (Fig. 6) (31). UCH-L1 may serve a key role in promoting proliferation and invasion of malignant B cells and regulating B cell adhesion by regulating the affinity of lymphocyte function-associated antigen 1 (65). Alternatively, UCH-L1 activates the PI3K/mTOR/Akt pathway by binding to eIF4F and bypassing mTORC1 expression, leading to development of B cell lymphoma (96). UCH-L1 is also an oncogenic biomarker of aggressive diffuse large B-cell lymphoma (97).
UCH-L1 promotes other tumors
UCH-L1 contributes to melanoma development by decreasing the stability of MITF as well as regulating the PI3K/Akt signaling pathway (64). A recent study suggested that UCH-L1 promotes uterine serous carcinoma by allowing cells to enter mitosis by upregulating expression of cyclin B, resulting in the proliferation of these cells (36). Therefore, UCH-L1 may be a novel prognostic marker for uterine serous carcinoma and a potential therapeutic target. UCH-L1 contributes to lymphatic metastasis by positively regulating growth arrest specific 2 protein levels, stimulating the migration ability of glioma (98). Furthermore, a study reported that UCH-L1 may support the development of distant metastasis in endometrial cancer (99). The metastasis of prostate cancer is also enhanced when EMT is promoted by UCH-L1 (100).
Metastasis is the primary cause of poor prognosis for patients with cancer. UCH-L serves a key role in promoting metastasis of some malignant tumors such as gastric cancer (69), breast cancer (86) and endometrial cancer (99). In 2009, UCH-L1 was identified as an essential factor contributing to tumor metastasis (33). Subsequently, it has been established that UCH-L1 overexpression further enhances metastasis or invasiveness of cells by altering cancer cell morphology and regulating the Akt signaling pathway (85). UCH-L1 is associated with cell proliferation, invasion and metastasis by activating the Erk1/2 and Akt pathways via deubiquitination in gastric cancer and liver metastatic tumor (69). Hypoxia-inducible factor-1 (HIF-1) supports cancer progression through a variety of mechanisms including angiogenesis, proliferation, invasion and metastasis of cells, cancer stem cell maintenance and treatment resistance (68,101). The downregulation of HIF-1 target gene expression is hypothesized to decrease with downregulation of UCH-L1 (102), demonstrating that inhibition of UCH-L1/HIF-1 pathway activity may be a method to treat distant metastasis and invasion of tumors.
6. UCH-L1 inhibitors
As aforementioned, UCH-L1 represents a unique therapeutic target for cancer. Research into UCH-L1 inhibitors will aid in treating malignancies that overexpress this protein. LDN-57444 has been widely used to downregulate expression of UCH-L1 in certain tumors, such as in NSCLC (19), oral squamous cell carcinoma (103) and neuroblastoma (37), therefore showing that it inhibits activity of metastatic tumor cells by reducing the deubiquitination activity of UCH-L1 (103). However, a number of investigations in recent years have indicated that this inhibitor has off-target toxicity and chemical instability, with LDN-57444 having limited binding to UCH-L1 in cells (104–107). MT16-001, a covalent inhibitor of UCH-L1 based on the thiazolyl cyanopyrrolidine backbone, binds to C90 in the active site of UCH-L1 (108). This inhibitor induces proliferation inhibition at sub-molar concentrations in B cell and lung cancer cell lines (which are known to be sensitive to UCH-L1 knockdown) and is more selective for UCH-L1 than other DUBs (108,109). However, based on the evidence reported to date (108), its selectivity profile has not been determined and further experiments are required to explore this. Fluorescent small molecule activity assay has shown that 6RK73 and 8RK59 effectively label UCH-L1 activity in vitro in cell lines and in vivo, with a higher inhibitory effect on UCH-L1 than LDN-57444 (110). However, as a UCH-L1 inhibitor, the cellular selectivity of 6RK73 and 8RK59 remains limited (110) It has also been demonstrated that 8RK59 binds only to the active site cysteine of UCH-L1 and not to catalytically inactive UCH-1 (110).
Recent studies have reported that IMP-1710, an UCH-L1 inhibitor, has higher quality and selectivity compared with LDN-57444 (111,112). IMP-1710 selectively labels C90 of UCH-L1 at nanomolar concentrations in a cell model of idiopathic pulmonary fibrosis to block the profibrotic response (112).
7. Discussion
In the present review, the molecular structure of UCH-L1 and its complex role in cancer were explained. UCH-L1, a multi-functional protein that is widely expressed in the brain, not only causes neurodegenerative disease but also serves a complex role in the occurrence and progression of cancer. UCH-L1 methylation is involved in development of various types of cancer, such as esophageal (21), gastric (22), ovarian (23) and renal cancer (24), head and neck squamous cell carcinoma (20), HCC (25), breast cancer (26), PNET (27) and nasopharyngeal cancer (28,67). When UCH-L1 expression is restored, UCH-L1 regulates key cyclin levels (such as p53), inhibits proliferation and promotes apoptosis of cancer cells (25,26). Notably, for cancer caused by epigenetic abnormality of UCH-L1, the CRISPR-Cas9 system, a specific genome editing technology, may effectively restore gene expression of UCH-L1 to normal levels and may be expected to be a future therapeutic option (81). However, this technology has numerous unsolved challenges, such as off-target effects and editing efficiency in clinical applications (113). Moreover, UCH-L1 functions as an oncogenic factor via PI3K/Akt, MAPK/Erk and other signaling pathways to induce tumorigeneses numerous types of cancer, including breast cancer (17,29,88), NSCLC (19), lymphoma (31), parathyroid carcinoma (32,114), melanoma (33), cutaneous squamous cell cancer (34), osteosarcoma (35), uterine serous carcinoma (36) and neuroblastoma (37) (Table I).
Table I.
UCH-L1 acts as a tumor suppressor or oncogenic factor in different tumors.
| A, Tumor suppressor | |||
|---|---|---|---|
|
| |||
| First author, year | Disease | Main mechanism | (Refs.) |
| Xiang et al, 2012 | Breast cancer | Stabilizes p53 and induces G0/G1 phase arrest and apoptosis | (26) |
| Mandelker et al, 2005 | Esophageal cancer | Blocks cell proliferation | (21) |
| Zhao et al, 2020 | Nasopharyngeal carcinoma | Increases K48-linked CTTN degradation to inhibit cell migration and invasion | (28) |
| Yu et al, 2008 | Hepatocellular carcinoma | Stabilizes p53 and induces G2/M phase arrest and apoptosis | (25) |
| Finnerty et al, 2019 and Moore et al, 2018 | Pancreatic neuroendocrine tumor | Stabilizes CHK2 and p21 and induces G0/G1 phase arrest | (27,82) |
| Okochi-Takada et al, 2006 | Ovarian cancer | Blocks cell proliferation | (23) |
| Kagara et al, 2008 | Renal carcinoma | Blocks cell proliferation | (24) |
|
| |||
| B, Oncogenic factor | |||
|
| |||
| First author, year | Disease | Main mechanism | (Refs.) |
|
| |||
| Jin et al, 2015 and Chen et al, 2020 | Breast cancer | Stabilizes EGRF | (88,90) |
| Kim et al, 2009 | Non-small cell lung cancer | Promotes upstream signaling of the Akt pathway | (33) |
| Kwan et al, 2020 | Uterine serous carcinoma | Stabilizes cyclin B and promotes cell cycle progression and tumor growth | (36) |
| Zheng et al, 2015 | Osteosarcoma | Regulates Akt and MAPK/Erk signaling pathways | (35) |
| Seo et al, 2017 | Melanoma | Decreases stability of MITF and modulates PI3K/Akt signaling | (64) |
| Hussain et al, 2010 | Lymphoma | Downregulates expression of PHLPP1, which activates the Akt pathway | (31) |
CTTN, cortactin; CHK2, checkpoint kinase 2; MITF, microphthalmia-related transcription factor; PHLPP1, PH domain and leucine rich repeat protein phosphatase 1; UCH-L1, ubiquitin C-terminal hydrolase-L1.
