Abstract
Objectives
There are some studies reporting the association between (manganese [Mn]) exposure to welding fume and neurological dysfunction. This study examined the relationship between Mn exposure and neurological behavior in Japanese male welders and non‐welders using biological samples, which to date has not been assessed in Japan.
Methods
A total of 94 male welders and 95 male non‐welders who worked in the same factories were recruited. The blood and urine samples were obtained from all the participants to measure Mn exposure levels. Neurological function tests were also conducted with all participants. The sampling of the breathing air zone using a personal sampler was measured for welders only.
Results
The odds ratios (ORs) for the Working Memory Index (WMI) scores were significantly higher among all participants in the low blood Mn concentration group than those in the high blood Mn concentration group (OR, 2.77; 95% confidence interval [CI], 1.24, 6.19; P = .013). The association of WMI scores and blood Mn levels in welders had the highest OR (OR, 3.73; 95% CI, 1.04, 13.38; P = .043). Although not statistically significant, a mild relationship between WMI scores and blood Mn levels was observed in non‐welders (OR, 2.09; 95% CI, 0.63, 6.94; P = .227).
Conclusions
The results revealed a significant positive relationship between blood Mn and neurological dysfunction in welders. Furthermore, non‐welders at the same factories may be secondarily exposed to welding fumes. Further research is needed to clarify this possibility.
Keywords: manganese, neurological function, welder, Working Memory Index
1. INTRODUCTION
Welding is the joining of metallic components by melting the metals using heat or pressure. Welding fumes contain various metals that are harmful to health. 1 In particular, adverse psychological performance and neurotoxicity from manganese (Mn) exposure in welders have been reported. 2 , 3 , 4 In Japan, there are approximately 180 000 metal welding and fusion cutting workers, and they account for 0.3% of the total working population. 5 The Japanese government had announced to partially revise laws and regulations in 2020 with the aim of strengthening measures to prevent welders' health hazards related to Mn and welding fumes. 6
Many reports on the toxicity of Mn relate to respiratory toxicity and neurotoxicity. Poor ventilation in the workplace was associated with decreased lung function among shipyard welders, although there was no relationship between Mn concentrations in the respiratory zone of the workplace and acute decreases in lung function. 7 Respiratory 30 630 symptoms such as nasal congestion and dry cough have been observed in welders. 8 Studies on neurotoxicity among those exposed to Mn are much more numerous than on respiratory toxicity. In a study of male workers at Korean shipbuilding companies, there was no relationship between the development of Parkinson's disease and airborne Mn levels. 9 However, there are many reports indicating chronic exposure to Mn is associated with Parkinsonism. 10 , 11 , 12 Park et al. 13 reported that a higher blood level of Mn reduced neurological functions in welders, such as the Working Memory Index (WMI), and verbal intelligence quotient. Among alloy manufacturing plant workers exposed to Mn, the workers in the high‐exposure group exhibited poorer performance in addition, symbol digit, finger tapping, and digit span tests.
In Japan, a few studies have been conducted on neurotoxicity or respiratory toxicity among welders exposed to Mn. A 56‐year‐old welder working for 30 years whose serum and urine Mn levels were high developed postural instability and writing clumsiness. 14 One study investigated the relationship between welding fume exposure and lung function among 143 male welders. 15 Another study investigated the relationship between respirable dust exposure and pneumoconiosis by examining of 1006 chest X‐ray films of workers including shipyard welders. 16 Unfortunately, two of these three studies did not measure metal concentrations in workers' biological samples but examined the strength of the residual magnetic field of externally magnetized lungs or environmental chemical concentrations and biological effects.
Therefore, this study examined the relationship between Mn exposure and neurological behavior in welders and non‐welders using biological samples which to date has not been assessed in Japan.
2. METHODS
2.1. Study participants
A total of 94 male welders from 7 factories in Japan were included in this study. These include one shipbuilding industry, an automobile manufacturing industry, two factories for manufacturing construction materials, and three steel industries. Forty‐eight workers treated high‐strength steel, 29 treated mild steel, 15 treated carbon steel, and two treated stainless steel as the base material. Three workers were engaged in Tungsten Inert Gas welding using Argon (Ar) gas for shielding gas, and 91 in Metal Active Gas (MAG) welding using CO2 gas for shielding gas. Sixteen workers occasionally engaged in MAG welding using CO2 and Ar gas for shielding gas. The welding wire used was Japanese Industrial Standards Z 3312 YGW11, YGW12, and YGW18.
Ninety‐five male non‐welders who worked in the same factories were recruited as control participants. Recruited non‐welders were not engaged in welding work at the time of this study, even if they had previously engaged in welding work. We recruited until the number of participants was almost the same as that of the welders. The non‐welders included 77 clerical workers, 6 manufacturing line workers, 3 product designers, 3 product inspectors, and 6 manufacturing managers. The welders and non‐welders were aged 20 years or older and were recruited from April 2021 to June 2022.
