Summary
Poor nutrition is one of the leading causes of non-communicable diseases (NCDs), especially in the WHO Region of the Americas (AMRO). In response, international organisations recommend front-of-pack nutrition labelling (FOPNL) systems that present nutrition information clearly to help consumers make healthier choices. In AMRO, all 35 countries have discussed FOPNL, 30 countries have formally introduced FOPNL, eleven have adopted FOPNL, and seven countries (Argentina, Chile, Ecuador, Mexico, Peru, Uruguay and Venezuela) have implemented FOPNL. FOPNL has gradually spread and evolved to better protect health by increasingly adopting larger warning labels, contrasting background devices for better salience, using “excess” instead of “high in” to improve efficacy, and adopting the Pan American Health Organization's (PAHO) Nutrient Profile Model to better define nutrient thresholds. Early evidence illustrates successful compliance, decreased purchases and product reformulation. Governments still discussing and waiting to implement FOPNL should follow these best practices to help reduce poor nutrition related NCDs.
Translated versions of this manuscript are available in Spanish and Portuguese in the supplementary material
Keywords: Front-of-pack nutrition labeling, Americas, Nutrition, Non-communicable diseases
Introduction
Poor nutrition is one of the leading causes of non-communicable diseases (NCDs), including cardiovascular disease, type 2 diabetes, high blood pressure, and some cancer(s) contributing to death and disability worldwide.1 The availability and overconsumption of ultra-processed foods have played a significant role in the global increases of overweight/obesity and NCD and nutrition-related diseases.2 To address the global NCD epidemic and nutrition-related diseases, the World Health Organization (WHO) recommends the implementation of effective front-of-pack nutrition labelling (FOPNL) systems that present clear nutrition information on the front of packaged foods and beverages.3 FOPNL systems aim to aid populations understand the products’ nutritional content, reduce consumption of ultra-processed and processed food products high in fats, sugars and/or salt, and ultimately help consumers make healthier choices.4 FOPNL systems may also induce reformulation of food portfolios.4
FOPNL systems are categorised according to their purpose and information provided. Nutrient-specific systems include interpretive labels, which provide nutrition information for one or more nutrients as guidance rather than specific facts and show judgment or recommendation (e.g., UK traffic light label, warning labels, ‘high in’ symbols). Some nutrient-specific systems also include non-interpretive labels, which show information, with no specific judgment or recommendation (e.g., Guideline Daily Amount - GDA, Facts Up Front - FUF). Summary indicator systems combine several criteria to establish one indication of the healthiness of a product and show judgment or recommendation (e.g., Health Star Ratings, Nutri-Score, and endorsement logos such as Choices, Keyhole, Healthier Choice).5,6
To date FOPNL systems worldwide have been outnumbered by the food and beverage industry's voluntary GDA and FUF systems, which provide only numeric information about nutrients and calories.5,6 However these systems have been proven to be ineffective because they do not provide interpretative information requiring consumers to employ significant cognitive effort and time to understand numeric nutrient information.7 These systems mislead consumer perceptions regarding the healthiness of processed and ultra-processed foods (e.g. providing misleading recommended cut-off points for critical nutrients, perceiving a product as healthy when they are excessive in sugars), and are rarely used by consumers even when they are aware of this information.7 In the last decade, food classification criteria (Nutrient Profile Models) have been developed in different regions (and countries) primarily based on the markets of food and drink products, including their composition, consumption and/or availability in those regions. The Pan American Health Organization's (PAHO) Nutrient Profile Model, established in 2016,8 was the first food classification tool to adopt WHO intake recommendations for critical nutrients of public health concern (i.e., sugars, sodium, total fats, saturated fats, trans fats) as a reference, instead of relying on the market of processed and ultra-processed products. PAHO's Nutrient Profile Model has been considered a best practice for the Region of the Americas' (AMRO), as it is based on WHO intake goals of critical nutrients, to support populations to meet these goals. It has been developed and used for designing and implementing FOPNL policies, as well as other regulatory strategies related to the prevention and control of obesity/overweight, including marketing restrictions, school food regulations, and taxation policies.8
More recent iterations of FOPNL systems vary in size, shape, and appearance, and can present information as non-interpretive, interpretive, or a combination of both such as multiple traffic lights and the health star rating system. Some interpretive labels and combined interpretive and non-interpretive labels have shown to be more effective than only non-interpretive labels at altering purchase intention, and consumption behaviours and improving knowledge of product healthfulness.9 Recent studies have shown that FOPNL warning label systems outperform traffic light, and summary grade systems (e.g. Health Star Rating, Nutri-Score) in capturing consumers’ attention, improving their ability to identify products high in critical nutrients, and increasing their intention to buy a relatively less harmful option.6
Over the past decade FOPNL policies have begun to spread rapidly worldwide,5 especially in AMRO. Mandatory FOPNL policy adoption in AMRO began in Chile (2012) followed by Peru (2013), Mexico (2014), Ecuador (2014), Bolivia (2017), Uruguay (2019), a re-designed FOPNL in Mexico (2020), Brazil (2020), Venezuela (2020, 2021), Colombia (2021) and Argentina (2021). Several other countries in the region are currently considering the adoption of a mandatory FOPNL.5 To date only a few studies have reported on the adoption of FOPNL policies,5,10 but no study has thoroughly documented the development, adoption, and implementation of FOPNL policies within a given region. This study has two objectives. First, it aims to trace the development of FOPNL in AMRO by using the policy cycle model to help document the stages of FOPNL in the region. Second, it aims to assess the key features of FOPNL systems implemented as of August 2022 to help best practices in the AMRO region.
Data collection
Between June 2021 and August 2022, we reviewed FOPNL policies in AMRO obtained from the Global database on the Implementation of Nutrition Action (GINA) and the World Cancer Research Fund International's NOURISHING database. We cross-checked these findings by reviewing publicly accessible documents in Google, including media reporting, government and inter-governmental reports, and individual country legislative and executive websites throughout AMRO. Standard snowball search methods were conducted in both English and Spanish beginning with key search terms, including “labelling”, “front-of-pack nutrition labelling”, and “warning label”.
Data analysis
We applied Knill and Tosun's application of the policy cycle model11 to analyse the development and progress of FOPNL policies in AMRO in five stages: 1) agenda setting, 2) policy formulation, 3) policy adoption, 4) implementation, and 5) evaluation. The policy cycle model has been used to identify best practices, gaps, patterns, and trends for developing effective public policies,11 a key purpose of this paper.
To assess FOPNL systems in AMRO, we used PAHO's best-practice policies for FOPNL described in PAHO's technical brief on FOPNL,6 which serves as a reference on this policy tool for the region, and in PAHO's Nutrition Profile Model,8 which serves as a benchmark for classifying food and drink products that are to be regulated by demand and offer reduction food policies, including FOPNL. The key features of FOPNL systems assessed included the label type of FOPNL, position/location, size, and nutrient criteria/thresholds in relation to PAHO's recommendations6 (Fig. 1).
Fig. 1.

Example of PAHO FOPNL benchmark design features, which include octagonal (shape) textual warning labels (type), with black colour background, white colour capital fonts (colour) and white borders, placed within a white fringe box (contrast), occupying at least 30% of the surface of the main display panel of the package for the most harmful products (size), located on the upper margin of the main panel of exhibition of the package (location).
