Abstract
Purpose
The long-term humoral immunity to COVID-19 is not well understood owing to the continuous emergence of new variants of concern, the evolving vaccine-induced and infection-induced immunity, and the limited duration of follow-up in previous studies. As the sole blood service in Québec (Canada), Héma-Québec established a COVID-19-focused biobank (‘PlasCoV’) in April 2021.
Participants
As of January 2022, the biobank included 86 483 plasma samples from 15 502 regular donors (age range=18–84 years, females=49.7%), for an average of 5.6 donations per donor. Nearly two-thirds (65.6%) of biobank donors made at least two donations, with many donors having provided samples prevaccination and postvaccination (3061 (19.7%)) or preinfection and postinfection (131 (0.8%)), thus allowing for longitudinal studies on vaccine-induced and infection-induced immunity.
Findings to date
A study that used PlasCoV samples revealed that previously infected individuals who received a single dose of the BNT162b2 COVID-19 vaccine exhibited the strongest immune response. By contrast, SARS-CoV-2-naïve individuals required two vaccine doses to produce a maximal immune response. Furthermore, the results of a four-phase seroprevalence study indicated that the antinucleocapsid (N) response wanes rapidly, so that up to one-third of previously infected donors were seronegative for anti-N.
Future plans
Donations from individuals who consented to participate before 1 October 2022 will be collected up until 31 March 2023. This plasma biobank will facilitate the conduct of longitudinal studies on COVID-19 immunity, thus helping to provide valuable insights into the anti-SARS-CoV-2 immune response and its persistence, and the effects of vaccination and variants on the specificity of the anti-SARS-CoV-2 immune response.
Keywords: Blood bank & transfusion medicine, Public health, COVID-19
STRENGTHS AND LIMITATIONS OF THIS STUDY.
To the best of our knowledge, this would be the largest biobank of plasma samples dedicated to COVID-19 research, with >80 000 samples from >15 000 donors; new samples will continue to be added until March 2023.
Furthermore, the large subset of donors with ≥2 samples (ie, 65.6%)—along with the high frequency of donations in this subset (ie, median: once every 29.0 days)—enables the conduct of longitudinal analyses on COVID-19 immunity.
The cost of establishing the biobank was minimised since the infrastructure and personnel required for sample collection were already in place at our blood collection sites.
Only plasma samples are available, such that the biobank cannot be used to study cell-mediated immunity.
Despite the large sample, the plasma donor population is not fully representative of the overall Québec population, as expected since plasma donors typically reflect the healthy adult population.
Introduction
Despite remarkable progress, our understanding of the long-term humoral immunity to COVID-19 is incomplete owing to a number of challenges. The emergence of new variants of concern, such as Omicron, continuously challenges prior assumptions and data on the effectiveness and persistence of COVID-19 immunity.1 2 Furthermore, amid mass vaccination, disentangling infection-induced from vaccine-induced immunity to SARS-CoV-2 has become increasingly difficult to inform public health authorities.3–5 Notably, the vast majority of published serological studies used a cross-sectional design, and hence do not provide longitudinally collected data on COVID-19 immunity.6 Lastly, the more limited number of longitudinal studies that have been conducted had follow-up periods <12 months7–18 and lacked long-term funding commitment. Clearly, new initiatives are needed to overcome these barriers.
Historically, biobanks have spurred research efforts on novel or rare diseases19 and would likely help address the aforementioned challenges for COVID-19 research. They do so using highly standardised, quality-controlled processes to analyse a large number of biological specimens which are made available to the research community. In addition, biobanks are subject to regulatory oversight to protect donors’ rights. Virtually all biobanks collect a ‘broad’ consent that allows for samples to be used for multiple research purposes.20 For researchers, this practice (while controversial21) alleviates the burden associated with seeking consent.
However, existing COVID-19-focused biobanks are suboptimal to longitudinally study COVID-19 immunity. The Biobanque québécoise de la COVID-19 (BCQ19) is a biobanking initiative in Québec (Canada) that includes blood samples from individuals with a negative (controls) or positive SARS-CoV-2 PCR test result, with samples collected at several fixed time points for up to 24 months postdiagnosis (for non-hospitalised individuals) or posthospitalisation (for hospitalised individuals).22 However, BCQ19 is limited in terms of sample size and population representativeness. Furthermore, the unavailability of samples prior to diagnosis or hospitalisation hinders the study of the early immune response to COVID-19.