The role of UCH-L1 in tumorigenesis remains incompletely defined. UCH-L1 impacts cell proliferation and progression of cancer such as breast, colorectal and nasopharyngeal carcinoma, possibly due to Ub ligase enzyme or DUB activity of UCH-L1 (28,50,88). UCH-L1 relies on this DUB activity to support the survival of breast (88) and colorectal cancer (50), uterine serous carcinoma (36), NSCLC (56) and lymphoma (31). As a tumor suppressor, UCH-L1 relies on DUB activity to inhibit proliferation of cancer cells in breast cancer (26), HCC (25) and PNET (27) but inhibition of NPC depends on its ligase activity (28). Therefore, most studies have concluded that UCH-L1 relies on its DUB activity to affect cancer (36,50,56,88). This has key implications in drug development as cancer may be treated by inhibiting or activating the ligase or deubiquitinating activity of UCH-L1. In addition, wild-type TP53 typically causes cancer cell apoptosis, while mutated TP53 promotes cell carcinogenesis (115). UCH-L1 may stabilize wild-type TP53 expression in cancer cells to suppress cancer or stabilize mutated TP53 to promote cancer. Moreover, UCH-L1-activated Akt and MAPK signaling phosphorylate MDM2, a negative regulator of p53, and downregulate p53 expression (116,117). Whether UCH-L1 activates upstream signaling of Akt needs further study. Aberrant expression of UCH-L1 may indirectly alter ubiquitination of oncogenes and tumor suppressors by affecting many ubiquitination-dependent cellular activities, resulting in abnormal protein degradation or altered protein function, ultimately inhibiting or promoting cancer. For example, UCH-L1 protects phorbol-12-myristate-13-acetate-inducedprotein 1, a pro-apoptotic protein, from proteasomal degradation by hydrolyzing Lys48-linked polyubiquitin chains and enhances the sensitivity of colorectal and melanoma cells to chemotherapy (118). Therefore, exploring the specific mechanism of UCH-L1 in cancer may provide a novel diagnostic marker and drug target.
The role of UCH-L1 in breast cancer remains controversial. Certain research shows that UCH-L1 inhibits breast cancer, but most studies support the hypothesis that it is an oncogenic factor and promotes invasion and metastasis (26,29,88,90). However, the specific mechanism for the dual role of UCH-L1 in breast cancer is still not fully understood, potentially due to its different effects on different types of breast cancer via diverse cell signaling pathways. Notably, UCH-L1 mutants have been found in some cases of neurodegenerative disease (119,120). The I93M mutant of UCH-L1 shows a significant decrease in hydrolase activity compared with the wild-type UCH-L1 (121). Changes in the enzymatic activity of UCH-L1 mutants may explain the differential effect of UCH-L1 dysregulation in cancer. Further studies on the effect of UCH-L1 will be beneficial in clarifying the ability of UCH-L1 in oncogenic pathways. These differences may be attributed to stage, grade and ER status of breast cancer as most research supporting the promotion of UCH-L1 in breast cancer is based on ER− and highly invasive breast cancer (29,85,90). UCH-L1 overexpression upregulates the expression of EGFR, which in turn allows hyperactivity of the MAPK and PI3K/Akt signaling pathway to inhibit transcription of ERα, enhancing breast cancer invasion, metastasis and MDR (88). The effect of negative regulation of UCH-L1 on the proliferation of breast cancer cells may also be associated with TP53 mutation. Previous studies have found that TP53 mutations occur in ~80% of triple-negative breast cancer cases (122,123).
In addition to affecting various types of tumors and neurological disease, UCH-L1 has also been reported as a neuron-derived biomarker for traumatic brain injury and sudden cardiac arrest (124). UCH-L1 accumulates in podocytes constituting one of the glomerular filtration membranes (44) and UCH-L1 is overexpressed in human membranous and lupus nephritis and diabetic nephropathy (125–128). More notably, UCH-L1 is vital in regulating vascular remodeling (52). UCH-L1 may lead to inhibition of vascular smooth muscle cell proliferation (129,130). UCH-L1 provides positive regulation of cardiac hypertrophy and remodeling by enhancing EGFR expression (131), which means that the addition of UCH-L1 may be a treatment method for certain types of cardiovascular disease associated with atherosclerosis.
In summary, UCH-L1 is a potential target for treating cancers and diseases. Further research on efficient and selective UCH-L1 inhibitors (such as IMP-1710) will be beneficial in treating UCH-L1-related cancer. To the best of our knowledge, however, there are few reports about UCH-L1 substrates thus far. Therefore, in future research, attention should be paid to developing high quality activity-based probes to identify UCH-L1 substrates. Unbiased UCH-L1 substrate analysis is key to establish the total spectrum of expression activity and phenotype of UCH-L1 and the development of UCH-L1 inhibitors.
UCH-L1 has both Ub hydrolase and ligase activity, as well as monomeric stabilization effects. The different enzymatic activities of UCH-L1 regulate stability of different signaling pathways and cell cycle proteins in cancer cells. UCH-L1 inhibits or promotes development in different types of cancer, but there is controversy about the effect of UCH-L1 on cancer and its mechanism is still not well understood. Therefore, other substrates of UCH-L1 and specific mechanisms of deubiquitination regulation are hot spots for further research and targeting UCH-L1 may be an effective strategy for the treatment of cancer.
Acknowledgements
Not applicable.
Funding Statement
The present review was supported by the National Natural Science Foundation of China (grant no. 13009038), Project of Science and Technology Department of Jiangxi Province (grant nos. 20192BAB215024 and 20202BABL206099).
Availability of data and materials
Not applicable.