FOPNL policies include not only what should be added to the label but also what should be eliminated. Hence, we also analysed FOPNL policy requirements to prohibit the use of persuasive elements such as marketing and promotional devices (e.g., cartoon characters), nutrition or health benefit claims, and endorsement logos.
Development of FOPNL in AMRO
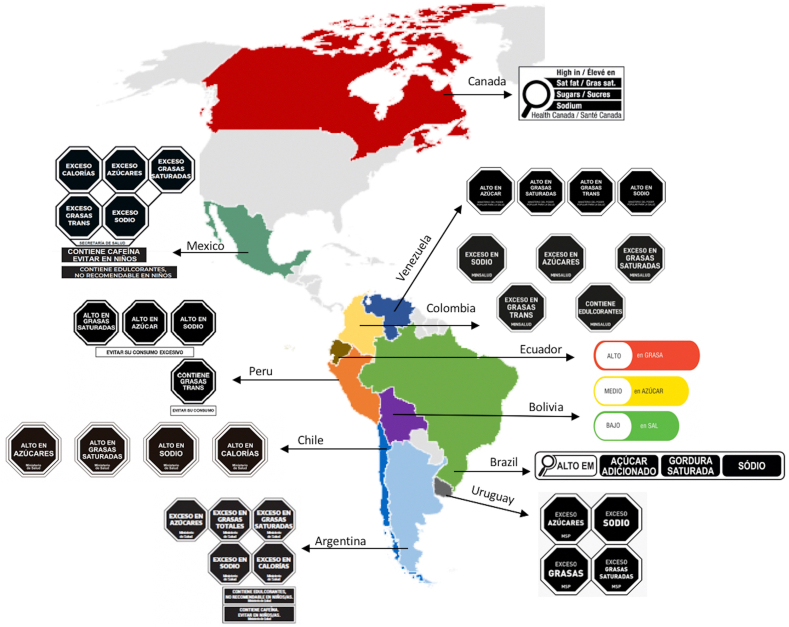
As of August 2022, eleven AMRO countries (Argentina, Bolivia, Brazil, Canada, Chile, Colombia, Ecuador, Mexico, Peru, Uruguay and Venezuela) had adopted a FOPNL system (Fig. 2). The results of the analysis on the development, adoption, and implementation of these FOPNL systems based on the Knill and Tosun's policy cycle model are described below (Table 1).
Fig. 2.
AMRO countries that had adopted a FOPNL system (as of August 2022).
Table 1.
Development of front-of-pack nutrition labeling in the Americas using the policy cycle model.
| Stage 1 (Agenda Setting) | Stage 2 (Policy Formulation) | Stage 3 (Policy Adoption) | Stage 4 (Implementation) | Stage 5 (Evaluation) |
|---|---|---|---|---|
| Identification of a societal problem based on social, economic, cultural, or ideological factors and selected by decision makers to create an agenda. | Exploration and discussion of multiple alternative paths of action to address identified issues where objectives are defined, and policy settings and instruments are determined. | Government institutions determining whether a policy is adopted or not. | Transformation of laws into action and application. | Knowledgeable experts evaluate processes and policy objectives creating a feedback loop which helps identify problems which then restarts the process of policy development. |
| Cuba Dominican Republic Honduras Nicaragua United States of America |
Costa Rica El Salvador Guatemala Panama Paraguay CARICOM |
Canada Colombia Brazil Bolivia |
Argentina Mexico Venezuela |
Chile Ecuador Peru Uruguay |
CARICOM: Antigua and Barbuda, Bahamas, Barbados, Belize, Dominica, Grenada, Guyana, Haiti, Jamaica, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines, Suriname, and Trinidad and Tobago.
Stage 1: Agenda setting
Although the policy cycle model is cyclical, agenda setting represents the first stage in the policy process. Agenda setting (stage 1) refers to the identification of a societal problem based on social, economic, cultural, or ideological factors. Based upon these factors, along with public interest and determining the viability of actions, decision-makers create an agenda to address identified problems.11 All of the countries in the region (n = 35) have entered the agenda-setting stage, identifying poor diets as a main driver of NCDs such as obesity and diabetes.12 Each of the AMRO countries have also discussed potential solutions to reduce these public health problems including marketing restrictions, school food regulations, taxation policies, and mandatory FOPNL systems, among others (Table 1). Additionally, PAHO has helped lead regional discussions and created action plans to prevent obesity among children and adolescents, which have included discussions around FOPNL systems.5,12
Stage 2: Policy formulation
After public issues have been identified and selected in the agenda-setting stage, policy formulation (stage 2) may include the identification, exploration, and discussion of multiple alternative paths of action to address identified issues11 (Table 2). It is in this stage that policy objectives are defined, and policy settings and instruments are determined.11 Policy formulation can occur either in the legislative or the executive branch of the government. In the legislative branch, a legislative bill is introduced and then discussed and debated in a committee (e.g. health), lower chamber (e.g. Deputies), upper chamber (e.g. Senate), and then prepared for a final vote of approval. Alternatively, policy formulation can occur in the executive branch, which typically consists of an executive branch member (e.g. president or minister) introducing and enacting a decree, ministerial order, or executive order.
Table 2.
Assessment of FOPNL regulations’ specifications in the WHO Region of the Americas (as of August 2022).