Blood services such as our institution (Héma-Québec—the only blood service operating in Québec) are ideally positioned to establish a biobank that would complement BCQ19 or other COVID-19-focused biobanks and better address researchers’ needs. Indeed, blood services routinely collect biological specimens from donors in the general population, thereby substantially alleviating the high cost normally associated with the establishment of a biobank. Blood services have been key partners in conducting COVID-19 research since the beginning of the pandemic23; involving them in biobanking efforts is therefore a sound approach to fuel additional research on COVID-19 immunity.
Here, we report on the establishment of a new biobank dedicated to COVID-19 research to inform public health in the province of Québec (Canada). The biobank consists of regular plasma donations collected throughout Québec, hence the name ‘PlasCoV’. Because plasma donors can donate every 6 days at our institution, the biobank includes a large number of repeat donors for whom longitudinal analyses are feasible.
Cohort description
Cohort establishment and overview
The project was initiated on 23 February 2021. A pilot phase was launched in March 2021, and the project was expanded to all of our fixed collection centres in April 2021. At that time, the majority of the Québec general population was unvaccinated,24 which enabled us to collect samples prevaccination and postvaccination for many donors.
Ten fixed blood centres—all located in large or medium size (≥100 000 population) areas—were designated to collect biobank-dedicated samples (table 1). The resulting donor cohort is therefore broadly representative of plasma donors living in urban and suburban areas throughout Québec.
Table 1.
Number of samples collected by each blood centre as of 24 January 2022
| Centre name | Metropolitan area | Health region (#-name) | Samples collected, N (%) |
| Globule—Centre Laval | Montréal | 13-Laval | 3706 (4.3) |
| Globule—Kirkland | Montréal | 06-Montréal | 2460 (2.8) |
| Globule—Quartier Dix30 | Montréal | 16-Montérégie | 4721 (5.5) |
| Globule—Place Versailles | Montréal | 06-Montréal | 3029 (3.5) |
| Globule—Quartier Lebourgneuf | Québec city | 03-Capitale Nationale | 10 108 (11.7) |
| Globule—Sainte-Foy | Québec city | 03-Capitale Nationale | 10 203 (11.8) |
| Plasmavie—Gatineau | Gatineau | 08-Outaouais | 10 614 (12.3) |
| Plasmavie—Saguenay | Saguenay | 02-Saguenay-Lac-St-Jean | 15 277 (17.7) |
| Plasmavie—Sherbrooke | Sherbrooke | 05-Estrie | 15 126 (17.5) |
| Plasmavie—Trois-Rivières | Trois-Rivières | 04-Mauricie et Centre du Québec | 11 239 (13.0) |
The biobank includes samples from a population-based cohort of healthy, voluntary, non-remunerated donors of plasma for fractionation who consented that their samples be used for research purposes. Specifically, the predonation questionnaire asks donors if they are willing to give part of their routine donation for research purposes. A donor’s consent is considered valid for all donations, and so those who consent are not asked this question at subsequent donations. A donor can decide at any time, simply by giving verbal notice, to withdraw his/her consent for the use of his/her samples and data. A 3 mL sample is collected from the plasma bag and frozen for biobanking, and aliquots of 200–500 µL of the thawed, biobanked sample can be provided on request (so that each sample can be used to prepare at least six aliquots on thawing).
