Abstract
Background and aims:
People living with HIV(HIV+) are surviving longer due to effective antiretroviral therapy. Cardiovascular disease is a leading cause of non-AIDS related clinical events. We determined HIV-related factors associated with coronary artery stenosis progression.
Methods:
We performed serial coronary CT angiography among HIV+ and HIV-uninfected (HIV−) men in the Multicenter AIDS Cohort Study. The median inter-scan interval was 4.5 years. Stenosis was graded as 0,1-29,30-49,50-69 or ≥70%. Progression was defined as an increase ≥2 categories. Suppressed HIV infection was consistent viral loads <50 copies/mL allowing 1 “blip”<500 copies/mL, otherwise considered viremic. Multivariable Poisson regression analysis assessed adjusted associations between HIV serostatus and viremia with coronary stenosis progression.
Results:
The sample included 310 HIV+ (31% viremic) and 234 HIV− men. The median age was 53 years, 30% Black and 23% current smokers. Viremic men were 2.3 times more likely to develop coronary stenosis progression than HIV− men (adjusted RR 2.30;95%CI, 1.32-4.00,p=0.003), with no difference in progression between HIV+ suppressed and HIV− men (RR 1.10;95%CI,0.70-1.74,p=0.67). There was a progressive increase in adjusted relative risk with greater viremia (p=0.03). Men with >1 viral load >500 copies/ml demonstrated greatest stenosis progression (RR 3.01;95%CI, 1.53-4.92, p=0.001 compared with HIV− men). Suppressed HIV+ men with suboptimal antiretroviral adherence had greater stenosis progression (RR 1.91; 95%CI 1.12-3.24,p=0.02) than HIV+ suppressed men with optimal adherence.
Conclusions:
Coronary artery stenosis progression was associated with suboptimal HIV RNA suppression and antiretroviral therapy adherence. Effective ongoing HIV virologic suppression and antiretroviral therapy adherence may mitigate risk for coronary disease events among people living with HIV.
Keywords: atherosclerosis, HIV, coronary artery disease, coronary CT angiography, epidemiology
1. Introduction:
The introduction of combination antiretroviral therapy (cART) in the mid-1990s transformed HIV infection from a fatal condition to a chronic disease(1). Viral suppression with cART dramatically improved HIV prognosis; however, multiple comorbidities continue to limit the health span of people living with HIV (HIV+)(2). Cardiovascular disease (CVD), including coronary artery disease, has emerged as a leading cause of non-AIDS related morbidity and mortality in people living with HIV(3). We previously demonstrated in the Multicenter AIDS Cohort Study (MACS) that HIV+ men had greater prevalence of coronary artery atherosclerosis than HIV-uninfected men (HIV−), and that coronary artery stenosis was associated with lower nadir CD4+ T cell count(4). The mechanisms leading to these findings are incompletely understood.
Untreated HIV infection is associated with heightened immune activation and markedly elevated circulating inflammatory markers (5, 6). The SMART study demonstrated that cART treatment interruption was associated with increased risk for CVD compared with continuous treatment(5). Viral “blips”, defined as transient detectable low-level viremia below 500 copies/mL in HIV RNA viral load (VL), occur commonly (~10%) among treated and suppressed HIV+ individuals(7). “Blips” are ascribed to measurement imprecision or technical issues, suboptimal adherence to cART, or viral release from reservoirs. Conflicting data exist regarding the clinical significance of “blips” and low level viremia (8, 9). In addition, the potential impact of viral “blips”, cART adherence, and magnitude of measured viremia on coronary artery progression is unknown.
The purpose of this study was to evaluate associations between degree of viral control and adherence to cART with progression of coronary artery stenosis(10).
2. Patients and methods:
HIV+ and HIV− men were recruited from the Multicenter AIDS Cohort Study (MACS). The MACS is a well-characterized longitudinal, prospective observational cohort study which includes HIV+ and at-risk (HIV−) gay and bisexual men in Baltimore, MD/Washington, DC, Chicago, IL, Los Angeles, CA, and Pittsburgh, PA (11). Men were recruited into the MACS in 1984-85, with additional enrollment in 1987-1990, 2001-2003 and 2010-2018. Men were seen semi-annually, with standardized interviews, physical examinations, and blood and urine collection.
All eligible MACS participants were invited to participate in the baseline MACS coronary CT angiography (CTA) study from 2010-2013. Enrollment criteria included age 40-70 years, weight < 300 pounds, and no history of cardiac surgery, coronary artery angioplasty or stent placement, since these procedures would interfere with assessment of atherosclerosis by CTA. Eligibility for contrast administration included lack of known contrast allergy and estimated glomerular filtration rate (GFR) ≥ 60 ml/min/1.73m2. Serum creatinine was measured at each visit and within 30 days prior to CT scanning. Although the CKD-epi formula is now the standard method, GFR was estimated using the Modification of Diet in Renal Disease (MDRD) equation to be consistent with the eligibility criteria used at baseline(12). Coronary CTA was completed in 765 men (453 HIV+ and 312 HIV−)(4). All surviving men with coronary CTA at baseline and ongoing eGFR > 60 ml/min/m2 were invited to undergo repeat CTA 3-6 years later (2015-2017). The median interscan interval was 4.5 years (IQR 3.9-4.9).
The study was approved by the Institutional Review Boards of all participating sites. All participants signed informed consent.
2.1. Coronary CT angiography and determination of stenosis progression
Coronary CT angiography scanning and analysis procedures have been described(13). CTA images were analyzed by trained, experienced readers masked to participant characteristics including HIV serostatus. Coronary artery segments were delineated using the American Heart Association coronary tree model (15-segment model at baseline; 18-segment model at follow-up)(14, 15). Using axial images, multi-planar reconstructions, and maximum intensity projections, readers assessed the degree of luminal narrowing (stenosis) in each assessable coronary segment. The most narrowed diameter in each segment was reported even when plaque was eccentric. Segment stenosis categories were defined as: none, 1-29% (minimal), 30-49% (mild), 50-69% (moderate), or ≥70% (severe). (In a prior study, inter-rater reliability for total stenosis score: correlation coefficients ranged from 0.91-0.93, among any 2 of 3 readers) (16). A clinical CTA report was sent to participants and their medical providers with participant permission; additional follow-up was determined by the participant and his medical providers.
