Abstract
Aging is marked by complex and progressive physiological changes, including in the glutamatergic system, that lead to a decline of brain function. Increased content of senescent cells in the brain, such as glial cells, has been reported to impact cognition both in animal models and human tissue during normal aging and in the context of neurodegenerative disease. Changes in the glutamatergic synaptic activity rely on the glutamate-glutamine cycle, in which astrocytes handle glutamate taken up from synapses and provide glutamine for neurons, thus maintaining excitatory neurotransmission. However, the mechanisms of glutamate homeostasis in brain aging are still poorly understood. Herein, we showed that mouse senescent astrocytes in vitro undergo upregulation of GLT-1, GLAST, and glutamine synthetase (GS), along with the increased enzymatic activity of GS and [3H]-D-aspartate uptake. Furthermore, we observed higher levels of GS and increased [3H]-D-aspartate uptake in the hippocampus of aged mice, although the activity of GS was similar between young and old mice. Analysis of a previously available RNAseq dataset of mice at different ages revealed upregulation of GLAST and GS mRNA levels in hippocampal astrocytes during aging. Corroborating these rodent data, we showed an increased number of GS + cells, and GS and GLT-1 levels/intensity in the hippocampus of elderly humans. Our data suggest that aged astrocytes undergo molecular and functional changes that control glutamate-glutamine homeostasis upon brain aging.
Keywords: astrocyte, glutamate-glutamine cycle, GLT-1, aging, hippocampus, senescence
Introduction
Astrocytes comprise a large and heterogeneous glial cell population in the central nervous system (CNS), playing key roles during brain development, adulthood, and aging. They form a non-overlapping and functional syncytium, through which they contact other cells, synapses, and blood vessels, thus maintaining the homeostasis of the CNS (Verkhratsky & Nedergaard, 2018). In addition, astrocytes actively integrate and control the synaptic environment, providing metabolic support to neurons and modulating synapse plasticity and transmission (Diniz et al., 2014). These cells uptake about 90% of the glutamate, the main CNS excitatory neurotransmitter, through the activity of excitatory amino acid transporters (EAATs), such as the glutamate-aspartate transporter (GLAST/EAAT1) and the glutamate transporter-1 (GLT-1/EAAT2) (Danbolt, 2001). Once inside the astrocytes, glutamate is readily converted to glutamine by the enzyme glutamine synthetase (GS) or oxidized in the Krebs cycle to supply energy demands (Sonnewald & Schousboe, 2016). Glutamine is then released into the extracellular space to be taken up by neurons and serves as a precursor for the synthesis of glutamate or γ-aminobutyric acid (GABA) (Mahmoud et al., 2019).
The glutamate-glutamine cycle plays important roles in brain energy metabolism and physiology, including regulating ammonia detoxification and synaptic transmission (Limón et al., 2021; Tani et al., 2014). In fact, the replenishment of presynaptic glutamate relies on the glutamate-glutamine cycle, in which the astrocytic glutamine supply is essential to synaptic activity and memory formation (Cheung et al., 2022). In contrast, impairments in this cycle are linked to elevated levels of extracellular glutamate and hyperactivation of glutamatergic neuronal receptors, which ultimately may lead to neuronal death. This process, known as glutamatergic excitotoxicity, is a hallmark of many brain disorders (Armada-Moreira et al., 2020).
Dysregulation of the levels and activity of glutamate transporters and GS have been linked to the pathogenesis of age-related neurodegenerative diseases, such as Alzheimer's disease (AD) and Parkinson's disease (PD) (Todd & Hardingham, 2020). Nevertheless, evidence has indicated that the glutamate-glutamine cycle control varies depending on the type and stage of disease and the experimental model. It has been reported that decreased levels of GLT-1 and/or GS in both AD human brain and mouse models of AD may contribute to glutamate excitotoxicity observed in the disease (Hoshi et al., 2018; Jacob et al., 2007; Kulijewicz-Nawrot et al., 2013; Olabarria et al., 2011). On the contrary, we recently demonstrated that astrocytes exposed to α-synuclein oligomers, the main neurotoxin in PD, showed increased glutamate uptake and upregulation of GLT-1 and GLAST, suggesting a neuroprotective mechanism by which astrocytes control glutamatergic excitotoxicity in early stages of PD (Diniz et al., 2020). Despite the evidence in pathological models, the regulation of the glutamate-glutamine cycle in physiological brain aging remains to be elucidated.
Aging is accompanied by changes in the phenotype and function of brain cells, which may contribute to cognitive decline and increased risk for neurodegenerative diseases (Matias et al., 2019). Among the age-related changes, the accumulation of senescent cells has been described as a harmful process to brain homeostasis and function (Guerrero et al., 2021; Shimabukuro et al., 2016). Recently, we showed that senescent astrocytes display morphological, molecular, and functional alterations, including nuclear deformations and impaired neuritogenic and synaptogenic capacity (Matias et al., 2022). It has been shown decreased levels of GS and glutamate transporters in different in vitro models of murine astrocyte senescence (Bellaver et al., 2017; Cao et al., 2019; Limbad et al., 2020). Nevertheless, the involvement of senescent astrocytes in controlling the glutamate-glutamine cycle during aging in rodents and humans remains elusive and somehow controversial.
Here, we investigated the molecular and functional profile of glutamate transporters and GS in an in vitro model of astrocyte senescence and in the rodent and human hippocampus upon aging. Our findings indicate an overall age-dependent upregulation of GS and glutamate transporters levels and activity in senescent astrocytes and in the hippocampus of old rodents and humans. We suggest that age-associated upregulation of glutamate-glutamine cycle-related proteins in mouse and human astrocytes may contribute to metabolic changes upon aging.
Experimental Procedures
Animals
Newborn (P0) Swiss mice were used for astrocyte cultures. For in vivo experiments, we used male C57Bl/6 mice, separated into two age groups: 2-3 months-old (young group) and 18–25 months-old (old group). All animals were housed at standard conditions with ad libitum access to food and water. Animal handling and experimental procedures were previously approved by the Animal Use Ethics Committee of the Federal University of Rio de Janeiro (CEUA-UFRJ, approval protocol A23/21-006-18). Experiments were performed according to the Brazilian Guidelines on Care and Use of Animals for Scientific and Teaching Purposes (DBCA).
Human Post-Mortem Brain Material
Human post-mortem brain tissue was obtained from the Biobank for Aging Studies (BAS), University of São Paulo Medical School. A written informed consent for a brain donation and the use of the material and clinical information for research purposes had been obtained by the BAS (CAAE number: 30038520.0.0000.5257, approval number 3.986.070). We used paraffin-embedded hippocampal tissue from 17 donors, classified into two age groups: middle-aged (ranged from 50–60 years, n = 10) and elderly (ranged from 76–93 years, n = 7). Only individuals without dementia were included in the analysis based on the medical history and pathological scoring (Supplementary Table 1).
Control and Senescent Mouse Astrocyte Cultures
Primary control and senescent astrocyte cultures were derived from newborn Swiss mice as previously described (Matias et al., 2022). Briefly, cerebral cortices were removed, the meninges carefully stripped off, and the tissues were maintained in Dulbecco's minimum essential medium (DMEM) and nutrient mixture F12 (DMEM/F12, Invitrogen), supplemented with 10% fetal bovine serum (FBS, Invitrogen). Cultures were incubated at 37°C in a humidified 5% CO2, 95% air chamber for approximately 7 days in vitro (DIV) until confluence and then treated with cytosine arabinoside (Ara C 10 μM, Sigma) in DMEM/F12 with 10% FBS for 48 h. After that, control cultures were washed and maintained in DMEM/F12 without FBS for 24 h until fixation or RNA extraction. Senescent astrocyte cultures were washed and maintained in DMEM/F12 supplemented with 10% FBS for 30–35 DIV, with medium exchange every two days. Twenty-four hours before fixation or RNA extraction, senescent astrocyte cultures were incubated in DMEM/F12 without FBS.
