Abstract
The use of antibiotics in fish production can induce bacterial populations to develop resistance to multiple antibiotics and transfer antibiotic resistance genes to other bacteria, including clinically relevant bacteria. This study evaluated the diversity of Enterobacterales in sediment from lagoons with fish farming activity and analyzed antibiotic resistance in the central region of Peru. Sediment samples were collected from four fish-active ponds and transported to the laboratory for analysis. Bacterial diversity was analyzed using DNA sequencing and antibiotic resistance was tested using the disk diffusion method. The results showed variability of bacterial diversity in the ponds with fish farming activity. Simpson's index indicated that the Habascocha lagoon is the most diverse in bacterial species of the order Enterobacterales (0.8), but the least dominant. The Shannon-Wiener index revealed that it is the most diverse (2.93) and the Margalef index revealed that species richness in this lagoon is high (5.72). Similarity percentage analysis (SIMPER) allowed the identification of the main Enterobacterales with the highest percentage contribution in the frequencies of individuals. In general, the Enterobacterales species isolated showed multi-resistance to the antibiotics used and Escherichia coli was the most resistant.
Keywords: Enterobacterales; Diversity; Lagoon sediment; Antibiotics, resistance; Fish production
Graphical Abstract
Highlights
-
•
Abundance of Plautia stali symbiont was lower in lake sediment with higher abundance of Escherichia coli.
-
•
E. coli was the pathogen with the highest percentage contribution to Enterobacterales order.
-
•
E. coli and Enterobacter sp. isolates from fish farming lagoons showed resistance to seven or more antibiotics.
1. Introduction
The quality of the aquatic environment has been undergoing changes due to the contribution of a range of pollutants from different sources [3], [7]. In recent years, the aquaculture sector has shown a growth trend in its production worldwide. According to the Food and Agriculture Organization of the United Nations 2020, aquaculture increased by 3.1% annually during 1961–2017 and exceeded the annual growth of the world population (1.6%) [41]. Fish production in ponds or cages in natural water bodies accumulates organogenic sediments continuously due to intensive feeding and fecal waste [69]. Under these conditions, these production media are often exposed to infectious agents that spread rapidly through the aqueous medium [25]. This situation leads to the direct use of prophylactic and therapeutic drugs to maintain the health of aquaculture specimens.
Fish production in high Andean lagoons is often conducted in cage systems, which allows free transfer of water from the cages to the surrounding water and sediment. The use of antibiotics to treat fish infections can cause the water and sediment to become reservoirs for antibiotic resistance genes [42], [52], [72], [75], [77]. The use of antibiotics in fish production can induce bacterial populations to develop resistance mechanisms to multiple antibiotics [2], [56], e.g. enzyme production, overexpression of the ejector pump, porin modification [15] and biofilm formation that reduce susceptibility to antibiotic activity [19]. Therefore, the use of antibiotics in fish production is of growing concern as a critical point for the enrichment and dissemination of antibiotic resistance genes through mobile genetic elements [86], [1]. Horizontal transfer of antibiotic resistance genes can occur and reach other bacteria and, in some cases, even human pathogens [78]. Therefore, bacterial genomes are constantly changing, and any segment of DNA in a large bacterial population could have the opportunity for horizontal transfer [74]. Many antibiotics used in human medicine are also used in aquaculture.
The presence of antibiotic residues and antibiotic resistance genes in the aquatic environment can affect the composition of the bacterial community that plays an important role in the ecosystem [24] and endangering water quality and human health [39]. Plasmids harboring resistance determinants to various antimicrobials are transferable from pathogenic bacteria of fish and aquatic bacteria to pathogenic bacteria such as Escherichia coli [60], [34], Vibrio parahaemolyticus, V. cholerae, Shigella and Salmonella [73]. The spread of antibiotic resistance among diverse bacterial populations is a major clinical problem that complicates the medical use of antibiotics. Currently, extended-spectrum β-lactamases (ESBL) and carbapenemases are the most common β-lactamases detected in enterobacteria, particularly in Escherichia coli and Klebsiella pneumoniae [71]. These enzymes endow bacteria with various resistances to most advanced generation antibiotics such as β-lactams, including penicillins, cephalosporins and aztreonam [51], but no cephamycins nor carbapenems [61]. Therefore, carbapenems have been used as the treatment of choice for infections due to ESBL-producing Enterobacteriaceae, resulting in the emergence of carbapenemasa-producing Enterobacteriaceae [81].
Due to the risk posed by the presence of antibiotics in the environment, many countries have already introduced the obligation to monitor this type of contamination in the aquatic environment. For example, the European Union Commission has established a watch list of substances for Union-wide monitoring in the field of water policy. Three antibiotics (erythromycin, clarithromycin and azithromycin) were included in the first watch list and are checked and reviewed every two years. In Peru, several clinical studies have been conducted on antibiotic resistance [23], [46], but studies of resistance to these antibacterial agents in the environment are limited [67], particularly in aquatic environments. Studies of antibiotic resistant bacterial diversity using DNA sequencing in continental lake environments with fish farming activity in the central region of Peru do not exist. Most studies are focused on evaluating the trophic status of these environments and others on monitoring heavy metals. Consequently, knowing the antibiotic resistant bacterial diversity in lake environments with intensive fish farming activity can help to understand the antibiotic consumption pattern and impact of intensive fish farming and contribute to address the epidemiology of antibiotic resistance. Therefore, the importance of this study, since the central region of Peru is one of the main regions in fish production for both domestic and foreign markets. In addition, bacteria of the order Enterobacterales often function as important vectors in the dissemination of β-lactamase genes in natural bacterial ecosystems [71]. Therefore, this research evaluated the diversity of Enterobacterales in sediment from lagoons with fish farming activity and analyzed antibiotic resistance in the central region of Peru.
2. Materials and methods
2.1. Study area and sampling
The study area is located in the Pomacocha, Habascocha, Tipicocha and Tranca Grande lagoons of glacial origin located in the upper basin of the Perené River, in the Central Andes of Peru, at an altitude between 4310 and 4330 m above sea level [47], [48] (Fig. 1). The area and depth of these ponds vary from 80 to 164 ha and from 10 to 28 m, respectively. Water temperature varies from 9.8 to 13.1 ºC and pH from 7.7 to 8.06 (Table S1). The four lagoons are used for the intensive culture of Oncorhynchus mykiss (rainbow trout) in large floating cages. In 2019, the number of cages per lagoon was 122 (Tranca Grande), 35 (Tipicocha), 13 (Habascocha) and 0 (Pomacocha) (Google, 2022). Three sampling sites were established at each lagoon and at each site 250 g of surface sediment (10 cm) was collected in triplicate in November 2020. A total of 36 sediment samples were collected from the study area. Sediment samples from each lagoon were conditioned in airtight plastic bags and transported in cold chain to the Molecular Biology Laboratory of the Universidad Nacional de Tumbes for analysis [49].
Fig. 1.
Location map of the study area in the central region of Peru.
2.2. Isolation and identification of Enterobacterales
Isolation of Enterobacterales was performed by mixing 1 g of sediment in 99 ml of sterile distilled water in a 250 ml Erlenmeyer flask, followed by shaking at 150 rpm for one hour at room temperature. Bacterial isolation was carried out from the supernatant by successive dilution methods and biochemical tests on Triple Sugar, Iron Agar (TSI) [8]. Dilutions of 10—1 to 10—5 were prepared and seeding of each dilution was performed on nutrient agar plates by surface streaking and incubation was performed at 37 °C for 24 h. Biochemical identification of individual colonies was performed by subculturing on TSI agar and molecular characterization using DNA sequencing.
