Abstract
Climate change and increased pollution caused by traditional petrochemical plastics made the biodegradable environment-friendly plastic (bioplastic) research more popular. Bioplastics can be manufactured from natural renewable ingredients and used as food packaging material without harming the environment. This research work focuses on developing bioplastic films from natural ingredients such as starch of tamarind seeds, and berry seeds, with licorice root. Attention has been paid to characterizing the material by biodegradability, mechanical testing, Fourier Transformed Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM), Thermogravimetric Analysis (TGA), Differential Scanning Calorimetry (DSC), antimicrobial analysis tests. Phenolic compounds present in the berry seeds starch increased the soil biodegradability as well as the mechanical and thermal properties of the bioplastic films. The FTIR spectra confirmed the presence of various bio-molecules. Improved antimicrobial performance is also obtained. The results of this research confirm that the prepared bioplastic samples can be used in packaging applications.
Keywords: Bioplastic, Food packaging application, Tamarind, Berry, Licorice
1. Introduction
The increased use of petrochemical plastics has become a great threat to human beings and living organisms' survival due to environmental pollution made by these petrochemical plastics. Emphasis is being paid and a lot of research work is going on to manufacture environmentally friendly biodegradable green plastics to replace the traditional harmful petrochemical plastics [[1], [2], [3], [4], [5]]. Recent years have seen increased research on the development of bioplastics and biocomposites [[6], [7], [8]]. The potentiality available in bioplastics can replace petrochemical plastics in a different home and industrial applications [[9], [10], [11], [12]]. Starch is an abundant, available, and low-cost source of raw material used to make bioplastics [13]. Starch is rich in glucan that has mainly two components namely amylose and amylopectin. Hydroxyl groups are available in starch which makes it hydrophilic. Linear molecules of glucose and bifurcated molecules are available in amylase and amylopectin respectively. Besides, hydrated starch is thermally conductive and biodegradable [14].
Tamarind is an evergreen tree that needs a dry climate to grow. Every part of tamarind is rich in nutritional value and is used as medicine. Its seeds are a good source of protein good for human health [15]. Tamarind seed can be used in paint and cosmetic industries due to its acidic value. Polysaccharides present in tamarind seeds are biodegradable and biocompatible in nature and can be extracted from the endosperm of the seed [16]. Tamarind seed polysaccharide is composed of monosaccharide, octasaccharide, heptasaccharide, hexasaccharide, and hendesaccharide [17]. Films produced from tamarind seeds polysaccharides can remain thermally stable up to the temperature range of 201.88 °C [18].
Berries are good for health due to the presence of antioxidant and organoleptic properties [19]. Berries are rich in phenolic compounds which contain different types of cyaniding, peonidin, etc at different percentages [20]. Berries are effective against bacteria, viruses, fungi, archaebacteria, and protozoa [21]. Besides, berries promote human health and can prevent different diseases such as cancers, neurodegenerative, heart diseases, etc. Bioactive compounds are also present in berries with dietary compounds [22].
Licorice is a valuable medicinal plant that has more than 30 species worldwide and is mainly found in Asia and the Mediterranean region [23]. Licorice root contains different types of bioactive compounds and as a result, it is used as medicine to treat diseases. It is expected that licorice root will be used to treat arthritis, asthma, and colitis. Licorice root contains glabridin which shows antibacterial effects on different types of pathogenic bacteria [24].
Here, the prime objective of this work is to show the usability of the developed bioplastic as a food packaging material to replace traditional petrochemical plastic. The focus was paid to evaluating its biodegradable, mechanical, morphological, thermal, and antibacterial performance.
In this study, two types of bioplastic samples were prepared, with berry seeds starch and without berry seeds starch keeping the licorice root percentages constant. In this study, we used 20 g licorice root. But in the previous study, we used 10 g licorice root. Here, the addition of berries increased biodegradation. Besides, the addition of berries developed mechanical properties. Moreover, the addition of berries increased antimicrobial properties.
2. Materials and methods
2.1. Materials
Tamarind seeds, berry seeds, licorice root, distilled water, glycerol, and white vinegar were used to fabricate the bioplastic samples. The agricultural lab of the Department of Agriculture of IUBAT provided the necessary seeds of berry and tamarind with licorice. Distilled water was collected from the environment lab of the Civil Engineering Department of IUBAT as well. We collected glycerol and white vinegar from a nearby shop.
2.2. Bioplastic sample preparation
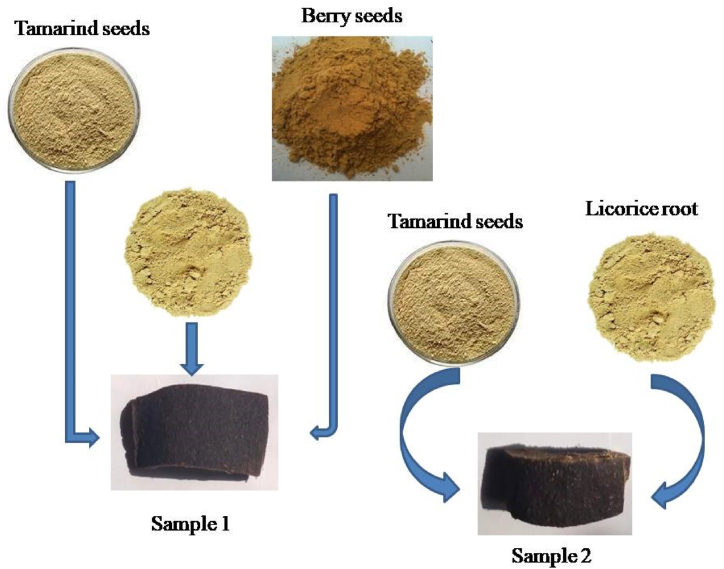
Dirt was removed from the solid ingredients by washing three times with distilled water followed by boiling and blending to get starch. Two types of samples were prepared where one sample contained tamarind seeds starch and licorice root and another sample contained starch of berry and tamarind with licorice shown in Fig. 1. The percentages of different ingredients can be seen in Table 1. The ingredients were weighed, mixed, blended, and stirred at 100 °C. Bioplastic was prepared after several minutes of heating which was poured on aluminum foil. The bioplastic films were obtained after several hours of cooling.
Fig. 1.
Production of bioplastic films.
Table 1.