Immunocytochemistry of Astrocyte Cultures
Astrocyte cultures were fixed with 4% PFA in PBS (pH 7.4) for 15 min, and nonspecific sites were blocked with 3% bovine serum albumin (BSA; Sigma-Aldrich), 5% normal goat serum (Sigma-Aldrich), and 0.2% Triton X-100 diluted in PBS for 1 h, before incubation with the primary antibodies: rabbit anti-GLT-1 (1:1,000; Abcam), mouse anti-glutamine synthetase (1:500; Millipore), rabbit anti-GLAST (1:500; Abcam) at 4°C overnight. Subsequently, the cells were washed with PBS and incubated with secondary antibodies at room temperature (RT) for 2 h. Secondary antibodies were Alexa Fluor 488-conjugated goat anti-rabbit IgG or goat anti-mouse IgG (1:300; Invitrogen). Nuclei were counterstained with DAPI (Sigma-Aldrich), and cells were observed with a TE2000 Nikon microscope.
Immunohistochemistry of Human Paraffin-Embedded Hippocampal Tissue
Immunohistochemistry of human paraffin-embedded tissue was done according to Matias et al., 2022. Paraffin sections were deparaffinized, rehydrated, and washed in distilled water, followed by PBS/0.05% Tween 20 for 30 min. After that, sections were submitted to antigen retrieval through exposure heating in a steamer in citrate buffer (10 mM citric acid, 0.05% Tween 20, pH 6.0; 98°C) for 20 min. After cooling down to RT, nonspecific sites were blocked with 5% normal goat serum (NGS, Sigma-Aldrich), 2% BSA (Sigma-Aldrich), 0.1% Triton X-100 diluted in PBS for 1 h before incubation with the primary antibodies: mouse anti-glutamine synthetase (1:500; Millipore) and rabbit anti-GLT-1 (1:1,000; Abcam) at 4°C overnight. The sections were washed in PBS and incubated with secondary antibody Alexa Fluor 594-conjugated goat anti-mouse |IgG (1,000; Molecular Probes) and Alexa Fluor 488-conjugated goat anti-rabbit IgG at RT for 2 h. Next, sections were washed in PBS and incubated in Sudan Black solution (0.3% Sudan Black in 70% ethanol) for 7 min to quench autofluorescence, then washed in 70% ethanol for 1 min, followed by an additional wash in PBS. Nuclei were counterstained with Hoechst 33528 or DAPI, and coverslips were mounted in a mounting medium (DakoCytomation) and imaged on a confocal microscope (Leica TCS SPE).
Western Blotting
Protein concentration in hippocampal extracts from mice and human tissue was measured using the BCA Protein Assay Kit (Cole-Parmer). Forty micrograms of protein/lane were electrophoretically separated on a 12% SDS polyacrylamide gel and electrically transferred onto a Hybond-P PVDF transfer membrane (Millipore) for 1 h. Membranes were blocked in PBS-milk 5% at RT for 1 h. Next, membranes were incubated in block solution overnight with the following primary antibodies: mouse anti-glutamine synthetase (1:500; Abcam), mouse anti-GAPDH (1:1,000; Abcam), rabbit anti-Cyclophilin B (1:1,000; Sigma) and rabbit anti-β-actin (1:1,000; Abcam). Membranes were incubated for 1 h with IRDye 680CW goat anti-mouse antibody, IRDye 800CW goat anti-mouse antibody, or IRDye 800CW goat anti-rabbit antibody (LI-COR, 1:20,000), then scanned with an Odyssey infrared imaging system (LI-COR) and analyzed using Un-Scan-It gel version 6.1 (Silk Scientific).
Quantitative RT-PCR (qPCR)
The astrocyte cultures were lysed with TRIzol® (Invitrogen), and total RNA was isolated and purified with Direct-zol™ MiniPrep Plus (Zymo Research, Irvine, CA, USA) according to the manufacturer's protocol. The RNA was quantified using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The total RNA (1-2 μg) was reverse transcribed with a GoScriptTM Reverse Transcriptase cDNA reverse transcription kit according to the manufacturer's instructions (Promega Corporation, an affiliate of Promega Biotecnologia do Brasil, Ltda). Primers were designed and synthesized by IDT-DNA (San Diego, CA, USA). The specific forward and reverse oligonucleotides were as follows: GLT-1: (F) CCT CCC TCT TAT CAT CTC CA, (R) CAC CTC GTC GTT CTT CTT C; GS (F): CCT GAG TGG AAC TTT GAT GG, (R) GCT CAC CAT GTC CAT TAT CC; GLAST: (F) CTG GTA ACC CGG AAG AAC CC, (R) GGG GAG CAC AAA TCT GGT GA; and the reference gene RPLP0 (ribosomal protein lateral stalk subunit P0): (F) CAG GTG TTT GAC AAC GGC AGC ATT, (R) ACT CAG TCT CCA CAG ACA ATG CCA. Quantitative real-time PCR was performed using Fast SYBR Green Master Mix qPCR Master Mix (Applied BiosystemTM); the cycling conditions were 95°C for 20 s, and 40 cycles of 95°C for 1 s, 60°C for 20 s in the Quant Studio 7 Flex System (Applied Biosystem TM). The relative expression levels of the genes were calculated using the 2 − ΔΔCT method (Livak & Schmittgen, 2001).
Glutamine Synthetase Activity
The enzymatic assay was performed as described by Minet et al. (1997) and slightly modified by (Busanello et al., 2014). Briefly, cell extracts from control and senescent astrocytes cultures and hippocampal homogenates from young and aged rodents were added to a reaction mixture containing 50 mM imidazole buffer, 50 mM hydroxylamine, 25 mM sodium arsenate (pH 6.8), 100 mM glutamine, 2 mM MgCl2 and 0.2 mM ADP and incubated for 15 min at 37 °C. The reaction was stopped by adding a solution containing 370 mM ferric chloride, 100 mM HCl, and 50 mM trichloroacetic acid. After centrifugation, the absorbance of the supernatant was measured at 540 nm and compared to a calibration curve of γ-glutamylhydroxamate treated with ferric chloride reagent. Results were expressed in μmol/hour/mg of protein or nmol/min/mg of protein.
[3H]-D-Aspartate Uptake
Astrocytes cultures or hippocampal tissues were incubated for 1 h in 1 mL of Hank's buffered at pH 7.4 at 37°C or 4°C (to block aspartate uptake) containing 1µCi of [3H]-D-Aspartate (35 Ci/mmol = 35.106 mCi). The incubation medium was removed, and the culture or tissue was washed three times with 3 mL of cold Hank's (128 mM NaCl, 4 mM KCl, 1 mM MgCl2, 3 mM CaCl2, 20 mM HEPES, 4 mM Glucose). This process was sufficient to wash the free radioactivity (not absorbed by the cultures/tissue). Then, 1 mL of water was added to disrupt cell membranes. Following repeated freeze-thaw cycles, cell radioactivity was assayed using a scintillation counter (Kubrusly et al., 2018). Protein concentration was estimated (Lowry et al., 1951), and results were expressed in pmol/mg/hour.
Evaluation of Mice Transcriptome Datasets
Single-cell RNAseq brain tissue data from young and aged mice was retrieved from GSE129788 (10.1038/s41593-019-0491-3). Data was explored, analyzed, and processed using CellDepot (DOI: 10.1016/j.jmb.2021.167425). Embedding plots were generated and cell classes colored (total of 6 classes). Expression levels are displayed for genes of interest. Bulk RNAseq of hippocampal astrocytes from mice in different ages was retrieved from BioProject accession number PRJNA417856 (10.1073/pnas.1800165115).
Densitometric Analysis of Astrocytes Culture
Densitometry for the immunocytochemistry images was based on the integrated density values generated by the ImageJ software (NIH, USA) and normalized by the number of cells per field. At least 10–17 images were acquired from duplicate coverslips per experimental condition. In the graphs where the control is set at 100%, each control culture was paired with its respective senescent culture and imaged independently from the other cultures. For each result, the exact number of astrocyte cultures per experimental group is indicated in the graph and legend.