2.3. Antibiotic sensitivity testing
Antibiotic sensitivity of bacterial isolates was determined by the disk diffusion method. Inocula of morphologically similar colonies were prepared in Tryptic Soy Broth and incubated at 35 °C until a turbidity equal to 0.5 McFarland (equivalent to 2 ×108 cells/ml) was obtained by UV/VIS spectrophotometer reading at 625 nm. Sterile swabs were then dipped into the inocula and spread on Mueller-Hinton agar plates (BD DifcoTM, USA). Antibiotic discs were placed on each of the plates equidistantly at 30 mm, and the plates were incubated at 35 °C for 24 h. After incubation, the diameters of the inhibition zones were measured and the bacterial isolates were classified as "S" (susceptible), "I" (intermediate) or "R" (resistant), according to the standards of the National Committee for Clinical Laboratory Standards [35]. The Escherichia coli ATCC 25922 strain was used as a control for the antibiotic sensitivity test due to its tolerance to different temperature ranges and its standardization for the determination of antimicrobial susceptibility of bacteria in the aquatic environment [50]. The antibiotic discs Aztreonam (ATM), Gentamicin (GM), Amikacin (MK), Ceftazidime (CAZ), Amoxicillin - Clavulanic Acid (AMC), Chloramphenicol (C), Ciprofloxacin (CIP), Cephalexin (CFL), Azithromycin (AZM), Tetracycline (TE) and Nalidixic Acid (NA) were purchased from Biodisc SAC.
2.4. Molecular characterization of bacterial isolates
2.4.1. DNA extraction
A set of colonies were randomly picked from each plate, cultured in brain heart infusion broth and incubated at 30 ºC, 150 rpm for 24 h. Bacterial genomic DNA extraction was performed using the PrestoTM Soil DNA Extraction Kit, following the manufacturer's instructions. Bacterial lysis was performed from 600 µl of the bacterial isolate and 750 µl of lysis buffer in a 2 ml tube with ceramic beads. Inhibitor removal was performed by transferring 600 µl of the supernatant from the tube to the silica column. The DNA was washed successively with SL3 buffer (one wash) and Wash buffer (two washes), with each wash step centrifuged at 16,000 x g for 30 s. Finally, the silica column was centrifuged at 16,000 x g for 2 min at room temperature. DNA elution was performed by applying 100 µl of elution buffer to the column, allowed to stand for 2 min and centrifuged at 16,000 x g for 2 min at room temperature. The eluted DNA was stored at − 20 °C until further analysis.
2.4.2. PCR amplification and sequencing
PCR amplification was performed using Gene One and GE Healthcare Life Sciences kits by mixing 1 µl of universal 16 S rRNA F primer, 1 µl of universal 16 S rRNA R primer, 22 µl of PCR mix (containing premix buffer, MgCl2, dNTPs and taqPolymerase) and 1 µl DNA sample for a total reaction volume of 25 µl. The primers 27 F (5′-AGAGAGTTTGATCCTGGCTCAG-3′) and 1492 R (5′-GGTTACCTTGTTACGACTT-3′) were used and amplified for a product of about 1465 bp [12]. The initial concentration of both primers was 10 pMol/µl equivalent to 10 µM, with a final concentration after incorporation into the amplification mixture of 0.4 µM. Bacterial 16 S rRNA amplicon sequencing of the V3 and V4 hypervariable regions was performed using the standard Illumina MiSeq v2 next generation protocol [18], [38], [68], [21]. The construction of the library was done commercially (ADMERA HEALTH LLC, USA).
2.4.3. Bioinformatic analysis of sequence reads
The raw Illumina paired reads were loaded as FASTQ files. Quality filtering was performed with the Trimmomatic v0.39 program [30] to remove primer regions, adapter regions and low quality reads (mean score < 20 and read length < 100 bp). MetaSPAdes v3.13 [59] was performed to assemble de novo DNA sequences. Amplicons were clustered in operational taxonomic units (OTU) using Kraken 2 v 2.1.2 [82] with the database SILVA v132 [66]. Data have been deposited with links to BioProject accession number PRJNA682317 in the NCBI BioProject database (https://www.ncbi.nlm.nih.gov/bioproject/).
2.5. Statistical analysis
The assessment of species diversity of the order Enterobacterales was performed using the number of taxa and diversity indices, such as Margalef (M) to determine species richness, Simpson (1-D) to determine dominance and Shannon-Wiener (H′) as diversity index [13], [53]. Diversity indices were considered only for the order Enterobacterales. Rényi diversity profiles were also used to classify Enterobacterales communities [28]. The diversity of the bacterial order studied was examined by rarefaction analysis to assess species richness [20]. Similarity percentage analysis (SIMPER) was used to evaluate the average percentage contribution of the individuals [14]. Analyses were calculated using PAST V4.08 software [26]. The number of species in relation to the gaps were inspected using the double clustering technique ("p" and "q") where the Euclidean dissimilarity index and the minimum variance method (Ward) were used [33]. The interpretation was based on clustering coefficients that reflect the distance at which groups merge. A large increase in the clustering coefficient suggests that two quite different clusters merged [11], for this study with a maximum distance value of 4000. The heat map was generated using R software, with the pheatmap package [36]. The antibiotic susceptibility analysis was performed with descriptive statistics (absolute frequencies) and principal component analysis with Euclidean distances with PAST software in order to determine the response of Enterobacterales to the antibiotics using the correlation ranges (0–0.1 null, 0.1–0.3 weak, 0.3–0.5 moderate and 0.5–1 strong) of the component scores for interpretation.
3. Results
3.1. Diversity of Enterobacterales in the sediment of lagoons with fish farming activity
The analysis of the diversity of species of the order Enterobacterales in lake sediment revealed variability in bacterial diversity according to lagoon (Table 1). In Habascocha, an important diversity of species was found, followed by Tipicocha, Pomacocha and Tranca Grande lagoons. However, the highest number of individuals was found in Tipicocha lagoon. The diversity indices mostly showed a positive relationship with each other according to richness or evenness. Simpson's index (1-D) indicated that the Habascocha lagoon is the most diverse in bacterial species of the order Enterobacterales (0.8), but the least dominant (0.2). This trend is due to the anthropogenic pressure associated with fish production [47], [48]. The Shannon index showed that the Habascocha lagoon is the most diverse (H′ = 2.293), while the other lagoons tend to present greater uniformity in the frequencies of species found. The Margalef Index (M = 5.719) revealed that Habascocha lagoon has a high species richness.
Table 1.
Species diversity index for Enterobacterales order in evaluated lagoons.
| Indices | Tranca Grande | Habascocha | Pomacocha | Tipicocha |
|---|---|---|---|---|
| Species | 34 | 44 | 35 | 36 |
| Individuals | 1226 | 1842 | 3837 | 5075 |
| Simpson (1-D) | 0.6413 | 0.8 | 0.585 | 0.7438 |
| Shannon-Wiener (H′) | 1.766 | 2.293 | 1.576 | 1.7 12 |
| Margalef (M) | 4.64 | 5.719 | 4.12 | 4.102 |
Fig. 2 shows the diversity profiles based on the exponential of the Rényi index. This index indicates the alpha diversity of species of the order Enterobacterales. The Habascocha lagoon showed a higher number of species in the diversity profile (higher values when α = 0), denoting that it is the most diverse for the order Enterobacterales. The results also show that in this lagoon the Shannon-Wiener index (α = 1) and inverse Simpson's index (α = 2) recorded slightly higher values than the values of these indices of the other lagoons. The low richness recorded in the other lagoons reveals a low uniformity.
Fig. 2.
Species diversity profiles of the order Enterobacterales in lake sediments. Diversity profile using the Rényi series representing. Alpha values: a = 0 richness; a = 1 Shannon index; a = 2 inverse Simpson index (1/D).