2.2. Coronary artery stenosis progression outcome definitions
We defined subclinical coronary artery stenosis progression in three ways (Table 1). The primary outcome, segment-level stenosis progression, measured the progression of coronary artery stenosis within coronary artery segments. This dichotomous outcome was defined as an increase in a segment’s stenosis grading by at least 2 categories, i.e., none to ≥ mild (30-49%), minimal (1-29%) to ≥ moderate (50-69%) or mild (30-49%) to severe (≥70%). Requiring a two-category increase avoids inclusion of small changes. Twelve coronary segments were defined identically in the baseline and follow-up CT readings; the remaining three segments were harmonized to their corresponding segments in the coronary tree at follow-up, with change in stenosis calculated for up to 14 coronary segments per person. All coronary artery segments visualized at both time points for which baseline stenosis was <50% were included in this analysis. We also evaluated two secondary outcomes at the individual-level: 1) incident (new) stenosis ≥50% captured progression to moderate or severe stenosis (stenosis burden ≥50% in any arterial segment) among individuals without significant stenosis at baseline; 2) vessel involvement progression measured an increase in the number of major coronary arteries affected (i.e. single, double, or triple vessel disease). For this analysis, each segment was assigned as left main, left anterior descending, circumflex or right coronary artery systems. The dichotomous outcome was defined as an increase in the number of major coronary arteries with stenosis ≥50% among men with ≤2 vessel (major coronary artery) stenosis at baseline.
Table 1. Outcome definitions:
The outcome definitions for Segment-level stenosis progression, incident stenosis and vessel involvement progression are listed.
|
Outcome Definition 1 (Primary outcome): Segment-Level Stenosis Progression Increase in stenosis by ≥ 2 stenosis categories Dichotomous: Yes/No Unit of analysis: coronary artery segment Among coronary artery segments with stenosis < 50% at baseline *Each participant may contribute up to 14 artery segments to the segment-level analysis |
| 5 Stenosis Categories: 0- no plaque present < 30%- minimal 30-49%- mild 50-69%- moderate ≥ 70%- severe |
|
Outcome Definition 2 (Secondary outcome): Incident Stenosis Incident (new) stenosis ≥ 50% in an individual participant Dichotomous: Yes/No Unit of analysis: individual Among individuals with stenosis <50% in all segments at baseline |
|
Outcome Definition 3 (Secondary outcome): Vessel Involvement Progression Increase in the number of major coronary arteries with stenosis ≥ 50% Dichotomous: Yes/No Unit of analysis: individual Among individuals with ≤ double vessel disease at baseline. |
| Vessel Involvement: None Single Vessel Disease Double Vessel Disease Triple Vessel Disease Four Vessel Disease |
2.3. HIV exposure definitions
Plasma HIV RNA viral load (VL) was measured semi-annually between the two CT scans (interscan interval) using assays with a lower limit of detection of 50 copies/ml or 20 copies/mL; suppressed VL was defined as <50 copies/ml. Durable HIV viral suppression was defined as VL<50 copies/ml at each interscan visit, allowing for one “blip” (50-500 copies/ml) during this period; men having any VL>500 copies/ml, or more than one “blip” during the interscan interval were considered “viremic”. The primary exposure variable of HIV serostatus and viral suppression had three categories: HIV− (reference), HIV+ suppressed, and HIV+ viremic.
2.4. CVD risk factors and HIV parameters
Standardized data collection at the semi-annual MACS visit was used to measure CVD risk factors and HIV parameters; participants completed a median of 9 visits (IQR 8-10) during the interscan interval. Race/ethnicity was self-reported. Participants self-reported antiretroviral medication use and the use of medications to treat hypertension, diabetes, or lipid abnormalities through standardized interview procedures that included the use of participants’ pharmacy lists and pill bottles to enhance recall. Glucose, total and high-density lipoprotein cholesterol (HDL), and triglycerides concentrations were measured. Low density lipoprotein cholesterol (LDL) concentration was calculated using the Friedewald equation or measured directly when triglycerides were >400 mg/dL or from nonfasting samples. Baseline atherosclerotic CVD (ASCVD) Risk Score (Pooled Cohort Equation) was calculated(17). Participants were screened prospectively for serum hepatitis C virus (HCV) antibody and, if positive, plasma HCV RNA was measured using a quantitative real-time polymerase chain reaction assay. Hepatitis C status was assessed at baseline CTA as there were no incident HCV cases between CTAs. Quality-controlled flow cytometry measured CD4+ T-cell counts, and medical record review confirmed history of AIDS-defining malignancies or opportunistic disease. Self-reported history of injection drug use, alcohol use averaging>14 drinks/week and any crack or cocaine use since the visit prior to the CTA were obtained. Cumulative duration of combination antiretroviral therapy use (cART) was updated at each visit. Adherence to cART was assessed at every visit by comparing self-reported number of pills taken to that prescribed over the prior 4 days for each medication and whether use in this 4-day period was typical for use since last study visit. Adherence was categorized as 100% (yes) if the participant reported complete adherence during the prior 4 days and this was typical of their adherence since the prior study visit; otherwise, adherence was categorized as <100% (no)(18). Complete adherence (yes) over the interscan period was defined as complete adherence at each visit; otherwise, adherence was <100% (no).
2.5. Statistics
The comparison of outcomes and participant characteristics by the three exposure groups, HIV−, HIV+ suppressed, and HIV+ viremic, were described using Kruskal Wallis and Chi-squared tests. Association between HIV exposure groups and segment-level stenosis progression was assessed using Poisson regression models, with a log link and an offset for the duration between scans (time at risk for stenosis to progress). This approach is preferable to logistic regression, which does not account for differences in the duration of at-risk time. We accounted for the statistical dependence between the repeated measures of stenosis progression (within multiple coronary artery segments) in the same individual using generalized estimating equation(19). We assumed an exchangeable correlation matrix and implemented the model with the GENMOD command with the REPEATED statement in SAS. Multivariable analyses adjusted for age, race (White, Black, Hispanic/other), education, CT scanning site, cohort recruitment wave (pre- or post-2001), systolic BP, use of hypertension medications, total and HDL cholesterol, use of cholesterol medications, fasting glucose, use of diabetes medications, cigarette smoking (pack-years) during the interscan period, and body mass index. These covariates were identified a priori for inclusion in the multivariable model irrespective of statistical significance. Sequentially adjusted models evaluated four additional factors: chronic hepatitis C infection, history of injection drug use, heavy alcohol use (>14 drinks/week on average at baseline CTA scan), and any use of cocaine since the visit prior to baseline CTA scan, as potential confounders of the association between HIV category and stenosis progression; statistically significant predictors were included in the multivariable model. Unless otherwise noted, covariate measures utilized data from all interscan study visits; averaging the values of continuous variables and defining medication use by its use in ≥20% of interscan visits. We conducted a sensitivity analysis that eliminated covariates with p-values >0.20.
