Abstract
Introduction
The lack of definitive means to prevent or treat cognitive impairment or dementia is driving intense efforts to identify causal mechanisms. Recent evidence suggests clinically meaningful declines in cognition might present as early as middle age. Studying cognitive changes in middle adulthood could elucidate modifiable factors affecting later cognitive and health outcomes, yet few cognitive ageing studies include this age group. The purpose of the MidCog study is to begin investigations of less-studied and potentially modifiable midlife determinants of later life cognitive outcomes.
Methods and analysis
MidCog is a prospective cohort study of adults ages 35–64, with two in-person interviews 2.5 years apart. Data will be collected from interviews, electronic health records and pharmacy fill data. Measurements will include health literacy, self-management skills, cognitive function, lifestyle and health behaviours, healthcare use, health status and chronic disease outcomes. Associations of health literacy and self-management skills with health behaviours and cognitive/health outcomes will be examined in a series of regression models, and moderating effects of modifiable psychosocial factors.
Finally, MidCog data will be linked to an ongoing, parallel cohort study of older adults recruited at ages 55–74 in 2008 (‘LitCog’; ages 70–90 in 2023), to explore associations between age, health literacy, self-management skills, chronic diseases, health status and cognitive function among adults ages 35–90.
Ethics and dissemination
The Institutional Review Board at Northwestern University has approved the MidCog study protocol (STU00214736). Results will be published in peer-reviewed journals and summaries will be provided to the funders of the study as well as patients.
Keywords: Dementia, HEALTH SERVICES ADMINISTRATION & MANAGEMENT, EPIDEMIOLOGIC STUDIES, PRIMARY CARE, PUBLIC HEALTH
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The MidCog will be the first longitudinal cohort study to investigate how health literacy and self-management skills, across the adult life course, evolve and influence—and, in turn, are influenced by—cognitive and health outcomes in middle and older age.
Linked to a parallel, ongoing LitCog cohort of older adults recruited in 2008 at ages 55–74 (ages 70–90 as of 2023), MidCog will provide a novel cognitive ageing dataset targeting midlife, with an expanse of patient-reported, performance-based and clinical data sources.
With a longitudinal cohort as young as 35 at baseline, we will be able to explore age-related associations of cognition, health literacy, self-management skills, healthcare utilisation, health outcomes and other understudied variables from ages 35 to 90.
The primary limitation of this longitudinal study will be lost to follow-up and missing data points that may introduce attrition bias.
Introduction
The world is rapidly ageing. In 2019, there were 703 million adults aged 65 and older, a number that is projected to more than double, reaching over 1.5 billion by 2050.1 With an ageing population, the number of people living with Alzheimer’s disease and related dementia (ADRD) is estimated to also increase from 57.4 million in 2019 to 152.8 million cases globally in 2050.2 At present, there are no confirmed disease-modifying therapies for ADRD, although aducanumab received controversial approval in 2021 as a first antiamyloid treatment, followed by a more recent approval of lecanemab.3–6 This lack of definitive means to prevent or treat cognitive impairment (CI) or ADRD is driving intense efforts to unveil underlying mechanisms.7–9 Recent evidence suggests clinically meaningful declines in cognition might present as early as middle age.10–14 Studying cognitive changes in middle age (ages 35–64) could elucidate modifiable factors affecting later cognitive, health outcomes.15
Many known or suspected risk factors for CI/ADRD manifest and become prevalent in middle age, such as: (1) chronic conditions (eg, hypertension, hyperlipidaemia, diabetes) that often are delayed in their detection, or inadequately managed due to poor treatment adherence; (2) undetected or uncorrected sensory impairments (vision, hearing); (3) entrenched lifestyle behaviours (eg, physical inactivity, poor diet, obesity, smoking, drug/alcohol use, poor sleep); (4) common biological (eg, pregnancy, menopause) and psychosocial stressors (eg, multiple caregiving roles, financial responsibilities, employment, depression/anxiety).16–33 Thus, greater attention is increasingly being paid to the importance of proper health self-management (SM) in midlife, defined as ‘the ability of the individual, in conjunction with family, community and healthcare professionals, to manage symptoms, treatments, lifestyle changes, and psychosocial, cultural, and spiritual consequences associated with a chronic illness or condition.’34 35
A large proportion of adults may reach middle age lacking proficient health literacy (HL), defined as ‘the cognitive and social skills which determine the motivation and ability of individuals to gain access to, understand and use information in ways which promote and maintain good health.’36 Research over the past three decades, almost exclusively among older adults, has found limited HL to be related to worse health knowledge, lower uptake of protective health behaviours, higher engagement in risk behaviours, poorer chronic disease outcomes, higher hospitalisation and mortality risk.37–39 HL is modifiable, by enhancing health knowledge and skills, but also by reducing treatment burden imparted by health systems.40–42 Promoting HL could improve SM, impart healthier lifestyle and healthcare use, thereby reducing one’s risk of later life impairment.
