Abstract
OBJECTIVES
We applied high-flow regional cerebral perfusion (HFRCP) for aortic arch reconstruction in neonates and infants by monitoring regional oxygen saturation of the thigh (rSO2T) using near-infrared spectroscopy to maintain peripheral perfusion. This study was designed to investigate the optimal perfusion flow of HFRCP for renal protection.
METHODS
From 2009 to 2021, 28 consecutive neonates and infants who underwent aortic arch reconstruction with HFRCP were enrolled. The median age of the patients was 27 days; the median body weight was 3.0 kg. In HFRCP, perfusion flow was targeted at ∼80–100 ml/kg/min and then lowered corresponding to brain rSO2 levels and blood gas data. Isosorbide dinitrate and chlorpromazine were administered to enhance peripheral perfusion flow. Regional oxygen saturation of the forehead and thighs were monitored. The stage of acute kidney injury (AKI) was classified based on the Kidney Disease Improving Global Outcomes criteria.
RESULTS
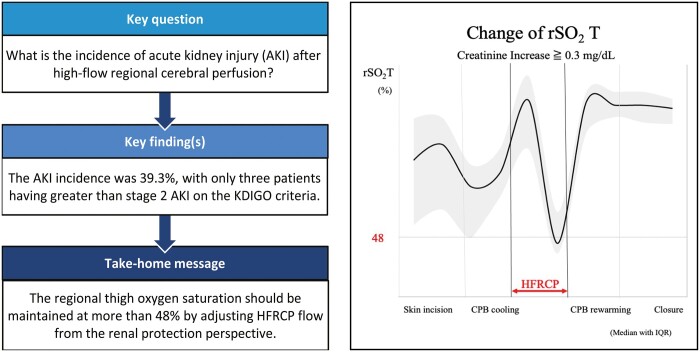
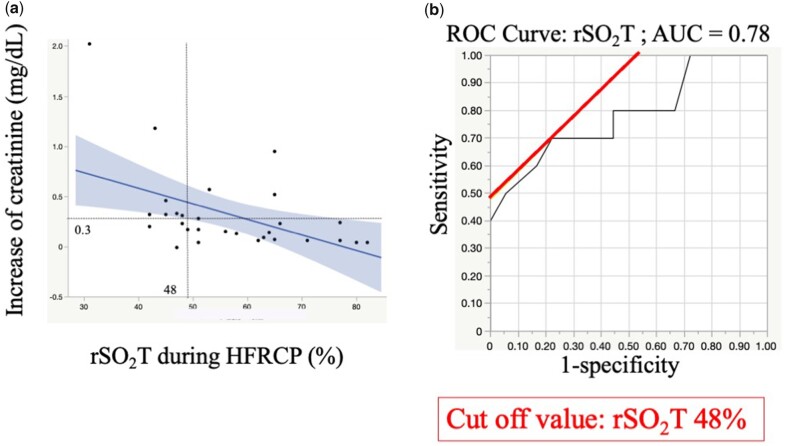
No patients had neurological events and peritoneal dialysis after surgery. The incidence of AKI was 39.3% with only 3 patients having greater than stage 2 AKI. The maximum postoperative serum creatinine concentration was negatively associated with the lowest rSO2T during HFRCP. The rSO2T during HFRCP was a predictive factor for the postoperative creatinine increase of ≧0.3 mg/dL. The area under receiver operating characteristic curve was 0.78 with the cut-off value of 48% for rSO2T.
CONCLUSIONS
The rSO2T during HFRCP is a potential predictor of postoperative renal function. To prevent AKI, the rSO2T should be preserved >48% by increasing HFRCP flow.
Keywords: Aortic arch repairs, Congenital heart surgery, Neonates, Infants
The perfusion strategy for neonatal and infant aortic arch reconstruction has been revolutionized to improve cerebral and organ protection, including the kidneys and liver, as deep hypothermic circulatory arrest (DHCA) for neonatal aortic arch reconstruction may carry a risk of brain and kidney injuries and neuro-developmental problems [1, 2].
INTRODUCTION
The perfusion strategy for neonatal and infant aortic arch reconstruction has been revolutionized to improve cerebral and organ protection, including the kidneys and liver, as deep hypothermic circulatory arrest (DHCA) for neonatal aortic arch reconstruction may carry a risk of brain and kidney injuries and neuro-developmental problems [1, 2]. Selective cerebral perfusion (SCP) with moderate hypothermia diminishes deep hypothermic arrest and protects the brain from hypoxic injury with adequate bypass flow and neurological monitoring using near-infrared spectroscopy (NIRS) [3]. However, this method raises some concerns of relatively low abdominal organ perfusion under moderate hypothermia, which may lead to renal dysfunction. We applied high-flow regional cerebral perfusion (HFRCP) in aortic arch reconstruction in neonates and infants to provide sufficient perfusion to the brain and peripheral organs, such as the kidneys and liver, through collateral arteries from the upper body with monitoring regional oxygen saturation of the thigh (rSO2T) using NIRS [4]. We previously reported that HFRCP during aortic arch reconstruction provides not only cerebral protection but also abdominal organ perfusion, indicated by postoperative low serum lactate concentration and other organ biomarkers [4, 5]. However, the precise renal outcomes after arch reconstruction using this method and the optimal flow of HFRCP from the perspective of renal function remain unveiled. Therefore, this study was designed to investigate the renal outcomes of arch reconstruction with HFRCP and the optimal perfusion flow of HFRCP to prevent postoperative renal dysfunction under the guidance of rSO2T levels.