Used raw materials to make bioplastic samples.
| Sample | White vinegar (ml) | Glycerol (ml) | Distilled water (ml) | Tamarind seed starch (g) | Berry seed starch (g) | Licorice Root(g) |
|---|---|---|---|---|---|---|
| S1 | 40 | 40 | 360 | 60 | 20 | 20 |
| S2 | 40 | 40 | 360 | 60 | – | 20 |
2.3. Characterization
2.3.1. Biodegradability test
The biodegradability of the prepared bioplastic samples was evaluated in the microorganism present soil. The bioplastic samples were cut at the dimension of 4 cm × 2 cm for the test. The prepared bioplastic samples were buried in the soil for different time periods. Each sample was measured for weight by precise electronic balance before burying. Each sample was cleaned carefully by running tap water followed by drying at room temperature under the sun after removing from the soil and weight was taken again. The weight loss data was obtained from the differences between the two values which indicated the soil biodegradability rate of the prepared bioplastic samples [25]. Three measurements were conducted for the samples considered the average value and the corresponding standard deviation was calculated. Weight loss was calculated by the following formula:
| (1) |
Here, W0 and W1 are the weights before and after test [[26], [27], [28], [29], [30], [31], [32]].
2.3.2. Mechanical test
ASTM-D638-14 standard was maintained to perform the mechanical test of the prepared bioplastic samples by a computer-controlled CMT-10 electronic universal testing machine. The experiments were conducted at room temperature at a 2 mm/min strain rate. The prepared samples for the mechanical test had 165 mm overall length with 57 mm length of the narrow section and 50 mm gage length. The narrow section had a 13 mm width and the overall thickness was 3.5 mm. Five experiments were conducted for each sample, the average value was considered and the corresponding standard deviation was calculated.
2.3.3. FTIR test
The FTIR profile from the bioplastic samples was obtained from Perkin Elmer Spectrometer. The presence of different functional groups was identified using the wave number ranging from 500 cm−1 to 4000 cm−1.
2.3.4. SEM test
The microstructure of the bioplastic films was analyzed by Hitachi S-4800 scanning electronic microscope. The influence of the berry seeds extract on the structure generated was determined. The samples were cut into 0.5 cm2 size, immersed in liquid nitrogen, curio fractured, randomly broken, and coated with gold palladium. The samples were observed at a 10 mm working distance, 10 kV accelerating voltage and different magnifications.
2.3.5. Thermal analysis
TA SDT 650 instrument was employed to perform TGA and DSC analysis of the bioplastic films at a weight rate of 10–25 mg, a heating rate of 5 °C, and from room temperature to 650 °C.
2.3.6. Antimicrobial assay
ASTM E2149-01 standard was followed to analyze the antimicrobial properties of the bioplastic films against the gram-positive S. aureus and gram-negative E. coli bacteria in the disk diffusion method.
3. Results and discussion
3.1. Soil biodegradation performance analysis
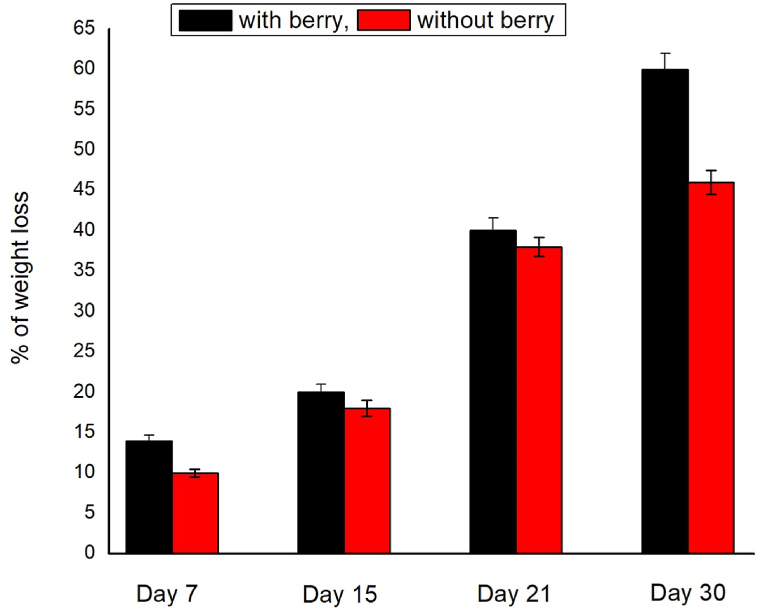
Fig. 2 (a to e) and 3 (a to e) shows the soil biodegradability of the prepared bioplastic samples with time variation. Fig. 4 shows the biodegradability comparison. From the beginning of soil burial, biodegradation was observed. After 7, 14, 21, and 30 days of observation, 14%, 20%, 40% and 60% biodegradation was observed from the sample S1. However, for the same time period, 10%, 18%, 38%, and 46% biodegradation were observed for Sample S2. The presence of microorganisms along with their microbial performance is the key to biodegradation [33,34]. Biodegradability increased due to the incorporation of berry starch with other ingredients. The phenolic compounds of berries are mainly responsible for fast biodegradation. Phenolic compounds are biodegradable in nature. It can be converted into quinone cross-linked to protein which later forms a new covalent cross-link at higher pH. A covalent bond in C–N is formed due to the reaction between quinones and amino polypeptides [35]. Besides, phenolic compounds are degraded to carbon dioxide by fermentation [36].
Fig. 2.
Soil biodegradability of the sample S1 after (a) 0 days (5 gm), (b) 7 days (14), (c) 15 days (20), (d) 21 days (40), (e) 30 days (60).
Fig. 3.
Soil biodegradability of the sample S2 after (a) 0 days (5 gm), (b) 7 days (10), (c) 15 days (18), (d) 21 days (38), (e) 30 days (46).
Fig. 4.
Biodegradability comparison of the prepared bioplastic samples.
3.2. Mechanical properties analysis
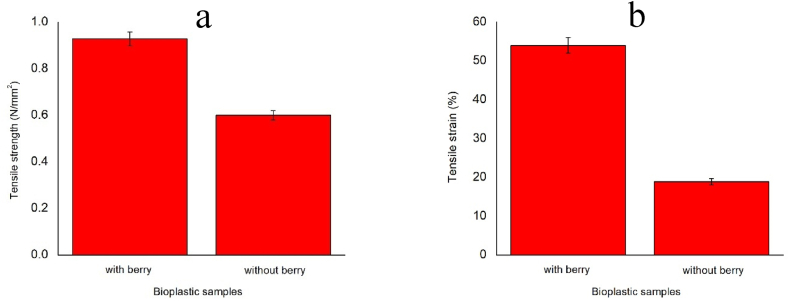
Fig. 5 shows the comparison in tensile strength of the developed bioplastic samples. Better tensile strength and tensile strain were obtained from the bioplastic sample after incorporating berry seeds starch which improved the mechanical properties. The interaction between the phenolic compound and protein is the reason for mechanical improvement which made the bioplastic sample more flexible. A stronger and stiffer film structure was formed due to the presence of phenolic compounds [37]. Ionic, covalent, hydrogen, and hydrophobic bonds are formed by phenolic compounds in the sample. A C–N covalent bond is formed due to berry seeds starch with free amino groups which are available in protein-forming quinones which results in a degree of protein cross-linking gradual increase. The mechanical properties are developed by the formation of a typical cross-link. Strong intermolecular connections reduced the free space of the polymer matrix which made the bioplastic sample stiffer, denser, and tightly packed with reduced molecular mobility [38,39]. Cross-links can be formed by the phenolic compounds among the proteins that react with several protein sites [40].