| Country | Type | Label | Purpose | Year in effect | Size | Location | Criteria/Threshold |
|---|---|---|---|---|---|---|---|
| Argentina | Octagon Warning Labels | Guarantee the right to health and adequate nutrition, promote healthy eating, provide simple and understandable nutritional information on packaged foods, promote assertive and active decision-making and safeguard the rights of consumers, warn consumers about excessive amounts of food components such as sugars, sodium, saturated fats, total fats and calories, providing clear, timely and truthful information, and promote the prevention of malnutrition in the population and the reduction of chronic non-communicable diseases. | 2022 – first phase of implementation 2023 – second phase of implementation |
Size of each octagon warning label if the main face of the product label is: > 10 cm2a < 15 cm2: 1.1 × 1.1 cm >= 15 cm2a < 20 cm2: 1.3 × 1.3 cm >= 20 cm2a < 25 cm2: 1.4 × 1.4 cm >= 25 cm2a < 30 cm2: 1.5 × 1.5 cm >= 30 cm2a < 35 cm2: 1.7 × 1.7 cm >= 35 cm2a < 40 cm2: 1.8 × 1.8 cm >= 40 cm2a < 50 cm2: 2.0 × 2.0 cm >= 50 cm2a < 60 cm2: 2.2 × 2.2 cm >= 60 cm2a < 80 cm2: 2.5 × 2.5 cm >= 80 cm2a < 100 cm2: 2.8 × 2.8 cm >= 100 cm2a < 125 cm2: 3.1 × 3.1 cm >= 125 cm2a < 150 cm2: 3.4 × 3.4 cm >= 150 cm2a < 200 cm2: 3.9 × 3.9 cm >= 200 cm2a < 250 cm2: 4.4 × 4.4 cm >= 250 cm2a ≤ 300 cm2: 4.8 × 4.8 cm > 300 cm2: 5% of the size of the main face of the product label |
On the upper right margin of the main face of the product label. In the case of cylindrical and conical containers, they should be placed on the top center margin of the main face of the product label. |
Phase 1 (in force) Energy ≥300 total kcal/100 g ≥50 total kcal/100 ml (for non-alcoholic beverages) Sugars ≥20% of total kcal from added sugars Saturated Fats ≥12% of total kcal from saturated fats Total Fat ≥35% of total kcal from total fat Sodium ≥5 mg/1 kcal or ≥600 mg/100 g ≥40 mg/100 ml (for non-caloric beverages) Phase 2 Energy ≥275 total kcal/100 g ≥25 total kcal/100 ml (for non-alcoholic beverages) Sugars ≥10% of total kcal from added sugars Saturated Fats ≥10% of total kcal from saturated fats Total Fat ≥30% of total kcal from total fat Sodium ≥1 mg/1 kcal or ≥300 mg/100 g ≥40 mg/100 ml (for non-caloric beverages) |
|
| Chile | Octagon Warning Labels | Inform the population about the nutrition composition of foods when they contain excessive amounts of saturated fats, sodium, sugars and energy. | 2016- first phase of implementation 2018- second phase of implementation 2020- Third and Final implementation Micro and small companies were only industry to comply from the third/last phase on. |
If the main face of the product label is: <30 cm2: Label on larger container 30-<60 cm2: 1.5 × 1.5 cm 60-<100 cm2: 2.0 × 2.0 cm 100-<200 cm2: 2.5 × 2.5 cm 200- <300 cm2: 3.0 × 3.0 cm ≥300 cm2: 3.5 × 3.5 cm |
Main face of the product label. When the size of the main face of the product label is 30-<60 cm2 the octagons can be placed in another face of the package that is visible. |
Phase 1: SOLID Energy: >350 kcal/100 g Sodium: >800 mg/100 g Total sugars: >22.5 g/100 g Saturated fats: >6 g/100 g LIQUID Energy: >100 kcal/100 ml Sodium: >100 mg/100 ml Total sugars: >6 g/100 ml Saturated fats: >3 g/100 ml Phase 2: SOLID Energy: >300 kcal/100 g Sodium: >500 mg/100 g Total sugars: >15 g/100 g Saturated fats: >5 g/100 g LIQUID Energy: >80 kcal/100 ml Sodium: >100 mg/100 ml Total sugars: >5 g/100 ml Saturated fats: >3 g/100 ml Phase 3 (final -in force): SOLID Energy: >275 kcal/100 g Sodium: >400 mg/100 g Added free sugars: >10 g/100 g Saturated fats: >4 g/100 g LIQUID Energy: >70 kcal/100 ml Sodium: >100 mg/100 ml Added free sugars: >5 g/100 ml Saturated fats: >3 g/100 ml |
|
| Ecuador | Textual Traffic Light |  |
Guarantee the constitutional right of people to timely, clear, accurate and non-misleading information about the content and characteristics of these foods, which allows the consumer the correct choice for their acquisition and consumption. | 2014 for medium and large companies and 2015 for micro and small companies Products already circulating 2015 |
If the total surface of the product label is: <19.4 cm2the label should be applied on external container. If the surface of the main exhibition face of the package is: 19.5-32 cm2: the label should measure 6.25 cm2 33-161 cm2: the label should occupy 20% of the surface of the main exhibition face ≥ 162 cm2: the label should occupy 15% of the surface of the main exhibition face |
Upper left corner of main or secondary panel from May 2014. |
SOLID LOW Sugars ≤5 g/100 g Sodium ≤120 mg/100 g Total Fat ≤3 g/100 g MEDIUM Sugars >5 g and <15 g/100 g Sodium >120 g and <600 mg/100 g Total Fat >3 g and <20 g/100 g HIGH Sugars ≥15 g/100 g Sodium ≥600 mg/100 g Total Fat ≥20 g/100 g LIQUID LOW Sugars ≤2.5 g/100 ml Sodium ≤120 ml/100 ml Total Fat ≤1.5 g/100 ml MEDIUM Sugars 2.5 g–7.5 g/100 ml Sodium 120 g-600 mg/100 ml Total Fat 1.5 g–10 g/100 ml HIGH Sugars ≥7.5 g/100 ml Sodium ≥600mg/100 ml Total Fat ≥10 g/100 ml |
| Mexico | Octagon Warning Labels |  |
“ …. which must warn clearly and truthfully about the content of critical nutrients and ingredients that pose risks to your health in excessive consumption.” | Law passed 2019 Phase I: Oct 1. 2020–Sept 30, 2023 Phase II: October 1, 2023–Sept 2025 Phase III: October 1, 2025 |
Size of each octagon warning label if package is: ≤5 cm2: At least 15% of main area 5 cm2-30cm2: 1 cm × 1.11 cm 30 cm2- 40 cm2: 1.5 cm × 1.66 cm 40 cm2- 60 cm2: 1.5 cm × 1.66 cm 60 cm2- 100 cm2: 2.0 cm × 2.22 cm 100 cm2- 200 cm2: 2.5 cm × 2.77 cm 200 cm2- 300 cm2: 3.0 cm × 3.32 cm > 300 cm2: 3.5 c2x 3.88 cm |
Upper right corner of the main panel of exhibition When the main panel of exhibition is smaller than a 60 cm2 the warnings can be placed in any other portion of the main panel of exhibition (not necessarily on the upper right corner). |
Phase 1 (in force) Energy ≥275 total kcal/100 g ≥70 total kcal or ≥10 kcal from free sugars/100 ml (for non-alcoholic beverages) Sugars ≥10% of total kcal from added free sugars Beverages containing less than <10 kcal from added free sugars are exempted from presenting this warning Saturated Fats ≥10% of total kcal from saturated fats Trans Fats ≥1% of total kcal from trans fats Sodium ≥350 mg/100 g ≥350mg/100 ml (for non-alcoholic beverages) ≥45mg/100 ml (for non-caloric beverages) Phase 2 Energy ≥275 total kcal/100 g ≥70 total kcal or ≥8 kcal from added free sugars/100 ml (for non-alcoholic beverages) Sugars ≥10% of total kcal from added free sugars Saturated Fats ≥10% of total kcal from saturated fats Trans Fats ≥1% of total kcal from trans fats Sodium ≥1mg/1 kcal or ≥300 mg/100 g ≥1mg/1 kcal or ≥300mg/100 ml (for non-alcoholic beverages) ≥45mg/100 ml (for non-caloric beverages) Phase 3 Thresholds are identical to Phase 2. But in this final phase the addition of any nutrient of concern (e.g. sugars, fats or sodium) makes the product subject to any of the warnings regardless of the nutrient the product was added with (e.g. A pre-packaged product added with sodium is a processed or ultra-processed product and if its content is above the thresholds for any of the nutrients of concern or calories it should include all corresponding warnings. Conversely, in phase 2, if a product is added with sodium, but not with other nutrients, it is not subject to the application of other warnings apart from sodium's). |
| Peru | Octagon Warning Labels |  |
“… the incorporation of advertising warnings on the front face of processed products facilitates the consumer to make informed decisions in the selection of products that are healthy. These warnings provide simple and easy-to-understand information on the content of critical nutrients such as sugar, saturated fat, trans fat or sodium content in processed products” | 2013- law passed (Law to Promote Healthy Eating for Children) 2017 – implementation of phase 1 2021 – implementation of phase 2 |
Size of each octagon warning label if front or main face of the product label is: <50 cm2: 3 × 3cm 50-100 cm2: 2 × 2cm 100-200 cm2: 2.5 × 2.5 cm >200 cm2: 3 × 3cm |
Upper right-hand side of the frontal face of the product label |