Fig. 3 shows the cluster of the distribution of species of the order Enterobacterales of the studied lagoons in two groups with relevant differences. The first cluster was formed by the Tipicocha lagoon and the second group by the Pomacocha, Tranca Grande and Habascocha lagoons. The diversity indices show a greater predominance of Plautia stali symbiont, Escherichia coli and Providencia alcalifaciens. Tranca Grande and Habascocha lagoons have a lower dominance of the Plautia stali symbiont. While in Pomacocha lagoon this symbiont tends to be more dominant. This would explain the low value of the Shannon-Wiener index for this lagoon despite having a higher species frequency. Providencia alcalifanciens is the second most frequent species in the studied lagoons. A higher frequency of this species was found in the Tipicocha lagoon, as well as Escherichia coli. The second cluster was able to classify three major groups. The first group indicates the dominance of Plautia stali symbiont in Pomacocha lagoon. The second group determines the positive relationship between the distribution and frequency of Escherichia coli and Providencia alcalafaris species, especially in Tipicocha and Pomacocha lagoons. The third group is made up of the remaining species of the order Enterobacterales, with a dominance of Raoultella ornithinolytica, Enterobacter hormaechei, Edwardsiella sp., Rahnella aquatilis, Candidatus Blochmannia floridanus, Serratia marcescens, Brenneria goodwinii, Candidatus Riesia sp., Pantoea rwandensis, Shimwellia blattae, Candidatus Ishikawaella capsulata, Edwardsiella ictaluri and Serratia rubidaea.
Fig. 3.
Heat map showing the cluster of Tranca Grande (TG), Habascocha (H), Pomacocha (P) and Tipicocha (T) lagoons and species of the order Enterobacterales identified by metagenomics.
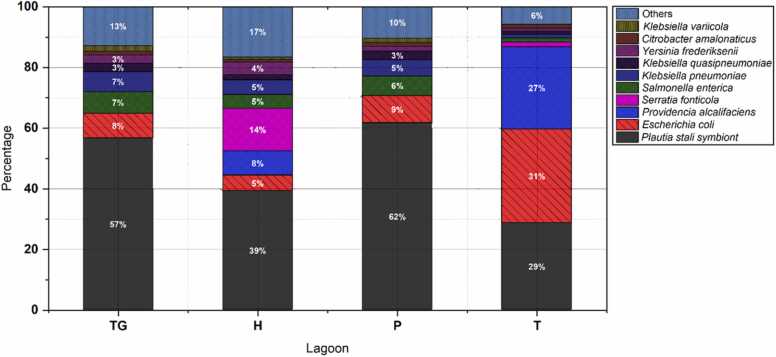
The SIMPER analysis allowed the identification of the main species of the order Enterobacterales that had a higher percentage contribution in the frequencies of individuals (Fig. 4). The analysis showed that the non-culturable bacteria Plautia stali symbiont was the most representative of the order studied, characteristic and obligatory symbiont of the insect Plautia stali [58]. These results revealed the presence of these or similar insects in the Peruvian Andean lagoons, especially in the Pomacocha (P) and Tranca Grande (TG) lagoons, with values of 62% and 57% contribution to the community of the order Enterobacterales, respectively. The second species of interest was Escherichia coli, present in all four lagoons, but more frequently in the Tipicocha lagoon (31%). Other species with an important frequency of contribution to the total number of Enterobacterales species were Providencia alcalifaciens (27%), characteristic of the intestinal flora of trout and salmon [6] in the Tipicocha lagoon, Serratia fonticola (14%), non-pathogenic species characteristic of trout skin mucosa, even beneficial for parasite control [10] in the Habascocha lagoon and the pathogenic species Klebsiella pneumoniae (7%) in the Tranca Grande lagoon [9].
Fig. 4.
Representativeness of individuals of the order Enterobacterales in sediment from Tranca Grande (TG), Habascocha (H), Pomacocha (P) and Tipicocha (T) lagoons according to SIMPER analysis.
3.2. Analysis of antibiotic resistance
Escherichia coli, Klebsiella pneumoniae, Enterobacter sp., Citrobacter sp. and Salmonella sp., were isolated and biochemically identified. Antibiotic resistance of these bacteria was tested by the disk diffusion method as described in the methods section. The percentages of isolates corresponding to susceptible, intermediate and resistant to each antibiotic are shown in Table 2. In the Pomacocha lagoon, the resistance of E. coli isolates (100%) was to ceftazidime and amoxicillin-clavulanic acid, those of Enterobacter sp. (84.62%) to aztreonam and amoxicillin-clavulanic acid and those of Klebsiella pneumoniae (100%) to amikacin, chloramphenicol and ciprofloxacin. All E. coli isolates were sensitive to gentamicin, Enterobacter sp., to ciprofloxacin and Klebsiella pneumoniae to aztreonam. In the Habascocha lagoon, the resistance of E. coli isolates (100%) was to aztreonam, amikacin and ceftazidime, those of Citrobacter sp. (100%) to amoxicillin-clavulanic acid and chloramphenicol and those of Salmonella sp. (66.67%) to chloramphenicol. In Tipicocha lagoon, the resistance of E. coli isolates (100%) was to aztreonam. The susceptibility of E. coli to antibiotics was very low, except for gentamicin and amikacin. In the Tranca Grande lagoon, the resistance of E. coli isolates (100%) was to aztreonam, gentamicin, ceftazidime and chloramphenicol and those of Enterobacter sp. (100%) to aztreonam and gentamicin. 100% of E. coli isolates were sensitive to azithromycin and 75% of Enterobacter sp., isolates were sensitive to amoxicillin-clavulanic acid and ciprofloxacin (Fig. 5).
Table 2.
Antibiotic susceptibility patterns of enterobacteria isolated from lake sediment identified by biochemical analysis.
| Isolation lagoon | Species/ Number of isolated | PS | Antibiotic |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATM | GM | MK | CAZ | AMC | C | CIP | CFL | AZM | TE | NA | |||
| Pomacocha |
Escherichia coli (9) |
S | 22.22 | 100 | 44.44 | 0 | 0 | 11.11 | 0 | 77.78 | 88.89 | 55.56 | 66.67 |
| I | 0 | 0 | 0 | 0 | 0 | 22.22 | 22.22 | 22.22 | 11.11 | 22.22 | 33.33 | ||
| R | 77.78 | 0 | 55.56 | 100 | 100 | 66.67 | 77.78 | 0 | 0 | 22.22 | 0 | ||
|
Enterobacter sp. (13) |
S | 0 | 0 | 23.08 | 23.08 | 15.38 | 69.23 | 100 | 84.62 | 53.85 | 69.23 | 76.92 | |
| I | 15.38 | 30.77 | 23.08 | 15.38 | 0 | 0 | 0 | 15.38 | 30.77 | 7.69 | 7.69 | ||
| R | 84.62 | 69.23 | 53.85 | 53.85 | 84.62 | 30.77 | 0 | 0 | 15.38 | 23.08 | 15.38 | ||
|
Klebsiella pneumoniae (5) |
S | 100 | 20 | 0 | 80 | 0 | 0 | 0 | 0 | 20 | 0 | 0 | |
| I | 0 | 20 | 0 | 20 | 60 | 0 | 0 | 20 | 0 | 0 | 0 | ||
| R | 0 | 60 | 100 | 0 | 40 | 100 | 100 | 80 | 80 | 100 | 100 | ||
| Habascocha |
Escherichia coli (5) |
S | 0 | 40 | 0 | 0 | 40 | 0 | 100 | 60 | 60 | 0 | 0 |
| I | 0 | 0 | 0 | 0 | 60 | 40 | 0 | 40 | 20 | 40 | 60 | ||
| R | 100 | 60 | 100 | 100 | 0 | 60 | 0 | 0 | 20 | 60 | 40 | ||
|
Citrobacter sp. (3) |
S | 33.33 | 0 | 0 | 33.33 | 0 | 0 | 33.33 | 33.33 | 100 | 66.67 | 33.33 | |
| I | 66.67 | 100 | 100 | 33.33 | 0 | 0 | 66.67 | 66.67 | 0 | 33.33 | 33.33 | ||
| R | 0 | 0 | 0 | 33.33 | 100 | 100 | 0 | 0 | 0 | 0 | 33.33 | ||
|
Salmonella sp. (6) |
S | 50 | 66.67 | 16.67 | 66.67 | 0 | 0 | 66.67 | 66.67 | 66.67 | 0 | 16.67 | |
| I | 33.33 | 33.33 | 33.33 | 33.33 | 50 | 33.33 | 33.33 | 16.67 | 0 | 50 | 50 | ||
| R | 16.67 | 0 | 50 | 0 | 50 | 66.67 | 0 | 16.67 | 33.33 | 50 | 33.33 | ||
| Tipicocha |
Escherichia coli (11) |
S | 0 | 100 | 63.64 | 0 | 0 | 0 | 36.36 | 27.27 | 0 | 45.45 | 0 |
| I | 0 | 0 | 9.09 | 18.18 | 36.36 | 18.18 | 0 | 27.27 | 36.36 | 18.18 | 27.27 | ||
| R | 100 | 0 | 27.27 | 81.82 | 63.64 | 81.82 | 63.64 | 45.45 | 63.64 | 18.18 | 72.73 | ||
| Tranca Grande |
Escherichia coli (5) |
S | 0 | 0 | 20 | 0 | 0 | 0 | 20 | 20 | 100 | 60 | 60 |
| I | 0 | 0 | 0 | 0 | 40 | 0 | 20 | 40 | 0 | 40 | 60 | ||
| R | 100 | 100 | 80 | 100 | 60 | 100 | 60 | 40 | 0 | 0 | 0 | ||
|
Enterobacter sp. (8) |
S | 0 | 0 | 25 | 37.5 | 75 | 50 | 75 | 62.5 | 37.5 | 25 | 25 | |
| I | 0 | 0 | 37.5 | 37.5 | 0 | 0 | 25 | 37.5 | 12.5 | 37.5 | 12.5 | ||
| R | 100 | 100 | 37.5 | 25 | 25 | 50 | 0 | 0 | 50 | 37.5 | 62.5 | ||
PS: Profile susceptibility, S: Sensitive, I: Intermediate, R: Resistant. ATM: Aztreonam, GM: Gentamicin, MK: Amikacin, CAZ: Ceftazidime, AMC: Amoxicillin – Clavulanic Acid, C: Chloramphenicol, CIP: Ciprofloxacin, CFL: Cephalexin, AZM: Azithromycin, TE: Tetracycline, NA: Nalidixic Acid.