Authors' contributions
TO conceived the study and revised the manuscript. XW drafted and revised the manuscript and constructed the figures. NZ revised the manuscript. ML, TH and MW reviewed the manuscript. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kulathu Y, Komander D. Atypical ubiquitylation-the unexplored world of polyubiquitin beyond Lys48 and Lys63 linkages. Nat Rev Mol Cell Bio. 2012;13:508–523. doi: 10.1038/nrm3394. [DOI] [PubMed] [Google Scholar]
- 2.Eldridge AG, O'Brien T. Therapeutic strategies within the ubiquitin proteasome system. Cell Death Differ. 2010;17:4–13. doi: 10.1038/cdd.2009.82. [DOI] [PubMed] [Google Scholar]
- 3.Mata-Cantero L, Lobato-Gil S, Aillet F, Lang V, Rodriguez MS. Springer; Netherlands, Dordrecht: 2014. The ubiquitin-proteasome system (UPS) as a cancer drug target: Emerging mechanisms and therapeutics; pp. 225–264. [Google Scholar]
- 4.Komander D, Clague MJ, Urbé S. Breaking the chains: Structure and function of the deubiquitinases. Nat Rev Mol Cell Bio. 2009;10:550–563. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 5.Das C, Hoang QQ, Kreinbring CA, Luchansky SJ, Meray RK, Ray SS, Lansbury PT, Ringe D, Petsko GA. Structural basis for conformational plasticity of the Parkinson's disease-associated ubiquitin hydrolase UCH-L1. Proc Natl Acad Sci USA. 2006;103:4675–4680. doi: 10.1073/pnas.0510403103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Setsuie R, Wada K. The functions of UCH-L1 and its relation to neurodegenerative diseases. Neurochem Int. 2007;51:105–111. doi: 10.1016/j.neuint.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Doran JF, Jackson P, Kynoch PA, Thompson RJ. Isolation of PGP 9.5, a new human neurone-specific protein detected by high-resolution two-dimensional electrophoresis. J Neurochem. 1983;40:1542–1547. doi: 10.1111/j.1471-4159.1983.tb08124.x. [DOI] [PubMed] [Google Scholar]
- 8.Day INM, Thompson RJ. UCHL1 (PGP 9.5): Neuronal biomarker and ubiquitin system protein. Prog Neurobiol. 2010;90:327–362. doi: 10.1016/j.pneurobio.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Bishop P, Rocca D, Henley JM. Ubiquitin C-terminal hydrolase L1 (UCH-L1): Structure, distribution and roles in brain function and dysfunction. Biochem J. 2016;473:2453–2462. doi: 10.1042/BCJ20160082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang KK, Yang Z, Sarkis G, Torres I, Raghavan V. Ubiquitin C-terminal hydrolase-L1 (UCH-L1) as a therapeutic and diagnostic target in neurodegeneration, neurotrauma and neuro-injuries. Expert Opin Ther Targets. 2017;21:627–638. doi: 10.1080/14728222.2017.1321635. [DOI] [PubMed] [Google Scholar]
- 11.Gong B, Leznik E. The role of ubiquitin C-terminal hydrolase L1 in neurodegenerative disorders. Drug News Perspect. 2007;20:365–370. doi: 10.1358/dnp.2007.20.6.1138160. [DOI] [PubMed] [Google Scholar]
- 12.Butterfield DA. Ubiquitin carboxyl-terminal hydrolase L-1 in brain: Focus on its oxidative/nitrosative modification and role in brains of subjects with Alzheimer disease and mild cognitive impairment. Free Radic Biol Med. 2021;177:278–286. doi: 10.1016/j.freeradbiomed.2021.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matuszczak E, Tylicka M, Komarowska MD, Debek W, Hermanowicz A. Ubiquitin carboxy-terminal hydrolase L1-physiology and pathology. Cell Biochem Funct. 2020;38:533–540. doi: 10.1002/cbf.3527. [DOI] [PubMed] [Google Scholar]
- 14.Fang Y, Shen X. Ubiquitin carboxyl-terminal hydrolases: Involvement in cancer progression and clinical implications. Cancer Metast Rev. 2017;36:669–682. doi: 10.1007/s10555-017-9702-0. [DOI] [PubMed] [Google Scholar]
- 15.Fang Y, Fu D, Shen X. The potential role of ubiquitin c-terminal hydrolases in oncogenesis. Biochim Biophys Acta. 2010;1806:1–6. doi: 10.1016/j.bbcan.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Ning K, Wang T, Sun X, Zhang P, Chen Y, Jin J, Hua D. UCH-L1-containing exosomes mediate chemotherapeutic resistance transfer in breast cancer. J Surg Oncol. 2017;115:932–940. doi: 10.1002/jso.24614. [DOI] [PubMed] [Google Scholar]
- 17.Luo Y, He J, Yang C, Orange M, Ren X, Blair N, Tan T, Yang JM, Zhu H. UCH-L1 promotes invasion of breast cancer cells through activating Akt signaling pathway. J Cell Biochem. 2018;119:691–700. doi: 10.1002/jcb.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma A, Liu H, Tobar-Tosse F, Chand Dakal T, Ludwig M, Holz FG, Loeffler KU, Wüllner U, Herwig-Carl MC. Ubiquitin carboxyl-terminal hydrolases (UCHs): Potential mediators for cancer and neurodegeneration. Int J Mol Sci. 2020;21:3910. doi: 10.3390/ijms21113910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding X, Gu Y, Jin M, Guo X, Xue S, Tan C, Huang J, Yang W, Xue M, Zhou Q, et al. The deubiquitinating enzyme UCHL1 promotes resistance to pemetrexed in non-small cell lung cancer by upregulating thymidylate synthase. Theranostics. 2020;10:6048–6060. doi: 10.7150/thno.42096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tokumaru Y, Yamashita K, Kim MS, Park HL, Osada M, Mori M, Sidransky D. The role of PGP9.5 as a tumor suppressor gene in human cancer. Int J Cancer. 2008;123:753–759. doi: 10.1002/ijc.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandelker DL, Yamashita K, Tokumaru Y, Mimori K, Howard DL, Tanaka Y, Carvalho AL, Jiang WW, Park HL, Kim MS, et al. PGP9.5 promoter methylation is an independent prognostic factor for esophageal squamous cell carcinoma. Cancer Res. 2005;65:4963–4968. doi: 10.1158/0008-5472.CAN-04-3923. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita K, Park HL, Kim MS, Osada M, Tokumaru Y, Inoue H, Mori M, Sidransky D. PGP9.5 methylation in diffuse-type gastric cancer. Cancer Res. 2006;66:3921–3927. doi: 10.1158/0008-5472.CAN-05-1511. [DOI] [PubMed] [Google Scholar]
- 23.Okochi-Takada E, Nakazawa K, Wakabayashi M, Mori A, Ichimura S, Yasugi T, Ushijima T. Silencing of the UCHL1 gene in human colorectal and ovarian cancers. Int J Cancer. 2006;119:1338–1344. doi: 10.1002/ijc.22025. [DOI] [PubMed] [Google Scholar]
