Abstract
Objectives
The aim of this study was to determine the risk factors associated with SARS-CoV-2 infection in a cohort of homeless people using survival analysis. Seroprevalence in the homeless community was also compared with that of the general population.
Design
Cohort study.
Setting
Data were collected across two testing sessions, 3 months apart, during which each participant was tested for anti-SARS-CoV-2 antibodies and completed a face-to-face survey.
Participants
All homeless adults sleeping rough, in slums or squats, in emergency shelters or transitional accommodation in Marseille were eligible.
Primary outcome measures
Occurrence of a seroconversion event defined as a biologically confirmed SARS-CoV-2 infection. Local data from a national seroprevalence survey were used for comparison between homeless people and the general population.
Results
A total of 1249 people were included. SARS-CoV-2 seroprevalence increased from 6.0% (4.7–7.3) during the first session to 18.9% (16.0–21.7) during the second one, compared with 3.0% (1.9–4.2) and 6.5% (4.5–8.7) in the general population. Factors significantly associated with an increased risk of COVID-19 infection were: having stayed in emergency shelters (1.93 (1.18–3.15)), being an isolated parent (1.64 (1.07–2.52)) and having contact with more than 5–15 people per day (1.84 (1.27–2.67)). By contrast, smoking (0.46 (0.32–0.65)), having financial resources (0.70 (0.51–0.97)) and psychiatric or addictive comorbidities (0.52 (0.32–0.85)) were associated with a lower risk.
Conclusion
We confirm that homeless people have higher infection rates than the general population, with increased risk in emergency shelters. There is growing evidence that, in addition to usual preventive measures, public policies should pay attention to adapt the type of accommodation and overall approach of precariousness.
Trial registration number
Keywords: EPIDEMIOLOGY, Public health, COVID-19
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Description of risk factors of SARS-CoV-2 infection in a large study population with high-quality and statistically robust methodology.
First surveillance data from a cohort of homeless people providing an incidence rate of seroconversion and comparing seroprevalence with the general population at two different time points.
Large number of people lost to follow-up and difficulties in following a cohort of homeless people who are highly mobile.
Sensitivity of detection by repeated serological tests.
Introduction
The crisis generated by the COVID-19 pandemic suddenly widened the gap in access to healthcare, especially for vulnerable populations.1 Before the pandemic, homelessness was already associated with higher health inequalities compared with the general population.2
Public policies had to devise new strategies to limit the impact of the evolving pandemic on healthcare systems and societies. For example, the French government imposed two stringent lockdowns in 2020. These restrictions were accompanied in most cities by a sheltering programme for homeless people, with allocation of extra emergency shelters, transitional accommodations and requisitioned hotels.3 In parallel, however, there was a rise of precariousness in France, with an increase in the number of homeless people.4 5 Studies show that homeless people are at high risk of developing SARS-CoV-2 infection and reinfection due to physical proximity, crowded emergency shelters and unsafe or unhygienic living conditions.6–8 In this context, data on the spread of the SARS-CoV-2 virus and immunity among the homeless are essential to inform policy stakeholders and to contain epidemic dynamics.
In France, in May 2020, a nationwide study in the general population estimated that seroprevalence ranged from 3.5% (South East of France) to 10.8% (North East of France),9 emphasising the need for regionally specific data. This seroprevalence reflected the regional heterogeneity at the beginning of the pandemic. A high prevalence rate of SARS-CoV-2 infection was reported in people living in homeless shelters,7 10 11 which also showed a high rate of severe COVID-19 symptoms, potentially due to a lack of access to the healthcare and a high prevalence of comorbidities such as lung or heart diseases.12–14 To our knowledge, there are no representative data of an entire homeless population to describe the dynamics of the prevalence of SARS-CoV-2 infection over time.
The aim of this study was to determine the risk factors associated with SARS-CoV-2 infection in a cohort of homeless people using survival analysis. Seroprevalence in the homeless community was also compared with that of the general population.
Methods
Study design
The present study was a descriptive and prospective cohort. Study design, participants and sampling were described in a previous study.15 Each participant receives individualised follow-up and repeated testing at the inclusion and 3 months later. There was no resampling.
Study area and population
The study area was the city of Marseille, the second largest city in France with 889 029 inhabitants, suffering from a high level of poverty.16
Eligible population
Data from the local orientation system for emergency and transitional accommodation (Services Intégrés de l’Accueil et de l’Orientation) and the non-governmental organisation (NGO) Doctors of the World estimated that in 2020, at the beginning of the COVID-19 outbreak, there were 2322 homeless adults living in emergency, transitional shelters or hostels and 619–817 living in squats or slums (online supplemental file 1). No point-in-time census was available for people living on the streets in Marseille.
bmjopen-2022-065734supp001.pdf (82KB, pdf)
Inclusion criteria
In order to focus on the homeless people the furthest from housing, we decided to select those characterised by the greatest residential instability: people sleeping rough, in squats or slums, in stabilisation shelters, in emergency shelters or hostels, respectively, corresponding to the following categories of the European Typology on Homelessness and Housing Exclusion (ETHOS): ETHOS 1, 2, 3 and 8.17
Participant selection
In the absence of a point-in-time count, random sampling was impossible. We set a 2-month inclusion period, during which we systematically offered all homeless people aged over 18 to participate in the study. Recruitment of participants was also facilitated by the ‘Accés aux Soins des Sans Abris (ASSAb) network’ of assistance to homeless people: 18 homeless outreach teams working in streets, hotels, squats or slums, 5 emergency shelters and 10 transitional accommodations.
Investigations
Two specific sessions of serological testing were conducted in order to assess seroprevalence. The first session lasted from 5 June to 5 August 2020, and the second from 11 September to 18 December 2020. At each session, each participant was tested using a rapid diagnostic serological test, and completed a face-to-face survey investigating: sociodemographic characteristics; comorbidities; past and current medical history of COVID-19; difficulties in access to care, water, food or hygiene supplies; and compliance with the preventive measures (social distancing, wearing a face mask and hand washing). Questions were asked by trained local interviewers in the participants’ native language to improve comprehension and to minimise the information bias.
Community engagement and medical care
Community awareness interventions were conducted during the two testing sessions to secure the commitment and participation of a majority of homeless people. Community engagement started by meetings with the community leaders or mediators but also the healthcare workers and the members of local institutions or NGOs implicated in health for homeless people with the help of a local network (ASSAb) in charge of coordination with these different stakeholders. Interviewers were sensitised to the study objectives, interventions and expected role of the community. A mobile team including an infectious disease specialist, a nurse and a community mediator followed the positive cases.