Fig. 5.
Tensile properties comparison of the bioplastic films (a) tensile strength, (b) tensile strain.
3.3. Morphological analysis
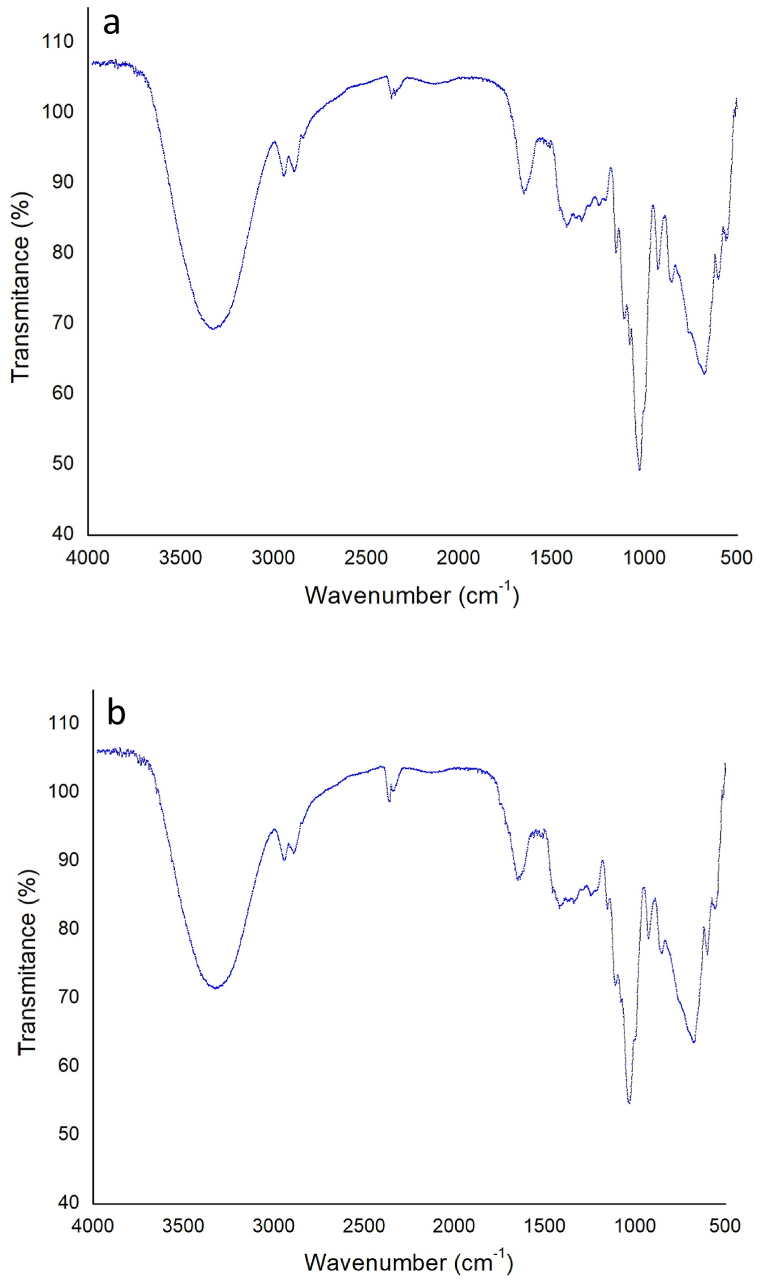
Fig. 6 (a and b) shows the FTIR spectra of the prepared bioplastic samples. The FTIR spectra show the presence of different functional groups in the prepared samples. The presence of berry, licorice root, and tamarind was confirmed by the characteristic peaks of the spectra. Similar characteristic peaks were observed from the bioplastic samples because of having similar constituents but at different intensities. The presence of licorice increases the intensity of the spectra. Medium stretching aliphatic primary amine N–H was identified by the broad absorption peak at 3325 cm−1. The intensity of N–H peaks shifted to 3323 cm−1 with greater intensity after the addition of berry seeds starch which signifies the excellent dispersion of berry seeds starch in the films. The peak at 2889 cm−1 is attributed to the medium stretching alkane C–H confirming the presence of polysaccharides [41]. The peak is shifted to 2943 cm−1 with berry seeds starch addition. Peaks in sample S2 at 2357 cm−1 denote strong stretching carbon dioxide CO2 which is shifted to 2362 cm−1 in sample S1. Peaks at 1647 cm−1 represent strong stretching Imine C–N. Peaks at 1417 cm−1 are attributed to medium stretching carboxylic acid OH which is shifted to 1409 cm−1 after berry seeds starch addition which indicates the formation of inter and intermolecular hydrogen bonds and confirms the presence of glabridin [42,43]. Medium bending amine group was identified at 1029 cm−1 by the absorption band. Sample S1 identified the presence of C–Br at 675 cm−1 and S2 at 677 cm−1. Table 2 shows various functional groups identified by the FTIR spectra.
Fig. 6.
FTIR analysis of the synthesized bioplastic (a) with berry seeds and (b) without berry seeds.
Table 2.
Presence of various functional groups identified by the FTIR spectra.