2.2. Questionnaire survey
Data on age, smoking and drinking habits, welding exposure‐years, current neurological findings (drooling, muscle twitching, numbness and tingling in hands and feet, and excessive sweating), and current respiratory symptoms (cough, shortness of breath, rhinorrhea, nasal congestion, wheezing, and sputum) were obtained through a self‐administered questionnaire. Regarding welding exposure‐years, we ascertained not only current welding experience, but past welding experience as well in addition to self‐administered questionnaire. Fatigue symptoms self‐awareness scores were determined using the Workers' Fatigue Accumulation Self‐Assessment Checklist. 17
2.3. Neurological function tests
2.3.1. Grip strength
Hand grip strength was measured in both the dominant and non‐dominant hands with a digital grip strength dynamometer (TKK5401; Takei Scientific Instruments Co., Ltd.). After holding the grip strength meter in an upright position and adjusting the second joint of the index finger to 90°, the measurement was repeated twice alternately with the dominant and non‐dominant hands. The dominant hand was determined by asking participants if they were right‐ or left‐handed. The mean value was recorded in kilograms. A rest period of at least 10 min was provided between grip strength and finger tapping measurements to prevent fatigue affecting the grip strength results. Abnormalities related to the skeletal muscles of the hands and arms were confirmed verbally before measuring grip strength. Two welders responded that there was an abnormality in their non‐dominant hands; thus, we assessed the non‐dominant hand grip strength of 92 welders.
2.3.2. Finger tapping
Finger tapping measures the maximum speed of repetitive finger movement. The fingers used are the index and middle fingers of the dominant and non‐dominant hands, respectively. Performance is evaluated as the mean number of taps during three 10‐s trails for each hand. 18
2.3.3. Working Memory Index
The Wechsler Adult intelligence Scale‐IV (WAIS‐IV) has subset WMI comprised of digit span forward, digit span backward, digit span sequencing, and arithmetic sections, and is recalculated considering the influence of age on these scores. The arithmetic section requires a participant to mentally solve arithmetic word problems, presented orally, within a specific time limit. 19 , 20 Ninety‐two welders underwent WMI because two welders refused to participate due to lack of time.
Grip strength, finger tapping, and WMI were performed before work to avoid fatigue.
2.4. Blood and urine sampling
The participants provided 8 mL blood and 10 mL urine samples at the end of their working shifts to medical doctors for measurement of metal concentration. The collected blood and urine were given to the staff of SRL (SRL, Inc.) within 2 h after sampling. Blood cadmium (Cd), nickel (Ni), Mn, chromium (Cr), and lead (Pb) and urine Cd, Mn, and Cr concentrations were determined at SRL. The detection limit for each metal were 0.2, 0.2, 0.2, 0.03, and 1.1 μg/dL for Cd, Ni, Mn, Cr, and Pb, respectively, and in the urine samples were 0.5, 1.1, and 0.3 μg/L for Cd, Mn, and Cr, respectively.
2.5. Breathing air zone sampling using a personal sampler for welders
Welders' breathing air zone sampling using a personal sampler was measured by a professional measurer from an external organization (Japan Industrial Safety & Health Association) in basic accordance with the guidelines for personal exposure measurements of chemical substances established by Japan Society for Occupational Health. 21 The Air Check 2000 sampler (SKC Inc.), NWPS‐254 sampler (Shibata), and TF98R PTFE binding filter (Shibata) with 2.5 L/min air flow rate were used to measure respirable dust concentration and total dust concentration during work. In order to determine Mn concentrations of welding fumes, the samples collected on the filters after extraction were analyzed using Agilent 7800 Quadrupole ICP‐MS (Agilent Technologies). Using the air sampling data, the 8‐h time‐weighted average (8 h‐TWA) of respirable dust, TWA of respirable Mn, and 8 h‐TWA of respirable Mn were calculated.
2.6. Statistical analyses
Two‐group comparisons were performed using the Mann–Whitney U test, Fisher's exact test, or multivariable logistic regression analyses. When metal concentration was not detected, we imputed the data by 1/10 for each detection limit. All participants were automatically divided into three groups (each containing about a third of the participants) according to metal concentrations in their blood and urine using a statistical software. The tertile 1 (T1) group contains participants with low levels of metal concentration, the tertile 2 (T2) group contains participants with intermediate levels of metal concentration, and the tertile 3 (T3) group contains participants with high levels of metal concentration. The odds ratio (OR) for neurological dysfunction risk and the corresponding 95% confidence intervals (CIs) were estimated after adjusting for the effects of age, body mass index (BMI), smoking habits, drinking habits, and factory, and welding exposure‐years. All statistical analyses were performed in STATA (StataCorp. LLC); statistical significance was P < .05 (two‐sided).
3. RESULTS
Table 1 presents the study population characteristics. The values are mean (standard deviation) or number (%). The welders had stronger grips, fewer numbers of finger tapping and lower WMI scores than non‐welders. Sixteen of the non‐welders had previous welding experience.
TABLE 1.
Study population characteristics.
