Summary
Background
As opioid overdoses surge, medications for opioid use disorder (MOUD) remain underutilized. MOUD is rarely offered in correctional facilities although individuals involved in the criminal justice system have higher rates of OUD and mortality relative to the general population.
Methods
A retrospective cohort design examined the effect of MOUD while incarcerated on 12 months post-release treatment engagement and retention, overdose mortality, and recidivism. Individuals (N = 1600) who participated in the Rhode Island Department of Corrections (RIDOC) MOUD program (the United States’ first statewide program) and were released from incarceration from December 1, 2016, to December 31, 2018, were included. The sample was 72.6% Male (27.4% female) and 80.8% White (5.8% Black, 11.4% Hispanic, 2.0% another race).
Findings
56% were prescribed methadone, 43% buprenorphine, and 1% naltrexone. During incarceration, 61% were continued on MOUD from the community, 30% were inducted onto MOUD upon incarceration, and 9% were inducted pre-release. At 30 days and 12 months post-release, 73% and 86% of participants engaged in MOUD treatment, respectively, and those newly inducted had lower post-release engagement than those who continued from the community. Reincarceration rates (52%) were similar to the general RIDOC population. Twelve overdose deaths occurred during the 12-month follow-up, with only one overdose death during the first two weeks post-release.
Interpretations
Implementing MOUD in correctional facilities, with seamless linkage to community care is a needed life-saving strategy.
Funding
Rhode Island General Fund, the NIH of Health HEAL Initiative, the NIGMS, and the NIDA.
Keywords: Access to care, Correctional facilities, Medications for opioid use disorder, Health services research, Opioid use disorder, Incarceration
Research in context.
Evidence before this study
Medications for opioid use disorder (MOUD) are the most effective evidence-based treatment for opioid use disorder (OUD) and are crucial to address the ongoing opioid overdose epidemic in the United States. MOUD remains underutilized, especially in justice-involved populations who are also at increased risk for overdose and death. MOUD engagement during incarceration and after release reduces opioid overdose mortality risk.
We searched PubMed for studies published in English between December 1, 2016, and June 1, 2022, using the title and abstract search terms (“methadone” OR “buprenorphine” OR “naltrexone”) AND medical subject heading (MeSH) term “prisoners”, with no restrictions on geographical location. This search resulted in 74 studies, of which n = 18 took place outside the United States, n = 22 did not report post-release outcomes of a MOUD program, n = 26 did not offer all three forms of FDA-approved MOUD, and n = 8 did not report statewide post-release outcomes.
Added value of this study
To our knowledge, this is the first study to evaluate MOUD treatment engagement, treatment retention, overdose mortality, and recidivism rates among participants in the nation's first statewide jail and prison system comprehensive MOUD program. Our real-world evidence is important because it combines evidence generated from clinical practice and bridges the gap between clinical research and practice. Evidence from the natural and uncontrolled environment as generated real-world evidence is key to addressing the ongoing opioid epidemic.
Implications of all the available evidence
Implementing comprehensive MOUD in correctional facilities, especially jails where the vast majority of those with MOUD are incarcerated, with seamless linkage to community care is a needed life-saving strategy, especially in jails where the vast majority of those with MOUD are incarcerated.
Introduction
Medications for opioid use disorder (MOUD) are the most effective evidence-based treatment for opioid use disorder (OUD) and are crucial to address the ongoing opioid overdose epidemic in the United States.1 As opioid overdose cases continue to rise, MOUD remains underutilized, resulting in a lack of life-saving care.2
The natural progression of OUD leads to increasing use despite adverse consequences, which often include involvement in the criminal legal system.3 Indeed, almost 1 in 5 incarcerated individuals suffer from OUD.4 Individuals with more severe OUD are more likely to become incarcerated.5 During incarceration, individuals with OUD generally decrease or stop their illicit opioid use. This results in a decreased tolerance to opioids, which, in turn, increases their risk for overdose and death after release with the highest risk during the first two weeks post-release compared to the general population.6,7 Given that MOUD is the most effective treatment to prevent fatal overdose,2,8 there is an impetus for consistent MOUD engagement during incarceration and the post-release period. However, fewer than 10% of US correctional facilities provide continuation or initiation of MOUD during incarceration or linkage to MOUD in the community.9,10
Rhode Island, compared to other states, has been particularly impacted by the opioid epidemic.11 The Governor of Rhode Island established a task force in 2015 to address the opioid crisis. The task force recognized the disproportionate burden of OUD on individuals who are incarcerated and thus included the development of a correctional-based MOUD program as a goal to reduce overdose deaths.12 The state's unified jail and prison system, the Rhode Island Department of Corrections (RIDOC), was given an additional annual budget of 2 million dollars to run a comprehensive MOUD program with OUD screening, MOUD treatment for those in need, and discharge planning with linkage to care after release for all incarcerated individuals.13,14 Notably, the program was designed to tailor treatment to meet the needs of the individual, which included consideration of past experiences with MOUD, addressing logistical issues of a post-release continuance of treatment (place of residence, transportation), and offering all three FDA-approved forms of MOUD: buprenorphine, methadone, and naltrexone. In addition, all post-release individuals were eligible to receive healthcare insurance that covered the cost of MOUD upon discharge due to Rhode Island's status as a Medicaid expansion state.
