Abstract
Aims
Rheumatoid arthritis (RA) is a common chronic immune disease. Berberine, as its main active ingredient, was also contained in a variety of medicinal plants such as Berberaceae, Buttercup, and Rutaceae, which are widely used in digestive system diseases in traditional Chinese medicine with anti-inflammatory and antibacterial effects. The aims of this article were to explore the therapeutic effect and mechanism of berberine on rheumatoid arthritis.
Methods
Cell Counting Kit-8 was used to evaluate the effect of berberine on the proliferation of RA fibroblast-like synoviocyte (RA-FLS) cells. The effect of berberine on matrix metalloproteinase (MMP)-1, MMP-3, receptor activator of nuclear factor kappa-Β ligand (RANKL), tumour necrosis factor alpha (TNF-α), and other factors was determined by enzyme-linked immunoassay (ELISA) kit. Transcriptome technology was used to screen related pathways and the potential targets after berberine treatment, which were verified by reverse transcription-polymerase chain reaction (RT-qPCR) and Western blot (WB) technology.
Results
Berberine inhibited proliferation and adhesion of RA-FLS cells, and significantly reduced the expression of MMP-1, MMP-3, RANKL, and TNF-α. Transcriptional results suggested that berberine intervention mainly regulated forkhead box O (FOXO) signal pathway, prolactin signal pathway, neurotrophic factor signal pathway, and hypoxia-inducible factor 1 (HIF-1) signal pathway.
Conclusion
The effect of berberine on RA was related to the regulation of RAS/mitogen-activated protein kinase/FOXO/HIF-1 signal pathway in RA-FLS cells.
Cite this article: Bone Joint Res 2023;12(2):91–102.
Keywords: Berberine, Traditional Chinese medicine, Rheumatoid arthritis, rheumatoid arthritis, fibroblast-like synoviocytes, MMP-3, Western blot, hypoxia, quantitative polymerase chain reaction, Enzyme-linked immunosorbent assay, matrix metalloproteinases (MMPs), arthritis, polymerase chain reaction
Article focus
This study is the first to demonstrate the anti-rheumatoid effect of berberine in rheumatoid arthritis fibroblast-like synoviocyte (RA-FLS) cells, and explore the potential mechanism through the biochemical index evaluation, transcriptomics, and molecular biotechnology.
Key messages
The RAS/mitogen-activated protein kinase (MAPK)/forkhead box O (FOXO)/hypoxia-inducible factor 1 (HIF-1) signal pathway is considered the most important pathway, and cyclin-dependent kinase 2, FOXO3, MAPK, and signal transducer and activator of transcription 3 were the key targets, which may provide data reference for clinical use and drug development in the future.
Strengths and limitations
This paper studied the effect of RA-FLS-cellular berberine on RA.
One limitation lies in the lack of animal experiments in vivo, and should be further studied in this direction in the future.
Introduction
Ranunculus Japonicus Thunb. is a perennial plant which is classified as a pungent, warm, and poisonous herb in traditional Chinese medicine (TCM). It was firstly recorded in ‘Zhouhou Beiji Fang’ with the main function of eliminating swelling, relieving pain, and preventing malaria. 1 It is commonly used clinically to treat jaundice, stomachache, malaria, asthma, toothache, migraine, lymphatic tuberculosis, rheumatoid arthritis (RA), and corneal opacity. 2
Berberine, one of the most effective ingredients, is widely distributed in many medicinal plants, such as Berberidaceae, Ranuculaceae, Rutaceae, and so on. In addition, berberine attracted extensive attention because of its significant bioactivity, including anti-inflammatory, 3 antibacterial, 4 antiviral, etc., which led to its widespread use in a variety of bacterial-induced digestive diseases for a long time. With the development of modern pharmacological research, berberine has proven to be effective in treating and preventing many common metabolic diseases including cardiovascular disease, 5,6 diabetes, 7 and neurodegenerative disease. 8 Furthermore, berberine can inhibit the development and progression of various cancers both in vitro and in vivo. 9
RA is a chronic autoimmune disease whose main clinical manifestations are synovitis and joint damage. 10-12 RA fibroblast-like synoviocytes (RA-FLSs) are highly specialized mesenchymal cells in sensitive joint synovium with strong invasive ability, and play an important role in the progression of RA. 13 RA-FLSs can secrete a variety of pathogenic mediators such as cytokines (tumour necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-23) and chemokines (receptor activator of nuclear factor kappa-Β ligand (RANKL), granulocyte-macrophage colony stimulating factor, and matrix metalloproteinases (MMPs)). They also induce the migration and differentiation of other cells in the synovium of RA, accelerating the disease’s progression. At present, the development of targeted drugs has become one of the main research directions in the prevention and treatment of RA, which work by inhibiting the phenotype of RA-FLSs. 14
Recently, medicinal chemistry and pharmacodynamics research has shown that berberine (Figure 1) from R. japonicus as a bioactive component may exert therapeutic effects in RA by inhibiting the aggressive phenotype of RA-FLSs. 15 Our previous wound healing assay results showed that the 5 μM berberine-treated group and the 150 μg/ml R. japonicus extract-treated group showed similar inhibition of RA-FLS migration expression. 16 Clinical studies have found that berberine shows excellent therapeutic effects on RA. 17 One published study suggested that berberine significantly reduced the incidence and severity of RA and inhibited the secretion of anti-collagen antibodies, interferon-gamma, and IL-17 in collagen-induced arthritis (CIA) mice. In addition, berberine could inhibit the formation and survival of osteoclasts by antagonizing the induction of nuclear factor κB (NF- κB) and AKT activation by RANKL. 18 Similarly, the results of our previous study found that berberine is a major ingredient isolated from Ranunculus, which exhibited a protective effect in RA treatment through biological processes, while the mechanism remained unclear. 16
Fig. 1.
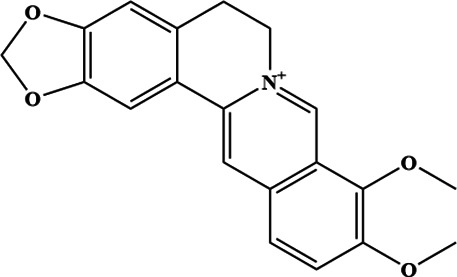
The molecular structure of berberine.
In view of the importance of RA-FLSs in the occurrence, prevention, and management of RA, RA-FLS cells were used to explore the protective effect of berberine on RA synovitis and its exact mechanism through the transcriptomics in our study, providing a reference for the clinical application of berberine for the RA.
Methods
Reagents
Berberine (633-65-8) was purchased from Chengdu Puth Biotechnology (China) with a purity of more than 98%. We also used: fetal bovine serum (FBS) (L20711 BI Transgen biotech, Australia); Dulbecco’s Modified Eagle Medium (DMEM) (2049231, BIOIND, Israel); Cell Counting Kit-8 (CCK-8) (DCM7126, Beijing Lambolid Trading, China); lipopolysaccharide (12190801, Beijing Trading, China); and TRIzol reagent (182806, Semel Fishi Technology, USA). MMP-1 (L2104850), MMP-3 (L201014164), RANKL (L201216066), and TNF-α (L201215056) were all purchased from Wuhan Yunkun Technology (China). We also used one-step removal of premixed reagent for first chain synthesis of genomic cDNA (U9126, Tiangen biochemical Technology, China) and PerfectStart Green qPCR SuperMix (+ Dye Ⅰ /+ Dye Ⅱ) (O10529, Beijing full Gold Biotechnology, China) in the RT-qPCR. Phosphorylase inhibitor (TargetMol, USA), bicinchoninic acid (BCA) protein concentration determination kit (Beijing Soleibao Technology, China), Oncogene homologue (GTPase Hras, HRAS) antibody (Beijing Boosen Biotechnology, China), cyclic adenine ribonucleotide dependent transcription factor 2 (ATF-2) antibody (Affinity, China), p-ATF-2 antibody (Affinity), p38 antibody (Beijing Boosen Biotechnology), p-p38 antibody (Affinity), MAPK1/2 antibody (Affinity); p-MAPK1/2 antibody (Affinity), FOXO3 antibody (Affinity), p-FOXO3 antibody (Affinity), HIF-1 α Antibody (Affinity), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Proteintech, China), and sheep anti-rabbit-peroxidase (Beijing Boosen Biotechnology) were used in the western blot experiment.
