Abstract
Although cannabis use during pregnancy is increasing widely, the effects of cannabis on developmental trajectories, such as whether its effects during pregnancy remain the same between time points or gradually increase, are unclear. This study aimed to examine whether cannabis use during pregnancy affects the process of change in cognition and brain volume. Data from two-time points measured longitudinally were analyzed. We used data from the Adolescent Brain and Cognitive Development Study. Participants included 11,876 children aged 9–11 years participated at baseline, and 10,414 participated at 2-year follow-up from 22 sites across the United States. We explored the associations between prenatal cannabis exposure and cognitive abilities and brain volumes developmental trajectories. Among 11,530 children with valid data for prenatal cannabis exposure, 10,833 had no prenatal cannabis use, and 697 had cannabis use during their pregnancy. There was a significant interaction between time points and cannabis use during pregnancy on visuo-perceptual processing ability (b = −0.019, p = .009) and intracranial volumes (b = −6338.309, p = .009). We found that the effects of exposure to cannabis during pregnancy are not uniform at all times and may gradually become more apparent and magnified as development progresses.
Abbreviation: THC, Δ(9)-tetrahydrocannabinol; EF, Executive function; LMT, Little man test; ICV, Intracranial volume; GMV, Gray matter volume; WMV, White matter volume
Keywords: Prenatal cannabis exposure, Neurodevelopment, Cognitive development, Longitudinal data, Marijuana
1. Introduction
As of September 2022, 37 states in the U.S. have legalized the medical use of cannabis, including 19 states that have legalized recreational use. A longitudinal study of cannabis use in the U.S. from 2002 to 2014 found that rates of use in the past year and daily use increased in all age groups except among 12- to 17-year-olds (Azofeifa et al., 2016). Similar results were observed in a longitudinal study from 2008 to 2016 (Cerdá et al., 2020). Additionally, an increasing number of pregnant women are using cannabis during pregnancy. The rate of cannabis use in the first, second, and third trimesters of pregnancy increased between 2002-2003 and 2016–2017 (Volkow et al., 2019). The idea that cannabis is a safe herb that improves nausea has induced the use of cannabis during pregnancy (Mark et al., 2017). Among pregnant women, 70% reported that cannabis was harmful to pregnancy and childbirth, and only 26% of pregnant women who used cannabis were aware of its harmfulness (Mark et al., 2017). Cannabis use during pregnancy is associated with the risk of birth and the physical development of the fetus. Controlling for tobacco and other drug use during pregnancy, cannabis use during pregnancy is associated with combined neonatal morbidity risk, low birth weight, and lower head circumference size (El Marroun et al., 2009, Metz et al., 2017). It has been suggested that maternal cannabis use during pregnancy may also be associated with long-term delays in children. Clarifying the effects of maternal cannabis use during pregnancy on children can help prevent substance abuse during pregnancy and protect the health of the mother and her child.
One of the earliest prospective cohorts, the Ottawa Prenatal Prospective Study, has been underway since 1978; this study has tested cognitive ability multiple times from infancy to adulthood. This study found that cannabis use during pregnancy was longitudinally associated with deficits in motor skills at age 3; vocabulary, memory, and verbal skills at age 4; visuo-spatial processing and cognitive stability scores at age 13; and executive function at ages 17–22 (Fried and Smith, 2001, Fried and Watkinson, 1988, Fried and Watkinson, 1990, Fried et al., 1992, Fried et al., 2003, Smith et al., 2016). The association of prenatal cannabis exposure with memory and verbal reasoning in preschool was found in a prospective cohort as well, the Maternal Health Practices and Child Development cohort, but was not replicated in another cohort, the Generation R cohort (Badowski and Smith, 2020). The Ottawa Prenatal Prospective Study found no association with visuo-spatial abilities from ages 5–9 (Fried et al., 1992, O’connell and Fried, 1991), but did identify an association with performance in tasks requiring visuo-perceptual and visuo-spatial abilities administered between ages 9–16 (Fried et al., 1998, Fried et al., 2003). For executive function, there was no significant association with inhibitory task performance at ages 13–16 or 18–22 (Fried et al., 2003, Smith et al., 2016), but functional brain imaging during the task at ages 18–22 showed altered activity in the posterior and superior frontal regions (Smith et al., 2016). Meanwhile, crystallized intelligence (IQ includes crystallized intelligence) was measured and was found not to be associated with cannabis exposure during pregnancy from 6 to 16 years of age (Fried et al., 1998, Fried et al., 2003, O’connell and Fried, 1991). Thus, existing results on exposure to cannabis during pregnancy are not always consistent, and several studies report no significant results. Almost all existing studies on the effects of cannabis use during pregnancy were conducted decades ago. The average Δ(9)-tetrahydrocannabinol (THC) content in cannabis products has increased from 3.4% in 1993 to 14% in 2019 (ElSohly et al., 2021, Mehmedic et al., 2010). Therefore, studies using more recent child development data are needed.
Scholars have suggested that structural brain development lies behind the development of cognitive function (Casey et al., 2000, Casey et al., 2005, Erus et al., 2015) and that exposure to cannabis during pregnancy may affect the development of the brain. The main component of cannabis, THC, is rapidly absorbed into the bloodstream and spreads throughout the body. THC interacts with the endocannabinoid system (ECS). The ECS is composed of cannabinoid receptors and endogenous ligands, both of which are involved in the regulation of neurogenesis, glial formation, synapse formation, and pruning and angiogenesis; thus, it has a key role in any stage of neural development (Bara et al., 2021, Maccarrone et al., 2014). THC passes through the placenta, enters the fetus’s bloodstream, and spreads to each tissue, including the brain (Daniel J. Corsi et al., 2020; Daniel J. Corsi, Murphy, and Cook, 2021). When THC was administered to pregnant rats in varying doses and frequency of administration, the plasma THC concentration in their fetuses was proportional to the amount of THC administered to the mothers and was particularly high in the group that received repeated doses (Hutchings et al., 1989). Prenatal THC exposure during fetal development is associated with decreased expression of Cnr1 mRNA, which is involved in the coding of CB1R, one of the cannabinoid receptors (Bara et al., 2021). In middle childhood, gray matter volume decreases, and white matter volume increases. Changes in gray matter volume include axon sprouting, dendritic branching, and synapse formation and pruning, neurogenesis, and angiogenesis; changes in white matter volume include changes in fiber organization, myelination of unmyelinated axons, changes in myelin thickness and morphology, astrocyte morphology or number, and angiogenesis (Zatorre et al., 2012). The above-mentioned finding suggests that exposure to cannabis during pregnancy may modulate the ECS, inhibit neurodevelopment (e.g., synaptogenesis and pruning), and affect brain volumes and phenotypic cognitive and behavioral development. Previous studies showed that mean head circumference at ages 9–12 was smaller in the group whose mothers smoked cannabis during pregnancy, even after controlling for tobacco and alcohol use (Fried et al., 1999). The purpose of this study was to examine the effects of changes in ECS exposure to cannabis during pregnancy on the developmental process by using the amount of change in gray matter (GMV), white matter (WMV), and intracranial volumes (ICV) between time points as dependent variables, along with cognitive task performance.