Variables
In addition to plasma samples, the biobank offers access to several variables that are collected as part of routine donor screening or linked to government registries. Variables collected as part of routine blood donation include demographic characteristics (eg, age, sex) and clinical characteristics (eg, blood type, recent bloodborne infections, diabetes), which (except for blood type) are all self-reported by the donor (table 2). Data on COVID-19 vaccination were obtained by linking in-house donor data with the Système d’information pour la protection des maladies infectieuses—a government registry that captures all COVID-19 vaccinations covered by the universal, public health insurance in Québec (ie, by the Régie de l’assurance maladie du Québec). Similarly, data on COVID-19 infections were obtained by linking in-house data with the Trajectoire de Santé Publique—a government registry that captures data on SARS-CoV-2 infections detected by PCR tests performed on nasopharyngeal swabs. These tests were freely available to anyone experiencing COVID-19-related symptoms at the beginning of the pandemic, but have been restricted to priority groups (ie, primarily healthcare workers and individuals aged ≥70) since 5 January 2022. If needed, additional variables (eg, tobacco use, medication use and occupational status) can be obtained through a supplemental questionnaire filled by donors during their donation.
Table 2.
Summary of the main variables* collected or derived from the cohort
| Variables | Information collected before vaccination/infection | Information collected after vaccination/infection | Information routinely collected at predonation screening |
| Internal data | |||
| Donor questionnaire | |||
| Demographics: age/sex/health region/ethnicity | X | X | X |
| BMI calculated with | X | X | X |
| Height | X | X | X |
| Weight | X | X | X |
| Blood pressure† | X | ||
| Diabetes profile and treatment (without insulin)‡ | X | X | X |
| COVID-19 vaccination status§ | X | X | X |
| History of PCR-confirmed infection§ | X | X | X |
| Laboratory tests | |||
| Haemoglobin level | X | X | X |
| Blood type (ABO and Rh genotype or phenotype) | X | X | X |
| External data | |||
| COVID-19 vaccination status¶ | X | X | X |
| History of PCR-detected infection** | X | X | X |
*Several other variables such as donor deferrals, infectious state, etc are routinely collected but are not presented in this table.
†Available only for a subset of blood donor before the COVID-19 pandemic.
‡Diabetes treated with insulin is an exclusion criterion for blood donation.
§Reported by the donor.
¶From the provincial vaccination registry Système d’information pour la protection des maladies infectieuses.
**From the provincial COVID-19 infection registry Trajectoire de Santé Publique.
BMI, body mass index.
Participant characteristics
As of 24 January 2022, 15 502 out of 17 298 (89.6%) donors consented to have a small volume of their donations used for research on COVID-19 (table 3). No donor recruitment campaign was specifically undertaken for this research initiative. The recruitment of new donors ended on 1 October 2022; however, donations from individuals who consented to participate before 1 October 2022 will be collected up until 31 March 2023.
Table 3.
Donor characteristics as of 24 January 2022
| Consenting donors (N=15 502) |
Non-consenting donors (N=1796) |
General, adult population* | |||
| Sex,† n (%) | |||||
| Female | 7709 | (49.7) | 831 | (46.3) | (50.2) |
| Age (years),†‡ mean±SD (median) | 43.9±16.8 (43.0) | 46.1±16.7 (48.0) | 50.2±18.8 (50.0) | ||
| Age (years),†‡, n (%) | |||||
| 18–29 | 4399 | (28.4) | 433 | (24.1) | (17.1) |
| 30–39 | 2556 | (16.5) | 269 | (15) | (16.0) |
| 40–49 | 2287 | (14.8) | 251 | (14) | (16.1) |