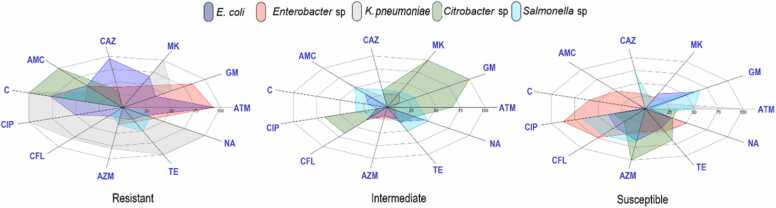
Fig. 5.
Radial classification diagram based on antimicrobial susceptibility testing (AST) differentiated by their reaction to antibiotics of species of the order Enterobacterales isolated from lake sediment. Where susceptible implies that the organism is likely to respond to treatment with the antibiotic at a standard dose, intermediate indicates that it may be effective if a higher dose can be used, and resistant implies that the organism is not likely to respond to therapy with that antibiotic.
Fig. 6 shows the principal component analysis of antibiotic susceptibility of species of the order Enterobacterales isolated from lake sediments. The percentage of total variance of antibiotic susceptibility was 49.69% for the first two components. The first component accounted for 28.82% of the total variance and the second component for 21.5%. This low value of variance reveals the response of the distribution in bacterial resistance and sensitivity to antibiotics in the gaps of this study, as there is no equivalent efficacy for most antibiotics against a single species in the four gaps. In general, the results reveal that few antibiotics have antibacterial activity, either inhibiting growth or causing bacterial death. E. coli and Enterobacter sp., are the most resistant species to the antibiotics aztreonam and ceftazidime, indicating their stronger correspondence with the first component. The results also show that few antibiotics have antibacterial activity, either inhibiting growth or causing bacterial death. Other results show a negative correlation of azithromycin (AZM) with the first component with a correlation load value of - 0.18 (Table S2). The correlation load values of E. coli with respect to the first component revealed a weak correlation according to the correlation ranges (0–0.1 null, 0.1–0.3 weak, 0.3–0.5 moderate and 0.5–1 strong).
Fig. 6.
Principal component analysis of antibiotic susceptibility of species of the order Enterobacterales isolated from lake sediment. In susceptibility profile, S: Sensitive, I: Intermediate, R: Resistant. ATM: Aztreonam, GM: Gentamicin, MK: Amikacin, CAZ: Ceftazidime, AMC: Amoxicillin - Clavulanic acid, C: Chloramphenicol, CIP: Ciprofloxacin, CFL: Cephalexin, AZM: Azithromycin, TE: Tetracycline, NA: Nalidixic acid.
4. Discussion
Trout production represents 54.63% (54878 MT) of Peruvian aquaculture production and is developed in ponds and cages with average densities of 20 kg/m3 and 14 kg/m3, respectively [64]. However, the poor sanitary conditions of the ponds or cages are critical points for enrichment, growth of pathogenic microorganisms and transfer of antibiotic resistance genes [55]. The results of the bacterial diversity indexes of the order Enterobacterales found in this study reveal that the Habascocha lagoon presented the highest bacterial richness and diversity. Tipicocha was the lagoon with the lowest diversity, but the highest abundance of individuals. The diversity of Enterobacteriaceae observed in this study would be determined by the intensified use of the water body in fish farming and the loading of nutrients not consumed by the fish. Surplus food favors selected groups of bacteria that have the ability to rapidly consume these available resources or selectively eliminate certain bacteria from the bacterial community [32], [44]. The results of this study reveal the variability of bacterial diversity of the order Enterobacterales in the studied lagoons. These results are supported by Defoirdt et al. (2011) [16] who refer that bacteria in aquatic environments can reach high densities of several species, which increases the risk of contagious diseases. The analysis of the species diversity profile of Enterobacterales corroborates that Habascocha is the most diverse and even more uniform than the other three lagoons. In addition, the species distribution cluster analysis and SIMPER revealed greater dominance and percentage contribution of P. stali symbiont, E. coli and P. alcalifaciens with respect to the rest of the Enterobacterales species found. However, little is known about P. stali symbiont, a non-culturable gamma-proteobacterium species (Kobayashi et al., 2011) that has evolved from environmental bacteria [31].
The antibiotic resistance profiles recorded in the fish-use lagoons in this study are similar to resistance gradients reported by other effluent, farm and aquaculture studies [19]. Our results also reveal that lagoons used in fish production represent an important source of antibiotic-resistant bacteria. Muziasari et al. [54] support our findings and indicate that wastewater from various sources act as a reservoir of antibiotic resistant bacteria and genes. In addition, other studies report that selective pressure of antibiotic loading favors and increases the acquisition/introduction of antibiotic resistant bacteria and genes through different resistance mechanisms in the aquaculture environment, highlighting antibiotic efflux as the main mechanism of resistance in sediments [65], [83], [76]. The use of medicated feeds in fish production and the frequent use of antibiotics to control infectious diseases has led to the emergence of reservoirs of antibiotic-resistant bacteria [24]. In general, aquatic bacteria are not different from other bacteria in their responses to antibiotic exposure, and are capable of transferring antibiotic resistance genes to other bacteria [79], [63]. Consequently, antibiotic resistance genes can be exchanged between bacteria from different environments [27]. Antibiotic resistance in fish pathogens, the transfer of their genetic determinants to terrestrial animal bacteria and human pathogens, and alterations in the bacterial microbiota of the aquatic environment constitute a threat to human and animal health. The present results indicate that E. coli is the enterobacteria with the highest frequency and multiple resistance to ceftazidime, aztreonam, amoxicillin-clavulanic acid, chloramphenicol, ciprofloxacin and tetracycline in the studied ponds. The emergence of bacteria resistant to multiple antibiotics is a growing threat to antibiotic therapy [43], as many antibiotics used in human medicine are used in species in aquatic environments. For example, in Chile, one of the main salmon producers in America, often applies amoxicillin, erythromycin, tetracycline, and ciprofloxacin in its production process [37]. In Vietnam, Philippines, Indonesia, India and Malaysia, sulfonamides, trimethoprim, five macrolides, lincomycin and three tetracyclines have been detected in farm water [70]. In Vietnam and China, although the use of antibiotics has been reduced in the last decade, ofloxacin, enrofluxacin, sulfamethoxazole, trimethoprim and azithromycin are still detected in aquaculture products [85], [45].