Field biological analysis
We used the rapid serological test ‘Biosynex COVID-19 BSS’ that detects immunoglobulin M (IgM) and G (IgG) in 10 min with high specificity and sensitivity (>95% and 90%, respectively).18
Data analysis
Descriptive analyses of sociodemographic characteristics were performed using numbers and percentage for categorical data, or medians and IQR for quantitative data. The seroprevalence of COVID-19 infection was investigated between 1 February 2020 and 18 December 2020. All the participants were considered to have a negative serology on 1 February 2020 before the first cases were detected in early March 2020 in Marseille. In the event of seroconversion, infection was reported as confirmed at the time of serological testing, with the possibility of overestimating the number of person-days before infection and regardless of the results of subsequent serological tests. This methodological choice was made in relation to the different predictive variables also collected at the time of the serological test. To assess seroprevalence rate according to presence or absence of symptoms, bootstrap resampling approach with a set of 1000 samples was used to create 95% CIs based on IgM/IgG sensitivity and specificity and their 95% CI. Kaplan-Meier methods along with the log rank test were used to establish statistical differences in seroprevalence rates between types of ETHOS accommodation.17 A survival analysis was carried out to address the spread of COVID-19 among the targeted population. The time (in months) was defined as follows: the starting date was 1 February 2020, the date at which non-positive cases were registered in Marseille, that is, when all participants could be considered to have a COVID-19-negative status. The event was a positive SARS-CoV-2 status, whatever a positive SARS-CoV-2 PCR or a positive serological test informed the diagnosis. His or her status was considered positive regardless of the results of subsequent serological tests. For those with a PCR test achieved, the date of the event corresponded to the PCR date, corrected with the date of the first symptoms when reported. For positive participant with a rapid serological test, the reported date of the first symptoms was considered. For participant with a positive serological test but with no history of symptoms, we considered the date of the testing strategy performed by the research team. In order to take into account in the analysis, in regard to the mobility of the participants, in terms of the place of residence and the possibility of changes in sociodemographic characteristics, we took the data at the time of the positive test for SARS-CoV-2 participants and at the time of the last test for negative patients throughout the follow-up. No additional corrections were made in absence of any informative data. Participants tested negative at the first testing wave but being lost to follow-up at the second testing wave were censored at the date of the last collection data. The cut-off date was 18 December 2020, precisely 11.2 months after the starting date. A Cox model was performed using both baseline covariates and time-dependent covariates. Time-dependent covariates were the following: type of ETHOS accommodation (ie, street, emergency shelters, hotels, transitional shelter or squat/slum), type of accommodation (private or shared room/area), number of contacts per day, having financial resources and having work. We fitted a multivariate Cox model by considering as eligible variables those that were significant in a univariate analysis at the 5% level, and considering all pairwise interactions. The covariate ‘number of contacts per day’ was forced into the model as it was considered to be a relevant variable. We tested the assumption of proportional hazards using Schoenfeld residuals. Then, we used the stepwise selection function in R, which starts with an empty model and adds/removes predictors according to Akaike Information Criterion (AIC) criteria. Unadjusted and adjusted HRs and 95% CI were given.
All of the statistical analyses were carried out using R software, and differences with p values of <0.05 were considered statistically significant.
Seroprevalence data of our study were compared with data from a representative sample of the general population living in Marseille, which were derived from a national seroprevalence survey (EpiCov).19 Results of seroprevalence in the general population were obtained from home self-samples of dried blood spots, in order to detect IgG antibodies (Euroimmun ELISA-S).19
All the confirmed cases of COVID-19 by positive SARS-CoV-2 PCR in Marseille registered from 1 January to 31 December 2020, by the French national monitoring department (SI-DEP) from Santé Publique France,20 were used to describe the local incidence rate of COVID-19 infection in cases per person-week.
Patient and public involvement
Public were involved in conduct (questionnaires were conducted by peer workers) and dissemination plans of this research (the results were presented to the public via photo and sound exhibitions and radio broadcasts in Marseille city).
Results
During the first session from 5 June to 5 August 2020, a total of 1241 people were included. Median age was 38 years (IQR 22), 70.40% were men (n=874) with 98 (8.1%) of participants living rough, 358 (29.5%) in emergency shelters, 197 (16.2%) in hostels, 196 (16.2%) in transitional shelters and 363 (29.9%) in squats and slums (table 1). A total of 30% of the participants in the cohort changed their place of residence during follow-up. Approximately 37% of eligible ETHOS 2, 3 and 8 participants were included in the study (online supplemental file 1). Around half of the participants, 52.2% (n=648/1241), had confirmed or possible risk factors for severe COVID-19 disease, including cancer, obesity, cardiac or pulmonary disease and severe renal insufficiency. In addition, half of the participants (52.0%, n=645) reported active tobacco consumption. A total of 58.1% (n=721) of the participants tested during the first session were also tested at the second session.
Table 1.
Population characteristics (n=1241)
| Baseline characteristics | n (%) or median (IQR) |
| Gender | |
| Men | 874 (70.4%) |
| Women | 367 (29.6%) |
| Age, median (years) | 38 (22) |
| Age ≤65 years | 1179 (95.0%) |
| French nationality | 222 (18.4%) |
| Country of birth | |
| France | 234 (18.9%) |
| Europe | 416 (33.5%) |
| Africa | 282 (22.7%) |
| Other | 279 (22.5%) |
| Missing | 30 (2.4%) |
| Educational attainment | |
| None | 560 (45.1%) |
| Lower secondary | 445 (35.9%) |
| Upper secondary or vocational | 122 (9.8%) |
| Missing | 114 (9.2%) |
| Household status | |
| Isolated adult | 660 (53.2%) |
| Isolated parent | 129 (10.4%) |
| Family | 411 (33.1%) |
| Missing | 41 (3.3%) |
| Health insurance | |
| No | 345 (27.8%) |
| Yes | 826 (66.6%) |
| Missing | 70 (5.6%) |
| Financial resources | |
| No | 448 (36.1%) |
| Yes | 730 (58.8%) |
| Missing | 63 (5.1%) |
| Working situation* | |
| No | 949 (76.5%) |
| Yes | 229 (18.5%) |
| Missing | 63 (5.1%) |
| Total length of homelessness | |
| ≤5 years | 775 (62.4%) |
| >5 years | 393 (31.7%) |
| Missing data | 73 (5.9%) |
| Typology ETHOS17 | |
| ETHOS 1: street | 98 (8.1%) |
| ETHOS 2: emergency shelters | 358 (29.5%) |
| ETHOS 2: hotels | 197 (16.2%) |
| ETHOS 3: transitional shelters | 196 (16.2%) |
| ETHOS 8: squats, slums | 363 (29.9%) |
| Type of accommodation | |
| Private room or area | 524 (42.2%) |
| Shared room or area | 648 (52.2%) |
| Missing data | 69 (5.6%) |
| Number of contacts per day | |
| ≤5 | 714 (58.0%) |
| 5–15 | 410 (33.3%) |
| >15 | 107 (8.7%) |
| Tobacco consumption | |
| No | 480 (38.7%) |
| Yes | 645 (52.0%) |
| Missing | 116 (9.3%) |
| Comorbidity | |
| Psychiatric or addictive comorbidities | 295 (23.8%) |
| Obesity | 72 (6.5%) |
| Diabetes | 91 (8.1%) |
| Chronic respiratory pathology | 99 (9.2%) |
| Cardiovascular pathology | 152 (14.1%) |
| Chronic renal failure with dialysis | 23 (2.1%) |
| Cancer | 24 (2.2%) |
*Declared or undeclared employment.