While the predominance of cognitive ageing studies have focused on adults 60 and older,43 researchers are increasingly seeking to study health, behaviours and cognitive function much earlier during middle age.15 The Midlife in the United States study is a nationally representative, longitudinal telephone survey of adults ages 42–92.10 The landmark Whitehall II study conducted in England included some cognitive testing in later phases among adults ages 45–70.11 In addition, the Coronary Artery Risk Development in Young Adults study since 1985 follows young men and women ages 18–30 from multiple.S sites. Also, the Dunedin Multidisciplinary Health and Development Study in New Zealand has an active middle age cohort recruited at birth in 1973 who have been administered IQ tests and supplement cognitive measures at age 45.12 13 Evidence from these studies suggest that baseline cognitive differences affect health outcomes. While studies are now emerging,14 existing research investigating cognitive function during middle age has mostly been limited to small or condition-specific samples, cross-sectional analyses or cohort studies with few follow-up periods, abbreviated cognitive tests, limited covariates or a lack of diversity in study samples. Furthermore, midlife investigations to date have not included objective measures of HL or SM skills, or adequately captured other salient, modifiable factors.
A conceptual framework
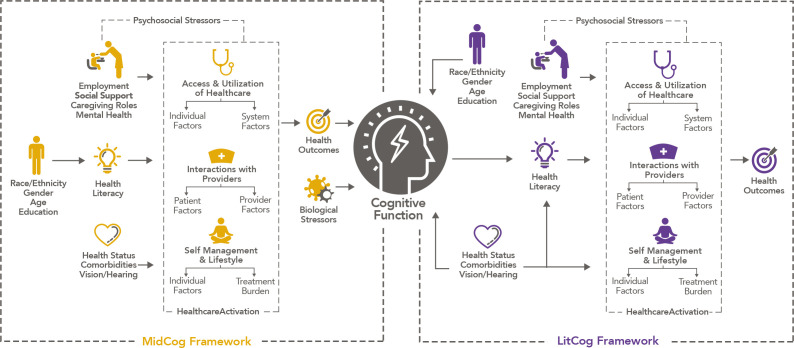
We propose a conceptual model (figure 1) derived from Paasche-Orlow and Wolf.44 Associations between cognition, HL and SM skills are likely bidirectional over the life course. Limited HL and SM skills in middle age may lead to unhealthy lifestyle, chronic disease and its inadequate management, suboptimal healthcare utilisation and interactions, ultimately increasing one’s risk of CI and/or more rapid declines. Biological and psychosocial stressors common in midlife may have both direct and indirect effects on cognitive function, the latter through their influences on lifestyle and health behaviours. Health activation, defined as ‘an individual’s willingness to take on the role of managing their health and healthcare’, has been found to independently be associated with health behaviours and functional health status through a separate pathway from HL and SM skills.45–49 Most of the evidence to date from HL research is based on older adults. The MidCog study will be the first to investigate how HL and SM skills, across the adult life course, evolve and influence—and, in turn, are influenced by—cognitive and health outcomes in middle and older age. We also will target other psychosocial factors in midlife that might independently affect health status and cognitive function in middle age, or plausibly offset the consequences of limited HL and SM skills.
Figure 1.
MidCog Conceptual Framework. In our ongoing LitCog cohort study of older adults, we hypothesised that age-related cognitive decline and impairment among older adults will translate to similar declines in health literacy (HL) and self-management (SM) skill, which have been associated with health knowledge and behaviours, adherence to medication regimens, physical and mental health, chronic disease outcomes, health services use, and mortality (the right side of figure). With MidCog, associations between cognition, HL and SM skills are likely bidirectional over the life course. Contrary to LitCog, limited HL and SM skills in middle age may lead to unhealthy lifestyle, chronic disease and its inadequate management, suboptimal healthcare utilisation and interactions, ultimately increasing one’s risk of cognitive impairment and dementia (the left side of figure). MidCog study will investigate how HL and SM skills, across the adult life course, evolve and influence—and, in turn, are influenced by—cognitive and health outcomes in middle and older age. We will also examine other psychosocial factors in midlife that might independently affect health status and cognitive function in middle age, or plausibly offset the consequences of limited HL and SM skills.
Study aims and hypotheses
The aims and hypotheses of this prospective study are summarised in table 1.
Table 1.
Aims and hypotheses
| Aim 1 | Characterise HL, SM skills and cognitive function in detail among middle age adults. |
| Aim 2 | Evaluate associations between HL, SM skills, health behaviours, healthcare use, health status, chronic disease outcomes and cognitive function over time among middle age adults. |
| H1 | Limited HL will be associated with inadequate SM skills; both will be associated with lower uptake of recommended health behaviours, infrequent healthcare use, worse health and poorer cognitive function. |
| H2 | Presence of uncontrolled chronic conditions, sensory impairments, physical inactivity, obesity, excess drug/ alcohol use, perimenopause, depression, stress and poor sleep will be associated with poorer cognition. |
| H3 | Among middle age adults with ≥1 chronic conditions, poor treatment adherence, infrequent healthcare use and worse health outcomes will mediate associations between HL, SM skills and cognitive function. |
| Aim 3 | Investigate whether certain modifiable, psychosocial factors (health activation, treatment burden, social support) moderate associations between HL, SM skills, health status and cognitive function. |
| Aim 4 | Using MidCog+LitCog data, explore associations between age, HL, SM skills, health status, presence and management of chronic disease, and cognitive function among adults ages 35–90. |
H, hypothesis; HL, health literacy; SM, self-management.
Methods and analysis
Study design
MidCog is a prospective, observational cohort study of middle age adults with two in-person interviews: a baseline (T1) and 2.5-year follow-up interview (T2). MidCog has been adapted from an ongoing cohort study (‘LitCog’; N=900) examining HL, SM skills and cognitive function in older adults (ages 55–74 at baseline; ages 70–90 in 2023) since 2008, leveraging similar methods and procedures.