Within the first year of implementation, preliminary analysis revealed that there was a 12% decrease in overdose deaths across the state and a 61% decrease in 12-month post-release overdose deaths.15 Three-quarters of patients self-reported post-release MOUD engagement.16
The purpose of this study is to better understand the rate of uptake of community-based MOUD and the risk factors for overdose fatalities among individuals who participated in the RIDOC MOUD program and were released from incarceration. Understanding trends in treatment and overdose fatalities will help inform points of intervention during the criteria period immediately following release from incarceration when an individual is at the highest risk of overdose. This paper extends and enhances the preliminary findings13 with a comprehensive analysis using multiple, linked, statewide datasets to evaluate MOUD treatment engagement, treatment retention, overdose mortality, and recidivism rates among the participants of the statewide MOUD program at the RIDOC.
Methods
Participants & setting
Individuals were included in the study if they enrolled in the MOUD program while incarcerated at the RIDOC after December 1, 2016, and were released on or before December 31, 2018. Changes in treatment status, reincarceration to RIDOC, and overdose deaths were coded for one year following release. The study protocol was approved by the Brown University Institutional Review Board, The Miriam Hospital Institutional Review Board, the Rhode Island Department of Health Institutional Review Board, the Federal Office for Human Research Protections, and the Medical Research Advisory Group at the Rhode Island Department of Corrections.
RIDOC is a unified (prison and jail) state-run correctional system housing about 3000 men and women with 13,000 intakes and releases annually. It is the sole correctional authority in the state. RIDOC's population is aged 18 years and older and is 94% male, 52% White, 23% Black, and 21% Hispanic. Before initiating the comprehensive MOUD program in 2016, RIDOC offered methadone, limited to pregnant women and individuals being tapered off methadone. Methadone for chronic pain is not included as MOUD.
The comprehensive statewide MOUD program includes (i) screening all individuals for OUD; (ii) continuing and initiating individuals on MOUD while incarcerated; (iii) offering all three types of FDA-approved MOUDs as appropriate; and (iv) linking individuals to MOUD in the community at release. Upon referral to the MOUD program and upon request, the patient meets with a provider who educates on the benefits and differences between each form of MOUD and answers questions regarding why a patient may prefer one form over another. Many patients have had prior experience with methadone and buprenorphine and have strong opinions about which they prefer, primarily because of which worked better for them and which made them feel better. Very few were interested in naltrexone. RIDOC covers all costs of MOUD treatment at rates similar to or below those paid by private and public insurers.14 There is no cost to the patient for treatment during incarceration.
Sources of data
Data were collected from the RIDOC administrative data, RIDOC electronic medical record data, the Rhode Island Behavioral Online Database (RI-BHOLD) which includes data from all state-regulated substance use treatment providers, the Rhode Island Prescription Drug Monitoring Program (PDMP), and the Office of the State Medical Examiner (OSME). A retrospective deterministic record linkage, using identifiers of first name, last name, and date of birth, including all aliases known to RIDOC, provided linkage across multiple data sources. All record linkages were conducted within the Stronghold Research Environment, Brown University's Health Insurance Portability and Accountability Act (HIPAA)-compliant computing environment, for data compliance.
Participant release dates and demographic data were collected through RIDOC's custody and control electronic record system. RIDOC uses a combined race and ethnicity variable where individuals identify as White, Black, Hispanic, Asian, American Indian, and another race. Asian, American Indian, and other races were combined for this study. The type of MOUD prescribed, start and stop dates, and medication discontinuations were collected from RIDOC's electronic medical record. Individuals were considered “continued from the community” if the medical team could confirm that MOUD was prescribed in the community prior to commitment. Individuals were considered “new inductions,” if they screened positive for OUD and were assessed to be appropriate for MOUD but were not on prescribed MOUD at the time of commitment. Sentenced individuals who requested MOUD prior to release were considered “pre-release inductions.” Reincarceration was defined as returning to RIDOC and is broken down to include pre-trial or sentenced reincarcerations.
The RI-BHOLD system is a portal into which substance use treatment providers licensed by the RI Department of Behavioral Health Developmental Disabilities and Hospitals are required to enter data on all individuals receiving services, regardless of pay source. Admission and discharge to MOUD programs combined with other data were used to determine the rate of uptake of MOUD post-release.