Recently, the Adolescent Brain Cognitive Development (ABCD) Study longitudinally measured the psychological and neurobiological development of more than 10,000 children in the U.S. from preadolescence to early adulthood. Using baseline data from this cohort, the study revealed that maternal cannabis use after knowledge of pregnancy was associated with an increased propensity for behavioral problems among children when controlling for potential covariates (Cioffredi et al., 2022, Fine et al., 2019, Paul et al., 2021). In contrast, the baseline data for this cohort showed no significant association between cannabis use during pregnancy and cognitive task performance after controlling for covariates (Cioffredi et al., 2022, Paul et al., 2021). Previous studies, including the ABCD Study, have only examined the association between cannabis use during pregnancy and cognitive task performance at a single time point and have not yet examined whether the amount of change between time points is affected by cannabis use during pregnancy. In other words, it is still unclear whether the effects of cannabis use during pregnancy persist, increase, or decrease over time. To uncover developmental differences, it is necessary to examine longitudinal trajectories (Haller et al., 2018, Reichenberg et al., 2010). In the present study, tasks measuring visuo-spatial ability, executive function, and crystallized intelligence were the dependent variables among the cognitive tasks measured in the ABCD Study.
The present study examined the association between exposure to cannabis during pregnancy and cognitive and brain development using data from two time points in the ABCD Study. The mean age at baseline in the ABCD Study was 9.91 years (SD = 0.62), and cognitive tasks and brain volumes were measured again at the 2-year follow-up (mean age = 12.00 years, SD = 0.66), an average of 2.09 years (SD = 0.22) later. Using these data, we aimed to (a) investigate the association between prenatal cannabis exposure and cognitive function and its intra-individual changes and (b) examine whether cannabis use during pregnancy is associated with the amount of change in brain volumes as one of the indicators reflecting the mediating role of the ECS on cellular processes in the developmental brain. A previous ABCD Study examined the association between exposure to cannabis during pregnancy and ICV, WMV, and GMV at the baseline; however, it did not find any significant associations based on the covariates (Paul et al., 2021). In the present study, we used the same index as this previous study to examine the change in volume up to the second time point.
2. Methods
2.1. Participants
Full recruitment details of the ABCD Study can be found in studies by Garavan et al. (2018) and Karcher and Barch (2021). Initially, the ABCD Study recruited a baseline sample of adolescents (N = 11,876; Mean age = 9.91 (Min = 8.91, Max = 11.08) years old; 47.8% female) and their parents or guardians. The cognitive tasks and MRI data were completed at two time points, at baseline and 2 year follow up (N = 10,414; Mean age = 12.00 (Min = 10.58, Max = 14.00) old 47.6% female) at the ABCD Study’s Data Release 4.0 (http://dx.doi.org/10.15154/1523041). The sample’s demographic characteristics were representative of the U.S., and data were measured at 22 nationally distributed research sites. Table 1 shows this sample characteristic. The institutional review board approval was obtained for each site before data collection. The Research Ethics Committee of the University of Fukui approved the analysis of the data (FU-20210067). All parents provided written informed consent, and all children provided assent. Demographic, clinical, and neurocognitive data were accessed from the National Institutes of Mental Health Data Archive (see Acknowledgments).
Table 1.
The demographic characteristics of the sample.
| Characteristic | Missing, N = 346 | No exposure, N = 10,833 | Pregnancy exposure, N = 697 |
|---|---|---|---|
| Age (year), Mean (SD) | 9.91 (0.60) | 9.92 (0.62) | 9.87 (0.64) |
| Interval (year), Mean (SD) | 2.10 (0.24) | 2.08 (0.22) | 2.09 (0.23) |
| Household income (USD), n (%) | |||
| ≦$49 999 | 103 (33) | 2762 (28) | 358 (56) |
| $50 000-$74 999 | 36 (11) | 1368 (14) | 95 (15) |
| $75 000-$99 999 | 53 (17) | 1446 (15) | 73 (12) |
| $100 000-$199 999 | 95 (30) | 3134 (32) | 85 (13) |
| ≧ $200 000 | 28 (8.9) | 1199 (12) | 23 (3.6) |
| Education (years), Mean (SD) | 14.38 (2.73) | 15.27 (2.62) | 14.06 (2.18) |
| Maternal age at birth (year), Mean (SD) | 27 (8) | 30 (6) | 25 (6) |
| Sex, n (%) | |||
| Female | 173 (50) | 5673 (52) | 350 (50) |
| Male | 172 (50) | 5160 (48) | 347 (50) |
| Birth weight (lbs), Mean (SD) | 6.69 (1.50) | 7.02 (1.46) | 6.96 (1.43) |
| Race | |||
| White, n (%) | 188 (54) | 8203 (76) | 413 (59) |
| Black, n (%) | 124 (36) | 2093 (19) | 301 (43) |
| Native American, n (%) | 24 (6.9) | 343 (3.2) | 39 (5.6) |
| Pacific Islander, n (%) | 3 (0.9) | 68 (0.6) | 8 (1.1) |
| Asian, n (%) | 40 (12) | 691 (6.4) | 20 (2.9) |
| Other, n (%) | 31 (9.0) | 722 (6.7) | 47 (6.7) |
| Ethnicity (Hispanic), n (%) | 64 (19) | 2215 (21) | 132 (19) |
2.2. Measures
Details of measures used in this study are described in Table S1.
2.2.1. Assessments of Prenatal Cannabis Exposure
The Developmental History Questionnaire was used to retrospectively assess prenatal exposure to cannabis through parents’ self-report (Kessler et al., 2009b, Kessler et al., 2009a, Merikangas et al., 2009). Categorical questions, “Before the biological mother/you found out she was pregnant, but while she/you might have been pregnant with this child, did she/you use cannabis?” and “Once you/biological mother knew you/she were pregnant, were you/biological mother using cannabis?” (0 = No, 1 = Yes, 999 = Don’t know) were asked. In total, 697 participants used cannabis at some point during their pregnancy. Of these, 452 participants only used cannabis before they discovered they were pregnant, 235 participants used cannabis both before and after they discovered they were pregnant, and ten participants only used cannabis after they discovered they were pregnant. In this study, parents who answered 1 (Yes) to either question were considered to be in the group using cannabis during pregnancy (dummy code = 1), parents who answered 0 (No) to both questions were considered to be in the non-using cannabis group, and parents who answered other than 0 or 1 to either question were treated as missing values. We created a pre (0/1) variable to compare participants who used cannabis before learning they were pregnant (N = 687) to those who did not use cannabis during that period, and a post (0/1) variable to compare participants who used cannabis after learning they were pregnant (N = 245) to those who did not. Pre and post are not mutually exclusive.
2.2.2. Cognitive ability
As in the previous study (Paul et al., 2021), the cognitive tasks included in the NIH Toolbox, which were performed using an iPad-based program, were included in the analysis. The NIH toolbox has three cognitive composite scores (total, fluid, crystallized), and although the total composite was the dependent variable in the previous study (Paul et al., 2021), not all tasks were performed at Time 2, and among the composite scores, crystallized composite score was only available at both baseline and Time 2. The crystallized composite was comprised of the picture vocabulary and oral reading task. In another previous study (Romer and Pizzagalli, 2021), the flanker and pattern comparison processing speed tests were combined to create a variable reflecting executive function (EF). In this study, both scores were z-scored and averaged to obtain a measure of EF.
In addition to the set of tasks in the NIH toolbox, the Little Man Test [LMT; Acker and Acker (1982)] was incorporated into the task battery in the ABCD Study measuring visuo-spatial processing abilities requiring mental rotation. This task performance quality is particularly vulnerable to alcohol and drug use (Luciana et al., 2018). The performance of LMT was defined by the index of efficiency, which is the percentage of correct answers divided by the average reaction time taken to obtain the correct answer.