Performance sites
MidCog will partner with the same clinical sites as LitCog for data collection, involving two large healthcare networks. The Northwestern Medicine Central Region Practices (NM) includes 12 practices across the greater Chicagoland area serving a diverse pool of 182 391 patients. Access Community Health Network (ACCESS) is a Public Health Service 330-funded network of 34 Federally Qualified Health Centres throughout the greater Chicagoland area, serving over 183 000 patients. The patient population is all low income, with 56% being Hispanic/Latino and 49% black.
Subject eligibility and recruitment
Individuals are eligible if they are (1) ages 35–64; (2) a patient receiving primary care services at least once in past 18 months at one of nine participating ACCESS clinics or at NM internal medicine practices with an upcoming visit in the next 6 months; (3) without uncorrectable vision/hearing impairments; (4) free of acute medical issue or severe CI and (5) English-speaking. Additionally, to be eligible for the follow-up interviews (T2), participants must have completed at least the first interview at baseline (T1) and must have been approximately 2.5 years since baseline.
Eligible patients (by age, clinic, healthcare utilisation over past 18 months, language spoken) will be first identified via electronic health record (EHR) queries. Patient lists will be generated for all primary care physicians at performance sites who will have the opportunity, without need for justification, to remove any patient from the list they believe would not be appropriate for participation. With physicians’ approval, those available to participate will be mailed a letter briefly introducing the MidCog study. It will state that they may receive a phone call in the next 10 days to introduce the project. If they wish to participate, a brief screener (vision, hearing, health, cognition) will be performed. If eligible, an interview will then be scheduled at the patient’s convenience. The letter will also allow them an opportunity to ‘opt-out’ by calling a toll-free number. A research coordinator will then delete them from the eligible patient list. A total of 1200 patients (n=600 NM; n=600 ACCESS) will be consecutively recruited from April 2022.
Data sources
Data will be gathered and merged from the following sources:
Interviews
Patients complete two interviews (in-person+telephone) each at a baseline (‘T1’) and 2.5-year follow-up (‘T2’). Interviews are split over 2 days to minimise participant fatigue and to allow data capture with actigraphy. Interview data will be stored in REDCap, a secure online data capture system. All patients are compensated US$70 for the in-person interview (2.5–3 hours in length), US$30 for the telephone interview (1 hour in length) and US$25 on return of actigraphy (see “Actigraphy”). An additional parking voucher or cash for public transit reimbursement is provided for each interview.
Actigraphy
Actigraphy has been validated for evaluation of sleep against polysomnography.50–54 At the end of the in-person interview at T1 and T2, patients will be fitted with a water-resistant, photosensor-integrated actigraphy (ActTrust2, Condor), with instructions to (1) wear the actigraphy on their non-dominant wrist for 14 days, (2) keep it always uncovered (for accurate light measure), (3) press the ‘event button’ at bedtime and on awakening, (4) fill out a daily sleep diary55 and (5) return the actigraphy and diary using the provided prepaid package on completion of 14-day recording. Deidentified data from returned actigraphy and diary will be uploaded to the secure institutional server using Condor ActStudio Software and visually inspected. If actigraphy data do not contain a minimum of five ‘valid days’, defined as 24-hour periods (12pm–12pm+1 day) with at least 20 hours of recording, patients will be invited to repeat the procedure. All actigraphy data will be manually scored for sleep by trained study personnel per standardised protocol.56 For quality control, 5% of the data will be randomly selected for blind, independent rescoring. Any 24-hour period with >4 hour of non-wear will be considered invalid and removed from analysis.
Electronic health record data
NM and ACCESS use the same EHR platform (Epic Systems, Verona, Wisconsin, USA), ensuring clinical and health services use data are collected in a similar manner. Data will be requested from each site. ICD-10 and CPT codes will be used to conform chronic conditions. Relevant date ranges will be selected for vitals, medications, laboratory values (Haemoglobin A1C, cholesterol, glomerular filtration rate), preventive services utilisation (cancer screening, immunisations), health services use (office visits, hospitalisation, emergency/urgent care visits) and clinical notes. EHR data will be collected for the entire study period and 1-year post-T2.
Patient measurements
Table 2 summarises collected measures.
Table 2.