Densitometric Analysis and Cell Count in Human Paraffin-Embedded Tissue
Human paraffin-embedded hippocampal tissues were immunostained for GS and GLT-1 and counterstained with Hoechst. The granule cell layer (GCL), polymorphic layer (PL), and molecular layer (ML) of the hippocampal dentate gyrus were imaged on a confocal microscope (Leica TCS SPE) using the same image parameters for middle-aged and elderly groups. Densitometry for the immunolabeling images was performed using integrated density values with the Fiji software (NIH, USA). The number of GS + cells per image was counted with the aid of the cell counter plugin from Fiji. The GS intensity and cell number values represent the mean of 10–12 images from the GCL, PL, and ML, from a total of 5-6 hippocampal tissue sections per donor. The GLT-1 intensity values represent the mean of 6 images from the GCL and ML, from a total of three hippocampal sections per donor. For each result, the exact number of donors is indicated in the graph and legend.
Statistical Analysis
Statistical analysis was done by Student's t-test, using GraphPad Prism version 8 (GraphPad Software, La Jolla, CA, USA). P-value <0.05 was considered statistically significant. Error bars represent the standard error of the mean (SEM). For each result, the exact number of experiments, animal samples, or human post-mortem hippocampal tissue donors are indicated in the graph and legend.
Results
Glutamate-Glutamine Cycle-Related Proteins Are Differentially Expressed in Mouse Astrocytes During Aging
The glutamate homeostasis in the healthy brain relies on the glutamate-glutamine cycle, in which synaptically-released glutamate is continuously recycled by astrocytes and intracellularly converted to glutamine by the enzymatic activity of glutamine synthetase (GS) (Mahmoud et al., 2019). However, it remains unknown the impact of brain aging in this cycle. To address this question, we first evaluated which cellular population in the mouse brain mainly expresses the molecular components of the glutamate-glutamine cycle.
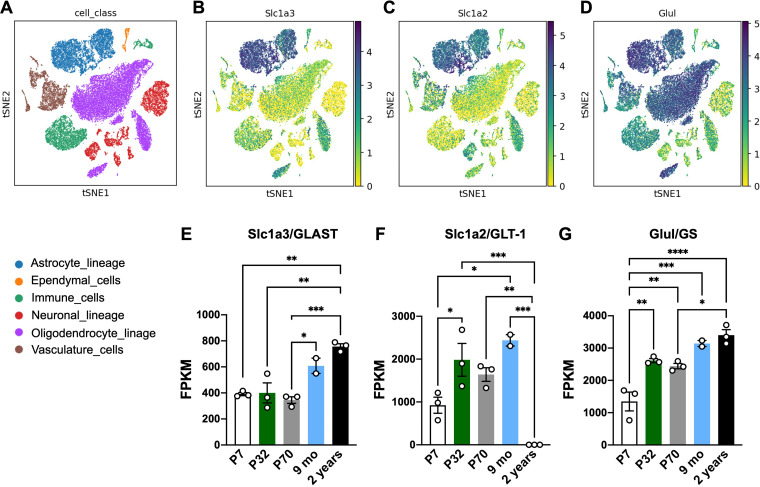
We analyzed Slc1a3/GLAST, Slc1a2/GLT-1, and Glul/GS expression in a single-cell RNAseq study (Ximerakis et al., 2019). As expected, we observed that astrocytes account as the main cellular population expressing GLAST, GLT-1, and GS (Figure 1(A–D)), although a significant amount of GS expression could also be detected in oligodendrocytes, vasculature, and immune cells. To better evaluate the effect of aging in the levels of these molecules in astrocytes, we assessed Slc1a3/GLAST, Slc1a2/GLT-1, and Glul/GS levels in a bulk-RNAseq dataset evaluating mice at different ages (Clarke et al., 2018). We observed that GLAST (Figure 1(E)) and GS (Figure 1(G)) levels are upregulated in hippocampal astrocytes during aging compared to young animals). Interestingly, GLT-1 expression increased from P32 to 9 months-old of age but drastically decreased in the astrocytes of 2-year-old mice (Figure 1(F)).
Figure 1.
Glutamate-glutamine cycle-related proteins are upregulated in mouse astrocytes during aging. (A–D) Embedding plots displaying reads of Slc1a3 (GLAST), Glul (GS), and Slc1a2 (GLT-1) in distinct cell classes in the brain of young (2-3 months) and aged (21-22 months) mice following single-cell RNAseq analysis (extracted from Ximerakis et al., 2019). Cell classes are indicated by color. (E–G) Bulk RNA-seq reads of (E) Slc1a3 (GLAST), (F) Slc1a2 (GLT-1) and (G) Glul (GS) in isolated astrocytes from hippocampi of mice in different ages (extracted from Clarke et al., 2018 dataset). Readings are plotted as FPKM (Fragments Per Kilobase Million); Individual data points are plotted and represent individual animals (n = 3 animals/group). One-way ANOVA followed by Tukey multiple comparison test was performed. ****: p < 0.001; ***: p < 0.005; **: p < 0.01; *: p < 0.05.
Together, these data indicate that in the aging brain, astrocytes express increasing levels of glutamate transporters (GLAST and GLT-1) and GS, while GLT-1 expression is differentially modulated by age, switching from a high level in adult mice to a steady reduction in advanced age.
Senescence-Associated Upregulation of the Glutamate Transporters and GS in Astrocytes In Vitro
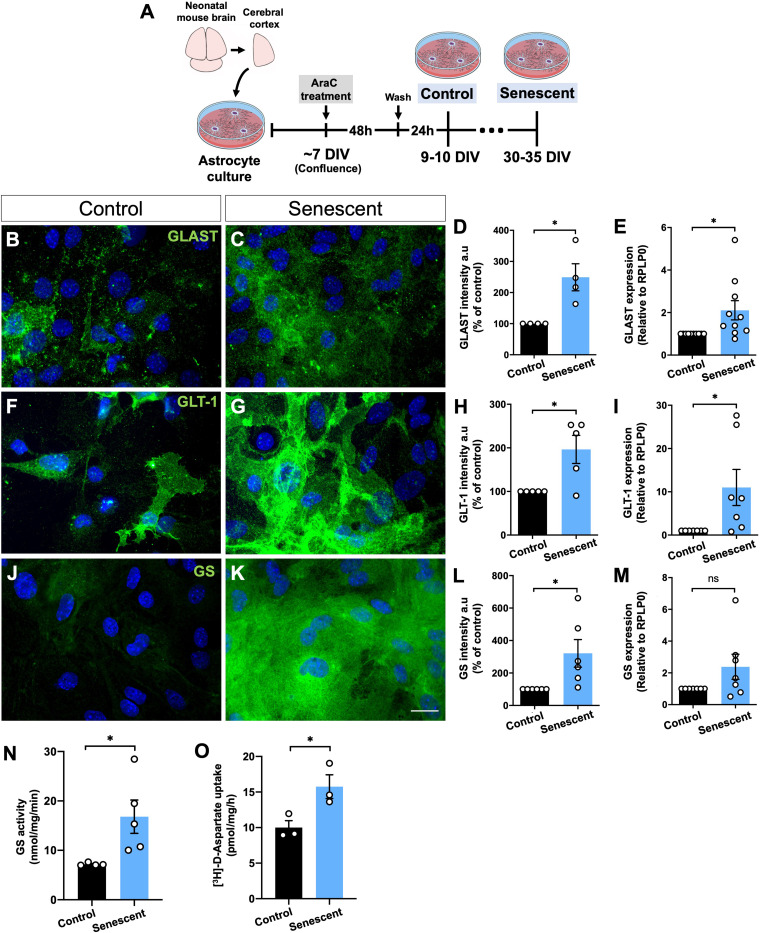
To further investigate the impact of aging in the glutamate transporters and GS in astrocytes, we used an in vitro model of senescent/aged astrocytes previously characterized (Matias et al., 2022). These cells presented senescence-associated secretory phenotype (SASP) and molecular and functional changes, including impaired neuritogenic and synaptogenic capacity (Matias et al., 2022). We observed that the immunofluorescence and mRNA expression of GLT-1 and GLAST were increased in senescent astrocytes compared to the control group (Figure 2(B–I)). GS immunostaining was also higher in senescent astrocytes (Figure 2(J–L)), although no significant difference in GS expression was observed between the experimental groups (Figure 2(M)).
Figure 2.