| Total (N = 189) | Non‐welders (N = 95) | Welders (N = 94) | P value | |
|---|---|---|---|---|
| Age (years) | 40.6 (12.9) | 40.2 (12.4) | 41.1 (13.3) | .810 a |
| Body mass index | 24.4 (3.5) | 25.1 (3.8) | 23.8 (3.0) | .019 a |
| Smoking habits | ||||
| Never/former | 103 (55%) | 57 (60%) | 46 (49%) | .145 b |
| Current | 86 (45%) | 38 (40%) | 48 (51%) | |
| Drinking habits | ||||
| No | 90 (48%) | 54 (57%) | 36 (38%) | .287 b |
| Yes | 99 (52%) | 41 (43%) | 58 (62%) | |
| Welding exposure‐years (years) | 10.6 (13.9) | 2.0 (6.8) | 19.2 (13.9) | <.001 a |
| Neurological findings c | ||||
| No | 137 (72%) | 69 (73%) | 68 (72%) | 1.000 b |
| Yes | 52 (28%) | 26 (27%) | 26 (28%) | |
| Respiratory symptoms d | ||||
| No | 126 (67%) | 68 (72%) | 58 (62%) | .167 b |
| Yes | 63 (33%) | 27 (28%) | 36 (38%) | |
| Fatigue symptoms self‐awareness score | ||||
| 0–4 | 0 (0%) | 0 (0%) | 0 (0%) | .546 b |
| 5–10 | 0 (0%) | 0 (0%) | 0 (0%) | |
| 11–20 | 121 (64%) | 63 (66%) | 58 (62%) | |
| 21– | 68 (36%) | 32 (34%) | 36 (26%) | |
| Hand grip strength (kg) | ||||
| Dominant hand | 45.5 (7.7) | 43.8 (6.9) | 47. 2 (8.1) | .009 a |
| Non‐dominant hand e | 43.3 (7.6) | 41.7 (6.4) | 44.9 (8.3) | .002 a |
| Finger tapping (number/10 s) | ||||
| Dominant hand | 37.6 (10.1) | 40.5 (8.7) | 34.6 (10.5) | <.001 a |
| Non‐dominant hand | 36.5 (9.0) | 38.8 (7.9) | 34.1 (9.4) | <.001 a |
| Working Memory Index e | 92.9 (15.4) | 97.6 (15.8) | 88.0 (13.5) | <.001 a |
Note: Values are mean (standard deviation) or number (%).
P values were obtained using Mann–Whitney U test.
P values were obtained using Fisher's exact test.
Drooling, muscle twitching, numbness and tingling in hands and feet, excessive sweating.
Cough, shortness of breath, rhinorrhea, nasal congestion, wheezing, sputum.
Number of welders = 92.
Table 2 shows the distribution of metal concentrations in non‐welders and welders. Urine Cd, blood and urine Mn, and urine Cr and blood Pb concentrations of the welders were high in our study (urine Cd, P < .001; blood and urine Mn, P < .001, P < .001, respectively; urine Cr, P < .001; blood Pb, P = .016). The percentage of participants having concentrations below the detection limits for metal concentrations in the biological samples was 89% for blood Cd, 58% for urine Cd, 79% for blood Ni, 0% for blood Mn, 91% for urine Mn, 93% for blood Cr, 3% for urine Cr, and 24% for blood Pb.
TABLE 2.
Distribution of metal concentrations by non‐welders and welders.
| Median (minimum and maximum) (μg/dL) | ||||
|---|---|---|---|---|
| Total (N = 189) | Non‐welders (N = 95) | Welders (N = 94) | P value a | |
| Cd | ||||
| Blood | 0.02 (0.02, 0.30) | 0.02 (0.02, 0.20) | 0.02 (0.02, 0.30) | .446 |
| Urine | 0.05 (0.05, 5.10) | 0.05 (0.05, 2.10) | 0.60 (0.05, 5.10) | <.001 |
| Ni | ||||
| Blood | 0.02 (0.02, 0.30) | 0.02 (0.02, 0.30) | 0.02 (0.02, 0.30) | .862 |
| Mn | ||||
| Blood | 1.00 (0.40, 2.80) | 0.90 (0.40, 1.50) | 1.20 (0.60, 2.80) | <.001 |
| Urine | 0.11 (0.11, 4.30) | 0.11 (0.11, 0.11) | 0.11 (0.11, 4.30) | <.001 |
| Cr | ||||
| Blood | 0.003 (0.003, 0.14) | 0.003 (0.003, 0.12) | 0.003 (0.003, 0.14) | .801 |
| Urine | 0.50 (0.03, 2.70) | 0.40 (0.03, 1.30) | 0.60 (0.03, 2.70) | <.001 |
| Pb | ||||
| Blood | 1.50 (0.11, 3.70) | 1.40 (0.11, 2.60) | 1.60 (0.11, 3.70) | .001 |
Note: When metal concentration was not detected, we recorded 1/10 of above the detection limits.
Abbreviations: Cd, cadmium; Cr, chromium; Mn, manganese; Ni nickel; Pb, lead.
P values were obtained using the Mann–Whitney U test.
Tables 3, 4, 5 show the results of the multivariable logistic analyses to estimate the risk of neurological dysfunction, grip strength reduction, the number of finger tapping, and WMI scores. There was no significant relationship between grip strength and finger tapping for both the dominant and non‐dominant hands and blood Mn concentrations (Tables 3 and 4). The OR for lower WMI scores were significantly higher among all participants in the high blood Mn group (T3) than those in the low blood Mn group (T1) (OR, 2.77; 95% CI, 1.24, 6.19; P = .013). Although not statistically significant, a mild relationship was observed between low WMI scores and high blood Mn levels in non‐welders (OR, 2.09; 95% CI: 0.63–6.94; P = .227). The association of WMI scores and blood Mn levels in welders had the highest OR, and the relationships were statistically significant (OR, 3.73; 95% CI, 1.04, 13.38; P = .043) (Table 5). In addition to blood Mn, urine Cr, and blood Pb were detectable in many participants, and a logistic analysis was conducted for these two metal concentrations. However, there was no statistically significant relationship between WMI and urine Cr and blood Pb concentrations (Tables S1 and S2).
TABLE 3.