2.2.3. Brain Imaging
The ABCD imaging protocol uses three 3 T scanners (Siemens Prisma, General Electric 750, and Philips) with multi-channel coils capable of multi-band echo-planar imaging (EPI). Images were acquired axially. For cortical and subcortical segmentation of the brain and the acquisition of each volume, a 3D T1-weighted magnetization preparation fast acquisition gradient echo scan was obtained (for more detailed parameters of T1-weighted image acquisition, please refer to the study by Casey et al., 2018).
All imaging data were preprocessed by the ABCD data team using a standardized processing pipeline Hagler et al. (2019). Of the 11,760 participants with baseline brain imaging data, 358 did not pass the FreeSurfer Quality Control and were excluded from the analysis. Of the 7827 participants with brain imaging data from the 2-year follow-up, 132 did not pass the Quality Control and were excluded. Total intracranial volume (ICV), total gray matter volume (GMV), and total white matter volume (WMV) were analyzed. Total GMVs were calculated by summing cortical, subcortical, and cerebellar volumes (https://surfer.nmr.mgh.harvard.edu/fswiki/MorphometryStats, Table S1). A similar calculation was applied to the WMVs. For ICV, the values provided in the ABCD Study were used (Table S1). The brainstem was not included in the totals because its volume varies depending on the field of view (https://surfer.nmr.mgh.harvard.edu/fswiki/MorphometryStats). In order to examine the relationship between cannabis use and the development of brain structure metrics other than volume, we also analyzed the three brain metrics of cortical thickness, cortical sulcus depth, and cortical area for the whole brain as dependent variables.
2.2.4. Covariates
Parents/guardians reported their child’s sex assigned at birth, age (in months), race,1 ethnicity, birth weight,2 maternal education and family income, and family history of psychopathology (depression; mania; issues with work, fights, or the police; problems with visions of others spying/plotting; alcohol problems; drug problems; nervous breakdowns), tobacco, alcohol, and vitamin use during pregnancy, maternal age at birth, whether the pregnancy was planned or not, the number of weeks when the pregnancy was detected, twin or triplet status, and the interval between time points. Children were asked whether they had ever consumed tobacco and alcohol. The following covariates were dummy coded by 0 or 1: race (White, Black, Pacific Islander, Native American, Asian, and Other Race), ethnicity (Hispanic/Latino/Latina or not), first-degree familial history of psychopathology, prenatal exposure to tobacco or alcohol, unplanned pregnancy, prenatal vitamin use, whether the child had tried alcohol or tobacco, child sex, and twin or triplet status. Annual household income was treated as a 5-level variable (1 = less than $49,999; 2 = $50,000-$74,999; 3 = $75,000-$99,999, 4 = $100,000-$199,999, 5 = $200,000 or more). The following covariates were included as continuous variables: birth weight, maternal age at birth, gestational age when pregnancy was discovered (weeks), child age, maternal educational level, and the interval between time points. In analyses that did not distinguish cannabis use during pregnancy before and after the discovery of pregnancy, alcohol and tobacco use during pregnancy were also included as “alcohol (or tobacco) use during pregnancy” variables, without distinguishing between before and after the knowledge of pregnancy. In the analysis distinguishing cannabis use during pregnancy before and after the knowledge of pregnancy, separate variables were created for alcohol and tobacco use during pregnancy before and after the knowledge of pregnancy and entered into the model. ICV was included as a covariate in models with GMV and WMV as outcomes, as in previous research (Paul et al., 2021). The above covariates were selected based on previous studies that examined the effects of cannabis exposure during pregnancy in the ABCD Study (Fine et al., 2019, Paul et al., 2021).
2.3. Statistical analyses
For all dependent variables, outliers were winsorized at 3 SD from the mean at each time point. For continuous covariates, outliers were also winsorized at 3 SD from the mean. A linear mixed-effect model was adapted to test the associations between prenatal cannabis exposure and childhood cognitive abilities and brain volumes. The categorical variable of prenatal cannabis exposure and measurement timing (baseline and the 2-year follow-up) were modeled as fixed effects. Individuals, siblings, and recruitment sites (or MRI scanner) were modeled as random intercepts and separately fed into the model. An interaction term between measurement timing and cannabis use during pregnancy was created and entered into the models. When the interaction was significant, a simple slope analysis was used to examine the extent to which the effect of measurement timing differed for each group. In the simple slope analysis, fixed effects were included as in the original model, except for the moderating variable, cannabis use during pregnancy. We also reported the results of the model, including the covariates mentioned above. Among the nine p-values for the independent variables of interest (time, cannabis use, time x cannabis use) for each domain (three cognitive tests and three brain volumes), p-values were corrected by the Benjamini-Hochberg correction to reduce the false discovery rate at the.05 level. All analyses were implemented using R 4.2.1 (R Core Team, 2022).
3. Results
3.1. Prenatal cannabis exposure and cognitive abilities
3.1.1. Figures
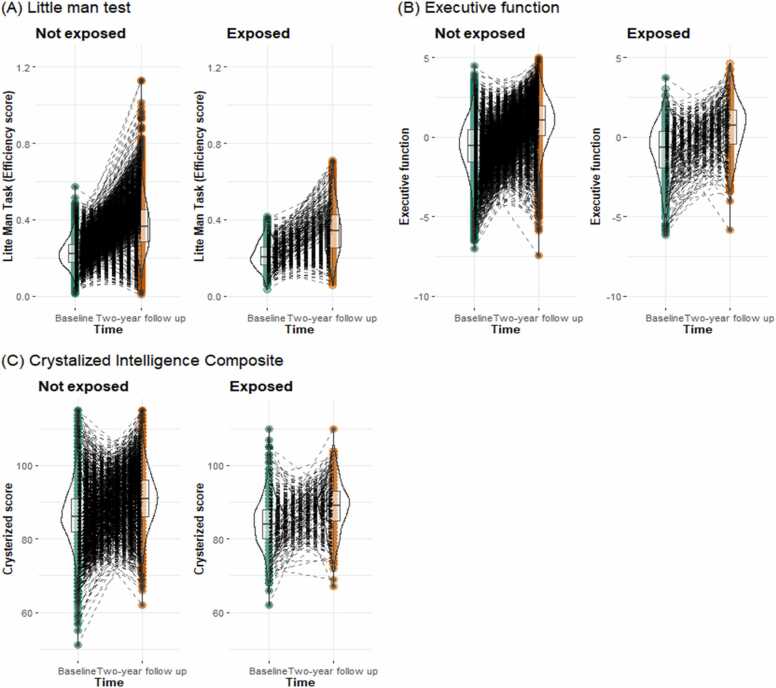
The results of the independent variables of interest in each model are shown in Fig. 1, Table 2, and Table S2-S4. The main effect of time was significant in each model even after controlling covariates (LMT: b = 0.151, 95%CI = [0.149, 0.153], pFDR =.004; EF: b = 1.573, 95%CI = [1.540, 1.605], pFDR <.001; crystallized: b = 4.154, 95%CI = [4.034, 4.273], pFDR <.001). Prenatal cannabis exposure was associated with lower cognitive abilities, but the effect was not significant after including covariates. The interaction of cannabis use during pregnancy and time points on the performance of the LMT was also significant after including covariates (b = −0.019, 95%CI = [−0.033, −0.005], pFDR =.021; Table 2). As a further test of this significant interaction, a simple slope analysis was performed with cannabis use during pregnancy as the moderating variable. A simple slope analysis indicated that although both groups improved their LMT performance over time, children with prenatal cannabis exposure showed smaller increases (i.e., more blunted development) in LMT compared to children without prenatal cannabis exposure (children with prenatal cannabis exposure: b = 0.135, 95%CI = [0.122, 0.149], p < .001; children without prenatally cannabis exposure: b = 0.154, 95%CI = [0.151, 0.157], p < .001).