The secondary individual-level outcomes of incident stenosis ≥ 50% and vessel involvement progression were evaluated using multivariable Poisson regression with an offset for duration between scans, adjusting for the same covariates as described above.
Additionally, we evaluated associations between segment-level stenosis progression and history of clinical AIDS, nadir CD4+ cell count, cumulative years and type of cART received, proportion of visits with suppressed VL, average CD4+ cell count between CTAs, and cART adherence, each in separate multivariable analyses among HIV+ men, adjusting for covariates specified above.
We also assessed the association between more specific viremic categories of 1)durable suppression with all viral loads <50 copies/ml; 2) one “blip” 50-500 copies/ml; 3)“low level viremia” defined as at least two viral loads 50-500 copies/ml among men with all viral loads <500 copies/ml; and 4) at least 1 viral load ≥500 copies/ml during the interscan period and segment-level stenosis, relative to HIV− men. We performed a global test for trend using the categories as a continuous variable. Analyses were performed with SAS 9.4.
3. Results:
The participant flow diagram is shown in Figure 1. A follow-up CTA was performed on 556 men (317 HIV+ and 239 HIV−), representing 70% and 77% of HIV+ men and HIV− men with baseline CTA data, respectively. The lower rate of follow-up CTA in HIV+ men was largely secondary to higher mortality (5% HIV+ and 2% HIV− men) and a greater incidence of reduced kidney function (low eGFR) between the baseline and follow-up CT visit (9% HIV+ and 3% HIV−), precluding eligibility for contrast. Regardless of HIV serostatus, men who did not complete a follow-up coronary CTA tended to have more traditional CVD risk factors than those who did (Supplementary Table S1). Nine men were excluded from these analyses since they were known to have had a coronary intervention (angioplasty and/or stent) during the follow-up period and 3 were excluded due to missing data.
Figure 1. Participant flow diagram.

The participant flow diagram is displayed. eGFR=estimate glomerular filtration rate; aparticipant excluded due to low eGFR documented between baseline CTA and enrollment screening for the follow-up CTA scan; bparticipant was enrolled for follow-up scan, but did not receive contrast CTA due to low eGFR at the study visit.
The segment-level stenosis progression analysis included 544 men who contributed a median of 13 coronary artery segments to the analysis (IQR 13-14) for a total of 7,196 coronary artery segments. The characteristics of this study population are shown in table 2, with HIV+ men stratified by viremic status. Differences were most pronounced between HIV− men and HIV+ men who were viremic during the interscan interval (31.6% of HIV+ men). HIV+ men and especially viremic men were younger, more likely to be co-infected with hepatitis C virus, Black or Hispanic, less educated, have higher average fasting glucose and triglycerides, and were more likely to be current smokers, crack/cocaine users, and to have recently injected drugs than HIV− men. They also had lower average systolic BP, and total, LDL and HDL cholesterol levels. Use of cholesterollowering medications was greatest among HIV+ suppressed men, and increased during the follow-up period in all three groups. The ASCVD risk score using the Pooled Cohort Equation was highest among men without HIV (7.6%; p=0.04), but did not differ significantly between HIV+ viremic and HIV+ suppressed men (6.5 % vs 5.9%,p=0.94). During the interscan interval, 21.2% of HIV+ suppressed men had one “blip” in VL, and the remaining HIV+ suppressed men had no “blips”. Among men categorized as viremic, 73.5% had ≥1 VL measurement ≥500 copies/mL while the remaining 26.5% had ≥2 VL of 50-499 copies/mL but none ≥500 copies/mL. Averaging VL measures across interscan visits for each person, the median average interscan VL among HIV+ viremic men was 857 (IQR 73, 11,121) copies/mL. HIV+ viremic men had lower CD4+ T cell counts and reported cART use during fewer visits during the interscan interval than HIV+ suppressed men. The latter were also less adherent when on cART, had a shorter durations of cART use, and were more likely to be receiving protease inhibitor-based cART. The use of integrase inhibitors at baseline was rare (n=13).