PDMP data on prescription records was used to obtain information on buprenorphine prescriptions filled, (e.g., using all relevant National Drug Codes to identify the subpopulation without opioid use disorder). The 12-month follow-up window began at the index release, the release associated with the first enrollment in the MOUD program during the study period. This is a preferred method for defining index release.17 Individuals who engaged in MOUD treatment for whom there was no RI-BHOLD discharge date (indicating having left a MOUD program) or had filled prescriptions for MOUD without more than a seven-day break according to PDMP within the 12-month follow-up period were defined as having sustained engagement. Individuals for whom there was no RI-BHOLD admission date and no filled MOUD prescriptions according to PDMP were coded as never engaged. Individuals for whom there was some evidence of engagement in MOUD in either the RI-BHOLD or PDMP but not sustained engagement were coded as intermittently engaged. For individuals reincarcerated during the study period, return to RIDOC was not considered a censoring event for treatment engagement because MOUD during incarceration was entered into the RI-BHOLD dataset.
The OSME surveillance data identified overdose decedents as persons whose death was pronounced in Rhode Island, whose final manner of death was an accident, whose a drug is listed on the death certificate as a cause of death or a significant contributing factor, and the Chief Medical Examiner reviewed and confirmed the accuracy of information sent to the Office of Vital Records for the final death certificate. For purposes of this study, an overdose decedent was defined as an individual who was last released from RIDOC in the study period and the death from an overdose was within one year of the index release.17
Statistical analysis
Exploratory data analysis was used to examine the dataset for normality, outliers, and missing data. Frequencies and descriptives of variables were examined and presented in univariate analyses. Independent samples t-test and chi-square analysis were used in a bivariate analysis examining the association between population characteristics versus release types. Non-parametric tests (Mann–Whitney U test) were used when indicated. Chi-square analysis was also used to examine MOUD continuance or induction vs. MOUD engagement. In supplementary analyses, multivariable logistic regression was used to examine unadjusted and adjusted odds of treatment engagement at 30 days post-release. Significance was set at α = 0.05. All analysis was performed with the Statistical Package for Social Sciences version 26.18
Role of the funding source
The study sponsors had no role in the study design, collection, analysis, interpretation of data, and writing of the report; or the decision to submit the paper for publication.
Results
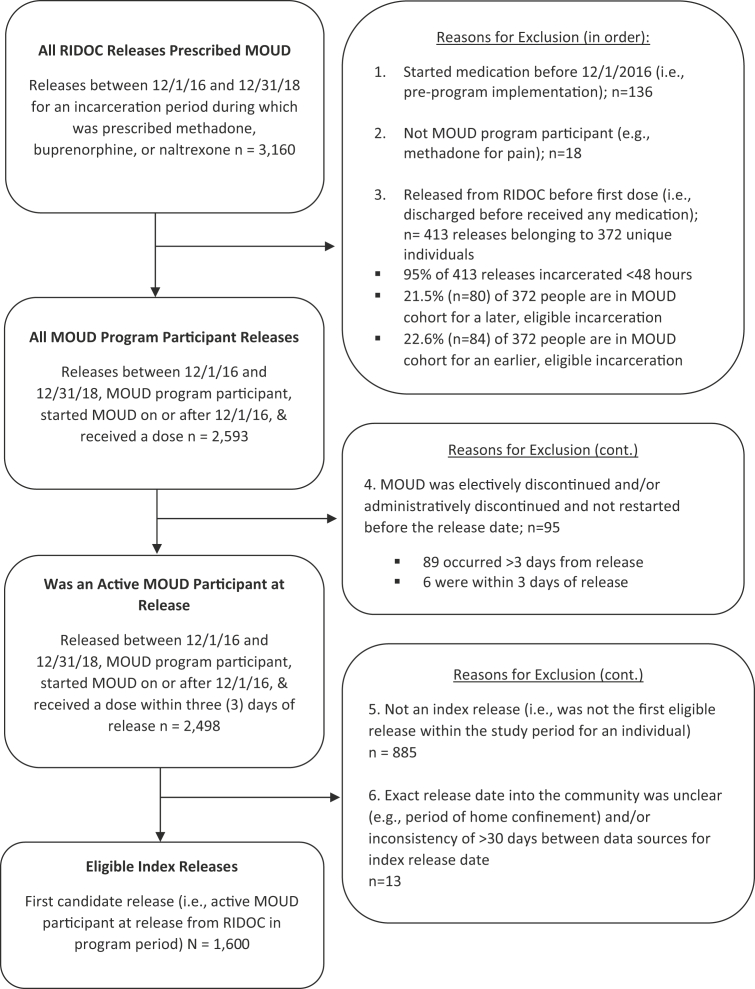
Among the 1600 unique individuals who met eligibility criteria and were enrolled in the MOUD program between December 1, 2016, and December 31, 2018, there were 2498 releases (Fig. 1). Eligible individuals were enrolled and received MOUD at RIDOC on or after December 1, 2016, and were released, at least once, between December 1, 2016, and December 31, 2018. Among the 1600 first releases that received MOUD during the study period, 66% were from the awaiting trial (jail) population (1,057) and 34% (543) were from the sentenced (prison) population. Demographic characteristics for the study sample presented in Table 1 compare those who were awaiting trial vs. those sentenced. There were more men enrolled from the sentenced population and awaiting trial, and the index incarceration was longer (in days) for the sentenced vs. awaiting trial population. There was minimal missing data (n = 4 were missing race/ethnicity and n = 1 was missing education).
