Summary
Background
Essential newborn care (ENC) covers optimal breastfeeding, thermal care, and hygienic cord care. These practices are fundamental to save newborn lives. Despite neonatal mortality remaining high in some parts of Peru, no comprehensive data on ENC is available. We sought to estimate the prevalence of ENC and assess differences between facility and home births in the remote Peruvian Amazon.
Methods
We used baseline data from a household census of rural communities of three districts in Loreto region, collected as part of the evaluation of a maternal-neonatal health (MNH) programme. Women between 15 and 49 years with a live birth in the last 12 months were invited to complete a questionnaire about MNH-related care and ENC. Prevalence of ENC was calculated for all births and disaggregated by place of birth. Adjusted prevalence differences (PD) were post-estimated from logistic regression models on the effect of place of birth on ENC.
Findings
All 79 rural communities with a population of 14,474 were censused. Among 324 (>99%) women interviewed, 70% gave birth at home, most (93%) without skilled birth assistance. Among all births, prevalence was lowest for immediate skin-to-skin contact (24%), colostrum feeding (47%), and early breastfeeding (64%). ENC was consistently lower in home compared to facility births. After adjusting for confounders, largest PD were found for immediate skin-to-skin contact (50% [95% CI: 38–62]), colostrum feeding (26% [16–36]), and clean cord care (23% [14–32]). ENC prevalence in facilities ranged between 58 and 93%; delayed bathing was lower compared to home births (−19% [−31 to −7]).
Interpretation
Low prevalence of ENC practices among home births in a setting with high neonatal mortality and difficult access to quality care in facilities suggests potential for a community-based intervention to promote ENC practices at home, along with promotion of healthcare seeking and simultaneous strengthening of routine facility care.
Funding
Grand Challenges Canada and Peruvian National Council of Science, Technology, and Technology Innovation.
Keywords: Essential newborn care, Prevalence, Peru, Indigenous, Amazon, Maternal-neonatal health, Thermal care, Cord care, Breastfeeding
Research in context.
Evidence before this study
Despite improvements on a national level in the last decades, neonatal mortality remains unacceptably high in some parts of Peru. Essential newborn care (ENC) is cost-effective, easy to implement, and fundamental to save newborn lives. After a comprehensive review of the literature (see Supplementary Material 1 for details), we did not identify any study that assessed a complete set of ENC indicators in Peru. The annual Peruvian Demographic and Health Survey (DHS) does not include a dedicated newborn care module and only provides estimates on early breastfeeding, skin-to-skin contact, and exclusive breastfeeding.
Added value of this study
To the best of our knowledge, this is the first study in Peru that provides prevalence estimates for a wide range of ENC indicators. We identified ENC with the lowest prevalence, disaggregated estimates by place of birth, and provided most accurate prevalence differences for ENC between home and facility births, using an effect model. Our findings help to determine the unmet need and develop context-specific strategies to improve newborn care in an underserved and hard-to-reach indigenous population with one of the highest neonatal mortality rates in the country.
Implications of all the available evidence
We found a large unmet need to improve key ENC practices, most prominently skin-to-skin contact, colostrum feeding, and early breastfeeding. Prevalence of ENC was consistently lower in home births compared to facility births and differences were unrelated to women's characteristics. ENC was also far from universal in facility births, calling for a context-specific intervention to improve birth outcomes in home and facility births alike. Our findings should encourage other subnational investigations in Latin America in similar indigenous settings with high neonatal mortality and difficult access to high-quality facility care to ensure that ENC is received by every newborn.
Introduction
Worldwide, 5.2 million children under the age of five die per year and of those, almost half are neonates.1 Most neonatal deaths are preventable; leading causes worldwide include prematurity, intrapartum-related events, and infections.2 Alongside the Millennium Development Goals (MDG) defined two decades ago, a large evidence base for effective interventions across the continuum of care to reduce neonatal mortality (NM) was established.3 Increasing access to antenatal care (ANC), skilled birth attendance (SBA), and early postnatal care in facilities was considered fundamental.4,5 Community-based delivery of care through Community Health Workers (CHW) or women's groups presented complementary approaches, assessed as being particularly effective in settings with high NM, low facility births, and weak health systems.6,7 While coverage of facility-based care indicators improved towards the end of the MDG era in some countries, NM reductions lagged behind.5 It was recognised that sustainable reductions in NM can only be achieved with simultaneous improvements in quality of care.8, 9, 10
Essential newborn care (ENC) is a set of preventative hygienic cord care, thermal care, and optimal breastfeeding practices that are implemented immediately and early after birth. These practices have been directly linked to reductions in NM and other benefits for the newborn and mother (see Panel 1). ENC practices are highly cost-effective, easy to implement and form an essential element of the Every Newborn Action Plan to achieve the Sustainable Development Goals.3,8 The World Health Organization (WHO) recommends ENC to be received by every newborn in any setting, at home or in facilities.11, 12, 13
Panel 1.
ENC practices: Overview of evidence on impact and mechanisms
| Concept | Indicator | Study | Impact on mortality and morbidity | Mechanisms and other benefits |
|---|---|---|---|---|
| Thermal care | Immediate drying | Lee 2011.14 Delphi panel on newborn assessment and stimulation (warming, drying, rubbing the back or flicking soles) | Reduction intrapartum NM: 10% Reduction NM preterm births: 10% |
Thermal regulation and prevention of hypothermia caused by heath loss through evaporation of amniotic fluid,15 stimulation and simple resuscitation of babies who do not breathe16 |
| Immediate skin-to-skin contact (SSC) | Moore 2017.17 Systematic review on RCTs comparing immediate (<10 min) or early SSC (10 min–24 h) vs usual hospital care, all settings. | Breastfeeding at 1–4 months: RR 1.24 (GRADE: moderate) Exclusive breastfeeding at 1 month: RR 1.30 (GRADE: moderate) |
Thermal regulation through conduction of mother’s body heat,15,18 regulation of cardiorespiratory parameters and stress levels (heart rate, cortisol, oxytocin),18,19 improvement of mother-infant interaction/ bonding18,19 | |
| Bathing delayed for at least 1 day | - | No evidence found | Maintaining vernix coating to avoid infection, prevention of hypothermia due to evaporation of water15 | |
| Hygienic cord care | Clean cord tying | Blencowe 2011.20 Delphi panel on clean birth practices (Handwashing, clean delivery surfaces, clean cord cutting/tying, hygienic cord care) and clean postnatal care practices (clean cord care) | Reduction NM sepsis: 5–60% Reduction NM tetanus: 5–80% |
Prevention of infections at the cord stump site due to increased hygiene21 |
| Clean cord cutting | ||||
| Clean cord care | Reduction NM sepsis: 10–60% Reduction NM tetanus: 5–70% |
|||
| Optimal breast-feeding | Colostrum feeding | - | No evidence found | Rich source of nutrients, anti-microbial and immune-stimulating agents, and muscular-skeletal repair and growth factors22 |
| Breastfeeding started ≤1 h after birth (EBF) | Smith 2017.23 Systematic review on breastfeeding initiation time and mortality and morbidity, all settings. | Late (2–23 h) vs early (≤1 h) breastfeeding: Increase all-cause NM: RR 1.33 (GRADE: moderate) | Exposure to colostrum and reduction of hypothermia through body contact with mother.23 | |
| Exclusive breastfeeding first 3 days | Sankar 2015.24 Systematic review on optimal breastfeeding practices, LMIC | Predominant, partial, no vs exclusive BF: Increase IM (0–5 months): RR 1.48, 2.84, 14.4 (GRADE: very low - low) | Reduction of risk of infection including for diarrhoea, pneumonia, neonatal sepsis, measles or malaria24 |
Overview based on a narrative review of key systematic and narrative reviews on impact and mechanisms of ENC practices.
Abbreviations: BF, breastfeeding; EBF, early breastfeeding; GRADE, Grading of Recommendations, Assessment, Development and Evaluations quality of evidence grading tool; IF, all-cause infant mortality 0–5 months; NM, all-cause neonatal mortality; RCT, Randomized controlled trial; RR, risk ratio; SSC, skin-to-skin contact.