Phase 1 SOLID Sugar ≥22.5 g/100 g Sodium ≥800 mg/100 g Saturated fats ≥6 g/100 g Trans fats Any amount (octagonal warning featuring “CONTAINS TRANS FATS”) LIQUID Sugar ≥6 g/100 ml Sodium ≥100 g/100 ml Sat fat ≥ 3 g/100 ml Trans fats Any amount (octagonal warning featuring “CONTAINS TRANS FATS”) Phase 2 (in force) SOLID Sugar ≥10 g/100 g Sodium ≥400 mg/100 g Saturated fats ≥4 g/100 g Trans fats Any amount (octagonal warning featuring “CONTAINS TRANS FATS”) LIQUID Sugar ≥5 g/100 ml Sodium ≥100 g/100 ml Sat fat ≥ 3 g/100 ml Trans fats Any amount (octagonal warning featuring “CONTAINS TRANS FATS”) Phase 3 (update44) SOLID and LIQUID Sugar ≥10% of total kcal from added sugars Saturated Fats ≥10% of total kcal from saturated fats Sodium ≥100 mg per 100 kcal of product Trans fats Any amount (octagonal warning featuring “CONTAINS TRANS FATS”) |
| Uruguay | Octagon Warning Labels |  |
-Provide simple nutrition information -Promote informed food selection -Favor changes in eating habits by reducing the consumption of products with excessive content of critical nutrients |
Approved 2018, implemented 2020 |
Size of each octagon warning label if main panel of the package is: <30 cm2: Secondary container must be labeled according to area of its main face. 30-60 cm2: 1.5 × 1.5 cm 60-100 cm2: 2.0 × 2.0 cm 100-200 cm2: 2.5 × 2.5 cm 200-300 cm2: 3.0 × 3.0 cm >300 cm2: 3.5 × 3.5 cm |
Main frontal face of the product container, preferably in the upper part |
SOLID Sodium: >500 mg/100 g Free sugars: >13 g/100 g Total fat: >13 g/100 g Saturated fats: >6 g/100 g LIQUID Sodium: >200mg/100 ml Free sugars: >3 g/100 ml >5 g/100 ml in products with non-caloric sweeteners >7 g in products that have up to 80% of calories from sugars and contain no non-caloric sweeteners Total fat: >4 g/100 ml Saturated fats: >3 g/100 ml |
| Venezuela | Octagon Warning Labels | To protect public health from the harmful consequences of the excessive intake of sodium, sugars, saturated fats and trans fats, providing better information to consumers. | 2022 – implementation of octagon warning labels for sodium 2026 – implementation of octagon warning labels for sodium, sugars, saturated fats and trans fats |
If the main face of the product label is: <30 cm2: Label on larger container 30-<60 cm2: 1.5 × 1.5 cm 60-<100 cm2: 2.0 × 2.0 cm 100-<200 cm2: 2.5 × 2.5 cm 200- <300 cm2: 3.0 × 3.0 cm ≥300 cm2: 3.5 × 3.5 cm |
Main face of the product label. |
Phase 1: SOLID Sodium: ≥600 mg/100 g LIQUID Sodium: ≥300 mg/100 ml Phase 2: SOLID Sodium: ≥600 mg/100 g Added sugars: ≥11 g/100 g Saturated fats: ≥5 g/100 g Trans fats: >0 g/100 g LIQUID Sodium: ≥300 mg/100 ml Added sugars: ≥5.5 g/100 ml Saturated fats: ≥3 g/100 ml Trans fats: >0 g/100 ml |
Bulk: “Foods that are marketed in bulk, portioned, fractionated and prepared at the request of the public, even if these were packaged at the time of sale”.
Of the 35 countries in AMRO, 30 countries - 16 countries (Argentina, Brazil, Bolivia, Canada, Chile, Colombia, Costa Rica, Ecuador, El Salvador, Guatemala, Mexico, Panama, Paraguay, Peru, Uruguay and Venezuela) and one integration mechanism, the Caribbean Community (CARICOM) which includes 14 countries - have formally introduced a bill, decree, standard or order either in the legislative or the executive branch (stage 2) thereby formulating a policy on FOPNL (Table 1). Each of these countries reiterated their concerns about the rise of obesity and NCDs when introducing FOPNL. Some specific examples include the Argentine government stressing 40% of children are overweight and that this was growing rapidly,13 the Chilean government concerned about 60% of the population (age 15–64) were overweight or obese with the second-highest per capita ultra-processed food sales in the region,14 and the Costa Rican government emphasizing that 34 out of every 100 students were overweight or obese.15
All of these governments have discussed different policy features throughout the past 20 years, including various types of FOPNL systems (e.g. traffic lights, warning labels), attempting to align approaches based on PAHO best practices, necessary implementation changes, and expected outcomes. Although similar discussions occurred in these countries, particular aspects evolved over time. For example, governments that began FOPNL discussions in the late 2000s and early 2010s considered the adoption of the GDA (Mexico) or traffic-light labelling (Bolivia, Chile, Ecuador) for their mandatory systems.16,17 Mexico adopted the GDA system in 2014, and Ecuador adopted a traffic-light-coloured system in 2014 followed by Bolivia in 2017. As the evidence evolved, governments such as Chile began developing a novel FOPNL system that could perform better in allowing consumers to identify products that were excessive in calories, sugars, saturated fats and sodium as it became the first country in AMRO to adopt a FOPNL warning label system in 2012.18 Following this success and international recommendations, from the late 2010s, governments (e.g. Argentina, Colombia, Mexico, Uruguay, Venezuela) have primarily focused on implementing FOPNL octagonal warning label systems.
Evidence and experience from implementing FOPNL continued to evolve and improvements to the application of octagonal warning labels persisted. This included new or amended provisions that required larger sizes for warning labels, contrasting background devices for the design and application of warning labels to provide better salience for warnings, using the word “excess” instead of “high in” for the warnings to improve efficacy, and using PAHO's Nutrient Profile Model to define the products that should feature warning labels.9,18 FOPNL legislation also evolved in terms of coherence by prohibiting persuasive elements in products that are excessive in sugars, fats, sodium or contain other substances of public health concern. Starting in Chile, in 2016, products with warning labels were no longer allowed to depict cartoon characters or other devices targeted at children on product labels. In 2020 and 2021, Mexico and Argentina respectively adopted additional restrictions for health or nutrition claims, endorsements, and other persuasive elements in products that feature warning labels.19,20
By the late 2010s, governments were no longer choosing FOPNL systems that could provide conflicting data (e.g. high, medium, low in different nutrients) or serve as an endorsement (e.g. green colour or endorsement logos) such as the traffic light labelling system and Nutri-Score, which could make consumers more vulnerable to deception and prevent them from making informed decisions. Brazil, Chile, and Mexico used emerging research to claim that GDA and traffic light labelling systems were not simple, time-consuming, and required consumer math calculations for nutrition decision making.18,21 Conversely, in Mexico, a modelling study projected that the warning labels could reduce the prevalence of obesity by 14.7% between 2018 and 2023, and save the country US$ 1.8 billion in obesity-related costs.22 In addition, it was indicated that the FOPNL regulation could improve information on packages of products and help consumers make healthier decisions by preventing most processed and ultra-processed foods from displaying health and nutrition claims.23
During this stage, policymakers also referred to the statement by the UN Special Rapporteur on the right to health on the adoption of front-of-package warning labelling to tackle NCDs, which added a rights basis to the evidence base. For example, in Costa Rica, policymakers argued that FOPNL is “constituted as a tool not only to protect consumers’ right to information, but also as a tool for the protection of the right to health.”24 Governments recently proposing FOPNL legislation also cited the success of the FOPNL warning label system adopted in Chile and most recently in Mexico and Uruguay. Although best practices and evidence favouring the use of warning signs accumulated in the past years, in 2019, the Brazilian authorities ultimately adopted a rectangular-shaped “high in” system that does not depict warning labels.21
Stage 3: Policy adoption
Policy adoption refers to whether a policy is accepted into law or not by the government. Typically, this is executed through the legislative branch where elected officials vote on proposals/bills/initiatives that have been discussed (as explained in stage 2), but can also occur through the executive branch where the president or ministry leaders (e.g. Minister of Health) can issue executive orders, ministerial orders or decrees, after being either formally or informally discussed amongst government sectors.