Table 2:
Characteristics of study participants, by HIV infection and viral suppression status
| HIV− (n=234) | HIV+ Suppressed (n=212) | HIV+ Viremic (n=98) | p-value | |
|---|---|---|---|---|
| Age, years | 55.3 (50.5, 62.8) | 52.2 (48.3, 57.3) | 49.2 (46.2, 54.2) | <0.001 |
| Race, white | 68.4% | 58.5% | 27.6% | <0.001 |
| Black | 23.9% | 25.9% | 52.0% | |
| Other | 7.7% | 15.6% | 20.4% | |
| Education, college or greater | 64.1% | 47.6% | 31.6% | <0.001 |
| Enrollment after 2001 | 34.6% | 49.1% | 68.4% | <0.001 |
| InterScan interval, years | 4.2 (3.6, 4.9) | 4.6 (4.0, 5.0) | 4.5 (3.9, 5.0) | 0.007 |
| Smoking, never | 23.2% | 30.3% | 24.5% | <0.001 |
| Former | 59.7% | 47.4% | 38.8% | |
| Current | 17.2% | 22.3% | 36.7% | |
| Cumulative pack-years up to baseline | 2.5 (0, 21.9) | 2.5 (0, 20.4) | 5.4 (0, 19.1) | 0.89 |
| Cumulative pack-years, interscan interval | 0(0,0) | 0(0,0.1) | 0(0,1.9) | <0.001 |
| Average body mass index, kg/m2 | 26.5 (23.9, 29.5) | 25.5 (23.2, 28.5) | 25.7 (23.3, 28.3) | 0.14 |
| Baseline hepatitis C viral infection | 2.6% | 6.7% | 14.3% | <0.001 |
| Ever Injection Drug use | 5.1% | 13.7% | 18.4% | <0.001 |
| Baseline recent injection drug use | 0% | 1.9% | 3.1% | <0.05 |
| Ever crack/cocaine use | 44.9% | 54.7% | 64.3% | 0.003 |
| Baseline recent crack/cocaine use (past 6 months) | 8.6% | 8.0% | 18.4% | 0.01 |
| Baseline recent alcohol use ≥ 14 drinks/week | 9.4% | 5.7% | 4.1% | 0.14 |
| Average systolic blood pressure, mmHg | 129 (122, 136) | 127 (119, 135) | 126 (115, 133) | 0.009 |
| Average diastolic blood pressure, mmHg | 78 (73, 82) | 79 (74, 83) | 78 (73, 83) | 0.25 |
| Hypertension medication usea | 41.5% | 40.6% | 34.7% | 0.501 |
| Average fasting glucose, mg/dL | 94 (88, 101) | 96 (91, 103) | 95 (90, 102) | 0.015 |
| Diabetes medication usea | 8.1% | 9.0% | 9.2% | 0.93 |
| Average total cholesterol, mg/dL | 184 (166, 207) | 185 (166, 205) | 169 (157, 189) | <0.001 |
| Average HDL cholesterol, mg/dL | 55 (45, 61) | 47 (39, 55) | 44 (39, 53) | <0.001 |
| Average LDL cholesterol, mg/dL | 108 (91,128) | 106 (86, 123) | 98 (80, 114) | <0.001 |
| Average triglycerides, mg/dL, median (IQR) | 101 (78, 127) | 134 (96,185) | 133 (92, 175) | <0.001 |
| Cholesterol medication usea | 43.6% | 54.2% | 35.7% | 0.005 |
| Baseline cholesterol medication use | 32.5% | 37.1% | 23.7% | 0.069 |
| Average HIV viral load, copies/mL, median (IQR) | 13 (10, 18) | 857 (73, 11,121) | <0.001 | |
| Baseline ASCVD risk score (pooled cohort equation) | 7.6 (4.1, 13.0) | 5.9 (3.8, 9.9) | 6.5 (3.7, 9.7) | 0.04 |
| Average percentage of interscan visits with HIV viral load>50, median (IQR) | 0.0% (0.0%, 0.0%) | 33.3% (20.0%, 60.0%) | <0.001 | |
| All HIV viral loads<50 copies/mL | 78.8% | 0% | <0.001 | |
| One HIV viral blip (50-500 copies/mL) | 21.2% | 0% | ||
| ≥ 2 blips (50-500 copies/mL) but no visits ≥ 500 copies/mL | 0% | 26.5% | ||
| ≥ 1 high HIV viral load (≥ 500 copies/mL) | 0% | 73.5% | ||
| Average CD4+ T cell count, cells/μL | 677 (529, 851) | 605 (383, 716) | <0.001 | |
| Nadir CD4+ T cell count, cells/μL | 291 (189, 418) | 330 (237, 450) | 0.09 | |
| Cumulative cART use up to baseline, years | 9.9 (7.2, 12.9) | 8.3 (3.4, 11.9) | <0.001 | |
| 100% of visits on cART | 79.6% | 52.0% | <0.001 | |
| Baseline cART | 95.3% | 78.6% | <0.001 | |
| Baseline cART regimen | <0.001 | |||
| None | 4.7% | 21.4% | ||
| Protease inhibitor-based cART | 38.7% | 54.1% | ||
| Non-nucleoside reverse transcriptase inhibitor based (NNRTI) cART |
50.5% | 19.4% | ||
| Nucleoside reverse transcriptase inhibitor-based (NRTI) cART |
<1% | <1% | ||
| Other | 4.7% | 4.1% | ||
| Self-reported 100% adherence to cART during interscan period |
50.2% | 17.3% | <0.001 | |
| History of clinical AIDS | 8.5% | 10.2% | 0.63 |
Medians and interquartile range or percentages shown. Average= mean across interscan visits, p-values from Kruskal Wallis or χ2 tests.
Cholesterol, hypertension and diabetes medication use was defined as user (≥20% of visits) or primarily non-user (<20% of visits) during the interscan interval.
HIV+ suppressed = HIV viral load consistently <50 copies/mL, one blip 50-500 copies/mL allowed; HIV+ viremic = detectable viremia >500 copies/mL or >1 blip; HIV−= HIV-uninfected.
AIDS=acquired immune deficiency syndrome, cART=combination antiretroviral therapy, CI=confidence interval, HDL=high-density lipoprotein, HIV=human immunodeficiency virus, LDL=low-density lipoprotein
Coronary artery stenosis ≥50% was seen in 13-15% of men at baseline, but very few of these men had more than single vessel coronary artery disease (Table 3). Table 4 shows the relative risk (RR) from the segment-level stenosis progression analysis, accounting for interscan interval length and adjusting for demographics and CVD risk factors. HIV+ viremic men were 2.3 times more likely to have segment-level stenosis progression than HIV− men (adjusted RR [aRR] 2.30; 95%CI, 1.32-4.00, p=0.003). In contrast, there was no significant difference in relative risk of segment-level stenosis progression between HIV+ suppressed and HIV− men (aRR 1.10, 95%CI 0.70-1.74, p=0.67). Relative to HIV+ suppressed men, HIV+ viremic men had 2.18 times the risk of stenosis progression (aRR, 2.18, 95%CI 1.26-3.79, p=0.006, data not shown). Traditional CVD risk factors were also independently associated with stenosis progression, including age, race, cigarette smoking (pack-years) during the interscan interval, total and HDL cholesterol levels. Use of cholesterol medications was associated with an increased risk of segment-level stenosis progression compared to non-use. Cocaine use was independently associated with progression. History of injection drug use, heavy alcohol use, and chronic hepatitis C virus infection were not associated with progression and were not included as covariates in the main model (p-values>0.20). In a sensitivity analysis with a parsimonious model excluding non-significant covariates, the magnitude and statistical significance of HIV viremia and the other significant predictors of stenosis progression remained consistent (Supplementary Tables S2–S4).