PATIENTS AND METHODS
Ethical statement
This retrospective single-institution study was approved by the Institutional Review Board of Kitasato University Hospital on 6 September 2021 (approved number: B21-152). Informed consent was obtained using a form of the opt-out approach on the website, which was approved by the Institutional Review Board.
Study design
We collected data on 28 consecutive neonates and infants who underwent aortic arch reconstruction with HFRCP from 2009 to 2021 from the institutional database system. Patients who underwent single-ventricle palliation with aortic arch reconstruction, those who underwent the Norwood procedure and repair of total anomalous venous connection and complete atrioventricular septal defect, those with preoperative renal dysfunction and those with mechanical circulatory support were excluded from this study. Twenty-five patients who underwent repair of coarctation of the aorta (89.3%) and 3 patients who underwent interrupted aortic arch repair (10.7%) were included in this study (Table 1). Eight of 28 patients had spontaneous ductus closure when they were diagnosed with arch anomaly. Soon after the diagnosis of aortic arch anomaly with closed ductus, they underwent arch repair at a median age of 35 days (IQR: 26–65 days) (Table 2). On the other hand, the remaining 20 patients, including those with ductus-dependent circulation, underwent arch repair at a median age of 18 days [interquartile range (IQR): 12–60 days]. Regarding concomitant surgeries, 25 patients underwent ventricular septal defect and/or atrial septal defect repairs, and 1 patient underwent pulmonary artery patch augmentation. The median age of the patients was 27 days (range: 7–360 days); the median body weight was 3.0 kg (range: 1.8–8.8 kg).
Table 1:
Patient characteristics
| Median | IQR | |
|---|---|---|
| Age (days) | 27 | 15–60 |
| Body weight (kg) | 3.0 | 2.6–4.3 |
| Male/female | 21/7 | |
| Diagnosis, n (%) | ||
| Coarctation of aorta | 25 (89.3) | |
| Interruption of aortic arch (type A) | 3 (10.7) | |
| Associated diseases | ||
| Ventricular septal defect | 25 | |
| Atrial septal defect | 21 | |
| 21 trisomy | 2 | |
| CHARGE syndrome | 1 | |
| Aorto-pulmonary window | 1 | |
| Left isomerism | 1 | |
IQR: interquartile range.
Table 2:
Preoperative renal functional parameters and background between ductus-dependent and nonductus-dependent patients
| Ductus-dependent patients (n = 16) | Nonductus-dependent patients (n = 12) | P-Value | |
|---|---|---|---|
| SCr (mg/dl) | 0.43 (0.35–0.51) | 0.35 (0.27–0.43) | 0.13 |
| BW at operation (kg) | 2.8 (2.4–3.3) | 3.4 (3.0–3.3) | 0.07 |
| Age at operation (days) | 18 (11–60) | 33 (23–65) | 0.13 |
| Neonates (%), n (%) | 10 (62.5) | 6 (37.5) | 0.25 |
| Preoperative AKI (%)a | 0 | 0 | N/A |
Numbers are expressed as median with interquartile range.
AKI has been defined according to the Kidney Disease Improving Global Outcomes criteria.
AKI: acute kidney injury; BW: body weight; SCr: serum creatinine, N/A: not applicable.
Surgery and cardiopulmonary bypass
The management of HFRCP and details of aortic arch reconstruction were reported previously [4, 6]. Briefly, surgery was performed via median sternotomy. A 3.5-mm GORE-TEX tube (W. L. Gore & Associates, Flagstaff, AZ, USA) was anastomosed to the right innominate artery and was connected to the arterial cannula. After bicaval cannulation, cardiopulmonary bypass (CPB) was initiated. After CPB was started, haematocrit was adjusted at a range of 27–30%. If the target haematocrit levels were not achieved, a pack of erythrocytes was transfused to increase haematocrit. The CPB system consisted of a low-prime reservoir (Baby-FX; Terumo, Tokyo, Japan), low-prime oxygenator and a distant roller pump [6]. The size of the extracorporeal tubing was selected according to the patient’s body weight. Blood gas was managed using the pH-stat strategy. After dissecting the aorta, the descending aorta and neck vessels were clamped, and HFRCP was started. Perfusion flow during HFRCP was targeted at approximately 80–100 ml/kg/min and lowered corresponding to brain rSO2 levels and blood gas data under moderate hypothermia (28–30°C). The pressure of the radial artery was maintained at 35–40 mmHg during total bypass, and the target maximum pressure of the radial artery during HFRCP was 45 mmHg. Isosorbide dinitrate (2.0 µg/kg/min) and chlorpromazine (3.0 mg/kg) were administered to decrease the resistance of peripheral vessels and enhance peripheral perfusion through collateral arteries of the upper body to the lower body. Lower body temperature was maintained at ∼28°C during HFRCP. We concentrated the patients’ blood according to the mixed venous saturation with the target range of 75–85%. If the target rSO2 T (>50%) and mixed venous saturation were not achieved despite an increase in CPB flow and inspired oxygen fraction with sufficient amount of vasodilator, the patients were cooled down to around 25% or lower. In such cases, the haematocrit level was adjusted at ∼25% to maintain blood viscosity in peripheral circulation. Crystalloid cardioplegia (10 ml/kg) was administered every 20 min. Ductal tissues were resected around the descending aorta, and aortic arch reconstruction with end-to-side anastomosis or extended end-to-end anastomosis was performed. After declamping the descending aorta and neck vessels, the patient was rewarmed. The targeted haematocrit level during the rewarming phase was 27–30%. After CPB was terminated, modified ultrafiltration was performed at 20 ml/kg/min for 10 min.