Cell culture
RA-FLSs, derived from synovial tissue of patients with RA, were purchased from Beijing Beina Chuanglian Biotechnology (China), and cultured with DMEM medium with 10% FBS in 37.5°C and 5% CO2 incubator.
Preparation of liquid medicine
The berberine was dissolved in dimethyl sulfoxide (DMSO) to prepare the stock solution. Before administration, the stock solution was diluted with fresh DMEM culture medium with various concentrations based on preliminary tests.
Cell proliferation and adhesion assay
The cells (3.5 × 105/ml) were cultured and treated with different concentrations of berberine in a 96-well plate for 24 hours, and the cell proliferation rate was detected by CCK-8 kit. The cells (3.5 × 105/ml) were inoculated into a 96-well plate coated with human recombinant laminin for two hours, subsequently incubated with 1% heat-denatured bovine serum albumin Ⅴ solution for one hour, and cultured for four hours. CCK-8 kit was used for determination of the adhesion rate (%) of each group according to formula.
ELISA
Cells growing in logarithmic phase were inoculated in a 100 mm cell culture dish with a cell density of 3.5 × 105 cells/ml for 24 hours. Subsequently, 10 ml of berberine with different concentrations was used to replace the cell culture medium for 24 hours. At the end of the experiments, the cell supernatant was collected from different groups, centrifuged at 4°C at 1,000 g/min for 20 minutes, and stored as the test sample. The expression levels of MMP-1, MMP-3, RANKL, and TNF-α in the supernatant of the cells were evaluated according to the instructions of each kit. The experiment was repeated three times for each index.
Gene expression detection
RNA was extracted with TRIzol reagent; genomic DNA was removed by DNase I (TaKara, Japan). The absorbance of RNA samples at 260 nm and 280 nm was read by NanoDrop 2000 (ThermoFisher Scientific, USA) to determine the content and purity of RNA samples. Agarose gel electrophoresis was used to detect the integrity of the samples. TruSeq RNA transcriptome library was prepared by RNA-seq sample preparation kit (TransGen Biotech, China). The paired terminal RNA-seq sequencing library was sequenced with Illumina Novaseq 6000 (2 × 150 bp reading length) by TBS380, and the sequencing work was completed by Shanghai Meiji Biotechnology (China). The end readings of the original pair were trimmed and quality-controlled through the default parameters of the SeqPrep website 19 and the default settings of the Sickle website. 20 The source of the reference genome was the Ensembl Project, 21 and the TopHatm was used to compare the clean reads with the reference genome in directional mode. 22
Gene differential expression analysis
The gene abundance was quantified by RSEM (University of Wisconsin-Madison, USA). The differential expression between groups was analyzed by the EdgeR package of R 3.6.3 software (R Foundation for Statistical Computing, Austria). The screening threshold was | log2FC | ≥ 1 and p < 0.05. Ggplot2 package (R Foundation for Statistical Computing) was used to visualize the volcano map of the transcripts from the different groups.
Enrichment analysis of GO and KEGG pathways
The differential transcripts of each group were uploaded to the Database for Annotation, Visualization and Integrated Discovery (DAVID) database (LHRI, USA) for enrichment analysis of gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. 23 The enrichment results of biological process (BP), cell component (CC), molecular function (MF), and KEGG pathways were downloaded into TXT format files, visualized by R3.6.3 software.
Core difference transcript screening
The differential transcripts of each group were uploaded to the String database for Protein-Protein Interaction analysis. 24 The confidence was set to 0.7. The analysis results were imported into Cytoscape 3.6.1 software (National Resource for Network Biology, USA).
Molecular docking
The 3D structures of small molecule compounds obtained from the PubChem database and the conformation of proteins collected from the Protein Data Bank (PDB) database (USA) were transferred into the PDB, Partial Charge, and Atom Type (PDBQT) format through Chem3D software. 25 Then, MGLTools was used to perform the protein processing, including removing water, adding hydrogen bonds, detecting the root, setting rotatable bonds, and computing the Gasteiger charge. Subsequently, the active pocket was determined according to the binding position of the protein and its inhibitor. Finally, molecular docking was performed using AutoDock Vina 1.1.2 (Scripps, USA), the results of which were visualized by PyMol (DeLano Scientific, USA). These methods have previously been reported in Du et al. 26
RT-qPCR
Total RNA from cells was extracted using TRIzol reagent. The sequences from reverse transcription to complementary DNA (cDNA) using kit one-step removal of premixed reagent for first chain synthesis of genomic cDNA, and q-PCR experiment using kit PerfectStart Green qPCR SuperMix (+ Dye Ⅰ/+ Dye Ⅱ) primers, are shown in Supplementary Table i.
Western blot
The cells were lysed with radioimmunoprecipitation assay (RIPA) buffer (Beyotime, China) containing 0.1 mmol/l phenylmethylsulfonyl fluoride (PMSF) on ice for 30 minutes. The supernatants were collected by centrifugation (12,000 rpm, 10 mins, 4°C) and then measured with the BCA assay. Aliquots of the samples were subjected to sodium dodecyl-sulphate polyacrylamide gel electrophoresis (SDS-PAGE), and transported on polyvinylidene difluoride (PVDF) membranes. After blocking with 5% skim milk for two hours, the PVDF membranes were incubated with primary antibodies and respective secondary antibodies. The protein bands were visualized with BeyoECL Plus Kit, and the grey values of the target bands and background in the exposure results were analyzed by ImageJ software (National Institutes of Health, USA). These methods have previously been reported in Zhao et al. 27
Statistical analysis
GraphPad Prism 7.0 software (USA) was used to determine whether the data were in accordance with normal distribution. Ordinary one-way analysis of variance (ANOVA) was used for statistical analysis. A p-value < 0.05 was considered to be statistically significant.
Results
Effect of berberine on RA-FLS cell proliferation
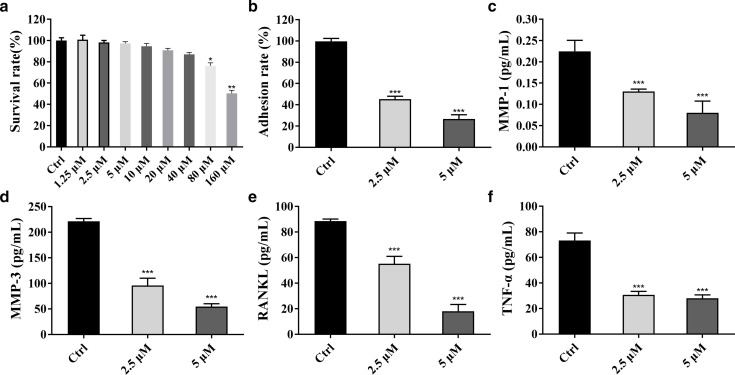
The effect of berberine on the viability of RA-FLS cells was evaluated by CCK-8 method. The results showed that the cell survival rate decreased with the increase of drug concentration (Figure 2a). Compared with the control group, berberine significantly inhibited the cell viability after 24-hour administration of 80 μM (p = 0.025) and 160 μM (p = 0.008).
