Graphical abstract
Recent years have seen notable progress in the use of deep eutectic solvents (DESs) in pharmaceutical applications. This is ascribed to the high preparation flexibility of DES mixtures and the ability to multi-tune their physicochemical properties and biopharmaceutical characteristics. The aim of this article is to provide perspective concerning the applications of DESs in pharmaceutical systems and their potential based on the current state of the field.
Notably, there is some disagreement about whether some mixtures are in fact DESs or ionic liquids (ILs) [1]. This indicates the need for more robust standards and definitions so as to avoid confusion within the scientific community. Many studies consider DESs to either constitute a subclass of ILs, analogs of ILs, or alternatives to ILs. In this regard, it would be best for the IUPAC to standardize DES classification and their similarity/dissimilarity with ILs.
To apply DESs in the pharmaceutical industry, it is imperative to elucidate their toxicology profiles [2,3]. This is in line with the requirements of REACH (i.e., Registration, Evaluation and Authorization of Chemicals) legislation. However, while toxicology profiles of DESs toward many prokaryotic and eukaryotic microorganisms have been reported, they have not yet been fully screened and toxicity data remain scarce. In addition, a claim of ‘greenness’ for a DES based on its individual components is no longer valid [2,4,5]. Therefore, evaluating the cytotoxicity and toxicity of DESs remains a fundamental need that must be addressed before applying DESs to health-related applications. In addition, the DESs being screened should also be registered for further preclinical and clinical trials.
Drug solubility, or lack thereof, is one of the main challenges faced by the pharmaceutical industry; accordingly, many solubilizing agents have come under study, including DESs. Significant progress has been made in this direction, with many research groups having investigated a variety of DES combinations and poorly-soluble drugs. The term therapeutic deep eutectic solvents (THEDESs) was recently coined to represent drug-based (active pharmaceutical ingredient, API) DESs. This class of DESs overcomes certain shortcomings, such as poor solubility, low absorption rate, and high toxicity. API-containing THEDES have dissolution capacities positions it as a property of the mixture of solvent and dissolved drug. Another promising application of THEDESs is in enhancing transdermal drug delivery. Three stages of experiments are needed to investigate the feasibility of THEDESs in this regard: first, conducting cytotoxicity assays; second, exploring absorption rates using artificial membranes; and third, the same exploration using mammalian skin membranes. The combined data from these three stages of characterization would provide a comprehensive overview on the DES mixtures and the feasibility of their use as transdermal drug carriers. Noteworthy, different research groups might produce controversial results or conclusions. Following standard protocols and taking into consideration the biological system, preparation method, storage, moisture content, acidity, and the stability of the DES under study have significant impact on the results obtained. Beyond transdermal delivery, the feasibility of using DESs in drug delivery generally has been explored. In this context, a number of terms for DES classes have been used interchangeably, including NADESs, THEDESs, pharmaceutical DESs (PDESs), hydrophobic DESs (HDESs), and polymerized drug based DESs. Regarding delivery methods, recent studies, but limited, have focused on oral, buccal mucosa, transdermal [6,7], intrajejunal injection, subcutaneous injection, and topical delivery [8] (Fig. S1). Other routes still merit investigation, such as nasal, ocular, pulmonary, sublingual, and vaginal/anal drug delivery.
DESs have been used to improve the surface chemistry of drug nano-carriers. Recently, the Hayyan group employed DESs as functionalizing agents for drug delivery applications (Table S1) [9]. It is vital to conduct robust characterization along with DESs formulations to avoid the misinterpretation of results. There remains a great deal to be done in this area, such as investigating various DES formulations as promising functionalizing agents, screening other carbonaceous and non-carbonaceous materials as potential drug carriers.
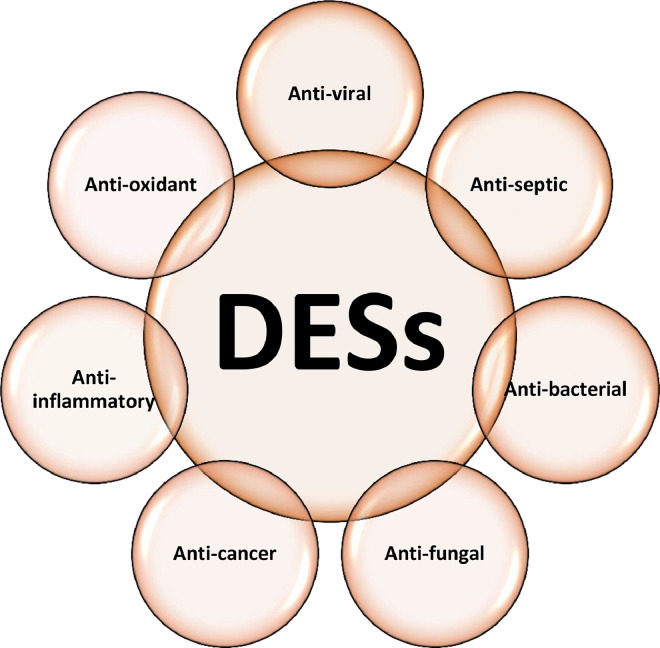
While DESs have been studied mainly as drug excipients, many are also promising candidates in their own right as agents having anti-septic, anti-inflammatory, anti-cancer, anti-bacterial, anti-fungal, anti-oxidant, and anti-viral activities (Fig. 1). Research to date has yielded remarkable results supporting the potential use of DESs as therapeutic agents; however, this area is still in its infancy and needs more attention from the scientific community.