| Band (cm−1) | Functional class | Assignment | Vibration type |
|---|---|---|---|
| With Berry seeds | |||
| 675 | Halo compound | C–Br | Strong stretching |
| 1029 | Amine | C–N | Medium bending |
| 1409 | Carboxylic acid | O–H | Medium bending |
| 1647 | Imine | C=N | Medium stretching |
| 2362 | Carbon dioxide | O=C=O | Strong stretching |
| 2943 | Alkane | C–H | Medium stretching |
| 3323 | Aliphatic primary amine | N–H | Medium stretching |
| Without Berry Seeds | |||
| 677 | Halo compound | C–Br | Strong stretching |
| 1029 | Amine | C–N | Medium bending |
| 1417 | Carboxylic acid | O–H | Medium bending |
| 1647 | Imine | C=N | Medium stretching |
| 2357 | Carbon dioxide | O=C=O | Strong stretching |
| 2889 | Alkane | C–H | Medium stretching |
| 3325 | Aliphatic primary amine | N–H | Medium stretching |
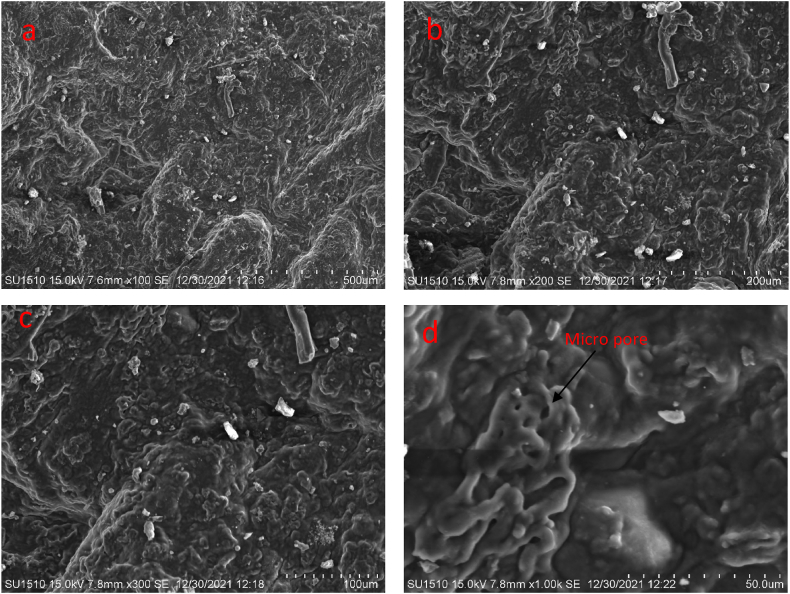
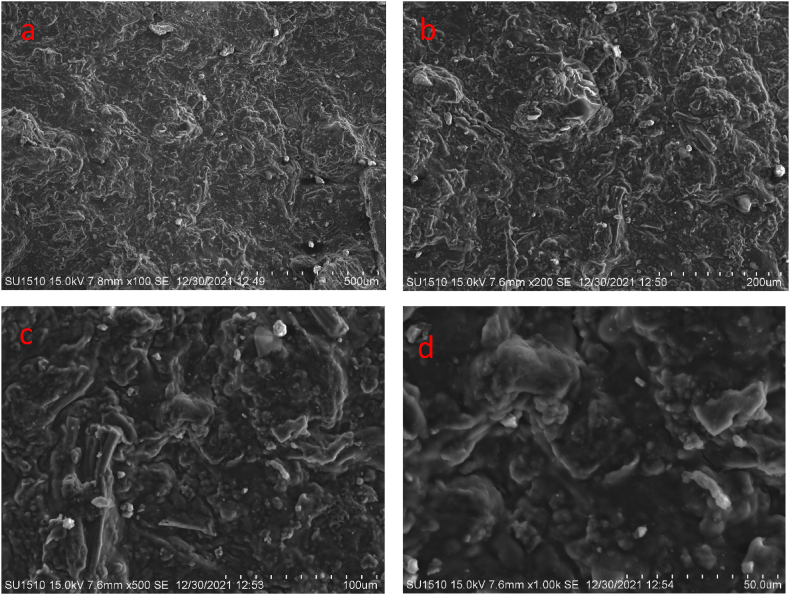
Fig. 7 (a to d) and 8 (a to d) shows the SEM surface morphology of the developed bioplastic samples. The previously observed results and the observed results by SEM analysis show similarities. Similar observations were found through the addition of berry seeds starch. A good number of micro-pores are observed from the analysis which helps to biodegrade the bioplastic samples interacting with the microorganism available in soil [44]. Both samples show good surface integrity and a homogeneous phase. Coarse, granules, irregularities, and flaws can be seen on the surface of both of the samples. The presence of foreign particles is observed on the surface of both samples.
Fig. 7.
SEM surface morphology of the bioplastic film prepared with berry seeds starch at (a) 500 μm and (b) 200 μm, (c) 100 μm, and (d) 50 μm.
Fig. 8.
SEM surface morphology of the bioplastic film prepared without berry seeds starch at (a) 500 μm and (b) 200 μm, (c) 100 μm, and (d) 50 μm.
3.4. Thermal analysis
3.4.1. TGA analysis
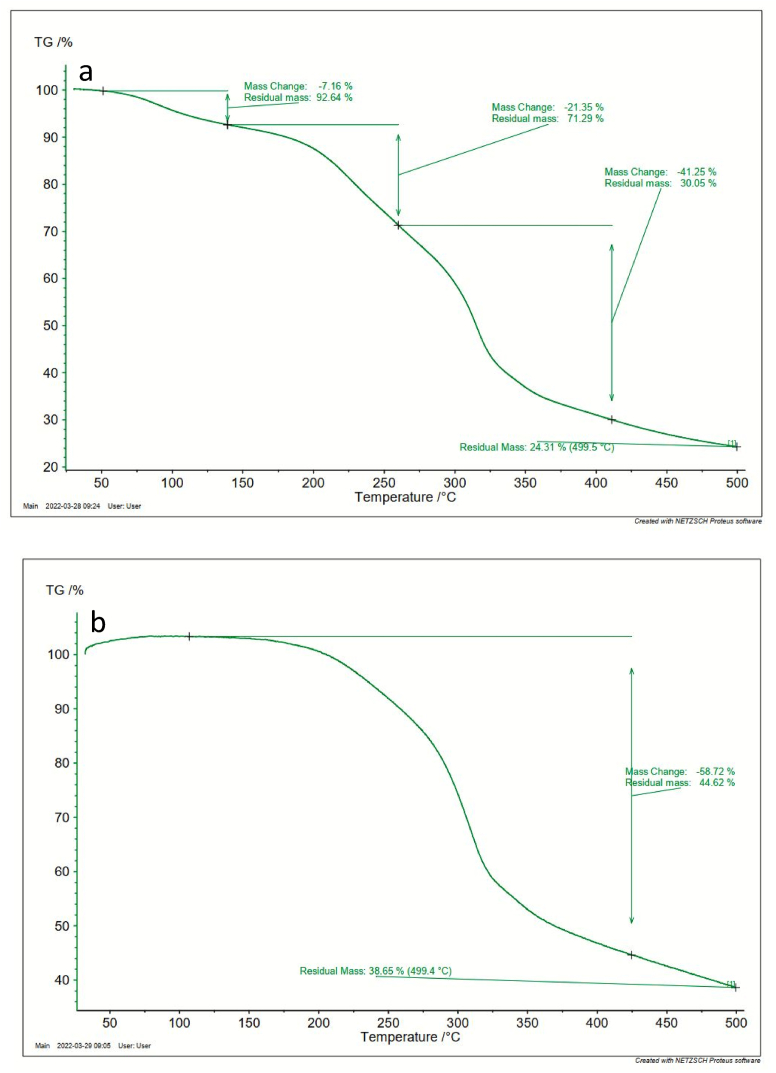
The thermal stability of the developed bioplastic samples prepared with and without berry seeds starch was analyzed by the Thermogravimetric Analysis (TGA) shown in Fig. 9 (a to b) and Differential Scanning Calorimetry (DSC) shown in Fig. 10 (a to b). Weight losses for sample S1 were observed at three different periods. 130 °C, 260 °C, and 410 °C. Evaporation of water made the first weight loss at 130 °C [45]. Phenolic compounds decompose thermally which made the second weight loss at 260 °C. At 200 °C, salvianolic acid as well as ferulic acid available at phenolic compounds decomposes. At 250 °C, decomposition occurs of protocatechuic acid present in the phenolic compound [46]. Starch degrades at 410 °C which is the third degradation [47]. On the other hand, only one weight loss is observed at sample S2 nearly at 425 °C as sample S2 does not contain phenolic compounds.