Monitoring and data measurement
Regional oxygen saturation (rSO2) on both sides of the forehead (rSO2H), flank (rSO2F) and bilateral thighs was monitored for cerebral and peripheral perfusion using the NIRS system (INVOS 5100; Somanetics, Troy, MI, USA) during HFRCP. The rSO2 values at each site were continuously collected from all patients through the surgeries. Blood samples for biomarkers of peripheral perfusion, such as aspartate transaminase, alanine transaminase, lactate dehydrogenase, creatinine kinase (CK), CK-myocardial band, blood urea nitrogen, creatinine, lactate and C-reactive protein, were measured before surgery, during admission to the paediatric intensive care unit (PICU) and after 1–3 days. The maximum serum creatinine and CK values were defined as the largest numbers in the first 3 postoperative days.
Criteria for acute kidney injury after aortic arch reconstruction
The stage of acute kidney injury (AKI) was classified based on the Kidney Disease Improving Global Outcomes (KDIGO) criterion, since most studies have used this criterion for the paediatric population in the meta-analysis of paediatric kidney injury [7, 8]. The AKI stages are as follows: stage 0, no AKI; stage 1, ≧0.3 mg/dl increase within 48 h or 1.5–1.9-fold increase in serum creatinine from baseline within 7 days or urine output of <0.5 ml/kg/h for >6 h; stage 2, 2–2.9-fold increase in serum creatinine from baseline within 7 days or <0.5 ml/kg/h for 12 h; stage 3, ≧3-fold increase in serum creatinine from baseline within 7 days or increase to ≧4 mg/dl with an acute increase of 0.5 mg/dl or initiation of dialysis or <0.3 ml/kg/h for 24 h or anuria for 12 h.
Statistical analysis
Continuous variables were expressed as means and standard deviations for normally distributed values or medians and IQRs (25th and 75th percentiles) for skewedly distributed values. Categorized variables were expressed as numbers and percentages. Mann–Whitney U-test was used to compare the groups for nonparametric data. To assess the effect of preoperative and perioperative factors on postoperative renal function, multivariate linear regression analysis of postoperative serum creatinine level increase (= postoperative maximum serum creatinine level − preoperative serum creatinine level) using the least-squares method was performed after the adjustment of factors associated with postoperative creatinine increase on the univariate linear regression analysis with a P-value of <0.05. The optimal cut-off level of rSO2 with the combination of the largest sensitivity and specificity for postoperative increase in serum creatinine of ≥0.3 mg/dl was analysed using the receiver operating characteristic curve (ROC). Statistical analysis was performed using JMP Pro (version 16; SAS institute, Cary, NC, USA). Differences with P-values of <0.05 were considered statistically significant.
RESULTS
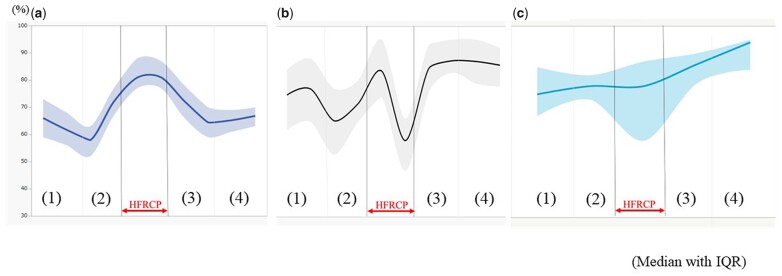
No surgical mortality was observed. No patient had neurological events, including seizures, after the surgery. The median duration of intubation after surgery was 6 days (IQR: 4–8.5 days), and the median duration of PICU stay was 9 days (IQR: 7–16 days). They had no significant difference in terms of preoperative serum creatinine level, body weight at operation, age at operation and preoperative AKI (Table 2) and were divided into 2 groups according to the preoperative status of ductus dependency. Detailed data on CPB management, such as CPB time, the total bypass time, the cross-clamp time of the ascending and descending aorta, the CPB flow rate, the HFRCP flow rate, systemic blood pressure during HFRCP, the pressure difference between circuit and radial artery during HFRCP, the lowest temperature during HFRCP and haematocrit during HFRCP, are outlined in Table 3. The serial changes in rSO2H and rSO2T during surgery are shown in Fig. 1. The rSO2H values rose from 57% (IQR: 52–63%) to 77% (IQR: 62–84%) along with cooling of body temperature and reached the highest values (82%, IQR: 65–88%) during HFRCP, followed by coming down to the baseline value during rewarming after the release of cross-clamp of the descending aorta. In contrast, after the rSO2T values increased to 85% (IQR: 74–95%) during the CPB cooling phase (Figure 1b (2), which dropped to the lowest value of 53% (IQR: 47–66%) during HFRCP (Figure 1b - HFRCP) and then recovered to the baseline levels in the rewarming period. The serial changes in rSO2F values showed apparent discrepancy with those in rSO2T values, especially during HFRCP. The rSO2F values increased slightly from 75% (IQR: 67–85%) to 78% (IQR: 73–82%) along with the cooling of body temperature. The rSO2F value was 78% (IQR: 58–87%) during HFRCP and then it increased to 86% (IQR: 79–90%) during the rewarming phase after declamping of the descending aorta.
Table 3:
Data of CPB management for aortic arch reconstruction in neonates and infants
| Median | IQR | |
|---|---|---|
| CPB time (min) | 134 | 113–155 |
| Cross-clamp time (min) | 61 | 54–76 |
| Descending aorta clamp time (min) | 36 | 29–44 |
| CPB flow (ml/min/kg) | 134.9 | 118–145.8 |
| HFRCP flow (ml/min/kg) | 85.2 | 70.7–102.1 |
| Systemic blood pressure during HFRCP (mmHg) | 44 | 42–44 |
| Lowest temperature during CPB (°C) | 28 | 26.6–28.3 |
| Lowest haematocrit during CPB (%) | 26.3 | 24.6–27.2 |
CPB: cardiopulmonary bypass; HFRCP: high-flow regional cerebral perfusion; IQR: interquartile range.