ETHOS, European Typology on Homelessness and Housing Exclusion.
A total of 74/1241 of participants had positive serology in the first campaign, with 2.5% of positive IgM tests, 5.2% positive IgG tests and 1.7% positive IgM and IgG tests. In the second campaign, 136/721 of participants had positive serology with 8.1% of positive IgM tests, 17.5% positive IgG tests and 6.8% positive IgM and IgG tests. Of the 74 participants with positive serology at the start of the study, 43 were able to be followed up and have a new serology 3 months later. A total of 69.8% (n=30) still had positive serology. Thus, in 30.2% of cases (n=13) there was a rapid negativation of serology.
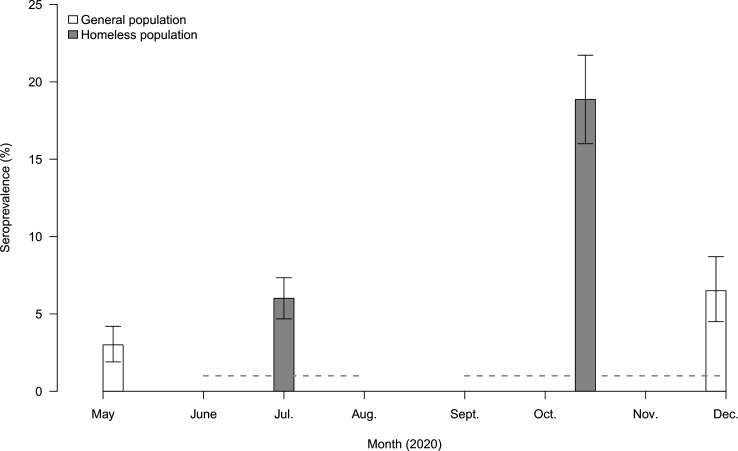
Seroprevalence was 6.0% (IQR 4.7–7.3) (n=1241) in the first campaign and 18.9% (IQR 16.0–21.7) (n=721) in the second campaign, and had significantly increased (p<0.005) (figure 1). In comparison, seroprevalence in the general population in Marseille (EpiCov-Marseille) was 3.0% (1.9–4.2) in May to June and 6.5% (95% CI 4.5%–8.7%) in November to December 2020 and had significantly increased (p<0.005) (figure 1).
Figure 1.
Seroprevalence rates during the two serological testing campaigns (Bootstrap resampling approach with a set of 1000 samples was used to create CIs, accounting for variability in the sensitivity and specificity of the serological assay) in homeless people cohorts and results for the general population from the EpiCov study in Marseille. General population seroprevalence rate data come from the EpiCov study in Marseille.
Factors associated with SARS-CoV-2 infection
A total of 180 participants presented a SARS-CoV-2 seroconversion defined by a positive serology result for SARS-CoV-2 (IgM or IgG); at inclusion (n=74/1241) or as part of the cohort follow-up (n=136/721). Average time of infection from 1 February 2020 was 230 days (IQR 162–277). Figure 2 shows the Kaplan-Meier curves according to the participant’s type of accommodation. Homeless people living in emergency shelters and hotels had a significantly higher risk of SARS-CoV-2 infection compared with their counterparts over the study follow-up period (p<0.001).
Figure 2.
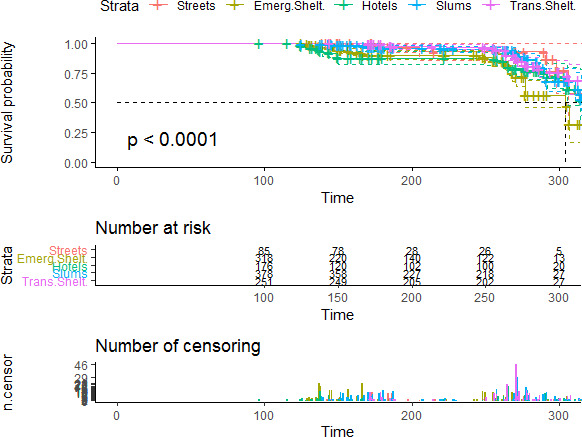
Risk of SARS-CoV-2 infection by type of housing for homeless participants estimated by the Kaplan-Meier method, including 95% CI. Censoring and number of participants at risk at different time points are indicated. Streets: people living rough (ETHOS 1); Emerg.Shelt: people living in emergency shelters (ETHOS 2); Hotels: people living in hostels (ETHOS 3); Slums: people living in slums or squats (ETHOS 8); Trans.Shelt: people living in transitional accommodation for the homeless (ETHOS 4). ETHOS, European Typology on Homelessness and Housing Exclusion.