Fig. 1.
Study inclusion flow chart for eligible index releases (N = 1600).
Table 1.
Demographic and incarceration characteristics for MOUD program participants released at least once between December 1, 2016, to December 31, 2018 by index release type (N = 1600).
| Characteristics | All Release Types (N = 1600) |
Awaiting Trial Release n = 1057 |
Sentenced Release n = 543 |
χ2 or t; p-value |
|---|---|---|---|---|
| % (n)/M(SD) | % (n)/M(SD) | % (n)/M(SD) | ||
| Sex | ||||
| Male | 72.6% (1165) | 70.1% (741) | 78.1% (424) | 11.54; p < .001 |
| Female | 27.4% (435) | 29.9% (316) | 21.9% (119) | |
| Age at release (years, M/SD) | 36.0 (9.3) | 35.8 (9.3) | 36.3 (9.2) | 0.90; p = .370 |
| Race/ethnicity | ||||
| Black | 5.8% (93) | 5.2% (55) | 7.0% (38) | 5.76; p = .124 |
| White | 80.8% (1289) | 82.2% (866) | 78.0% (423) | |
| Hispanic | 11.4% (189) | 10.4% (110) | 13.3% (72) | |
| Another race | 2.0% (32) | 2.2% (23) | 1.7% (9) | |
| Educational attainment | ||||
| Less than high school | 31.8% (509) | 30.4% (321) | 34.6% (188) | 3.67; p = .160 |
| High school | 50.3% (805) | 50.9% (537) | 49.4% (268) | |
| Some college or more | 17.8% (285) | 18.8% (198) | 16.0% (87) | |
| Index incarceration length (days, M/SD) | 82.8 (224.4) | 27.6 (45.5) | 190.2 (356.4) | 83,026a; p < .001 |
| Returned, sentenced or AT | 51.8% (829) | 50.0% (528) | 55.4% (301) | 4.32; p = .038 |
| Days from release to return (M/SD) | 136.5 (101.9) | 136.4 (102.6) | 136.7 (101.0) | 0.04; p = .968 |
| Returned, AT only | 23.6% (378) | 25.8% (273) | 19.3% (105) | 8.38; p = .004 |
| Days from release to return, not sentenced only (M/SD) | 167.1 (105.0) | 157.3 (105.2) | 192.4 (100.7) | 2.94; p = .004 |
| Returned, sentenced | 28.2% (451) | 24.1% (255) | 36.1% (196) | 25.40; p < .001 |
| Days from release to sentenced date (M/SD) | 155.2 (104.9) | 164.9 (108.2) | 142.4 (99.5) | 2.27; p = .024 |
| Total days reincarcerated during the 365-day period after index release (M/SD) | 37.7 (68.1) | 33.8 (64.5) | 45.4 (74.2) | 3.24; p = .001 |
| Total days reincarcerated during the 365-day period after index release among those who returned (n = 829; M/SD) | 72.8 (80.0) | 67.6 (77.7) | 81.9 (83.3) | 2.47; p = .014 |
MOUD: medications for opioid use disorder. AT = awaiting trial; M = mean. SD = standard deviation. Return to incarceration outcomes were assessed at 365 days from the index release date. Missing and invalid responses <5% were excluded from this table for educational attainment and race/ethnicity.
Index incarceration length variable was skewed, so a non-parametric test (Mann–Whitney U) was used to evaluate statistical significance.
MOUD medication at RIDOC
Of the 1600 MOUD participants, 894 (56%) were prescribed methadone, 684 (43%) were prescribed buprenorphine, and 22 (1%) were prescribed extended-release naltrexone during their first incarceration of the study period (the index incarceration). A total of 976 (61%) were continued on MOUD from the community upon entry to RIDOC, 484 (30%) were inducted onto MOUD upon commitment, and 140 (9%) were inducted onto MOUD before sentence release (Table 2). Additional details of gender and race/ethnicity are presented in supplemental materials. Black and Hispanic program participants were less likely to come into the carceral facility already on buprenorphine compared to White program participants. However, for those who initiated treatment in the facility, Black and Hispanic program participants were more likely to initiate buprenorphine than White program participants (Supplementary Table S2). Post-release treatment engagement rates at 30 days were not significantly different for Black and Hispanic program participants relative to White program participants (Supplementary Table S3).
Table 2.