Fig. 1.
Potential therapeutic activities of DESs.
Conclusion and future directions
Future studies should strive to better identify DES formulations that can serve as drug vehicles. In addition, it is anticipated that DESs will come to occupy a notable place in pharmaceutical applications due to their ability to take on multiple roles, such as acting as therapeutic-based mixtures, improving drug penetration, boosting drug dissolution, carrying drugs for targeted delivery, extracting bioactive compounds, and enhancing toxicology profiles. However, most research studies, whether in vitro, in vivo or ex vivo, have focused on the preclinical angle. Moving this research into clinical stages is highly recommended to foster the industrial scale of DESs in this domain and facilitate the transition of these materials from research mode into tangible applications. In addition, with drug-resistant diseases having become commonplace, it would be interesting to investigate the implications of DESs in relation to such diseases. Also of interest is the application of DESs to therapeutic diagnosis, along with their use in vaccination. Due to the theoretically unlimited number of DES formulations and other complex factors involved, in silico tools are important for benchmarking; they are also important in interpreting experimental results. It is key for future studies to conduct full characterization of DESs, elucidate the effects of altering composition and molar ratio, determine toxicology profiles and biodegradability, and study interactions with biosystems. These endeavors should be supported with micro research on toxicokinetics and pharmacokinetics to assess whether the DES under study is a potential toxicant or pharmaceutical. Notably, research outcomes relating to toxicology profiles may contradict each other for a variety of methodological reasons; accordingly, protocol standardization represents a critical step in the process of exploring how benign a DES before proceeding with pharmaceutical applications. The DES puzzle still features many missing parts, and its completion by researchers active in this domain is highly welcomed.
Conflicts of interest
None.
Acknowledgment
The author would like to thank the Ministry of Higher Education, Research, and Innovation (MoHERI) for supporting this research through TRC block funding.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajps.2023.100780.
Appendix. Supplementary materials
References
- 1.Rogers RD, Gurau G. Is "choline and geranate" an ionic liquid or deep eutectic solvent system? Proc Natl Acad Sci USA. 2018;115(47):E10999. doi: 10.1073/pnas.1814976115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayyan M, Hashim MA, Hayyan A, et al. Are deep eutectic solvents benign or toxic? Chemosphere. 2013;90(7):2193–2195. doi: 10.1016/j.chemosphere.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Mbous YP, Hayyan M, Hayyan A, Wong WF, Hashim MA, Looi CY. Applications of deep eutectic solvents in biotechnology and bioengineering—promises and challenges. Biotechnol Adv. 2017;35(2):105–134. doi: 10.1016/j.biotechadv.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Zainal-Abidin MH, Hayyan M, Ngoh GC, Wong WF, Looi CY. Emerging frontiers of deep eutectic solvents in drug discovery and drug delivery systems. J Control Release. 2019;316:168–195. doi: 10.1016/j.jconrel.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Marchel M, Cieśliński H, Boczkaj G. Deep eutectic solvents microbial toxicity: current state of art and critical evaluation of testing methods. J Hazard Mater. 2022;425 doi: 10.1016/j.jhazmat.2021.127963. [DOI] [PubMed] [Google Scholar]
- 6.Qu W, Qader IB, Abbott AP. Controlled release of pharmaceutical agents using eutectic modified gelatin. Drug Deliv Transl Res. 2022;12(5):1187–1194. doi: 10.1007/s13346-021-00998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanner EEL, Curreri AM, Balkaran JPR, et al. Design principles of ionic liquids for transdermal drug delivery. Adv Mater. 2019;31(27) doi: 10.1002/adma.201901103. [DOI] [PubMed] [Google Scholar]
- 8.Dharamdasani V, Mandal A, Qi QM, Suzuki I, Bentley MVLB, Mitragotri S. Topical delivery of siRNA into skin using ionic liquids. J Control Release. 2020;323:475–482. doi: 10.1016/j.jconrel.2020.04.038. [DOI] [PubMed] [Google Scholar]
- 9.Zainal-Abidin MH, Hayyan M, Ngoh GC, Wong WF, Tok TT. Derivation of an anti-cancer drug nanocarrier using a malonic acid-based deep eutectic solvent as a functionalization agent. J Drug Deliv Sci Technol. 2022;75 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.