Table 3:
Characterization of baseline coronary artery stenosis and progression outcomes, by HIV serologic and viremic status
| HIV− | HIV+ Suppressed | HIV+ Viremic | |
|---|---|---|---|
| Individuals contributing to the segment-level progression analysis, N | 234 | 212 | 98 |
| Coronary artery stenosis ≥50% at baseline | 31 (13.2%) | 32 (15.1%) | 13 (13.3%) |
| Vessel involvement among those with coronary artery stenosis ≥50% at baseline | |||
| Single Vessel Disease | 25 (80.6%) | 26 (81.3%) | 11 (84.6%) |
| Double Vessel Disease | 2 (6.5%) | 5 (15.6%) | 1 (7.7%) |
| Triple Vessel Disease | 4 (12.9%) | 1 (3.1%) | 1 (7.7%) |
| AHA coronary artery segments included in the analysis, per person, median (IQR) | 13.5 (13.0, 14.0) | 13.0 (13.0, 14.0) | 13.0 (13.0, 14.0) |
| Total AHA coronary artery segments included in the progression analysis, N | 3,104 | 2,793 | 1,299 |
| Extent of coronary artery stenosis at baseline | |||
| None | 2563 (82.6%) | 2274 (81.4%) | 1082 (83.3%) |
| Minimal (<30%) | 449 (14.5%) | 382 (13.7%) | 174 (13.4%) |
| Mild (30-49%) | 92 (3.0%) | 137 (4.9%) | 43 (3.3%) |
| Primary Outcome: Segment-level progression | 61 (2.0%) | 72 (2.6%) | 47 (3.6%) |
| SECONDARY OUTCOMES | |||
| Vessel involvement, N | 230 | 211 | 97 |
| Vessel involvement progression | 16 (7.0%) | 21 (10.0%) | 13 (13.4%) |
| Coronary artery stenosis incidence, N | 203 | 180 | 85 |
| Incident coronary artery stenosis ≥50% | 10 (4.9%) | 15 (8.3%) | 10 (11.8%) |
Numbers reported are counts and (percentages), unless otherwise specified. IQR=interquartile range. AHA coronary artery segments with baseline stenosis ≥50% were excluded from the segment-level analysis. The study population for vessel involvement progression excluded 6 men with triple vessel stenosis or greater at baseline. The study population for incident stenosis ≥50% excluded 76 men with prevalent stenosis ≥50% at baseline.
Table 4.
Adjusted relative risk of segment-level stenosis progression.
| Adjusted RR (95% CI) | p-value | |
|---|---|---|
| HIV infection/viral suppression status- HIV− | 1.0 (ref) | |
| HIV+ viremic | 2.30 (1.32,4.00) | 0.003 |
| HIV+ suppressed | 1.10 (0.70,1.74) | 0.67 |
| Baseline age, per 10 years | 1.41 (1.04,1.91) | 0.03 |
| Race-White | 1.0 (ref) | |
| Black | 0.42 (0.22,0.82) | 0.01 |
| Hispanic/other | 0.55 (0.24,1.26) | 0.16 |
| Interval cigarette smoking (per pack-year) | 1.14 (1.03,1.26) | 0.015 |
| Fasting glucosea (per 10 mg/dl) | 1.02 (0.93,1.11) | 0.69 |
| Systolic blood pressurea (per 10 mmHg) | 1.06 (0.87,1.30) | 0.57 |
| Total cholesterola (per 10 mg/dl) | 1.13 (1.05,1.22) | 0.002 |
| HDL cholesterola (per 10 mg/dl) | 0.75 (0.62,0.92) | 0.006 |
| Lipid lowering medicationsb | 1.97 (1.22,3.18) | 0.006 |
| Baseline cocaine use | 1.84 (1.10,3.08) | 0.02 |
Averaged value among measurements across interscan visits.
Use of lipid-lowering medication at 20% or more interscan visits. N=7196 segments/544 men. Model adjusted for all variables shown above in addition to study site, cohort recruitment wave (pre or post 2001), education level, average body mass index across interscan visits, and use of hypertension medications, and diabetes medication at more than 20% of visits during the interscan period (all p > 0.10 except for study site). The generalized estimating equation model accounted for correlation between segments within individuals, and the interscan interval duration was a model offset. Abbreviations: CI=confidence interval, HDL=high-density lipoprotein, HIV=human immunodeficiency virus, RR=relative risk.
Among HIV+ men, stenosis progression was not significantly associated with cART duration (aRR 0.95, 0.75-1.23, p=0.69), cART type (aRR 1.04, 0.57-1.90, p=0.89 for protease-inhibitor based cART; aRR 1.05, 0.60-1.84, p=0.86 for non-nucleoside reverse transcriptase inhibitor-based cART; both compared to nucleoside reverse transcriptase inhibitor-based/other), history of clinical AIDS (aRR 1.32, 0.63-2.75, p=0.46), nadir CD4 (aRR 0.99, 0.98-1.01, p=0.31), or average interscan CD4+ T cell count(aRR 1.00, 0.99-1.01, p=0.48), independent of HIV viremic status and the other covariates in the multivariable models.
We also evaluated the association between HIV serologic and viremic categories and two secondary outcomes, incidence of coronary artery stenosis ≥50%, and progression of the number of major coronary arteries with stenosis ≥ 50% (i.e. single, double, or triple vessel disease), shown in Figure 2. The observed incidence of coronary stenosis ≥ 50% was 1.2/100 person-years among HIV− men, 1.9/100 person-years among HIV+ suppressed men, and 2.6/100 person-years among HIV+ viremic men. The incidence of new coronary artery stenosis ≥ 50% among men without moderate or severe stenosis at baseline was more than 4 times greater in HIV+ viremic men than HIV− men (aRR 4.63, 1.70- 12.59, p= 0.003). There was no significant difference in incident stenosis among HIV+ suppressed compared with HIV− men (aRR 1.82, 0.74-4.44, p=0.19). HIV+ viremic men had a significantly greater risk of progression of vessel involvement than HIV− men (aRR 2.68, 1.18-6.09, p=0.019). There was no significant difference in progression of vessel involvement in HIV+ suppressed men compared with HIV− men (aRR 1.29, 0.63- 2.65, p=0.49).