| 50–59 | 2440 | (15.7) | 335 | (18.7) | (16.1) |
| 60–70 | 3178 | (20.5) | 408 | (22.7) | (18.3) |
| ≥71 | 642 | (4.1) | 100 | (5.6) | (16.4) |
| Self-reported race/ethniticity,†§ n (%) | |||||
| White | 14 517 | (93.6) | 1667 | (92.8) | NA |
| Other | 985 | (6.4) | 129 | (7.2) | NA |
| Health region (#-name),†‡ n (%) | |||||
| 02-Saguenay-Lac-Saint-Jean | 2234 | (14.4) | 299 | (16.6) | (3.3) |
| 03-Capitale-Nationale | 2811 | (18.1) | 351 | (19.5) | (9.0) |
| 04-Mauricie et Centre-du-Québec | 1771 | (11.4) | 213 | (11.9) | (6.2) |
| 05-Estrie | 2282 | (14.7) | 277 | (15.4) | (5.8) |
| 06-Montréal | 1518 | (9.8) | 103 | (5.7) | (24.4) |
| 07-Outaouais | 1729 | (11.2) | 263 | (14.6) | (4.6) |
| 12-Chaudière-Appalaches | 744 | (4.8) | 100 | (5.6) | (5.0) |
| 13-Laval | 353 | (2.3) | 24 | (1.3) | (5.1) |
| 14-Lanaudière | 252 | (1.6) | 18 | (1) | (6.0) |
| 15-Laurentides | 333 | (2.1) | 22 | (1.2) | (7.4) |
| 16-Montérégie | 1259 | (8.1) | 108 | (6) | (16.6) |
| Regions without fixed centres§ | 126 | (0.8) | <11 | (<0.70)¶ | (6.4) |
| Unknown or outside Québec | 90 | (0.6) | <11 | (<0.70)¶ | NA |
| No of vaccine doses received,†** n (%) | |||||
| 0 | 254 | (1.6) | NA | NA | |
| ≥1 | 14 939 | (96.4) | NA | NA | (91.6) |
| ≥2 | 14 836 | (95.7) | NA | NA | (88.7) |
| >3 | 9561 | (61.7) | NA | NA | (47.1) |
| Missing data†† | 309 | (2.0) | NA | NA | NA |
| Documented COVID-19 infection,†**‡‡ n (%) | |||||
| Yes | 1662 | (10.7) | NA | NA | (5.6) |
| No | 13 531 | (87.3) | NA | NA | (94.4) |
| Missing data†† | 309 | (2.0) | NA | NA | NA |
*For the general population of Québec, various data sources were used to capture variables of interest.
†Demographics (ie, sex, age, self-reported ethnicity and health region) are reported as of 24 January 2022. Data on prior vaccination and documented infection are reported as of 24 January 2022.
‡For the general population, data come from the following: Ministère de la Santé et des Services sociaux du Québec. Estimations et projections de population par territoire sociosanitaire. Available from: https://publications.msss.gouv.qc.ca/msss/document-001617/. Accessed on 10 August 2022.
§Includes 01-Bas-Saint-Laurent, 08-Abitibi-Témiscamingue, 09-Côte-Nord, 10-Nord-du-Québec, 11-Gaspésie-Îles-de-la-Madeleine, 17-Nunavik.
¶The exact number is lower than 11 and is thus not reported to preserve donor anonymity.
**For the general population, data come from the following: Institut national de santé publique du Québec. Données COVID-19 par âge et sexe - Évolution des cas. Available from: https://www.inspq.qc.ca/COVID-19/donnees/age-sexe/evolution-cas. Accessed on 3 January 2022.
††Missing data are due to mismatches between donor information recorded in our institution’s database and that recorded in governmental databases (N=309).
‡‡Data for the general population are underestimated since the age groups used in publicly available data (see footnote 7) exclude individuals aged 18–19 years.
NA, not available.
Donor characteristics were assessed as of 24 January 2022. Among consenting donors, median age was 43.0 years (table 3). The six health regions where donors most often lived were Capitale-Nationale (18.1%), Estrie (14.7%), Saguenay-Lac-Saint-Jean (14.4%), Mauricie et Centre-du-Québec (11.4%), Outaouais (11.2%) and Montréal (9.8%). The cohort predominantly included donors who self-identified as white (93.6%). Relative to non-consenting donors, consenting donors tended to be younger (ie, median age: 43.0 vs 48.0 years) and included slightly more female donors (ie, 49.7% vs 46.3%) and more residents of urban areas (eg, Montreal-Laval region: 12.1% vs 7.0%). Relative to the Québec general population, consenting donors had a similar age and sex distribution, but the densely populated region of Montreal-Laval was under-represented (12.1% vs 29.5%).