Results of the multivariable analysis for the relationships between hand grip strength and blood manganese (Mn) concentrations.
| Blood Mn (μg/dL) | All | Non‐welders | Welders | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≧Mean a | <Mean | OR (95% CI) | P value b | ≧Mean a | <Mean | OR (95% CI) | P value b | ≧Mean a | <Mean | OR (95% CI) | P value b | |
| Dominant hand | ||||||||||||
| T1 (0.5–0.8) | 26 | 29 | 1.00 (referent) | 18 | 23 | 1.00 (referent) | 8 | 6 | 1.00 (referent) | |||
| T2 (0.9–1.1) | 30 | 37 | 1.01 (0.46–2.21) | .972 | 11 | 24 | 1.36 (0.49–3.76) | .554 | 19 | 13 | 0.94 (0.22–4.07) | .930 |
| T3 (1.2–2.8) | 35 | 32 | 0.89 (0.40–1.99) | .777 | 11 | 8 | 0.50 (0.15–1.74) | .279 | 24 | 24 | 1.31 (0.32–5.34) | .711 |
| P for trend | .936 | P for trend | .330 | P for trend | .805 | |||||||
| Non‐dominant hand | ||||||||||||
| T1 (0.5–0.8) | 26 | 28 | 1.00 (referent) | 18 | 23 | 1.00 (referent) | 8 | 5 | 1.00 (referent) | |||
| T2 (0.9–1.1) | 32 | 35 | 1.06 (0.49–2.33) | .877 | 9 | 26 | 1.99 (0.70–5.67) | .196 | 23 | 9 | 0.64 (0.14–2.90) | .559 |
| T3 (1.2–2.8) | 41 | 25 | 0.74 (0.33–1.67) | .468 | 11 | 8 | 0.60 (0.18–2.01) | .410 | 30 | 17 | 0.79 (0.19–3.33) | .753 |
| P for trend | .613 | P for trend | .177 | P for trend | .832 | |||||||
Abbreviations: CI, confidence interval; OR, odds ratio; T, tertile.
Mean of dominant hand is 45.5 (kg), non‐dominant hand is 43.3 (kg).
P values were obtained using multivariable logistic regression analysis adjusted for age, body mass index, smoking habits, drinking habits, factory, and welding exposure‐years.
TABLE 4.
Results of the multivariable analysis for the relationships between finger tapping and blood manganese (Mn) concentrations.
| Blood Mn (μg/dL) | All | Non‐welders | Welders | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≧Mean a | <Mean | OR (95% CI) | P value b | ≧Mean a | <Mean | OR (95% CI) | P value b | ≧Mean a | <Mean | OR (95% CI) | P value b | |
| Dominant hand | ||||||||||||
| T1 (0.5–0.8) | 34 | 21 | 1.00 (referent) | 27 | 14 | 1.00 (referent) | 7 | 7 | 1.00 (referent) | |||
| T2 (0.9–1.1) | 37 | 30 | 1.25 (0.58–2.73) | .568 | 25 | 10 | 0.85 (0.30–2.42) | .756 | 12 | 20 | 1.53 (0.36–6.43) | .561 |
| T3 (1.2–2.8) | 30 | 37 | 1.48 (0.66–3.28) | .339 | 12 | 7 | 1.11 (0.33–3.77) | .870 | 18 | 30 | 1.36 (0.35–5.28) | .661 |
| P for trend | .632 | P for trend | .916 | P for trend | .845 | |||||||
| Non‐dominant hand | ||||||||||||
| T1 (0.5–0.8) | 32 | 23 | 1.00 (referent) | 27 | 14 | 1.00 (referent) | 5 | 9 | 1.00 (referent) | |||
| T2 (0.9–1.1) | 37 | 30 | 1.04 (0.48–2.25) | .919 | 21 | 14 | 1.19 (0.43–3.28) | .735 | 16 | 16 | 0.40 (0.09–1.81) | .233 |
| T3 (1.2–2.8) | 33 | 34 | 1.13 (0.51–2.51) | .760 | 13 | 6 | 1.00 (0.28–3.60) | .995 | 20 | 28 | 0.50 (0.12–2.13) | .350 |
| P for trend | .952 | P for trend | .939 | P for trend | .475 | |||||||
Abbreviations: CI, confidence interval; OR, odds ratio; T, tertile.
Mean of dominant hand is 37.6 (number/10 s), non‐dominant hand is 36.5 (number/10 s).
P values were obtained using multivariable logistic regression analysis adjusted for age, body mass index, smoking habits, drinking habits, factory, and welding exposure‐years.
TABLE 5.
Results of the multivariable analysis for the relationships between WMI and blood manganese (Mn) concentrations.
| Blood Mn (μg/dL) | All | Non‐welders | Welders | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≧Mean a | <Mean | OR (95% CI) | P value b | ≧Mean a | <Mean | OR (95% CI) | P value b | ≧Mean a | <Mean | OR (95% CI) | P value b | |
| WMI | ||||||||||||
| T1 (0.5–0.8) | 35 | 20 | 1.00 (referent) | 27 | 14 | 1.00 (referent) | 8 | 6 | 1.00 (referent) | |||
| T2 (0.9–1.1) | 29 | 37 | 2.14 (0.99–4.64) | .054 | 17 | 18 | 2.38 (0.87–6.49) | .092 | 12 | 19 | 2.10 (0.56–7.82) | .269 |
| T3 (1.2–2.8) | 22 | 44 | 2.77 (1.24–6.19) | .013 | 9 | 10 | 2.09 (0.63–6.94) | .227 | 13 | 34 | 3.73 (1.04–13.38) | .043 |
| P for trend | .033 | P for trend | .184 | P for trend | .111 | |||||||
Abbreviations: CI, confidence interval; OR, odds ratio; T, tertile; WMI, working memory index.