Table 2 shows univariate and multivariate analyses of the factors associated with the SARS-CoV-2 seroprevalence. Univariate analysis identified an association between positive serological results and participants coming from Africa (2.51 (1.45–4.33)) or those applying physical distancing (1.61 (1.14–2.27)). These two variables were not retained in the final model. Difficult access to hygiene products was also associated with lower seroprevalence in univariate analysis (0.72 (0.52–0.96)) but not in multivariate analysis. Being an isolated parent (1.64 (1.07–2.52)), spending more than 33% (1.70 (1.11–2.62)) or 66% (1.93 (1.18–3.15)) of time living in an emergency shelter during follow-up and having between 5 and 15 daily contacts (1.84 (1.27–2.67)) were associated with SARS-CoV-2 infection in multivariate analysis. By contrast, having financial resources (1.64 (1.07–2.52)), being a smoker at the time of the survey (0.46 (0.32–0.65)) and having psychiatric or addictive comorbidities (0.52 (0.32–0.85)) were associated with a lower risk of SARS-CoV-2 seroprevalence. Figure 3 summarises the Cox multivariable regression analysis. Model remains unchanged even by adjusting on age and sex (online supplemental file 2).
Table 2.
Univariate and multivariate analyses of the seroprevalence of SARS-CoV-2 infection (n=180/1241) between February and December 2020 in homeless people living in Marseille
| Results | Univariate analysis | Multivariate analysis* | |
| HR (95% CI)‡ | P value | Adjusted HR (95% CI) | |
| Gender | |||
| Men | Ref | ||
| Women | 0.97 (0.70 to 1.34) | 0.900 | |
| Age (years) | |||
| ≤65 | Ref | ||
| >65 | 1.66 (0.99 to 2.77) | 0.050 | |
| Country of birth | |||
| France | Ref | ||
| Europe | 1.45 (0.83 to 2.55) | 0.193 | |
| Africa | 2.51 (1.45 to 4.33) | 0.001* | |
| Other | 2.80 (1.63 to 4.79) | <0.001 | |
| Educational attainment | |||
| None | Ref | ||
| Lower secondary | 1.32 (0.96 to 1.82) | 0.090 | |
| Upper secondary or vocational | 1.14 (0.63 to 2.05) | 0.670 | |
| Household status | |||
| Isolated adult | Ref | Ref | |
| Isolated parent | 1.78 (1.18 to 2.67) | 0.006 | 1.64 (1.07 to 2.52) |
| Family | 0.78 (0.55 to 1.11) | 0.168 | 0.78 (0.50 to 1.20) |
| Health insurance | |||
| No | Ref | ||
| Yes | 0.96 (0.69 to 1.34) | 0.800 | |
| Having financial resources | |||
| No | Ref | Ref | |
| Yes | 0.64 (0.47 to 0.86) | 0.003 | 0.70 (0.51 to 0.97) |
| Having work | |||
| No | Ref | ||
| Yes | 0.71 (0.45 to 1.12) | 0.110 | |
| Total length of homelessness (years) | |||
| ≤5 | Ref | ||
| >5 | 0.95 (0.69 to 1.30) | 0.700 | |
| Per cent of time spent in emergency shelters† | |||
| <33% | Ref | Ref | |
| 33%–66% | 1.68 (1.15 to 2.46) | 0.007 | 1.70 (1.11 to 2.62) |
| >66% | 2.45 (1.59 to 3.76) | <0.001 | 1.93 (1.18 to 3.15) |
| Number of daily contacts | |||
| ≤5 per day | Ref | Ref | |
| >5 to ≤15 per day | 1.21 (0.88 to 1.65) | 0.100* | 1.84 (1.27 to 2.67) |
| >15 per day | 0.68 (0.33 to 1.40) | 0.200 | 1.45 (0.69 to 3.04) |
| Wearing mask | |||
| No, somewhat no | Ref | ||
| Yes, somewhat yes | 1.23 (0.85 to 1.78) | 0.300 | |
| Physical distancing | |||
| No, somewhat no | Ref | ||
| Yes, somewhat yes | 1.61 (1.14 to 2.27) | 0.007* | |
| Hand washing | |||
| No, somewhat no | Ref | ||
| Yes, somewhat yes | 1.43 (0.99 to 2.06) | 0.060 | |
| Difficult access to hygiene products | |||
| No, never, rarely | Ref | Ref | |
| Yes, always, often | 0.72 (0.52 to 0.96) | 0.040 | 0.75 (0.50 to 1.12) |
| Difficult access to water | |||
| No, never, rarely | Ref | ||
| Yes, always, often | 0.76 (0.54 to 1.09) | 0.100 | |
| Difficult access to food | |||
| No, never, rarely | Ref | ||
| Yes, always, often | 0.83 (0.62 to 1.12) | 0.200 | |
| Smoking status | |||
| Non-smoker | Ref | Ref | |
| Smoker | 0.39 (0.28 to 0.53) | <0.001 | 0.46 (0.32 to 0.65) |
| Psychiatric or addictive comorbidity | |||
| No | Ref | Ref | |
| Yes | 0.46 (0.30 to 0.69) | <0.001 | 0.52 (0.32 to 0.85) |
The values noted in bold are significant
*We fitted a multivariable model containing all variables that were significant in a univariate analysis at the 10% level. We used the stepwise selection function in R (a mix between forward and backward selection), which starts with an empty model and adds predictors according to AIC criteria. Accordingly, ‘Country of birth’ and ‘physical distancing’ were considered in the multivariate model and removed. In addition, ‘number of contacts’ was forced into the model as a relevant variable.
†Percentage was calculated on the basis of each participant’s exposed time until the event or until the end of the follow-up in the absence of an event.
‡Total participants in the analysis: 1241; missing values exist for some of the independent variables; for example, smoking status (n=116), educational attainment (n=114), health insurance (n=70), self-reported financial resources (n=63) or household status (n=41).
§
¶
**
AIC, Akaike Information Criterion.
Figure 3.
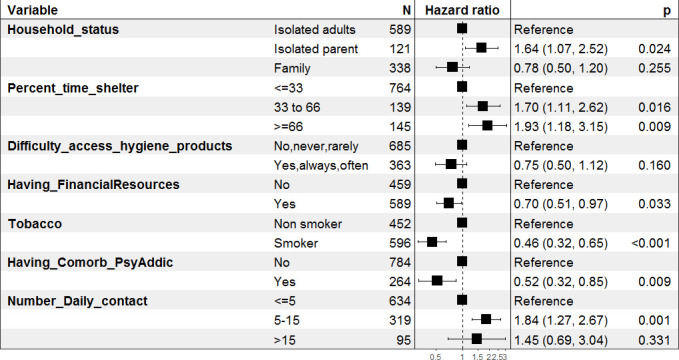
Cox multivariable logistic regression analysis of risk factors of SARS-CoV-2 seroprevalence in homeless people in Marseille. Having_Comorb_PsyAddic: psychiatric or addictive comorbidities.
bmjopen-2022-065734supp002.pdf (79.8KB, pdf)
Other potential risk factors, such as educational attainment, gender, age, total length of homelessness in the life of participants, wearing a mask, hand washing, difficult access to water or not having health insurance, were not associated with SARS-CoV-2 prevalence.