Percentage engaged in MOUD at 30 days and 12-Months Post-Release by MOUD continuance or induction for individuals who enrolled in MOUD (methadone or buprenorphine) and were released, December 1, 2016 to December 31, 2018 (N = 1578).
| Total |
30 days post-release |
365 days post-release |
|||||
|---|---|---|---|---|---|---|---|
| Ever engaged |
Never Engaged |
χ2; p-value | Ever engaged |
Never Engaged |
χ2; p-value | ||
| % (n) | % (n) | % (n) | % (n) | % (n) | |||
| Methadone | |||||||
| Continued | 68.0% (608) | 87.0% (529) | 13.0% (79) | 86.67; p < .001 | 91.8% (558) | 8.2% (50) | 16.78; p < .001 |
| Induction | 32.0% (286) | 59.4% (170) | 40.6% (116) | 82.5% (236) | 17.5% (50) | ||
| Total | 56.7% (894) | 78.2% (699) | 21.8% (195) | 88.8% (794) | 11.2% (100) | ||
| Buprenorphine | |||||||
| Continued | 53.5% (366) | 76.5% (280) | 23.5% (86) | 44.29; p < .001 | 83.9% (307) | 16.1% (59) | 2.14; p = .144 |
| Induction | 46.5% (318) | 52.2% (166) | 47.8% (152) | 79.6% (253) | 20.4% (65) | ||
| Total | 43.3% (684) | 65.2% (446) | 34.8% (238) | 81.9% (560) | 18.1% (124) | ||
| Buprenorphine or Methadone | |||||||
| Continued | 61.7% (974) | 83.1% (809) | 16.9% (165) | 140.89; p < .001 | 88.8% (865) | 11.2% (109) | 18.86; p < .001 |
| Induction | 38.3% (604) | 55.6% (336) | 44.4% (268) | 81.0% (489) | 19.0% (115) | ||
| Total | 100% (1578) | 72.6% (1145) | 27.4% (433) | 85.8% (1354) | 14.2% (224) | ||
MOUD: medications for opioid use disorder. Continued = continued MOUD from the community; Induction = MOUD induction at pre-release or at commitment.
Post-release treatment engagement
Table 2 presents MOUD disposition and medication by 30- and 365-day post-release treatment engagement among individuals who received methadone or buprenorphine while incarcerated. At both time points after the index release (first release during the study period) from RIDOC, the majority of MOUD patients engaged in MOUD treatment in the community (72.6% at 30 days and 85.8% at 365 days). Only 224 (14%) never engaged in MOUD treatment post-release at one year, although at 30 days over a quarter had not engaged in treatment in the community. People who newly started on MOUD had lower post-release engagement than those who were continued on MOUD from the community at 30 days post-release (55% vs 83%), but the engagement was similar at 365 days after release, 81% vs 89%, respectively. By 365 days post-release, the difference between those initiated on the inside (induction) vs. continued on medication was no longer statistically different for buprenorphine but remained for methadone, perhaps reflecting the more widespread availability of buprenorphine in the community.
Re-incarceration
Fifty-two per cent of MOUD participants (n = 829) were reincarcerated, either pre-trial or sentenced, at least once during the 12-month follow-up period. MOUD participants were reincarcerated on average 1.56 times (SD = 1.39). See Table 1. Twenty-four per cent (24%; n = 378) were recommitted to RIDOC for an average of 73 days and 28% (451) were sentenced to prison during the 12-month follow-up period.
Overdose deaths
There were 12 overdose deaths during the study period (see Table 3). At the time of release, all decedents were receiving MOUD (5 received methadone, 7 received buprenorphine). Days from index release to death ranged from 4 days to 343 days (Mean = 200.00, SD = 122.21). Days from the last release to death ranged from 4 days to 299 days (Mean = 149.25, SD = 91.78). Eight decedents were newly inducted onto MOUD (2 pre-release) and four were already receiving MOUD in the community and were continued at RIDOC. After their index release, eight received MOUD in the community, but only two were engaged in MOUD treatment at the time of their death.
Table 3.
Characteristics and factors associated with twelve individuals who died by accidental drug overdose within 365-days of index release date.
| Participant | Age at death | Sex | Days from Index Incarceration to Death if > 1 incarceration | Days Incarcerated Index Incarceration if > 1 incarceration |
MOUD Days index incarceration RIDOC if > 1 incarceration | Days from most recent incarceration to death | Days Incarcerated Prior to Deatha |
MOUD days at last RIDOC incarceration before death | Accessed MOUD in community post-release | Days from last MOUD treatment (community or RIDOC) and death | Last Release Type | MOUD Method | MOUD Type |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35–54 | Male | 323 | 6 | 6 | 243 | 75 | 66 | Yes | 108 | Pre-Trial | C | M |
| 2 | 55–64 | Male | 282 | 9 | 8 | No | 283 | Pre-Trial | C | M | |||
| 3 | 35–54 | Female | 77 | 15 | 6 | No | 78 | Sentenced | I | B | |||
| 4 | 35–54 | Male | 29 | 15 | 7 | Yes | 25 | Pre-Trial | I | M | |||
| 5 | 55–64 | Male | 285 | 29 | 16 | 160 | 48 | 16 | Yes | 160 | Sentenced | I | M |
| 6 | 25–34 | Male | 4 | 30 | 14 | No | 4 | Pre-Trial | I | B | |||
| 7 | 35–54 | Female | 343 | 3 | 3 | 111 | 171 | 167 | Yes | 66 | Pre-Trial | C | B |
| 8 | 35–54 | Female | 156 | 20 | 17 | Yes | 156 | Pre-Trial | C | B | |||
| 9 | 55–64 | Male | 170 | 1286 | 7 | Yes | 139 | Sentenced | P | M | |||
| 10 | 35–54 | Male | 313 | 371 | 15 | 141 | 424 | 52 | Yes | 1 | Sentenced | P | B |
| 11 | 35–54 | Male | 299 | 10 | 7 | No | 300 | Pre-Trial | I | B | |||
| 12 | 35–54 | Male | 119 | 63 | 49 | Yes | 49 | Pre-Trial | I | B |
I = Induction.