Fig. 1.
Cognitive scores by each time point and prenatal cannabis exposure group.
Table 2.
Results of mixed-effects models with time point, cannabis exposure during pregnancy, and their interactions as independent variables for the cognitive tasks.
| Unstandardized |
Standardized |
R squared | p value | FDR p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| coefficient | 95% CI |
coefficients | 95% CI |
||||||
| lower | upper | lower | upper | ||||||
| Without covariate | |||||||||
| Little man task | |||||||||
| Time | 0.151 | 0.149 | 0.153 | 0.589 | 0.580 | 0.598 | 0.348 | < .001 | < .001 |
| Cannabis | -0.009 | -0.017 | -0.001 | -0.029 | -0.042 | -0.017 | 0.027 | 0.035 | |
| Time: Cannabis | -0.015 | -0.024 | -0.005 | -0.013 | -0.023 | -0.004 | 0.004 | 0.007 | |
| Executive function | |||||||||
| Time | 1.573 | 1.540 | 1.605 | 0.459 | 0.449 | 0.468 | 0.204 | < .001 | < .001 |
| Cannabis | -0.134 | -0.252 | -0.016 | -0.024 | -0.039 | -0.009 | 0.026 | 0.035 | |
| Time: Cannabis | -0.082 | -0.220 | 0.056 | -0.006 | -0.015 | 0.004 | 0.244 | 0.244 | |
| Crystalized composite | |||||||||
| Time | 4.220 | 4.122 | 4.317 | 0.284 | 0.278 | 0.291 | 0.080 | < .001 | < .001 |
| Cannabis | -1.479 | -2.024 | -0.934 | -0.052 | -0.069 | -0.035 | < .001 | < .001 | |
| Time: Cannabis | -0.321 | -0.736 | 0.094 | -0.005 | -0.012 | 0.001 | 0.129 | 0.145 | |
| With covariate | |||||||||
| Little man task | |||||||||
| Time | 0.154 | 0.151 | 0.157 | 0.600 | 0.589 | 0.612 | 0.398 | < .001 | < .001 |
| Cannabis | 0.005 | -0.008 | 0.017 | -0.008 | -0.027 | 0.012 | 0.469 | 0.604 | |
| Time: Cannabis | -0.019 | -0.033 | -0.005 | -0.017 | -0.030 | -0.004 | 0.009 | 0.021 | |
| Executive function | |||||||||
| Time | 1.556 | 1.517 | 1.596 | 0.455 | 0.444 | 0.467 | 0.284 | < .001 | < .001 |
| Cannabis | -0.033 | -0.208 | 0.142 | -0.005 | -0.027 | 0.017 | 0.714 | 0.803 | |
| Time: Cannabis | -0.010 | -0.196 | 0.176 | -0.001 | -0.014 | 0.012 | 0.916 | 0.916 | |
| Crystalized composite | |||||||||
| Time | 4.154 | 4.034 | 4.273 | 0.279 | 0.271 | 0.287 | 0.340 | < .001 | < .001 |
| Cannabis | 0.528 | -0.198 | 1.255 | 0.009 | -0.013 | 0.032 | 0.154 | 0.231 | |
| Time: Cannabis | -0.501 | -1.062 | 0.059 | -0.008 | -0.017 | 0.001 | 0.08 | 0.144 | |
Note. The FDR was corrected for the number of three (Time, Cannabis, and Time: Cannabis) x three (LMT, EF, and Crystallized).
Similar to the results above, the interaction between cannabis use during pregnancy and time on the efficiency of LMT was significant (b = −0.024, 95%CI = [−0.040, −0.008], p = 0.004) in the analysis comparing the groups that used cannabis before they learned their pregnancy to those that did not (Table S8). However, when comparing the cannabis use and non-use groups after learning of pregnancy, the main effect of cannabis use during pregnancy and the interaction with time on LMT were not significant.
3.2. Prenatal cannabis exposure and brain volumes
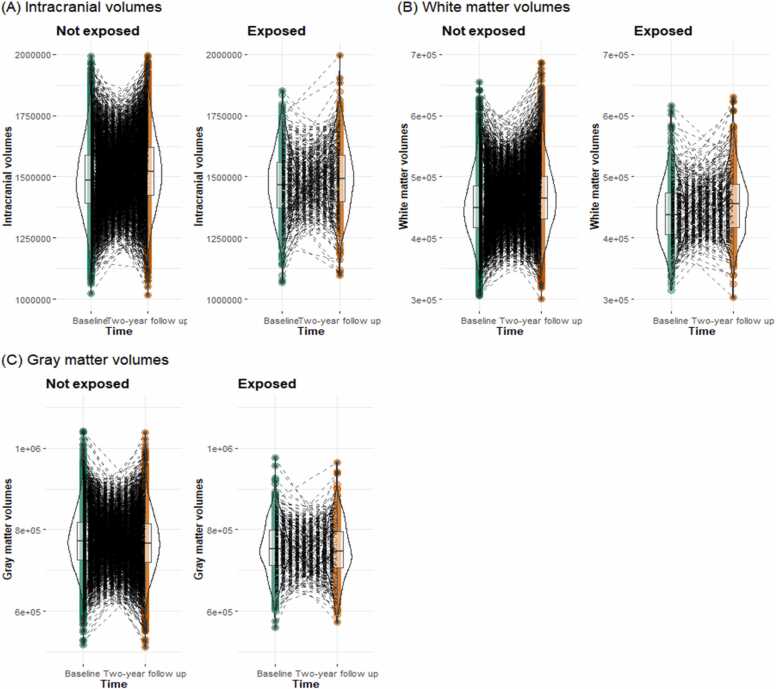
The results of the independent variables of interest in each model are shown in Fig. 2, Table 3, and Table S5-S7. The main effects of time were significant in all models, and these effects remained after including covariates (ICV: b = 29,683.910, 95%CI = [28,814.313, 30,553.508], pFDR <.001; WMV: b = 5869.002, 95%CI = [5590.628, 6147.376], pFDR <.001; GMV: b = - −20,796.640, 95%CI = [−21,232.186, −20,361.094], pFDR <.001; Fig. 2 and Table 3). ICV was entered as a covariate in the model with WMV and GMV as dependent variables (see the Methods section for descriptions of the other covariates). Furthermore, the main effect of cannabis use during pregnancy was also significant in all models, but not after controlling covariates. For ICV, the interaction of time with cannabis use during pregnancy was significant after including covariates (b = −6338.309, 95%CI = [−11,066.588, −1610.029], pFDR =.019). Simple slope analysis indicated that although both groups showed increasing ICV volumes over time, children with prenatal cannabis exposure showed smaller increases (i.e., more blunted development) in ICV compared to children without prenatal cannabis exposure (Children with prenatal cannabis exposure: b = 23601.208, 95%CI = [18974.982, 28227.434], p < .001; Children without prenatally cannabis exposure: b = 29939.517, 95%CI = [28904.834, 30974.200], p < .001).
Fig. 2.
Brain volumes by each time point and prenatal cannabis exposure group.
Table 3.