Symptomatology of participants with positive SARS-CoV-2 serological status
Among participants with SARS-CoV-2 infection (positive IgM or IgG or both, n=180), 67.6% reported no symptoms (table 3). Among participants with symptomatic SARS-CoV-2 infections, the most common symptoms were fever, cough, headache and fatigue. Even if participants with a positive serological status reported COVID-19 syndrome (fever, cough, anosmia, headache notably) significantly more often than participants without serological immunity (table 3), the frequency of symptoms reported did not appear to be strictly specific to SARS-CoV-2 infection.
Table 3.
Symptoms reported in the last 3 months prior the serological test according to serological status (n=1241)
| Negative serological status n (%) |
95% CI* | Positive serological status n (%) |
95% CI* | P value | |
| Individuals missing symptom data (n=303) | 302 (24.3) | 1 (0.1) | |||
| Individuals with symptom data (n=938) | 759 (75.7) | 179 (99.9) | |||
| Asymptomatic patient | 656 (86.4) | 84.7 to 91.4 | 121 (67.6) | 65.8 to 71.5 | <0.001 |
| Participants with symptoms | 103 (13.6) | 11.7 to 14.3 | 58 (32.4) | 30.6 to 34.1 | |
| Fever | 42 (4.0) | 2.1 to 4.1 | 36 (18.0) | 16.1 to 18.9 | <0.001 |
| Cough | 38 (3.7) | 1.8 to 3.8 | 28 (14.0) | 12.1 to 14.6 | <0.001 |
| Dyspnoea | 16 (1.5) | 0.0 to 1.5 | 8 (4.0) | 2.1 to 4.1 | 0.040 |
| Headache | 41 (3.9) | 2.0 to 4.0 | 36 (18.0) | 16.2 to 18.9 | <0.001 |
| Anosmia | 15 (1.4) | 0.0 to 1.4 | 21 (10.5) | 8.6 to 10.9 | <0.001 |
| Rhinitis | 39 (3.7) | 1.8 to 3.8 | 21 (10.5) | 8.6 to 10.9 | <0.001 |
| Fatigue | 35 (3.4) | 1.5 to 3.5 | 38 (19.0) | 17.1 to 20.0 | <0.001 |
| Diarrhoea | 15 (1.4) | 0.0 to 1.4 | 15 (7.5) | 5.6 to 7.8 | <0.001 |
| Joint pain | 15 (1.4) | 0.0 to 1.4 | 19 (9.5) | 7.6 to 10.0 | <0.001 |
| Odynophagia | 22 (2.1) | 0.0 to 2.1 | 14 (7.0) | 5.1 to 7.3 | <0.001 |
| Chills | 21 (2.0) | 0.0 to 2.0 | 17 (8.5) | 6.6 to 8.8 | <0.001 |
| Mottling | 1 (0.1) | 0.0 to 0.1 | 0 (0) | 0.0 to 0.1 | 0.999 |
| Skin rash | 1 (0.1) | 0.0 to 0.1 | 3 (1.5) | 0.0 to 1.5 | 0.014 |
| Conjunctivitis | 9 (0.8) | 0.0 to 0.7 | 8 (4.0) | 2.1 to 4.1 | 0.002 |
| Other | 5 (0.5) | 0.0 to 0.4 | 2 (1.0) | 0.0 to 0.9 | 0.316 |
*An exact test of a simple null hypothesis about the probability of success in a Bernoulli experiment was performed, with confidence level for the returned CI.
Discussion
The present study is the first to describe the dynamics of SARS-CoV-2 seroprevalence among a large cohort of 1241 homeless people living in Marseille, France. Analysis of data from homeless participants with positive serology results over time revealed a high prevalence of asymptomatic infection and significant associations between positive serology and the lack of financial resources, being an isolated parent, having between 5 and 15 daily contacts and the time spent in emergency shelters. Repeated seroprevalence studies enabled to estimate the cumulative incidence of SARS-CoV-2 infection in both asymptomatic and symptomatic people, offering valuable data to inform public health policy makers.7 21 In the general population, asymptomatic individuals represent up to 68% of SARS-CoV-2 infections7 22 and contribute to the rapid spread of the disease.1 In our study, the estimated prevalence of SARS-CoV-2 increased from 6.01% (4.68–7.34) in June to August to 18.86% (16.00–21.72) in September to December and remained higher than in the general population. Indeed, a cross-sectional study evaluating the seroprevalence of SARS-CoV-2 antibodies across the general population in Marseille in May and November 2020 found 3.0% (1.9–4.2) and 6.5% (95% CI 4.5%–8.7%), respectively. The increasing gap in seroprevalence between the general population and the homeless population may be due to a potential breakdown of protective measures for people in the most precarious situations.5 The available data on homeless people come from cross-sectional studies, that mainly found high seroprevalence.7 10 11 However, the testing approach was different and concerned a population selected from one type of accommodation (mainly emergency shelters) and results also depend on the intensity of the local epidemic at the time of the survey. A lower infection rate with increasing age was reported in several population-based serological studies, which is not consistent with our findings.23 24 We observed no differences, in univariate analysis, in estimated seropositivity for older participants or for participants who had comorbidities. These results suggest that aged homeless people at risk of severe COVID-19 disease may be infected by SARS-CoV-2 at the same rate as other adults. The pandemic has played an important role in amplifying health inequalities that already existed.25 26 Increasing evidence has emerged, highlighting that COVID-19 mortality is higher for those who are socioeconomically deprived. We reported in our findings, in addition to the poorest condition of homelessness, that not having financial resources during the pandemic crisis was also a risk factor of SARS-CoV-2 seroprevalence. The link between socioeconomic status and development of infectious disease is well documented, and the main mechanisms reported to be associated with higher occurrence rates of communicable diseases included poor housing, lack of education, nutritional deficiencies, poor work conditions and hygiene.27
African homeless immigrants had higher SARS-CoV-2 prevalence rates in our study compared with other nationalities. This is despite the fact that North and sub-Saharan Africans are grouped together for analysis in our study. These findings were consistent with French, English or US studies which reported higher seroprevalence rates and mortality in black ethnic groups.9 28 29 The homeless are a heterogeneous population. Even if homeless people already face disparity in health outcomes in the current COVID-19 pandemic, African immigrants are a subgroup at even more risk. Thus, it could be important to generate accessible health information and preventive measures for this subgroup, adapted to their literacy and specific needs.