Levels and activity of glutamate transporters and GS are upregulated in senescent astrocytes in vitro. (A) Primary murine astrocyte cultures were maintained for 10 DIV (control group) or 30–35 DIV (senescent group) followed by immunocytochemical and PCR assays of the glutamate transporters, GLT-1 and GLAST, and the enzyme, GS, and evaluation of GS activity and aspartate uptake assays. (B–E) Increased immunostaining intensity and expression of GLAST (B–E) and GLT-1 (F–I) in senescent astrocyte cultures compared to the control group. (J–M) Increased immunolabeling intensity of GS in senescent astrocytes. (N-O) Upregulated GS activity and [3H]-D-aspartate uptake in senescent astrocytes compared to control cultures. Scale bar, 20 μm. Individual data points are plotted and represent individual cultures (n = 3–10 cultures/group). Significance was determined using the unpaired t-test. Error bars represent ± SEM. **: p < 0.01; *: p < 0.05. RPLP0: ribosomal protein lateral stalk subunit P0.
To address whether the upregulation of GLT-1 and GLAST functionally affects the glutamate-glutamine cycle, we quantified the enzymatic activity of GS and EAATs. Interestingly, senescent astrocytes showed a 2.4-fold increase in GS activity (Figure 2(N)). In addition, we quantified the EAATs activity through the [3H]-D-Aspartate uptake assay in astrocyte cultures under 37°C (normal assay temperature) or 4°C (to block aspartate uptake). As expected, [3H]-D-Aspartate uptake at a lower temperature (4°C) was abolished entirely in both control and senescent astrocytes (data not shown). Conversely, at 37°C, senescent astrocytes displayed a 1.5-fold increase of [3H]-D-Aspartate uptake compared to the control cultures (Figure 2(O)).
Altogether, these results suggest a cell-autonomous mechanism of aged astrocytes by enhancing the expression and function of the glutamate-glutamine cycle-related proteins.
Age-Related Increase in the Level of GS and Glutamate Uptake in the Mouse Hippocampus
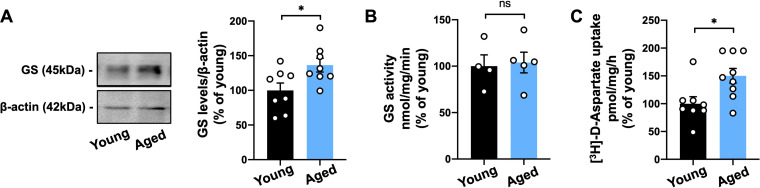
To validate the effects of aging in glutamate transporters and GS levels and functions in vivo, we first investigated GS protein levels in hippocampal extracts from young (2-3 months-old) and aged (18–25 months-old) C57Bl/6 mice. We observed an increase of approximately 33% of GS levels in aged mice compared to young ones (Figure 3(A)). Additionally, we also found increased levels of GS in the hippocampus of aged BALB/c mice and Wistar rats (Supplementary Figure 1). Interestingly, higher levels of GS in the aged hippocampus were not followed by increased enzyme activity in neither species (Figure 3 and Supplementary Figure 1).
Figure 3.
GS levels and glutamate uptake are increased in the old mouse hippocampus. (A) Western blot analysis of GS levels in the hippocampus of 2-3-month-old mice (Young) compared to 18–25 months-old mice (Aged) revealed increased protein levels upon aging. (B) Unchanged enzymatic activity of GS was observed between young and aged mice. (C) Augmented [3H]-D-aspartate uptake in the hippocampus of aged mice compared to young mice. Individual data points are plotted and represent individual animals (n = 4–10 animals/group). Significance was determined using the unpaired t-test. Error bars represent ± SEM. *: p < 0.05. GS: glutamine synthetase.
To further evaluate the function of glutamate transporters in aging, we analyzed D-Aspartate uptake in hippocampal extracts from young and old animals. As previously shown in cultured aged astrocytes (Figure 2(O)), we observed a 50% increase of [3H]-D-Aspartate uptake in the hippocampus of aged mice compared to the young group (Figure 3(C)).
Therefore, these results corroborate our in vitro data and suggest an age-related increase of GS levels in rodent aging, which is associated with higher activity of the astrocytic glutamate transporters in the aged mouse hippocampus.
Human Hippocampal Aging Is Associated with Increased Levels of GS and GLT-1
Human astrocytes are highly heterogeneous, morphologically, molecularly, and functionally compared with their murine counterparts (Oberheim et al., 2009). Therefore, to investigate whether the modulation of GS and glutamate transporters upon rodent aging also applies to human aging, we evaluated post-mortem hippocampal tissue from non-demented middle-aged and elderly donors. Clinic-pathological information of all donors is present in Table S1.
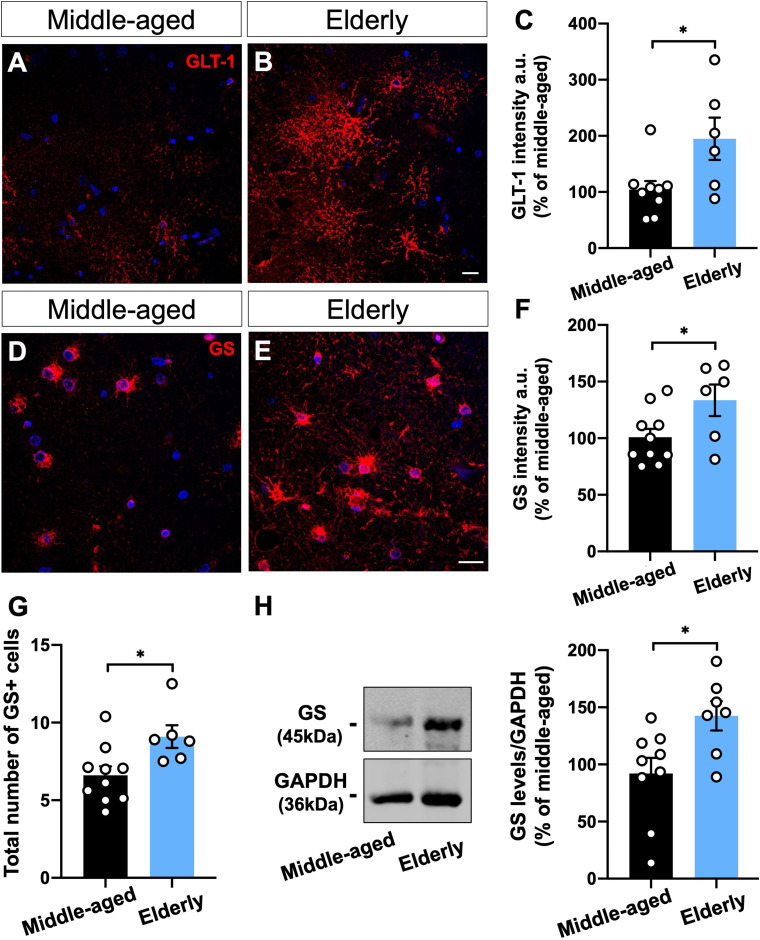
Densitometric quantification of GLT-1 intensity revealed an increase of about 90% in the dentate gyrus, including the granule cell layer (GCL) and molecular layer (ML), of elderly donors (Figure 4(A–C))). Similarly, GS immunostaining showed an overall increase of 33% in the GCL, ML, and polymorphic layer (PL) of the dentate gyrus from aged donors compared to the middle-aged ones (Figure 4(D–F)). In addition, the individualized analysis of GS immunostaining at the GCL, ML, and PL revealed a comparable increased intensity in all regions (Supplementary Figure 2).
Moreover, we reported an age-associated increment in the number of GS + cells at the dentate gyrus of elderly donors compared with middle-aged subjects (Figure 4(G)). Interestingly, normalization of GS immunostaining by the number of GS + cells indicated no difference between middle-aged and elderly donors, suggesting that the increased GS intensity observed in the aged dentate gyrus is due to a higher proportion of GS + cells (Supplementary Figure 3). Furthermore, Western blotting analysis revealed a 42% increase in GS level at the dentate gyrus of elderly donors compared with middle-aged subjects (Figure 4(G, H))).
Figure 4.