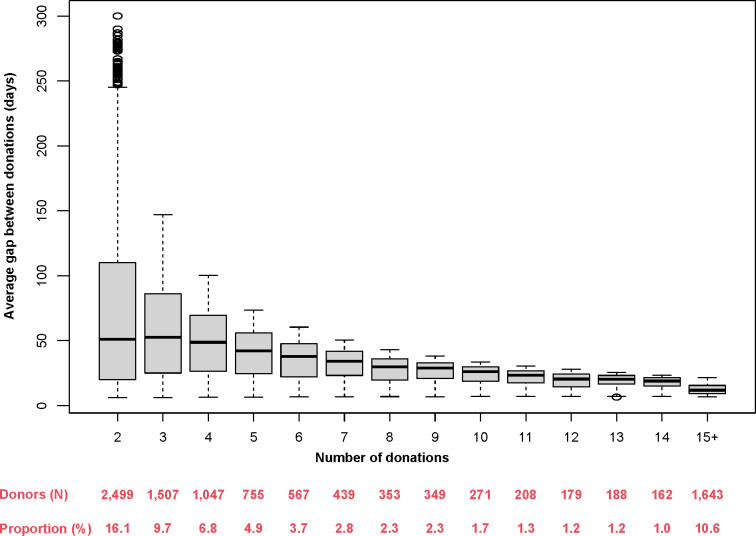
A key feature of this biobank is the availability of multiple, longitudinally collected plasma samples and associated data. As of 24 January 2022, the biobank had collected 86 483 samples from 15 502 donors, for an average of 5.6 donations per donor. At least two donations are available for nearly two thirds of the cohort (ie, 65.6%), thereby enabling the conduct of longitudinal analyses (figure 1). Most of these repeat donors appeared to donate regularly, with a median gap between donations ranging from 51.0 days for those with 2 donations to 11.8 days for those with ≥15 donations (median=29.0 days among those with ≥2 donations; figure 1).
Figure 1.
Average number of days between donations as a function of the number of donations in PlasCoV.
The cohort includes 3061 donors with samples collected prevaccination and postvaccination, thereby enabling the study of vaccine-induced immunity in a real-world population of vaccine-naïve individuals. The cohort also includes 10 330 donors with samples collected only postvaccination. Finally, the cohort includes 1326 donors with sample collected only prevaccination and 564 donors with no vaccination history or missing information.
The vast majority (ie, 96.4%) of donors had received ≥1 vaccine dose, and 95.7% had received ≥2 doses; only 1.6% were unvaccinated. In Québec, the second vaccine dose was delayed for up to 16 weeks to optimise population-level immunity amid concerns over limited vaccine supply. As a result, a large proportion of plasma donors (ie, 14.6%) received their second dose 85–112 days after their first dose, but some received it within the ⁓3-week gap recommended by the manufacturer (ie, 1.9% within 4 weeks). This notable feature of our biobank may be used to study the impact of vaccine dose intervals on the immune response, as done by Tauzin et al 25 (described in more details further down).
The following vaccines have been administered in Québec: ChAdOx1-S, BNT162b2 and mRNA-1273. Some individuals have received combinations of these vaccines, which was allowed by public health authorities to help fast-track the vaccination campaign.
Overall, 1662 (10.7%) donors had a documented infection as of 24 January 2022 (table 3), including 131 (7.9%) donors with available samples before and after a documented infection. This seemingly low number of donors who contracted SARS-CoV-2 between two donations is largely driven by the low number of at-risk patients (ie, either because they donated only once (N=712 (42.8%)) or because the timing of their infection left little time for a donation before 24 January 2022).
Funding
The project is entirely funded by the COVID-19 Immunity Task Force (CITF), a government-funded working group dedicated to advancing knowledge and research on COVID-19 in Canada and to inform public health authorities. CITF has provided a long-term funding commitment (ie, 2 years) for this biobank project, which will enable the conduct of long-term studies on infection-induced and vaccine-induced immunity to COVID-19.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Findings to date
Longitudinal assessment of COVID-19 immunity
A study by Tauzin et al used samples from our biobank to study the immune response to the mRNA vaccine BNT162b2 among SARS-CoV-2-naïve and previously infected individuals.25 The authors found that, in previously infected individuals, the immune response was strengthened after the first dose of vaccine and minimally declined thereafter. The administration of a second dose did not further increase the strength of the immune response. By contrast, in SARS-CoV-2-naïve individuals, the administration of a second dose further strengthened the immune response (except for antibody-mediated cell cytotoxicity (ADCC), which appeared more transient).