P = Pre-release Induction.
C = Continuation.
M = Methadone.
B = Buprenorphine.
Total days for all incarcerations during study period.
Discussion
This is the first multi-year description of outcomes from the nation's first comprehensive statewide correctional MOUD program and the findings are striking. Out of 1600 people in the program, there were only 12 overdose deaths within a year of release from incarceration, with only one overdose death (8%) during the first two weeks after release. This is in contrast to previous studies that have found 16%7 and 26%19 of the deaths within a year occur in the first two weeks after release. This is especially striking given that our evaluation period was during a dramatic worsening of opioid overdose deaths due to an influx of illicitly manufactured fentanyl into the state. The most logical explanation for this low overdose rate is that MOUD exposure during incarceration with linkage to treatment after release is effective at reducing overdose deaths in a high-risk population at a high-risk time. This is consistent with the known effect of MOUD on reducing overdose in the community.2 This should serve as an impetus to the 90% of correctional facilities in the country that do not offer MOUD to implement this life-saving treatment, particularly those facilities that do not at least continue community treatment, which we demonstrate to have high rates of treatment retention after release.9
Several components of this comprehensive MOUD program likely led to the limited number of overdose-related deaths among people enrolled in the program. First, all individuals who were incarcerated, both pretrial and sentenced, at RIDOC were screened for opioid use disorder. Those who were diagnosed with OUD were offered appropriate MOUD treatment. Second, offering the choice of medication and patient-centred shared decision-making likely improved engagement inside corrections and in the community after release, as demonstrated in prior studies.20, 21, 22, 23, 24 All three MOUD work by blocking the effects of additional opioids, but they only work if they are taken. Therefore, allowing individuals to choose the medication, that they want to and will take, is perhaps the most crucial factor in maintaining long-term engagement and adherence. Third, the program prioritized the “warm hand-off”, i.e., connecting individuals while incarcerated to treatment in the community post-release. Rhode Island has a unique advantage in this respect due to its small size and the fact that the statewide correctional system is unified and comprises both the prisons and jails on a single campus. However, this could certainly be replicated in county jails throughout the nation, where the vast majority of people with OUD who are detained are incarcerated and released each year.
Providing MOUD at correctional facilities with linkage to treatment in the community upon release is a tremendous challenge, but some progress has already begun.9 For prison systems, this has happened either through policy changes, legislative directives, or litigation, either through advocacy groups such as the ACLU Prison Project or through the Department of Justice as violations of the Americans with Disabilities Act (ADA). For jails, which have approximately 10 times as many people with OUD passing through them than prisons, the challenges and rewards are greater. The same strategies can be utilized, but will likely require local efforts, county by county. Moreover, a critical part of success is to have MOUD treatment programs in the community accept patients returning to the community who have begun treatment during incarceration. It would be very helpful for sheriffs to embrace a treatment rather than punishment approach, which will make their jobs easier and their communities safer. They can help advocate for treatment in the community, something that is now possible with much of the opioid settlement funds going directly to local counties.
Within the context of the overall low number of overdose deaths, it is noteworthy that two-thirds of the deaths occurred among individuals newly initiated on MOUD. The group newly initiated on MOUD comprises only 38% of the total and had a lower retention rate. This suggests that individuals new to MOUD may particularly benefit from additional support following release. Additionally, eleven of the 12 decedents were aged 35 years or older. Further research could help identify risk factors for overdose upon release. Age, perhaps, suggests more severe disease persisting for a longer period or less exposure to newer dangerous adulterants and contaminants in the drug supply (e.g., fentanyl). These coincide with trends identifying an increase in the absolute number of overdose deaths in an ageing population.25
MOUD uptake in the community was not immediate for many, as over 25% had not engaged in treatment in the community after one month. This demonstrates a continued need to support individuals immediately following release, one of the most dangerous times in terms of overdose risk. While treatment initiation clearly has a positive impact, treatment retention likely requires other wrap-around services and supports including transitional housing, transportation, and employment. Many may leave jail or prison with a plethora of social challenges; this study highlights that other factors may serve as barriers to treatment retention upon release, not just immediate medication access. Indeed, of the 12 decedents in the study, only two were engaged in MOUD treatment at the time of their death. This underscores the importance of continued engagement in MOUD and recovery support and that community treatment retention efforts may offer additional life-saving benefits to increased MOUD access.