Figure 1:
Serial change in rSO2H (forehead) (%), rSO2T (thigh) and rSO2F (flank) at skin incision, CPB cooling, HFRCP, CPB rewarming and chest closure. Values are expressed as median with interquartile range. (a) rSO2H, (b) rSO2T and (c) rSO2F. (1) Skin incision, (2) CPB cooling, (3) CPB rewarming and (4) chest closure. CPB: cardiopulmonary bypass; HFRCP: high-flow regional cerebral perfusion.
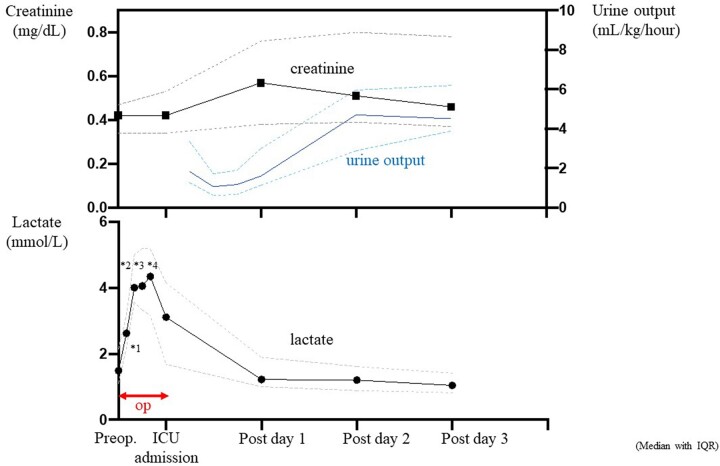
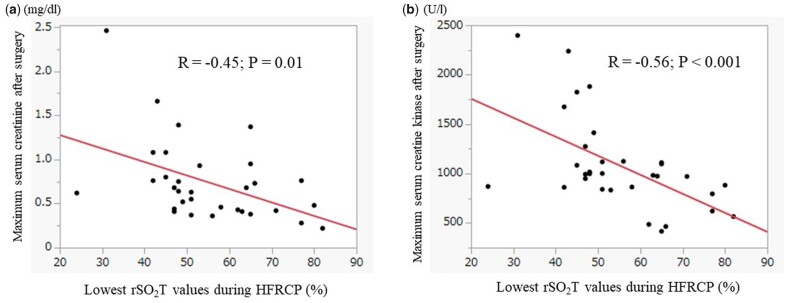
Serial changes in biomarkers of peripheral perfusion and renal function are summarized in Fig. 2. Serum creatinine concentration did not change during PICU admission compared with the preoperative values and increased from 0.41 mg/dl (IQR: 0.34–0.52 mg/dl) to the largest value of 0.55 mg/dl (IQR: 0.37–0.76 mg/dl) 1 day after surgery. The serum creatinine level decreased to 0.45 mg/dl (IQR: 0.37–0.73 mg/dl) 3 days after surgery. The urine output reduced from 1.7 ml/kg/h (IQR: 1.28–3.3 ml/kg/h) to 1.1 ml/kg/h (IQR: 0.6–1.7 ml/kg/h) 6–12 h after surgery. Then, it gradually recovered from 18 h after surgery to postoperative day 2. The plasma lactate concentration had the largest values of 3.0 mmol/l (IQR: 1.6–4.0 mmol/l) during PICU admission, reduced to 1.2 mmol/l (IQR: 1.0–2.0 mmol/l) on postoperative day 1 and gradually decreased through postoperative day 3. The maximum postoperative serum creatinine concentration was negatively associated with the lowest rSO2T values during HFRCP (R = −0.45; P = 0.01), and the maximum serum CK value also showed a negative correlation with the lowest rSO2T values during HFRCP (R = −0.56; P < 0.001) (Fig. 3). The incidence of AKI was 39.3% (11/28) with only 3 patients (10.7%) having greater than AKI stage 2 of the KDIGO criterion (Table 4). No significant difference in the incidence of postoperative AKI was observed between ductus-dependent patients and non-ductus-dependent patients. No patients required peritoneal dialysis after arch reconstruction surgeries. All patients recovered to AKI stage 0 (no AKI) within the first week after surgery.
Figure 2:
Serial change in serum creatinine (mg/dl) and urine output (ml/kg/h) (upper graph) and change in plasma lactate concentration (lower graph) at preoperative, surgery, intensive care unit admission and postoperative days 1–3. Serum creatinine concentration rose to a peak level during the first 24 h after surgery, whereas the urine output declined through the first 12 h after surgery and recovered after 18 h after surgery. Plasma lactate increased to over 4 mmol/l during HFRCP and gradually decreased to baseline levels during the first 24 h after surgery. Values are expressed as median with interquartile range. *1: cardiopulmonary bypass cooling; *2: HFRCP; *3: rewarming; *4: termination of cardiopulmonary bypass. HFRCP: high-flow regional cerebral perfusion; op: operation.
Figure 3:
Correlation of the lowest rSO2T values with postoperative maximum serum creatinine and creatine kinase. (a) The lowest rSO2T values during HFRCP were negatively correlated with the maximum postoperative serum creatinine concentration (R = −0.45, P = 0.01). (b) The lowest rSO2T values were also negatively correlated with the maximum serum creatine kinase (R = −0.56, P < 0.001). HFRCP: high-flow regional cerebral perfusion; rSO2T: regional oxygen saturation of the thigh.