MidCog measurements
| Variable | Instrument(s) or measure(s) | Data source | |
| Interview | EHR | ||
| Sociodemographic | Age, sex, education, race, ethnicity, income, household composition, employment, marital status, housing, insurance, occupation | X | X |
| Health status and self-care regimen complexity | |||
| Medications | Medication reconciliation (prescription and OTC), pharmacy use | X | X |
| Chronic conditions | Self-report, ICD-10 codes | X | X |
| Treatment burden | Medication Regimen Complexity Index | X | |
| Health literacy and self-management skills | |||
| Health literacy | Test of functional health literacy in adults, Newest Vital Sign | X | |
| Self-management | Comprehensive Health Activities Scale | X | |
| Cognitive function | |||
| Working memory | NIHTB (List Sorting, FNAME), Size Judgement Span | X | |
| Executive function | NIHTB (Dimensional Change Card Sort), ETS Letter Sets, Flanker, Visual Reasoning | X | |
| Attention/processing Speed | NIHTB (Flanker, Pattern Comparison), symbol digit, pattern comparison | X | |
| Episodic memory | NIHTB (picture sequence), NYU Paragraph Recall | X | |
| Verbal/crystalised abilities | NIHTB (Oral Reading, Picture Vocabulary), American National Adult Reading Test | X | |
| Global cognition | MoCA (objective), ECOG (subjective) | X | |
| Function | |||
| Vision | Rosenbaum Eye Chart, Corrective Eyewear | X | |
| Hearing | Hearing Handicap Inventory Screener, Hearing Aids | X | |
| Physical function | 6 min walking test, grip strength | X | |
| Sleep and wake | |||
| Sleep health | Actigraphy (objective), PROMIS Sleep Disturbance, RUSATED, Insomnia Severity Index (subjective) | X | |
| Sleep apnoea risk | STOP-BANG | X | |
| Chronotype | Micro-Munich ChronoType Questionnaire (µMCTQ) | X | |
| Psychosocial and biological factors | |||
| Psychosocial | Cohen’s Perceived Stress Scale, Financial Chronic Stress Scale, Total Economic Problems, Caregiver Burden Scale |
X | |
| Patient activation | Consumer Health Activation Index | X | |
| Biological | Menopause Rating Scale, NHANES reproductive survey | X | X |
| Behavioural factors | |||
| Adherence | ASK-12 | X | |
| Preventive services | BRFSS Colon and Breast Cancer Screening, Vaccinations and Immunisations | X | X |
| Health risk behaviours | BRFSS Tobacco Use, Alcohol Use Disorders Identification Test, Drug Abuse Screen Test | X | |
| Protective health behaviours | NHANES Dietary Screener Questionnaire, Physical Activity (actigraphy) | X | |
| Technology use | Portal use | X | |
| Social factors | |||
| Healthcare support | Tangible Social Support | X | |
| Social networks and support | Three-Item Loneliness Scale, PROMIS Instrumental Support, Social Isolation, Emotional Support | X | |
| Routine | Martin and Park Environmental Demands | X | |
| Patient-reported outcomes | |||
| Mental health | WHO Well-being Index, PROMIS Depression/Anxiety | X | |
| Physical health | PROMIS Physical Function | X | |
| Health services use | |||
| Primary care | Routine clinic visits, sick/problem-specific visits, RUI | X | X |
| Specialty care | Medical Subspeciality Visits, Cognition (neuropsychological, behavioural, neurology, etc) related referral, RUI | X | X |
| Urgent/acute care | ED/urgent care visits, RUI, hospitalisations | X | X |
| Clinical outcomes | |||
| Chronic disease outcomes | Haemoglobin A1c, Blood pressure, lipid panel, glomerular filtration rate, body mass index | X | X |
ASK-12, Adherence Starts with Knowledge 12; BRFSS, Behavioural Risk Factor Surveillance System; ECOG, Everyday Cognition; ED, Emergency Department; EHR, Electronic Health Record; ETS, Educational Testing Service; FNAME, Face Name Associative MEmory-cued first letter; ICD-10, International Classification of Diseases, Tenth Revision; MoCA, Montreal Cognitive Assessment; NHANES, National Health and Nutrition Examination Survey; NIHTB, NIH Toolbox for the Assessment of Neurological Behavioural and Function Cognition Battery; OTC, over-the-counter; PROMIS, Patient-Reported Outcomes Measurement Information System; RUI, resource use inventory.
Health status and self-care regimen complexity
Medications (prescription and over-the-counter), pharmacy use and list of chronic conditions will be collected from self-report and EHR. This will allow us to calculate Medication Regimen Complexity Index57 at each time point, as a measure of treatment burden.
HL and SM skills
HL will be measured with the Test of Functional Health Literacy in Adults and the Newest Vital Sign.58–60 The Comprehensive Health Activities Scale measures SM skills necessary to navigate healthcare.61 The Consumer Health Activation Index measures health activation, level of motivation to engage in healthcare decisions/actions.46
Cognitive function
We will use both paper-and-pencil tests and iPad-based NIH Toolbox Cognition Battery62 to target fluid (working memory, executive function, attention, processing speed, episodic memory) and crystallised (verbal) abilities of cognitive function. Domain-specific paper-and-pencil tests include: working memory (Size Judgment Span),63 executive function (Educational Testing Service (ETS) Letter Sets),64 attention/processing speed (Symbol Digit,62 Pattern Comparison63), episodic memory (New York University Paragraph)65 and verbal ability (American-National Adult Reading Test).66 In addition, Objective (Montreal Cognitive Assessment)67 and subjective (Everyday Cognition)68 global cognition will be assessed.
Sensory function
Vision and hearing will be assessed using Rosenbaum Eye Chart and the Hearing Handicap Inventory for the Elderly Screener,69 respectively. The use of corrective eyewear and hearing aids will be collected.
Physical Function will be assessed with grip strength and 6 min walk test.70 71
Sleep and wake
Objective sleep-wake variables will be derived from actigraphy data.72 Self-reported measures of sleep health will be collected using the RUSATED,45 Insomnia Severity Index73 and Patient-Reported Outcomes Measurement Information System (PROMIS) Sleep Disturbance subscale.74 The risk of sleep apnoea is defined as STOP-BANG ≥3.75 The Munich ChronoType Questionnaire will be used to assess individual’s preferred sleep time.76
Psychosocial and biological factors
Cohen’s 10-item Perceived Stress Scale77 assess overall level of stress. Psychosocial stressors will be measured with Financial Chronic Stress, Total Economic Problems and Caregiver Burden Scale.78 Biological stressors will be assessed with National Health and Nutrition Examination Survey (NHANES) Pregnancy Survey and Menopause Rating Scale.79–81 The Consumer Health Activation Index (CHAI) measures health activation, level of motivation to engage in healthcare decisions/actions.46
Behavioural factors
Medication adherence will be examined via self-report (Adherence Starts with Knowledge 12 scale (ASK-12)).31 Preventive service use will be assessed with Behavioural Risk Factor Surveillance Service (BRFSS)82 items on cancer screening, vaccinations and immunisations. Health risk behaviours will be assessed with BRFSS items on tobacco use, Alcohol Use Disorders Identification Test and Drug Abuse Screen Test. Protective health behaviours will be examined with NHANES, and actigraphy-measured activity level.82–85 Health-information seeking behaviour and technology use will be assessed via patient portal use.