Fig. 2.
The effect of berberine on proliferation and adhesion of rheumatoid arthritis fibroblast-like synoviocytes (RA-FLSs). a) The results of Cell Counting Kit-8. b) Cell adhesion test results. c) to f) Changes of matrix metalloproteinase (MMP)-1, MMP-3, receptor activator of nuclear factor kappa-Β ligand (RANKL), and tumour necrosis factor alpha (TNF-α) (*p = 0.05, **p = 0.010, ***p < 0.001, n = 3).
Berberine inhibits RA-FLSs cell adhesion
The adhesion test was used to evaluate the effect of berberine on the adhesion ability of RA-FLSs (Figure 2b). Compared with the control cells, 2.5 μM and 5 μM berberine significantly inhibited the adhesion ability of RA-FLSs cells in a concentration-dependent manner (p < 0.001).
ELISA
Enzyme-linked immunosorbent assay (ELISA) detection kits were used to detect the effect of berberine on the expression of MMP-1, MMP-3, RANKL, and TNF- α in the supernatant of RA-FLS cells. The results showed that 2.5 μM and 5 μM berberine significantly decreased the expression of MMP-1, MMP-3, RANKL, and TNF-α in the supernatant of RA-FLS cells (p < 0.001) in a dose-dependent manner (Figures 2c to 2f).
Results of differential analysis of gene expression
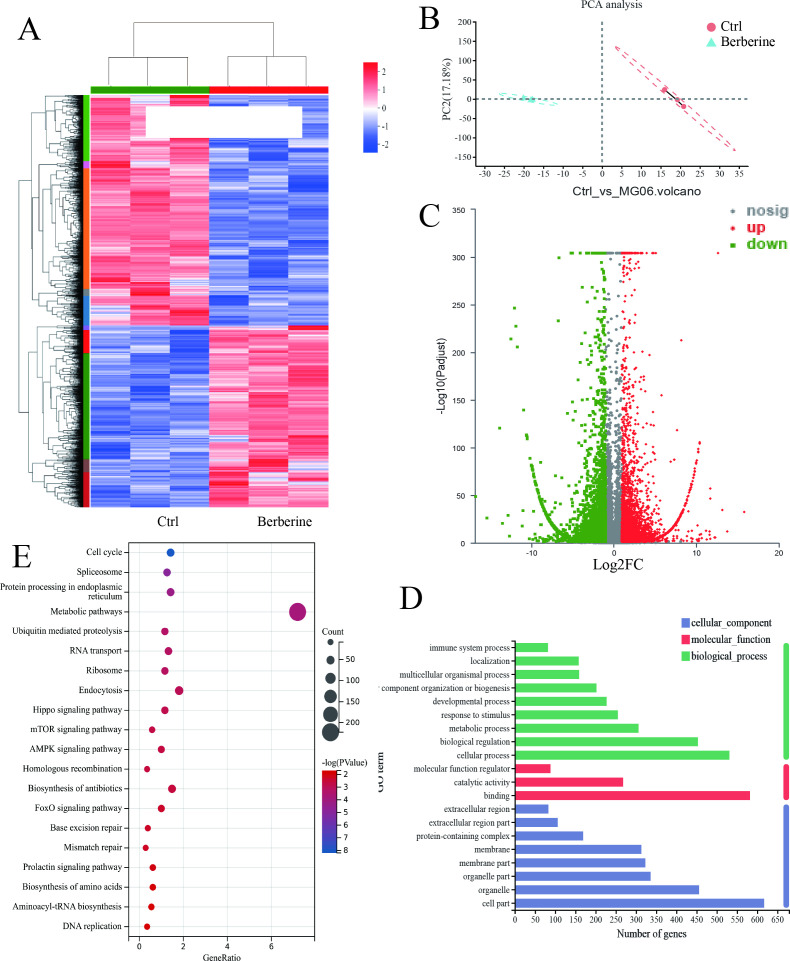
A total of 28,114 expressed genes were detected, including 27,706 known genes and 408 new genes. PCA analysis model revealed a significant separation between the control and treatment groups (Figure 3b). EdgeR was used to analyze the differential transcripts between the berberine intervention group and the control group with the screening criteria of | log2FC | ≥ 1 and p > 0.05, and the transcript was visualized in the form of a volcanic map (Figure 3c). It was found that berberine could regulate 3,270 differential transcripts, including 1,765 upregulated transcripts and 1,505 downregulated transcripts. Furthermore, the differential transcripts of each group were visualized in the form of a cluster heat map (Figure 3a), which showed that the berberine-treated group could be distinguished from the control group by hierarchical clustering of differential genes.
Fig. 3.
a) Clustering heat map of differential transcripts. b) The principal component analysis (PCA). c) Volcanic map of berberine-regulated transcripts. d) Gene ontology (GO) bar chart of differential transcripts. e) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway bubble diagram. AMPK, AMP-activated protein kinase; FOXO, forkhead box O.
Functional enrichment analysis of differential transcripts
In total, 4,240 differential transcripts affected by berberine were analyzed by GO enrichment analysis using the DAVID database. The first ten entries of BP, CC, and MF were visualized (Figure 3d). The main changes in biological processes in which they participate were concentrated in cellular process, metabolism process, biological regulation, and other processes. The main changes of differentially expressed genes in cell components were located in cell part, organelles, and cell membranes. In molecular functional analysis, gene expression level changes showed various functions, mainly including binding, catalytic activity, and molecular function regulation. Furthermore, 4,240 differential transcripts of berberine were analyzed by KEGG pathway enrichment analysis by DAVID database, 60 KEGG pathways were enriched, and the first 20 KEGG pathways were visualized, as shown in Figure 3e. The enrichment results suggest that berberine intervention mainly regulated Hippo signal pathway, mammalian rapamycin target protein signal pathway, adenylate-activated protein kinase signal pathway, FOXO signal pathway, HIF-1 signal pathway, and Wnt signal pathway. The previously predicted cross-analysis of transcriptome results with network pharmacology analysis highlighted four shared pathways including FOXO signal pathway, prolactin signal pathway, neurotrophic signal pathway, and HIF-1 signal pathway. 16
Screening of core differential transcripts
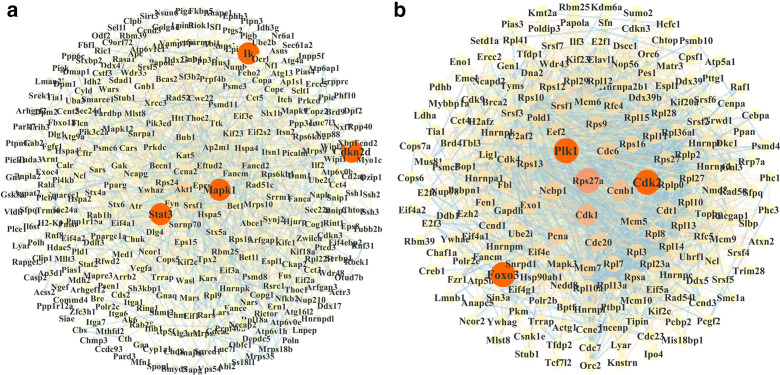
The String database was used to analyze and screen the key differential genes from the different treatment groups (0.7 high confidence), which was mapped by Cytoscape 3.6.1 software. The results showed that 1,127 upregulated and 961 downregulated differential transcripts were selected. Furthermore, 349 nodes with more than 9° of freedom were considered as the core nodes of berberine upregulated transcripts (Figure 4a), and 294 nodes for downregulated transcripts (Figure 4b).