Table 4:
The incidence of acute kidney injury after arch reconstruction with high-flow regional cerebral perfusion based on the Kidney Disease Improving Global Outcomes criterion
| Total | Ductus-dependent patients (n = 16) | Non-ductus-dependent patients (n = 12) | P-Value | |
|---|---|---|---|---|
| Incidence of AKI | 11/28 (39.3%) | 7/16 (43.8%) | 4/12 (33.3%) | 0.30 |
| Stage 0 | 17/28 (60.7%) | 9/16 (56.3%) | 8/12 (66.7%) | |
| Stage 1 | 8/28 (28.6%) | 4 (25.0%) | 4/12 (33.3%) | |
| Stage 2 | 2/28 (7.1%) | 2 (12.5%) | 0/12 (0%) | |
| Stage 3 | 1/28 (3.6%) | 1 (6.3%) | 0/12 (0%) |
AKI: acute kidney injury.
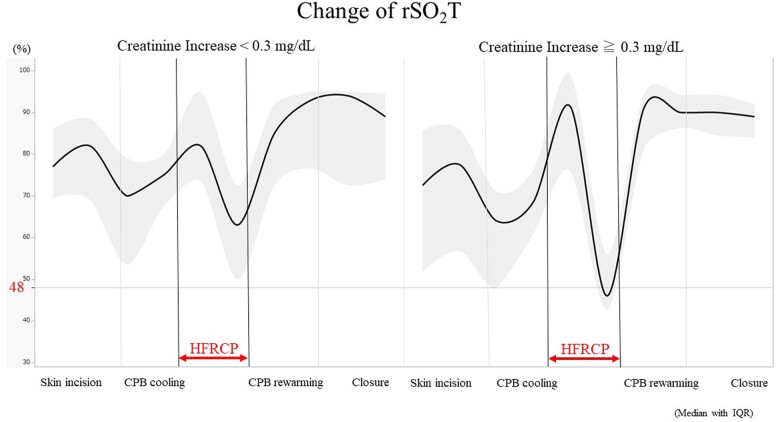
To assess which variables affected postoperative renal function after aortic arch repair under HFRCP, we performed a univariate linear regression analysis to investigate factors associated with the postoperative increase in serum creatinine by entering the following variables: age at operation, lowest rSO2T, descending aortic clamp time, CPB time, HFRCP flow, serum lactate at CPB termination and the lowest haematocrit during CPB (Table 5). Then, we performed a multivariate linear regression analysis of the postoperative increase of serum creatinine, which revealed that lowest rSO2T and longer CPB time were independent risk factors (Table 6). The predictive factor of rSO2T during HFRCP was determined for postoperative increases in serum creatinine of ≥0.3 mg/dl compared with preoperative levels (greater than stage 1 AKI on KDIGO criteria). The area under the curve of ROC was 0.78, with an rSO2T cut-off value of 48%, a sensitivity of 0.70 and a specificity of 0.78 (Fig. 4). The comparisons of rSO2T between the increased creatinine group and the lower change in creatinine group are shown in Fig. 5. RSO2T levels were observed to significantly decrease to <48% in the group with a creatinine increase of ≥0.3 mg/dl during HFRCP. Furthermore, among the patients who had rSO2T ≤48% during HFRCP, a longer time spent (≤48% of rSO2T) during HFRCP was observed in the group with a creatinine increase of ≥0.3 mg/dl after surgery than in that with a creatinine increase of <0.3 mg/dl (20 min [IQR: 13–29 min] versus 8 min [IQR: 2–13 min]; P = 0.04).
Table 5:
Univariate analysis of variables associated with the postoperative serum creatinine level increase
| Coefficient | 95% CI | P-Value | |
|---|---|---|---|
| Age at operation (days) | −0.002 | −0.004 to −0.001 | 0.18 |
| Lowest rSO2T during HFRCP (%) | −0.015 | −0.027 to −0.003 | 0.01 |
| Descending aortic clamp time (min) | 0.008 | −0.005 to −0.021 | 0.23 |
| CPB time (min) | 0.006 | 0.002 to 0.009 | 0.002 |
| HFRCP flow (ml/kg/min) | 0.002 | −0.007 to 0.010 | 0.68 |
| Serum lactate at the termination of CPB (mmol/l) | 0.011 | −0.002 to 0.023 | 0.08 |
| Lowest haematocrit during CPB (%) | 0.028 | −0.071 to 0.128 | 0.56 |
CI: confidence interval; CPB: cardiopulmonary bypass; HFRCP: high-flow regional cerebral perfusion; rSO2T: regional oxygen saturation of the thigh.
Table 6:
Multivariate analysis of variables associated with the postoperative serum creatinine level increase
| Coefficient | 95% CI | P-Value | |
|---|---|---|---|
| Lowest rSO2T during HFRCP (%) | −0.011 | −0.022 to −0.001 | 0.03 |
| CPB time (min) | 0.005 | 0.002 to 0.008 | 0.004 |
CI: confidence interval; CPB: cardiopulmonary bypass; HFRCP: high-flow regional cerebral perfusion; rSO2T: regional oxygen saturation of the thigh.
Figure 4:
(a) The correlation between rSO2T values during HFRCP and the increase in serum creatinine after aortic arch reconstruction. (b) ROC analysis of rSO2T to predict the increase in creatinine of ≧0.3 mg/dl after arch reconstruction. The AUC was 0.79 with a sensitivity of 0.73 and a specificity of 0.81. A cut-off value of rSO2T of ≦48% was a predictor of postoperative increase in the creatinine of ≧0.3 mg/dl. AUC: area under the ROC; HFRCP, high-flow regional cerebral perfusion; ROC: receiver operating curve; rSO2T: regional oxygen saturation of the thigh.