Mean of WMI is 92.9.
P values were obtained using multivariable logistic regression analysis adjusted for age, body mass index, smoking habits, drinking habits, factory, and welding exposure‐years.
Bold values indicate P < 0.05.
The distribution of individual sampler results of median (min and max) (mg/m3) were 8 h‐TWA of respirable dust; 1.02 (0.01, 10.24), TWA of respirable Mn; 0.189 (0.0001, 2.818), 8 h‐TWA of respirable Mn; 0.094 (0.00007, 1.538). Figure 1 shows the distribution of 8 h‐TWA of respirable dust, TWA of respirable Mn, and 8 h‐TWA of respirable Mn by blood Mn concentration among welders. In the results of all individual sampler results, the respirable dust and Mn concentrations were higher in the group with high blood Mn concentration (8 h‐TWA of respirable dust, P < .001; TWA of respirable Mn, P < .001; 8 h‐TWA of respirable Mn, P < .001, by covariance analysis after adjusting for the effects of age, BMI, smoking habits, drinking habits, factory, and welding exposure‐years).
FIGURE 1.
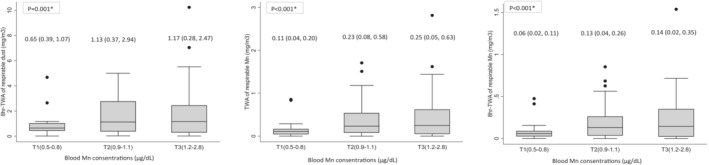
Distribution for individual sampler results of welders by blood manganese (Mn) concentration sub‐groups (tertiles). Values are Median (mg/m3) (25th and 75th percentiles). *P values were obtained using an analysis of covariance adjusted for age, body mass index, smoking habits, drinking habits, factory, and welding exposure‐years. T, tertile; TWA, time‐weighted average.
4. DISCUSSION
Compared to non‐welders, welders had higher concentrations of urine Cd, blood Mn, urine Mn, urine Cr, and blood Pb. Lower WMI scores were observed in the high Mn blood concentration group (T3) than in the low Mn blood concentration group (T1) in welders. Although not statistically significant, a mild relationship between WMI scores and blood Mn concentrations was observed in non‐welders.
Cd, Ni, Mn, Cr, and Pb are well‐known metals contained in welding fumes. 4 , 22 , 23 These metals are found in the blood and urine of welders due to occupational exposure. 23 , 24 In this study, urinary Ni concentration was not greater in welders than in non‐welders, and there was no difference between them. Ni is a metal often used in welding using stainless steel as a base material. 24 Since this time the study was conducted in factories using high‐strength, mild, or carbon steel, whose raw material is iron as the base metal, it is possible that Ni was not significantly detected in the population of welders in this study.
In this study, we found a relationship between welders with a high blood Mn concentration and a lower WMI score. Similar to previous studies, there was an association between blood or urine Mn concentrations and WAIS‐related tests in welders. 13 , 18 One study reported 9.6 μg/L (range 5.1–15.3) for the mean blood Mn of welders and their WMI scores was reduced by exposure after considering dilation of employment. 13 Another study demonstrated blood Mn and urine Mn of workers exposed Mn ranged from 4 to 18 μg/L and from 0.7 to 7 μg/L, respectively. 18 The high‐concentration exposure group had reduced finger tapping and digit span scores. 18 Our results had similar or slightly higher values compared to these Mn concentrations. Therefore, it would be acceptable to consider whether there is a relationship between blood Mn levels and neurological dysfunction in the welders of our study.
Although not statistically significant, a mild relationship between WMI scores and blood Mn concentrations was observed in non‐welders that were presumably not directly occupationally exposed to high Mn levels. The blood Mn concentration in adult males was 1.3 μg/dL (median) in Japan in a previous report using general population data, 25 therefore, the blood Mn concentration of non‐welders was not high in comparison. However, the effect of Mn exposure on neuronal function cannot be clarified using its relationship with blood concentrations of Mn at any time point. 13 Mn concentrations in the environment, duration of Mn exposure, and usage of personal protective equipment (PPE) to prevent Mn exposure are important factors in determining whether Mn exposure affects neurological function. 13 In an Italian study targeting residents exposed to Mn, the Mn dust concentration near the ferroalloy industries factories was high, and the residents living near the factories had a high incidence of Parkinson's disease. 26 In a study of residents near an Mn manufacturing plant, the group with higher blood Mn levels (median 7.5 μg/L) showed decreased neurological function, such as poorer learning and recall. 27 In our study, non‐welders who had been working at the current factory for 15 years (median) and did not wear PPE during work. Depending on the job, non‐welders will also be in and out of the factory. Concerning these facts, it is considered that non‐welders, like the residents near factories in previous reports, were in an environment where they were likely to be secondarily exposed to Mn from factories for a long period of time. Our findings suggest that secondary exposure to Mn from factories may have decreased the WMI of the non‐welders.