GS and GLT-1 are upregulated in the human brain upon aging. (A-C) Densitometric analysis of GLT-1 (A-B) and GS (D-E) immunostaining at the hippocampal dentate gyrus, including granule cell layer (CGL), molecular layer (ML), and polymorphic layer (PL), from human post-mortem tissue of middle-aged and elderly donors revealed an overall increased intensity of both proteins upon aging (A–F). Images represent the ML (A, B) and PL (D, E). (G) Immunostaining revealed a higher number of GS + cells in the elderly dentate gyrus than in the middle-aged ones. (H) GS protein level was increased in the dentate gyrus of elderly donors compared with middle-aged cases. Scale bars, 20 μm. Individual data points are plotted and represent individual donors (n = 6–10 donors/group). Significance was determined using the unpaired t-test. Error bars represent ± SEM. *: p < 0.05.
Taken together, our results suggest that age-dependent upregulation of GS and GLT-1 is a conserved feature between mouse and human hippocampal astrocytes.
Discussion
Here, we described the age-related modulation of proteins associated with the glutamate-glutamine cycle in senescent astrocytes in vitro and in the hippocampus of old rodents and humans. Using an in vitro model of murine astrocyte senescence and human post-mortem brain tissues, we showed that astrocytes undergo upregulation of GLT-1, GLAST, and GS, along with the increased enzymatic activity of GS and [3H]-D-aspartate uptake. Our data suggest that aged astrocytes undergo molecular and functional changes that control glutamate-glutamine homeostasis upon brain aging.
Aging is accompanied by progressive alterations of functional capabilities, usually associated with cognitive and motor-coordination decline (Yeoman et al., 2012). Among the causal mechanisms of brain aging, the increasing number of senescent cells, including glial cells, has been linked to the chronic inflammation observed in advanced age (Salminen et al., 2011) and neurodegeneration in both animal models and AD and PD human brain (Bhat et al., 2012; Bussian et al., 2018; Chinta et al., 2018). Nevertheless, our knowledge about the phenotype and function of senescent glial cells in normal aging is still incipient.
The glutamatergic synaptic activity relies on the glutamate-glutamine cycle, in which astrocytes handle glutamate taken up from synapses and provide glutamine for neurons, thus maintaining excitatory neurotransmission (Tani et al., 2014). The mechanisms that underly glutamate homeostasis in normal brain aging are controversial and have been subject of discussion. It has been suggested that the downregulation of astrocytic glutamate transporters is a feature of CNS aging, although several studies indicate that it depends on the experimental model and brain region (Gasiorowska et al., 2021). The basal levels of glutamate and aspartate increase during development and young adulthood in most brain areas, including the hippocampus and cerebral cortex, but gradually decrease upon aging in mice (Saransaari & Oja, 1995). Similarly, a meta-analysis reported that glutamate concentration, measured by proton spectroscopy (1HMRS), declines in different cortical areas during human aging, whereas glutamine level is significantly higher in older adults (Roalf et al., 2020), suggesting a modulation of the molecular components of the glutamate-glutamine cycle upon aging.
While some studies reported lower levels of glutamate transporters, such as GLT-1 and GLAST, along with reduced glutamate uptake in the cerebral cortex and hippocampus of aged rats (Potier et al., 2010; Vatassery et al., 1998; Wheeler & Ondo, 1986), unchanged glutamate-glutamine profile or glutamate uptake between young and old rats have also been described (Najlerahim et al., 1990; Segovia et al., 2001). Conversely, by analyzing a recent RNAseq dataset of mouse hippocampal astrocytes at different ages (Clarke et al., 2018), we noticed an upregulation of GLT-1, GLAST, and GS transcripts throughout aging. However, GLT-1 showed a drastic downregulation in the hippocampus of 2-year-old mice, while GLAST and GS transcripts remained high at the same age. It is noteworthy that the transcript levels do not necessarily reflects protein levels or activity. Moreover, although it remains to be investigated the cause and consequence of GLT-1 loss in aged mouse astrocytes, this event might be compensated by the upregulation of GLAST at the same age.
In line with these results, here, we also described an age-dependent increase of GS levels in the hippocampus of aged mice and rats and a higher [3H]-D-aspartate uptake in old mice. Although we did not observe differences in GS activity between young and old rodents, increased function of this enzyme has already been reported in the brain of old rats (Cao Danh et al., 1985). Interestingly, in a recent single-cell RNAseq analysis, Lee et al. identified a new cluster of astrocytes in the aged mouse hippocampus that significantly upregulates genes involved in astrocyte reactivity, synapse modulation, and cell homeostasis, including Slc1a2 (GLT-1) and Slc1a3 (GLAST) (Lee et al., 2022). Therefore, the higher expression of astrocytic glutamate transporters and glutamate uptake might be at least partially involved in the lower concentration of glutamate previously observed in the brain of old animals (Saransaari & Oja, 1995).
Astrocytes in the aging brain undergo senescence, which changes their molecular and functional profile, including their capacity to regulate synapse formation (Matias et al., 2022). We have recently identified that senescent astrocytes have an abnormal nuclear morphology, represented by the loss of lamin-B1 and increased nuclear lamina invaginations, along with upregulation of pro-inflammatory cytokines and reactive oxygen/nitrogen species. Furthermore, these cells lose their neuritogenic and synaptogenic capacity (Matias et al., 2022), pivotal functions of healthy astrocytes in controlling neural circuit formation (Diniz et al., 2019). Therefore, it is likely that senescent astrocytes are involved in age-related synaptic dysfunction and cognitive decline.
It has been shown that hippocampal astrocyte cultures from adult rats display decreased GLAST and GS levels and reduced GS activity. However, the levels of GLT-1 increased in the aged astrocyte cultures compared with those derived from newborn rats (Bellaver et al., 2017). Similarly, Limbad et al. reported lower GLT-1 and GLAST transcripts levels in an in vitro model of X-irradiation-induced senescent human astrocytes. This downregulation was associated with neuronal death in co-culture assays of neurons and senescent astrocytes exposed to glutamate (Limbad et al., 2020). In contrast, here, by using a long-term astrocyte culture model for senescence, which recapitulates key features of the senescent phenotype (Matias et al., 2022), we demonstrated that senescent astrocytes present upregulation of GLT-1, GLAST, and GS, along with increased GS activity and [3H]-D-aspartate uptake. Corroborating our results, mouse cortical astrocytes cultured for 90 DIV displayed an increase in the basal glutamate uptake, followed by a higher GLAST level, compared to control cultures (Pertusa et al., 2007). Curiously, when astrocytes were exposed to H2O2, glutamate uptake was inhibited in a concentration-dependent manner, an effect potentiated in 90 DIV cultures (Pertusa et al., 2007).
In line with our data, Lee et al. recently showed that, in the aged mouse hippocampus, a subtype of autophagy-dysregulated astrocytes (APDAs) exhibits upregulation of several genes, including GLT-1 and GLAST transcripts. Although it was not investigated if APDAs exhibit the senescence phenotype, these cells display impairments in the secretion of synaptogenic molecules and a deficit in the control of synapse formation in aged animals (Lee et al., 2022). Together with ours, these data support the concept that increased levels of glutamate transporters are a hallmark of aged astrocytes.
Altogether, the above-mentioned studies suggest that astrocytes exposed to toxic/damaged stimuli have a differential glutamate shuttle response compared to the long-term culture models. Furthermore, our data is in line with recent studies pointing to the upregulation of glutamate transporters and GS in aged astrocytes, suggesting a cell-autonomous mechanism of senescent astrocytes in vitro.
Human astrocytes are highly heterogeneous, morphologically, molecularly, and functionally compared with their murine counterparts (Oberheim et al., 2009). Therefore, in our study, we took advantage of analyzing human post-mortem brain samples from non-demented middle-aged and elderly donors to better elucidate the underlying mechanisms of aging, compared with the rodent data. Here, we observed a higher intensity of GLT-1 and GS in the dentate gyrus of elderly donors compared to middle-aged ones. This was followed by an increased number of GS + cells and GS levels in the same hippocampal region of elderly subjects. In agreement with our findings, extracted data from a recent meta-analysis study revealed a trend of increase in GS expression in the prefrontal cortex of male and female humans during aging (Wruck & Adjaye, 2020). Therefore, our results suggest that the age-associated upregulation of GS and GLT-1 is a conserved feature between mouse and human hippocampal astrocytes. In fact, an interesting RNAseq study (Zhang et al., 2016) revealed that mature human astrocytes derived from the temporal lobe cortex are the primary cell type expressing GS, GLAST, and GLT-1.