Fig. 4.
Analysis of the key targets of berberine against rheumatoid arthritis. a) Berberine core upregulated transcriptional protein-protein interaction (PPI) diagram. b) Berberine core downregulated transcriptional PPI diagram.
As can be seen from Figure 4, polo-like kinase-1 (PLK1), cyclin-dependent kinase-2 (CDK2), mitogen-activated protein kinase 1 (MAPK1), signal transducer and activator of transcription-3 (STAT3), forkhead box 3 (FOXO3), and inhibitor of nuclear factor kappa-B kinase subunit β (IKBKB) were important nodes in the network, and they were all located in the FOXO signal pathway and HIF-1 signal pathway. Berberine administration could significantly affect FOXO signal pathway and HIF-1 signal pathway, so PLK1, CDK2, MAPK1, STAT3, FOXO3, IKBKB, and their related genes in FOXO signal pathway and HIF-1 signal pathway were selected for experimental verification (Figure 5).
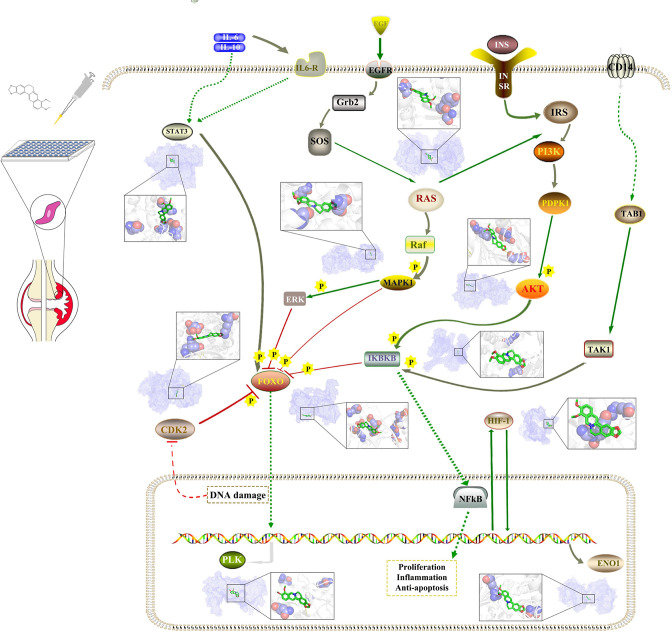
Fig. 5.
The pathways and molecular docking diagrams of berberine with the key targets. CDK2, cyclin-dependent kinase 2; EGFR, estimated glomerular filtration rate; ENO1, enolase 1; ERK, extracellular signal-regulated kinase; Grb2, growth factor receptor-bound protein 2; FOXO, forkhead box O; HIF-1, hypoxia-inducible factor 1; IKBKB, inhibitor of nuclear factor kappa-B kinase subunit β; IL6-R, interleukin-6 receptor; INS, insulin preproprotein; IRS, insulin receptor substrate; NFkB, nuclear factor kappa B; PLK, polo-like kinase-1; RAS, renin-angiotensin system; TAB1, TAK1-binding protein 1; TAK1, TGF-activated kinase 1; SOS, salt overly sensitive; STAT3, signal transducer and activator of transcription 3.
Molecular docking
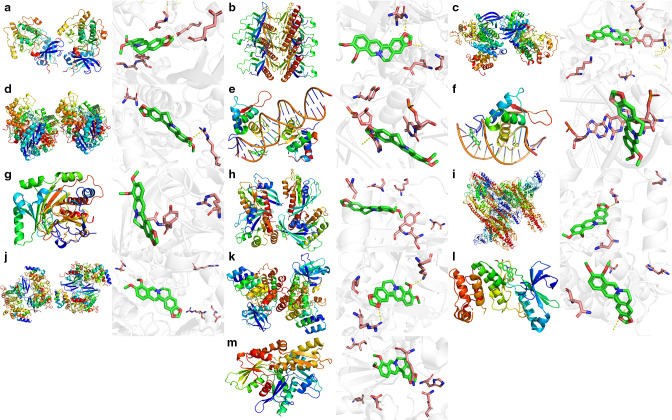
In order to confirm the potential key targets and related pathways of berberine against RA, molecular docking was used to verify the reliability of the prediction results above. The results showed that most of the targets had good binding activity with berberine, including MAPK1, STAT3, CDK2, IKBKB, FOXO3, PLK1, HRAS, AKT2, AKT3, FOXO4, PLK4, ENO1, and HIF-1 α (Figure 6).
Fig. 6.
The binding energies for the key targets docked into the berberine. a) AKT2-berberine (-9.1); b) AKT3 -berberine (-7.0); c) cyclin-dependent kinase 2 (CDK2)-berberine (-10.1); d) enolase 1 (ENO1)-berberine (-7.1); e) forkhead box 3 (FOXO3)-berberine (-8.9); f) FOXO4-berberine (-9.0); g) hypoxia-inducible factor 1 alpha (HIF-1α)-berberine (-6.0); h) HRAS-berberine (-7.3); i) inhibitor of nuclear factor kappa-B kinase subunit β (IKBKB)-berberine (-7.7); j) mitogen-activated protein kinase 1 (MAPK1)-berberine (-9.1); k) polo-like kinase-1 (PLK1)-berberine (-9.0); l) PLK4-berberine (-6.6); and m) signal transducer and activator of transcription 3 (STAT3)-berberine (-7.9).
RT-qPCR
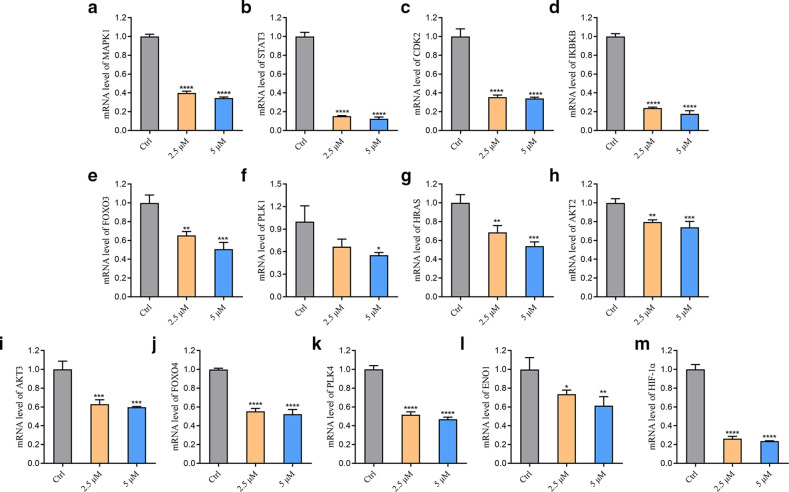
The regulatory effect of berberine on the transcriptional levels of core transcripts and key genes in FOXO signal pathway and HIF-1 signal pathway was verified by reverse transcription quantitative polymerase chain reaction (RT-qPCR). The results are shown in Figure 7. The results showed that berberine downregulated MAPK1, STAT3, CDK2, IKBKB, HRAS, FOXO3, RAC serine/threonine protein kinase 2 (AKT2), AKT3, FOXO4, polo-like kinase 4 (PLK4), enolase 1 (ENO1), and HIF-1 α (p < 0.001). With the increase of drug concentration, the inhibitory effect on the transcriptional level of the above genes was enhanced.
Fig. 7.