- 24.Kagara I, Enokida H, Kawakami K, Matsuda R, Toki K, Nishimura H, Chiyomaru T, Tatarano S, Itesako T, Kawamoto K, et al. CpG hypermethylation of the UCHL1 gene promoter is associated with pathogenesis and poor prognosis in renal cell carcinoma. J Urol. 2008;180:343–351. doi: 10.1016/j.juro.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 25.Yu J, Tao Q, Cheung KF, Jin H, Poon FF, Wang X, Li H, Cheng YY, Röcken C, Ebert MPA, et al. Epigenetic identification of ubiquitin carboxyl-terminal hydrolase L1 as a functional tumor suppressor and biomarker for hepatocellular carcinoma and other digestive tumors. Hepatology. 2008;48:508–518. doi: 10.1002/hep.22343. [DOI] [PubMed] [Google Scholar]
- 26.Xiang T, Li L, Yin X, Yuan C, Tan C, Su X, Xiong L, Putti TC, Oberst M, Kelly K, et al. The ubiquitin peptidase UCHL1 induces G0/G1 cell cycle arrest and apoptosis through stabilizing p53 and is frequently silenced in breast cancer. PLoS One. 2012;7:e29783. doi: 10.1371/journal.pone.0029783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finnerty BM, Moore MD, Verma A, Aronova A, Huang S, Edwards DP, Chen Z, Seandel M, Scognamiglio T, Du YN, et al. UCHL1 loss alters the cell-cycle in metastatic pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2019;26:411–423. doi: 10.1530/ERC-18-0507. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y, Lei Y, He SW, Li YQ, Wang YQ, Hong XH, Liang YL, Li JY, Chen Y, Luo WJ, et al. Hypermethylation of UCHL1 promotes metastasis of nasopharyngeal carcinoma by suppressing degradation of cortactin (CTTN) Cells. 2020;9:559. doi: 10.3390/cells9030559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, González-Prieto R, Zhang M, Geurink PP, Kooij R, Iyengar PV, van Dinther M, Bos E, Zhang X, Le Dévédec SE, et al. Deubiquitinase activity profiling identifies UCHL1 as a candidate oncoprotein that promotes TGFβ-induced breast cancer metastasis. Clin Cancer Res. 2020;26:1460–1473. doi: 10.1158/1078-0432.CCR-19-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimada Y, Kudo Y, Maehara S, Matsubayashi J, Otaki Y, Kajiwara N, Ohira T, Minna JD, Ikeda N. Ubiquitin C-terminal hydrolase-L1 has prognostic relevance and is a therapeutic target for high-grade neuroendocrine lung cancers. Cancer Sci. 2020;111:610–620. doi: 10.1111/cas.14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hussain S, Foreman O, Perkins SL, Witzig TE, Miles RR, van Deursen J, Galardy PJ. The de-ubiquitinase UCH-L1 is an oncogene that drives the development of lymphoma in vivo by deregulating PHLPP1 and Akt signaling. Leukemia. 2010;24:1641–1655. doi: 10.1038/leu.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howell VM, Gill A, Clarkson A, Nelson AE, Dunne R, Delbridge LW, Robinson BG, Teh BT, Gimm O, Marsh DJ. Accuracy of combined protein gene product 9.5 and parafibromin markers for immunohistochemical diagnosis of parathyroid carcinoma. J Clin Endocrinol Metab. 2009;94:434–441. doi: 10.1210/jc.2008-1740. [DOI] [PubMed] [Google Scholar]
- 33.Kim HJ, Kim YM, Lim S, Nam YK, Jeong J, Kim HJ, Lee KJ. Ubiquitin C-terminal hydrolase-L1 is a key regulator of tumor cell invasion and metastasis. Oncogene. 2009;28:117–127. doi: 10.1038/onc.2008.364. [DOI] [PubMed] [Google Scholar]
- 34.Mastoraki A, Ioannidis E, Patsouris E, Safioleas M, Aroni K. PGP 9.5 expression in cutaneous keratoacanthomas and squamous cell carcinomas. Arch Dermatol Res. 2009;301:653–658. doi: 10.1007/s00403-009-0962-6. [DOI] [PubMed] [Google Scholar]
- 35.Zheng S, Qiao G, Min D, Zhang Z, Lin F, Yang Q, Feng T, Tang L, Sun Y, Zhao H, et al. Heterogeneous expression and biological function of ubiquitin carboxy-terminal hydrolase-L1 in osteosarcoma. Cancer Lett. 2015;359:36–46. doi: 10.1016/j.canlet.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Kwan SY, Au-Yeung CL, Yeung TL, Rynne-Vidal A, Wong KK, Risinger JI, Lin HK, Schmandt RE, Yates MS, Mok SC, Lu KH. Ubiquitin carboxyl-terminal hydrolase L1 (UCHL1) promotes uterine serous cancer cell proliferation and cell cycle progression. Cancers (Basel) 2020;12:118. doi: 10.3390/cancers12010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gu Y, Lv F, Xue M, Chen K, Cheng C, Ding X, Jin M, Xu G, Zhang Y, Wu Z, et al. The deubiquitinating enzyme UCHL1 is a favorable prognostic marker in neuroblastoma as it promotes neuronal differentiation. J Exp Clin Canc Res. 2018;37:258. doi: 10.1186/s13046-018-0931-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luchansky SJ, Lansbury PT, Jr, Stein RL. Substrate recognition and catalysis by UCH-L1. Biochemistry. 2006;45:14717–14725. doi: 10.1021/bi061406c. [DOI] [PubMed] [Google Scholar]
- 39.Leroy E, Boyer R, Auburger G, Leube B, Ulm G, Mezey E, Harta G, Brownstein MJ, Jonnalagada S, Chernova T, et al. The ubiquitin pathway in Parkinson's disease. Nature. 1998;395:451–452. doi: 10.1038/26652. [DOI] [PubMed] [Google Scholar]
- 40.Sekiguchi S, Kwon J, Yoshida E, Hamasaki H, Ichinose S, Hideshima M, Kuraoka M, Takahashi A, Ishii Y, Kyuwa S, et al. Localization of ubiquitin C-terminal hydrolase L1 in mouse ova and its function in the plasma membrane to block polyspermy. Am J Pathol. 2006;169:1722–1729. doi: 10.2353/ajpath.2006.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Day IN, Thompson RJ. Molecular cloning of cDNA coding for human PGP 9.5 protein. A novel cytoplasmic marker for neurones and neuroendocrine cells. FEBS Lett. 1987;210:157–160. doi: 10.1016/0014-5793(87)81327-3. [DOI] [PubMed] [Google Scholar]
- 42.Larsen CN, Krantz BA, Wilkinson KD. Substrate specificity of deubiquitinating enzymes: Ubiquitin C-terminal hydrolases. Biochemistry. 1998;37:3358–3368. doi: 10.1021/bi972274d. [DOI] [PubMed] [Google Scholar]
- 43.Esteve-Rudd J, Campello L, Herrero MT, Cuenca N, Martin-Nieto J. Expression in the mammalian retina of parkin and UCH-L1, two components of the ubiquitin-proteasome system. Brain Res. 2010;1352:70–82. doi: 10.1016/j.brainres.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Wu J, Wu H, Wang T, Gan H, Zhang X, Liu Y, Li R, Zhao Z, Chen Q, et al. UCH-L1 expression of podocytes in diseased glomeruli and in vitro. J Pathol. 2009;217:642–653. doi: 10.1002/path.2511. [DOI] [PubMed] [Google Scholar]