Despite this, important coverage gaps persist, especially in low-middle income (LMIC) countries. Pagel et al.25 conducted a secondary analysis of data from control arms of four large, cluster-randomized controlled trials in rural Bangladesh, Eastern India, and Nepal with study areas characterized by poor maternal education and high (>75%) proportion of home births. While coverage gaps in ENC were largest among unskilled home births, they also found that provision of ENC was not universal in facility births. This suggests that expanding access to institutional births does not necessarily guarantee full access to WHO recommended ENC, and thus important opportunities to reduce mortality and morbidity might be missed.9,26 Likewise, promotion of ENC for births at home might offer a large scope for improvement and survival gains, especially in settings with high NM and weak health systems.3 To scope the need and develop context-specific strategies however, it is fundamental to understand the prevalence of ENC disaggregated by place of birth, data which is often unavailable.27
In Peru, remarkable progress has been made in neonatal health during the last decades. From 2000 to 2020, NM was reduced by more than half from 16 to 7 per 1000 live births.28 Success factors included expansion of health insurance and policies to improve service delivery in a context of political stability and economic growth.29,30 However, despite improvements on a national level, not all regions have benefited equally by this progress. Large inequalities still persist between rural and urban areas, especially in the Amazon region that has a population with a large ethnic diversity scattered in vast rural areas.30,31 Availability of regional data is poor; based on the 2010–2012 DHS datasets, the jungle region of Loreto had a NM of 19 per 1000 live births,30 with 55% under-registration of neonatal deaths.32
While Peru has a continuous DHS survey producing yearly population-based health data, it does not cover the full set of ENC indicators.33,34 Available indicators, including early breastfeeding and skin-to-skin contact, do not correlate with other immediate ENC practices and therefore cannot be used as proxy.35,36 A comprehensive literature review (see Supplementary Material 1) did not identify any other Peruvian study assessing a complete set of ENC indicators; most studies only reported estimates on early initiation of breastfeeding or skin-to-skin contact, most frequently from hospital settings.37, 38, 39, 40, 41, 42, 43, 44, 45, 46
Responding to the important knowledge gap around ENC in Peru, we use baseline programme evaluation data from a household census in rural areas of three underserved districts in Loreto to estimate the prevalence of ENC. We further set out to assess differences in ENC by place of birth to determine the unmet needs for improvement and to aid development of context-specific intervention strategies for facility and home births.
Methods
Study design & participants
This study is a secondary analysis of household census data conducted during the evaluation of a maternal and neonatal health programme called Mamás del Río (MDR).47,48 The area of programme implementation included all 79 rural river-bound communities in the districts Nauta, Parinari, and Saquena. Only rural communities were included as needs were anticipated to be more pronounced as opposed to urban towns. To gain an understanding of the prevalence of ENC, data from the baseline census conducted before programme implementation was used. As part of the census, questionnaires were administered to women between 15 and 49 years who had a singleton live birth in the last 12 months prior to census date. For the current analysis, women with caesarean births and women who gave birth during transit to a facility were excluded since likely receiving different care and therefore less comparable to the rest of the women. Ethical approval was received by the London School of Hygiene & Tropical Medicine (ID: 100419) and the Universidad Peruana Cayetano Heredia (ID: 16071).
Setting and programmatic background
A detailed description of the study setting has been reported previously.49 Briefly, the region of Loreto is located in the north of Peru within the Amazon basin, with about a third of its population living in remote rural areas. Loreto is one of the regions with the poorest maternal-neonatal health (MNH) indicators in Peru30,32; in some rural communities 80% of women give birth at home,50 basic infrastructure and hygienic conditions are poor,51 and infections are a leading cause of neonatal death.52
The MDR programme aims to improve ENC and healthcare seeking in rural areas of three districts (Nauta, Parinari, and Saquena) of Loreto. These are inhabited predominantly by native communities of Kokama-Kokamilla ethnicity, which are dispersed along the Amazon River tributaries within dense tropical rainforest. The people's main occupation is subsistence farming and fishing; most live in extreme poverty with a monthly income of less than 50 USD.53 Running water, electricity, and sanitation are mostly lacking. The study area comprises of 18 health posts and three health centres where medical care is free through a basic insurance scheme (Seguro Integral de Salud). All facilities provide basic routine antenatal and postnatal care as well as attendance of imminent births. Emergency obstetric care is only available at health centres and the regional hospital, located in the capital of Iquitos outside the study area. Health facilities, and in particular health posts, are often poorly staffed and lack basic infrastructure and supplies.49 Perceptions of mistreatment and inadequate facility care among women are common.51 Transport to facilities via motorized canoe is expensive and time-consuming due to long distances and slow travel speed. Travel by night or during high river levels is dangerous and often avoided.
Data collection
All communities within the programme implementation area were eligible for the census. Prior permission for the census was obtained from community authorities who then announced activities to foster participation among inhabitants. Three interview teams were deployed, with three female interviewers and one supervisor each. All had health-related degrees, underwent a 4-day classroom and field training. Interview teams used draft maps of communities based on publicly available data,54 which were updated as required after systematic numbering of each inhabited house in clockwise direction from the landing point.
House-to-house enumeration consisted of identification of the head of household or next person in charge over 18 years, who was asked to list all persons usually residing in the household, their family relationships, and their ages. Households were revisited twice to maximise encounters. Eligibility for participation in questionnaires was ascertained directly from women, and if eligible, the purpose of the questionnaire was explained and written informed consent testified by an independent witness was obtained. Electronic questionnaires were administered using mobile devices and covered women's characteristics, their healthcare seeking and ENC during pregnancy, birth, and after birth as well as household characteristics.
ENC indicators
Nine ENC indicators were selected as outcomes for this study which were assessed by questionnaires in Spanish language through maternal self-report (see Panel 1). To minimize measurement error, comprehension of questions was confirmed using concurrent probing during face-to-face interviews and subsequent cultural adaptation of questions, based on a sample of n = 22 women from adjacent communities that was required to reach saturation (unpublished). The final ENC module of the questionnaire in English, operational definitions, and response options required for classification of care received or not received for each indicator can be found in Supplementary Material 2. Cord tying and cutting practices were collected for home births only due to known difficulties in recall for mothers who give birth in facilities.55
Definition of variables
All variables, except for distance to nearest facility, were ascertained using maternal self-report. Place of birth was defined by the location (at a health facility or at home), type of birth attendant was defined by level of training (skilled health staff, traditional birth attendants with practical experience, or family members or other lay persons), and persons physically present during birth included previously mentioned persons plus the women's husband/partner and the newborn's godparent. Education was collapsed into two levels to avoid small groups sizes; higher education includes any education beyond secondary school and university degrees. Monthly household income was categorized into three levels according to poverty levels for the rural jungle region in Peru at the time of data collection.53 ANC contacts were coded into four levels (≥8, 6–7, 1–5, and 0), reflecting WHO recommendations, Peruvian recommendations, insufficient contact, and no contact, respectively. Travel distance via river in kilometres from the participant's community to the nearest health centre was estimated based on Geographic Positioning System (GPS) coordinates using ArcGIS 10.5 (Environmental Systems Research Institute, Redlands, California) and recoded into percentiles.
Conceptual framework
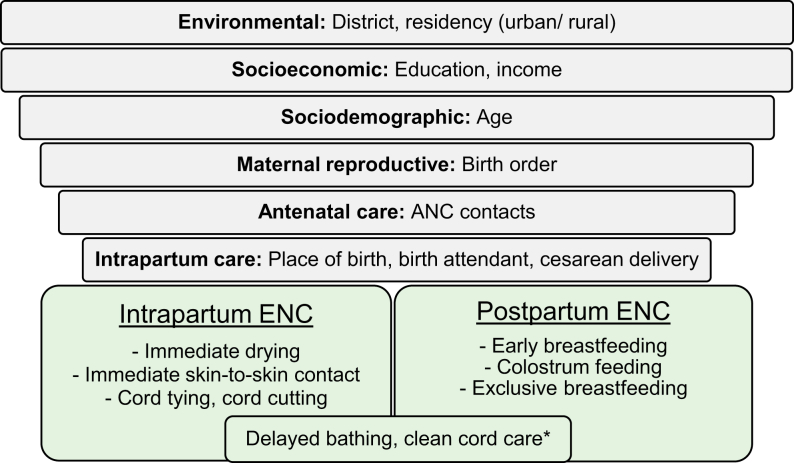
Newborn care practices are embedded within the larger social, cultural, economic, and political context and are determined by a complex, multilevel network of different actors.56,57 While birth attendants are usually the direct implementers of practices during the intrapartum-period, such as drying, cord cutting, and newborn placement; mothers often have more agency over practices such as breastfeeding and cord care in the postpartum period, especially those with previous childbirth experience and during home births.58, 59, 60 Other relevant setting-specific actors, such as the person who traditionally becomes the godparent after cutting the cord in the Peruvian Amazon51 or other decision makers, might further influence ENC practices, directly or indirectly. Women's education level, age, ANC contacts, district, birth order and type of birth attendant were determinants of ENC most consistently identified in a review of quantitative studies (Fig. 1).61, 62, 63, 64, 65, 66, 67, 68, 69
Fig. 1.
Conceptual hierarchical framework of determinants of ENC practices. Figure shows independent determinants (grey bars) of ENC practices (green boxes) identified from international literature. Determinants are ordered in a hierarchical way to depict their causal proximity to the outcomes in a simplified way. The largest grey bar on top presents determinants expected to be most distantly related while the smallest grey bar presents those that are expected to be most proximal related to ENC practices. For the purpose of this framework, intrapartum period is defined as the period immediately after birth, while postpartum period is defined from the first hour to the first week after birth. ∗Practices corresponding to both intrapartum and postpartum periods.
Statistical analysis
We cross-tabulated women's characteristics for all births and stratified by place of birth. We calculated column percentages and used Pearson's chi-squared test to assess differences between home and facility births. Prevalence of ENC overall and by place of birth was calculated as the proportion of women who received each care practice among all women and among women giving birth at a facility or at home, respectively.