Fig. 9.
TGA graph of the prepared bioplastics samples (a) with berry seeds starch, (b) without berry seeds starch.
Fig. 10.
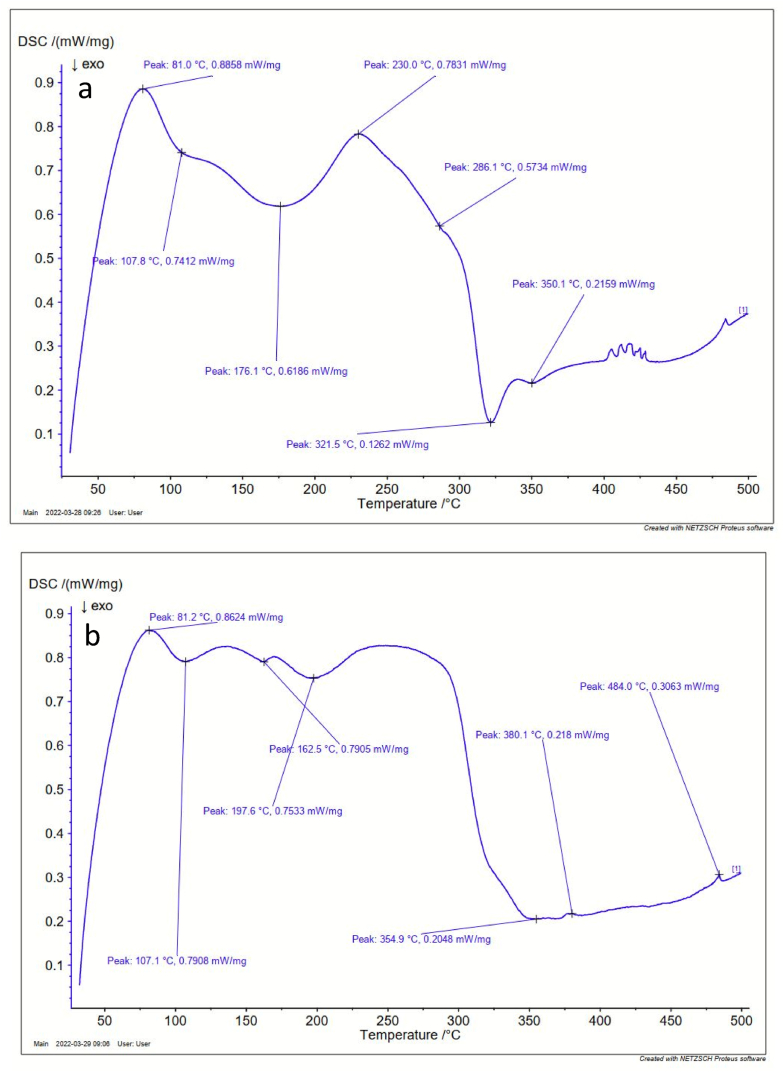
DSC graph of the prepared bioplastics samples (a) with berry seeds starch, (b) without berry seeds starch.
3.4.2. DSC analysis
Exothermic and endothermic curves of the developed bioplastic samples analyzed by DSC are shown in Fig. 10 (a and b). The first exothermic peak determined the glass transition temperature which is also termed starch gelatin temperature. Good mechanical properties with flexibility can be obtained from developing bioplastic at this temperature from starch. At this temperature, the mechanical properties of the prepared bioplastic films change from elastic to brittle due to changes in chain mobility. The incorporation of berry seeds starch resulted in minimal change in this parameter. The results indicate that this temperature is suitable for bioplastic casting. Glass transition temperature was observed at 81.0 °C for sample S1 and 81.2 °C for sample S2. Moreover, glass-like characteristics can be observed in terms of strength, brittleness, and stiffness from the developed bioplastic samples as the glass transition temperature was observed above room temperature [48]. Besides, above the glass transition temperature, the bioplastic polymer chains have high mobility and can form ordered arrangements due to having high energy as well as undergoing crystallization.
3.5. Antibacterial performance analysis
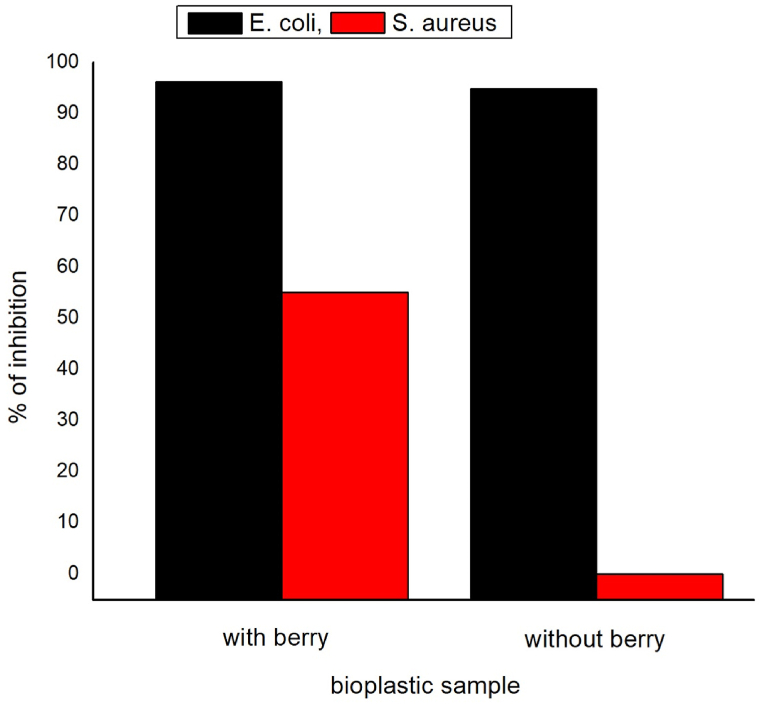
Fig. 11 (a and b) and 12 show the antibacterial performance of the developed bioplastic samples against both gram-positive and gram-negative bacteria strain S. aureus and E. coli respectively. E. coli is naturally available in food items and the environment. It causes diarrhea, sepsis, and urinary infections [49]. S. aureus causes various clinical infections such as skin and soft tissue-related infections, pleuropulmonary and pleuropulmonary [50]. Bacterial inhibition was characterized as % for the developed bioplastic samples. Sample S1 showed better bacterial inhibition against both bacteria strains. However, both samples worked better against E. coli. A thick polypeptide layer having a thickness of 55 nm without any outer lipid membrane is possessed by the gram-positive bacteria which is difficult to break. On the other hand, a thin polypeptide layer having only a 2 nm thickness wall with an outer lipid membrane is possessed by the gram-negative bacteria which is easy to disrupt and kill the bacteria strain [51]. Moreover, Phenolic compounds show good antibacterial properties against bacterial strains [52]. That is why; the incorporation of berry seed extracts increased the antibacterial strain of the synthesized bioplastic films.