Tauzin et al additionally investigated the impact of using an extended interval between the two vaccine doses in SARS-CoV-2-naïve individuals. With the exception of ADCC, all assays were consistent with a better immune response when administering the two doses ~16 weeks apart compared with ~4 weeks apart.
Serosurvey
A publicly available report from our institution used biobank samples to estimate the seroprevalence of anti-SARS-CoV-2 antibodies in Québec from June 2021 to July 2021.3 This was the third phase of a serosurvey initiated in May 2020. Phases 1 (May–June 2020) and 2 (January–March 2021) only included whole blood donors rather than plasma donors from the biobank, since the PlasCoV project had not yet been launched. Phase 3 assessed both anti-receptor binding domain (RBD) and antinucleocapsid (N) seroprevalence, since >80% of the population had received at least one vaccine dose at the time of the serosurvey.24
The anti-RBD seroprevalence was 89.6%, consistent with the widespread vaccination coverage during phase 3. However, the anti-N seroprevalence was only 6.4%, which was lower than the anti-RBD seroprevalence among unvaccinated blood donors included in phase 2 (ie, 10.5%). This unexpected result was likely driven by seroreversion, which occurs faster for anti-N than anti-spike (or anti-RBD) antibodies.26–28 Indeed, nearly 40% of previously infected donors in phase 3 were seronegative for anti-N, likely because of seroreversion. This apparent rapid waning of anti-N antibodies was also observed in a separate cohort of 54 previously infected donors of convalescent plasma used in the CONCOR-1 clinical trial.29 After more than 200 days of follow-up, anti-N seroreversion occurred in 33.3% of these donors, whereas anti-RBD seroreversion occurred in only 11.1% of donors.
Taken together, these results suggest anti-N seroprevalence may only be adequate to capture relatively recent infections, thereby questioning its usefulness in serosurveys analysed using conventional analytical approaches (ie, using a hard threshold applied at a single time point to determine seropositivity).5 At the time of writing this manuscript, the fourth phase of this study is underway and uses PlasCoV samples to compare anti-N responses preinfection and postinfection—a new approach to estimate the incidence of recent infections in the context of the Omicron wave.
Future plans
The plasma biobank described here will help conduct longitudinal studies on COVID-19 immunity. The resulting evidence will provide valuable insights on several aspects of the immune response to COVID-19, including its persistence, its specificity against variants, and the effects of vaccination.
Future studies using this biobank are already planned or underway. Our organisation will continue using PlasCoV samples for an ongoing anti-SARS-CoV-2 serosurvey up until March 2023 (see above description). The biobank will also prove useful to address other research questions in public health such as hybrid immunity, and the immune response to emerging variants or new vaccine formulations. For example, another study will soon be conducted to estimate the impact of post-COVID-19 syndrome (a.k.a., long COVID) in a healthy, adult population using this large, prospective longitudinal cohort.
Strengths and limitations
The biobank described here has several strengths. To the best of our knowledge, this would be the largest biobank of plasma samples dedicated to COVID-19 research, with >80 000 samples from >15 000 donors and new samples continually being added until March 2023. Furthermore, the large subset of donors with ≥2 samples (65.6%)—along with the high frequency of donations in this subset (ie, median: once every 29.0 days)—enables the conduct of longitudinal analyses on COVID-19 immunity. Another strength is that donors provided a broad consent, which allows researchers to recontact them for other projects (eg, supplemental questionnaire). Lastly, the cost of establishing the biobank was minimised since the infrastructure and personnel required for sample collection were already in place at our blood collection sites. Given these strengths, our biobank may serve as a model for other blood operators and government partners who would be interested in reproducing our initiative elsewhere.