In cross-sectional studies, odds ratios are difficult to interpret when outcomes are frequent.70 Use of prevalence ratios or prevalence differences (PD) as absolute effect measures are therefore preferable. To estimate adjusted PD between facility and home births, we first built separate logistic regression models for each ENC outcome. We then used a post-estimation approach71, 72, 73 to transform odds ratios obtained by logistic models to PD. We calculated 95% confidence intervals (95% CI) for PD based on linearized standard errors and used a linear test of equivalence for significance testing.71 Further details on the statistical procedures can be found in Supplementary Material 3.
Separate logistic regression models were built examining the effect of the main exposure place of birth on each ENC outcome, accounting for clustering of observations at the community level. We considered mother's age, education, and district of residence as a priori confounders and included them as covariates in all models. Apart from their association with ENC, there is ample evidence for their effect on facility use for births.74 We used a stepwise forward selection approach to examine other potential confounders for each model, which were retained if they resulted in a change-in-estimate of ≥10%, commonly considered as an important cut-off.75,76 After fitting all models, only ethnicity was added as an additional covariate for the models of delayed bathing and early breastfeeding (see Supplementary Material 4 for details of covariate selection procedure). All analyses were conducted using Stata 14.2 (Stata Corp, College Station, Texas).
Role of the funding source
This manuscript has been prepared as part of the implementation and evaluation of the Mamás del Río programme, funded by the Peruvian National Council of Science, Technology, and Technology Innovation, Grant Number 135-2016 and Grand Challenges Canada, Grant Number 0816-05. The funders had no role in study design, data collection and analysis, decision to publish, preparation of the manuscript, or any other aspect pertinent to the study.
Results
Census
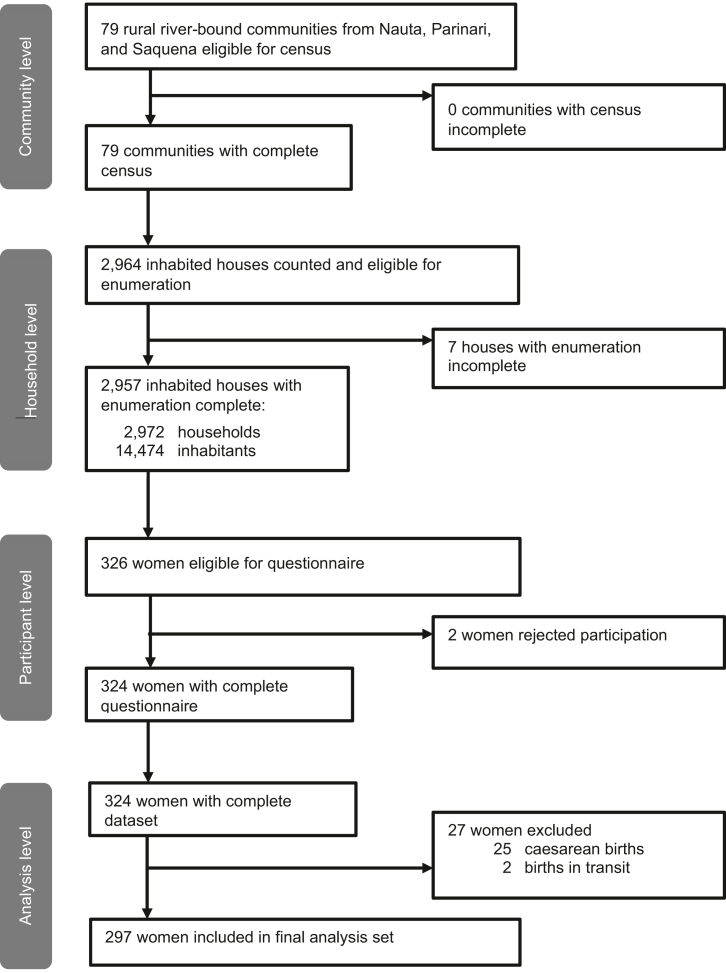
The census was conducted in all 79 communities eligible for participation between November 2018 and January 2019 (Fig. 2). Enumeration was complete in 2957 (>99%) of all inhabited houses, covering a population of 14,474 with a very young age distribution (44% <15 years). Out of 326 eligible women with a live birth in the last 12 months, 324 (>99%) completed the questionnaire and 297 were included in the final dataset, after 25 women with caesarean births and two women with births during transit were excluded.
Fig. 2.
Flowchart of study participants.
Characteristics of women
Most women were between 20 and 34 years of age; about half had primary education only and were living in extreme poverty (Table 1). About half (47%) considered themselves as pertaining to Kokama-Kokamilla ethnicity. Only 26% of women had eight or more ANC contacts as recommended by WHO and 37% had five or more children. More than two thirds of women (70%) gave birth at home. Among those, SBA was very low (7%) as most home births were attended by Traditional birth attendants (TBAs, 63%). Among facility births, only 19% of women gave birth in a hospital. Women's partners, godparents, and other family members were significantly less often present during facility births compared to home births (p ≤ 0.001 for all, except for godparent p < 0.015, Table 1).
Table 1.
Characteristics of women between 15 and 49 years with a live birth in the last 12 months, overall and by facility and home births.
| Variable | All births (n = 297) |
Facility birth (n = 89) |
Home birth (n = 208) |
p-value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| District | |||||||
| Nauta | 186 | 63 | 43 | 48 | 143 | 69 | 0.066 |
| Parinari | 61 | 21 | 25 | 28 | 36 | 17 | |
| Saquena | 50 | 17 | 21 | 24 | 29 | 14 | |
| Age | |||||||
| 15–19 | 50 | 17 | 14 | 16 | 36 | 17 | 0.556 |
| 20–34 | 184 | 62 | 59 | 66 | 125 | 60 | |
| 35–49 | 63 | 21 | 16 | 18 | 47 | 23 | |
| Highest educationa | |||||||
| No education/primary education | 157 | 53 | 39 | 44 | 118 | 57 | 0.063 |
| Secondary/higher education | 138 | 47 | 49 | 56 | 89 | 43 | |
| Household incomeb | |||||||
| <S/150 extreme poverty | 153 | 52 | 44 | 50 | 109 | 53 | 0.039 |
| S/151–250 poverty | 84 | 29 | 19 | 22 | 65 | 31 | |
| >S/250 above poverty line | 58 | 20 | 25 | 28 | 33 | 16 | |
| Ethnicity | |||||||
| Kokama/Kokamilla | 135 | 47 | 35 | 41 | 100 | 50 | 0.207 |
| Mixed-race | 120 | 42 | 42 | 49 | 78 | 39 | |
| Other/None | 32 | 11 | 8 | 9 | 24 | 12 | |
| Birth order | |||||||
| 1 | 42 | 14 | 15 | 17 | 27 | 13 | 0.147 |
| 2–4 | 146 | 49 | 49 | 55 | 97 | 47 | |
| ≥5 | 109 | 37 | 25 | 28 | 84 | 40 | |
| Distance to nearest health centre | |||||||
| ≤0–10 km | 106 | 36 | 43 | 48 | 63 | 30 | 0.052 |
| 11–22 km | 93 | 31 | 23 | 26 | 70 | 34 | |
| 23–51 km | 98 | 33 | 23 | 26 | 75 | 36 | |
| Antenatal care contactsc | |||||||
| 0 | 25 | 9 | 3 | 3 | 22 | 11 | 0.180 |
| 1–5 | 100 | 34 | 30 | 34 | 70 | 34 | |
| 6–7 | 94 | 32 | 33 | 37 | 61 | 30 | |
| ≥8 | 77 | 26 | 23 | 26 | 54 | 26 | |
| Type of facility for birth | |||||||
| Hospital | 17 | 8 | 17 | 19 | – | – | – |
| Health centre | 40 | 14 | 40 | 45 | – | – | |
| Health post | 32 | 11 | 32 | 36 | – | – | |
| Type of birth attendant | |||||||
| Skilled birth attendant | 130 | 44 | 88 | 99 | 15 | 7 | <0.001 |
| Traditional birth attendant | 103 | 35 | 1 | 1 | 129 | 62 | |
| Family member/other lay persons | 64 | 21 | 0 | 0 | 64 | 31 | |
| Persons present during birthd | |||||||
| Traditional birth attendant | 149 | 50 | 3 | 3 | 146 | 70 | <0.001 |
| Health staff | 101 | 34 | 83 | 93 | 18 | 9 | <0.001 |
| Husband/Partner | 163 | 55 | 31 | 35 | 132 | 64 | <0.001 |
| Godparent | 26 | 9 | 2 | 2 | 24 | 12 | 0.015 |
| Other family member | 159 | 54 | 30 | 34 | 129 | 62 | <0.001 |
Two (n = 2) women who responded with “don't know”. Two (n = 2) women had no primary education.
Two (n = 2) women who responded with “don't know”. Currency is Peruvian Soles (PEN, S/), at the time of write-up 1 PEN was 0.27 USD, with categories translating to <41 USD extreme poverty, 41–68 USD poverty, and >68 USD above poverty line.