Fig. 11.
Antibacterial reduction performance of the prepared bioplastic samples (a) with berry seed starch and (b) without berry seeds starch.
Fig. 12.
Bacterial reduction performance comparison.
The prepared bioplastic samples are the ideal candidate for food packaging applications as this material posses the characteristics that can improve foods' shelf life. It can be said from the results that samples S1 possesses characteristics that can protect the food improving the physical barrier and blocking bacterial pass [[53], [54], [55], [56]].
4. Conclusion
Fabrication and characterization of bioplastic from natural sources for food packaging applications have been reported here. Berry seeds starch was incorporated with tamarind seeds starch and licorice root to develop the bioplastic samples. Improved soil biodegradable, tensile, antimicrobial, thermal, and morphological performance was observed from the prepared bioplastic samples. The properties improved due to the increased crosslinking of berry seeds starch with other materials. The incorporation of berry seeds starch improved the properties remarkably and behaved similarly to the materials used for food packaging applications. Therefore, the results indicate that the prepared bioplastic films especially sample S1 can be used as biodegradable alternatives for food packaging applications. Other natural starch can be used to improve the mechanical properties of bioplastic films.
Author contribution statement
Mohammad Asaduzzaman Chowdhury: Conceived and designed the experiments.
Nayem Hossain: Analyzed and interpreted the data; Wrote the paper.
Md. Badrudduza: Performed the experiments.
Md. Masud Rana: Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Contributor Information
Mohammad Asaduzzaman Chowdhury, Email: asad@duet.ac.bd, asadzmn2014@yahoo.com.
Nayem Hossain, Email: nayem.hossain@iubat.edu.
M.D. Badrudduza, Email: badrudduza1978@gmail.com.
Md. Masud Rana, Email: masudranadeepto@gmail.com.
References
- 1.Shruti V.C., Kutralam-Muniasamy G. Bioplastics: missing link in the era of microplastics. Sci. Total Environ. 2019;697 doi: 10.1016/j.scitotenv.2019.134139. [DOI] [PubMed] [Google Scholar]
- 2.Goliszek M., Podko′scielna B., Fila K., Riazanova A.V., Aminzadeh S., Sevastyanova O., Gun’ko V.M. Synthesis and structure characterization of polymeric nanoporous microspheres with lignin. Cellulose. 2018;25:5843–5862. doi: 10.1007/s10570-018-2009-7. [DOI] [Google Scholar]
- 3.Goliszek M., Podko′scielna B. Synthesis and characterization of polymer biocomposites with lignin. Physicochem. Probl. Min. Process. 2019;55:1375–1381. doi: 10.5277/ppmp19055. [DOI] [Google Scholar]
- 4.Goliszek M., Podko′scielna B., Sevastyanova O., Fila K., Chabros A., Pączkowski P. Investigation of accelerated aging of lignin-containing polymer materials. Int. J. Biol. Macromol. 2019;123:910–922. doi: 10.1016/j.ijbiomac.2018.11.141. [DOI] [PubMed] [Google Scholar]
- 5.Podko′scielna Beata, Gargol Mateusz, Goliszek Marta, Klepka Tomasz, Sevastyanova Olena. Degradation and flammability of bioplastics based on PLA and lignin. Polym. Test. 2022;111 doi: 10.1016/j.polymertesting.2022.107622. [DOI] [Google Scholar]
- 6.Wang X.-Z., He J., Weng Y.-X., Zeng J.-B., Li Y.-D. Structure-property relationship in fully biobased epoxidized soybean oil thermosets cured by dicarboxyl terminated polyamide 1010 oligomer with different carboxyl/epoxy ratios. Polym. Test. 2019;79 doi: 10.1007/s11746-012-2102-2. [DOI] [Google Scholar]
- 7.Taufik D., Reinders M.J., Molenveld K., Onwezen M.C. The paradox between the environmental appeal of bio-based plastic packaging for consumers and their disposal behaviour. Sci. Total Environ. 2020;705 doi: 10.1016/j.scitotenv.2019.135820. [DOI] [PubMed] [Google Scholar]
- 8.Farias da Silva J.M., Soares B.G. Epoxidized cardanol-based prepolymer as promising biobased compatibilizing agent for PLA/PBAT blends. Polym. Test. 2021;93 doi: 10.1016/j.polymertesting.2020.106889. [DOI] [Google Scholar]
- 9.Santos I.S., Nascimento B.L., Marino R.H., Sussuchi E.M., Matos M.P., Griza S. Influence of drying heat treatments on the mechanical behavior and physicochemical properties of mycelial biocomposite. Compos. B Eng. 2021;217 doi: 10.1016/j.compositesb.2021.108870. [DOI] [Google Scholar]
- 10.Chegdani F., El Mansori M., Chebbi A.-A. Cutting behavior of flax fibers as reinforcement of biocomposite structures involving multiscale hygrometric shear. Compos. B Eng. 2021;211 doi: 10.1016/j.compositesb.2021.108660. [DOI] [Google Scholar]
- 11.Aminoroaya A., Neisiany R.E., Khorasani S.N., Panahi P., Das O., Madry H., Cucchiarini M., Ramakrishna S. A review of dental composites: challenges, chemistry aspects, filler influences, and future insights. Compos. B Eng. 2021;216 doi: 10.1016/j.compositesb.2021.108852. [DOI] [Google Scholar]
- 12.Perroud Th′eo, Shanmugam Vigneshwaran, Mensah Rhoda Afriyie, Jiang Lin, Xu Qiang, Neisiany Rasoul Esmaeely, Gabriel Sas, Forsth Michael, Kim Nam Kyeun, Hedenqvist Mikael S., Das Oisik. Testing bioplastic containing functionalised biochar. Polym. Test. 2022;113 doi: 10.1016/j.polymertesting.2022.107657. [DOI] [Google Scholar]
- 13.Acquavia M.A., Pascale R., Martelli G., Bondoni M., Bianco G. Natural polymeric materials: a solution to plastic pollution from the agro-food sector. Polymers. 2021;13(158) doi: 10.3390/polym13010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behera Lopamudra, Mohanta Monalisha, Thirugnanam Arunachalam. Intensification of yam-starch based biodegradable bioplastic film with bentonite for food packaging application. Environ. Technol. Innovat. 2022;25 doi: 10.1016/j.eti.2021.102180. [DOI] [Google Scholar]