There are limitations to the present study that are important to highlight here. First, RIDOC is one of six unified correctional systems in the United States. Even among unified systems, RIDOC is unique due to its small size and the proximity of all correctional facilities (all facilities are within a one mile radius). The challenges of implementing a MOUD program in larger state systems and local detention centres may differ. Obtaining commitment from the leadership of the many jails and prisons in the United States is a formidable challenge particularly given the challenges of providing continuity of health care between fragmented correctional systems across city, county, state and federal jurisdictions. Secondly, while representative of the RIDOC population and the state as a whole, the demographics of the sample are not representative of the population of incarcerated people nationally, given that the population in this study is predominantly White. This is especially important to recognize given the known geographic and racial inequality in the availability of MOUD in the community, as well as the recent upsurges in overdose deaths among people of colour in the US. However, given the extreme racial disparities in the incarcerated population throughout the US and the racial disparities in access to MOUD among people of colour in the community, it is possible that a program such as this, which offers MOUD to all, may, if done correctly, be able to offset some of the racial disparities in engagement in treatment in the community. The increase in initiation of buprenorphine among the Black and Hispanic study subjects suggests this may be possible (see Supplementary material). Of course, that is no substitute for fighting to eliminate racial disparities in the justice and treatment systems. Ideally, economic status and other social determinants of health should be tracked and utilized as tools to effectively address similar disparities. Additionally, there have been substantial disruptions in incarceration, and generally worsening of the opioid epidemic since the COVID pandemic began, which these data pre-date. However, it is likely that the benefit of continuation or initiation of MOUD during incarceration and linkage to care after release are even more important now.
Third, the proportion of people “not engaged” with treatment may be falsely high as it did not include people who may have received treatment out of state. Fourth, ethnicity and race differences could not be examined because source data from RIDOC is a combined race and ethnicity variable. Fifth, because the MOUD program was comprehensive, we are unable to examine outcomes in a comparison group of individuals who screened positive for OUD but were not offered MOUD. Finally, the linkage of data sources could result in an underestimation of overdose deaths and treatment engagement post-release. OSME overdose death data are limited to deaths that occurred within the state of RI. Overdose deaths that may have occurred in other jurisdictions are not known. Similarly, it is possible that post-release treatment engagement may be underestimated - as treatment admission/discharge and prescription refills not identified as matches to participant identifiers are counted as non-engagement.
In the US, with criminalization as the major approach to the disease of opioid use disorder, it is essential that individuals with criminal-legal involvement have access to being diagnosed, treated with MOUD and linked to care in the community upon release. MOUD clearly can offer a life-saving intervention to a high-risk population, vulnerable to overdose upon release. Rather than rely on a carceral system to provide such life-saving treatment, increased community services, rather than punishment by the criminal legal system, may offer improved health outcomes and a clear path to addressing the opioid overdose crisis. In the meantime, implementing comprehensive MOUD in correctional facilities, especially jails where the vast majority of those with MOUD are incarcerated, with linkage to care in the community is a strategy that will save many lives.
Contributors
RM had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. RM, JC, JDR, JB, and LH conceptualized and designed the study. Acquisition, analysis, or interpretation of data was done by RM, RC, AK, and AH. RM and EK drafted the manuscript. JC, AK, NA-S, and JDR provided critical revision of the manuscript for important intellectual content. RM, JDR and JC obtained funding for the study.
Data sharing statement
According to data protection rules set out by the RI Department of Corrections, RI Department of Health, and RI Department of Behavioral Health, Developmental Disabilities, and Hospitals, the authors cannot publicly release the data from the RIDOC electronic medical record data, the Rhode Island Behavioral Online Database (RI-BHOLD) or the prescription drug monitoring program.
Declaration of interests
We declare no competing interests.
Acknowledgements
RM, JC, NA-S, JB, RC, AK, and AH are funded by the National Institutes of Health through the NIH HEAL Initiative under award number U01 DA050442-01. JR and EK are funded by NIH grants P20 GM125507 and R21 DA043487.
Funding: This work was supported by the Rhode Island General Fund through the Department of Corrections, the NIH of Health HEAL Initiative, (U01 DA050442-01), the NIGMS (P20 GM125507), and the NIDA (R21 DA043487 and K23DA055695).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2022.100419.