Effect of berberine on core transcripts of forkhead box O (FOXO) and hypoxia-inducible factor 1 (HIF-1) signalling pathway. a) to m) The effect of berberine on the transcriptional levels of mitogen-activated protein kinase 1 (MAPK1), signal transducer and activator of transcription 3 (STAT3), cyclin-dependent kinase 2 (CDK2), inhibitor of nuclear factor kappa-B kinase subunit β (IKBKB), FOXO3, polo-like kinase 1 (PLK1), HRAS, RAC serine/threonine protein kinase 2 (AKT2), AKT3, FOXO4, PLK4, enolase 1 (ENO1), and HIF-1 α after 24-hour administration (*p < 0.050, **p < 0.0, ***p < 0.001, ****p < 0.0001; n = 3). mRNA, messenger RNA.
Western blot
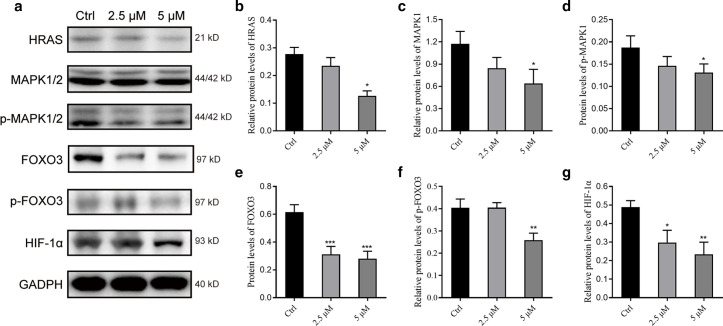
Western blot method was used to verify the regulatory effect of berberine on the expression of core proteins in the FOXO signal pathway and HIF-1 signal pathway, as shown in Figure 8. After 24-hour administration, berberine at 2.5 and 5 μM could significantly reduce the protein expression of HIF-1 α in a dose-dependent manner, while 5 μM berberine could significantly decrease the protein expression level of HRAS, MAPK1/2, p-MAPK1/2, FOXO3, and p-FOXO3 (p = 0.035).
Fig. 8.
Effect of berberine on the expression of core proteins in forkhead box O (FOXO) and hypoxia-inducible factor 1 (HIF-1) signalling pathway. a) Western blot-related bands of related proteins. b) to g) Bar charts representing the relative quantitative results of HRAS, mitogen-activated protein kinase 1 (MAPK1), p-MAPK1, FOXO3, p-FOXO3, and HIF-1 α protein expression levels in turn (*p < 0.05, **p < 0.01, ***p < 0.001; n = 3). GADPH, glyceraldehyde 3-phosphate dehydrogenase.
Discussion
Clinically, RA is the most prevalent severe chronic inflammatory autoimmune disease which, along with pain, swelling, and damage of synovial joints, brings great inconvenience to the lives of patients and their families. 28 At present, the commonly used clinical treatment options for RA are wide use of some drugs, including non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, disease-modifying antirheumatic drugs (DMARDs), and novel biological agents to alleviate or inhibit the development of the disease. However, the adverse reactions of the drugs are of common concern to clinicians and patients.
TCM has long been used as a complementary and alternative treatment for certain complex diseases including RA, with the benefit of fewer adverse reactions and side-effects. There is growing interest in TCM among researchers, who believe that new and more effective drugs could be developed from the medicinal plants.
Berberine, an isoquinoline alkaloid, is the major effective compound isolated from various medicinal plants, such as Rhizoma Coptidis (Chinese name: Huang-Lian). Emerging evidence has demonstrated that berberine exerts some excellent effects in the clinical treatment of digestive diseases, such as bacterial gastroenteritis and dysentery. In addition, berberine has been reported to exhibit anti-inflammatory effects and reduce blood lipid and glucose. In recent years, modern pharmacology has shown that berberine has remarkable effects on heart failure, arrhythmia, cholesterol reduction, vascular smooth muscle proliferation, and insulin resistance. One recent clinical study showed that berberine has excellent therapeutic effects on patients with RA. 9 The previous experiment of our research group found that berberine has anti-RA effects in RA-FLS cells, which play an important role in RA development and were selected in this research. 16
Based on the previous study results, 2.5 and 5 μM berberine were initially selected as an experimental concentration to confirm the biological effect in RA-FLSs; the results showed that berberine can significantly inhibit the proliferation and adhesion of RA-FLS cells.
Accumulating evidence has demonstrated a correlation between the RA process and a variety of MMPs, which were released from RA-FLS cells and associated with the degradation of articular cartilage and bone. Clinical data found that the secretion of MMP-1 and MMP-3 in serum of patients with early RA was significantly upregulated, and their expression was positively correlated with joint swelling index, ESR, and CRP. Therefore, it is suggested that the detection of the expression level of MMP-1 and MMP-3 was helpful to judge the degree of joint destruction and monitor disease progression in RA patients; downregulating the expression of MMP-1 and MMP-3 was beneficial to the prevention and treatment of RA. 29,30 More importantly, compared with FLS cells from osteoarthritis (OA) and without vascular necrosis, RA-FLSs showed stronger invasiveness and were positively correlated with the expression of MMP-1, MMP-3, and MMP-10.
Furthermore, the tumour necrosis factor superfamily TNF-α played an important role in the inflammation and bone destruction of RA, since it could promote the release of downstream inflammatory cytokines and inflammatory response, leading to tissue damage. RANKL, osteoclast differentiation factor, and osteoprotegerin ligand were bound up with the disease progression of RA. 31 RA-FLSs can activate macrophages and osteoclasts by secreting RANKL, and then induce osteoclast formation. 32 At present, biological agents targeting TNF superfamily have been used to treat RA. In view of the positive regulation of MMP-1, MMP-3, TNF-α, and RANKL on RA-FLS migration, ELISA assay was used to detect the effect of berberine on the secretion of MMP-1 and MMP-3 in the supernatant of RA-FLS cells. The results showed that berberine could significantly reduce the secretion of MMP-1, MMP-3, TNF-α, and RANKL in the supernatant of RA-FLS cells, thus effectively inhibiting the migration and invasion of RA-FLS cells, and preventing RA synovitis.
In recent years, omics technology has been widely used to investigate the mechanism of drug action and screen the target of action, with a view to providing more detailed biological response information. Therefore, in this paper transcriptome assessment was performed to describe the detailed characteristics of change profile from the transcriptional level after the intervention of berberine in RA-FLSs.
The results showed that berberine could cause significant changes in cell transcription. Compared with the control group, berberine treatment caused significant difference in 3,270 differential transcripts (| log2FC | > 1and p < 0.05), including 1,765 rising and 1,505 falling. In order to further clarify the effects of the above differential genes on biological events, GO and KEGG analyses were used to elucidate the potential pathway of berberine in the treatment of RA. The results showed that the pathway affected by berberine include the positive regulation of cell migration, the positive regulation of RNA polymerase II promoter transcription, protein phosphorylation, and other biological processes, mainly affecting FOXO signal pathway and HIF-1 signal pathway.
Ras family homologous proteins (i.e. HRAS, KRAS, and NRAS) were expressed in RA synovium and RA-FLSs, and preferentially in intimal lining. Each Ras family homologous protein has a unique contribution and overlapping effect on the activation of FLS. Research has shown that silencing the expression of three Ras proteins could significantly inhibit the disease severity and joint destruction of CIA mice, while activation of each Ras homologue could promote the secretion of IL-6 and MMP-3 induced by IL-1β, but only HRAS can increase the production of interleukin 8 induced by IL-1β. 33 In RA-FLSs, HRAS-specific Ras guanine nucleotide releasing factor 1 was activated to promote the secretion of endogenous MMP-3, indicating that negative regulation of HRAS expression could inhibit the invasive phenotype of RA-FLS cells. 34 In this experiment, the downregulation of Ras expression as well as the related protein were observed, indicating that the protective effect of berberine against RA was related to the extensive inhibition of Ras and its related proteins.