Certain limitations should nonetheless be considered when using our biobank samples. To begin, the large number of samples is made possible in part because donors are free to donate whenever they want, that is, they did not agree to participate in a research that involves a rigid protocol with scheduled visits. As a result, samples are not collected systematically after an event of interest, such as vaccination or infection. This limitation is nonetheless mitigated by the large number of samples as well as the participation of frequent donors who regularly provide samples, thereby increasing the number of samples collected early after vaccination or infection. Furthermore, only plasma samples are available, so that the biobank cannot be used to study cell-mediated immunity. Researchers interested in studying cell-mediated immunity may want to contact BCQ19, which routinely collects peripheral blood mononuclear cells.22 Moreover, despite the large sample, the plasma donor population is not fully representative of the overall Québec population, as expected since plasma donors are typically more representative of the healthy adult population. All exclusion criteria for plasma donations were also exclusion criteria for the biobank, including immunodeficiencies, active infection and recent cancer among other chronic diseases. In addition, the database associated with our biobank does not include information on disease severity, such as hospitalisation or intensive care unit admission, mortality and comorbidities. Lastly, the database does not include information on socioeconomic status, such as income and education. However, the six-digit zip code can be used to generate a proxy index for socioeconomic status.
Collaboration
All researchers in Canada or elsewhere may apply to access the PlasCoV biobank. Researchers will be asked to fill a form with high-level information on their project’s objectives, methods, novelty and relevance to the biobank’s objectives. They will also be asked to justify the sample size needed and any particular inclusion criteria, and to obtain ethics approval for their project.
Supplementary Material
Acknowledgments
Medical writing assistance was provided by Samuel Rochette, an employee of Héma-Québec.
Footnotes
Contributors: MG, AL, RB and CR conceived and designed the study. AL, AB, JP and YG collected the data. AL and YG analysed the data, with input from MG, RB, MD and CR. MG, AL, YG and RB drafted the manuscript and MD, AB, CR and JP critically revised it for important intellectual content. All authors approved the final version to be published. MG acts as the guarantor for this work.
Funding: The project is entirely funded by the COVID-19 Immunity Task Force (CITF), which is supported by the Public Health Agency of Canada (PHAC).
Disclaimer: The views expressed here do not necessarily reflect the views of the PHAC.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request. Details on the PlasCoV biobank and the application process can be obtained through Héma-Québec’s website at https://www.hema-quebec.qc.ca/coronavirus/hema-quebec-en-contexte-de-pandemie/etude-plascov.en.html. Enquiries can be sent by email to BiobanqueCOVID@hema-quebec.qc.ca, or alternately to MG (Marc.Germain@hema-quebec.qc.ca), the biobank director.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
Ethics approval
This study involves human participants and was approved by #B6-2021-003. Participants gave informed consent to participate in the study before taking part.
References
- 1. Cevik M, Grubaugh ND, Iwasaki A, et al. COVID-19 vaccines: keeping pace with SARS-CoV-2 variants. Cell 2021;184:5077–81. 10.1016/j.cell.2021.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hie B, Zhong ED, Berger B, et al. Learning the language of viral evolution and escape. Science 2021;371:284–8. 10.1126/science.abd7331 [DOI] [PubMed] [Google Scholar]
- 3. Héma-Québec . Phase 3 de l’étude sur la séroprévalence des anticorps dirigés contre le SRAS-CoV-2 au québec. Available: https://www.hema-quebec.qc.ca/userfiles/file/coronavirus/rapport-seroprevalence-phase-3.pdf [Accessed 14 Feb 2023].