Social factors
Healthcare support will be measured by the Tangible Social Support scale.86 Social Networks and Support will be assessed by the Loneliness Scale87 and PROMIS subscales on Social Isolation, Instrumental Support and Emotional Support.88 Self-reported busyness and routine will be measured with Martin and Park Environmental Demands.89
Patient-reported outcomes on mental and physical health will be collected with the WHO Well-Being Index and PROMIS subscales on depression, anxiety and physical function.88 90
Health services use
The Resource Use Inventory will capture healthcare resource utilisation and cost.91 We will also capture primary care (routine and sick visits), specialty care and urgent/acute care (emergency department/Urgent Care, hospitalisations) use data from EHRs. The principal International Classification of Diseases (ICD) code will be used to identify hospitalisations for ambulatory care sensitive conditions, defined as those that are often avoidable if patients receive appropriate outpatient care.92–94
Clinical outcomes
Chronic disease outcomes will be assessed with blood pressure, haemoglobin A1c, cholesterol panel, glomerular filtration rate and other relevant data from EHRs. Additionally, blood pressure will be measured during in-person interviews per NHANES procedures.95
Analysis plan
Analyses, per aim, are described below.
Aim 1: characterise HL, SM skills and cognitive function in detail among middle age adults
We will assess HL, SM skills, cognitive function and other psychosocial (eg, health activation, social support) and health factors (eg, physical, mental function, sleep, physical activity, comorbidity) and healthcare use for all MidCog participants. Using means and SDs for each outcome, we will create estimates and 95% CIs for the entire sample, and specifically by sex as a biological variable. HL, SM skills, cognitive function and other psychosocial and health-related covariates of primary interest will also be described by other demographics (education, marital status, socioeconomic status and comorbidity).
Aim 2: evaluate associations between HL, SM skills, health behaviours, healthcare use, health status, chronic disease outcomes and cognitive function over time among middle age adults
For aim 2, as this is a cohort study, and any direct comparisons may be impacted by confounding, we will first use stepwise regression to determine the statistically best fitting models for each outcome, and mixed effects models to examine potential random effects of service provider or site. If those resulting, statistically determined models do not include sex or SES as a biological variable, those will be included in ‘final models’. Those final models will be used to determine the unique sets of covariates for each regression model.
H1: HL will be positively associated with SM skills; both will be associated with lower uptake of recommended health behaviours, infrequent healthcare use, worse health and poorer cognitive function
For H1, we will examine linear regression models, first predicting HL using SM skills (adjusting for covariates found to predict HL), and second, a series of four overall regression model sets predicting recommended health behaviours, healthcare use, health status and cognitive function, sequentially, using HL and SM skills as predictors of interest, after adjusting for covariates identified as above. Each model will be fit using a Bonferroni adjusted type I error rate, so that the analysis of each outcome maintains a consistent type I error rate of 5%.
H2: presence of uncontrolled chronic conditions, sensory impairments, physical inactivity, obesity, excess drug/alcohol use, perimenopause, depression, stress and poor sleep will be associated with poorer cognition
To address H2, we will fit a series of regression models predicting the 6+2 domains of cognitive function using the presence of ≥1 uncontrolled chronic conditions, physical inactivity, obesity, excess drug/alcohol use, menopause, depression, stress or poor sleep quality as individual predictors to avoid a table 2 fallacy.96
H3: among middle-aged adults with ≥1 chronic conditions, poor treatment adherence, infrequent healthcare use and worse health outcomes might mediate associations between HL, SM skills and cognitive function
To address H3, mediation models will be fit using methods by Iacobucci.97 Using analogous adaptations of methods to Preachers’ methods,98 we will be able to test for moderation and mediation together using joint tests. Depending on the distribution of the variables under consideration, we will fit linear or logistic regression models to estimate regression parameters a (HL and SM skills and the relationship to potential mediators) and b (mediator relationships to cognitive function). Iacobucci shows that a normally distributed test statistic can be constructed from standardised regression parameters Za=a/SEa such that Z(mediation)=Za*Zb/ (sqrt(Za2+Zb2 + 1)) can be used to test for mediation. Further, we can examine if there is additional moderation of these models such that any patient covariate could moderate the relationship between HL, SM skills and cognitive function.
Aim 3: investigate whether certain modifiable, psychosocial factors (health activation, treatment burden, social support) moderate associations between HL, SM skills, health status and cognitive function
For aim 3, additional models will be fit to test for moderation, using interactions; potential moderating effect of health activation, treatment burden and social support on the relationship between HL, SM skills, health status or cognitive function will be examined.