- 45.Johnston SC, Larsen CN, Cook WJ, Wilkinson KD, Hill CP. Crystal structure of a deubiquitinating enzyme (human UCH-L3) at 1.8 A resolution. EMBO J. 1997;16:3787–3796. doi: 10.1093/emboj/16.13.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grabbe C, Husnjak K, Dikic I. The spatial and temporal organization of ubiquitin networks. Nat Rev Mol Cell Biol. 2011;12:295–307. doi: 10.1038/nrm3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang M, Cai F, Zhang S, Zhang S, Song W. Overexpression of ubiquitin carboxyl-terminal hydrolase L1 (UCHL1) delays Alzheimer's progression in vivo. Sci Rep. 2014;4:7298. doi: 10.1038/srep07298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suong DN, Thao DT, Masamitsu Y, Thuoc TL. Ubiquitin carboxyl hydrolase L1 significance for human diseases. Protein Pept Lett. 2014;21:624–630. doi: 10.2174/0929866521666140403125959. [DOI] [PubMed] [Google Scholar]
- 49.Wilkinson KD, Lee KM, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989;246:670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- 50.Zhong J, Zhao M, Ma Y, Luo Q, Liu J, Wang J, Yuan X, Sang J, Huang C. UCHL1 acts as a colorectal cancer oncogene via activation of the β-catenin/TCF pathway through its deubiquitinating activity. Int J Mol Med. 2012;30:430–436. doi: 10.3892/ijmm.2012.1012. [DOI] [PubMed] [Google Scholar]
- 51.Bheda A, Yue W, Gullapalli A, Whitehurst C, Liu R, Pagano JS, Shackelford J. Positive reciprocal regulation of ubiquitin C-terminal hydrolase L1 and beta-catenin/TCF signaling. PLoS One. 2009;4:e5955. doi: 10.1371/journal.pone.0005955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takami Y, Nakagami H, Morishita R, Katsuya T, Cui TX, Ichikawa T, Saito Y, Hayashi H, Kikuchi Y, Nishikawa T, et al. Ubiquitin carboxyl-terminal hydrolase L1, a novel deubiquitinating enzyme in the vasculature, attenuates NF-kappaB activation. Arterioscler Thromb Vasc Biol. 2007;27:2184–2190. doi: 10.1161/ATVBAHA.107.142505. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Sun Y, Hu R, Luo W, Mao X, Zhao Z, Chen Q, Zhang Z. The regulation of the UCH-L1 gene by transcription factor NF-κB in podocytes. Cell Signal. 2013;25:1574–1585. doi: 10.1016/j.cellsig.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 54.Nagata A, Itoh F, Sasho A, Sugita K, Suzuki R, Hinata H, Shimoda Y, Suzuki E, Maemoto Y, Inagawa T, et al. The evolutionarily conserved deubiquitinase UBH1/UCH-L1 augments DAF7/TGF-β signaling, inhibits dauer larva formation, and enhances lung tumorigenesis. J Biol Chem. 2020;295:9105–9120. doi: 10.1074/jbc.RA119.011222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y, Fallon L, Lashuel HA, Liu Z, Lansbury PT., Jr The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson's disease susceptibility. Cell. 2002;111:209–218. doi: 10.1016/S0092-8674(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 56.Chuma M, Sakamoto M, Yasuda J, Fujii G, Nakanishi K, Tsuchiya A, Ohta T, Asaka M, Hirohashi S. Overexpression of cortactin is involved in motility and metastasis of hepatocellular carcinoma. J Hepatol. 2004;41:629–636. doi: 10.1016/j.jhep.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 57.Osaka H, Wang YL, Takada K, Takizawa S, Setsuie R, Li H, Sato Y, Nishikawa K, Sun YJ, Sakurai M, et al. Ubiquitin carboxy-terminal hydrolase L1 binds to and stabilizes monoubiquitin in neuron. Hum Mol Genet. 2003;12:1945–1958. doi: 10.1093/hmg/ddg211. [DOI] [PubMed] [Google Scholar]
- 58.Kabuta T, Mitsui T, Takahashi M, Fujiwara Y, Kabuta C, Konya C, Tsuchiya Y, Hatanaka Y, Uchida K, Hohjoh H, Wada K. Ubiquitin C-terminal hydrolase L1 (UCH-L1) acts as a novel potentiator of cyclin-dependent kinases to enhance cell proliferation independently of its hydrolase activity. J Biol Chem. 2013;288:12615–12626. doi: 10.1074/jbc.M112.435701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hussain S, Bedekovics T, Ali A, Zaid O, May DG, Roux KJ, Galardy PJ. A cysteine near the C-terminus of UCH-L1 is dispensable for catalytic activity but is required to promote AKT phosphorylation, eIF4F assembly, and malignant B-cell survival. Cell Death Discov. 2019;5:152. doi: 10.1038/s41420-019-0231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fulga TA, Elson-Schwab I, Khurana V, Steinhilb ML, Spires TL, Hyman BT, Feany MB. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat Cell Biol. 2007;9:139–148. doi: 10.1038/ncb1528. [DOI] [PubMed] [Google Scholar]
- 61.Xie M, Han Y, Yu Q, Wang X, Wang S, Liao X. UCH-L1 inhibition decreases the microtubule-binding function of tau protein. J Alzheimers Dis. 2016;49:353–363. doi: 10.3233/JAD-150032. [DOI] [PubMed] [Google Scholar]
- 62.Yu Q, Zhang H, Li Y, Liu C, Wang S, Liao X. UCH-L1 inhibition suppresses tau aggresome formation during proteasomal impairment. Mol Neurobiol. 2018;55:3812–3821. doi: 10.1007/s12035-017-0558-7. [DOI] [PubMed] [Google Scholar]
- 63.Bheda A, Gullapalli A, Caplow M, Pagano JS, Shackelford J. Ubiquitin editing enzyme UCH L1 and microtubule dynamics: Implication in mitosis. Cell Cycle. 2010;9:980–994. doi: 10.4161/cc.9.5.10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seo EY, Jin SP, Sohn KC, Park CH, Lee DH, Chung JH. UCHL1 regulates melanogenesis through controlling MITF stability in human melanocytes. J Invest Dermatol. 2017;137:1757–1765. doi: 10.1016/j.jid.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 65.Rolén U, Freda E, Xie J, Pfirrmann T, Frisan T, Masucci MG. The ubiquitin C-terminal hydrolase UCH-L1 regulates B-cell proliferation and integrin activation. J Cell Mol Med. 2009;13:1666–1678. doi: 10.1111/j.1582-4934.2008.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lohmann F, Sachs M, Meyer TN, Sievert H, Lindenmeyer MT, Wiech T, Cohen CD, Balabanov S, Stahl RA, Meyer-Schwesinger C. UCH-L1 induces podocyte hypertrophy in membranous nephropathy by protein accumulation. Biochim Biophys Acta. 2014;1842:945–958. doi: 10.1016/j.bbadis.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 67.Li L, Tao Q, Jin H, van Hasselt A, Poon FF, Wang X, Zeng MS, Jia WH, Zeng YX, Chan AT, Cao Y. The tumor suppressor UCHL1 forms a complex with p53/MDM2/ARF to promote p53 signaling and is frequently silenced in nasopharyngeal carcinoma. Clin Cancer Res. 2010;16:2949–2958. doi: 10.1158/1078-0432.CCR-09-3178. [DOI] [PubMed] [Google Scholar]