Antibiotics, in and of themselves, do not cause resistance, but frequent and high exposure of antibiotics to bacteria creates selection pressure that triggers bacterial resistance mechanisms. Antibiotic resistance is a worldwide concern due to the prevalence of resistant bacteria carrying antibiotic resistance genes [80], [57]. Many studies indicate that the selection pressures imposed by antibiotics such as fluoroquinolones and the evolutionary principle of survival of the fittest have led many pathogens to evolve a variety of evasion mechanisms [17]. Resistant bacteria are increasingly difficult to treat and require less available and more toxic antibiotics [5]. 100% of Klebsiella pneumoniae isolates showed resistance to amikacin, chloramphenicol, ciprofloxacin and tetracycline, 80% showed resistance to cephalexin and azithromycin, 60% to gentamicin and 40% to Amoxicillin-clavulanic acid.
The increasing trend toward the emergence of resistance to multiple antibiotics is a threat to the management of infections in humans [29]. The fact that some bacteria that cause infections in fish belong to the same genera as bacteria that cause infections in humans is likely to increase the likelihood of spread of antibiotic resistance from aquatic environments to humans [4]. Other studies supporting the multi-resistance recorded in our study have shown that plasmids harboring antibiotic resistance determinants are transferable from fish pathogens and aquatic bacteria, not only to other bacteria of the same genus, but also to E. coli and Salmonella [40], [84]. Salmonella resistance to ciprofloxacin observed in this study is consistent with that of foodborne Salmonella worldwide [22]. The results obtained also reveal resistance of Salmonella to other antibiotics, including azithromycin, which are used for the treatment of salmonellosis; however, resistance to azithromycin has already been reported.
The emergence of resistance in microorganisms is a natural process [5]. However, the increasing use of antibiotics and the existence of reservoirs of antibiotic resistance in the environment may greatly accelerate the evolution of multidrug-resistant bacteria [62] and compromise the therapeutic potential of antibiotics. Our findings corroborate the antibiotic resistance observed in other studies [69]. Consequently, it is essential to take into account that the use of antibiotics in fish production impacts on bacterial diversity and promotes bacteria to evolve rapidly not only by mutation and rapid multiplication, but also by DNA transfer [74]. One control strategy is the use of specific antibiotics rather than broad-spectrum antibiotics, in order to avoid affecting beneficial bacteria [63].
5. Conclusions
Our study showed that the highest bacterial richness and diversity of Enterobacterales was recorded in the Habascocha lagoon and the lowest richness and diversity in Tipicocha. Sensitivity tests were applied to six bacterial species against 11 antibiotics using the disk diffusion method. E. coli was the most resistant bacterium against the antibiotics used. Therefore, is necessary to emphasize that the environmental occurrence of antibiotics and antibiotic resistant bacteria is a global concern. Therefore, it is urgent to undertake practices that minimize the use of antibiotics in fish farms and control the spread of antibiotic resistance. This study also shows that fish farms impact aquatic bacterial diversity and underscores the value of analyzing antibiotic resistance by providing a baseline for future studies and fish farm management in the use of antibiotics. Likewise, the antibiotics authorized for use in Peruvian aquaculture production are amoxicillin, chlorotetracycline, enrofloxacin, florfenicol, flumequine, erythromycin, oxytetracycline, oxolinic acid and sulfonamides. However, there are no records available on the amount of antibiotics used in the fish farms studied. This constitutes an important knowledge gap on antibiotic use and antibiotic-resistant bacterial diversity in inland water fish production. This limitation is particularly acute for some of the most commonly cultured fish species such as Oncorhynchus mykiss.
CRediT authorship contribution statement
M. Custodio: Investigation, Conceptualization, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. R. Peñaloza: Investigation, Data curation, Visualization, Writing – review & editing. A. Ordinola: Investigation, Formal analysis, Visualization, Writing – review & editing. T. Peralta: Investigation, Formal analysis, Writing – review & editing. H. Sánchez: Investigation, Formal analysis, Writing – review & editing. E. Vieyra: Investigation, Formal analysis, Writing – review & editing. H. De la Cruz: Investigation, Formal analysis, Writing – review & editing. J. Alvarado: Investigation, Visualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by CONCYTEC-FONDECYT under the call E041-01 [contract number 76-2018- FONDECYT-BM-IADT-MU] and Universidad Nacional del Centro del Perú through FEDU/012021861223. The authors wish to express their gratitude to the Molecular Biology Laboratory of the Universidad Nacional de Tumbes and for facilitating the use of the equipment and instruments in the first stage of the study.
Handling Editor: Dr. L.H. Lash
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2023.02.002.
Appendix A. Supplementary material
Supplementary material.
.
Data availability
Data will be made available on request.
References
- 1.Ahmad A., Kurniawan S.B., Abdullah S.R.S., Othman A.R., Hasan H.A. Contaminants of emerging concern (CECs) in aquaculture effluent: Insight into breeding and rearing activities, alarming impacts, regulations, performance of wastewater treatment unit and future approaches. Chemosphere. 2022;290 doi: 10.1016/j.chemosphere.2021.133319. [DOI] [PubMed] [Google Scholar]
- 2.Aktan Y., Tan S., Icgen B. Characterization of lead-resistant river isolate Enterococcus faecalis and assessment of its multiple metal and antibiotic resistance. Environ. Monit. Assess. 2013;185:5285–5293. doi: 10.1007/s10661-012-2945-x. [DOI] [PubMed] [Google Scholar]
- 3.Alam M.Z., Carpenter-Boggs L., Rahman A., Haque M.M., Miah M.R.U., Moniruzzaman M., Qayum M.A., Abdullah H.M. Water quality and resident perceptions of declining ecosystem services at Shitalakka wetland in Narayanganj city. Sustain. Water Qual. Ecol. 2016 doi: 10.1016/j.swaqe.2017.03.002. [DOI] [Google Scholar]
- 4.Asaduzzaman M., Rousham E., Unicomb L., Islam M.R., Amin M.B., Rahman M., Hossain M.I., Mahmud Z.H., Szegner M., Wood P., Islam M.A. Spatiotemporal distribution of antimicrobial resistant organisms in different water environments in urban and rural settings of Bangladesh. Sci. Total Environ. 2022;831 doi: 10.1016/j.scitotenv.2022.154890. [DOI] [PubMed] [Google Scholar]
- 5.Aslam B., Wang W., Arshad M.I., Khurshid M., Rasool M.H., Nisar M.A., Aslam M.A., Qamar M.U. Antibiotic resistance: a rundown of a global crisis. Infect. Drug Resist. 2018;11:1645–1658. doi: 10.2147/IDR.S173867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin B., Al‐Zahrani A.M.J. The effect of antimicrobial compounds on the gastrointestinal microflora of rainbow trout, Salmo gairdneri Richardson. J. Fish. Biol. 1988;33:1–14. doi: 10.1111/j.1095-8649.1988.tb05444.x. [DOI] [Google Scholar]