In addition to Mn, working memory of the participants in our study may have been affected by chemical factors, such as metals and chemical substances 28 , 29 , 30 and other factors, such as occupation, task difficulty, fatigue, stress, and sleep quality 31 , 32 , 33 , 34 , 35 This time the participants work with high‐strength, mild, or carbon steel, with iron as the base material. Excessive iron in select regions of the brain may be involved in the etiology of neurodegenerative disorders and has been reported to be associated with memory impairment. 36 , 37 , 38 The aluminum contained in the wire flux affects memory by modifying hippocampal calcium signal pathways. 28 Long‐term exposure to carbon monoxide generated by carbon dioxide gas arc welding in the welding process may cause health problems, such as deterioration of memory in the welders. 29 , 30 However, our study did not examine the concentrations of these chemical factors in biological or environmental samples. We analyzed the relationship between fatigue symptoms, self‐awareness scores, occupations, and WMI. There was no statistically significant difference between them and WMI in our study. However, even among welders in the same factory, work processes and task difficulty are widely different at individual levels. These differences might be possibly related to WMI. In addition, we have not obtained the data of stress level, sleep quality, and working forms, such as night shifts. In the future, before concluding that there is a relationship between Mn exposure and WMI, it is necessary to examine various factors that are related to WMI, and carefully examine whether there is a relationship between Mn exposure and WMI.
Blood Mn concentration increased by approximately 1 μg/L for each mg/m3 × month of (unprotected) cumulative exposure in welders. 13 In the general population, blood Mn levels are affected by diet, especially tea, nuts, and vegetables. 39 According to Figure 1, there was a relationship between the blood Mn concentration and the Mn in the breathing area of the welders. Therefore, the source of blood Mn concentrations for welders was likely to be Mn exposure from factories. However, there was no significant difference between the likes and dislikes of vegetables and blood Mn concentration (P = .934) (data not shown). Although this was not clear because we did not conduct a detailed dietary survey, it is possible that the source of Mn exposure in participants was not due to the diets.
In the Ordinance on Prevention of Hazards Due to Specified Chemical Substances, doctors can order the measurement of Mn concentration in urine or other biological samples of welders for secondary health checkup. However, the main excretion route of Mn is through the liver and feces, and excretion into the urine is small. 40 Our study also found few participants had detectable urine Mn, and participants with high blood Mn did not necessarily have high urine Mn levels detected. Blood Mn has been reported to correlate more sensitively with neurological findings than urine, 18 thus when examining the relationship between Mn and biological effects, blood concentration should be measured rather than urine.
This study has several limitations. First, in this study, we focused solely on Mn as the metal in welding fumes that affected WMI. There is a need to measure other factors, which could be possibly related to WMI. Second, we did not measure the breathing air zone samples by personal sampler for non‐welders. Factory workers who work near a welding site may be secondarily exposed to welding fumes from the welding site. In the future, it will be necessary to conduct studies in which personal samplers are obtained for welders and non‐welders working near the welding site. Third, we did not conduct detailed dietary surveys, thus we could not accurately determine whether there was an effect of Mn oral exposure from food. Information on dietary Mn concentrations is necessary to determine whether occupational exposure is involved. Fourth, our study involved a small sample size. Although not statistically significant, higher Mn concentrations may be associated with the lower grip strength of non‐welders. Since skeletal muscle abnormalities affect grip strength measurements, we verbally confirmed the absence of skeletal muscle abnormalities among the study subjects before measuring grip strength in this study. However, it is desirable to confirm the absence of skeletal muscle abnormalities with a specialist. In the future, it is necessary to increase the sample size and investigate the effects of secondary exposure in detail.
5. CONCLUSION
There was a significant relationship between blood Mn concentrations and lower WMI scores in welders. Furthermore, non‐welders at the same factories may be secondarily exposed to welding fumes. Further research is needed to clarify this possibility.
AUTHOR CONTRIBUTIONS
Mayumi Tsuji, Chihaya Koriyama, Yasuhiro Ishihara, Hajime Hori, Kazuhiro Yatera, and Susumu Ueno contributed to the study's conception. Mayumi Tsuji, Toyohi Isse, Tsunetoshi Ishizuka, Wataru Hasegawa, Motohide Goto, Rie Tanaka, Noriaki Kakiuchi, Naoki Kunugita, Toshihide Sakuragi, Yoshiko Yasumura, and Maori Kono contributed to the study design and recruitment. Mayumi Tsuji, Yasuhiro Ishihara, Megumi Yamamoto, Mami Kuwamura, Kyoko Kitagawa, and Susumu Ueno contributed to experimental design and recruitment. Mayumi Tsuji and Chihaya Koriyama analyzed the data and wrote the article. Hajime Hori, Tsunetoshi Ishizuka, Wataru Hasegawa, and Susumu Ueno reviewed the article and provided advice. All authors have read and approved the final manuscript.
DISCLOSURE
Approval of the research protocol: This study was approved by the Institutional Ethics Committee at the University of Occupational and Environmental Health in 2020 (R2‐011). Informed consent: Written informed consent was obtained from all participants. Registry and the registration no. of the study/trial: N/A. Animal studies: N/A. Conflict of interest statement: The authors declare that there is no conflict of interest.
Supporting information
Table S1.
ACKNOWLEDGMENTS
This work was supported by MHLW Program Grant Number JPMH 200501. We would like to thank Editage (http://www.edita ge.jp) for English language editing.