- 15.Kuru Pinar. Tamarindus indica and its health related effects. Asian Pac. J. Trop. Biomed. 2014;4(9):676–681. doi: 10.12980/APJTB.4.2014APJTB-2014-0173. [DOI] [Google Scholar]
- 16.Paul Suprio R., Nayak Suraj K., Yogalakshmi Yamini, Singh Vinay K. Archana rath, indranil banerjee, arfat anis, kunal pal. Understanding the effect of tamarind gum proportion on the properties of tamarind gum-based hydroethanolic physical hydrogels. Polym. Plast. Technol. Eng. 2018;57(6):540–547. doi: 10.1080/03602559.2017.1329435. [DOI] [Google Scholar]
- 17.Brailson Mansingh B., Binoj J.S., Prem Sai N., Abu Hassan Shukur, Siengchin Suchart, Sanjay M.R., Liu Y.C. Sustainable development in utilization of Tamarindus indica L. and its by-products in industries: a review. Curr. Res. Green Sustain. Chem. 2021;4 doi: 10.1016/j.crgsc.2021.100207. [DOI] [Google Scholar]
- 18.Chandra Mohan C., Harini K., Vajiha Aafrin B., Lalitha priya U., Maria jenita P., Babuskin S., Karthikeyan S., Sukumar M. Extraction and Characterization of polysaccharides from tamarind seeds, rice mill residue, okra waste and sugarcane bagasse for its Bio-thermoplastic properties. Carbohydr. Polym. 2018;186(1):394–401. doi: 10.1016/j.carbpol.2018.01.057. [DOI] [PubMed] [Google Scholar]
- 19.Okatan V. Antioxidant properties and phenolic profile of the most widely appreciated cultivated berry species: a comparative study. Folia Hort. 2020;32:79–85. doi: 10.2478/fhort-2020-0008. [DOI] [Google Scholar]
- 20.Zhang J., Zhang C., Chen X., Quek S.Y. Effect of spray drying on phenolic compounds of cranberry juice and their stability during storage. J. Food Eng. 2020;269 doi: 10.1016/j.jfoodeng.2019.109744. [DOI] [Google Scholar]
- 21.Burgos-Edwards A., Ferna′ ndez-Romero A., Carmona M., Thuissard-Vasallo I., Schmeda-Hirschmann G., Larrosa M. Effects of gastrointestinal digested polyphenolic enriched extracts of Chilean currants (Ribes magellanicum and Ribes punctatum) on in vitro fecal microbiota. Food Res. Int. 2020;129 doi: 10.1016/j.cofs.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Navindra P. Seeram, recent trends and advances in berry health benefits research. J. Agric. Food Chem. 2010;58(7):3869–3870. doi: 10.1021/jf902806j. [DOI] [PubMed] [Google Scholar]
- 23.Sharifi-Rad J., Quispe C., Herrera-Bravo J., Bel′ en L.H., Kaur R., et al. Glycyrrhiza genus: enlightening phytochemical components for pharmacological and health-promoting abilities. Oxid. Med. Cell. Longev. 2021 doi: 10.1155/2021/7571132. 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Chun-xiao, Li Tian-hao, Zhu Mei, Jiang Lai. Zhi-peng Wu, Pharmacological properties of glabridin (a flavonoid extracted from licorice): a comprehensive review. J. Funct.Foods. 2021;85 doi: 10.1016/j.jff.2021.104638. [DOI] [Google Scholar]
- 25.Nigam Shashwat, Apurba K. Das, Mukesh Kumar Patidar, Synthesis, characterization and biodegradation of bioplastic films produced from Parthenium hysterophorus by incorporating a plasticizer (PEG600) Environ. Chall. 2021;5 doi: 10.1016/j.envc.2021.100280. [DOI] [Google Scholar]
- 26.Chowdhury Mohammad Asaduzzaman, Badrudduza M.D., Hossain Nayem, Rana Md. Masud. Development and characterization of natural sourced bioplastic synthesized from tamarind seeds, berry seeds and licorice root. Appl. Surf. Sci. Adv. 2022;11 doi: 10.1016/j.apsadv.2022.100313. [DOI] [Google Scholar]
- 27.Shanmathy Mrithula, Mohanta Monalisha, Thirugnanam A. Development of biodegradable bioplastic films from Taro starch reinforced with bentonite. Surf. Polym. Technol. Appl. 2021;(2) doi: 10.1016/j.carpta.2021.100173. [DOI] [Google Scholar]
- 28.Marichelvam M., Manimaran P., Sanjay M., Siengchin S., Geetha M., Kandakodeeswaran K., Boonyasopon P., Gorbatyuk S. Extraction and development of starch-based bioplastics from Prosopis Juliflora Plant: eco-friendly and sustainability aspects. Curr. Res. Green Sustain. Chem. 2022;5 doi: 10.1016/j.crgsc.2022.100296. [DOI] [Google Scholar]
- 29.Extraction and development of starch-based bioplastics from ProsopisJuliflora Plant: eco-friendly and sustainability aspects. Curr. Res. Green Sustain. Chem. 2022;5 doi: 10.1016/j.crgsc.2022.100296. [DOI] [Google Scholar]
- 30.Behera Lopamudra, Mohanta Monalisha, Thirugnanam Arunachalam. Intensification of yam-starch based biodegradable bioplastic film with bentonite for food packaging application. Environ. Technol. Innovat. 2022;25 doi: 10.1016/j.eti.2021.102180. [DOI] [Google Scholar]
- 31.Baidurah Siti, Takada Seiya, Shimizu Kazuki, Ishida Yasuyuki, Yamane Tsuneo, Ohtani Hajime. Evaluation of biodegradation behavior of poly(butylene succinate-co-butylene adipate) with lowered crystallinity by thermally assisted hydrolysis and methylation-gas chromatography. J. Anal. Appl. Pyrol. 2013;103:73–77. doi: 10.1016/j.jaap.2012.08.011. [DOI] [Google Scholar]
- 32.Mai-Prochnow A., Clauson M., Hong J., Murphy A.B. Gram positive andGram-negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016;6(1) doi: 10.1038/srep38610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Méité N., Konan L.K., Tognonvi M.T., Doubi B.I.H.G., Gomina M., Oyetola S. Properties of hydric and biodegradability of cassava starch-based bioplastics reinforced with thermally modified kaolin. Carbohydr. Polym. 2021;254 doi: 10.1016/j.carbpol.2020.117322. [DOI] [PubMed] [Google Scholar]
- 34.Chowdhury Mohamman Asaduzzaman, Hossain Nayem, Islam Noman Tauhidul, Ali Hasan, Alam Shafiul, Abul Kashem Mohammod. Biodegradable, physical and microbial analysis of tamarind seed starch infused eco-friendly bioplastics by different percentage of Arjuna powder. Results Eng. 2022;13 doi: 10.1016/j.rineng.2022.100387. [DOI] [Google Scholar]
- 35.Strauss G., Gibson S.M. Plant phenolic as cross-linkers of gelatin gels and gelatin-based coacervates for use as food ingredients. Food Hydrocolloids. 2004;18:81–89. doi: 10.1016/S0268-005X(03)00045-6. [DOI] [Google Scholar]