1 missing values.
Multiple answers possible.
Women giving birth at home had lower income (p = 0.039) and lived further away from a health centre (p = 0.052, Table 2). There was weak evidence that women giving birth at home were less educated (p = 0.063) and living in Nauta compared to other districts (p = 0.066).
Table 2.
Prevalence differences of essential newborn care practices between facility and home births.
| Variable | All births (n = 297) |
Facility birth (n = 89) |
Home birth (n = 208) |
Difference (unadjusted) |
Difference (adjusted)b |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | 95%CI | n | % | 95%CI | n | % | 95%CI | % | 95%CI | p-value | % | 95%CI | p-value | |
| Thermal care | |||||||||||||||
| Immediate drying after birth | 230 | 77 | 71–83 | 76 | 85 | 75–92 | 154 | 74 | 67–80 | 11 | 1–21 | 0.030 | 12 | 3–21 | 0.015 |
| Immediate skin-to-skin contact | 71 | 24 | 19–30 | 52 | 58 | 47–69 | 19 | 9 | 6–14 | 49 | 38–61 | <0.001 | 50 | 38–62 | <0.001 |
| Bathing delayed ≥1 day | 226 | 76 | 71–81 | 60 | 67 | 57–77 | 166 | 80 | 75–84 | −12 | −23 to −2 | 0.025 | −19 | −31 to −7 | 0.003 |
| Cord care | |||||||||||||||
| Clean cord tyinga | – | – | – | – | – | – | 159 | 76 | 69–82 | – | – | – | – | – | – |
| Clean cord cuttinga | – | – | – | – | – | – | 167 | 80 | 72–87 | – | – | – | – | – | – |
| Clean cord care | 195 | 66 | 59–72 | 72 | 81 | 72–88 | 123 | 59 | 52–66 | 22 | 13–31 | <0.001 | 23 | 14–32 | <0.001 |
| Feeding practices | |||||||||||||||
| Colostrum feeding | 139 | 47 | 41–53 | 58 | 65 | 58–72 | 81 | 39 | 31–47 | 26 | 17–36 | <0.001 | 26 | 16–36 | <0.001 |
| Breastfeeding started ≤1 h after birth | 191 | 64 | 58–70 | 65 | 73 | 63–81 | 126 | 61 | 53–68 | 13 | 1–24 | 0.025 | 11 | 0–23 | 0.053 |
| Exclusive breastfeeding for first 3 days | 264 | 89 | 84–92 | 83 | 93 | 86–97 | 181 | 87 | 81–91 | 6 | −1 to 14 | 0.110 | 7 | 0–15 | 0.067 |
Not collected for facility births due to high likelihood for misclassification.
Adjusted for age, education, and district as known confounders in all logistic regression models. Models for delayed bathing and early breastfeeding also adjusted for ethnicity after stepwise-forward selection using a 10% change-in-estimate criterion.
Prevalence of ENC among all births
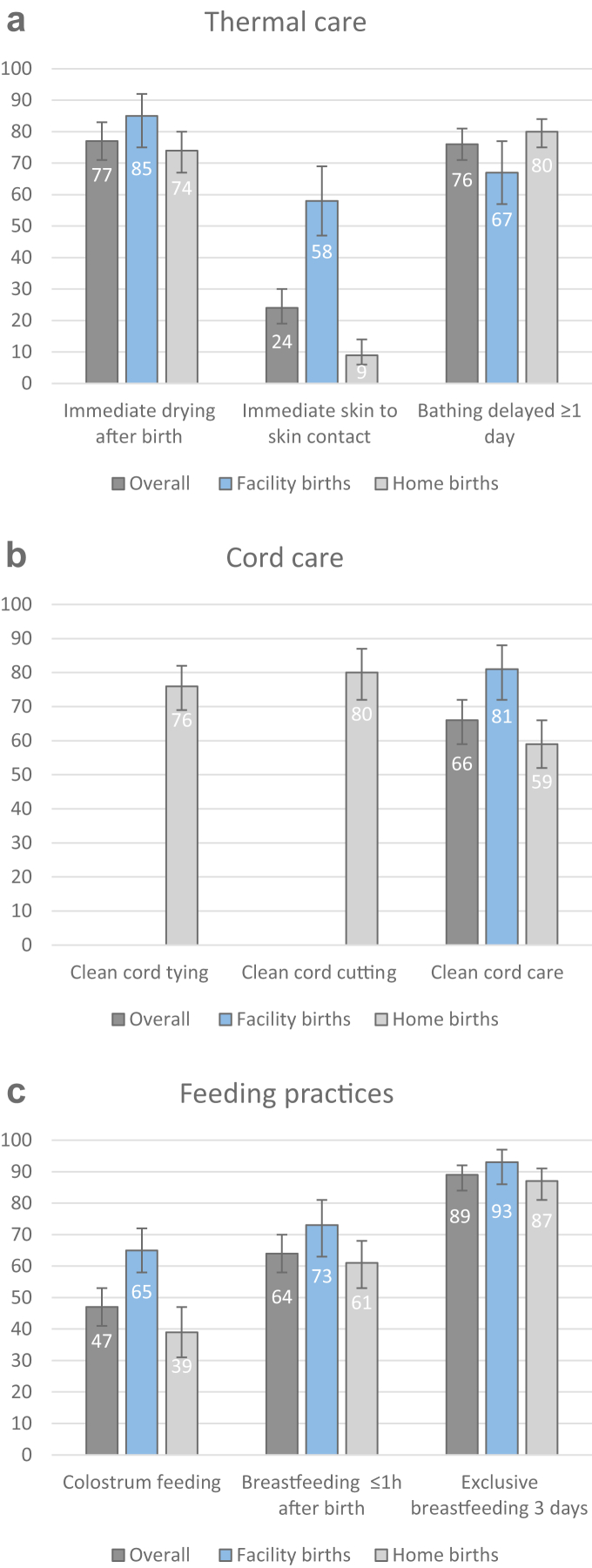
Among all births, fewest women reported immediate skin-to-skin contact (24% [95% CI: 19–30]), colostrum feeding (47% [41–53]), and early breastfeeding (64% [58–70] Fig. 3, Table 2). ENC most frequently practiced included exclusive breastfeeding after birth (89% [84–92]), immediate drying (77% [71–83]), and delayed bathing (76% [71–81]).
Fig. 3.
ENC practices reported by women between 15 and 49 years, for all births and by place of birth, for a) thermal care, b) cord care, and c) feeding practices. Numbers represent percentages, error bars represent corresponding 95% Confidence intervals. Clean cord tying and cord cutting not assessed for facility births and therefore only presented for home births
Prevalence of ENC in home births
Among home births, very few women practiced immediate skin-to-skin contact (9% [6–14]) while other thermal care practices of immediate drying and delayed bathing ranged between 74% [67–80] and 80% [75–84], respectively (Table 2). A similar proportion of women reported clean cord tying and cutting practices while only 59% [52–66] of women practiced clean cord care without application of harmful substances. Only 39% [31–47] of women fed colostrum and 61% [53–68] started breastfeeding early, while most (87% [81–91]) reported exclusive breastfeeding after birth.
Prevalence of ENC in facility births
Among facility births, prevalence of thermal care ranged between 58% [47–69] and 85% [75–92] for immediate skin-to-skin contact and immediate drying, respectively (see Table 2). Most women (81% [72–88]) reported clean cord care. Among feeding practices, colostrum feeding was least frequent (65% [58–72]) while 73% [63–81] of women started breastfeeding early and the majority (93% [86–97]) reported exclusive breastfeeding in the first 3 days after birth.
Prevalence differences
In the unadjusted models, five out of seven ENC practices available for all births were significantly more prevalent in facility births compared to home births (Table 2). Largest differences were seen in immediate skin-to-skin contact (PD: 49% [95% CI: 38–61]), colostrum feeding (PD: 26% [17–36]), and clean cord care (PD: 22% [13–31]). Differences were smaller for early breastfeeding and immediate drying, while no differences were found for exclusive breastfeeding after birth. Delayed bathing was the only practice that was less common in facility births compared to home births (PD: −12% [−23 to −2]).
After adjusting for confounders, the prevalence of immediate drying, immediate skin-to-skin contact, clean cord care, colostrum feeding, and early breastfeeding remained significantly higher in facility compared to home births with no or very little changes in estimates (Table 2). Delayed bathing was still less common in facility births compared to home births; however, with a more pronounced difference after adjusting (PD: −19% [−31 to −7]).
Discussion
Key findings
Most women in our study area give birth at home without skilled birth attendance. ENC prevalence is overall lowest for skin-to-skin contact, colostrum feeding, and early breastfeeding. Home births have a consistently lower prevalence of ENC compared to facility births; immediate skin-to-skin contact, colostrum feeding, and clean cord care are among the indicators with the largest gaps. Despite being recommended by the WHO, easy to implement, and low-cost, coverage of ENC practices is also not universal in facility births. Surprisingly, delayed bathing for at least 1 day has a lower prevalence in facility compared to home births. Our findings highlight the need for context-specific strategies to promote ENC practices among attendants of home and facility births alike.