Tsuji M, Koriyama C, Ishihara Y, et al. Associations between welding fume exposure and neurological function in Japanese male welders and non‐welders. J Occup Health. 2023;65:e12393. doi: 10.1002/1348-9585.12393
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Antonini JM, Lewis AB, Roberts JR, Whaley DA. Pulmonary effects of welding fumes: review of worker and experimental animal studies. Am J Ind Med. 2003;43(4):350‐360. doi: 10.1002/ajim.10194 [DOI] [PubMed] [Google Scholar]
- 2. Iregren A. Psychological test performance in foundry workers exposed to low levels of manganese. Neurotoxicol Teratol. 1990;12(6):673‐675. doi: 10.1016/0892-0362(90)90085-q [DOI] [PubMed] [Google Scholar]
- 3. Racette BA, Criswell SR, Lundin JI, et al. Increased risk of parkinsonism associated with welding exposure. Neurotoxicology. 2012;33(5):1356‐1361. doi: 10.1016/j.neuro.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rahmani A, Golbabaei F, Dehghan SF, Mazlomi A, Akbarzadeh A. Assessment of the effect of welding fumes on welders' cognitive failure and health‐related quality of life. Int J Occup Saf Ergon. 2016;22(3):426‐432. doi: 10.1080/10803548.2016.1164499 [DOI] [PubMed] [Google Scholar]
- 5. Statistics Bureau of Japan . Population and Households of Japan. 2015. Accessed November 6, 2022. https://www.e‐stat.go.jp/dbview?sid=0003209883 (in Japanese)
- 6. Ministry of Health, Labour and Welfare . Amendments to ordinance on prevention of hazards from specified chemical substances, work environment measurement standard. Accsessed November 6, 2022. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000099121_00001.html (in Japanese)
- 7. Akbar‐Khanzadeh F. Short‐term respiratory function changes in relation to workshift welding fume exposures. Int Arch Occup Environ Health. 1993;64(6):393‐397. doi: 10.1007/bf00517944 [DOI] [PubMed] [Google Scholar]
- 8. Hedmer M, Karlsson JE, Andersson U, Jacobsson H, Nielsen J, Tinnerberg H. Exposure to respirable dust and manganese and prevalence of airways symptoms, among Swedish mild steel welders in the manufacturing industry. Int Arch Occup Environ Health. 2014;87(6):623‐634. doi: 10.1007/s00420-013-0896-3 [DOI] [PubMed] [Google Scholar]
- 9. Park J, Yoo CI, Sim CS, et al. A retrospective cohort study of Parkinson's disease in Korean shipbuilders. Neurotoxicology. 2006;27(3):445‐449. doi: 10.1016/j.neuro.2006.01.005 [DOI] [PubMed] [Google Scholar]
- 10. Kissani N, Naji Y, Mebrouk Y, Chraa M, Ghanima A. Parkinsonism and chronic manganese exposure: Pilot study with clinical, environmental and experimental evidence. Clin Park Relat Disord. 2020;3:100057. doi: 10.1016/j.prdoa.2020.100057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dlamini WW, Nelson G, Nielsen SS, Racette BA. Manganese exposure, parkinsonian signs, and quality of life in South African mine workers. Am J Ind Med. 2020;63(1):36‐43. doi: 10.1002/ajim.23060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rolle‐McFarland D, Liu Y, Mostafaei F, et al. The association of bone and blood manganese with motor function in Chinese workers. Neurotoxicology. 2022;88:224‐230. doi: 10.1016/j.neuro.2021.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park RM, Bowler RM, Roels HA. Exposure‐response relationship and risk assessment for cognitive deficits in early welding‐induced manganism. J Occup Environ Med. 2009;51(10):1125‐1136. doi: 10.1097/JOM.0b013e3181bd8114 [DOI] [PubMed] [Google Scholar]
- 14. Sato K, Ueyama H, Arakawa R, Kumamoto T, Tsuda T. [A case of welder presenting with parkinsonism after chronic manganese exposure]. Rinsho Shinkeigaku. 2000;40(11):1110‐1115. (in Japanese). [PubMed] [Google Scholar]
- 15. Nakadate T, Aizawa Y, Yagami T, Zheg YQ, Kotani M, Ishiwata K. Change in obstructive pulmonary function as a result of cumulative exposure to welding fumes as determined by magnetopneumography in Japanese arc welders. Occup Environ Med. 1998;55(10):673‐677. doi: 10.1136/oem.55.10.673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takigawa T, Kishimoto T, Nabe M, et al. The current state of workers' pneumoconiosis in relationship to dusty working environments in Okayama Prefecture, Japan. Acta Med Okayama. 2002;56(6):303‐308. doi: 10.18926/amo/31694 [DOI] [PubMed] [Google Scholar]
- 17. Ministry of Health, Labour and Welfare . Workers' fatigue accumulation self‐assessment checklist. Accessed November 6, 2022. https://www.mhlw.go.jp/topics/2004/06/tp0630‐1.html (in Japanese)
- 18. Lucchini R, Selis L, Folli D, et al. Neurobehavioral effects of manganese in workers from a ferroalloy plant after temporary cessation of exposure. Scand J Work Environ Health. 1995;21(2):143‐149. doi: 10.5271/sjweh.1369 [DOI] [PubMed] [Google Scholar]
- 19. Wechsler D. WAIS‐IV Administration and Scoring Manual. The Psychological Corporation; 2008. [Google Scholar]