- 68.Goto Y, Zeng L, Yeom CJ, Zhu Y, Morinibu A, Shinomiya K, Kobayashi M, Hirota K, Itasaka S, Yoshimura M, et al. UCHL1 provides diagnostic and antimetastatic strategies due to its deubiquitinating effect on HIF-1α. Nat Commun. 2015;6:6153. doi: 10.1038/ncomms7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gu Y, Yang M, Zhao M, Luo Q, Yang L, Peng H, Wang J, Huang SK, Zheng ZX, Yuan XH, et al. The de-ubiquitinase UCHL1 promotes gastric cancer metastasis via the Akt and Erk1/2 pathways. Tumour Biol. 2015;36:8379–8387. doi: 10.1007/s13277-015-3566-0. [DOI] [PubMed] [Google Scholar]
- 70.Wang G, Zhang W, Zhou B, Jin C, Wang Z, Yang Y, Wang Z, Chen Y, Feng X. The diagnosis value of promoter methylation of UCHL1 in the serum for progression of gastric cancer. Biomed Res Int. 2015;2015:741030. doi: 10.1155/2015/741030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seliger B, Handke D, Schabel E, Bukur J, Lichtenfels R, Dammann R. Epigenetic control of the ubiquitin carboxyl terminal hydrolase 1 in renal cell carcinoma. J Transl Med. 2009;7:90. doi: 10.1186/1479-5876-7-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mitsui Y, Shiina H, Hiraki M, Arichi N, Hiraoka T, Sumura M, Honda S, Yasumoto H, Igawa M. Tumor suppressor function of PGP9.5 is associated with epigenetic regulation in prostate cancer-novel predictor of biochemical recurrence after radical surgery. Cancer Epidemiol Biomarkers Prev. 2012;21:487–496. doi: 10.1158/1055-9965.EPI-11-0970. [DOI] [PubMed] [Google Scholar]
- 73.Brait M, Maldonado L, Noordhuis MG, Begum S, Loyo M, Poeta ML, Barbosa A, Fazio VM, Angioli R, Rabitti C, et al. Association of promoter methylation of VGF and PGP9.5 with ovarian cancer progression. PLoS One. 2013;8:e70878. doi: 10.1371/journal.pone.0070878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abdelmaksoud-Dammak R, Saadallah-Kallel A, Miladi-Abdennadher I, Ayedi L, Khabir A, Sallemi-Boudawara T, Frikha M, Daoud J, Mokdad-Gargouri R. CpG methylation of ubiquitin carboxyl-terminal hydrolase 1 (UCHL1) and P53 mutation pattern in sporadic colorectal cancer. Tumour Biol. 2016;37:1707–1714. doi: 10.1007/s13277-015-3902-4. [DOI] [PubMed] [Google Scholar]
- 75.Nanok C, Jearanaikoon P, Proungvitaya S, Limpaiboon T. Aberrant methylation of HTATIP2 and UCHL1 as a predictive biomarker for cholangiocarcinoma. Mol Med Rep. 2018;17:4145–4153. doi: 10.3892/mmr.2017.8319. [DOI] [PubMed] [Google Scholar]
- 76.Jaferian S, Soleymaninejad M, Daraee H. Verapamil (VER) enhances the cytotoxic effects of docetaxel and vinblastine combined therapy against non-small cell lung cancer cell lines. Drug Res (Stuttg) 2018;68:146–152. doi: 10.1055/s-0043-117895. [DOI] [PubMed] [Google Scholar]
- 77.Ge N, Yang GS, Zhang TY, Chang N, Kang YH, Zhou Q, Fan PS. Upregulation of KCNMA1 facilitates the reversal effect of verapamil on the chemoresistance to cisplatin of esophageal squamous cell carcinoma cells. Eur Rev Med Pharmacol Sci. 2021;25:1869–1880. doi: 10.26355/eurrev_202102_25082. [DOI] [PubMed] [Google Scholar]
- 78.Li P, Zhong D, Gong PY. Synergistic effect of paclitaxel and verapamil to overcome multi-drug resistance in breast cancer cells. Biochem Biophys Res Commun. 2019;516:183–188. doi: 10.1016/j.bbrc.2019.05.189. [DOI] [PubMed] [Google Scholar]
- 79.Yang G, Fan G, Zhang T, Ma K, Huang J, Liu M, Teng X, Xu K, Fan P, Cheng D. Upregulation of ubiquitin carboxyl-terminal hydrolase L1 (UCHL1) mediates the reversal effect of verapamil on chemo-resistance to adriamycin of hepatocellular carcinoma. Med Sci Monit. 2018;24:2072–2082. doi: 10.12659/MSM.908925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang WJ, Li QQ, Xu JD, Cao XX, Li HX, Tang F, Chen Q, Yang JM, Xu ZD, Liu XP. Over-expression of ubiquitin carboxy terminal hydrolase-L1 induces apoptosis in breast cancer cells. Int J Oncol. 2008;33:1037–1045. doi: 10.3892/ijo_00000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maroufi F, Maali A, Abdollahpour-Alitappeh M, Ahmadi MH, Azad M. CRISPR-mediated modification of DNA methylation pattern in the new era of cancer therapy. Epigenomics. 2020;12:1845–1859. doi: 10.2217/epi-2020-0110. [DOI] [PubMed] [Google Scholar]
- 82.Moore MD, Finnerty B, Gray KD, Hoda R, Liu Y, Soong L, Beninato T, Rao R, Zarnegar R, Fahey TJ., III Decreased UCHL1 expression as a cytologic biomarker for aggressive behavior in pancreatic neuroendocrine tumors. Surgery. 2018;163:226–231. doi: 10.1016/j.surg.2017.04.040. [DOI] [PubMed] [Google Scholar]
- 83.Scully OJ, Bay BH, Yip G, Yu Y. Breast cancer metastasis. Cancer Genomics Proteomics. 2012;9:311–320. [PubMed] [Google Scholar]
- 84.Miyoshi Y, Nakayama S, Torikoshi Y, Tanaka S, Ishihara H, Taguchi T, Tamaki Y, Noguchi S. High expression of ubiquitin carboxy-terminal hydrolase-L1 and -L3 mRNA predicts early recurrence in patients with invasive breast cancer. Cancer Sci. 2006;97:523–529. doi: 10.1111/j.1349-7006.2006.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schröder C, Milde-Langosch K, Gebauer F, Schmid K, Mueller V, Wirtz RM, Meyer-Schwesinger C, Schlüter H, Sauter G, Schumacher U. Prognostic relevance of ubiquitin C-terminal hydrolase L1 (UCH-L1) mRNA and protein expression in breast cancer patients. J Cancer Res Clin. 2013;139:1745–1755. doi: 10.1007/s00432-013-1496-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang W, Zou L, Zhou D, Zhou Z, Tang F, Xu Z, Liu X. Overexpression of ubiquitin carboxyl terminal hydrolase-L1 enhances multidrug resistance and invasion/metastasis in breast cancer by activating the MAPK/Erk signaling pathway. Mol Carcinog. 2016;55:1329–1342. doi: 10.1002/mc.22376. [DOI] [PubMed] [Google Scholar]
- 87.Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84. doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 88.Jin Y, Zhang W, Xu J, Wang H, Zhang Z, Chu C, Liu X, Zou Q. UCH-L1 involved in regulating the degradation of EGFR and promoting malignant properties in drug-resistant breast cancer. Int J Clin Exp Patho. 2015;8:12500–12508. [PMC free article] [PubMed] [Google Scholar]