Results of mixed-effects model with time point, cannabis exposure during pregnancy, and their interactions as independent variables for the brain volumes.
| Unstandardized |
Standardized |
R squared | p value | FDR p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| coefficients | 95% CI |
coefficients | 95% CI |
||||||
| lower | upper | lower | upper | ||||||
| Without covariate | |||||||||
| Intracranial volume | |||||||||
| Time | 29,683.910 | 28,814.313 | 30,553.508 | 0.101 | 0.098 | 0.104 | 0.011 | < .001 | < .001 |
| Cannabis | -19,360.978 | -30,063.711 | -8658.244 | -0.035 | -0.052 | -0.018 | < .001 | 0.001 | |
| Time: Cannabis | -4938.278 | -8470.297 | -1406.259 | -0.004 | -0.007 | -0.001 | 0.006 | 0.008 | |
| White matter volume | |||||||||
| Time | 5869.002 | 5590.628 | 6147.376 | 0.057 | 0.055 | 0.060 | 0.639 | < .001 | < .001 |
| Cannabis | -2637.851 | -4638.176 | -637.525 | -0.011 | -0.020 | -0.002 | 0.01 | 0.012 | |
| Time: Cannabis | 582.143 | -485.575 | 1649.861 | 0.001 | -0.001 | 0.004 | 0.285 | 0.285 | |
| Gray matter volume | |||||||||
| Time | -20,796.640 | -21,232.186 | -20,361.094 | -0.152 | -0.155 | -0.149 | 0.675 | < .001 | < .001 |
| Cannabis | -6529.182 | -8947.759 | -4110.606 | -0.021 | -0.029 | -0.012 | < .001 | < .001 | |
| Time: Cannabis | 1262.279 | -443.614 | 2968.171 | 0.002 | -0.001 | 0.005 | 0.147 | 0.162 | |
| With covariate | |||||||||
| Intracranial volume | |||||||||
| Time | 29,939.517 | 28,904.834 | 30,974.200 | 0.102 | 0.098 | 0.105 | 0.271 | < .001 | < .001 |
| Cannabis | 2688.782 | -11,613.667 | 16,991.232 | -0.000 | -0.023 | 0.023 | 0.713 | 0.773 | |
| Time: Cannabis | -6338.309 | -11,066.588 | -1610.029 | -0.005 | -0.009 | -0.001 | 0.009 | 0.019 | |
| White matter volume | |||||||||
| Time | 7729.754 | 7436.208 | 8023.300 | 0.075 | 0.072 | 0.078 | 0.616 | < .001 | < .001 |
| Cannabis | -3036.717 | -6458.079 | 384.646 | -0.014 | -0.029 | 0.002 | 0.082 | 0.148 | |
| Time: Cannabis | 175.226 | -1013.692 | 1364.144 | 0.000 | -0.002 | 0.003 | 0.773 | 0.773 | |
| Gray matter volume | |||||||||
| Time | -19,359.974 | -19,848.143 | -18,871.805 | -0.141 | -0.145 | -0.138 | 0.727 | < .001 | < .001 |
| Cannabis | 2839.766 | -760.075 | 6439.608 | 0.012 | -0.001 | 0.024 | 0.122 | 0.183 | |
| Time: Cannabis | 1035.433 | -1065.623 | 3136.489 | 0.002 | -0.002 | 0.005 | 0.334 | 0.43 | |
Note. The FDR was corrected for the three (Time, Cannabis, and Time: Cannabis) x three (ICV, WMV, and GMV).
In an analysis comparing cannabis use before the knowledge of pregnancy to the non-use group, the interaction between cannabis use and time on the ICV was significant (b = −9176.673, 95%CI = [−14734.169, −3619.177], p = .001). However, a comparison of cannabis use after the knowledge of pregnancy with the non-use group showed no significant effects on any of the dependent variables (Table S11-S13).
The results of the model with cortical thickness, cortical sulcus depth, and cortical area as dependent variables are shown in Table S14. The same covariates used in the previous models were also used in this model. The main effects of cannabis use during pregnancy and the interactions between cannabis use during pregnancy and time were not significant.
4. Discussion
This study examined how cannabis use during pregnancy was associated with cognitive function and brain volume. According to our results, each cognitive index showed developmental improvement over two years, but the degree of development was associated with prenatal exposure to cannabis in the LMT. Furthermore, we found that exposure to cannabis during pregnancy was associated with an increase in intracranial volumes. Although several cohort studies have examined the effects of cannabis use during pregnancy on children’s cognitive performance and brain structure (Badowski and Smith, 2020; Daniel J. Corsi et al., 2020; Fried and Smith, 2001), to the best of our knowledge, this is the first attempt to examine the association with developmental changes using large longitudinal data.
Among the cognitive tasks measured longitudinally in the ABCD Study, LMT showed an interaction between cannabis use during pregnancy and time points. This interaction remained significant even after controlling for covariates. LMT measures visuo-spatial processing ability (Luciana et al., 2018). Interestingly, this task has previously been used to investigate the neurobehavioral effects of substance use in adults (Luciana et al., 2018). It is also notable that adolescent rats treated with THC performed worse on radial maze tasks requiring visuo-spatial abilities (Rubino et al., 2009). Meanwhile, another study found that adolescents who were regular cannabis users (i.e., they used cannabis at least once a week) performed significantly more poorly on the four measures of cognitive functioning, reflecting attention, spatial working memory, and learning (Harvey et al., 2007). Previous studies using data from the Ottawa Prenatal Prospective Study have indicated the effect of prenatal cannabis exposure on tasks measuring visuo-spatial cognitive abilities at 9–12 and 13–16 years old during a single time point (Fried and Smith, 2001, Fried et al., 1998, Fried et al., 2003). For example, Fried et al. (1998) showed that cannabis exposure during pregnancy worsens performance on the WISC block design and object assembly tasks at ages 9–12. Both tasks require visual-spatial processing and visual-motor coordination. In a later study, Fried et al. (2003) found that at ages 13–16, the group exposed to cannabis during pregnancy showed delayed reaction times on the Abstract Designs task, which requires memorizing and analyzing abstract figures. On the other hand, other studies have found that cannabis use during pregnancy had no significant effect on the child’s ability to process visual-spatial information measured by the perceptual component of the McCarthy scale at 6–7 years of age (Fried et al., 1992, Fried et al., 1992). Moreover, visuo-spatial skills, as measured by the Test of Visual Perceptual Skills, were not significantly affected by cannabis exposure during pregnancy at 6–9 years (O’connell and Fried, 1991). Thus, exposure to prenatal cannabis may have similar neurological effects in children as it does in adults who use it themselves; notably, it may interfere with the child’s long-term cognitive development. Additionally, these findings suggest that the adverse effects of cannabis exposure during pregnancy on visuo-spatial ability may not be significant until preadolescence and may become apparent through adolescence. The baseline of this study involved children aged approximately 9–10 years, with a second-time point approximately two years later, consistent with the finding in the Ottawa Prenatal Prospective Study that no effect on visuospatial ability was found in analyses up to age 9.