Altered glutamate homeostasis has been linked to the pathogenesis of age-related neurodegenerative diseases, including AD and PD (Todd & Hardingham, 2020). However, data remain controversial depending on the disease and experimental model. Reduced GS levels and GS + cell density have been reported in the hippocampus and prefrontal cortex of aged triple transgenic mouse model of AD (3x-Tg-AD) (Kulijewicz-Nawrot et al., 2013; Olabarria et al., 2011). In agreement, downregulation of GLT-1 and GLAST has been observed in the human hippocampus and medial frontal gyrus already at early stages of AD (Hoshi et al., 2018; Jacob et al., 2007). Therefore, the disturbance in glutamate homeostasis may represent a key contributor to glutamate excitotoxicity in AD.
Similarly, dysregulation of glutamate-glutamine balance has been implicated in PD pathogenesis, in which variations in glutamate transporters and GS levels and/or activity have been linked to the disease stage. Downregulation of glutamate transporters has been reported in animal models for PD (Chung et al., 2008; Zhang et al., 2017), and the glutamate uptake is reduced by 50% in platelets of PD patients (Ferrarese et al., 2001). In contrast, we recently showed that, in an early sporadic model of synucleinopathy, astrocytes exposed to α-synuclein oligomers displayed increased glutamate uptake and upregulation of GLT-1 and GLAST, suggesting a neuroprotective mechanism by which astrocytes may control glutamatergic excitotoxicity in early stages of PD (Diniz et al., 2020).
Therefore, our study is the first to integrate an in vitro experimental model, rodent, and human data to elucidate the astrocytic control of the glutamate-glutamine cycle during normal brain aging. The overall upregulation of the molecular components of this cycle upon aging strongly supports the involvement of astrocytes in regulating glutamate homeostasis and possibly preventing glutamate excitotoxicity in physiological brain aging. Due to glutamate's broad effect in different processes in the brain (McKenna, 2013), additional metabolic impacts such as effects on GABA and/or glutathione synthesis and oxidation should also be considered. Although apparently controversial, we suggest that the increase in the molecular machinery that controls glutamate levels might represent an early neuroprotective tentative mechanism of old healthy brain against glutamatergic excitotoxicity observed in several neurodegenerative diseases.
Supplemental Material
Supplemental material, sj-docx-1-asn-10.1177_17590914231157974 for Age-Associated Upregulation of Glutamate Transporters and Glutamine Synthetase in Senescent Astrocytes In Vitro and in the Mouse and Human Hippocampus by Isadora Matias, Luan Pereira Diniz, Ana Paula Bergamo Araujo, Isabella Vivarini Damico, Pâmella de Moura, Felipe Cabral-Miranda, Fabiola Diniz, Belisa Parmeggiani, Valeria de Mello Coelho, Renata E. P. Leite, Claudia K. Suemoto, Gustavo Costa Ferreira, Regina Célia Cussa Kubrusly and Flávia Carvalho Alcantara Gomes in ASN Neuro
Supplemental material, sj-docx-2-asn-10.1177_17590914231157974 for Age-Associated Upregulation of Glutamate Transporters and Glutamine Synthetase in Senescent Astrocytes In Vitro and in the Mouse and Human Hippocampus by Isadora Matias, Luan Pereira Diniz, Ana Paula Bergamo Araujo, Isabella Vivarini Damico, Pâmella de Moura, Felipe Cabral-Miranda, Fabiola Diniz, Belisa Parmeggiani, Valeria de Mello Coelho, Renata E. P. Leite, Claudia K. Suemoto, Gustavo Costa Ferreira, Regina Célia Cussa Kubrusly and Flávia Carvalho Alcantara Gomes in ASN Neuro
Acknowledgments
We thank Marcelo Meloni for technical assistance. This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (IM, IVD, FCAG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (IM, LPD), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (IM, FCAG), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (CKS), Departamento de Ciência e Tecnologia, Ministério da Saúde (Decit-MS) (IM, LPD, APBA, FCAG), Fiocruz-MS-Servier Award (FCAG), Instituto Nacional de Neurociência Translacional (INCT-INNT) (FCAG).
Abbreviations
- AD
Alzheimer's disease
- Ara C
cytosine arabinoside
- BSA
bovine serum albumin
- CNS
central nervous system
- DIV
days in vitro
- DMEM
Dulbecco's minimum essential medium
- EAAT
excitatory amino acid transporter
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GCL
granule cell layer
- GFAP
glial fibrillary acidic protein
- GLAST
glutamate-aspartate transporter
- GLT-1
glutamate transporter-1
- GS
glutamine synthetase
- ML
molecular layer
- PBS
phosphate-buffered saline
- PD
Parkinson's disease
- PL
polymorphic layer.
Footnotes
Author Contributions: Conceptualization: IM, FCAG; Research design: IM, LPD, RCCK, FCAG; Experimental performance: IM, LPD, IVD, APBA, PM, FCM; FD; BP; Data analysis: IM, LPD, IVD, APBA, PM, FCM, FD, BP; Supervision: GCF, RCCK, FCAG; Writing-original draft preparation: IM; Reviewing and editing: IM; FCAG; Resources: VMC, REPL, CKS, GCF, RCCK, FCAG; Funding acquisition: FCAG.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from Departamento de Ciência e Tecnologia, Ministério da Saúde (Decit-MS), Fiocruz-MS-Servier Award, Instituto Nacional de Neurociência Translacional (INCTINNT),Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
ORCID iD: Flávia Carvalho Alcantara Gomes https://orcid.org/0000-0003-2966-0638
Supplemental Material: Supplemental material for this article is available online.