- 4. Jones JM, Stone M, Sulaeman H, et al. Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020-May 2021. JAMA 2021;326:1400–9. 10.1001/jama.2021.15161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bolotin S, Tran V, Osman S, et al. SARS-CoV-2 seroprevalence survey estimates are affected by anti-nucleocapsid antibody decline. J Infect Dis 2021;223:1334–8. 10.1093/infdis/jiaa796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arora RK, Joseph A, Van Wyk J, et al. SeroTracker: a global SARS-CoV-2 seroprevalence dashboard. Lancet Infect Dis 2021;21:e75–6. 10.1016/S1473-3099(20)30631-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021;371:eabf4063. 10.1126/science.abf4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doria-Rose N, Suthar MS, Makowski M, et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for COVID-19. N Engl J Med 2021;384:2259–61. 10.1056/NEJMc2103916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ebinger JE, Fert-Bober J, Printsev I, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med 2021;27:981–4. 10.1038/s41591-021-01325-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goel RR, Apostolidis SA, Painter MM, et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci Immunol 2021;6:eabi6950. 10.1126/sciimmunol.abi6950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med 2020;383:1724–34. 10.1056/NEJMoa2026116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He Z, Ren L, Yang J, et al. Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in Wuhan, China: a longitudinal, population-level, cross-sectional study. Lancet 2021;397:1075–84. 10.1016/S0140-6736(21)00238-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krammer F, Srivastava K, Alshammary H, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med 2021;384:1372–4. 10.1056/NEJMc2101667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N Engl J Med 2021;385:e84. 10.1056/NEJMoa2114583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of SARS-CoV-2 variant delta to antibody neutralization. Nature 2021;596:276–80. 10.1038/s41586-021-03777-9 [DOI] [PubMed] [Google Scholar]
- 16. Röltgen K, Powell AE, Wirz OF, et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol 2020;5:eabe0240. 10.1126/sciimmunol.abe0240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang H, Yuan Y, Xiao M, et al. Dynamics of the SARS-CoV-2 antibody response up to 10 months after infection. Cell Mol Immunol 2021;18:1832–4. 10.1038/s41423-021-00708-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perreault J, Tremblay T, Fournier M-J, et al. Waning of SARS-CoV-2 RBD antibodies in longitudinal convalescent plasma samples within 4 months after symptom onset. Blood 2020;136:2588–91. 10.1182/blood.2020008367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malsagova K, Kopylov A, Stepanov A, et al. Biobanks—A platform for scientific and biomedical research. Diagnostics (Basel) 2020;10:485. 10.3390/diagnostics10070485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Allen C, Joly Y, Moreno PG. Data sharing, biobanks and informed consent: a research paradox. McGill JL Health 2013;7:85. [Google Scholar]
- 21. Caulfield T, Murdoch B. Genes, cells, and biobanks: Yes, there’s still a consent problem. PLoS Biol 2017;15:e2002654. 10.1371/journal.pbio.2002654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tremblay K, Rousseau S, Zawati MH, et al. The biobanque québécoise de la COVID-19 (BQC19)—A cohort to prospectively study the clinical and biological determinants of COVID-19 clinical trajectories. PLoS One 2021;16:e0245031. 10.1371/journal.pone.0245031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O’Brien SF, Lieshout-Krikke RW, Lewin A, et al. Research initiatives of blood services worldwide in response to the COVID-19 pandemic. Vox Sang 2021;116:296–304. 10.1111/vox.12995 [DOI] [PubMed] [Google Scholar]
- 24. Institut national de santé publique du québec. Données de vaccination contre la COVID-19 au québec. Available: https://www.inspq.qc.ca/covid-19/donnees/vaccination [Accessed 17 Nov 2021].
- 25. Tauzin A, Gong SY, Beaudoin-Bussières G, et al. Strong humoral immune responses against SARS-CoV-2 spike after BNT162b2 mRNA vaccination with a 16-week interval between doses. Cell Host Microbe 2022;30:97–109. 10.1016/j.chom.2021.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Whitcombe AL, McGregor R, Craigie A, et al. Comprehensive analysis of SARS-CoV-2 antibody dynamics in New Zealand. Clin Transl Immunology 2021;10:e1261. 10.1002/cti2.1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ripperger TJ, Uhrlaub JL, Watanabe M, et al. Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity 2020;53:925–33. 10.1016/j.immuni.2020.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cohen KW, Linderman SL, Moodie Z, et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep Med 2021;2:100354. 10.1016/j.xcrm.2021.100354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bégin P, Callum J, Heddle NM, et al. Convalescent plasma for adults with acute COVID-19 respiratory illness (CONCOR-1): study protocol for an international, multicentre, randomized, open-label trial. Trials 2021;22. 10.1186/s13063-021-05235-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request. Details on the PlasCoV biobank and the application process can be obtained through Héma-Québec’s website at https://www.hema-quebec.qc.ca/coronavirus/hema-quebec-en-contexte-de-pandemie/etude-plascov.en.html. Enquiries can be sent by email to BiobanqueCOVID@hema-quebec.qc.ca, or alternately to MG (Marc.Germain@hema-quebec.qc.ca), the biobank director.