The study also showed a group difference in brain volume changes with cannabis use during pregnancy, and the degree of increase in intracranial volumes was modest. During this period of adolescence, the brain is still developing, with a general decrease in gray matter volumes and an increase in intracranial and white matter volumes (Sgouros et al., 1999, Vijayakumar et al., 2018). Ingestion and inhalation of cannabinoids, such as THC, cannabidiol, and cannabinol, interact with receptors in the endogenous cannabinoid signaling system (Daniel J. Corsi et al., 2021). Cannabis use during pregnancy inhibits the endogenous cannabinoid signaling system in the fetus (Daniel J. Corsi et al., 2021; Richardson et al., 2016). The endogenous cannabinoid signaling system is present in almost all brain structures from early pregnancy and plays an essential regulatory role in early embryonic and prenatal brain development (Richardson et al., 2016). Extreme prenatal environmental conditions have been shown to affect ICV in adulthood (Hulshoff Pol et al., 2000). ICV is relatively stable in adulthood, increasing from childhood to mid-to-late adolescence (Mills et al., 2016). ICV contains neurons of the central nervous system, glial cells, and cerebrospinal fluid. The dysfunction of the endogenous cannabinoid signaling system during prenatal life may have inhibited the children’s development and been associated with the reduction of the amount of ICV growth.
Following previous studies, we included multiple potentially confounding covariates in our models. In these models, the dependent variables are generally predicted by the participants’ races, genders, ages at participation, and parental education and income levels. Scholars have suggested that variances in brain structure and cognitive function may be explained by race and gender (Chee et al., 2011). Parental income and education have been shown to positively predict child achievement (Davis-Kean, 2005) and positively predict brain surface area (Noble et al., 2015). Higher socioeconomic status has also been shown to increase cannabis use (Humensky, 2010, Patrick et al., 2012); therefore, there would be a complex confounding between the use of cannabis during pregnancy, socioeconomic status, and child development. Therefore, in future studies, analyses that control for as many confounding variables as possible will be critical when assessing the effects of cannabis use during pregnancy.
This study has several strengths and limitations. One strength is that the ABCD Study data are large, collected at multiple sites in the U.S., and the sample is representative of the population (Garavan et al., 2018). Furthermore, the ABCD Study has not yet completed data collection, and longitudinal data on development over ten years will be collected in the future. The increased time points will allow examinations to be conducted at different ages and the modeling of non-linear developmental processes. This will allow further exploration of the idea of examining the amount of change presented in this study. Second, since the need to control for covariates has been proposed when examining the effects of cannabis use during pregnancy (D. J. Corsi, 2020), we controlled for potentially confounding covariates.
One of the limitations of this study is the limited time window of the participating children’s age. In the present study, the effect of cannabis use during pregnancy was found only in LMT among the cognitive tasks. However, it is impossible to determine whether the results are task- or time-specific. Previous studies have shown that executive function is impaired in adult subjects by prenatal cannabis use (Smith et al., 2016), and the same analysis conducted at an older age would likely yield different results than the present study. Future studies must examine the longer-term effects of cannabis use during pregnancy on these abilities and their developmental processes. The second limitation is that substance use during pregnancy was answered retrospectively at age 9–10, not objectively screened and prospectively tracked. Therefore, it is possible that cannabis use during pregnancy, which is socially undesirable, may not have been answered accurately, and the effects of cannabis use during pregnancy may be underestimated. Furthermore, there are no detailed data on when and how much cannabis was used during the gestational period. For example, the Ottawa Prenatal Prospective Study interviewed women about their marijuana use and dosage during pregnancy (Fried and Smith, 2001). Future studies should include longitudinal data and track the timing and amount of cannabis use during pregnancy. Third, because the ABCD Study is an observational cohort study without intervention, it is not possible to assume a causal relationship for all the relationships observed here. To elucidate causal relationships, it would be useful to examine, in an animal model, whether the administration of THC to pregnant dams adversely affects the pup’s learning and acquisition of new skills. Fourth, cognitive tasks and the age of participants included in our analyses were limited, and since we only had two time points, we could only examine a linear change. With more time points in the future, it would be possible to model linear and non-linear developmental processes. Since non-significant results neither confirm nor deny the null hypothesis, we could not make any arguments regarding the domain-specificity of the effect of cannabis use during pregnancy. Finally, the number of mothers in this cohort who used cannabis during pregnancy was much smaller than that of non-users, especially as only 245 of the 11,876 participants continued to use cannabis after the discovery of pregnancy. In this study, the analysis in which the cannabis-use-during-pregnancy group was divided into groups before and after the knowledge of pregnancy showed results consistent with the main results for comparing the pre-and non-use groups. CB1R is expressed in humans at least as early as the ninth week of gestation, which coincides with the early stages of cortical development (Zurolo et al., 2010). In our sample, the mean time at which the participants learned they were pregnant was 6.56 weeks (SD = 5.16); accordingly, there is a possibility that cannabis intake in early pregnancy may have altered the already existing CB1 binding and function of the ECS. However, no significant interaction was found for the comparison using the cannabis use group after the knowledge of pregnancy. This study cannot determine whether cannabis use before the knowledge of pregnancy is more critical than use after the discovery of pregnancy or whether it is a matter of the power of the test due to the sample size. Since it would be difficult to collect a sample size more than that of the ABCD Study to increase the power of the test for cannabis use during pregnancy, it would be necessary to obtain more reliable data by acquiring data from the gestational period, as mentioned above, or by using objective methods to determine whether or not a woman is using cannabis.
In this study, we analyzed longitudinal data from adolescents who participated in the ABCD Study and found that cannabis use during pregnancy resulted in group differences in the degree of individual cognitive and brain development. The pattern of developmental risk factor emergence is assumed to be either an initial effect with a parallel trajectory, a gradual emergence of risk, or a gradual loss of the initial difference (Haller et al., 2018, Richardson et al., 2016), and the effect of cannabis use during pregnancy has not been elucidated to date. In the present study, we found a negative effect on the development of visuo-spatial abilities and an effect on the growth of intracranial volume, at least in the two years between ages 9 and 11. Quantitative analysis of the developmental risks of cannabis use during pregnancy and understanding the detailed mechanisms will help educate people about the risks of cannabis use during pregnancy and understand and support the developmental characteristics of at-risk children.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Data were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041025, U01DA041028, U01DA041048, U01DA041089, U01DA041093, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/nih-collaborators. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators.html. The ABCD data used in this report came from https://nda.nih.gov/study.html?id=721. The authors have declared that they have no competing or potential conflicts of interest. This work was supported by JSPS KAKENHI (21K13548, 21K02380) and Research Grants from the University of Fukui (AY 2022).
Footnotes
The parents were asked to select their children's races from 16 categories. Based on these, we created variables for White, Black, Pacific Islander, Native American, Asian, and Other Race, which were not mutually exclusive.
The parents reported their children’s birth weights with two variables using pounds and ounces as units. Ounces were converted to pounds (if the ounces variable was blank, it was treated as 0), and the summed values were used as birth weight (lbs). See also Table S1.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2023.101209.
Contributor Information
Akemi Tomoda, Email: atomoda@u-fukui.ac.jp.
Yoshifumi Mizuno, Email: mizunoy@u-fukui.ac.jp.
Appendix A. Supplementary material
Supplementary material.
.
Data Availability
The data used in this manuscript is available at the ABCD Study’s Data Release 4.0 (http://dx.doi.org/10.15154/1523041). The Rmarkdown code used to analyze and create the manuscript is available at https://osf.io/t7y3s/?view_only=e8d6cd5f3b3a4a1897885ff28178bc9c.
References
- Acker, W., & Acker, C. (1982). Bexley maudsley automated processing screening and bexley maudsley category sorting test manual. Windsor, England: NFER-Nelson. (Original work published).