Furthermore, MAPK, PI3K/AKT, and other signal pathways were the downstream targets of Ras signal pathway and activated in RA. MAPK signal pathway was involved in the migration and invasion of RA-FLSs and the recombination of actin cytoskeleton. 35 Specifically, the protein expression of MAPK1 and JNK in synovial fluid of patients with RA was significantly increased, as were the phosphorylated proteins of these proteins. 36 As the catalytic subunit of IκB kinase complex (IKK), IKK β participates in the activation of NF-κB pathway, while inactivation of MAPK/NF- κB, and use of MAPK1/2 phosphorylation inhibitors could result in the inhibition of proliferation, migration, invasion, and inflammation of RA-FLS cells. 37 In addition, several novel p38 inhibitors can effectively reduce the severity of the disease. Therefore, the development of drugs targeting the MAPK signal pathway may be an effective strategy to prevent and treat the progression of RA. 38
HIF-1, highly expressed in the synovium of RA, plays a core role in the hypoxic environment of RA. 18 The number of HIF-1-positive cells affects angiogenesis in RA synovium. Downregulation of HIF-1 expression could significantly inhibit angiogenesis induced by VEGF in RA-FLSs, indicating that the HIF-1/VEGF axis could exert important regulation of the angiogenesis process of RA. 39 Furthermore, the infiltration level of myeloid cells at the inflammatory site, and the degree of foot swelling and disease progression, were inhibited in CIA mice lacking HIF-1. 40 However, overexpression of HIF-1 could promote the expansion of FLS-mediated inflammatory Th1 and Th17 cells in the synovium of RA, resulting in a significant increase in the secretion of interferon-γ and IL-17. 41 HIF-1 could also promote the expression of MMP-1 and MMP-13 mediated by IL-1β, and the secretion of MMP-2 and MMP-9 mediated by IL-17/TNF- α, thus enhancing the migration and invasion ability of FLS. 42 HIF-1 could also induce osteoclasts to rapidly produce adenosine triphosphate under hypoxia, leading to pathological bone injury. 43
In the FLS of synovial layer and synovial lining of RA patients, the phosphorylation level of FOXO3 was significantly upregulated, suggesting its inhibition effect in RA. 44 In RA, the upregulation of FOXO3 and promotion of inflammatory response was found after inhibiting the expression of transforming growth factor-β 1. 45 Cytoplasmic STAT3 was associated with RA synoviocyte survival by interacting with autophagy-related signal molecules such as FOXO1 and FOXO3. Studies have suggested that expression of the STAT3 gene has been found to correlate with synovitis and modulation of Th17 differentiation in RA patients, 46 while STAT3 inhibition has been reported to mediate chemokine expression in RA synoviocytes. 47 Meanwhile, CDK is a member of the Ser/Thr kinase system corresponding to cell cycle progression and CDK2 can regulate the expression of FOXO. Fu et al’s 48 study has shown that miR-124a suppresses cell proliferation and chemokine secretion in FLS via its direct target genes CDK2 and MCP-1. The inhibition of CDK2 could produce a significantly protective effect during infection and acute systemic inflammation. 49 ATF-2 is the downstream target of JNK and p38 in MAPK signal pathway and a member of activating protein 1 transcription factor family. 50 Additionally, another study has provided evidence that reactive oxygen species and inflammatory cytokines such as TNF-α could promote the phosphorylation of ATF-2, which in turn leads to its activation. 51 Similar results have been validated in a clinical study, which found that peripheral blood mononuclear leucocytes (PBMCs) and ATF-2 expressed by FLS in RA patients have potential proinflammatory activity. 52 In addition, in synovial tissue of RA patients the protein expression and phosphorylation of ATF-2 were significantly upregulated. Furthermore, in RA-FLSs the high expression and phosphorylation of ATF-2 significantly promoted the migration and invasion of RA-FLSs. Knocking down ATF-2 could significantly inhibit the migration and invasion of RA-FLSs, reduce the secretion of IL-1β and MMP-13, and inhibit the expression of IL-6 induced by TNF- α. 50
ENO1 is an important glycolytic enzyme that plays a key role in aerobic glycolysis. Bae et al 53 reported that PBMCs from RA patients expressed more ENO1 on their surface compared with PBMCs from healthy controls (HCs) or patients with OA, and those cells elicited an enhanced inflammatory response after stimulation with anti-ENO1 antibody. Moreover, apoB aggravated arthritis by potentiating the inflammatory response via its interaction with ENO1 expressed on the surface of immune cells. 54
In order to further confirm the regulatory mechanism of berberine on RA-FLSs, RT-qPCR was used to confirm the transcriptional level of core differential transcripts regulated by berberine, and western blot method was used to verify the expression level and phosphorylation level of proteins related to key pathways. The results showed that berberine could significantly reduce the transcriptional levels of MAPK1, STAT3, CDK2, IKBKB, FOXO3, HRAS, AKT2, AKT3, FOXO4, PLK4, ENO1, and HIF-1 α in RA-FLS cells in a concentration-dependent manner. Similarly, western blot results showed that protein expression of HRAS, MAPK1, FOXO3, and HIF-1 α, and the phosphorylation level of MAPK1 and FOXO3 in RA-FLS cells, were significantly downregulated by berberine.
In conclusion, berberine may inhibit the proliferation, adhesion, and inflammation of RA-FLSs by regulating RAS/MAPK/FOXO/HIF-1 signal pathway to exert the therapeutic role in RA synovitis.
Author contributions
Z. Li: Investigation, Formal analysis, Writing – original draft.
M. Chen: Investigation, Formal analysis.
Z. Wang: Investigation.
Q. Fan: Investigation.
Z. Lin: Investigation.
X. Tao: Investigation.
J. Wu: Writing – review & editing.
Z. Liu: Methodology.
R. Lin: Funding acquisition, Methodology, Writing – original draft, Writing – review & editing.
C. Zhao: Methodology.
Funding statement
The authors disclose receipt of the following financial or material support for the research, authorship, and/or publication of this article: this work was supported by National Science and Technology Major Project of the Ministry of Science and Technology of China (No. 2018ZX09735005), Natural Science Foundition of China (No.82204753), Special Scientific Research for Traditional Chinese Medicine of State Administration of Traditional Chinese Medicine of China (No. 201507004), Scientific Research Staring Foundation for the new teachers of Beijing University of Chinese Medicine (2020-JYB-XJSJJZ), and The Key Research Project of Beijing University of Chinese Medicine (2020-JYB-ZDGG-035). The funders had no role in the study design, data collection, data analysis, interpretation, or writing of the report.
ICMJE COI statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data sharing
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
We are grateful to the Beijing Key Laboratory of Traditional Chinese Medicine Quality Evaluation for providing experimental instruments and equipment. We thank the Beijing University of Chinese Medicine for providing the laboratory platform.
Open access funding
The open access fee for this study was provided by the funding mentioned above.
Supplementary material
Table showing primer details of reverse transcription quantitative polymerase chain reaction.
Contributor Information
Zhiqi Li, Email: lizhiqi1998@126.com.
Meilin Chen, Email: 1658968275@qq.com.
Zhaoyi Wang, Email: zywang6834@126.com.
Qiqi Fan, Email: ki_ki1998@163.com.
Zili Lin, Email: 543646724@qq.com.
Xiaoyu Tao, Email: 13365544971@163.com.
Jiarui Wu, Email: exogamy@163.com.
Zhenquan Liu, Email: lzqbzy@sina.com.