- 7.Bartley P.S., Domitrovic T.N., Moretto V.T., Santos C.S., Ponce-Terashima R., Reis M.G., Barbosa L.M., Blanton R.E., Bonomo R.A., Perez F. Antibiotic resistance in enterobacteriaceae from surface waters in Urban Brazil highlights the risks of poor sanitation. Am. J. Trop. Med. Hyg. 2019;100:1369–1377. doi: 10.4269/ajtmh.18-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bou G., Fernández-Olmos A., García C., Sáez-Nieto J.A., Valdezate S. Methods of bacterial identification in the microbiology laboratory. Enferm. Infecc. Microbiol. Clin. 2011;29:601–608. doi: 10.1016/j.eimc.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Capkin E., Terzi E., Altinok I. Occurrence of antibiotic resistance genes in culturable bacteria isolated from Turkish trout farms and their local aquatic environment. Dis. Aquat. Organ. 2015;114:127–137. doi: 10.3354/dao02852. [DOI] [PubMed] [Google Scholar]
- 10.Carbajal-González M.T., Fregeneda-Grandes J.M., González-Palacios C., Aller-Gancedo J.M. Adhesion to brown trout skin mucus, antagonism against cyst adhesion and pathogenicity to rainbow trout of some inhibitory bacteria against Saprolegnia parasitica. Dis. Aquat. Organ. 2013;104:35–44. doi: 10.3354/dao02582. [DOI] [PubMed] [Google Scholar]
- 11.Chavez M.D., Berentsen P.B.M., Oude Lansink A.G.J.M. Creating a typology of tobacco farms according to determinants of diversification in Valle de Lerma (Salta-Argentina) Span. J. Agric. Res. 2010;8:460–471. doi: 10.5424/sjar/2010082-1201. [DOI] [Google Scholar]
- 12.Chen Y.L., Lee C.C., Lin Y.L., Yin K.M., Ho C.L., Liu T. Obtaining long 16S rDNA sequences using multiple primers and its application on dioxin-containing samples. BMC Bioinforma. 2015;16:1–11. doi: 10.1186/1471-2105-16-S18-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chernov T.I., Tkhakakhova A.K., Kutovaya O.V. Assessment of diversity indices for the characterization of the soil prokaryotic community by metagenomic analysis. Eurasian J. Soil Sci. 2015;48(4):410–415. doi: 10.1134/S1064229315040031. [DOI] [Google Scholar]
- 14.Clarke K.R., Somerfield P.J., Gorley R.N. Testing of null hypotheses in exploratory community analyses: similarity profiles and biota-environment linkage. J. Exp. Mar. Biol. Ecol. 2008 doi: 10.1016/j.jembe.2008.07.009. [DOI] [Google Scholar]
- 15.De Angelis G., Giacomo P., Del, Posteraro B., Sanguinetti M., Tumbarello M. Molecular mechanisms, epidemiology, and clinical importance of β-lactam resistance in enterobacteriaceae. Int. J. Mol. Sci. 2020;21:1–22. doi: 10.3390/ijms21145090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Defoirdt T., Sorgeloos P., Bossier P. Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr. Opin. Microbiol. 2011;14:251–258. doi: 10.1016/j.mib.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Deku J.G., Deku John, Gameli, Duedu K.O., Ativi E., Kpene E., Feglo P.K. Burden of fluoroquinolone resistance in clinical isolates of Escherichia coli at the Ho Teaching Hospital, Ghana. Ethiop. J. Health Sci. 2022;32:93–102. doi: 10.4314/ejhs.v32i1. 11 Received: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fadrosh D.W., Bing Ma P.G., Sengamalay N., Ott S., Brotman R.M., Ravel J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome. 2014;2:1–7. doi: 10.1186/2049-2618-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felis E., Kalka J., Sochacki A., Kowalska K., Bajkacz S., Harnisz M., Korzeniewska E. Antimicrobial pharmaceuticals in the aquatic environment - occurrence and environmental implications. Eur. J. Pharmacol. 2020;866 doi: 10.1016/j.ejphar.2019.172813. [DOI] [PubMed] [Google Scholar]
- 20.Feng B.W., Li X.R., Wang J.H., Hu Z.Y., Meng H., Xiang L.Y., Quan Z.X. Bacterial diversity of water and sediment in the Changjiang estuary and coastal area of the East China Sea. FEMS Microbiol. Ecol. 2009;70:236–248. doi: 10.1111/j.1574-6941.2009.00772.x. [DOI] [PubMed] [Google Scholar]
- 21.Fouhy F., Clooney A.G., Stanton C., Claesson M.J., Cotter P.D. 16S rRNA gene sequencing of mock microbial populations-impact of DNA extraction method, primer choice and sequencing platform. BMC Microbiol. 2016;16:1–13. doi: 10.1186/s12866-016-0738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gambino D., Gargano V., Butera G., Sciortino S., Pizzo M., Oliveri G., Cardamone C., Piraino C., Cassata G., Vicari D., Costa A. Food is reservoir of MDR Salmonella: prevalence of ESBLs profiles and resistance genes in strains isolated from food. Microorganisms. 2022;10:780. doi: 10.3390/microorganisms10040780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzáles Mendoza J., Maguiña Vargas C., Gonzáles Ponce F., de M. Antibiotic resistance: a very serious problem. Acta Med. Peru. 2019;36:145–151. doi: 10.35663/amp.2019.362.816. [DOI] [Google Scholar]
- 24.Grenni P., Ancona V., Barra Caracciolo A. Ecological effects of antibiotics on natural ecosystems: a review. Microchem. J. 2018;136:25–39. doi: 10.1016/j.microc.2017.02.006. [DOI] [Google Scholar]
- 25.Halwart, M., Soto, D., Arthur, J.R. (Eds., 2007. Cage aquaculture Regional reviews and global overview, FAO Fisheries Technical Paper.
- 26.Hammer Ø., Harper D., Ryan P. PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001:4. [Google Scholar]
- 27.Hanna N., Purohit M., Diwan V., Chandran S.P., Riggi E., Parashar V., Tamhankar A.J., Lundborg C.S. Monitoring of water quality, antibiotic residues, and antibiotic-resistant Escherichia coli in the kshipra river in india over a 3-year period. Int. J. Environ. Res. Public Health. 2020;17:1–22. doi: 10.3390/ijerph17217706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harta I., Simon B., Vinogradov S., Winkler D. Collembola communities and soil conditions in forest plantations established in an intensively managed agricultural area. J. For. Res. 2021;32:1819–1832. doi: 10.1007/s11676-020-01238-z. [DOI] [Google Scholar]
- 29.Henriksson P.J.G., Rico A., Troell M., Klinger D.H., Buschmann A.H., Saksida S., Chadag M.V., Zhang W. Unpacking factors influencing antimicrobial use in global aquaculture and their implication for management: a review from a systems perspective. Sustain. Sci. 2018;13:1105–1120. doi: 10.1007/s11625-017-0511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernández M., Quijada N.M., Rodríguez-Lázaro D., Eiros J.M. Bioinformatics of next generation sequencing in clinical microbiology diagnosis. Rev. Argent. Microbiol. 2020;52:150–161. doi: 10.1016/j.ram.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Hosokawa T., Ishii Y., Nikoh N., Fujie M., Satoh N., Fukatsu T. Obligate bacterial mutualists evolving from environmental bacteria in natural insect populations. Nat. Microbiol. 2016;1:1–7. doi: 10.1038/nmicrobiol.2015.11. [DOI] [PubMed] [Google Scholar]
- 32.Hu A., Ju F., Hou L., Li J., Yang X., Wang H., Mulla S.I., Sun Q., Bürgmann H., Yu C.P. Strong impact of anthropogenic contamination on the co-occurrence patterns of a riverine microbial community. Environ. Microbiol. 2017;19:4993–5009. doi: 10.1111/1462-2920.13942. [DOI] [PubMed] [Google Scholar]
- 33.Ivanisevic J., Benton H.P., Rinehart D., Epstein A., Kurczy M.E., Boska M.D., Gendelman H.E., Siuzdak G. An interactive cluster heat map to visualize and explore multidimensional metabolomic data. Metabolomics. 2015;11(4):1029–1034. doi: 10.1007/s11306-014-0759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamruzzaman M., Shoma S., Naymul Bari S.M.N., Ginn A.N., Wiklendt A.M., Partridge S.R., Faruque S.M., Iredell J.R. Genetic diversity and antibiotic resistance in Escherichia coli from environmental surface water in Dhaka City, Bangladesh. Diagn. Microbiol. Infect. Dis. 2013;76:222–226. doi: 10.1016/j.diagmicrobio.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Kiehlbauch J.A., Hannett G.E., Salfinger M., Archinal W., Monserrat C., Carlyn C. Use of the National Committee for Clinical Laboratory Standards guidelines for disk diffusion susceptibility testing in New York State Laboratories. J. Clin. Microbiol. 2000;38:3341–3348. doi: 10.1128/jcm.38.9.3341-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolde, R., 2019. Package ‘pheatmap’: pretty heat map 1–8.