- 20. Wechsler D. WAIS‐IV Technical and Interpretive Manual. The Psychological Corporation; 2008. [Google Scholar]
- 21. Japan Society for Occupational Health . Guidelines for personal exposure measurement of chemicals. Sangyo Eiseigaku Zasshi. 2015;57(2):A13‐A60. (in Japanese). [Google Scholar]
- 22. Yu KM, Topham N, Wang J, et al. Decreasing biotoxicity of fume particles produced in welding process. J Hazard Mater. 2011;185(2–3):1587‐1591. doi: 10.1016/j.jhazmat.2010.09.083 [DOI] [PubMed] [Google Scholar]
- 23. Baloch S, Kazi TG, Baig JA, Afridi HI, Arain MB. Occupational exposure of lead and cadmium on adolescent and adult workers of battery recycling and welding workshops: Adverse impact on health. Sci Total Environ. 2020;720:137549. doi: 10.1016/j.scitotenv.2020.137549 [DOI] [PubMed] [Google Scholar]
- 24. Li N, Taneepanichskul N. Associations between welding fume exposure and blood hemostatic parameters among workers exposed to welding fumes in confined space in Chonburi, Thailand. PLoS One. 2021;16(11):e0260065. doi: 10.1371/journal.pone.0260065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ministry of the Environment . The exposure to chemical compounds in the Japanese people. Accessed November 6, 2022. https://warp.da.ndl.go.jp/info:ndljp/pid/11656271/www.env.go.jp/chemi/dioxin/pamph/cd/2017en_full.pdf (in Japanese)
- 26. Lucchini RG, Albini E, Benedetti L, et al. High prevalence of Parkinsonian disorders associated to manganese exposure in the vicinities of ferroalloy industries. Am J Ind Med. 2007;50(11):788‐800. doi: 10.1002/ajim.20494 [DOI] [PubMed] [Google Scholar]
- 27. Mergler D, Baldwin M, Bélanger S, et al. Manganese neurotoxicity, a continuum of dysfunction: results from a community based study. Neurotoxicology. 1999;20(2–3):327‐342. [PubMed] [Google Scholar]
- 28. Klotz K, Weistenhöfer W, Neff F, Hartwig A, van Thriel C, Drexler H. The health effects of aluminum exposure. Dtsch Arztebl Int. 2017;114(39):653‐659. doi: 10.3238/arztebl.2017.0653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tamura T, Sugihara G, Takahashi H. Memory impairment and hippocampal volume after carbon monoxide poisoning. Arch Clin Neuropsychol. 2021;36(1):145‐148. doi: 10.1093/arclin/acaa050 [DOI] [PubMed] [Google Scholar]
- 30. Ministry of Health, Labour and Welfare . Prevention of carbon monoxide poisoning in arc welding work. Accessed January 25, 2023. https://www.jaish.gr.jp/anzen/hor/hombun/hor1‐45/hor1‐45‐33‐1‐0.htm (in Japanese)
- 31. Van der Elst W, Van Boxtel MP, Jolles J. Occupational activity and cognitive aging: a case‐control study based on the Maastricht Aging Study. Exp Aging Res. 2012;38(3):315‐329. doi: 10.1080/0361073x.2012.672137 [DOI] [PubMed] [Google Scholar]
- 32. Galy E, Mélan C. Effects of cognitive appraisal and mental workload factors on performance in an arithmetic task. Appl Psychophysiol Biofeedback. 2015;40(4):313‐325. doi: 10.1007/s10484-015-9302-0 [DOI] [PubMed] [Google Scholar]
- 33. Jain S, Nataraja NP. The effect of fatigue on working memory and auditory perceptual abilities in trained musicians. Am J Audiol. 2019;28(2S):483‐494. doi: 10.1044/2019_aja-ind50-18-0102 [DOI] [PubMed] [Google Scholar]
- 34. Yuan Y, Leung AW, Duan H, et al. The effects of long‐term stress on neural dynamics of working memory processing: An investigation using ERP. Sci Rep. 2016;6:23217. doi: 10.1038/srep23217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xie W, Berry A, Lustig C, Deldin P, Zhang W. Poor sleep quality and compromised visual working memory capacity. J Int Neuropsychol Soc. 2019;25(6):583‐594. doi: 10.1017/s1355617719000183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang X, Zhang J, Zhou L, et al. Long‐term iron exposure causes widespread molecular alterations associated with memory impairment in mice. Food Chem Toxicol. 2019;130:242‐252. doi: 10.1016/j.fct.2019.05.038 [DOI] [PubMed] [Google Scholar]
- 37. Hirsch EC. Iron transport in Parkinson's disease. Parkinsonism Relat Disord. 2009;15(suppl 3):S209‐S211. doi: 10.1016/s1353-8020(09)70816-8 [DOI] [PubMed] [Google Scholar]
- 38. Shi Z, Li M, Wang Y, Liu J, El‐Obeid T. High iron intake is associated with poor cognition among Chinese old adults and varied by weight status‐a 15‐y longitudinal study in 4852 adults. Am J Clin Nutr. 2019;109(1):109‐116. doi: 10.1093/ajcn/nqy254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barceloux DG. Manganese. J Toxicol Clin Toxicol. 1999;37(2):293‐307. doi: 10.1081/clt-100102427 [DOI] [PubMed] [Google Scholar]
- 40. U.S. Environmental Protection Agency . Drinking water criteria document for manganese. Accessed November 6, 2022. https://www.epa.gov/sites/default/files/2018‐12/documents/dw‐criteria‐manganese.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