Home births and ENC
Finding lower prevalence of ENC practices in home compared to facility births was not surprising, given the fact that SBA at home is virtually absent in this setting: most births are attended by TBAs, family members or other lay persons from the community. While TBAs in this region are usually older women with substantial experience in attending births, we are not aware of previous formal training on ENC. Traditional beliefs and practices around childbirth and newborn care are pronounced among indigenous populations in the Amazon setting.51,77,78 During extensive qualitative work that we conducted with community actors in the study area,51 a common belief around colostrum was that it can make the baby sick and throw up. Perceptions about the colostrum being harmful and associated discarding are not uncommon in other rural LMIC settings.27,79 Similarly, skin-to-skin contact has been reported to be rare, especially in home births.27,80 Recent guidance by the WHO defines immediate skin-to-skin contact between the mother and newborns as “ventral-to-ventral skin-to-skin contact, less than 10 min after birth, uninterrupted for at least 60 min.”81 This procedure is usually not part of traditional newborn care.82,83 Indeed, our qualitative work suggests that newborns are usually cleaned and dressed before being handed to the mother,51 impeding timely contact as well as direct skin contact with the mother. Clean cord care that we operationalized as dry care, cleaning with water and soap, or application of alcohol, was also less common in home births compared to facility births. Some of the other substances applied included iodine, oxygenated water or antibacterial creams, as well as powder from the local Pona tree or violet flower leaves. Application of substances is often believed to have curative effects rather than being associated with risk for infections.27,84
Clean cord cutting had a high prevalence among home births, most women stated that the cord was cut with newly bought or boiled scissor, less frequently with a new razor blade. This matches with findings from our qualitative work suggesting scissors being the predominant tool for cord cutting.51 A unique characteristic of our setting is the role of the godparent in cutting the cord, which can result in the baby placed aside until the godparent arrives and then only subsequently cleaned, dried, and dressed.51 We only found small differences in immediate drying between home and facility births, suggesting that the godparent's role in cutting the cord is less important or has less impact on the delay of drying the baby than expected.
Facility births and ENC
Comprehensive newborn care as recommended by the WHO is not received by all newborns – ENC practiced during facility births was far from universal. Facility births, other than home births, are exclusively attended by health staff working in facilities, which we assumed to be “skilled” providers. While no further data on the type of staff was collected due to known difficulties around women's self-reporting of specific cadres,21 we found that one third of facility births took place in health posts. According to national norms, health posts should be minimally staffed with an obstetrician, a nurse, and a nurse technician or assistant to provide routine birth and newborn care.85 In practice, only just above half of health posts in the study area meet these criteria, as many are only staffed with a single nurse technician (unpublished data). Nurse technicians in Peru undergo a 3-year training for their supportive role to health staff; as such they likely do not meet WHO's definition of skilled health personnel for childbirth care86 and therefore cannot be expected to provide comprehensive ENC.
Among facility births, immediate skin-to-skin contact, and colostrum feeding were among the practices with the lowest coverage. In Peru, immediate skin-to-skin contact has been part of the national norm on newborn care since 2012,85 matching WHO's operational definition. While we did not cover health staff in our qualitative investigation and are not aware of any literature on skin-to-skin contact in facilities in Peru, commonly cited barriers from other LMIC settings are staffing and time constraints, interference with clinical routine, and acceptability among mothers.80,87, 88, 89 While early breastfeeding is recommended as part of Peruvian norms85; colostrum feeding is not explicitly mentioned. This, together with prevailing negative beliefs and women's preference of discarding the colostrum, might explain our findings of low proportion of colostrum feeding in facility births.
The finding of lower prevalence of delayed bathing among facility births was somewhat unexpected. While Peruvian guidance recommends immediate drying with a pre-warmed towel,85 dressing of the newborn after finishing uninterrupted and prolonged skin-to-skin contact, and subsequent medical examination; there is no explicit mentioning that bathing should be avoided.90 Consequently, lower prevalence of delayed bathing in facility births could point towards a harmful procedure exclusively practiced by health staff, warranting specific focus during training.
Strengths and limitations of this study
Census-based data collection, along with a participation rate of >99% among eligible women make us confident that our results are representative of women from the rural district of Nauta, Parinari, and Saquena. The Loreto region comprises of 53 districts and is inhabited by 26 different indigenous ethnicities.91 While our study population is slightly younger but otherwise comparable to rural Loreto, our population's access to maternal care is poorer (see comparison with DHS populations in Supplementary Material 5) and the principal ethnicity is Kokama-Kokamilla. Although we think that our findings provide a good indication for other remote Amazonian communities, we cannot claim overall representativeness for rural Loreto or the Peruvian Amazonas.
We undertook a rigorous development of the ENC module in our questionnaire, including testing of comprehension, cultural adaptation of terminology, and pilot testing in the target population. We found that the question on immediate skin-to-skin contact, as per original wording from the Peruvian DHS, had much better understanding after being split up into separate questions. This is consistent with findings by other authors, who could reduce overestimation when formulating immediate skin-to-skin contact as a two-item question.92 This could also explain the discrepancy with the Peruvian DHS from 2019, where the prevalence of immediate skin-to-skin contact was 36% (95% CI: 29–44) for rural Loreto and thus higher compared to our estimate. We adapted the question on early breastfeeding to reflect actual feeding initiation rather than feeding intents, which might have reduced overestimation93 and could also explain the higher prevalence estimates (87%, 95% CI: 81–92) from the Peruvian DHS. While DHS surveys have a recall period of up to 5 years, our census had a recall period of only 1 year, which might have reduced recall error. Nevertheless, ENC indicators are solely based on maternal self-report and as such have important limitations in their validity.92,94, 95, 96, 97 In the most comprehensive validation study of MNH care indicators comparing exit-surveys to birth observations in hospitals in Bangladesh, Nepal, and Tanzania; immediate drying was the only indicator with acceptable accuracy; while skin-to-skin contact, cord care, and early breastfeeding had low validity.97 Recall might be clouded by strenuous birth experience especially for facility births,55,96 sequence and exact timing of events might be misreported especially for longer recall periods resulting in recall error,95,96 and social desirability bias might lead to over-reporting.93 The results of this study therefore need to be interpreted with caution, especially smaller prevalence differences might in fact be attributed to measurement error.
We used an effect modelling approach to minimize confounding of PD estimates of ENC between facility and home births. Based on a thorough review of the literature on determinants of ENC practices and choice of place of birth, we selected three variables for a priori adjustment and also employed a change-in-estimate approach to explore other possible confounders in the data set. Ethnicity was the only additional variable that, after inclusion as covariate, resulted in important changes in effect estimates for early breastfeeding and delayed bathing. Although not statistically associated with the exposure or outcomes, it is plausible to assume that ethnicity mediated through unique customs and beliefs has an effect on newborn care practices, specifically those that are principally determined by the mother and family members. This matches with a secondary analysis of 2018 Peruvian DHS data where authors found that, amongst others, women who ethnically self-identified as “native” had lower odds for early breastfeeding.43
Lastly, small sample sizes did not permit stratification by type of health facility or birth attendant; however, insights from such analyses could produce even further tailored strategies for improving ENC.25,98 Similarly, we excluded women with caesarean births and did not attempt subgroup analysis due to small sample size. However, caesarean section is commonly associated with non-receipt of some ENC practices43,99 and should therefore be explored in future studies.
Implications for intervention
The findings show that there is a large unmet need to improve ENC practices. While the prevalence of ENC is consistently lower among home births, coverage of ENC practices is also not universal in facility births; calling for an intervention that takes into account different actors and processes involved, allowing to improve childbirth outcomes in both home and facility contexts.
Since most women give birth at home, an intervention would require a strong community-based platform targeting TBAs as the direct implementers of intrapartum ENC practices with promotional messages. Mothers, family members, and the wider community that directly or indirectly influence ENC, especially postpartum practices such as bathing, cord care, and exclusive breastfeeding that happen at home even for facility births, will need to be targeted.100 Given the presence of a large but mostly inactive cadre of Community health workers (CHW) with no prior training on ENC in the study area, and existing evidence for effectiveness from other settings,6,7 training of CHW to conduct home visits to women and their family members to promote ENC, in combination with sensitization of TBAs on ENC, is deemed a viable option. To improve ENC in facilities, simultaneous training for health staff is required, with a focus on practices such as colostrum feeding and avoidance of bathing that are currently not explicitly covered in national norms and were found among the lowest.
Our findings also suggest an urgent need to shift from home to facility births; however, we are unsure whether a focus on such an approach would be successful in the short-term and without fundamental, structural changes. Access to facilities in our study area is geographically difficult, time-consuming, and expensive. Apart from the largest health centres, facilities are not equipped to receive women in the early stages of labour and most health posts are understaffed to even provide routine birth care; encouraging women to give birth in such facilities with low capability might be unethical.9 Nevertheless, we agree that increasing access to SBA in facilities is the most effective strategy to avoid both neonatal and maternal deaths and a future intervention should include the promotion of healthcare seeking for antenatal, intra-partum, and post-partum care alongside training of health staff.