Of the 30, (16 countries and CARICOM, one integration mechanism of 14 countries) that have formally introduced FOPNL legislation, eleven countries (Argentina, Bolivia, Brazil, Canada, Chile, Colombia, Ecuador, Mexico, Peru, Uruguay and Venezuela) have adopted legislation on mandatory FOPNL systems (stage 3). Five countries (Brazil, Canada, Ecuador, Uruguay and Venezuela) have gone through the executive route, in which the national health authority (Brazil, Canada, Ecuador and Venezuela) has issued a regulation or ministerial decree, or the president (Uruguay) has issued a presidential decree. The remaining seven countries have gone through the legislative branch. In the case of Colombia, both the executive and legislative routes were taken in parallel, but ultimately the legislative route was the latest to be adopted and is hierarchically superior. Some countries took a relatively long time to pass FOPNL since its formal introduction, including Chile14 (∼5 years), Canada25,26 (∼4 years), Uruguay27 (∼3.5 years), Brazil21 (∼3 years), Bolivia (∼3 years),28 Colombia (2 years),29 and Ecuador30 (2 years), while other countries approved FOPNL more quickly, including Argentina19 (∼1.5 years), Peru31 (∼1 year) and Mexico20 (6 months).
Stage 4: Implementation
The implementation stage refers to the transformation of laws or policies into programs or actions and applications.11 Of the eleven countries that have approved FOPNL, seven (Argentina, Chile, Ecuador, Mexico, Peru, Uruguay and Venezuela) have implemented FOPNL. Venezuela has adopted an octagonal warning label for sodium in a separate and earlier ministry of health regulation published in January 2020, which is already being implemented since April 2020,32 and in 2021 a new regulation was approved for the additional warnings for sugars, saturated fats and trans fats, and its implementation is due to start by 2024.33 Argentina recently approved FOPNL in November 2021 and its regulation was published in February 2022 setting the beginning of implementation for August 2022,34 Brazil published FOPNL in October 2020 and scheduled the implementation for October 2022, while Canada published FOPNL in June 2022 and scheduled the implementation for January 2026. Bolivia is still awaiting to publish their regulations as of August 2022. Implementation of FOPNL has varied where each government has agreed to roll out FOPNL in stages. For example, in Mexico, FOPNL is rolled out in three phases.20
In terms of implementation and enforcement of FOPNL, compliance appears to be high from early reports in Chile.35 For example, inspections reported compliance with the law was approximately 75% between June and December 2016 with compliance increasing again in 2018 to reach over 80% when 2600 inspections occurred.35 Similar figures were found in Peru, where 78% of a sample of products available at different points of sales were complying with the legislation.36 A study examining schools found some cases of product advertising violating the Peruvian law by allowing the usage of animated and public characters to promote the consumption of these foods.37 In Mexico, compliance also appears to be high. For instance, a survey on a sample of 10 supermarkets showed that the use of cartoon characters in products with warning labels decreased, particularly in breakfast cereals and in packed flavoured milk and that some products have been reformulated to avoid having a warning label.38
The manufacturers, as well as importers, distributors and retailers are responsible for complying with FOPNL policies and are all subject to sanctions for violating respective FOPNL laws and regulations. Sanctions are essentially established by overarching laws that provide enforcement and sanction power to health authorities (e.g. Ministry of Health). The types of sanctions can vary from admonition and fines to confiscation of products, business closure and permit cancelations, depending on the extent, severity, and recurrence of violations.
Stage 5: Evaluation
Evaluation represents the final stage of the policy cycle model where often times knowledgeable experts evaluate the policy, its process, and impact. This evaluation creates a feedback loop to help identify problems in the policy design (design evaluation) or throughout the implementation (process evaluation), or the impact that the intervention is having on a determined outcome. This stage is key to improving and developing a policy into a strong foundation so communities can progress towards positive outcomes. Some of the expected outcomes of interest of FOPNL policies include improving populations’ correct understanding of packaged products' content, perception about the products' harmfulness, and effectively improving purchasing and consumption patterns towards increasing unprocessed and minimally processed foods consumption and limiting ultra-processed food products, thus improving population diet and health.
In Ecuador, a study conducted to determine the response from young children's mothers to the FOPNL policy found that after a year of its implementation, the regulation was well-known regardless of socio-economic status. Another study conducted with 21 focus groups and 178 participants to understand the attitudes towards the FOPNL traffic light labelling system concluded that participants understood the information, but not all changed their attitudes and practices.39 It is noteworthy, that being aware of or understanding a FOPNL label may not suffice to effectively improve food purchase and consumption patterns. It has been demonstrated that the traffic light has not been able to reduce purchases of carbonated soft drinks, especially those high in sugar in Ecuador.40 In this case, the impact of the policy is not yet seen in a defined outcome, and therefore shows the weakness of traffic light systems and the need for such policies to upgrade their FOPNL system to warning labels.6
The warning labels have consistently influenced most peoples’ purchase decisions in Chile and have proven to effectively reduce sales of products high in calories, sugars, sodium, and saturated fats.35,41 In addition, studies have documented that families recognise that regulation is driving the shift towards healthier eating within their families and that younger children tend to have the most positive attitudes towards the regulation. Although FOPNL and marketing restrictions are different policy instruments some countries have tied them together as a comprehensive approach. The FOPNL policy in Chile and Argentina is part of a comprehensive policy which also addresses marketing restrictions and school food environment regulations, and has helped decrease the amount of child-directed marketing for unhealthy food products. In Chile, a study found that the percentage of cereal packages “high in” calories, sugars, sodium, or saturated fats that featured marketing strategies targeted at children decreased from 43% before (February–March 2015) the regulation was implemented (June 2016) to 15% after implementation (January–February 2017).42 Studies have also demonstrated that octagonal warning labels have shown to benefit populations equally across different socioeconomic groups and have not negatively impacted the economy.10,41
In Uruguay, an online study using two surveys of Facebook users (prior to and after enforcement) found that there were high levels of awareness and self-reported use of the warning labels.43 Consumers also reported that the presence of warning labels increased their ability to use nutritional labels to compare and identify which products contain excessive amounts of critical nutrients.43
Assessing of FOPNL policies
This section assesses the content and features of the FOPNL systems implemented, as of August 2022, as mandatory policies in seven AMRO countries, including Argentina, Chile, Ecuador, Mexico, Peru, Uruguay and Venezuela. We assess label type, size, position/location, and nutrient criteria/thresholds, in relation to PAHO's recommendations.