- Azofeifa A., Mattson M.E., Schauer G., McAfee T., Grant A., Lyerla R. National estimates of marijuana use and related indicators - national survey on drug use and health, united states, 2002-2014. Morb. Mortal. Wkly. Report. Surveill. Summ. 2016;65(11):1–25. doi: 10.15585/mmwr.ss6511a1. [DOI] [PubMed] [Google Scholar]
- Badowski S., Smith G. Cannabis use during pregnancy and postpartum. Can. Fam. Physician Med. De. Fam. Can. 2020;66(2):98–103. [PMC free article] [PubMed] [Google Scholar]
- Bara A., Ferland J.M.N., Rompala G., Szutorisz H., Hurd Y.L. Cannabis and synaptic reprogramming of the developing brain. Nat. Rev. Neurosci. 2021;22(7):423–438. doi: 10.1038/s41583-021-00465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Giedd J.N., Thomas K.M. Structural and functional brain development and its relation to cognitive development. Biol. Psychol. 2000;54(1–3):241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Tottenham N., Liston C., Durston S. Imaging the developing brain: What have we learned about cognitive development. Trends Cogn. Sci. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Cannonier T., Conley M.I., Cohen A.O., Barch D.M., Heitzeg M.M., ABCD Imaging Acquisition Workgroup The adolescent brain cognitive development (ABCD) study: Imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 2018;32(May 2017):43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdá M., Mauro C., Hamilton A., Levy N.S., Santaella-Tenorio J., Hasin D., Martins S.S. Association between recreational marijuana legalization in the united states and changes in marijuana use and cannabis use disorder from 2008 to 2016. JAMA Psychiatry. 2020;77(2):165–171. doi: 10.1001/jamapsychiatry.2019.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M.W.L., Zheng H., Goh J.O.S., Park D., Sutton B.P. Brain structure in young and old east asians and westerners: Comparisons of structural volume and cortical thickness. J. Cogn. Neurosci. 2011;23(5):1065–1079. doi: 10.1162/jocn.2010.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioffredi L.-A., Anderson H., Loso H., East J., Nguyen P., Garavan H., Potter A. Prenatal cannabis exposure predicts attention problems, without changes on fMRI in adolescents. Neurotoxicol. Teratol. 2022;91 doi: 10.1016/j.ntt.2022.107089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi D.J. Epidemiological challenges to measuring prenatal cannabis use and its potential harms. BJOG: Int. J. Obstet. Gynaecol. 2020;127(1):17. doi: 10.1111/1471-0528.15985. [DOI] [PubMed] [Google Scholar]
- Corsi Daniel J., Donelle J., Sucha E., Hawken S., Hsu H., El-Chaâr D., Walker M. Maternal cannabis use in pregnancy and child neurodevelopmental outcomes. Nat. Med. 2020;26(10):1536–1540. doi: 10.1038/s41591-020-1002-5. [DOI] [PubMed] [Google Scholar]
- Corsi Daniel J., Murphy M.S.Q., Cook J. The effects of cannabis on female reproductive health across the life course. Cannabis Cannabinoid Res. 2021;6(4):275–287. doi: 10.1089/can.2020.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Kean P.E. The influence of parent education and family income on child achievement: The indirect role of parental expectations and the home environment. J. Fam. Psychol.: JFP: J. Div. Fam. Psychol. Am. Psychol. Assoc. 2005;19(2):294–304. doi: 10.1037/0893-3200.19.2.294. [DOI] [PubMed] [Google Scholar]
- El Marroun H., Tiemeier H., Steegers E.A.P., Jaddoe V.W.V., Hofman A., Verhulst F.C., Huizink A.C. Intrauterine cannabis exposure affects fetal growth trajectories: The generation R study. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48(12):1173–1181. doi: 10.1097/CHI.0b013e3181bfa8ee. [DOI] [PubMed] [Google Scholar]
- ElSohly M.A., Chandra S., Radwan M., Majumdar C.G., Church J.C. A comprehensive review of cannabis potency in the united states in the last decade. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 2021;6(6):603–606. doi: 10.1016/j.bpsc.2020.12.016. [DOI] [PubMed] [Google Scholar]
- Erus G., Battapady H., Satterthwaite T.D., Hakonarson H., Gur R.E., Davatzikos C., Gur R.C. Imaging patterns of brain development and their relationship to cognition. Cereb. Cortex. 2015;25(6):1676–1684. doi: 10.1093/cercor/bht425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine J.D., Moreau A.L., Karcher N.R., Agrawal A., Rogers C.E., Barch D.M., Bogdan R. Association of prenatal cannabis exposure with psychosis proneness among children in the adolescent brain cognitive development (ABCD) study. JAMA Psychiatry. 2019;76(7):762–764. doi: 10.1001/jamapsychiatry.2019.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried P.A., Smith A.M. A literature review of the consequences of prenatal marihuana exposure - an emerging theme of a deficiency in aspects of executive function. Neurotoxicol. Teratol. 2001;23(1):1–11. doi: 10.1016/S0892-0362(00)00119-7. [DOI] [PubMed] [Google Scholar]
- Fried P.A., Watkinson B. 12- and 24-month neurobehavioural Follow-Up of children prenatally exposed to marihuana, cigarettes and alcohol. Neurotoxicol. Teratol. 1988;10(4):305–313. doi: 10.1016/0892-0362(88)90032-3. [DOI] [PubMed] [Google Scholar]
- Fried P.A., Watkinson B. 36- and 48-month neurobehavioral follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol. J. Dev. Behav. Pediatr. JDBP. 1990;11(2):49–58. doi: 10.1097/00004703-199004000-00003. [DOI] [PubMed] [Google Scholar]
- Fried P.A., O’Connell C.M., Watkinson B. 60- and 72-month follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol: Cognitive and language assessment. J. Dev. Behav. Pediatr.: JDBP. 1992;13(6):383–391. [PubMed] [Google Scholar]
- Fried P.A., Watkinson B., Gray R. A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes, and alcohol. Neurotoxicol. Teratol. 1992;299(5):299–311. doi: 10.1016/0892-0362(92)90036-A. [DOI] [PubMed] [Google Scholar]
- Fried P.A., Watkinson B., Gray R. Differential effects on cognitive functioning in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol. Teratol. 1998;20(3):293–306. doi: 10.1016/S0892-0362(97)00091-3. [DOI] [PubMed] [Google Scholar]
- Fried P.A., Watkinson B., Gray R. Growth from birth to early adolescence in offspring prenatally exposed to cigarettes and marijuana. Neurotoxicol. Teratol. 1999;21(5):513–525. doi: 10.1016/S0892-0362(99)00009-4. [DOI] [PubMed] [Google Scholar]
- Fried P.A., Watkinson B., Gray R. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol. Teratol. 2003;25(4):427–436. doi: 10.1016/S0892-0362(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Garavan H., Bartsch H., Conway K., Decastro A., Goldstein R.Z., Heeringa S., Zahs D. Recruiting the ABCD sample: Design considerations and procedures. Dev. Cogn. Neurosci. 2018;32(August 2017):16–22. doi: 10.1016/j.dcn.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler D.J., Hatton S., Cornejo M.D., Makowski C., Fair D.A., Dick A.S., Dale A.M. Image processing and analysis methods for the adolescent brain cognitive development study. NeuroImage. 2019;202(5) doi: 10.1016/j.neuroimage.2019.116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S.P.W., Mills K.L., Hartwright C.E., David A.S., Cohen Kadosh K. When change is the only constant: The promise of longitudinal neuroimaging in understanding social anxiety disorder. Dev. Cogn. Neurosci. 2018;33(May):73–82. doi: 10.1016/j.dcn.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey M.A., Sellman J.D., Porter R.J., Frampton C.M. The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev. 2007;26(3):309–319. doi: 10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol H.E., Hoek H.W., Susser E., Brown A.S., Dingemans A., Schnack H.G., Kahn R.S. Prenatal exposure to famine and brain morphology in schizophrenia. Am. J. Psychiatry. 2000;157(7):1170–1172. doi: 10.1176/appi.ajp.157.7.1170. [DOI] [PubMed] [Google Scholar]