Conclusions
Low prevalence of ENC practices among home births in a setting with high neonatal mortality and difficult access to quality care in facilities suggests potential for a community-based intervention to promote ENC practices at home in case facility births are not feasible or desired, accompanied by promotion of healthcare seeking and simultaneous strengthening of routine care in facilities.
Contributors
S.R. conducted the literature search and is the lead author of the manuscript. S.R. and C.R. conceived the study. S.R., M.M.B., M.N., L.H., and C.R. contributed to the study design. S.R. and M.M.B. supervised data collection. S.R. analysed data and M.M.B. verified underlying data. M.M.B. acquired funding for the project. S.R., M.M.B., M.N., L.H., and C.R. critically reviewed and gave final approval for publication.
Data sharing statement
De-identified data is available from the corresponding author upon reasonable request.
Declaration of interests
M.M.B. received support from Grand Challenges Canada to attend conferences on Global Health. M.N. is supported by an award jointly funded by the UK Medical Research Council (MRC) and the UK Foreign, Commonwealth and Development Office (FCDO) under the MRC/FCDO Concordat agreement and is also part of the EDCTP2 programme supported by the European Union: Grant Ref: MR/R010161/1. M.N. is participant and/or independent statistician for PRIZE (Peer support for mental illness in South Africa), Participatory adolescent groups, youth leadership training and livelihood promotion to improve school attendance, dietary diversity and mental health among adolescent girls in rural eastern India, ANRS 12388 PREVENIR-PEV & ANRS 12397 PROMISE EPI, and PVACS (Preventing Violence Against Children in Schools).
Acknowledgments
Funding: Grand Challenges Canada and Peruvian National Council of Science, Technology, and Technology Innovation.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2022.100404.
Appendix A. Supplementary data
References
- 1.United Nations Inter-agency Group for Child Mortality Estimation (UNIGME) Levels and trends in child mortality 2020. https://www.unicef.org/media/79371/file/UN-IGME-child-mortality-report-2020.pdf URL:
- 2.Liu L., Oza S., Hogan D., et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the sustainable development goals. Lancet. 2016;388(10063):3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darmstadt G.L., Bhutta Z.A., Cousens S., et al. Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet. 2005;365(9463):977–988. doi: 10.1016/S0140-6736(05)71088-6. [DOI] [PubMed] [Google Scholar]
- 4.Bhutta Z.A., Das J.K., Bahl R., et al. Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? Lancet. 2014;384(9940):347–370. doi: 10.1016/S0140-6736(14)60792-3. [DOI] [PubMed] [Google Scholar]
- 5.Lawn J.E., Blencowe H., Oza S., et al. Every Newborn: progress, priorities, and potential beyond survival. Lancet. 2014;384(9938):189–205. doi: 10.1016/S0140-6736(14)60496-7. [DOI] [PubMed] [Google Scholar]
- 6.Hanson C., Kujala S., Waiswa P., Marchant T., Schellenberg J. Community-based approaches for neonatal survival: meta-analyses of randomized trial data. Bull World Health Organ. 2017;95(6):453–464C. doi: 10.2471/BLT.16.175844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gogia S., Sachdev H.P. Home-based neonatal care by community health workers for preventing mortality in neonates in low- and middle-income countries: a systematic review. J Perinatol. 2016;36 Suppl 1:S55–S73. doi: 10.1038/jp.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mason E., McDougall L., Lawn J.E., et al. From evidence to action to deliver a healthy start for the next generation. Lancet. 2014;384(9941):455–467. doi: 10.1016/S0140-6736(14)60750-9. [DOI] [PubMed] [Google Scholar]
- 9.Campbell O.M., Calvert C., Testa A., et al. The scale, scope, coverage, and capability of childbirth care. Lancet. 2016;388(10056):2193–2208. doi: 10.1016/S0140-6736(16)31528-8. [DOI] [PubMed] [Google Scholar]
- 10.Grove J., Claeson M., Bryce J., et al. Maternal, newborn, and child health and the sustainable development goals--a call for sustained and improved measurement. Lancet. 2015;386(10003):1511–1514. doi: 10.1016/S0140-6736(15)00517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO) Integrated management of pregnancy and childbirth. Pregnancy, childbirth, postpartum and newborn care: a guide for essential practice. Third edition. 2015. https://apps.who.int/iris/handle/10665/249580 URL: [PubMed]
- 12.World Health Organization (WHO) A global review of the key interventions related to reproductive, maternal, newborn and child health (Rmnch). Geneva, Switzerland: PMNCH. 2014. https://www.who.int/pmnch/knowledge/publications/201112_essential_interventions/en/ URL:
- 13.World Health Organization (WHO) Western Pacific Region Early essential newborn care. First Embrace. A clinical practice pocket guide. 2014. https://apps.who.int/iris/bitstream/handle/10665/208158/9789290616856_eng.pdf URL:
- 14.Lee A.C., Cousens S., Wall S.N., et al. Neonatal resuscitation and immediate newborn assessment and stimulation for the prevention of neonatal deaths: a systematic review, meta-analysis and Delphi estimation of mortality effect. BMC Public Health. 2011;11 Suppl 3:S12. doi: 10.1186/1471-2458-11-S3-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar V., Shearer J.C., Kumar A., Darmstadt G.L. Neonatal hypothermia in low resource settings: a review. J Perinatol. 2009;29(6):401–412. doi: 10.1038/jp.2008.233. [DOI] [PubMed] [Google Scholar]
- 16.Wall S.N., Lee A.C., Niermeyer S., et al. Neonatal resuscitation in low-resource settings: what, who, and how to overcome challenges to scale up? Int J Gynaecol Obstet. 2009;107 Suppl 1:S47–S62. doi: 10.1016/j.ijgo.2009.07.013. S3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore E.R., Bergman N., Anderson G.C., Medley N. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev. 2016;11:CD003519. doi: 10.1002/14651858.CD003519.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta N., Deierl A., Hills E., Banerjee J. Systematic review confirmed the benefits of early skin-to-skin contact but highlighted lack of studies on very and extremely preterm infants. Acta Paediatr. 2021;110(8):2310–2315. doi: 10.1111/apa.15913. [DOI] [PubMed] [Google Scholar]
- 19.Ionio C., Ciuffo G., Landoni M. Parent-Infant skin-to-skin contact and stress regulation: a systematic review of the literature. Int J Environ Res Public Health. 2021;18(9):4695. doi: 10.3390/ijerph18094695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blencowe H., Cousens S., Mullany L.C., et al. Clean birth and postnatal care practices to reduce neonatal deaths from sepsis and tetanus: a systematic review and Delphi estimation of mortality effect. BMC Public Health. 2011;11 Suppl 3:S11. doi: 10.1186/1471-2458-11-S3-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radovich E., Benova L., Penn-Kekana L., Wong K., Campbell O.M.R. 'Who assisted with the delivery of (NAME)?' Issues in estimating skilled birth attendant coverage through population-based surveys and implications for improving global tracking. BMJ Glob Health. 2019;4(2) doi: 10.1136/bmjgh-2018-001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uruakpa F.O., Ismond M.A.H., Akobundu E.N.T. Colostrum and its benefits: a review. Nutr Res. 2002;22(6):755–767. [Google Scholar]
- 23.Smith E.R., Hurt L., Chowdhury R., et al. Delayed breastfeeding initiation and infant survival: a systematic review and meta-analysis. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0180722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sankar M.J., Sinha B., Chowdhury R., et al. Optimal breastfeeding practices and infant and child mortality: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):3–13. doi: 10.1111/apa.13147. [DOI] [PubMed] [Google Scholar]
- 25.Pagel C., Prost A., Hossen M., et al. Is essential newborn care provided by institutions and after home births? Analysis of prospective data from community trials in rural South Asia. BMC Pregnancy Childbirth. 2014;14:99. doi: 10.1186/1471-2393-14-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryce E., Mullany L.C., Khatry S.K., Tielsch J.M., LeClerq S.C., Katz J. Coverage of the WHO's four essential elements of newborn care and their association with neonatal survival in southern Nepal. BMC Pregnancy Childbirth. 2020;20(1):540. doi: 10.1186/s12884-020-03239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bee M., Shiroor A., Hill Z. Neonatal care practices in sub-Saharan Africa: a systematic review of quantitative and qualitative data. J Health Popul Nutr. 2018;37(1):9. doi: 10.1186/s41043-018-0141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.United Nations Inter-agency Group for Child Mortality Estimation (UNIGME) Neonatal mortality rate for Peru. 2020. https://childmortality.org/data/Peru URL:
- 29.Dickson K.E., Simen-Kapeu A., Kinney M.V., et al. Every newborn: health-systems bottlenecks and strategies to accelerate scale-up in countries. Lancet. 2014;384(9941):438–454. doi: 10.1016/S0140-6736(14)60582-1. [DOI] [PubMed] [Google Scholar]