Appendix A. Supplementary data
References
- 1.Seth P., Scholl L., Rudd R.A., Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants—United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018;67(12):349. doi: 10.15585/mmwr.mm6712a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Academies of Sciences E, Medicine . In: Medications for opioid use disorder save lives. Leshner A.I., Mancher M., editors. The National Academies Press; Washington, DC: 2019. p. 174. [PubMed] [Google Scholar]
- 3.Degenhardt L., Bucello C., Mathers B., et al. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 2011;106(1):32–51. doi: 10.1111/j.1360-0443.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- 4.Joudrey P.J., Khan M.R., Wang E.A., et al. A conceptual model for understanding post-release opioid-related overdose risk. Addiction Sci Clin Pract. 2019;14(1) doi: 10.1186/s13722-019-0145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkelman T.N.A., Chang V.W., Binswanger I.A. Health, polysubstance use, and criminal justice involvement among adults with varying levels of opioid use. JAMA Netw Open. 2018;1(3) doi: 10.1001/jamanetworkopen.2018.0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binswanger I.A., Blatchford P.J., Mueller S.R., Stern M.F. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013;159(9):592–600. doi: 10.7326/0003-4819-159-9-201311050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranapurwala S.I., Shanahan M.E., Alexandridis A.A., et al. Opioid overdose mortality among former North Carolina inmates: 2000-2015. Am J Publ Health. 2018;108(9):1207–1213. doi: 10.2105/AJPH.2018.304514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkow N.D., Blanco C. The changing opioid crisis: development, challenges and opportunities. Mol Psychiatr. 2021;26(1):218–233. doi: 10.1038/s41380-020-0661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jail and prison opioid Project – medication for opioid use disorder in the criminal legal system (JPOP) 2022. http://prisonopioidproject.org/ Retrieved August 23, 2022, from.
- 10.Klein A.R., Association N.S. National Sheriffs' Association; 2018. America USo, Care NCoCH, America USo. Jail-based Medication-assisted Treatment: Promising Practices, Guidelines, and Resources for the Field. [Google Scholar]
- 11.Ahmad F.B., Rossen L.M., Sutton P. National Center for Health Statistics; 2021. Provisional drug overdose death counts. [Google Scholar]
- 12.Raimondo G.M. 2019. Rhode Island overdose prevention and intervention task force action plan. [Google Scholar]
- 13.Clarke J.G., Martin R.A., Gresko S.A., Rich J.D. American Public Health Association; 2018. The first comprehensive program for opioid use disorder in a US statewide correctional system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin R.A., Alexander-Scott N., Wendelken J., Clarke J.G. In: A public health guide to ending the opioid epidemic. Butler Jay C., Fraser Michael R., editors. Oxford University Press; 2019. Collaborating to address substance use disorder in correctional settings: the Rhode Island experience. [Google Scholar]
- 15.Green T.C., Clarke J., Brinkley-Rubinstein L., et al. Postincarceration fatal overdoses after implementing medications for addiction treatment in a statewide correctional system. JAMA Psychiatr. 2018;75(4):405–407. doi: 10.1001/jamapsychiatry.2017.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin R.A., Gresko S.A., Brinkley-Rubinstein L., Stein L., Clarke J.G. Post-release treatment uptake among participants of the Rhode Island Department of Corrections comprehensive medication assisted treatment program. Prev Med. 2019;128 doi: 10.1016/j.ypmed.2019.105766. [DOI] [PubMed] [Google Scholar]
- 17.Merrall E.L.C., Dhami M.K., Bird S.M. Exploring methods to investigate sentencing decisions. Eval Rev. 2010;34(3):185–219. doi: 10.1177/0193841X10369624. [DOI] [PubMed] [Google Scholar]
- 18.SPSS Reference: IBM Corp. Released. IBM Corp; Armonk, NY: 2019. IBM SPSS Statistics for Windows, Version 26.0. [Google Scholar]
- 19.Binswanger I.A., Stern M.F., Deyo R.A., et al. Release from prison--a high risk of death for former inmates. N Engl J Med. 2007;356(2):157–165. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joosten E. Effect of shared decision-making on therapeutic alliance in addiction health care. Patient Prefer Adherence. 2008:277. doi: 10.2147/ppa.s4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joosten E.A.G., De Jong C.A.J., De Weert-Van Oene G.H., Sensky T., Van Der Staak C.P.F. Shared decision-making reduces drug use and psychiatric severity in substance-dependent patients. Psychother Psychosom. 2009;78(4):245–253. doi: 10.1159/000219524. [DOI] [PubMed] [Google Scholar]
- 22.Kaplowitz E., Truong A., Berk J., et al. Treatment preference for opioid use disorder among people who are incarcerated. J Subst Abuse Treat. 2021 doi: 10.1016/j.jsat.2021.108690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teruya C., Schwartz R.P., Mitchell S.G., et al. Patient perspectives on buprenorphine/naloxone: a qualitative study of retention during the starting treatment with agonist replacement therapies (START) study. J Psychoact Drugs. 2014;46(5):412–426. doi: 10.1080/02791072.2014.921743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedrichs A., Spies M., Härter M., Buchholz A. Patient preferences and shared decision making in the treatment of substance use disorders: a systematic review of the literature. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0145817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lippold K., Jones C., Olsen E., Giroir B. Racial/ethnic and age group differences in opioid and synthetic opioid–involved overdose deaths among adults aged ≥18 Years in metropolitan areas — United States, 2015–2017. MMWR Morb Mortal Wkly Rep. 2019;68:967–973. doi: 10.15585/mmwr.mm6843a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.