- 37.Kovalakova P., Cizmas L., McDonald T.J., Marsalek B., Feng M., Sharma V.K. Occurrence and toxicity of antibiotics in the aquatic environment: a review. Chemosphere. 2020;251 doi: 10.1016/j.chemosphere.2020.126351. [DOI] [PubMed] [Google Scholar]
- 38.Kozich J.J., Westcott S.L., Baxter N.T., Highlander S.K., Schloss P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the miseq illumina sequencing platform. Appl. Environ. Microbiol. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar M., Jaiswal S., Sodhi K.K., Shree P., Singh D.K., Agrawal P.K., Shukla P. Antibiotics bioremediation: perspectives on its ecotoxicity and resistance. Environ. Int. 2019;124:448–461. doi: 10.1016/j.envint.2018.12.065. [DOI] [PubMed] [Google Scholar]
- 40.Lal Gupta C., Kumar Tiwari R., Cytryn E. Platforms for elucidating antibiotic resistance in single genomes and complex metagenomes. Environ. Int. 2020;138 doi: 10.1016/j.envint.2020.105667. [DOI] [PubMed] [Google Scholar]
- 41.Lastauskienė E., Valskys V., Stankevičiūtė J., Kalcienė V., Gėgžna V., Kavoliūnas J., Ružauskas M., Armalytė J. The impact of intensive fish farming on pond sediment microbiome and antibiotic resistance gene composition. Front. Vet. Sci. 2021;8:1–12. doi: 10.3389/fvets.2021.673756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazăr V., Gheorghe I., Curutiu C., Savin I., Marinescu F., Cristea V.C., Dobre D., Popa G.L., Chifiriuc M.C., Popa M.I. Antibiotic resistance profiles in cultivable microbiota isolated from some romanian natural fishery lakes included in Natura 2000 network. BMC Vet. Res. 2021;17:1–11. doi: 10.1186/s12917-021-02770-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X.Z., Plésiat P., Nikaido H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol. Rev. 2015;28:337–418. doi: 10.1128/CMR.00117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long Y., Jiang J., Hu X., Hu J., Ren C., Zhou S. The response of microbial community structure and sediment properties to anthropogenic activities in Caohai wetland sediments. Ecotoxicol. Environ. Saf. 2021;211 doi: 10.1016/j.ecoenv.2021.111936. [DOI] [PubMed] [Google Scholar]
- 45.Lulijwa R., Rupia E.J., Alfaro A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: a review of the top 15 major producers. Rev. Aquac. 2020;12:640–663. doi: 10.1111/raq.12344. [DOI] [Google Scholar]
- 46.Marcos-Carbajal P., Galarza-Perez M., Huancahuire-Vega S., Otiniano-Trujillo M., Soto-Pastrana J. Comparison of Escherichia coli antibiotic-resistance profiles and incidence of betalactamase phenotypes in three private health facilities in Peru. Biomedica. 2020;40:139–147. doi: 10.7705/biomedica.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mariano M., Huaman P., Mayta E., Montoya H., Chanco M.C. Contamination produced by intensive fish farming in Andean lagoons of Junin, Peru. Rev. Peru. Biol. 2011;17:137–140. doi: 10.15381/rpb.v17i1.63. [DOI] [Google Scholar]
- 48.Mariano M., Huaman P., Mayta E., Montoya H., Chanco M.C. Contaminación producida por piscicultura intensiva en lagunas andinas de Junín, Perú. Rev. Peru. Biol. 2011;17:137–140. doi: 10.15381/rpb.v17i1.63. [DOI] [Google Scholar]
- 49.Miller D.N., Bryant J.E., Madsen E.L., Ghiorse W.C. Evaluation and optimization of DNA extraction and purification procedures for soil and sediment samples. Appl. Environ. Microbiol. 1999;65:4715–4724. doi: 10.1128/aem.65.11.4715-4724.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller R.A., Walker R.D., Carson J., Coles M., Coyne R., Dalsgaard I., Gieseker C., Hsu H.M., Mathers J.J., Papapetropoulou M., Petty B., Teitzel C., Reimschuessel R. Standardization of a broth microdilution susceptibility testing method to determine minimum inhibitory concentrations of aquatic bacteria. Dis. Aquat. Organ. 2005;64:211–222. doi: 10.3354/dao064211. [DOI] [PubMed] [Google Scholar]
- 51.Mills M.C., Lee J. The threat of carbapenem-resistant bacteria in the environment: evidence of widespread contamination of reservoirs at a global scale. Environ. Pollut. 2019;255 doi: 10.1016/j.envpol.2019.113143. [DOI] [PubMed] [Google Scholar]
- 52.Moretto V.T., Cordeiro S.M., Bartley P.S., Silva L.K., Ponce-Terashima R., Reis M.G., Blanton R.E., Barbosa L.M. Antimicrobial-resistant enterobacteria in surface waters with fecal contamination from urban and rural communities. Rev. Soc. Bras. Med. Trop. 2021;54:1–6. doi: 10.1590/0037-8682-0724-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mulya H., Santosa Y., Hilwan I. Comparison of four species diversity indices in mangrove community. Biodiversitas. 2021;22(9):3648–3655. doi: 10.13057/BIODIV/D220906. [DOI] [Google Scholar]
- 54.Muziasari W.I., Pitkänen L.K., Sørum H., Stedtfeld R.D., Tiedje J.M., Virta M. The resistome of farmed fish feces contributes to the enrichment of antibiotic resistance genes in sediments below baltic sea fish farms. Front. Microbiol. 2017;7:1–10. doi: 10.3389/fmicb.2016.02137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muziasari W.I., Pärnänen K., Johnson T.A., Lyra C., Karkman A., Stedtfeld R.D., Tamminen M., Tiedje J.M., Virta M. Aquaculture changes the profile of antibiotic resistance and mobile genetic element associated genes in Baltic Sea sediments. FEMS Microbiol. Ecol. 2016;92:1–7. doi: 10.1093/femsec/fiw052. [DOI] [PubMed] [Google Scholar]
- 56.Najar I.N., Sherpa M.T., Das Sayak, Das Saurav, Thakur N. Diversity analysis and metagenomic insights into the antibiotic resistance and metal resistances among Himalayan hot spring bacteriobiome-insinuating inherent environmental baseline levels of antibiotic and metal tolerance. J. Glob. Antimicrob. Resist. 2020;21:342–352. doi: 10.1016/j.jgar.2020.03.026. [DOI] [PubMed] [Google Scholar]
- 57.Navarro A., Sanseverino I., Cappelli F., Lahm A., Niegowska M., Fabbri M., Paracchini V., Petrillo M., Skejo H., Valsecchi S., Pedraccini R., Guglielmetti S., Frattini S., Villani G., Lettieri T. Study of antibiotic resistance in freshwater ecosystems with low anthropogenic impact. Sci. Total Environ. 2023;857 doi: 10.1016/j.scitotenv.2022.159378. [DOI] [PubMed] [Google Scholar]
- 58.Nishide Y., Onodera N.T., Tanahashi M., Moriyama M., Fukatsu T., Koga R. Aseptic rearing procedure for the stinkbug Plautia stali (Hemiptera: Pentatomidae) by sterilizing food-derived bacterial contaminants. Appl. Entomol. Zool. 2017;52:407–415. doi: 10.1007/s13355-017-0495-y. [DOI] [Google Scholar]
- 59.Nurk S., Meleshko D., Korobeynikov A., Pevzner P.A. MetaSPAdes: a new versatile metagenomic assembler. Genome Res. 2017;27:824–834. doi: 10.1101/gr.213959.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olaniran A.O., Naicker K., Pillay B. Antibiotic resistance profiles of Escherichia coli isolates from river sources in Durban, South Africa. World J. Microbiol. Biotechnol. 2009;25:1743–1749. doi: 10.1007/s11274-009-0071-x. [DOI] [Google Scholar]
- 61.Ouchar Mahamat O., Kempf M., Lounnas M., Tidjani A., Hide M., Benavides J.A., Carrière C., Bañuls A.L., Jean-Pierre H., Ouedraogo A.S., Dumont Y., Godreuil S. Epidemiology and prevalence of extended-spectrum β-lactamase- and carbapenemase-producing Enterobacteriaceae in humans, animals and the environment in West and Central Africa. Int. J. Antimicrob. Agents. 2021;57 doi: 10.1016/j.ijantimicag.2020.106203. [DOI] [PubMed] [Google Scholar]
- 62.Perron G.G., Whyte L., Turnbaugh P.J., Goordial J., Hanage W.P., Dantas G., Desai M.M. Functional characterization of bacteria isolated from ancient arctic soil exposes diverse resistance mechanisms to modern antibiotics. PLoS One. 2015;10:1–19. doi: 10.1371/journal.pone.0069533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Preena P.G., Swaminathan T.R., Kumar V.J.R., Singh I.S.B. Antimicrobial resistance in aquaculture: a crisis for concern. Biologia. 2020;75:1497–1517. doi: 10.2478/s11756-020-00456-4. [DOI] [Google Scholar]
- 64.PRODUCE, 2020. Supreme Decree that amends the Regulations of the General Law of Aquaculture, approved by Supreme Decree N° 003–2016-PRODUCE, El Peruano Official Newspaper.