Figure 2. Adjusted relative risk of stenosis progression by HIV viremic group.
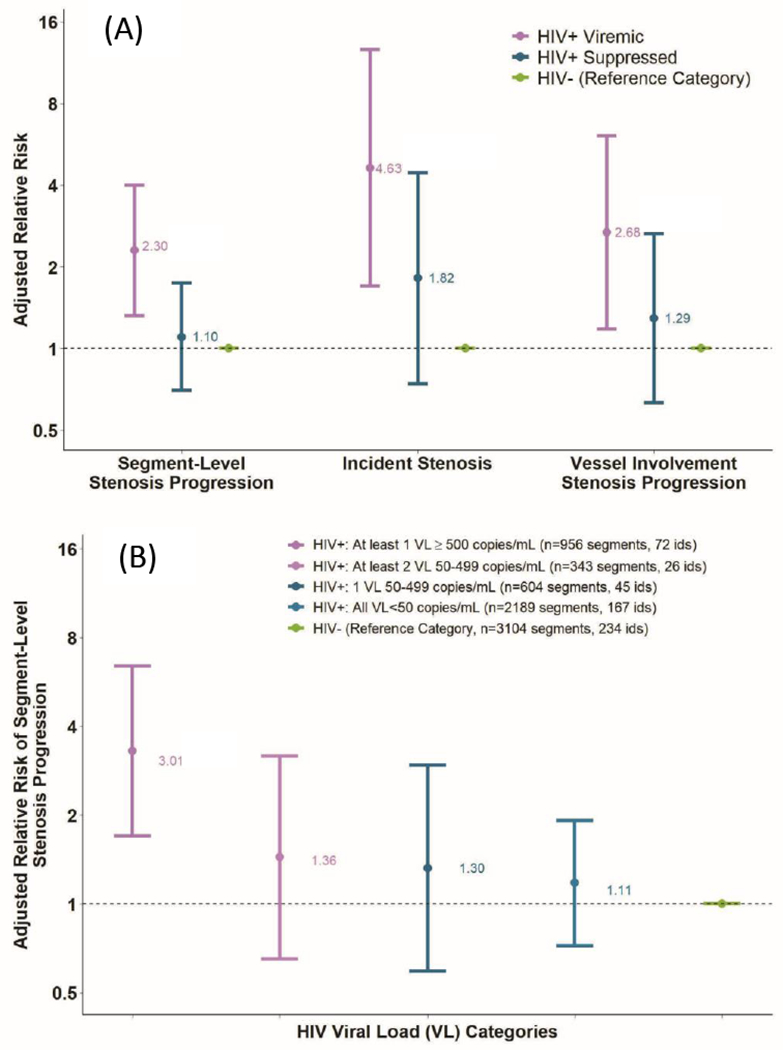
The adjusted relative risks for coronary artery stenosis progression are presented. Relative risks are adjusted for age, race, education, study site, cohort enrollment date, interval scan time, CVD risk factors and cocaine use. Upper panel (A) sample sizes by outcome: 1) segment-level stenosis, HIV+ Viremic=1,299 segments/98 ids, HIV+ suppressed=2,793 segments/212 ids, and HIV−=3,104 segments/234 ids, 2) incident stenosis, HIV+ Viremic=85, HIV+ Suppressed=180, and HIV−=203, and 3) vessel-involvement progression, HIV+ Viremic=97, HIV+ Suppressed=211, and HIV−=230. HIV=human immunodeficiency virus, RR=relative risk, VL=viral load.
In order to assess the risks associated with gradations of viremia during the interscan interval, HIV+ participants were divided into four categories: 1) all viral loads <50 copies/ml; 2) one “blip” 50-499 copies/ml; 3) at least two viral loads 50-499 copies/ml with no measurement ≥500 copies/ml; and 4) at least 1 viral load ≥500 copies/ml [group median (IQR) VL 4457 (542-22,412)]. As shown in figure 2, the adjusted relative risk for segment-level stenosis progression increased as the extent of viremia increased, relative to HIV− men (trend test p=0.02); however, for individual categories of viremia, only the highest level (men with at least 1 viral load ≥500 copies/ml) was statistically significantly different than HIV− men (aRR 3.01, (1.53-5.92); p=0.001). Excluding men with viral load ≥500 copies/ml, stenosis progression among the other HIV+ men (three groups combined) was elevated but not statistically significantly different compared to HIV− men (aRR 1.17, (0.76,1.81); p=0.48). We also conducted a sensitivity analysis among HIV+ men to assess the association between the percentage of visits with detectable HIV viral load and the progression of segment-level coronary stenosis. Each increase of 10 percentage points in visits with detectable viral load during the interscan interval was associated with a 16% higher risk of stenosis progression in the multivariable model.
Among men with viral suppression (allowing for one blip), 50% reported complete (100%) adherence with cART during the interscan period. We further divided the men with viral suppression by adherence status, using incomplete (<100%) cART adherence as a marker for potential exposure to low-level viral replication. Men with viral suppression and incomplete adherence, were less likely to be on statins or other lipid-lowering medications (42% versus 66%) than men with complete adherence. There were also trends towards a greater proportion of non-white men and mildly elevated glucose (Supplementary Table S5). After adjusting for interscan interval, demographics, CVD risk factors, and HIV disease progression markers, men with viral suppression who reported less than 100% adherence had an aRR of 1.91 (95%CI, 1.12-3.24, p=0.02) for segment-level stenosis progression compared with virally suppressed HIV+ men who reported complete adherence to cART. Viremic men had an aRR of 2.75 (95%CI, 1.51-5.01, p=0.001), compared to HIV+ men with viral suppression with complete adherence.