Figure 5:
Intraoperative change in rSO2T between the group with a postoperative creatinine increase of ≧0.3 and <0.3 mg/dl. Data are expressed as median with interquartile range. CPB: cardiopulmonary bypass; HFRCP: high-flow regional cerebral perfusion; rSO2T: regional oxygen saturation of the thigh.
DISCUSSION
This study demonstrated acceptable outcomes of renal function after aortic arch reconstruction with HFRCP in neonates and infants with an incidence rate of AKI of 38.7% and no requirement of peritoneal dialysis for postoperative intensive care. Previously, we reported that the oxygen delivery ratio (DO2R) was sensitive to peripheral perfusion during HFRCP and could predict postoperative low lactate concentration after aortic arch construction [9]. However, calculating DO2R values during CPB was difficult for perfusionists, and they needed practical cut-off values to adjust pump flow and vasodilators in clinical setting. Therefore, we sought the optimal flow of HFRCP under moderate hypothermia during clamping of the descending aorta for aortic arch anastomosis from the perspective of postoperative renal function under the guidance of rSO2T values. Looking at Fig. 1, while rSO2H continued to increase and maintained at high saturated levels after clamping of the descending aorta, rSO2T values increased to the highest value after HFRCP initiation and declined but maintained baseline rSO2T values. This suggests that collateral arteries arising from the upper body keep the blood flow to the lower body even after clamping of the descending aorta. To maintain peripheral flow on rSO2T, in addition to increasing pump flow of HFRCP, we administered vasodilators, including isosorbide dinitrate and chlorpromazine, to decrease the resistance of the brain and abdominal organs to increase oxygen delivery to these organs and enhance collateral flows from the arterial circle of Willis, internal mammary and intercostal arteries, resulting in higher urine output after surgery than that without the administration of vasodilators [4, 5]. We also kept haematocrit at 27–30% by transfusing erythrocytes to maintain rSO2H and rSO2T. Furthermore, monitoring radial artery pressure and limiting bypass flow to under 45 mmHg of the radial artery pressure are important to avoid excess cerebral blood flow and subsequent brain oedema. Based on the results of this study, we should maintain rSO2T at >48% during HFRCP along with CPB to preserve renal function.
Although different AKI criteria in studies make determining the superiority of cerebral and renal protection difficult among different perfusion techniques for neonatal arch reconstruction, our results regarding AKI incidence seem to be comparable with other techniques, such as double aortic cannulation under moderate hypothermia [10–12]. DHCA is a traditionally used technique for arch reconstruction in neonates and is still routinely used among many centres in North America [13]. DHCA lowers oxygen consumption and metabolic demand, which increase tolerance to hypoxia in the brain and provide a bloodless operative field; however, the disadvantages of this method were neurological impairment, reperfusion injury to organs and coagulopathy [2, 11, 14]. Therefore, the strategy of prefusion during aortic arch reconstruction shifted to the avoidance of DHCA [15]. SCP with moderate hypothermia leads to favourable outcomes of cerebral and renal function compared with DHCA; however, there remain some concerns about visceral perfusion during aortic arch anastomosis on SCP alone [11, 15]. Moreover, whether SCP alone is superior to DHCA for arch reconstruction has been debateable, and the result, especially in renal outcomes, varies among studies possibly because the target of cooling temperature for SCP during cross-clamping of the descending aorta differs from 18°C to 28°C among centres [12, 14, 16]. Alternatively, distal perfusion with cannulation to the descending aorta provides continuous flow to visceral organs and prevents the occurrence of postoperative AKI in addition to the advantage of SCP alone regarding brain protection [10, 11, 14, 17]. Böttcher et al. have shown a lower incidence of stage 2 and 3 AKI in the SCP with descending aortic cannulation group than that in the DHCA group (12.2% vs 29.4%; P = 0.02) [18]. A retrospective study comparing between SCP alone and SCP with descending aortic cannulation has shown SCP with descending aortic cannulation had significantly lower incidence of AKI stage 2 (4.8%) than SCP alone (19%) (P = 0.032) in addition to the lower requirement of renal replacement therapy in SCP with descending aortic cannulation than that in SCP alone (28.6% vs 49.4%; P = 0.002) [11]. Kulyabin et al. [10] have reported higher renal oxygen saturation during descending aorta cross-clamping in the SCP with descending aortic cannulation group than that in the SCP with moderate hypothermia and DHCA groups in a pilot prospective randomized study. Although SCP with descending aortic cannulation did not reduce the incidence of postoperative AKI in this prospective randomized study, this could be explained as the sample size in this study was too small to detect the difference between the 3 techniques. Our results showed comparable low incidence of AKI with published data on SCP with descending aortic cannulation. Furthermore, our perfusion method raises no concerns regarding bleeding around the cannulated descending aorta, provides a simpler operative field without additional cannula and does not require dissection around the descending aorta, which may be challenging in small neonates and may increase the risk of chylothorax.
As several centres use HFRCP routinely at lower temperatures, often in the DHCA range, the question can arise that HFRCP combined with a lower DHCA temperature range can reduce the incidence of postoperative AKI. Patients are not routinely cooled down to the DHCA range during HFRCP; therefore, there are no data about this. As a subanalysis of this study, patients were divided into 2 groups: patients cooled down under 27° (n = 9) and patients maintained over 27° during HFRCP (n = 19). Nine of 19 patients (47.3%) who were maintained over 27° (median, 28°; IQR, 27.8–29.6°) during HFRCP had a postoperative creatinine increase of ≥0.3 mg/dl, while only 1 of 9 patients (11.1%) cooled down under 27° (median, 25°, IQR; 24.7–26.7°) during HFRCP had a postoperative creatinine increase of ≥0.3 mg/dl. However, this result was not statistically significant (P = 0.10). It could be suggested that HFRCP with the maintenance of lower temperature during aortic arch repair was better than higher temperature during HFRCP from the postoperative renal function perspective, if the sample size of the lower temperature group increased.