Our study reported lower SARS-CoV-2 infection rates in participants with mental disorders or substance abuse. This is surprising since substance use disorders have frequently been reported to increase the risk of infectious diseases and mental illness to impact awareness of vulnerability to infection and help seeking when symptoms of COVID-19 develop.30 31 Since social contacts are the way in which the infection is spread, this lower seroprevalence could be interpreted as a sign of exclusion of these particularly stigmatised people.32 It should also be noted that in Marseille there are specific healthcare mobile teams for people suffering from mental disorders and substance abuse.33 This type of specific programme has previously reported positive results in pandemic context.34
In line with the findings of other studies, we observed a considerable proportion of positive subjects (67.6%) with asymptomatic infection.22 35 36 Some symptoms (fever, cough, headache or fatigue notably) were significantly associated with positive SARS-CoV-2 serology and should be repeated to people during interventions on prevention and information.
As previously described, smoking prevalence was lower in seropositive SARS-CoV-2 participants in our study.37 Even if prevalence was lower, smoking was associated with an increased risk of hospitalisation and morbidity.38 In our study, a substantial proportion of participants reported alcohol and tobacco consumption. In homeless populations a large proportion of deaths are therefore substance attributable.39
Our study reinforces the negative role of overcrowded types of accommodation for homeless people, which increase SARS-CoV-2 transmission. In the USA and France, emergency shelters and their high population density appear to increase the risk of infection.7 11 Indeed, the emergency shelters are short-term shelters that can accommodate several hundred people in Marseille with common sanitary facilities.40 Collective transitional shelters are longer term, smaller facilities offering more consistent social work. The first French lockdown was ordered on 17 March 2020, as emergency shelters are already full in normal times, hotels were required. In these hotels, people did not have kitchen facilities and often found themselves in high-density grouping areas, especially at mealtimes or in the few outdoor spaces available.40 Shelters should be considered as high-risk environments and stays there should be limited to the minimum. Providing adequate housing with individual bathroom facilities could be the most effective strategy for mitigating SARS-CoV-2 transmission in homeless communities, as was reported in a modelling study and by field healthcare workers.41 42 Our findings also show an association with a high prevalence in shelters and hostels, which highlights the limits of individual preventive measures in transitional collective accommodations. These studies illustrate a good compliance with preventive measures, notably in collective accommodations, but these are clearly insufficient to limit the spread of infection. Homeless people in the pandemic must face concurrent risks: the risk of SARS-CoV-2 infection in shelters and collective accommodation, and the risk of the lack of access to food, water or hygiene products in more insecure housing conditions.43
Throughout the course of the pandemic, healthcare and housing programmes for homeless people have been modified. However, our results suggest that overcrowded and large emergency shelters or transitional accommodation including hostels increase the risk of SARS-CoV-2 transmission which pleads to adapt social and public health infrastructures towards good-quality, smaller and semiprivate accommodation. Holistic action (food, hygiene and financial support, health insurance, specific vaccination programme…) must also be taken to ensure that the needs of these individuals are met sufficiently for them to be able to limit viral spread, survive this pandemic and be well enough equipped to endure the following economic crisis.
In addition, our findings highlight two other risk factors linked to socioeconomic inequalities: the lack of financial resource and being an isolated parent. Furthermore, people with low financial resources or single parents with one child are potentially more likely to seek outside support which may increase the risk of viral exposure, as it was previously described for people who have to work outside.44
Strengths and limitations
Our study has a number of strengths. Although studies have shown an increased risk of SARS-CoV-2 infection in homeless people, this is the first surveillance data from a cohort providing an incidence rate of seroconversion and comparing seroprevalence with the general population at two different time points. Our study described risk factors of SARS-CoV-2 infection in a large study population with high-quality and statistically robust methodology. Our findings quantify the excess risk associated with staying in emergency shelters. We also found evidence of SARS-CoV-2 infection among the most economically vulnerable (lack of financial resources, isolated parent) highlighting the need for a comprehensive and proactive approach including financial aid, food, water, adequate housing, mobile healthcare and social assistance for this vulnerable population.
Our study has also some limitations. Although it is representative of different homelessness categories (living in the street, slum, squat, emergency shelter or transitional accommodation), the sample was not randomly enrolled and therefore our findings may not reflect true seroprevalence, as a potential selection bias cannot be excluded. Characteristics of study participants on inclusion and at the end of follow-up were also different, roofless and younger populations were most frequently lost to follow-up because of a higher mobility. We may also underestimate seroprevalence and false negative results cannot be excluded.45 46 Finally, the serological tests between the general population and the homeless population were not exactly the same. This may account for some of the observed differences in seroprevalence rates between the two populations.
Policy implications and further research
There is growing evidence that, in addition to usual preventive measures, public policies should pay attention to adapt the type of accommodation and overall approach of precariousness. The findings of this study can guide European and other governments’ disease control planning; to find optimal solutions to house people in less crowded accommodations, prioritising individual rather than collective settings and a global approach, thus restricting transmission. To complete these results, future studies in this vulnerable population should assess the morbidity and mortality associated with SARS-CoV-2.
Conclusions
The longitudinal cohort of homeless people in Marseille revealed an increase in the seroprevalence of SARS-CoV-2 infection. This was higher than that observed in the general population and reflects precarious living conditions and inadequate types of accommodation for this vulnerable population.
Supplementary Material
Acknowledgments
We would like to thank all the homeless people who participated in this study by providing their samples and questionnaires. We would also like to thank also the nurses and mediators involved in the ‘COVID homeless’ project: Maud Landreau, Anne Ranise and Nathalie Vuagniaux, Grâce Inegbeze, Snjezana Huette, Marko Sava, Lisa Hasse, Mathieu Ledu, Alejandro Vernet and Drisse Mekkaoui; the ASSAb network; the homeless outreach teams: ADDAP13, AMPIL, ASUD, Bus 31/32, Equipe Mobile d’Aide (EMA) Saralogisol, Equipe Mobile santé ADJ Marceau, Equipe Mobile Gare et Connexion ADJ Marceau, MARSS-APHM, Nouvelle Aube, Mission Bidonville Médecins du monde, PASS adulte APHM, Réseau Santé Marseille Sud (RSMS); the emergency shelters: UHU La Madrague-ville, St Louis, Sleep’in (groupe SOS Solidarité), Forbin (Fondation St Jean de Dieu), Marius Macias (AAJT); and the people involved in providing collective accommodation for homeless people: Marius Macias (AAJT); William Booth (Armée du Salut); Le Marabout, le Mascaret (Habitat Alternatif Social); Foyer Honorat (Hospitalité pour les Femmes); Jane Pannier (Maison de la Jeune Fille); SHAS (Saralogisol) Coco Velten, La Selonne (groupe SOS Solidarité); Forbin (St Jean de Dieu); Village Club du Soleil, and Prospective Coopération for their work in this study and improving the care management of homeless people in Marseille.