Ruichao Lin, Email: 971710061@qq.com.
Chongjun Zhao, Email: 1014256537@qq.com.
References
- 1. Nala . Advances in research of chemistry and pharmacology of ranunculus japonicus. Asia-Pacific Traditional Medicine. 2015. [Google Scholar]
- 2. Guo X, Luo S, Zhao G. Research progress on chemical constituents and pharmacological action of ranunculus. Journal of Chengde Medical College. 2014;31(5):4. [Google Scholar]
- 3. Zhao Y, Huang J, Li T, Zhang S, Wen C, Wang L. Berberine ameliorates aGVHD by gut microbiota remodelling, TLR4 signalling suppression and colonic barrier repairment for NLRP3 inflammasome inhibition. J Cellular Molecular Medi. 2022;26(4):1060–1070. 10.1111/jcmm.17158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tong J, Hou X, Cui D, et al. . A berberine hydrochloride-carboxymethyl chitosan hydrogel protects against staphylococcus aureus infection in A rat mastitis model. Carbohydr Polym. 2022;278:118910. 10.1016/j.carbpol.2021.118910 [DOI] [PubMed] [Google Scholar]
- 5. Sheida A, Taghavi T, Shafabakhsh R, et al. . Potential of natural products in the treatment of myocardial infarction: focus on molecular mechanisms. Crit Rev Food Sci Nutr. 2022;2022:1–18. 10.1080/10408398.2021.2020720 [DOI] [PubMed] [Google Scholar]
- 6. Cao RY, Zhang Y, Feng Z, et al. . The effective role of natural product berberine in modulating oxidative stress and inflammation related atherosclerosis: Novel insights into the gut-heart axis evidenced by genetic sequencing analysis. Front Pharmacol. 2021;12:764994. 10.3389/fphar.2021.764994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guo J, Chen H, Zhang X, et al. . The effect of berberine on metabolic profiles in type 2 diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Oxid Med Cell Longev. 2021;2021:2074610. 10.1155/2021/2074610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fan D, Liu L, Wu Z, Cao M. Combating neurodegenerative diseases with the plant alkaloid berberine: molecular mechanisms and therapeutic potential. Curr Neuropharmacol. 2019;17(6):563–579. 10.2174/1570159X16666180419141613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu M, Ren L, Fan J, et al. . Berberine inhibits gastric cancer development and progression by regulating the JAK2/STAT3 pathway and downregulating IL-6. Life Sci. 2022;290:120266. 10.1016/j.lfs.2021.120266 [DOI] [PubMed] [Google Scholar]
- 10. Holm-Glad T, Røkkum M, Röhrl SM, Roness S, Godang K, Reigstad O. A randomized controlled trial comparing two modern total wrist arthroplasties: improved function with stable implants, but high complication rates in non-rheumatoid wrists at two years. Bone Joint J. 2022;104-B(10):1132–1141. 10.1302/0301-620X.104B10.BJJ-2022-0201.R2 [DOI] [PubMed] [Google Scholar]
- 11. Luo P, Wang P, Xu J, et al. . Immunomodulatory role of T helper cells in rheumatoid arthritis: a comprehensive research review. Bone Joint Res. 2022;11(7):426–438. 10.1302/2046-3758.117.BJR-2021-0594.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ji M, Ryu HJ, Hong JH. Signalling and putative therapeutic molecules on the regulation of synoviocyte signalling in rheumatoid arthritis. Bone Joint Res. 2021;10(4):285–297. 10.1302/2046-3758.104.BJR-2020-0331.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grzelecki D, Walczak P, Szostek M, Grajek A, Rak S, Kowalczewski J. Blood and synovial fluid calprotectin as biomarkers to diagnose chronic hip and knee periprosthetic joint infections. Bone Joint J. 2021;103-B(1):46–55. 10.1302/0301-620X.103B1.BJJ-2020-0953.R1 [DOI] [PubMed] [Google Scholar]
- 14. Leo DG, Green G, Eastwood DM, Bridgens A, Gelfer Y. Development of a core outcome set for the orthopaedic management of spinal dysraphism: a study protocol. Bone Jt Open. 2022;3(1):54–60. 10.1302/2633-1462.31.BJO-2021-0157.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zou Y, Zhang X, Liang J, et al. . Mucin 1 aggravates synovitis and joint damage of rheumatoid arthritis by regulating inflammation and aggression of fibroblast-like synoviocytes. Bone Joint Res. 2022;11(9):639–651. 10.1302/2046-3758.119.BJR-2021-0398.R2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Z-Y, Chu F-H, Gu N-N, et al. . Integrated strategy of LC-MS and network pharmacology for predicting active constituents and pharmacological mechanisms of Ranunculus japonicus Thunb. for treating rheumatoid arthritis. J Ethnopharmacol. 2021;271:113818. 10.1016/j.jep.2021.113818 [DOI] [PubMed] [Google Scholar]
- 17. Ghorbani N, Sahebari M, Mahmoudi M, Rastin M, Zamani S, Zamani M. Berberine inhibits the gene expression and production of proinflammatory cytokines by mononuclear cells in rheumatoid arthritis and healthy individuals. Curr Rheumatol Rev. 2021;17(1):113–121. 10.2174/1573397116666200907111303 [DOI] [PubMed] [Google Scholar]
- 18. Johanna W, Grietje M, KCG M. Hypoxia-inducible factor-1 as regulator of angiogenesis in rheumatoid arthritis - therapeutic implications. Current medicinal chemistry. 2010;17(3). [DOI] [PubMed] [Google Scholar]
- 19. No authors listed . SeqPrep. GitHub Inc. https://github.com/jstjohn/SeqPrep (date last accessed 21 December 2022).
- 20. No authors listed . Sickle. GitHub Inc. https://github.com/najoshi/sickle (date last accessed 21 December 2022).
- 21. No authors listed . Mouse (GRCm39). Ensembl Project. http://www.ensembl.org/Mus_musculus/Info/Index (date last accessed 21 December 2022).
- 22. No authors listed . Top Hat: a spliced read mapper for RNA-Seq. Center for Computational Biology. http://ccb.jhu.edu/software/tophat/index.shtml (date last accessed 22 December 2022).
- 23. Xiaochen L, Li Z, Xiaoqing S, Taiyang L, Nongshan Z, Yifan G, et al. . MicroRNA-10a-3p improves cartilage degeneration by regulating CH25H-CYP7B1-RORα mediated cholesterol metabolism in knee osteoarthritis rats. Front Pharmacol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. STRING Consortium . Protein-protein interaction networks functional enrichment analysis. https://string-db.org/ (date last accessed 21 December 2022).
- 25. No authors listed . 3D Chem.com - Chemistry, Structure & 3D Molecules. https://www.3dchem.com/index.html (date last accessed 22 December 2022).