In this study, rSO2T was used to predict postoperative AKI on ROC analysis, whereas rSO2 of the flank did not show a correlation with postoperative renal function. The same phenomenon was observed that rSO2T rather than rSO2 of the flank had a cut-off value of 67% to predict the development of postoperative AKI within 24 h after adult cardiac surgeries [19]. In the review article on NIRS application by Mu et al. [20], the association of haemodynamic and regional oxygen saturation on different sites, including the brain, anterior abdomen, kidneys and legs, depends on ages, diseases, surgery types and the degrees of maturity in children. They have suggested that the site of NIRS probe placement to detect haemodynamic during surgery should be decided by targeted tissue bed allocated by the patient population and the types of surgical interventions. Presumably, our results could explain that rSO2T is more sensitive in detecting peripheral perfusion than rSO2 of the flank, which could involve monitoring of other abdominal organs, such as the intestines and liver, due to the range of waveform from NIRS probes and the position of patients, whereas probes of rSO2T detect waveforms mainly reflected by monotonous skeletal muscles of the legs.
Limitations
Our renal outcomes after arch reconstruction were acceptable and comparable with those after other perfusion techniques for arch reconstruction in children, such as SCP with descending aortic cannulation; however, concluding that our method is superior to others was difficult. First, we did not compare outcomes among different perfusion techniques, including DHCA, HFRCP and SCP with descending aortic cannulation, as we had not used other techniques, besides HFRCP, for aortic arch reconstruction in children. Second, the comparison of the incidence of AKI between our results and those of other studies may not be accurate since different AKI criteria, such as the AKIN and paediatric RIFLE criteria, were used in each article.
CONCLUSIONS
The occurrence of more than moderate AKI (more than AKI stage 2) after aortic arch reconstruction with HFRCP was low. The rSO2T during HFRCP is a potential predictor of postoperative renal function. To prevent acute renal dysfunction, rSO2T should be maintained at >48% along with increasing HFRCP flow and administering vasodilators.
Conflict of interest: none declared.
Glossary
ABBREVIATIONS
- AKI
Acute kidney injury
- CK
Creatinine kinase
- CPB
Cardiopulmonary bypass
- DHCA
Deep hypothermic circulatory arrest
- HFRCP
High-flow regional cerebral perfusion
- IQR
Interquartile range
- KDIGO
Kidney Disease Improving Global Outcomes
- NIRS
Near-infrared spectroscopy
- PICU
Paediatric intensive care unit
- ROC
Receiver operating characteristic curve
- rSO2T
Regional oxygen saturation of the thigh
- SCP
Selective cerebral perfusion
Contributor Information
Fumiaki Shikata, Department of Cardiovascular Surgery, Kitasato University, Kanagawa, Japan.
Kagami Miyaji, Department of Cardiovascular Surgery, Kitasato University, Kanagawa, Japan.
Satoshi Kohira, Department of Medical Engineering, Kitasato University Hospital, Kanagawa, Japan.
Hiroshi Goto, Department of Cardiovascular Surgery, Kitasato University, Kanagawa, Japan.
Shinzo Torii, Department of Cardiovascular Surgery, Kitasato University, Kanagawa, Japan.
Tadashi Kitamura, Department of Cardiovascular Surgery, Kitasato University, Kanagawa, Japan.
Toshiaki Mishima, Department of Cardiovascular Surgery, Kitasato University, Kanagawa, Japan.
Masaomi Fukuzumi, Department of Cardiovascular Surgery, Kitasato University, Kanagawa, Japan.
Shunichiro Fujioka, Department of Cardiovascular Surgery, Kitasato University, Kanagawa, Japan.
Akihiro Sasahara, Department of Cardiovascular Surgery, Kitasato University, Kanagawa, Japan.
Haruna Araki, Department of Cardiovascular Surgery, Kitasato University, Kanagawa, Japan.
Data availability
All relevant data are included in the manuscript. The data underlying this article will be shared upon reasonable request to the corresponding author.
Author contributions
Fumiaki Shikata: Project administration; Writing—original draft. Kagami Miyaji: Supervision. Satoshi Kohira: Conceptualization; Data curation; Formal analysis; Investigation. Hiroshi Goto: Data curation; Investigation. Shinzo Torii: Data curation; Investigation. Tadashi Kitamura: Data curation; Supervision. Toshiaki Mishima: Conceptualization; Data curation. Masaomi Fukuzumi: Data curation; Validation. Shunichiro Fujioka: Data curation; Formal analysis. Akihiro Sasahara: Data curation; Visualization. Haruna Araki: Data curation; Validation.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Erle H. Austin, Emre Belli and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Presented at the Oral Session of the Fifty-eighth Annual Meeting of the Society of Thoracic Surgeons, Miami, Florida, 29–31 January 2022.