Footnotes
Contributors: EMos, SL, EMon and AT were involved in the first draft of the manuscript. EMos, SL, EMon, AA, JL, LN, TB, MM, SNW, JW and AT contributed to writing of the manuscript. EMos, TB and MM collected the data and samples. EMos, LN and MM performed biological analysis. EMos, SL, AA and AT were involved in data analysis. EMos, SL, TB, PA and AT were involved in study design. All authors contributed to subsequent drafts and have reviewed and agreed with the content of the final manuscript. AT, as Principal Investigator of Covidhomless project, declare to be guarantor of this study.
Funding: This work was supported by the French directorate of healthcare facilities (DGOS)—research grant PHRC COVID-19 (COVID-homeless-0047).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The data sets generated and analysed during the current study are not publicly available due to special authorisation to transfer databases given by the CNIL. Upon prior authorisation by the CNIL, the data set would be available from the corresponding author upon reasonable request. Additionally, the study protocol is available upon request. All data requests should be addressed to the corresponding author.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was approved by the French Ethics Committee of Ile-de-France VI on 28 May 2020 (CPP IDF VI, No 44-20; ID: 2020-AO1398-31). Participants gave informed consent to participate in the study before taking part.
References
- 1.Baggett TP, Gaeta JM. COVID-19 and homelessness: when crises intersect. The Lancet Public Health 2021;6:e193–4. 10.1016/S2468-2667(21)00022-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang SW, Burns T. Health interventions for people who are homeless. Lancet 2014;384:1541–7. 10.1016/S0140-6736(14)61133-8 [DOI] [PubMed] [Google Scholar]
- 3.DIHL . Hébergement d’urgence les Hôtels mobilisés pour l’hebergement des personnes sans-abri. gouvernement.fr, 2020. Available: https://www.gouvernement.fr/hebergement-d-urgence-les-hotels-mobilises-pour-l-hebergement-des-personnes-sans-abri
- 4.FAP . 26e rapport sur l’état du mal-logement en france 2021. fondation abbé pierre, 2021. Available: https://www.fondation-abbe-pierre.fr/actualites/26e-rapport-sur-letat-du-mal-logement-en-france-2021
- 5.ALERTE P. Ultra-précarité en région PACA, l’avertissement du collectif ALERTE PACA. uriopss PACA et corse. 2020. Available: https://www.uriopss-pacac.fr/actualites/ultra-precarite-en-region-paca-lavertissement-du-collectif-alerte-paca [Accessed 30 Nov 2020].
- 6.Karb R, Samuels E, Vanjani R, et al. Homeless shelter characteristics and prevalence of SARS-cov-2. West J Emerg Med 2020;21:1048–53. 10.5811/westjem.2020.7.48725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roederer T, Mollo B, Vincent C, et al. Seroprevalence and risk factors of exposure to COVID-19 in homeless people in Paris, France: a cross-sectional study. The Lancet Public Health 2021;6:e202–9. 10.1016/S2468-2667(21)00001-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bean DJ, Monroe J, Turcinovic J, et al. Severe acute respiratory syndrome coronavirus 2 reinfection associates with unstable housing and occurs in the presence of antibodies. Clin Infect Dis 2022;75:e208–15. 10.1093/cid/ciab940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warszawski J, Bajos N, Meyer L. En mai 2020, 4, 5% de la population en france métropolitaine a développé des anticorps contre le SARS-cov-2. premiers résultats de l’enquête nationale epicov 2020.
- 10.Baggett TP, Keyes H, Sporn N, et al. Prevalence of SARS-cov-2 infection in residents of a large homeless shelter in Boston. JAMA 2020;323:2191–2. 10.1001/jama.2020.6887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosites E, Parker EM, Clarke KEN, et al. Assessment of SARS-cov-2 infection prevalence in homeless shelters - four U.S. cities, march 27-april 15, 2020. MMWR Morb Mortal Wkly Rep 2020;69:521–2. 10.15585/mmwr.mm6917e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doran KM, Ragins KT, Iacomacci AL, et al. The revolving hospital door: hospital readmissions among patients who are homeless. Med Care 2013;51:767–73. 10.1097/MLR.0b013e31829fafbb [DOI] [PubMed] [Google Scholar]
- 13.Russolillo A, Moniruzzaman A, Parpouchi M, et al. A 10-year retrospective analysis of hospital admissions and length of stay among a cohort of homeless adults in Vancouver, Canada. BMC Health Serv Res 2016;16:60. 10.1186/s12913-016-1316-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Shakarchi NJ, Evans H, Luchenski SA, et al. Cardiovascular disease in homeless versus housed individuals: a systematic review of observational and interventional studies. Heart 2020;106:1483–8. 10.1136/heartjnl-2020-316706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loubière S, Monfardini E, Allaria C, et al. Seroprevalence of SARS-CoV-2 antibodies among homeless people living rough, in shelters and squats: a large population-based study in France. PLoS One 2021;16:e0255498. 10.1371/journal.pone.0255498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunner A, Maurin L. Rapport sur La pauvreté en France, édition 2020-2021. Observatoire Des Inégalités 2020:104. [Google Scholar]
- 17.Ethos typology on homelessness and housing exclusion. 2021. Available: https://www.feantsa.org/en/toolkit/2005/04/01/ethos-typology-on-homelessness-and-housing-exclusion
- 18.Vauloup-Fellous C, Maylin S, Périllaud-Dubois C, et al. Performance of 30 commercial SARS-CoV-2 serology assays in testing symptomatic COVID-19 patients. Eur J Clin Microbiol Infect Dis 2021;40:2235–41. 10.1007/s10096-021-04232-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warszawski J, Bajos N, Barlet M. A national mixed-mode seroprevalence random population-based cohort on SARS-CoV-2 epidemic in France: the socio-epidemiological EpiCov study. Public and Global Health 2021. [Google Scholar]
- 20.SPF . Taux d’incidence de l’épidémie de COVID-19 (SI-DEP) - data.gouv.fr, 2021. Available: /fr/datasets/taux-dincidence-de-lepidemie-de-covid-19/