- 89.Li QQ, Wang WJ, Xu JD, Cao XX, Chen Q, Yang JM, Xu ZD. Up-regulation of CD147 and matrix metalloproteinase-2, −9 induced by P-glycoprotein substrates in multidrug resistant breast cancer cells. Cancer Sci. 2007;98:1767–1774. doi: 10.1111/j.1349-7006.2007.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen XS, Wang KS, Guo W, Li LY, Yu P, Sun XY, Wang HY, Guan YD, Tao YG, Ding BN, et al. UCH-L1-mediated down-regulation of estrogen receptor α contributes to insensitivity to endocrine therapy for breast cancer. Theranostics. 2020;10:1833–1848. doi: 10.7150/thno.39814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rochefort H, Glondu M, Sahla ME, Platet N, Garcia M. How to target estrogen receptor-negative breast cancer? Endocr Relat Cancer. 2003;10:261–266. doi: 10.1677/erc.0.0100261. [DOI] [PubMed] [Google Scholar]
- 92.Mondal M, Conole D, Nautiyal J, Tate EW. UCHL1 as a novel target in breast cancer: Emerging insights from cell and chemical biology. Br J Cancer. 2022;126:24–33. doi: 10.1038/s41416-021-01516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu J, Yu S, Jia M, Sun PL, Gao H. Ubiquitin C-terminal hydrolase-L1 expression in non-small-cell lung cancer and its association with clinicopathological features and prognosis. Virchows Arch. 2022;480:577–585. doi: 10.1007/s00428-021-03199-y. [DOI] [PubMed] [Google Scholar]
- 94.Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: From enhancement to normalization. Cell. 2018;175:313–326. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mao R, Tan X, Xiao Y, Wang X, Wei Z, Wang J, Wang X, Zhou H, Zhang L, Shi Y. Ubiquitin C-terminal hydrolase L1 promotes expression of programmed cell death-ligand 1 in non-small-cell lung cancer cells. Cancer Sci. 2020;111:3174–3183. doi: 10.1111/cas.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hussain S, Bedekovics T, Liu Q, Hu W, Jeon H, Johnson SH, Vasmatzis G, May DG, Roux KJ, Galardy PJ. UCH-L1 bypasses mTOR to promote protein biosynthesis and is required for MYC-driven lymphomagenesis in mice. Blood. 2018;132:2564–2574. doi: 10.1182/blood-2018-05-848515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bedekovics T, Hussain S, Feldman AL, Galardy PJ. UCH-L1 is induced in germinal center B cells and identifies patients with aggressive germinal center diffuse large B-cell lymphoma. Blood. 2016;127:1564–1574. doi: 10.1182/blood-2015-07-656678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sui R, Piao HZ. UCHL1 enhances the malignant development of glioma via targeting GAS2. Eur Rev Med Pharmacol Sci. 2020;24:6195–6203. doi: 10.26355/eurrev_202006_21515. [DOI] [PubMed] [Google Scholar]
- 99.Nakao K, Hirakawa T, Suwa H, Kogure K, Ikeda S, Yamashita S, Minegishi T, Kishi H. High expression of ubiquitin C-terminal hydrolase L1 Is associated with poor prognosis in endometrial cancer patients. Int J Gynecol Cancer. 2018;28:675–683. doi: 10.1097/IGC.0000000000001201. [DOI] [PubMed] [Google Scholar]
- 100.Jang MJ, Baek SH, Kim JH. UCH-L1 promotes cancer metastasis in prostate cancer cells through EMT induction. Cancer Lett. 2011;302:128–135. doi: 10.1016/j.canlet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 101.Rashid M, Zadeh LR, Baradaran B, Molavi O, Ghesmati Z, Sabzichi M, Ramezani F. Up-down regulation of HIF-1α in cancer progression. Gene. 2021;798:145796. doi: 10.1016/j.gene.2021.145796. [DOI] [PubMed] [Google Scholar]
- 102.Li X, Hattori A, Takahashi S, Goto Y, Harada H, Kakeya H. Ubiquitin carboxyl-terminal hydrolase L1 promotes hypoxia-inducible factor 1-dependent tumor cell malignancy in spheroid models. Cancer Sci. 2020;111:239–252. doi: 10.1111/cas.14236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kobayashi E, Hwang D, Bheda-Malge A, Whitehurst CB, Kabanov AV, Kondo S, Aga M, Yoshizaki T, Pagano JS, Sokolsky M, Shakelford J. Inhibition of UCH-L1 deubiquitinating activity with two forms of LDN-57444 has anti-invasive effects in metastatic carcinoma cells. Int J Mol Sci. 2019;20:3733. doi: 10.3390/ijms20153733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu Y, Lashuel HA, Choi S, Xing X, Case A, Ni J, Yeh LA, Cuny GD, Stein RL, Lansbury PT., Jr Discovery of inhibitors that elucidate the role of UCH-L1 activity in the H1299 lung cancer cell line. Chem Biol. 2003;10:837–846. doi: 10.1016/j.chembiol.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 105.Hussain S, Bedekovics T, Chesi M, Bergsagel PL, Galardy PJ. UCHL1 is a biomarker of aggressive multiple myeloma required for disease progression. Oncotarget. 2015;6:40704–40718. doi: 10.18632/oncotarget.5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.D'Arcy P, Wang X, Linder S. Deubiquitinase inhibition as a cancer therapeutic strategy. Pharmacol Ther. 2015;147:32–54. doi: 10.1016/j.pharmthera.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 107.Mermerian AH, Case A, Stein RL, Cuny GD. Structure-activity relationship, kinetic mechanism, and selectivity for a new class of ubiquitin C-terminal hydrolase-L1 (UCH-L1) inhibitors. Bioorg Med Chem Lett. 2007;17:3729–3732. doi: 10.1016/j.bmcl.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 108.Panyain N, Godinat A, Thawani AR, Lachiondo-Ortega S, Mason K, Elkhalifa S, Smith LM, Harrigan JA, Tate EW. Activity-based protein profiling reveals deubiquitinase and aldehyde dehydrogenase targets of a cyanopyrrolidine probe. RSC Med Chem. 2021;12:1935–1943. doi: 10.1039/D1MD00218J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Krabill AD, Chen H, Hussain S, Feng C, Abdullah A, Das C, Aryal UK, Post CB, Wendt MK, Galardy PJ, Flaherty DP. Ubiquitin C-terminal hydrolase L1: Biochemical and cellular characterization of a covalent cyanopyrrolidine-based inhibitor. Chembiochem. 2020;21:712–722. doi: 10.1002/cbic.201900434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kooij R, Liu S, Sapmaz A, Xin BT, Janssen GMC, van Veelen PA, Ovaa H, Dijke PT, Geurink PP. Small-molecule activity-based probe for monitoring ubiquitin C-terminal hydrolase L1 (UCHL1) activity in live cells and zebrafish embryos. J Am Chem Soc. 2020;142:16825–16841. doi: 10.1021/jacs.0c07726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Berkers CR, van Leeuwen FW, Groothuis TA, Peperzak V, van Tilburg EW, Borst J, Neefjes JJ, Ovaa H. Profiling proteasome activity in tissue with fluorescent probes. Mol Pharm. 2007;4:739–748. doi: 10.1021/mp0700256. [DOI] [PubMed] [Google Scholar]