Label type
Each of the seven countries that have implemented FOPNL policies has adopted an interpretive system. Six of these countries have adopted warning labels (Argentina, Chile, Mexico, Peru, Uruguay and Venezuela), while one country (Ecuador) has implemented a textual traffic light labelling system.
Size
All the identified countries provided guidelines for FOPNL label sizes based on the surface size of the main display panel of the product label. The smallest noted main display panel size that still required a FOPNL was ≤5 cm2 by Mexico. The largest required FOPNL label was found in Argentina (6 × 6 cm or more depending on the size of the main panel of exhibition), followed by Mexico (3.5 × 3.88 cm), for main panels of exhibition over 300 cm,2 and by Chile, Uruguay and Venezuela (3.5 × 3.5 cm) for main panels of exhibition greater than or equal to 300 cm.2 The evidence on other unhealthy commodities and PAHO's guidance indicate that the full set of warning labels (i.e. when the product is excessive in all nutrients) should occupy at least 30% of the surface of the main display panel.6,20 This means if a product is required to apply all five warning labels in Mexico (e.g. the product content is above the thresholds for calories and all nutrients), the labels together should occupy at least 30% of the main panel of the exhibition. Although Mexico's legislation requires relatively large warning icons in the region, depending on the size of the main display panel of a product, even if the product has five warning signs, they would still occupy less than 30% of the surface of the main display panel. For instance, a package with a main display panel of 300 cm2 would have only around 17% of this surface occupied by five warning labels. For smaller packages, the proportional occupation of warnings relative to the main panel of exhibition is higher but still below 30%.20 Argentina's policy provides the best size requirements which result in an occupation of at least 30% of the surface of the main face of the product label when such products feature the maximum number of five octagon warning labels.34
Position/location
Argentina, Chile, Mexico, Peru, Uruguay and Venezuela have clear guidelines that mandate all FOPNL labels be presented prominently on the front or on the main face of a product label while Ecuador modified its regulations in 2014 to allow the label to be applied on any part of the package. Based on the experience and evidence accumulated and on PAHO's guidance, countries have been providing more detailed specifications to the location requiring (Argentina, Mexico and Peru) or suggesting (Uruguay) the label to be placed in the upper margin of the main display panel of the package, as this is where consumers tend to focus their attention the most when screening packages at the point of sale.6,20
Criteria/thresholds
Of the seven implemented FOPNL policies, Mexico was the first country to adopt and implement PAHO's Nutrient Profile Model published in 2016, to define the products that should be subject to warning labels.20 Argentina also adopted it recently and started implementing.34 Chile and Ecuador developed their own criteria since the adoption of their legislation preceded the publication of PAHO's Nutrient Profile Model. Uruguay first adopted a decree establishing criteria that aimed at progressively achieving PAHO's guidelines, but modifications were made following recommendations of technicians from the Ministry of Industry moving the criteria away from the PAHO's Nutrient Profile Model.44 The law in Peru required the warnings to be applied following PAHO's recommendations, but the regulation issued implemented a weakened version of Chile's criteria, which was also the case in Venezuela. Recently a judicial decision has required the Peruvian regulation to update the criteria following PAHO's recommendations.45
Policy implications
Over the past decade important progress in the development and implementation of FOPNL in the AMRO has occurred. Every country in the region has entered the agenda-setting stage, and 30 of the 35 countries have formally introduced FOPNL legislation. Eleven of these countries have adopted FOPNL, which accounts for approximately 60% of the population living in AMRO and 90% of the population of Latin America.46 As of August 2022, seven of these eleven countries have implemented FOPNL.
Some of the success factors that enabled these countries to adopt FOPNL include the evidence presented to policymakers when setting the agenda (both on the health consequences of the lack of an appropriate food labelling and on the efficacy of FOPNL in improving consumers' ability to make healthier decisions).13, 14, 15 This also included arguing the relevance of FOPNL as an instrument to accomplish a population's right to information and to fulfil the right to health and children's right to be protected from harmful products.13, 14, 15 Additional key factors include the openness to policy innovation and sharing of lessons learned between countries.
PAHO's guidance also played an important role in reducing the time taken by countries to adopt policies and select warning label systems that are more effective. For instance, prior to the publication of PAHO's Nutrient Profile Model, countries struggled, especially during the implementation stage, to reach a decision on the criteria to define products to be regulated because they were basing their assessment on a changing and distorted market of processed and ultra-processed products, not on public health parameters. Providing an objective definition based on WHO recommendations PAHO's food classification tool helped countries substantially reduce the time taken to decide on the products that should be regulated as evidenced by the accelerated rate of implementation in Mexico and Argentina, which all have adopted FOPNL warning label systems. This is a clear example of the role intergovernmental agencies have on policy agendas, and avoiding delays in policy implementation, and an important lesson for other global regions trying to execute similar policies. It also highlights the relevance of this resource in achieving policy coherence, as PAHO's Nutrient Profile Model applies to different regulatory policies, such as taxation, marketing restrictions, and regulation of school food environments to reduce the demand for unhealthy food products.8
Countries that have implemented FOPNL and have reached the evaluation stage have the opportunity to discuss the need for improvements to keep up with the evolution of evidence and recommendations generated by regional assistance and best practices. As the policy cycle is not linear, these evaluations may take part in different stages as well, establishing baselines, reviewing new evidence and experience, and tracking the impact of the policy from proximal and shorter-term (e.g. change in consumer behaviour) to more distal and longer-term outcomes (e.g. change in diets and in health outcomes). For example, following best practices and regional recommendations the Argentinian legislation was adopted quickly, requiring precautionary labels for non-sugar sweeteners, and FOPNL was introduced as part of comprehensive legislation that also regulates marketing and the school food environment.13
It is noteworthy that, FOPNL is one of many policy tools for the consumption end of food value chains, that are needed to reshape the food environment to favour healthier diets, along with fiscal policies, marketing restrictions, and school food regulations, among other population approaches to change food demand.8 In this sense, Chile has pioneered the adoption of a comprehensive approach that includes FOPNL as an instrumental component that facilitates a coherent regulation of the marketing and food school environment under the same law.14 Peru and Argentina have also taken the same route, while others have dealt with the different regulations using separate normative instruments.13,31
Although countries who adopted earlier versions of FOPNL had fewer resources and guidance like PAHO's Nutrition Profile Model at their disposal, some have still managed to adjust their regulations to meet the highest available standards to protect public health. For example, Mexico improved its policy from a GDA system to warning labels following the PAHO food classification criteria and further required a precautionary label for non-sugar sweeteners.20 In Peru, the criteria used to require the application of warning labels are also being updated to align with PAHO recommendations.31,45 Given the constant evolution of updating regulations this could eventually lead to further reductions in the presence of ultra-processed food and drink products in diets and food systems regionally.
Since 19 of the 30 countries that have formally introduced FOPNL still have not adopted this policy, future research should explore barriers that exist in preventing further policy diffusion. Given that the food and beverage industry was heavily involved in the development of earlier FOPNL systems (e.g. GDA) that were weaker and ineffective, an analysis of industry political and legal tactics to block, weaken and delay FOPNL is warranted to better understand the barriers that are preventing these best practices from spreading regionally and globally.44 While this study offered the breadth of FOPNL in the region, future research should examine in-depth case studies to better understand the opportunities and challenges faced with approving, implementing, and evaluating FOPNL. To date, the majority of this research has been done in Chile and Uruguay, but more studies are needed to understand the evolving dynamics of FOPNL. Recent FOPNL implementation in Mexico and Argentina could be a good starting point. Finally, future research should examine FOPNL in other regions, most notably Europe, Africa, and Asia, to offer comparisons on the development and assessment of FOPNL.