- Humensky J.L. Are adolescents with high socioeconomic status more likely to engage in alcohol and illicit drug use in early adulthood. Subst. Abus. Treat., Prev., Policy. 2010;5:19. doi: 10.1186/1747-597X-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings D.E., Martin B.R., Gamagaris Z., Miller N., Fico T. Plasma concentrations of delta-9-tetrahydrocannabinol in dams and fetuses following acute or multiple prenatal dosing in rats. Life Sci. 1989;44(11):697–701. doi: 10.1016/0024-3205(89)90380-9. [DOI] [PubMed] [Google Scholar]
- Karcher N.R., Barch D.M. The ABCD study: understanding the development of risk for mental and physical health outcomes. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 2021;46(1):131–142. doi: 10.1038/s41386-020-0736-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Avenevoli S., Costello E.J., Green J.G., Gruber M.J., Heeringa S., Zaslavsky A.M. Design and field procedures in the US national comorbidity survey replication adolescent supplement (NCS-A. Int. J. Methods Psychiatr. Res. 2009;18(2):69–83. doi: 10.1002/mpr.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Avenevoli S., Costello E.J., Green J.G., Gruber M.J., Heeringa S., Zaslavsky A.M. National comorbidity survey replication adolescent supplement (NCS-A): II. Overview and design. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48(4):380–385. doi: 10.1097/CHI.0b013e3181999705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M., Bjork J.M., Nagel B.J., Barch D.M., Gonzalez R., Nixon S.J., Banich M.T. Adolescent neurocognitive development and impacts of substance use: overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Dev. Cogn. Neurosci. 2018;32(July 2017):67–79. doi: 10.1016/j.dcn.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M., Guzmán M., Mackie K., Doherty P., Harkany T. Programming of neural cells by (endo)cannabinoids: From physiological rules to emerging therapies. Nat. Rev. Neurosci. 2014;15(12):786–801. doi: 10.1038/nrn3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark K., Gryczynski J., Axenfeld E., Schwartz R.P., Terplan M. Pregnant women’s current and intended cannabis use in relation to their views toward legalization and knowledge of potential harm. J. Addict. Med. 2017;11(3):211–216. doi: 10.1097/ADM.0000000000000299. [DOI] [PubMed] [Google Scholar]
- Mehmedic Z., Chandra S., Slade D., Denham H., Foster S., Patel A.S., ElSohly M.A. Potency trends of 9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J. Forensic Sci. 2010;55(5):1209–1217. doi: 10.1111/j.1556-4029.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- Merikangas K.R., Avenevoli S., Costello E.J., Koretz D., Kessler R.C. National comorbidity survey replication adolescent supplement (NCS-A): I. Background and measures. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48(4):367–379. doi: 10.1097/CHI.0b013e31819996f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz T.D., Allshouse A.A., Hogue C.J., Goldenberg R.L., Dudley D.J., Varner M.W., Silver R.M. Maternal marijuana use, adverse pregnancy outcomes, and neonatal morbidity. Am. J. Obstet. Gynecol. 2017;217(4):478.e1–478.e8. doi: 10.1016/j.ajog.2017.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K.L., Goddings A.L., Herting M.M., Meuwese R., Blakemore S.J., Crone E.A., Tamnes C.K. Structural brain development between childhood and adulthood: convergence across four longitudinal samples. NeuroImage. 2016;141:273–281. doi: 10.1016/j.neuroimage.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Brito N.H., Bartsch H., Kan E., Kuperman J.M., Sowell E.R. Family income, parental education and brain structure in children and adolescents. Nat. Neurosci. 2015;18(5):773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’connell C.M., Fried P.A. Prenatal exposure to cannabis: a preliminary report of postnatal consequences in School-Age children I. Plan. Perspect. 1991;PP, 631:639. doi: 10.1016/0892-0362(91)90047-z. [DOI] [PubMed] [Google Scholar]
- Patrick M.E., Wightman P., Schoeni R.F., Schulenberg J.E. Socioeconomic status and substance use among young adults: a comparison across constructs and drugs. J. Stud. Alcohol Drugs. 2012;73(5):772–782. doi: 10.15288/jsad.2012.73.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S.E., Hatoum A.S., Fine J.D., Johnson E.C., Hansen I., Karcher N.R., Bogdan R. Associations between prenatal cannabis exposure and childhood outcomes: results from the ABCD study. JAMA Psychiatry. 2021;78(1):64–76. doi: 10.1001/jamapsychiatry.2020.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2022). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. (Original work published).
- Reichenberg A., Caspi A., Harrington H., Houts R., Keefe R.S.E., Murray R.M., Moffitt T.E. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am. J. Psychiatry. 2010;167(2):160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson K.A., Hester A.K., McLemore G.L. Prenatal cannabis exposure - the “first hit” to the endocannabinoid system. Neurotoxicol. Teratol. 2016;58:5–14. doi: 10.1016/j.ntt.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Romer A.L., Pizzagalli D.A. Is executive dysfunction a risk marker or consequence of psychopathology? A test of executive function as a prospective predictor and outcome of general psychopathology in the adolescent brain cognitive development study. Dev. Cogn. Neurosci. 2021;51 doi: 10.1016/j.dcn.2021.100994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T., Realini N., Braida D., Guidi S., Capurro V., Viganò D., Parolaro D. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19(8):763–772. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- Sgouros S., Goldin J.H., Hockley A.D., Wake M.J., Natarajan K. Intracranial volume change in childhood. J. Neurosurg. 1999;91(4):610–616. doi: 10.3171/jns.1999.91.4.0610. [DOI] [PubMed] [Google Scholar]
- Smith A.M., Mioduszewski O., Hatchard T., Byron-Alhassan A., Fall C., Fried P.A. Prenatal marijuana exposure impacts executive functioning into young adulthood: An fMRI study. Neurotoxicology Teratol. 2016;58(January 2001):53–59. doi: 10.1016/j.ntt.2016.05.010. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N., Mills K.L., Alexander-Bloch A., Tamnes C.K., Whittle S. Structural brain development: a review of methodological approaches and best practices. Dev. Cogn. Neurosci. 2018;33(February 2017):129–148. doi: 10.1016/j.dcn.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Han B., Compton W.M., McCance-Katz E.F. Self-reported medical and nonmedical cannabis use among pregnant women in the united states. JAMA: J. Am. Med. Assoc. 2019;322(2):167–169. doi: 10.1001/jama.2019.7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre R.J., Fields R.D., Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat. Neurosci. 2012;15(4):528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurolo E., Iyer A.M., Spliet W.G.M., Van Rijen P.C., Troost D., Gorter J.A., Aronica E. CB1 and CB2 cannabinoid receptor expression during development and in epileptogenic developmental pathologies. Neuroscience. 2010;170(1):28–41. doi: 10.1016/j.neuroscience.2010.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Data Availability Statement
The data used in this manuscript is available at the ABCD Study’s Data Release 4.0 (http://dx.doi.org/10.15154/1523041). The Rmarkdown code used to analyze and create the manuscript is available at https://osf.io/t7y3s/?view_only=e8d6cd5f3b3a4a1897885ff28178bc9c.