- 112.Panyain N, Godinat A, Lanyon-Hogg T, Lachiondo-Ortega S, Will EJ, Soudy C, Mondal M, Mason K, Elkhalifa S, Smith LM, et al. Discovery of a potent and selective covalent inhibitor and activity-based probe for the deubiquitylating enzyme UCHL1, with antifibrotic activity. J Am Chem Soc. 2020;142:12020–12026. doi: 10.1021/jacs.0c04527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roy B, Zhao J, Yang C, Luo W, Xiong T, Li Y, Fang X, Gao G, Singh CO, Madsen L, et al. CRISPR/cascade 9-mediated genome editing-challenges and opportunities. Front Genet. 2018;9:240. doi: 10.3389/fgene.2018.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Takano T, Miyauchi A, Matsuzuka F, Yoshida H, Nakata Y, Kuma K, Amino N. PGP9.5 mRNA could contribute to the molecular-based diagnosis of medullary thyroid carcinoma. Eur J Cancer. 2004;40:614–618. doi: 10.1016/j.ejca.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 115.Sabapathy K, Lane DP. Therapeutic targeting of p53: All mutants are equal, but some mutants are more equal than others. Nat Rev Clin Oncol. 2018;15:13–30. doi: 10.1038/nrclinonc.2017.151. [DOI] [PubMed] [Google Scholar]
- 116.Chibaya L, Karim B, Zhang H, Jones SN. Mdm2 phosphorylation by Akt regulates the p53 response to oxidative stress to promote cell proliferation and tumorigenesis. Proc Natl Acad Sci USA. 2021;118:e2003193118. doi: 10.1073/pnas.2003193118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.De S, Campbell C, Venkitaraman AR, Esposito A. Pulsatile MAPK signaling modulates p53 activity to control cell fate decisions at the G2 checkpoint for DNA damage. Cell Rep. 2020;30:2083–2093.e5. doi: 10.1016/j.celrep.2020.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brinkmann K, Zigrino P, Witt A, Schell M, Ackermann L, Broxtermann P, Schüll S, Andree M, Coutelle O, Yazdanpanah B, et al. Ubiquitin C-terminal hydrolase-L1 potentiates cancer chemosensitivity by stabilizing NOXA. Cell Rep. 2013;3:881–891. doi: 10.1016/j.celrep.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 119.Kabuta T, Setsuie R, Mitsui T, Kinugawa A, Sakurai M, Aoki S, Uchida K, Wada K. Aberrant molecular properties shared by familial Parkinson's disease-associated mutant UCH-L1 and carbonyl-modified UCH-L1. Hum Mol Genet. 2008;17:1482–1496. doi: 10.1093/hmg/ddn037. [DOI] [PubMed] [Google Scholar]
- 120.Kabuta T, Furuta A, Aoki S, Furuta K, Wada K. Aberrant interaction between Parkinson disease-associated mutant UCH-L1 and the lysosomal receptor for chaperone-mediated autophagy. J Biol Chem. 2008;283:23731–23738. doi: 10.1074/jbc.M801918200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nishikawa K, Li H, Kawamura R, Osaka H, Wang YL, Hara Y, Hirokawa T, Manago Y, Amano T, Noda M, et al. Alterations of structure and hydrolase activity of parkinsonism-associated human ubiquitin carboxyl-terminal hydrolase L1 variants. Biochem Biophys Res Commun. 2003;304:176–183. doi: 10.1016/S0006-291X(03)00555-2. [DOI] [PubMed] [Google Scholar]
- 122.Duffy MJ, Synnott NC, Crown J. Mutant p53 in breast cancer: Potential as a therapeutic target and biomarker. Breast Cancer Res Treat. 2018;170:213–219. doi: 10.1007/s10549-018-4753-7. [DOI] [PubMed] [Google Scholar]
- 123.Sporikova Z, Koudelakova V, Trojanec R, Hajduch M. Genetic markers in triple-negative breast cancer. Clin Breast Cancer. 2018;18:e841–e850. doi: 10.1016/j.clbc.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 124.Bazarian JJ, Biberthaler P, Welch RD, Lewis LM, Barzo P, Bogner-Flatz V, Gunnar Brolinson P, Büki A, Chen JY, Christenson RH, et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): A multicentre observational study. Lancet Neurol. 2018;17:782–789. doi: 10.1016/S1474-4422(18)30231-X. [DOI] [PubMed] [Google Scholar]
- 125.Meyer-Schwesinger C, Meyer TN, Sievert H, Hoxha E, Sachs M, Klupp EM, Münster S, Balabanov S, Carrier L, Helmchen U, et al. Ubiquitin C-terminal hydrolase-l1 activity induces polyubiquitin accumulation in podocytes and increases proteinuria in rat membranous nephropathy. Am J Pathol. 2011;178:2044–2057. doi: 10.1016/j.ajpath.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fang Y, Li F, Qi C, Mao X, Xu Y, Zhao Z, Wu H, Zhang Z. Plakoglobin is involved in cytoskeletal rearrangement of podocytes under the regulation of UCH-L1. Biochem Biophys Res Commun. 2020;529:112–118. doi: 10.1016/j.bbrc.2020.05.093. [DOI] [PubMed] [Google Scholar]
- 127.Cui JH, Xie X. UCH-L1 expressed by podocytes: A potentially therapeutic target for lupus nephritis? Inflammation. 2017;40:657–665. doi: 10.1007/s10753-017-0512-x. [DOI] [PubMed] [Google Scholar]
- 128.Xu Y, Gao H, Hu Y, Fang Y, Qi C, Huang J, Cai X, Wu H, Ding X, Zhang Z. High glucose-induced apoptosis and necroptosis in podocytes is regulated by UCHL1 via RIPK1/RIPK3 pathway. Exp Cell Res. 2019;382:111463. doi: 10.1016/j.yexcr.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 129.Ichikawa T, Li J, Dong X, Potts JD, Tang D, Li DQ, Li DS, Cui T. Ubiquitin carboxyl terminal hydrolase L1 negatively regulates TNFalpha-mediated vascular smooth muscle cell proliferation via suppressing ERK activation. Biochem Biophys Res Commun. 2010;391:852–856. doi: 10.1016/j.bbrc.2009.11.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gao X, Wu L, Wang K, Zhou X, Duan M, Wang X, Zhang Z, Liu X. Ubiquitin carboxyl terminal hydrolase L1 attenuates TNF-α-mediated vascular smooth muscle cell migration through suppression of NF-κB activation. Int Heart J. 2018;59:1409–1415. doi: 10.1536/ihj.17-541. [DOI] [PubMed] [Google Scholar]
- 131.Bi HL, Zhang XL, Zhang YL, Xie X, Xia YL, Du J, Li HH. The deubiquitinase UCHL1 regulates cardiac hypertrophy by stabilizing epidermal growth factor receptor. Sci Adv. 2020;6:eaax4826. doi: 10.1126/sciadv.aax4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.