4. Discussion:
People living with HIV are at increased risk for atherosclerotic CVD(3). We found that progression of coronary artery stenosis, assessed by coronary CTA, was associated with the presence and magnitude of HIV viremia, traditional cardiovascular risk factors, cocaine use, and suboptimal cART adherence. There was an ordered increase in the risk for coronary artery stenosis progression with progressively greater levels of viremia during the interscan interval, from consistently suppressed, to low-level viremia “blips” <500 copies/mL, to a viral load >500 copies/mL, though only the highest exposure group was significantly different from the other categories. The greatest risk for coronary artery disease progression was seen with the highest levels of HIV viremia.
Historically, ART was initiated when there was evidence of immune deficiency, specifically with CD4+ T cell counts beneath specific threshold values. The importance of viral suppression to prevent HIV disease progression and co-morbidity occurrence (5), such as CVD, has led to current recommendations to initiate cART promptly after HIV diagnosis regardless of CD4+ T cell counts, and to focus efforts on optimization of cART adherence and retention in care. We found that men with detectable viremia differed from those who were suppressed. They were more likely to be younger, Black, have less education, currently smoke, use cocaine, and report imperfect adherence to cART; they also had lower SBP and lower total and LDL cholesterol. Our finding that the risk of stenosis progression was significantly higher among HIV+ virally suppressed men with suboptimal adherence relative to HIV+ virally suppressed men reporting perfect adherence suggests that in people living with HIV viral suppression below the limits of detection of commercial assays may contribute to progression of coronary atherosclerosis. Suboptimal adherence among virally suppressed individuals is associated with higher systemic concentrations of inflammatory biomarkers than those who report complete adherence(18). Although viral blips and low-level viremia were not associated with progression compared with HIV− men, the small but progressive increase in risk with gradations of inter-current viremia suggests that further research is needed to determine risk associated with low levels of HIV viremia and blips.
The risk for CVD events in people living with HIV is likely multifactorial, including 1) a high prevalence of traditional CVD risk factors, 2) heightened inflammatory response and immune dysregulation, and 3) metabolic side effects of some older ART agents (3). People living with HIV were randomized to ART treatment interruption compared with continuous ART in the SMART study, which demonstrated the association between ART disruption and viremia, the association between inflammation and CVD events(20), and the increased risk for CVD events with viremia and treatment interruption(5, 21). The START study randomized people living with HIV to immediate versus deferred ART based on CD4+ T cell counts. Immediate therapy led to a lower risk of AIDS and non-AIDS serious events and mortality; however, there was no association with CVD events, likely due to limited power(22). The heightened immune activation and inflammation present in the setting of HIV viremia demonstrated in these studies and others (23, 24), are likely drivers contributing to coronary plaque progression and coronary artery stenosis. In addition, in vitro and animal studies suggest that specific viral proteins may have effects on endothelial function and contribute to the pathogenesis of coronary atherosclerosis.(25, 26) The present study results support early and uninterrupted ART for people living with HIV to achieve durable viral suppression, which may reduce the risk of coronary artery stenosis progression, with vigilance required to address occurrences of suboptimal viral suppression and less than optimal adherence to cART. Although the number of people living with HIV who are prescribed ART and are virally suppressed has increased in recent years, there are still significant age and racial disparities, which need to be addressed (27–29). It is also possible that men with viral suppression had better cardiovascular outcomes in part due to access and behaviors related to other predictors of slower atherosclerosis progression, such as diet, exercise, avoidance of unhealthy behaviors, and health care utilization that were not evaluated in our study. For example, men with viral suppression and adherence to cART, were more likely to receive lipid lowering medications than men less adherent to cART. Systemic factors that limit the ability to optimally address CVD risk factor modification and healthy behaviors need to be addressed.
There are limitations to this study. We observed a relatively small differential follow-up by HIV serostatus, with the HIV+ participants less likely to have a follow-up CT scan, mostly due to a higher proportion of deaths and incident kidney function decline below the eligibility threshold to receive intravenous contrast. This potential selection effect could lead to an underestimation of the relative risk of coronary disease progression among men with HIV, since those excluded due to kidney function decline and death likely had a higher risk for stenosis progression. Due to the limited number of clinical events in this cohort, we assessed predictors of coronary artery stenosis progression on coronary CTA, a clinically relevant outcome. In addition, the possibility of residual confounding exists due to unmeasured or inadequately measured factors. The MACS includes only gay and bisexual men; therefore, results may not be generalizable to women or other men living with HIV.
To our knowledge, this is the largest prospective serial CTA study to date in a cohort that includes people living with and without HIV; however, the number of men in this study with uncontrolled viremia was relatively small. A larger sample size could have led to greater precision in the estimates of risk. In addition, a larger sample size would be needed to determine with greater certainty that there is no difference in coronary stenosis progression risk in HIV+ suppressed men compared to HIV− men. The comparison group consisted of well-characterized HIV− men at risk for HIV. We found strong and consistent associations between HIV viremia and each of the three dimensions of stenosis progression assessed. The longitudinal nature of this study allowed for detailed prospective characterization and analysis of viral “blips”, low level viremia, and cART adherence, and included time-varying assessment of cardiovascular risk factors during the interscan interval.
In summary, coronary artery stenosis progression was associated with incomplete HIV viral suppression, suboptimal cART adherence, traditional CVD risk factors, and cocaine use. Effective ongoing HIV viral suppression, cART adherence, and control of CVD risk factors, including healthy behaviors, may mitigate increased risk for coronary disease events among people living with HIV. Additional studies are needed to ascertain the significance of isolated viral “blips” and low level viremia for the risk for CVD and to address ongoing disparities that exist among racial/ethnic groups.
Supplementary Material
Highlights:
The extent of HIV viremia and traditional CVD risk factors are associated with progression of coronary artery stenosis.
Improved adherence to combination antiretroviral therapy may mitigate this increased risk.
Disparities exist in control of HIV viremia which require thoughtful interventions.