- 30.Huicho L., Huayanay-Espinoza C.A., Herrera-Perez E., et al. Examining national and district-level trends in neonatal health in Peru through an equity lens: a success story driven by political will and societal advocacy. BMC Public Health. 2016;16 Suppl 2:796. doi: 10.1186/s12889-016-3405-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huicho L., Segura E.R., Huayanay-Espinoza C.A., et al. Child health and nutrition in Peru within an antipoverty political agenda: a countdown to 2015 country case study. Lancet Glob Health. 2016;4(6):e414–e426. doi: 10.1016/S2214-109X(16)00085-1. [DOI] [PubMed] [Google Scholar]
- 32.Ministerio de Salud (MINSA) Ministerio de Salud, Dirección General de Epidemiología. Mortalidad Neonatal en el Peru y sus departamentos 2011-2012. Lima, Peru. 2013. http://www.dge.gob.pe/portal/docs/Mortalidad_neonatal11_12.pdf URL:
- 33.Demographic and Health Surveys (DHS) Newborn care module DHS8. https://dhsprogram.com/pubs/pdf/DHSQM/DHS8-Module-Newborn-Care-Qnnaire-EN-24Jan2020-DHSQM.pdf URL:
- 34.Instituto Nacional de Estadística e Informática (INEI) Encuesta demográfica y de salud familiar (ENDES) 2019. Lima-Perú: INEI. 2020. http://iinei.inei.gob.pe/microdatos/ URL:
- 35.Sitrin D., Perin J., Vaz L.M., et al. Evidence from household surveys for measuring coverage of newborn care practices. J Glob Health. 2017;7(2):020503. doi: 10.7189/jogh.07.020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tahsina T., Hossain A.T., Ruysen H., et al. Immediate newborn care and breastfeeding: EN-BIRTH multi-country validation study. BMC Pregnancy Childbirth. 2021;21(Suppl 1):237. doi: 10.1186/s12884-020-03421-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Velasquez Hurtado J.E., Solis Alcedo L., Vigo Valdez W.E., et al. [Evaluating maternal child care practices in extreme poverty areas in Peru, 2012] Rev Peru Med Exp Salud Pública. 2014;31(2):243–253. [PubMed] [Google Scholar]
- 38.Binfa L., Pantoja L., Ortiz J., et al. Midwifery practice and maternity services: a multisite descriptive study in Latin America and the Caribbean. Midwifery. 2016;40:218–225. doi: 10.1016/j.midw.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Villarreal Verde C., Placencia Medina M.D., Nolberto Sifuentes V.A. 2020. Exclusive breastfeeding and associated factors in mothers who attend Health Establishments of Lima, Peru (unpublished). Revista URP - Revista de la Facultad de Medicina Humana. [Google Scholar]
- 40.Matias S.L., Nommsen-Rivers L.A., Creed-Kanashiro H., Dewey K.G. Risk factors for early lactation problems among Peruvian primiparous mothers. Matern Child Nutr. 2010;6(2):120–133. doi: 10.1111/j.1740-8709.2009.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berger-Larranaga M., Bustamante-Abuid C., Diaz-Vergara S., Tresierra-Cabrera J., Mayta-Tristan P., Segura E.R. [Breastfeeding problems and other factors associated with excessive neonatal weight loss in a social security hospital in Lima, Peru] Nutr Hosp. 2015;32(5):2062–2070. doi: 10.3305/nh.2015.32.5.9462. [DOI] [PubMed] [Google Scholar]
- 42.Matias S.L., Nommsen-Rivers L.A., Dewey K.G. Determinants of exclusive breastfeeding in a cohort of primiparous periurban peruvian mothers. J Hum Lact. 2012;28(1):45–54. doi: 10.1177/0890334411422703. [DOI] [PubMed] [Google Scholar]
- 43.Hernandez-Vasquez A., Chacon-Torrico H. Determinants of early initiation of breastfeeding in Peru: analysis of the 2018 demographic and family health survey. Epidemiol Health. 2019;41 doi: 10.4178/epih.e2019051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee G., Paredes Olortegui M., Rengifo Pinedo S., et al. Infant feeding practices in the Peruvian Amazon: implications for programs to improve feeding. Rev Panam Salud Publica. 2014;36(3):150–157. [PubMed] [Google Scholar]
- 45.Boccolini C.S., Perez-Escamilla R., Giugliani E.R., Boccolini P.D.M. Inequities in milk-based prelacteal feedings in Latin America and the Caribbean: the role of cesarean section delivery. J Hum Lact. 2015;31(1):89–98. doi: 10.1177/0890334414559074. [DOI] [PubMed] [Google Scholar]
- 46.Blouin B., Penny M.E., Casapia M., et al. Effect of a two-component intervention to change hospital practice from early to delayed umbilical cord clamping in the Peruvian Amazon. Rev Panam Salud Publica. 2011;29(5):322–328. [PubMed] [Google Scholar]
- 47.Reinders S., Blas M.M., Lange I.L., Ronsmans C. London School of Hygiene & Tropical Medicine; London: 2021. Study protocol: evaluation of the Mamás del Río programme - a community-based, maternal and neonatal health intervention in Rural Amazonian Peru. [Google Scholar]
- 48.Castro-Arroyave D., Bautista M. Mama River program: an initiative beyond borders. World Health Organization & UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, Geneva: Social Innovation in Health Initiative. 2020. https://socialinnovationinhealth.org/wp-content/uploads/2021/08/SIHI-Case-Study-MAMA-RIVER.pdf URL:
- 49.Reinders S., Alva A., Huicho L., Blas M.M. Indigenous communities' responses to the COVID-19 pandemic and consequences for maternal and neonatal health in remote Peruvian Amazon: a qualitative study based on routine programme supervision. BMJ Open. 2020;10(12) doi: 10.1136/bmjopen-2020-044197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Limaye N.P., Blas M.M., Alva I.E., Carcamo C.P., Garcia P.J. The Amazon Hope: a qualitative and quantitative assessment of a mobile clinic ship in the Peruvian Amazon. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0196988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Del Mastro N.I., Tejada-Llacsa P.J., Reinders S., et al. Home birth preference, childbirth, and newborn care practices in rural Peruvian Amazon. PLoS One. 2021;16(5) doi: 10.1371/journal.pone.0250702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warren J., Lambert W., Fu R., Anderson J., Edelman A. Global neonatal and perinatal mortality: a review and case study for the Loreto Province of Peru. Dove Med Press. 2012;2012(2):103–113. [Google Scholar]
- 53.Instituto Nacional de Estadística e Informática (INE) Evolución de la Pobreza monetaria 2009-2020. Informe tecnico. Lima, Peru. 2020. https://www.inei.gob.pe/media/MenuRecursivo/publicaciones_digitales/Est/pobreza2020/Pobreza2020.pdf URL:
- 54.Instituto Nacional de Estadística e Informática (INEI) Sistema de información geográfica. Sistema de consulta de centros poblados. http://sige.inei.gob.pe/test/atlas/ URL:
- 55.Moran A.C., Kerber K., Sitrin D., et al. Measuring coverage in MNCH: indicators for global tracking of newborn care. PLoS Med. 2013;10(5) doi: 10.1371/journal.pmed.1001415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar V., Mohanty S., Kumar A., et al. Effect of community-based behaviour change management on neonatal mortality in Shivgarh, Uttar Pradesh, India: a cluster-randomised controlled trial. Lancet. 2008;372(9644):1151–1162. doi: 10.1016/S0140-6736(08)61483-X. [DOI] [PubMed] [Google Scholar]
- 57.Kumar V., Kumar A., Darmstadt G.L. Behavior change for newborn survival in resource-poor community settings: bridging the gap between evidence and impact. Semin Perinatol. 2010;34(6):446–461. doi: 10.1053/j.semperi.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 58.Iganus R., Hill Z., Manzi F., et al. Roles and responsibilities in newborn care in four African sites. Trop Med Int Health. 2015;20(10):1258–1264. doi: 10.1111/tmi.12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mukunya D., Nankabirwa V., Ndeezi G., et al. Key decision makers and actors in selected newborn care practices: a community-based survey in northern Uganda. Int J Environ Res Public Health. 2019;16(10):1723. doi: 10.3390/ijerph16101723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shamba D.D., Schellenberg J., Penfold S.C., et al. Clean home-delivery in rural southern Tanzania: barriers, influencers, and facilitators. J Health Popul Nutr. 2013;31(1):110–117. doi: 10.3329/jhpn.v31i1.14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akter T., Dawson A., Sibbritt D. The determinants of essential newborn care for home births in Bangladesh. Public Health. 2016;141:7–16. doi: 10.1016/j.puhe.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 62.Saaka M., Ali F., Vuu F. Prevalence and determinants of essential newborn care practices in the Lawra District of Ghana. BMC Pediatr. 2018;18(1):173. doi: 10.1186/s12887-018-1145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waiswa P., Peterson S., Tomson G., Pariyo G.W. Poor newborn care practices - a population based survey in eastern Uganda. BMC Pregnancy Childbirth. 2010;10:9. doi: 10.1186/1471-2393-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Menezes M.A.S., Gurgel R., Bittencourt S.D.A., Pacheco V.E., Cipolotti R., Leal M.D.C. Health facility structure and maternal characteristics related to essential newborn care in Brazil: a cross-sectional study. BMJ Open. 2018;8(12) doi: 10.1136/bmjopen-2017-021431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baqui A.H., Williams E.K., Darmstadt G.L., et al. Newborn care in rural Uttar Pradesh. Indian J Pediatr. 2007;74(3):241–247. doi: 10.1007/s12098-007-0038-6. [DOI] [PubMed] [Google Scholar]