Aim 4: using MidCog and LitCog data, explore associations between age, HL, SM skills, health status, presence and management of chronic disease, and cognitive function among adults ages 35–90
For aim 4, we will examine these associations between and across the MidCog (T1) and LitCog populations. HL scores, SM skills and each of six cognitive domains of overall ‘fluid’ and ‘crystallised’ cognitive function abilities will be transformed into standardised z-scores. Health status, and presence and management of chronic disease will be treated as ordinal variables, and age will be treated continuously. We will graphically depict these relationships, fitting a spline to age to determine if an assumption of linearity and/or a monotone increasing or decreasing relationship, is reasonable, and if so, estimate Pearson or Spearman’s correlation coefficients. The splines can be estimated with and without the inclusion of the MidCog data to determine if there is a temporal impact on the relationship of age to HL, SM skills and cognitive function in a more rigorous manner than allowed for in aim 1. Additionally, the longitudinal nature of LitCog will enable us to examine the trajectories of HL, SM skills and cognitive function over time. With the trajectory and spline results together, we will have the ability to not simply adjust for the time differences of the cohorts, but also provide predictions of the trajectories of these outcomes beginning in middle age that can be further tested as our cohort ages.
Sample size estimation
The total sample size is large enough to detect even the smallest of associations (eg, a total sample size of 1200 subjects will provide over 80% power to detect correlations as small as 0.113 at a type I error rate of 0.002; meaning that the variation in one factor explains just 1% of the variation in another factor), For aim 1, the MidCog sample of 1200 alone will provide enough information to create 95% CIs with half-widths of 0.057. With the addition of the 563 participants in LitCog, who were between 55 and 64 at baseline, we would have enough information to create 95% CIs with half-widths of 0.047. Assuming a similar distribution of sex as a biological variable, we expect approximately 68% females and 32% males, which would provide information to estimate 95% CIs with half-widths of 0.057 and 0.083, respectively. For aim 2, the sample of 1200 will provide 80% power to detect an increase in R-squared in multiple linear regression of 0.005 at the Bonferroni-adjusted type I error rate of 0.6%, assuming that we have already accounted for 50% of the variability in outcome while adjusting for 5 independent covariates. For mediation analyses, per Fritz and Mackinnon,99 a sample of 667 provides 80% power at a type I error rate of 5% to detect S-S mediation pathways (s=effect size of 0.14); even with an adjusted type I error of 0.006 (or 0.002), we will have adequate power to test mediation models. Sample size is usually restrictive in testing moderation, however, according to Gelman,100 a good rule of thumb is 16 times the sample size of a main effect. As 1/16 of 1200 is 75, we anticipate having 80% power to detect a standardised interaction slope of 0.32.
Plans to address sex as a biological variable
Across all aims, we will explicitly examine sex differences in cognitive function and decline, HL and SM skills, as well in relevant outcomes of interest.
Strategies to deal with bias/missing data/attrition and lost to follow-up
Baseline data will be used to understand the extent to which subjects who complete T2 differ from those who do not. If differences are found, pattern-mixture models will classify participant drop-out type or pattern (eg, completed, refused, lost to follow-up, death). This variable will then be added to models to account for differences.101 We will examine missing data rates for all variables within interviews and determine whether these vary by patient characteristics. Analyses will indicate the extent to which survey non-response and missing data could bias results. In extreme cases, we will employ sensitivity analyses using propensity scoring to impute missing data for patients, with no less than five imputed datasets. We will estimate overall averaged effects in models above with corresponding SEs. Relevant model estimates will be tested on these imputation model-estimated parameters to examine sensitivity of inferences made in previous analyses ignoring missing data.99
Patient and public involvement
Members of the public were involved in designing recruitment strategies and refining MidCog procedures. Specifically, individuals who met study eligibility criteria were recruited locally for a stakeholder discussion group; the study protocol and list of assessments were reviewed. Acceptability of study participation, compensation and other means for optimising recruitment were reviewed. As the MidCog study progresses, summaries of study results will also be disseminated to study participants via email and/or mailed newsletters.
Methodological issues
The primary limitation of this longitudinal study is lost to follow-up and missing data points that would challenge the internal validity of reported results. However, our research team has extensive experience in achieving excellent retention (80%) with the parallel, LitCog study. We will employ the same strategies to minimise lost to follow-up, including calling a week and a day before the scheduled interview to confirm the appointment, sending confirmation text messages that contain the location, date and time of their interview, collecting multiple forms of contact information, sending birthday cards reminding them of their study participation, strong interpersonal skills of study personnel, and providing compensation for the participant’s time and travel. Additional strategies to promote retention include offering weekend and outside of regular business hour appointments to accommodate participants with traditional work schedules and ride share services for those with limited access to transportation.
Data storage and security
Data will be stored on institutional network drives with firewalls and security measures in place. Paper records will be stored in a locked cabinet in a secure location. Access to records and data will be limited to study personnel. Study data will be deidentified and a password-protected, encrypted master linking log with identifiers will be kept and stored separately from the data.
Ethics and dissemination
This study was approved by Northwestern University Institutional Review Board (STU00214736). Results will be published in international peer-reviewed journals and summaries will be provided to the funders of the study.
Supplementary Material
Footnotes
Twitter: @moonk321
Contributors: MW conceptualised the study and obtained funding and MMK wrote the statistical analysis plan. MK, MMK, SCB, PCZ, MB, RO'C, LMC, RL, AR, PCZ and MW contributed to the final selection of measurements. MB, PCZ, HQL, PC and PA administered the project and MW, MK and JYB supervised the project administration. LMC, SH and FY cleaned and analysed data. MK wrote the original draft and revised the paper. MMK, SCB, JYB, PCZ, MB, HQL, PC, PA, RO'C, LMC, SH, FY, RL, AR, YL, PCZ and MW reviewed and edited the draft paper.