Limitations
This is an overview of the development and progress of FOPNL in AMRO, and does not seek to give an in-depth analysis of each case study and as such, we do not describe the detailed step-by-step process of FOPNL in each country. Furthermore, policymaking is not linear and generally, stages are iterative therefore the policy cycle model is limited in capturing these details. However, one of the article's strengths is providing the breadth of FOPNL across the region to trace the evolution of FOPNL in AMRO.
Conclusions
The diffusion of FOPNL has gradually spread in AMRO, gaining momentum in the past few years and evolving to align with evidence and PAHO's best-practice policies for FOPNL. Governments still discussing and waiting to implement FOPNL policies should follow such practices to improve the uptake and impact of the policy to help reduce poor nutrition related NCDs in the Americas.
Contributors
EC and AC conceptualized the study. JO and AC collected the raw data and EC and AC prepared the first draft of the manuscript. EC, SH, JO, SRGP, AC and FG contributed to the subsequent drafts of the manuscript and to the revisions of the paper. FG is a staff member of the Pan American Health Organization. The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the decisions or policies of the Pan American Health Organization.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
EC, SH, JO, SRGP, AC and FG have nothing to declare.
Acknowledgements
We thank Carla G. Spinillo and Carlos Felipe Urquizar Rojas for helping with the design of mock-up product image illustrated in the paper.
Funding: This work was supported by the University of Nevada, Reno. The university played no role in the conduct of the research or the preparation of this article.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2022.100400.
Appendix A. Supplementary data
References
- 1.Micha R., Shulkin M.L., Penalvo J.L., et al. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: systematic reviews and meta-analyses from the nutrition and chronic diseases expert group (nutricode) PLoS One. 2017;12 doi: 10.1371/journal.pone.0175149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askari M., Heshmati J., Shahinfar H., et al. Ultra-processed food and the risk of overweight and obesity: a systematic review and meta-analysis of observational studies. Int J Obes. 2020;44:2080–2091. doi: 10.1038/s41366-020-00650-z. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . 2021. Implementing nutrition Labelling Policies: A Review of Contextual Factors.https://www.who.int/publications/i/item/9789240035089 Available at: [Google Scholar]
- 4.Croker H., Packer J., Russell S.J., et al. Front of pack nutritional labelling schemes: a systematic review and meta-analysis of recent evidence relating to objectively measured consumption and purchasing. J Hum Nutr Diet. 2020;33:518–537. doi: 10.1111/jhn.12758. [DOI] [PubMed] [Google Scholar]
- 5.Kanter R., Vanderlee L., Vandevijvere S. Front-of-Package nutrition labelling policy: global progress and future directions. Publ Health Nutr. 2018;21:1399–1408. doi: 10.1017/S1368980018000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan American Health Organization Front-of-Package labeling as a policy tool for the prevention of noncommunicable diseases in the Americas. 2020. https://iris.paho.org/bitstream/handle/10665.2/52740/PAHONMHRF200033_eng.pdf?sequence=6&isAllowed=y Available at:
- 7.Rincón Gallardo Patiño S., Carriedo A., Tolentino-Mayo L., et al. Front-of-Pack warning labels are preferred by parents with low education level in four Latin American countries. World Nutr. 2019;10:11–26. [Google Scholar]
- 8.Pan American Health Organization Nutrient profile model. June 2017. https://iris.paho.org/bitstream/handle/10665.2/18621/9789275118733_eng.pdf?sequence=9&isAllowed=y Available at:
- 9.Cabrera M., Machin L., Arrua A., et al. Nutrition warnings as front-of-pack labels: influence of design features on healthfulness perception and attentional capture. Publ Health Nutr. 2017;20:3360–3371. doi: 10.1017/S136898001700249X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taillie L.S., Reyes M., Colchero M.A., et al. An evaluation of Chile's law of food labeling and advertising on sugar-sweetened beverage purchases from 2015 to 2017: a before-and-after study. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knill C., Tosun J. Policy making. 2008. https://kops.uni-konstanz.de/bitstream/handle/123456789/3885/WorkingPaper2008_01.pdf Available at:
- 12.Pan American Health Organization Plan of action for the elimination of industrially produced trans-fatty acids 2020-2025. 2020. https://iris.paho.org/bitstream/handle/10665.2/51965/PlanofAction-ELIMINATE-IPTFA-EN.pdf Available at:
- 13.Ministerio de Salud de Argentina Encuesta Nacional De Nutrición Y Salud - Indicadores Priorizados. 2021. Available at: https://bancos.salud.gob.ar/recurso/2deg-encuesta-nacional-de-nutricion-y-salud-indicadores-priorizados.
- 14.Food and Agriculture Organization Approval of a new food act in Chile: process summary. 2017. https://www.paho.org/chi/dmdocuments/Approval%20of%20a%20new%20food%20act%20in%20Chile.pdf Available at:
- 15.de Salud de Costa Rica Ministerio. Primer censo escolar peso - talla. 2016. https://www.mep.go.cr/sites/default/files/page/adjuntos/informe-ejecutivo-censo-escolar-peso-cortofinal.pdf Available at:
- 16.de Salud de Ecuador Ministerio. Reglamento sanitario de etiquetado de alimentos procesados para El consumo humano. 2014. https://www.controlsanitario.gob.ec/wp-content/uploads/downloads/2014/08/REGLAMENTO-SANITARIO-DE-ETIQUETADO-DE-ALIMENTOS-PROCESADOS-PARA-EL-CONSUMO-HUMANO-junio-2014.pdf Available at:
- 17.La Asamblea Legislativa de Bolivia . 2016. Ley De Promoción De Alimentación Saludable.https://www.paho.org/bol/dmdocuments/Ley775gaceta.pdf Available at: [Google Scholar]
- 18.Instituto de Nutricion y Tecnologia de Alimentos Estudio sobre evaluación de mensajes de advertencia de nutrientes críticos en El rotulado de alimentos. 2012. https://www.ciperchile.cl/pdfs/2014/11/alimentos/INFORME-FINAL-MENSAJES-INTA.pdf Available at:
- 19.La Asamblea Legislativa de Argentina . 2021. Ley 27642: Promoción De La Alimentación Saludable.https://www.boletinoficial.gob.ar/detalleAviso/primera/252728/20211112?busqueda=2 Available at: [Google Scholar]
- 20.Secretaria de Economia de Mexico Modificación a La norma oficial mexicana nom-051-scfi/ssa1-2010, especificaciones generales de etiquetado para alimentos Y bebidas No alcohólicas preenvasados-información comercial Y sanitaria. 2020. http://dof.gob.mx/2020/SEECO/NOM_051.pdf Available at:
- 21.Agência Nacional de Vigilância Sanitária Relatório de análise de impacto regulatório sobre rotulagem nutricional. 2019. http://antigo.anvisa.gov.br/documents/10181/3882585/%281%29Relat%C3%B3rio+de+An%C3%A1lise+de+Impacto+Regulat%C3%B3rio+sobre+Rotulagem+Nutricional/3e2c2728-b55a-4296-b5af-6c7960fd6efa Available at:
- 22.Basto-Abreu A., Torres-Alvarez R., Reyes-Sanchez F., et al. Predicting obesity reduction after implementing warning labels in Mexico: a modeling study. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cruz-Casarrubias C., Tolentino-Mayo L., Vandevijvere S., et al. Estimated effects of the implementation of the Mexican warning labels regulation on the use of health and nutrition claims on packaged foods. Int J Behav Nutr Phys Activ. 2021;18:76. doi: 10.1186/s12966-021-01148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.La Asamblea Legislativa de la República de Costa Rica Ley de etiquetado frontal de alimentos Y bebidas No alcohólicas, para facilitar La comprensión sobre El contenido de ingredientes que, pro su consumo excesivo, representen riesgos para La salud de las personas. San jose, Costa Rica. 2020. https://d1qqtien6gys07.cloudfront.net/wp-content/uploads/2021/03/22065.pdf Available at:
- 25.Health Canada Consultation document. Toward front-of-package nutrition labels for Canadians. 2016. https://www.canada.ca/en/health-canada/programs/front-of-package-nutrition-labelling/consultation-document.html Available at:
- 26.Canada Gazette . Canada Gazette; 2022. Regulations Amending the Food and Drug Regulations (nutrition Symbols, Other Labelling Provisions, Vitamin D and Hydrogenated Fats or Oils): SOR/2022-168.https://canadagazette.gc.ca/rp-pr/p2/2022/2022-07-20/html/sor-dors168-eng.html Part II, Volume 156, Number 15, June 2022. Available at: [Google Scholar]
- 27.Ministerio de Salud de Uruguay, Decreto Rotulado Alimentos Envasados 2018. https://www.gub.uy/ministerio-salud-publica/sites/ministerio-salud-publica/files/documentos/publicaciones/msp_decreto_rotulado_alimentos_envasados_0.pdf Available at:
- 28.de Diputados de Bolivia Cámara. 2016. Ley N. 775: Se Establece Lineamientos Y Mecanismos Para Promover Hábitos Alimentarios Saludables En La Población Boliviana, a Fin De Prevenir Las Enfermedades Crónicas Relacionadas Con La Dieta.https://www.diputados.bo/leyes/ley-n%C2%B0-775 Available at: [Google Scholar]
- 29.El Congreso de Colombia . 2021. Ley 2120 De 2021: Por Medio De La Cual Se Adoptan Medidas Para Fomentar Entornos Alimentarios Saludables Y Prevenir Enfermedades No Transmisibles Y Se Adoptan Otras Disposiciones.http://www.secretariasenado.gov.co/senado/basedoc/ley_2120_2021.html Available at: [Google Scholar]
- 30.de Salud Pública de Ecuador Ministerio. 2014. Reglamento De Etiquetado De Alimentos Procesados Para Consumo Humano.https://www.gob.ec/sites/default/files/regulations/2018-11/00005103.pdf Available at: [Google Scholar]
- 31.El Congreso de Peru . 2013. Ley N. 30021: Ley De Promoción De La Alimentación Saludable Para Niños, Niñas Y Adolescentes.https://www2.congreso.gob.pe/sicr/tradocestproc/Expvirt_2011.nsf/visbusqptramdoc/01038?opendocument Available at: [Google Scholar]
- 32.Gaceta Oficial de la República Bolivariana de Venezuela . Gaceta Oficial de la República Bolivariana de Venezuela No 41804; Caracas: 2020. Ministerio del Poder Popular para la Salud. Resolución No 011. [Google Scholar]
- 33.Gaceta Oficial de la República Bolivariana de Venezuela . 2021. Ministerio del Poder Popular para la Salud. Resolución No 137. Caracas: Gaceta Oficial de la República Bolivariana de Venezuela No 42271. [Google Scholar]
- 34.Poder Ejecutivo Nacional . 2022. Decreto 151/2022. Apruébase la Reglamentación de la Ley N° 27.642.https://www.boletinoficial.gob.ar/detalleAviso/primera/259690/20220323 Available at: [Google Scholar]
- 35.de Salud de Chile Ministerio. 2019. Evaluación Ley De Alimentos N. 20.606.https://www.minsal.cl/wp-content/uploads/2019/08/EVALUACION-LEY-DE-ALIMENTOS_julio-2019_02.pdf Available at: [Google Scholar]
- 36.Pan American Health Organization . 2021. Cumplimiento De La Normativa De Inclusión De Advertencias Publicitarias En Envases De Productos Alimenticios En El Perú.https://iris.paho.org/bitstream/handle/10665.2/54421/OPSNMHRF210011_spa.pdf?sequence=5&isAllowed=y Available at: [Google Scholar]
- 37.Saavedra-Garcia L., Meza-Hernandez M., Hernández-Vazquez A., et al. Oferta Y publicidad de alimentos Y bebidas en instituciones educativas Y entornos escolares de Lima metropolitana. Un estudio exploratorio. Rev Peruana Med. 2020;37:372–378. doi: 10.17843/rpmesp.2020.374.5838. [DOI] [PubMed] [Google Scholar]
- 38.El Poder del Consumidor . 2021. Con 56% De Productos Envasados Reformulados, El Etiquetado Frontal De Advertencia Muestra Que Funciona.https://elpoderdelconsumidor.org/wp-content/uploads/2021/12/b-2112-etiquetado-reformulacion-d-productos-vf.pdf Available at: [Google Scholar]
- 39.Freire W.B., Waters W.F., Rivas-Marino G., et al. A qualitative study of consumer perceptions and use of traffic light food labelling in Ecuador. Publ Health Nutr. 2017;20:805–813. doi: 10.1017/S1368980016002457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandoval L.A., Carpio C.E., Sanchez-Plata M. The effect of 'traffic-light' nutritional labelling in carbonated soft drink purchases in Ecuador. PLoS One. 2019;14 doi: 10.1371/journal.pone.0222866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taillie L.S., Bercholz M., Popkin B., et al. Changes in food purchases after the Chilean policies on food labelling, marketing, and sales in schools: a before and after study. Lancet Planet Health. 2021;5:e526–e533. doi: 10.1016/S2542-5196(21)00172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mediano Stoltze F., Reyes M., Smith T.L., et al. Prevalence of child-directed marketing on breakfast cereal packages before and after Chile's food marketing law: a pre- and post-quantitative content analysis. Int J Environ Res Publ Health. 2019;16 doi: 10.3390/ijerph16224501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ares G., Antunez L., Curutchet M.R., et al. Immediate effects of the implementation of nutritional warnings in Uruguay: awareness, self-reported use and increased understanding. Publ Health Nutr. 2021;24:364–375. doi: 10.1017/S1368980020002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ares G., Antunez L., Cabrera M., et al. Analysis of the policy process for the implementation of nutritional warning labels in Uruguay. Publ Health Nutr. 2021;24:5927–5940. doi: 10.1017/S1368980021002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Salud Ministerio. 2022. República del Perú. Resolución Ministerial N° 526-2022-MINSA.https://www.gob.pe/institucion/minsa/normas-legales/3281514-526-2022-minsa Available at: [Google Scholar]
- 46.United Nations . 2022. World Population Prospects 2022: Summary of Results.https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf Available at: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.