- 21.Murhekar MV, Bhatnagar T, Selvaraju S, et al. SARS-CoV-2 antibody seroprevalence in India, August-September, 2020: findings from the second nationwide household serosurvey. Lancet Glob Health 2021;9:e257–66. 10.1016/S2214-109X(20)30544-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ralli M, Morrone A, Arcangeli A, et al. Asymptomatic patients as a source of transmission of COVID-19 in homeless shelters. Int J Infect Dis 2021;103:243–5. 10.1016/j.ijid.2020.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrat F, Touvier M, Severi G, et al. Incidence and risk factors of COVID-19-like symptoms in the French general population during the lockdown period: a multi-cohort study. BMC Infect Dis 2021;21:169. 10.1186/s12879-021-05864-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stringhini S, Wisniak A, Piumatti G, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. The Lancet 2020;396:313–9. 10.1016/S0140-6736(20)31304-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorn Avan, Cooney RE, Sabin ML. COVID-19 exacerbating inequalities in the US. Lancet 2020;395:1243–4. 10.1016/S0140-6736(20)30893-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bambra C, Riordan R, Ford J, et al. The COVID-19 pandemic and health inequalities. J Epidemiol Community Health 2020;74:jech-2020-214401–8. 10.1136/jech-2020-214401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sommer I, Griebler U, Mahlknecht P, et al. Socioeconomic inequalities in non-communicable diseases and their risk factors: an overview of systematic reviews. BMC Public Health 2015;15:914. 10.1186/s12889-015-2227-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anand S, Montez-Rath M, Han J. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet Published Online First 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aldridge RW, Lewer D, Katikireddi SV, et al. Black, Asian and minority ethnic groups in England are at increased risk of death from COVID-19: indirect standardisation of NHS mortality data. Wellcome Open Res 2020;5:88. 10.12688/wellcomeopenres.15922.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serota DP, Barocas JA, Springer SA. Infectious complications of addiction: a call for a new subspecialty within infectious diseases. Clin Infect Dis 2020;70:968–72. 10.1093/cid/ciz804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neto MLR, de Souza RI, Quezado RMM, et al. When basic supplies are missing, what to do? Specific demands of the local street population in times of coronavirus - a concern of social psychiatry. Psychiatry Res 2020;288:112939. 10.1016/j.psychres.2020.112939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mejia-Lancheros C, Lachaud J, O'Campo P, O’Campo P, et al. Trajectories and mental health-related predictors of perceived discrimination and stigma among homeless adults with mental illness. PLoS One 2020;15:e0229385. 10.1371/journal.pone.0229385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tinland A, Loubière S, Boucekine M, et al. Effectiveness of a housing support team intervention with a recovery-oriented approach on hospital and emergency department use by homeless people with severe mental illness: a randomised controlled trial. Epidemiol Psychiatr Sci 2020;29:e169. 10.1017/S2045796020000785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin C, Andrés P, Bullón A, et al. COVID pandemic as an opportunity for improving mental health treatments of the homeless people. Int J Soc Psychiatry 2021;67:335-343. 10.1177/0020764020950770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milazzo L, Lai A, Pezzati L. Dynamics of the seroprevalence of SARS-cov-2 antibodies among healthcare workers at a COVID-19 referral hospital in Milan, Italy. Occup Environ Med 2021. [DOI] [PubMed] [Google Scholar]
- 36.Rivett L, Sridhar S, Sparkes D, et al. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife 2020;9. doi: 10.7554/eLife.58728. [Epub ahead of print: 11 May 2020]. 10.7554/eLife.58728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guan W-J, Ni Z-Y, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med n.d.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cattaruzza MS, Zagà V, Gallus S, et al. Tobacco smoking and COVID-19 pandemic: old and new issues. A summary of the evidence from the scientific literature. Acta Biomed 2020;91:106–12. 10.23750/abm.v91i2.9698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baggett TP, Chang Y, Singer DE, et al. Tobacco-, alcohol-, and drug-attributable deaths and their contribution to mortality disparities in a cohort of homeless adults in Boston. Am J Public Health 2015;105:1189–97. 10.2105/AJPH.2014.302248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allaria C, Loubière S, Mosnier E, et al. "Locked down outside": Perception of hazard and health resources in COVID-19 epidemic context among homeless people. SSM Popul Health 2021;15:100829. 10.1016/j.ssmph.2021.100829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baggett TP, Scott JA, Le MH, et al. Clinical outcomes, costs, and cost-effectiveness of strategies for adults experiencing sheltered homelessness during the COVID-19 pandemic. JAMA Netw Open 2020;3:e2028195. 10.1001/jamanetworkopen.2020.28195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MIM . 120 sans-abri relogés dans le village club du soleil la belle de mai. made in marseille, 2020. Available: https://madeinmarseille.net/67072-120-personnes-sans-abri-relogees-dans-le-village-club-du-soleil-a-la-belle-de-mai/
- 43.Locked down outside”: perception of hazard and health resources in COVID-19 epidemic context among homeless people. 2021. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8160278 [Accessed 31 May 2021]. [DOI] [PMC free article] [PubMed]
- 44.Bajos N, Franck J-E, Counil E. Social inequalities and dynamics of the COVID-19 epidemic: evidence from france. EPICOV study, social inequalities and dynamics of the COVID-19 epidemic: evidence from france. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin Y, Wang M, Zuo Z, et al. Diagnostic value and dynamic variance of serum antibody in coronavirus disease 2019. Int J Infect Dis 2020;94:49–52. 10.1016/j.ijid.2020.03.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brandstetter S, Roth S, Harner S, et al. Symptoms and immunoglobulin development in hospital staff exposed to a SARS‐CoV‐2 outbreak. Pediatr Allergy Immunol 2020;31:841–7. 10.1111/pai.13278 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-065734supp001.pdf (82KB, pdf)
bmjopen-2022-065734supp002.pdf (79.8KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data sets generated and analysed during the current study are not publicly available due to special authorisation to transfer databases given by the CNIL. Upon prior authorisation by the CNIL, the data set would be available from the corresponding author upon reasonable request. Additionally, the study protocol is available upon request. All data requests should be addressed to the corresponding author.