Funding: This work was supported by grants from the National Institutes of Health (R01AG070212, R01AG030711), with institutional support from UL1TR001422 and from the Claude D. Pepper Older Americans Independence Center at Northwestern University Feinberg School of Medicine (P30AG059988).
Disclaimer: The funding agency played no role in the study design, collection of data, analysis, or interpretation of data.
Competing interests: SCB reports grants from the NIH, Merck, Pfizer, Gordon and Betty Moore Foundation, Retirement Research Foundation for Aging, Lundbeck, Gilead, and Eli Lilly via her institution and personal fees from Sanofi, Pfizer, University of Westminster, Lundbeck, Gilead, and Luto UK outside the submitted work. PCZ reports grants from the NIH and Vanda via her institution and personal consulting fees from Jazz, Eisai, Harmony and Sleep Number that are outside the submitted work. MW reports grants from the NIH, Gordon and Betty Moore Foundation, and Eli Lilly, and personal fees from Pfizer, Sanofi, Luto UK, University of Westminster, and Lundbeck outside the submitted work. RO’C is supported by a training grant from the National Institute on Aging (K01AG070107). All the other authors report no conflict of interest.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.United Nations Department of Economic and Social Affairs PD . World population aging 2019 highlights; 2019.
- 2.Nichols E, Steinmetz JD, Vollset SE. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health 2022;7:e105–25. 10.1016/S2468-2667(21)00249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhillon S. Aducanumab: first approval. Drugs 2021;81:1437–43. 10.1007/s40265-021-01569-z [DOI] [PubMed] [Google Scholar]
- 4.Hane FT, Robinson M, Lee BY, et al. Recent progress in Alzheimer’s disease research, part 3: diagnosis and treatment. J Alzheimers Dis 2017;57:645–65. 10.3233/JAD-160907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration . FDA grants accelerated approval for alzheimer’s disease treatment. Available: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-disease-treatment [Accessed 10 Jan 2023].
- 6.Larkin HD. Lecanemab gains FDA approval for early Alzheimer disease. JAMA 2023;329:363. 10.1001/jama.2022.24490 [DOI] [PubMed] [Google Scholar]
- 7.Shah H, Albanese E, Duggan C, et al. Research priorities to reduce the global burden of dementia by 2025. Lancet Neurol 2016;15:1285–94. 10.1016/S1474-4422(16)30235-6 [DOI] [PubMed] [Google Scholar]
- 8.Pickett J, Bird C, Ballard C, et al. A roadmap to advance dementia research in prevention, diagnosis, intervention, and care by 2025. Int J Geriatr Psychiatry 2018;33:900–6. 10.1002/gps.4868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daviglus ML, Bell CC, Berrettini W, et al. NIH state-of-the-science conference statement: preventing alzheimer’s disease and cognitive decline. In: NIH Consens State Sci Statements, 27. 2010: 1–30. [PubMed] [Google Scholar]
- 10.Hughes ML, Agrigoroaei S, Jeon M, et al. Change in cognitive performance from midlife into old age: findings from the midlife in the United States (MIDUS) study. J Int Neuropsychol Soc 2018;24:805–20. 10.1017/S1355617718000425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh-Manoux A, Kivimaki M, Glymour MM, et al. Timing of onset of cognitive decline: results from whitehall II prospective cohort study. BMJ 2012;344:d7622. 10.1136/bmj.d7622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moffitt TE, Caspi A, Rutter M, et al. Sex differences in antisocial behaviour: conduct disorder, delinquency, and violence in the dunedin longitudinal study. New York: Cambridge Univ Press, 2001. [Google Scholar]
- 13.Rasmussen LJH, Caspi A, Ambler A, et al. Association of neurocognitive and physical function with gait speed in midlife. JAMA Netw Open 2019;2:e1913123. 10.1001/jamanetworkopen.2019.13123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batty GD, Deary IJ, Shipley MJ. Association of change in cognitive function from early adulthood to middle age with risk of cause-specific mortality: the Vietnam experience study. J Epidemiol Community Health 2019;73:712–6. 10.1136/jech-2019-212377 [DOI] [PubMed] [Google Scholar]
- 15.Lachman ME. Mind the gap in the middle: a call to study midlife. Res Hum Dev 2015;12:327–34. 10.1080/15427609.2015.1068048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng Y, Jin Y, Unverzagt FW, et al. The relationship between cholesterol and cognitive function is homocysteine-dependent. Clin Interv Aging 2014;9:1823–9. 10.2147/CIA.S64766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anstey KJ, Ashby-Mitchell K, Peters R. Updating the evidence on the association between serum cholesterol and risk of late-life dementia: review and meta-analysis. J Alzheimers Dis 2017;56:215–28. 10.3233/JAD-160826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim DJ, Yu JH, Shin MS, et al. Hyperglycemia reduces efficiency of brain networks in subjects with type 2 diabetes. PLoS One 2016;11:e0157268. 10.1371/journal.pone.0157268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duning T, van den Heuvel I, Dickmann A, et al. Hypoglycemia aggravates critical illness-induced neurocognitive dysfunction. Diabetes Care 2010;33:639–44. 10.2337/dc09-1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iadecola C, Yaffe K, Biller J, et al. Impact of hypertension on cognitive function: a scientific statement from the American heart association. Hypertension 2016;68:e67–94. 10.1161/HYP.0000000000000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaffe K. Prevention of cognitive impairment with intensive systolic blood pressure control. JAMA 2019;321:548–9. 10.1001/jama.2019.0008 [DOI] [PubMed] [Google Scholar]