REFERENCES
- 1. Lodge AJ, Andersen ND, Turek JW.. Recent advances in congenital heart surgery: alternative perfusion strategies for infant aortic arch repair. Curr Cardiol Rep 2019;21:13. [DOI] [PubMed] [Google Scholar]
- 2. Wypij D, Newburger JW, Rappaport LA, duPlessis AJ, Jonas RA, Wernovsky G. et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg 2003;126:1397–403. [DOI] [PubMed] [Google Scholar]
- 3. Fraser CD Jr, Andropoulos DB.. Principles of antegrade cerebral perfusion during arch reconstruction in newborns/infants. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2008;11:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miyaji K, Miyamoto T, Kohira S, Itatani K, Tomoyasu T, Inoue N. et al. Regional high-flow cerebral perfusion improves both cerebral and somatic tissue oxygenation in aortic arch repair. Ann Thorac Surg 2010;90:593–9. [DOI] [PubMed] [Google Scholar]
- 5. Miyamoto T, Miyaji K, Okamoto H, Kohira S, Tomoyasu T, Inoue N. et al. Higher cerebral oxygen saturation may provide higher urinary output during continuous regional cerebral perfusion. J Cardiothorac Surg 2008;3:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miyaji K, Miyamoto T, Kohira S, Nakashima K, Inoue N, Sato H. et al. Miniaturized cardiopulmonary bypass system in neonates and small infants. Interact Cardiovasc Thorac Surg 2008;7:75–9. [DOI] [PubMed] [Google Scholar]
- 7. Van den Eynde J, Cloet N, Van Lerberghe R, Sa M, Vlasselaers D, Toelen J. et al. Strategies to prevent acute kidney injury after pediatric cardiac surgery: a network meta-analysis. Clin J Am Soc Nephrol 2021;16:1480–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jetton JG, Askenazi DJ.. Acute kidney injury in the neonate. Clin Perinatol 2014;41:487–502. [DOI] [PubMed] [Google Scholar]
- 9. Sughimoto K, Kohira S, Hayashi H, Torii S, Kitamura T, Horai T. et al. Markers of peripheral perfusion during high-flow regional cerebral perfusion for aortic arch repair. J Thorac Cardiovasc Surg 2018;156:2251–7. [DOI] [PubMed] [Google Scholar]
- 10. Kulyabin YY, Bogachev-Prokophiev AV, Soynov IA, Omelchenko AY, Zubritskiy AV, Gorbatykh YN.. Clinical assessment of perfusion techniques during surgical repair of coarctation of aorta with aortic arch hypoplasia in neonates: a pilot prospective randomized study. Semin Thorac Cardiovasc Surg 2020;32:860–71. [DOI] [PubMed] [Google Scholar]
- 11. Kulyabin YY, Gorbatykh YN, Soynov IA, Zubritskiy AV, Voitov AV, Bogachev-Prokophiev AV.. Selective antegrade cerebral perfusion with or without additional lower body perfusion during aortic arch reconstruction in infants. World J Pediatr Congenit Heart Surg 2020;11:49–55. [DOI] [PubMed] [Google Scholar]
- 12. Kornilov IA, Sinelnikov YS, Soinov IA, Ponomarev DN, Kshanovskaya MS, Krivoshapkina AA. et al. Outcomes after aortic arch reconstruction for infants: deep hypothermic circulatory arrest versus moderate hypothermia with selective antegrade cerebral perfusion. Eur J Cardiothorac Surg 2015;48:e45-50–e50. [DOI] [PubMed] [Google Scholar]
- 13. Meyer DB, Jacobs JP, Hill K, Wallace AS, Bateson B, Jacobs ML.. Variation in perfusion strategies for neonatal and infant aortic arch repair: contemporary practice in the STS congenital heart surgery database. World J Pediatr Congenit Heart Surg 2016;7:638–44. [DOI] [PubMed] [Google Scholar]
- 14. Fernández-Doblas J, Ortega-Loubon C, Pérez-Andreu J, Linés M, Fernández-Molina M, Abella RF.. Selective visceral perfusion improves renal flow and hepatic function in neonatal aortic arch repair. Interact Cardiovasc Thorac Surg 2018;27:395–401. [DOI] [PubMed] [Google Scholar]
- 15. Asou T, Kado H, Imoto Y, Shiokawa Y, Tominaga R, Kawachi Y. et al. Selective cerebral perfusion technique during aortic arch repair in neonates. Ann Thorac Surg 1996;61:1546–8. [DOI] [PubMed] [Google Scholar]
- 16. Gupta B, Dodge-Khatami A, Tucker J, Taylor MB, Maposa D, Urencio M. et al. Antegrade cerebral perfusion at 25°C for arch reconstruction in newborns and children preserves perioperative cerebral oxygenation and serum creatinine. Transl Pediatr 2016;5:114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hammel JM, Deptula JJ, Karamlou T, Wedemeyer E, Abdullah I, Duncan KF.. Newborn aortic arch reconstruction with descending aortic cannulation improves postoperative renal function. Ann Thorac Surg 2013;96:1721–6. [DOI] [PubMed] [Google Scholar]
- 18. Bottcher W, Weixler V, Redlin M, Murin P, Dehmel F, Schmitt K. et al. Acute kidney injury after neonatal aortic arch surgery: deep hypothermic circulatory arrest versus moderate hypothermia with distal aortic perfusion. World J Pediatr Congenit Heart Surg 2021;12:573–80. [DOI] [PubMed] [Google Scholar]
- 19. Sakaki K, Kitamura T, Kohira S, Torii S, Mishima T, Hanayama N. et al. Regional thigh tissue oxygen saturation during cardiopulmonary bypass predicts acute kidney injury after cardiac surgery. J Artif Organs 2020;23:315–20. [DOI] [PubMed] [Google Scholar]
- 20. Mu DL, Wang DX, Meng L.. Incremental value of noncerebral somatic tissue oxygenation monitoring for patients undergoing surgery. Curr Opin Anaesthesiol 2019;32:50–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are included in the manuscript. The data underlying this article will be shared upon reasonable request to the corresponding author.