References
- Armada-Moreira A., Gomes J. I., Pina C. C., Savchak O. K., Gonçalves-Ribeiro J., Rei N., Pinto S., Morais T. P., Martins R. S., Ribeiro F. F., Sebastião A. M., Crunelli V., Vaz S. H. (2020). Going the extra (Synaptic) mile: Excitotoxicity as the road toward neurodegenerative diseases. Frontiers in Cellular Neuroscience, 14. 10.3389/fncel.2020.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellaver B., Souza D. G., Souza D. O., Quincozes-Santos A. (2017). Hippocampal astrocyte cultures from adult and aged rats reproduce changes in glial functionality observed in the aging brain. Molecular Neurobiology, 54, 2969–2985. 10.1007/s12035-016-9880-8 [DOI] [PubMed] [Google Scholar]
- Bhat R., Crowe E. P., Bitto A., Moh M., Katsetos C. D., Garcia F. U., Johnson F. B., Trojanowski J. Q., Sell C., Torres C. (2012). Astrocyte senescence as a component of Alzheimer’s disease. PLoS One, 7, e45069. 10.1371/journal.pone.0045069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busanello E. N. B., Fernandes C. G., Martell R. V., Lobato V. G. A., Goodman S., Woontner M., de Souza D. O. G., Wajner M. (2014). Disturbance of the glutamatergic system by glutaric acid in striatum and cerebral cortex of glutaryl-CoA dehydrogenase-deficient knockout mice: Possible implications for the neuropathology of glutaric acidemia type I. Journal of the Neurological Sciences, 346, 260–267. 10.1016/j.jns.2014.09.003 [DOI] [PubMed] [Google Scholar]
- Bussian T. J., Aziz A., Meyer C. F., Swenson B. L., van Deursen J. M., Baker D. J. (2018). Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature, 562, 578–582. 10.1038/s41586-018-0543-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P., Zhang J., Huang Y., Fang Y., Lyu J., Shen Y. (2019). The age-related changes and differences in energy metabolism and glutamate-glutamine recycling in the d-gal-induced and naturally occurring senescent astrocytes in vitro. Experimental Gerontology, 118, 9–18. 10.1016/j.exger.2018.12.018 [DOI] [PubMed] [Google Scholar]
- Cao Danh H., Strolin Benedetti M., Dostert P. (1985). Age-related changes in glutamine synthetase activity of rat brain, liver and heart. Gerontology, 31, 95–100. 10.1159/000212686 [DOI] [PubMed] [Google Scholar]
- Cheung G., Bataveljic D., Visser J., Kumar N., Moulard J., Dallérac G., Mozheiko D., Rollenhagen A., Ezan P., Mongin C., Chever O., Bemelmans A.-P., Lübke J., Leray I., Rouach N. (2022). Physiological synaptic activity and recognition memory require astroglial glutamine. Nature Communications, 13, 753. 10.1038/s41467-022-28331-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta S. J., Woods G., Demaria M., Rane A., Zou Y., McQuade A., Rajagopalan S., Limbad C., Madden D. T., Campisi J., Andersen J. K. (2018). Cellular senescence is induced by the environmental neurotoxin paraquat and contributes to neuropathology linked to Parkinson’s disease. Cell Reports, 22, 930–940. 10.1016/j.celrep.2017.12.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E. K. Y., Chen L. W., Chan Y. S., Yung K. K. L. (2008). Downregulation of glial glutamate transporters after dopamine denervation in the striatum of 6-hydroxydopamine-lesioned rats. Journal of Comparative Neurology, 511, 421–437. 10.1002/cne.21852 [DOI] [PubMed] [Google Scholar]
- Clarke L. E., Liddelow S. A., Chakraborty C., Münch A. E., Heiman M., Barres B. A. (2018). Normal aging induces A1-like astrocyte reactivity. Proceedings of the National Academy of Sciences of the United States of America, 115, E1896–E1905. 10.1073/pnas.1800165115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt N. C. (2001). Glutamate uptake. Progress in Neurobiology, 65, 1–105. 10.1016/S0301-0082(00)00067-8 [DOI] [PubMed] [Google Scholar]
- Diniz L. P., Araujo A. P. B., Matias I., Garcia M. N., Barros-Aragão F. G. Q., de Melo Reis R. A., Foguel D., Braga C., Figueiredo C. P., Romão L., Gomes F. C. A. (2020). Astrocyte glutamate transporters are increased in an early sporadic model of synucleinopathy. Neurochemistry International, 138, 104758. 10.1016/j.neuint.2020.104758 [DOI] [PubMed] [Google Scholar]
- Diniz L. P., Matias I., Siqueira M., Stipursky J., Gomes F. C. A. (2019). Astrocytes and the TGF-β1 pathway in the healthy and diseased brain: A double-edged sword. Molecular Neurobiology, 56, 4653–4679. 10.1007/s12035-018-1396-y [DOI] [PubMed] [Google Scholar]
- Diniz L. P., Matias I. C. P., Garcia M. N., Gomes F. C. A. (2014). Astrocytic control of neural circuit formation: Highlights on TGF-beta signaling. Neurochemistry International, 78, 18–27. 10.1016/j.neuint.2014.07.008 [DOI] [PubMed] [Google Scholar]
- Ferrarese C., Tremolizzo L., Rigoldi M., Sala G., Begni B., Brighina L., Ricci G., Albizzati M. G., Piolti R., Crosti F., Dalprà L., Frattola L. (2001). Decreased platelet glutamate uptake and genetic risk factors in patients with Parkinson’s disease. Neurological Sciences, 22, 65–66. [DOI] [PubMed] [Google Scholar]
- Gasiorowska A., Wydrych M., Drapich P., Zadrozny M., Steczkowska M., Niewiadomski W., Niewiadomska G. (2021). The biology and pathobiology of glutamatergic, cholinergic, and dopaminergic signaling in the aging brain. Frontiers in Aging Neuroscience, 13, Available at: https://www.frontiersin.org/articles/10.3389/fnagi.2021.654931 [Accessed August 19, 2022]. 10.3389/fnagi.2021.654931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero A., De Strooper B., Arancibia-Cárcamo I. L. (2021). Cellular senescence at the crossroads of inflammation and Alzheimer’s disease. Trends in Neurosciences, 44, 714–727. 10.1016/j.tins.2021.06.007 [DOI] [PubMed] [Google Scholar]
- Hoshi A., Tsunoda A., Yamamoto T., Tada M., Kakita A., Ugawa Y. (2018). Altered expression of glutamate transporter-1 and water channel protein aquaporin-4 in human temporal cortex with Alzheimer’s disease. Neuropathology and Applied Neurobiology, 44, 628–638. 10.1111/nan.12475 [DOI] [PubMed] [Google Scholar]
- Jacob C. P., Koutsilieri E., Bartl J., Neuen-Jacob E., Arzberger T., Zander N., Ravid R., Roggendorf W., Riederer P., Grünblatt E. (2007). Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer’s disease. Journal of Alzheimer's Disease: JAD, 11, 97–116. 10.3233/JAD-2007-11113 [DOI] [PubMed] [Google Scholar]
- Kubrusly R. C. C., Günter A., Sampaio L., Martins R. S., Schitine C. S., Trindade P., Fernandes A., Borelli-Torres R., Miya-Coreixas V. S., Rego Costa A. C., Freitas H. R., Gardino P. F., de Mello F. G., Calaza K. C., Reis R. A. M. (2018). Neuro-glial cannabinoid receptors modulate signaling in the embryonic avian retina. Neurochemistry International, 112, 27–37. 10.1016/j.neuint.2017.10.016 [DOI] [PubMed] [Google Scholar]
- Kulijewicz-Nawrot M., Syková E., Chvátal A., Verkhratsky A., Rodríguez J. J. (2013). Astrocytes and glutamate homoeostasis in Alzheimer’s disease: A decrease in glutamine synthetase, but not in glutamate transporter-1, in the prefrontal cortex. ASN Neuro, 5, 273–282. 10.1042/AN20130017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E., Jung Y.-J., Park Y. R., Lim S., Choi Y.-J., Lee S. Y., Kim C. H., Mun J. Y., Chung W.-S. (2022). A distinct astrocyte subtype in the aging mouse brain characterized by impaired protein homeostasis. Nat Aging, 2, 726–741. 10.1038/s43587-022-00257-1 [DOI] [PubMed] [Google Scholar]
- Limbad C., Oron T. R., Alimirah F., Davalos A. R., Tracy T. E., Gan L., Desprez P.-Y., Campisi J. (2020). Astrocyte senescence promotes glutamate toxicity in cortical neurons. PLOS ONE, 15, e0227887. 10.1371/journal.pone.0227887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limón I. D., Angulo-Cruz I., Sánchez-Abdon L., Patricio-Martínez A. (2021). Disturbance of the glutamate-glutamine cycle, secondary to hepatic damage, compromises memory function. Frontiers in Neuroscience, 15, 578922. 10.3389/fnins.2021.578922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951). Protein measurement with the folin phenol reagent. Journal of Biological Chemistry, 193, 265–275. 10.1016/S0021-9258(19)52451-6 [DOI] [PubMed] [Google Scholar]