- 66.Komakech H., Lubogo D., Nabiwemba E., Orach C.G. Essential newborn care practices and determinants amongst mothers of infants aged 0-6 months in refugee settlements, Adjumani district, west Nile, Uganda. PLoS One. 2020;15(4) doi: 10.1371/journal.pone.0231970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mallick L., Yourkavitch J., Allen C. Trends, determinants, and newborn mortality related to thermal care and umbilical cord care practices in South Asia. BMC Pediatr. 2019;19(1):248. doi: 10.1186/s12887-019-1616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gul S., Khalil R., Yousafzai M.T., Shoukat F. Newborn care knowledge and practices among mothers attending pediatric outpatient clinic of a hospital in Karachi, Pakistan. Int J Health Sci. 2014;8(2):167–175. doi: 10.12816/0006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takahashi K., Ganchimeg T., Ota E., et al. Prevalence of early initiation of breastfeeding and determinants of delayed initiation of breastfeeding: secondary analysis of the WHO Global Survey. Sci Rep. 2017;7 doi: 10.1038/srep44868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barros A.J., Hirakata V.N. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Norton E.C., Miller M.M., Kleinman L.C. Computing adjusted risk ratios and risk differences in Stata. STATA J. 2013;13(3):492–509. [Google Scholar]
- 72.Kleinman L.C., Norton E.C. What's the risk? A simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Serv Res. 2009;44(1):288–302. doi: 10.1111/j.1475-6773.2008.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. STATA J. 2012;12(2):308–331. [Google Scholar]
- 74.Gabrysch S., Campbell O.M. Still too far to walk: literature review of the determinants of delivery service use. BMC Pregnancy Childbirth. 2009;9:34. doi: 10.1186/1471-2393-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weng H.Y., Hsueh Y.H., Messam L.L., Hertz-Picciotto I. Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol. 2009;169(10):1182–1190. doi: 10.1093/aje/kwp035. [DOI] [PubMed] [Google Scholar]
- 76.Greenland S., Daniel R., Pearce N. Outcome modelling strategies in epidemiology: traditional methods and basic alternatives. Int J Epidemiol. 2016;45(2):565–575. doi: 10.1093/ije/dyw040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yajahuanca R.D.S.A., Fontenele C.V., Sena B.F., Diniz S.G. Birth at the health center or at home: an analysis of birthing care among the Kukamas Kukamirias women of Peru. J Hum Growth Dev. 2013;23:322–330. [Google Scholar]
- 78.Medina I.A., Mayca P.J. Creencias y costumbres relacionadas con el embarazo, parto y puerperio en comunidades nativas Awajun y Wampis. Rev Peru Med Exp Salud Pública. 2006;23:22–32. [Google Scholar]
- 79.Sharma I.K., Byrne A. Early initiation of breastfeeding: a systematic literature review of factors and barriers in South Asia. Int Breastfeed J. 2016;11:17. doi: 10.1186/s13006-016-0076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abdulghani N., Edvardsson K., Amir L.H. Worldwide prevalence of mother-infant skin-to-skin contact after vaginal birth: a systematic review. PLoS One. 2018;13(10) doi: 10.1371/journal.pone.0205696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.WHO 2017 . World Health Organization; Geneva: 2017. Guideline: protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services. Licence: CC BY-NC-SA 3.0 IGO. [PubMed] [Google Scholar]
- 82.Degefie T., Amare Y., Mulligan B. Local understandings of care during delivery and postnatal period to inform home based package of newborn care interventions in rural Ethiopia: a qualitative study. BMC Int Health Hum Right. 2014;14:17. doi: 10.1186/1472-698X-14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dhingra U., Gittelsohn J., Suleiman A.M., et al. Delivery, immediate newborn and cord care practices in Pemba Tanzania: a qualitative study of community, hospital staff and community level care providers for knowledge, attitudes, belief systems and practices. BMC Pregnancy Childbirth. 2014;14:173. doi: 10.1186/1471-2393-14-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coffey P.S., Brown S.C. Umbilical cord-care practices in low- and middle-income countries: a systematic review. BMC Pregnancy Childbirth. 2017;17(1):68. doi: 10.1186/s12884-017-1250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ministerio de Salud (MINSA) Directiva Sanitaria para la Evaluación de las funciones obstétricas y neonatales en los establecimientos de salud. Directiva Sanitaria N° 001- MINSA/DGSP-V.02. Lima, Peru. 2012. http://bvs.minsa.gob.pe/local/MINSA/3199.pdf URL:
- 86.World Health Organization (WHO) World Health Organization; Geneva: 2018. Definition of skilled health personnel providing care during childbirth: the 2018 joint statement by who, UNFPA, UNICEF, ICM, ICN, FIGO and ipa. [Google Scholar]
- 87.Abdulghani N., Edvardsson K., Amir L.H. Health care providers' perception of facilitators and barriers for the practice of skin-to-skin contact in Saudi Arabia: a qualitative study. Midwifery. 2020;81:102577. doi: 10.1016/j.midw.2019.102577. [DOI] [PubMed] [Google Scholar]
- 88.Alenchery A.J., Thoppil J., Britto C.D., de Onis J.V., Fernandez L., Suman Rao P.N. Barriers and enablers to skin-to-skin contact at birth in healthy neonates - a qualitative study. BMC Pediatr. 2018;18(1):48. doi: 10.1186/s12887-018-1033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mbalinda S., Hjelmstedt A., Nissen E., Odongkara B.M., Waiswa P., Svensson K. Experience of perceived barriers and enablers of safe uninterrupted skin-to-skin contact during the first hour after birth in Uganda. Midwifery. 2018;67:95–102. doi: 10.1016/j.midw.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 90.Ministerio de Salud (MINSA) Norma técnica de salud para la atencíon integral de salud neonatal. R.M. N° 828 – 2013/MINSA. Lima, Peru. 2013. http://bvs.minsa.gob.pe/local/minsa/3281.pdf URL:
- 91.INEI III Censo de Comunidades nativas 2017. Resultados definitivos. Tomo 1. Instituto Nacional de Estadistica e Informatica. Lima, Diciembre de 2018 [Internet] 2018. https://www.inei.gob.pe/media/MenuRecursivo/publicaciones_digitales/Est/Lib1598/TOMO_01.pdf Available from:
- 92.Blanc A.K., Warren C., McCarthy K.J., Kimani J., Ndwiga C., RamaRao S. Assessing the validity of indicators of the quality of maternal and newborn health care in Kenya. J Glob Health. 2016;6(1) doi: 10.7189/jogh.06.010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Day L.T., Sadeq-Ur Rahman Q., Ehsanur Rahman A., et al. Assessment of the validity of the measurement of newborn and maternal health-care coverage in hospitals (EN-BIRTH): an observational study. Lancet Glob Health. 2021;9(3):e267–e279. doi: 10.1016/S2214-109X(20)30504-0. [DOI] [PubMed] [Google Scholar]
- 94.Stanton C.K., Rawlins B., Drake M., et al. Measuring coverage in MNCH: testing the validity of women's self-report of key maternal and newborn health interventions during the peripartum period in Mozambique. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0060694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bhattacharya A.A., Allen E., Umar N., et al. Monitoring childbirth care in primary health facilities: a validity study in Gombe State, northeastern Nigeria. J Glob Health. 2019;9(2) doi: 10.7189/jogh.09.020411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McCarthy K.J., Blanc A.K., Warren C.E., Kimani J., Mdawida B., Ndwidga C. Can surveys of women accurately track indicators of maternal and newborn care? A validity and reliability study in Kenya. J Glob Health. 2016;6(2) doi: 10.7189/jogh.06.020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ameen S., Siddique A.B., Peven K., et al. Survey of women's report for 33 maternal and newborn indicators: EN-BIRTH multi-country validation study. BMC Pregnancy Childbirth. 2021;21(Suppl 1):238. doi: 10.1186/s12884-020-03425-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Waiswa P., Akuze J., Peterson S., et al. Differences in essential newborn care at birth between private and public health facilities in eastern Uganda. Glob Health Action. 2015;8:24251. doi: 10.3402/gha.v8.24251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tran H.T., Murray J.C.S., Sobel H.L., et al. Early essential newborn care is associated with improved newborn outcomes following caesarean section births in a tertiary hospital in Da Nang, Vietnam: a pre/post-intervention study. BMJ Open Qual. 2021;10(3) doi: 10.1136/bmjoq-2020-001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hill Z., Manu A., Tawiah-Agyemang C., et al. How did formative research inform the development of a home-based neonatal care intervention in rural Ghana? J Perinatol. 2008;28 Suppl 2:S38–S45. doi: 10.1038/jp.2008.172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.