- 36.Biodegradation of phenolic compounds and their metabolites in contaminated groundwater using microbial fuel cells. Bioresour. Technol. 2016;200:426–434. doi: 10.1016/j.biortech.2015.09.092. [DOI] [PubMed] [Google Scholar]
- 37.Prodpran T., Benjakul S., Phatcharat S. Effect of phenolic compounds on protein cross-linking and properties of film from fish myofibrillar protein. Int. J. Biol. Macromol. 2012 doi: 10.1016/j.ijbiomac.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Makishi G.L.A., Lacerda R.S., Bittante A.M.Q.B., Chambi H.N.M., Costa P.A., Gomide C.A., et al. Films based on castor bean (Ricinus communis L.) proteins crosslinked with glutaraldehyde and glyoxal. Ind. Crop. Prod. 2013;50:375–382. doi: 10.1590/1678-4324-2020190141. [DOI] [Google Scholar]
- 39.Choi I., Lee S.E., Chang Y., Lacroix M., Han J. Effect of oxidized phenolic compounds on cross-linking and properties of biodegradable active packaging film composed of turmeric and gelatin. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2018 doi: 10.1016/j.lwt.2018.03.065. [DOI] [Google Scholar]
- 40.Nuthong Pornpot, Benjakul Soottawat, Prodpran Thummanoon. Effect of phenolic compounds on the properties of porcine plasma protein-based film. Food Hydrocolloids. 2009;23:736–741. doi: 10.1016/j.foodhyd.2008.08.003. [DOI] [Google Scholar]
- 41.Coates J. John Wiley & Sons Ltd; Chichester: 2000. Interpretation of Infrared Spectra, a Practical Approach; pp. 10815–10837. (Encyclopedia of Analytical Chemistry). [DOI] [Google Scholar]
- 42.Shanmathy Mrithula, Mohanta Monalisha, Thirugnanam A. Development of biodegradable bioplastic films from Taro starch reinforced with bentonite. Carbohydr. Polym. Technol. Appl. 2021;2 http://hdl.handle.net/2080/3452 [Google Scholar]
- 43.He Y., Pang Y., Liu L., Li X., Wang K. Physicochemical characterization of rice straw pretreated with sodium hydroxide in the solid state for enhancing biogas production. Energy Fuels. 2008;22:2775–2781. doi: 10.1021/ef8000967. [DOI] [Google Scholar]
- 44.Rohmawati B., Atikah Nata Sya’idah F., Rhismayanti R., Alighiri D., Tirza Eden W. Synthesis of bioplastic-based renewable cellulose acetate from teak wood (Tectona grandis) biowaste using glycerol-chitosan plasticizer. Orient. J. Chem. 2018;34:1810–1816. doi: 10.13005/ojc/3404014. [DOI] [Google Scholar]
- 45.Guzman-Puyol Susana, Hierrezuelo Jesús, Benítez José J., Tedeschi Giacomo, Porras-Vazquez José M., Heredia Antonio, Athanassiou Athanassia, Romero Diego, Heredia-Guerrero José A. Transparent, UV-blocking, and high barrier cellulose-based bioplastics with naringin as active food packaging materials. Int. J. Biol. Macromol. 2022;209:1985–1994. doi: 10.1021/acsnano.0c03224. [DOI] [PubMed] [Google Scholar]
- 46.Cheng Yan, Xu Qingzhong, Liu Jing, Zhao Changsheng, Xue Fumin, Zhao Yuzhong. Decomposition of five phenolic compounds in high temperature water. J. Braz. Chem. Soc. 2014;25(11):2102–2107. doi: 10.5935/0103-5053.20140201. [DOI] [Google Scholar]
- 47.Kaczmarska K., Grabowska B., Grabowski G., et al. Thermal decomposition of binder based on etherified starch to use in foundry industry. J. Therm. Anal. Calorim. 2017;130:285–290. doi: 10.1007/s10973-017-6451-9. [DOI] [Google Scholar]
- 48.Smith K.E., Sawicki S., Hyjek M.A., Downey S., Gall K. The effect of the glass transition temperature on the toughness of photopolymerizable (meth)acrylate networks under physiological conditions. Polymer. 2009;50:5112–5123. doi: 10.1016/j.polymer.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaper J.B., Nataro J.P., Mobley H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 50.Steven Y. C. Tong, Davis Joshua S., Eichenberger Emily, Holland Thomas L., Fowler Vance G., Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations and management. Clin. Microbiol. Rev. 2015:28. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mai-Prochnow A., Clauson M., Hong J., Murphy A.B. Gram positive andGram-negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016;6(1) doi: 10.1038/srep38610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maddox C.E., Laur L.M., Tian L. Antibacterial activity of phenolic compounds against the phytopathogen Xylella fastidiosa. Curr. Microbiol. 2010 Jan;60(1):53–58. doi: 10.1007/s00284-009-9501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu M., You D., Zhuang J., Lin S., Dong L., Weng S., Zhang B., Cheng K., Weng W., Wang H. Controlled release of naringin in metal-organic framework-loaded mineralized collagen coating to simultaneously enhance osseointegration and antibacterial activity. ACS Appl. Mater. Interfaces. 2017:19698–19705. doi: 10.1021/acsami.7b05296. [DOI] [PubMed] [Google Scholar]
- 54.Laorenza Y., Harnkarnsujarit N. Carvacrol, citral and α-terpineol essential oil incorporated biodegradable films for functional active packaging of Pacific white shrimp. Food Chem. 2021;363 doi: 10.1016/j.foodchem.2021.130252. [DOI] [PubMed] [Google Scholar]
- 55.Klinmalai P., Srisa A., Laorenza Y., Katekhong W., Harnkarnsujarit N. Antifungal and plasticization effects of carvacrol in biodegradable poly(lactic acid) and poly (butylene adipate terephthalate) blend films for bakery packaging. LWT. 2021;152 doi: 10.1016/j.lwt.2021.112356. [DOI] [Google Scholar]
- 56.Guzman-Puyol Susana, Hierrezuelo Jesús, Benítez José J., Tedeschi Giacomo, Porras-Vazquez José M., Heredia Antonio, Athanassiou Athanassia, Romero Diego, Heredia-Guerrero José A. Transparent, UV-blocking, and high barrier cellulose-based bioplastics with naringin as active food packaging materials. Int. J. Biol. Macromol. 2022;209:1985–1994. doi: 10.1021/acsnano.0c03224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.