- Mahmoud S., Gharagozloo M., Simard C., Gris D. (2019). Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells, 8, E184. 10.3390/cells8020184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias I., Diniz L. P., Damico I. V., Araujo A. P. B., Neves L. S., Vargas G., Leite R. E. P., Suemoto C. K., Nitrini R., Jacob-Filho W., Grinberg L. T., Hol E. M., Middeldorp J., Gomes F. C. A. (2022). Loss of lamin-B1 and defective nuclear morphology are hallmarks of astrocyte senescence in vitro and in the aging human hippocampus. Aging Cell, 21, e13521. 10.1111/acel.13521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias I., Morgado J., Gomes F. C. A. (2019). Astrocyte heterogeneity: Impact to brain aging and disease. Frontiers in Aging Neuroscience, 11, 59. 10.3389/fnagi.2019.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna M. C. (2013). Glutamate pays its own way in astrocytes. Frontiers in Endocrinology, 4, Available at: http://journal.frontiersin.org/article/10.3389/fendo.2013.00191/abstract [Accessed January 16, 2023]. 10.3389/fendo.2013.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minet R., Villie F., Marcollet M., Meynial-Denis D., Cynober L. (1997). Measurement of glutamine synthetase activity in rat muscle by a colorimetric assay. Clinica Chimica Acta, 268, 121–132. [DOI] [PubMed] [Google Scholar]
- Najlerahim A., Francis P. T., Bowen D. M. (1990). Age-related alteration in excitatory amino acid neurotransmission in rat brain. Neurobiology of Aging, 11, 155–158. 10.1016/0197-4580(90)90049-6 [DOI] [PubMed] [Google Scholar]
- Oberheim N. A., Takano T., Han X., He W., Lin J. H. C., Wang F., Xu Q., Wyatt J. D., Pilcher W., Ojemann J. G., Ransom B. R., Goldman S. A., Nedergaard M. (2009). Uniquely hominid features of adult human astrocytes. Journal of Neuroscience, 29, 3276–3287. 10.1523/JNEUROSCI.4707-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olabarria M., Noristani H. N., Verkhratsky A., Rodríguez J. J. (2011). Age-dependent decrease in glutamine synthetase expression in the hippocampal astroglia of the triple transgenic Alzheimer’s disease mouse model: Mechanism for deficient glutamatergic transmission? Molecular Neurodegeneration, 6, 55. 10.1186/1750-1326-6-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertusa M., García-Matas S., Rodríguez-Farré E., Sanfeliu C., Cristòfol R. (2007). Astrocytes aged in vitro show a decreased neuroprotective capacity. Journal of Neurochemistry, 101, 794–805. 10.1111/j.1471-4159.2006.04369.x [DOI] [PubMed] [Google Scholar]
- Potier B., Billard J.-M., Rivière S., Sinet P.-M., Denis I., Champeil-Potokar G., Grintal B., Jouvenceau A., Kollen M., Dutar P. (2010). Reduction in glutamate uptake is associated with extrasynaptic NMDA and metabotropic glutamate receptor activation at the hippocampal CA1 synapse of aged rats. Aging Cell, 9, 722–735. 10.1111/j.1474-9726.2010.00593.x [DOI] [PubMed] [Google Scholar]
- Roalf D. R., Sydnor V. J., Woods M., Wolk D. A., Scott J. C., Reddy R., Moberg P. J. (2020). A quantitative meta-analysis of brain glutamate metabolites in aging. Neurobiology of Aging, 95, 240–249. 10.1016/j.neurobiolaging.2020.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Ojala J., Kaarniranta K., Haapasalo A., Hiltunen M., Soininen H. (2011). Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. European Journal of Neuroscience, 34, 3–11. 10.1111/j.1460-9568.2011.07738.x [DOI] [PubMed] [Google Scholar]
- Saransaari P., Oja S. S. (1995). Age-related changes in the uptake and release of glutamate and aspartate in the mouse brain. Mechanisms of Ageing and Development, 81, 61–71. 10.1016/0047-6374(95)01583-L [DOI] [PubMed] [Google Scholar]
- Segovia G., Del Arco A., Prieto L., Mora F. (2001). Glutamate-glutamine cycle and aging in striatum of the awake rat: Effects of a glutamate transporter blocker. Neurochemical Research, 26, 37–41. 10.1023/A:1007624531077 [DOI] [PubMed] [Google Scholar]
- Shimabukuro M. K., Langhi L. G. P., Cordeiro I., Brito J. M., Batista C. M. C., Mattson M. P., de Mello Coelho V. (2016). Lipid-laden cells differentially distributed in the aging brain are functionally active and correspond to distinct phenotypes. Scientific Reports, 6, 23795. 10.1038/srep23795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnewald U., Schousboe A. (2016). Introduction to the glutamate–glutamine cycle. In Schousboe A., Sonnewald U. (Eds.), The glutamate/GABA-glutamine cycle. Advances in neurobiology (pp. 1–7). Springer International Publishing. Available at: http://link.springer.com/10.1007/978-3-319-45096-4_1 [Accessed January 16, 2023]. [DOI] [PubMed] [Google Scholar]
- Tani H., Dulla C. G., Farzampour Z., Taylor-Weiner A., Huguenard J. R., Reimer R. J. (2014). A local glutamate-glutamine cycle sustains synaptic excitatory transmitter release. Neuron, 81, 888–900. 10.1016/j.neuron.2013.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd A. C., Hardingham G. E. (2020). The regulation of astrocytic glutamate transporters in health and neurodegenerative diseases. International Journal of Molecular Sciences, 21, E9607. 10.3390/ijms21249607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatassery G. T., Lai J. C., Smith W. E., Quach H. T. (1998). Aging is associated with a decrease in synaptosomal glutamate uptake and an increase in the susceptibility of synaptosomal vitamin E to oxidative stress. Neurochemical Research, 23, 121–125. 10.1023/A:1022495804817 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A., Nedergaard M. (2018). Physiology of astroglia. Physiological Reviews, 98, 239–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D. D., Ondo J. G. (1986). Time course of the aging of the high affinity L-glutamate transporter in rat cortical synaptosomes. Experimental Gerontology, 21, 159–168. 10.1016/0531-5565(86)90069-0 [DOI] [PubMed] [Google Scholar]
- Wruck W., Adjaye J. (2020). Meta-analysis of human prefrontal cortex reveals activation of GFAP and decline of synaptic transmission in the aging brain. Acta Neuropathologica Communications, 8, 26. 10.1186/s40478-020-00907-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ximerakis M., Lipnick S. L., Innes B. T., Simmons S. K., Adiconis X., Dionne D., Mayweather B. A., Nguyen L., Niziolek Z., Ozek C., Butty V. L., Isserlin R., Buchanan S. M., Levine S. S., Regev A., Bader G. D., Levin J. Z., Rubin L. L. (2019). Single-cell transcriptomic profiling of the aging mouse brain. Nature Neuroscience, 22, 1696–1708. 10.1038/s41593-019-0491-3 [DOI] [PubMed] [Google Scholar]
- Yeoman M., Scutt G., Faragher R. (2012). Insights into CNS ageing from animal models of senescence. Nature Reviews Neuroscience, 13, 435–445. 10.1038/nrn3230 [DOI] [PubMed] [Google Scholar]
- Zhang Y., He X., Meng X., Wu X., Tong H., Zhang X., Qu S. (2017). Regulation of glutamate transporter trafficking by Nedd4-2 in a Parkinson’s disease model. Cell Death & Disease, 8, e2574. 10.1038/cddis.2016.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Sloan S. A., Clarke L. E., Caneda C., Plaza C. A., Blumenthal P. D., Vogel H., Steinberg G. K., Edwards M. S., Li G., Duncan J. A., 3rd, Cheshier S. H., Shuer L. M., Chang E. F., Grant G. A., Gephart M. G., Barres B. A. (2016). Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron, 89, 37–53. 10.1016/j.neuron.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-asn-10.1177_17590914231157974 for Age-Associated Upregulation of Glutamate Transporters and Glutamine Synthetase in Senescent Astrocytes In Vitro and in the Mouse and Human Hippocampus by Isadora Matias, Luan Pereira Diniz, Ana Paula Bergamo Araujo, Isabella Vivarini Damico, Pâmella de Moura, Felipe Cabral-Miranda, Fabiola Diniz, Belisa Parmeggiani, Valeria de Mello Coelho, Renata E. P. Leite, Claudia K. Suemoto, Gustavo Costa Ferreira, Regina Célia Cussa Kubrusly and Flávia Carvalho Alcantara Gomes in ASN Neuro
Supplemental material, sj-docx-2-asn-10.1177_17590914231157974 for Age-Associated Upregulation of Glutamate Transporters and Glutamine Synthetase in Senescent Astrocytes In Vitro and in the Mouse and Human Hippocampus by Isadora Matias, Luan Pereira Diniz, Ana Paula Bergamo Araujo, Isabella Vivarini Damico, Pâmella de Moura, Felipe Cabral-Miranda, Fabiola Diniz, Belisa Parmeggiani, Valeria de Mello Coelho, Renata E. P. Leite, Claudia K. Suemoto, Gustavo Costa Ferreira, Regina Célia Cussa Kubrusly and Flávia Carvalho Alcantara Gomes in ASN Neuro