Financial support:
The MACS coronary CT angiography studies are funded by National Heart Lung and Blood Institute (NHLBI) RO1 HL095129 (Post) and R01 HL125053 (Post). Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS), now the MACS/WIHS Combined Cohort Study (MWCCS). U01-HL146201, U01-HL146193, U01-HL146245, U01-HL146240, U01-HL146333, U01-HL146208. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding NICHD, NIDCR, NIAID, NINDS, NIMH, NIDA, NINR, NCI, NIAAA, NIDCD, NIDDK, NIMHD. MWCCS data collection is also supported by UL1TR003098 (JHU ICTR), UL1- TR001881 (UCLA-CTSI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest:
TTB has served as a consultant to Gilead Sciences, ViiV Healthcare, Janssen, Merck, and Theratechnologies. FJP is a consultant and/or on the Speakers’ Bureau for Gilead, Janssen, ViiV and Merck. The remaining authors do not have any relationship with industry.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- 1.Wada N, Jacobson LP, Cohen M, French A, Phair J, Munoz A. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984-2008. American journal of epidemiology. 2013;177(2):116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lerner AM, Eisinger RW, Fauci AS. Comorbidities in Persons With HIV: The Lingering Challenge. JAMA. 2019. [DOI] [PubMed] [Google Scholar]
- 3.Feinstein MJ, Hsue PY, Benjamin LA, Bloomfield GS, Currier JS, Freiberg MS, et al. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation. 2019;140(2):e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Post WS, Budoff M, Kingsley L, Palella FJ Jr., Witt MD, Li X, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160(7):458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strategies for Management of Antiretroviral Therapy Study G, El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, et al. CD4+ count-guided interruption of antiretroviral treatment. The New England journal of medicine. 2006;355(22):2283–96. [DOI] [PubMed] [Google Scholar]
- 6.Fahey JL, Taylor JM, Detels R, Hofmann B, Melmed R, Nishanian P, et al. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. The New England journal of medicine. 1990;322(3):166–72. [DOI] [PubMed] [Google Scholar]
- 7.Sorstedt E, Nilsson S, Blaxhult A, Gisslen M, Flamholc L, Sonnerborg A, et al. Viral blips during suppressive antiretroviral treatment are associated with high baseline HIV-1 RNA levels. BMC Infect Dis. 2016;16:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming J, Mathews WC, Rutstein RM, Aberg J, Somboonwit C, Cheever LW, et al. Low-level viremia and virologic failure in persons with HIV infection treated with antiretroviral therapy. Aids. 2019;33(13):2005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grennan JT, Loutfy MR, Su D, Harrigan PR, Cooper C, Klein M, et al. Magnitude of virologic blips is associated with a higher risk for virologic rebound in HIV-infected individuals: a recurrent events analysis. J Infect Dis. 2012;205(8):1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, et al. Diagnostic performance of coronary angiography by 64-row CT. The New England journal of medicine. 2008;359(22):2324–36. [DOI] [PubMed] [Google Scholar]
- 11.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR Jr. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. American journal of epidemiology. 1987;126(2):310–8. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54. [DOI] [PubMed] [Google Scholar]
- 13.Hacioglu Y, Gupta M, Choi TY, George RT, Deible CR, Jacobson LP, et al. Use of cardiac CT angiography imaging in an epidemiology study - the Methodology of the Multicenter AIDS Cohort Study cardiovascular disease substudy. Anadolu kardiyoloji dergisi : AKD = the Anatolian journal of cardiology. 2013;13(3):207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51(4 Suppl):5–40. [DOI] [PubMed] [Google Scholar]
- 15.Raff GL, Abidov A, Achenbach S, Berman DS, Boxt LM, Budoff MJ, et al. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2009;3(2):122–36. [DOI] [PubMed] [Google Scholar]
- 16.Pagali SR, Madaj P, Gupta M, Nair S, Hamirani YS, Min JK, et al. Interobserver variations of plaque severity score and segment stenosis score in coronary arteries using 64 slice multidetector computed tomography: a substudy of the ACCURACY trial. J Cardiovasc Comput Tomogr. 2010;4(5):312–8. [DOI] [PubMed] [Google Scholar]
- 17.Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73. [DOI] [PubMed] [Google Scholar]
- 18.Castillo-Mancilla JR, Brown TT, Erlandson KM, Palella FJ Jr., Gardner EM, Macatangay BJ, et al. Suboptimal Adherence to Combination Antiretroviral Therapy Is Associated With Higher Levels of Inflammation Despite HIV Suppression. Clin Infect Dis. 2016;63(12):1661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LIANG K-Y, ZEGER SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 20.Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7(9):e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strategies for Management of Antiretroviral Therapy Study G, Lundgren JD, Babiker A, El-Sadr W, Emery S, Grund B, et al. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the SMART study: role of CD4+ Cell counts and HIV RNA levels during follow-up. J Infect Dis. 2008;197(8):1145–55. [DOI] [PubMed] [Google Scholar]
- 22.Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. The New England journal of medicine. 2015;373(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ Jr., Kingsley LA, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis. 2015;211(8):1219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bahrami H, Budoff M, Haberlen SA, Rezaeian P, Ketlogetswe K, Tracy R, et al. Inflammatory Markers Associated With Subclinical Coronary Artery Disease: The Multicenter AIDS Cohort Study. Journal of the American Heart Association. 2016;5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sviridov D, Mukhamedova N, Makarov AA, Adzhubei A, Bukrinsky M. Comorbidities of HIV infection: role of Nef-induced impairment of cholesterol metabolism and lipid raft functionality. Aids. 2020;34(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovacs L, Bruder-Nascimento T, Greene L, Kennard S, Belin de Chantemèle EJ. Chronic Exposure to HIV-Derived Protein Tat Impairs Endothelial Function via Indirect Alteration in Fat Mass and Nox1-Mediated Mechanisms in Mice. Int J Mol Sci. 2021;22(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beer L, Bradley H, Mattson CL, Johnson CH, Hoots B, Shouse RL, et al. Trends in Racial and Ethnic Disparities in Antiretroviral Therapy Prescription and Viral Suppression in the United States, 2009–2013. J Acquir Immune Defic Syndr. 2016;73(4):446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley H, Althoff KN, Buchacz K, Brooks JT, Gill MJ, Horberg MA, et al. Viral suppression among persons in HIV care in the United States during 2009-2013: sampling bias in Medical Monitoring Project surveillance estimates. Ann Epidemiol. 2019;31:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradley H, Mattson CL, Beer L, Huang P, Shouse RL, Medical Monitoring P. Increased antiretroviral therapy prescription and HIV viral suppression among persons receiving clinical care for HIV infection. Aids. 2016;30(13):2117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