- 65.Qu J., Wu Y., Liu Y., Cui Y., Zhao M., Zhu H., Zhang Q. Journal of Physics: Conference Series. IOP Publishing Ltd; 2021. Metagenomics reveals the taxonomy and resistance mechanism of antibiotic resistance genes in bacterial communities of an aquaculture pond. [DOI] [Google Scholar]
- 66.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013;41:590–596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rivera-Jacinto M., Rodríguez-Ulloa C., Huayán-Dávila G., Mercado-Martínez P. Beta-lactam susceptibility and extended-spectrum beta-lactamase (ESBL) resistance in Enterobacteriaceae isolated from environmental reservoirs of a general hospital in Cajamarca. Peru. Rev. Med. Hered. 2011;22:69–75. doi: 10.20453/rmh.v22i2.1105. [DOI] [Google Scholar]
- 68.Salipante S.J., Kawashima T., Rosenthal C., Hoogestraat D.R., Cummings L.A., Sengupta D.J., Harkins T.T., Cookson B.T., Hoffman N.G. Performance comparison of Illumina and Ion Torrent next-generation sequencing platforms for 16S rRNA-based bacterial community profiling. Appl. Environ. Microbiol. 2014;80:7583–7591. doi: 10.1128/AEM.02206-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santos L., Ramos F. Antimicrobial resistance in aquaculture: current knowledge and alternatives to tackle the problem. Int. J. Antimicrob. Agents. 2018;52:135–143. doi: 10.1016/j.ijantimicag.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 70.Shimizu A., Takada H., Koike T., Takeshita A., Saha M., Rinawati, Nakada N., Murata A., Suzuki T., Suzuki S., Chiem N.H., Tuyen B.C., Viet P.H., Siringan M.A., Kwan C., Zakaria M.P., Reungsang A. Ubiquitous occurrence of sulfonamides in tropical Asian waters. Sci. Total Environ. 2013;452–453:108–115. doi: 10.1016/j.scitotenv.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 71.Singh R., Singh A.P., Kumar S., Giri B.S., Kim K.H. Antibiotic resistance in major rivers in the world: a systematic review on occurrence, emergence, and management strategies. J. Clean. Prod. 2019;234:1484–1505. doi: 10.1016/j.jclepro.2019.06.243. [DOI] [Google Scholar]
- 72.Suzuki Y., Nazareno P.J., Nakano R., Mondoy M., Nakano A., Bugayong M.P., Bilar J., Perez M.V., Medina E.J., Saito-Obata M., Saito M., Nakashima K., Oshitani H., Yano H. Environmental presence and genetic characteristics of carbapenemase-producing enterobacteriaceae from hospital sewage and river water in the philippines. Appl. Environ. Microbiol. 2020;86:1–10. doi: 10.1128/AEM.01906-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tafoukt R., Touati A., Leangapichart T., Bakour S., Rolain J.M. Characterization of OXA-48-like-producing Enterobacteriaceae isolated from river water in Algeria. Water Res. 2017;120:185–189. doi: 10.1016/j.watres.2017.04.073. [DOI] [PubMed] [Google Scholar]
- 74.Thomas C.M., Nielsen K.M. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 2005;3:711–721. doi: 10.1038/nrmicro1234. [DOI] [PubMed] [Google Scholar]
- 75.Thornton C.N., Tanner W.D., Vanderslice J.A., Brazelton W.J. Localized effect of treated wastewater effluent on the resistome of an urban watershed. Gigascience. 2020;9:1–13. doi: 10.1093/gigascience/giaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Goethem M.W., Pierneef R., Bezuidt O.K.I., Van De Peer Y., Cowan D.A., Makhalanyane T.P. A reservoir of “historical” antibiotic resistance genes in remote pristine Antarctic soils. Microbiome. 2018;6:1–12. doi: 10.1186/s40168-018-0424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vital P.G., Zara E.S., Paraoan C.E.M., Dimasupil M.A.Z., Abello J.J.M., Santos I.T.G., Rivera W.L. Antibiotic resistance and extended-spectrum beta-lactamase production of Escherichia coli isolated from irrigationwaters in selected urban farms in Metro Manila, Philippines. Water. 2018;10:1–11. doi: 10.3390/w10050548. [DOI] [Google Scholar]
- 78.Wang J., Ben W., Yang M., Zhang Y., Qiang Z. Dissemination of veterinary antibiotics and corresponding resistance genes from a concentrated swine feedlot along the waste treatment paths. Environ. Int. 2016;92–93:317–323. doi: 10.1016/j.envint.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 79.Watts J.E.M., Schreier H.J., Lanska L., Hale M.S. The rising tide of antimicrobial resistance in aquaculture: sources, sinks and solutions. Mar. Drugs. 2017;15:1–16. doi: 10.3390/md15060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.WHO . Global Report on Surveillance. World Health Organization; Geneva: 2014. Antimicrobial Resistance. [Google Scholar]
- 81.Wilson H., Török M.E. Extended-spectrum β-lactamase-producing and carbapenemase-producing Enterobacteriaceae. Microb. Genom. 2018:4. doi: 10.1099/mgen.0.000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wood D.E., Lu J., Langmead B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019;20:1–13. doi: 10.1186/s13059-019-1891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiao K.Q., Li B., Ma L., Bao P., Zhou X., Zhang T., Zhu Y.G. Metagenomic profiles of antibiotic resistance genes in paddy soils from South China. FEMS Microbiol. Ecol. 2016;92:3–8. doi: 10.1093/femsec/fiw023. [DOI] [PubMed] [Google Scholar]
- 84.Xu Y., Wang X., Tan L., Mao D., Luo Y. Metal impacts on the persistence and proliferation of β-lactam resistance genes in Xiangjiang River, China. Environ. Sci. Pollut. Res. 2019;26:25208–25217. doi: 10.1007/s11356-019-05698-7. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Ruiling, Pei J., Zhang Ruijie, Wang S., Zeng W., Huang D., Wang Yi, Zhang Y., Wang Yinghui, Yu K. Occurrence and distribution of antibiotics in mariculture farms, estuaries and the coast of the Beibu Gulf, China: bioconcentration and diet safety of seafood. Ecotoxicol. Environ. Saf. 2018;154:27–35. doi: 10.1016/j.ecoenv.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 86.Zhao W., Wang B., Yu G. Antibiotic resistance genes in China: occurrence, risk, and correlation among different parameters. Environ. Sci. Pollut. Res. 2018;25:21467–21482. doi: 10.1007/s11356-018-2507-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Data Availability Statement
Data will be made available on request.