- 26. Du H, Wang Y, Zeng Y, et al. . Tanshinone IIA suppresses proliferation and inflammatory cytokine production of synovial fibroblasts from rheumatoid arthritis patients induced by TNF-α and attenuates the inflammatory response in AIA mice. Front Pharmacol. 2020;11:568. 10.3389/fphar.2020.00568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao Z-W, Zhang M, Zou J, et al. . TIGAR mitigates atherosclerosis by promoting cholesterol efflux from macrophages. Atherosclerosis. 2021;327:76–86. 10.1016/j.atherosclerosis.2021.04.002 [DOI] [PubMed] [Google Scholar]
- 28. Luo P, Cheng S, Zhang F, et al. . A large-scale genetic correlation scan between rheumatoid arthritis and human plasma protein. Bone Joint Res. 2022;11(2):134–142. 10.1302/2046-3758.112.BJR-2021-0270.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xianyao L, Yao Z, Jingtao D. Correlation between serum MMP-3 level and bone erosion in rheumatoid arthritis. Journal of Chinese Physician. 2021;23:227–230. [Google Scholar]
- 30. Shuang F. Study on the expression of MMP-1 and MMP-3 in early rheumatoid arthritis and their correlation with clinic [Master’s thesis]. Hebei Medical University, 2006. [Google Scholar]
- 31. Yokota K, Sato K, Miyazaki T, et al. . Characterization and function of tumor necrosis factor and interleukin-6-induced osteoclasts in rheumatoid arthritis. Arthritis Rheumatol. 2021;73(7):1145–1154. 10.1002/art.41666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jones DH, Kong YY, Penninger JM. Role of RANKL and RANK in bone loss and arthritis. Ann Rheum Dis. 2002;61 Suppl 2(Suppl 2):ii32–9. 10.1136/ard.61.suppl_2.ii32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Launay D, Vreijling J, Hartkamp LM, et al. . Silencing the expression of Ras family GTPase homologues decreases inflammation and joint destruction in experimental arthritis. Am J Pathol. 2010;177(6):3010–3024. 10.2353/ajpath.2010.091053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abreu JR, de Launay D, Sanders ME, et al. . The Ras guanine nucleotide exchange factor RasGRF1 promotes matrix metalloproteinase-3 production in rheumatoid arthritis synovial tissue. Arthritis Res Ther. 2009;11(4):R121. 10.1186/ar2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu F, Feng XX, Zhu SL, et al. . Sonic hedgehog signaling pathway mediates proliferation and migration of fibroblast-like synoviocytes in rheumatoid arthritis via MAPK/ERK signaling pathway. Front Immunol. 2018;9:2847. 10.3389/fimmu.2018.02847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schett G, Tohidast-Akrad M, Smolen JS, et al. . Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal-regulated kinase, c-JUN N-terminal kinase, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis. Arthritis Rheum. 2000;43(11):2501–2512. [DOI] [PubMed] [Google Scholar]
- 37. Wang Z, Li J, Zhang J, Xie X. Sodium tanshinone IIA sulfonate inhibits proliferation, migration, invasion and inflammation in rheumatoid arthritis fibroblast-like synoviocytes. Int Immunopharmacol. 2019;73:370–378. 10.1016/j.intimp.2019.05.023 [DOI] [PubMed] [Google Scholar]
- 38. Haller V, Nahidino P, Forster M, Laufer SA. An updated patent review of p38 MAP kinase inhibitors (2014-2019). Expert Opin Ther Pat. 2020;30(6):453–466. 10.1080/13543776.2020.1749263 [DOI] [PubMed] [Google Scholar]
- 39. del Rey MJ, Izquierdo E, Caja S, et al. . Human inflammatory synovial fibroblasts induce enhanced myeloid cell recruitment and angiogenesis through a hypoxia-inducible transcription factor 1alpha/vascular endothelial growth factor-mediated pathway in immunodeficient mice. Arthritis Rheum. 2009;60(10):2926–2934. 10.1002/art.24844 [DOI] [PubMed] [Google Scholar]
- 40. Cramer T, Yamanishi Y, Clausen BE, et al. . HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112(5):645–657. 10.1016/s0092-8674(03)00154-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hu F, Mu R, Zhu J, et al. . Hypoxia and hypoxia-inducible factor-1α provoke toll-like receptor signalling-induced inflammation in rheumatoid arthritis. Ann Rheum Dis. 2014;73(5):928–936. 10.1136/annrheumdis-2012-202444 [DOI] [PubMed] [Google Scholar]
- 42. Li G, Zhang Y, Qian Y, et al. . Interleukin-17A promotes rheumatoid arthritis synoviocytes migration and invasion under hypoxia by increasing MMP2 and MMP9 expression through NF-κB/HIF-1α pathway. Mol Immunol. 2013;53(3):227–236. 10.1016/j.molimm.2012.08.018 [DOI] [PubMed] [Google Scholar]
- 43. Morten KJ, Badder L, Knowles HJ. Differential regulation of HIF-mediated pathways increases mitochondrial metabolism and ATP production in hypoxic osteoclasts. J Pathol. 2013;229(5):755–764. 10.1002/path.4159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brandstetter B, Dalwigk K, Platzer A, et al. . FOXO3 is involved in the tumor necrosis factor-driven inflammatory response in fibroblast-like synoviocytes. Lab Invest. 2019;99(5):648–658. 10.1038/s41374-018-0184-7 [DOI] [PubMed] [Google Scholar]
- 45. Jabbari N, Eftekhari Z, Roodbari NH, Parivar K. Evaluation of encapsulated eugenol by chitosan nanoparticles on the aggressive model of rheumatoid arthritis. Int Immunopharmacol. 2020;85:106554. 10.1016/j.intimp.2020.106554 [DOI] [PubMed] [Google Scholar]
- 46. Krause A, Scaletta N, Ji JD, Ivashkiv LB. Rheumatoid arthritis synoviocyte survival is dependent on Stat3. J Immunol. 2002;169(11):6610–6616. 10.4049/jimmunol.169.11.6610 [DOI] [PubMed] [Google Scholar]
- 47. Rosengren S, Corr M, Firestein GS, Boyle DL. The JAK inhibitor CP-690,550 (tofacitinib) inhibits TNF-induced chemokine expression in fibroblast-like synoviocytes: autocrine role of type I interferon. Ann Rheum Dis. 2012;71(3):440–447. 10.1136/ard.2011.150284 [DOI] [PubMed] [Google Scholar]
- 48. Fu X, Song G, Ni R, et al. . LncRNA-H19 silencing suppresses synoviocytes proliferation and attenuates collagen-induced arthritis progression by modulating miR-124a. Rheumatology (Oxford). 2021;60(1):430–440. 10.1093/rheumatology/keaa395 [DOI] [PubMed] [Google Scholar]
- 49. Hsu AY, Wang D, Liu S, et al. . Phenotypical microRNA screen reveals a noncanonical role of CDK2 in regulating neutrophil migration. Proc Natl Acad Sci U S A. 2019;116(37):18561–18570. 10.1073/pnas.1905221116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang X, Zhang D, Wang Q, et al. . Sprouty2 inhibits migration and invasion of fibroblast-like synoviocytes in rheumatoid arthritis by down-regulating ATF2 expression and phosphorylation. Inflammation. 2021;44(1):91–103. 10.1007/s10753-020-01311-z [DOI] [PubMed] [Google Scholar]
- 51. Watson G, Ronai ZA, Lau E. ATF2, a paradigm of the multifaceted regulation of transcription factors in biology and disease. Pharmacol Res. 2017;119:347–357. 10.1016/j.phrs.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Poolman TM, Gibbs J, Walker AL, et al. . Rheumatoid arthritis reprograms circadian output pathways. Arthritis Res Ther. 2019;21(1):47. 10.1186/s13075-019-1825-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bae S, Kim H, Lee N, et al. . α-Enolase expressed on the surfaces of monocytes and macrophages induces robust synovial inflammation in rheumatoid arthritis. J Immunol. 2012;189(1):365–372. 10.4049/jimmunol.1102073 [DOI] [PubMed] [Google Scholar]
- 54. Lee JY, Kang MJ, Choi JY, et al. . Apolipoprotein B binds to enolase-1 and aggravates inflammation in rheumatoid arthritis. Ann Rheum Dis. 2018;77(10):1480–1489. 10.1136/annrheumdis-2018-213444 [DOI] [PubMed] [Google Scholar]