- 22.Zeki Al Hazzouri A, Vittinghoff E, Zhang Y, et al. Use of a pooled cohort to impute cardiovascular disease risk factors across the adult life course. Int J Epidemiol 2019;48:1004–13. 10.1093/ije/dyy264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahinrad S, Kurian S, Garner CR, et al. Cumulative blood pressure exposure during young adulthood and mobility and cognitive function in midlife. Circulation 2020;141:712–24. 10.1161/CIRCULATIONAHA.119.042502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burnier M, Egan BM. Adherence in hypertension. Circ Res 2019;124:1124–40. 10.1161/CIRCRESAHA.118.313220 [DOI] [PubMed] [Google Scholar]
- 25.Lemstra M, Nwankwo C, Bird Y, et al. Primary nonadherence to chronic disease medications: a meta-analysis. Patient Prefer Adherence 2018;12:721–31. 10.2147/PPA.S161151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenbaum L, Shrank WH. Taking our medicine -- improving adherence in the accountability era. N Engl J Med 2013;369:694–5. 10.1056/NEJMp1307084 [DOI] [PubMed] [Google Scholar]
- 27.Dawes P, Dickinson C, Emsley R, et al. Vision impairment and dual sensory problems in middle age. Ophthalmic Physiol Opt 2014;34:479–88. 10.1111/opo.12138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallaway PJ, Miyake H, Buchowski MS, et al. Physical activity: a viable way to reduce the risks of mild cognitive impairment, Alzheimer’s disease, and vascular dementia in older adults. Brain Sci 2017;7:22. 10.3390/brainsci7020022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards Iii GA, Gamez N, Escobedo G, et al. Modifiable risk factors for alzheimer’s disease. Front Aging Neurosci 2019;11:146. 10.3389/fnagi.2019.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673–734. 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 31.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 2011;10:819–28. 10.1016/S1474-4422(11)70072-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber MT, Maki PM, McDermott MP. Cognition and mood in perimenopause: a systematic review and meta-analysis. J Steroid Biochem Mol Biol 2014;142:90–8. 10.1016/j.jsbmb.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies SJ, Lum JA, Skouteris H, et al. Cognitive impairment during pregnancy: a meta-analysis. Med J Aust 2018;208:35–40. 10.5694/mja17.00131 [DOI] [PubMed] [Google Scholar]
- 34.Wilkinson A, Whitehead L. Evolution of the concept of self-care and implications for nurses: a literature review. Int J Nurs Stud 2009;46:1143–7. 10.1016/j.ijnurstu.2008.12.011 [DOI] [PubMed] [Google Scholar]
- 35.Richard AA, Shea K. Delineation of self-care and associated concepts. J Nurs Scholarsh 2011;43:255–64. 10.1111/j.1547-5069.2011.01404.x [DOI] [PubMed] [Google Scholar]
- 36.Sørensen K, Van den Broucke S, Fullam J, et al. Health literacy and public health: a systematic review and integration of definitions and models. BMC Public Health 2012;12:80. 10.1186/1471-2458-12-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berkman ND, Sheridan SL, Donahue KE, et al. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med 2011;155:97–107. 10.7326/0003-4819-155-2-201107190-00005 [DOI] [PubMed] [Google Scholar]
- 38.Baker DW, Wolf MS, Feinglass J, et al. Health literacy, cognitive abilities, and mortality among elderly persons. J Gen Intern Med 2008;23:723–6. 10.1007/s11606-008-0566-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolf MS, Gazmararian JA, Baker DW. Health literacy and functional health status among older adults. Arch Intern Med 2005;165:1946–52. 10.1001/archinte.165.17.1946 [DOI] [PubMed] [Google Scholar]
- 40.Koh HK, Brach C, Harris LM, et al. A proposed “health literate care model” would constitute a systems approach to improving patien’s’ engagement in care. Health Aff (Millwood) 2013;32:357–67. 10.1377/hlthaff.2012.1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koh HK, Rudd RE. The Arc of health literacy. JAMA 2015;314:1225–6. 10.1001/jama.2015.9978 [DOI] [PubMed] [Google Scholar]
- 42.Sheridan SL, Halpern DJ, Viera AJ, et al. Interventions for individuals with low health literacy: a systematic review. J Health Commun 2011;16 Suppl 3:30–54. 10.1080/10810730.2011.604391 [DOI] [PubMed] [Google Scholar]
- 43.Medicine NAo . Annual report 2015; 2016.
- 44.Paasche-Orlow MK, Wolf MS. The causal pathways linking health literacy to health outcomes. Am J Health Behav 2007;31 Suppl 1:S19–26. 10.5555/ajhb.2007.31.supp.S19 [DOI] [PubMed] [Google Scholar]
- 45.Smith SG, Curtis LM, Wardle J, et al. Skill set or mind set? Associations between health literacy, patient activation and health. PLoS One 2013;8:e74373. 10.1371/journal.pone.0074373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolf MS, Smith SG, Pandit AU, et al. Development and validation of the consumer health activation index. Med Decis Making 2018;38:334–43. 10.1177/0272989X17753392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gwynn KB, Winter MR, Cabral HJ, et al. Racial disparities in patient activation: evaluating the mediating role of health literacy with path analyses. Patient Educ Couns 2016;99:1033–7. 10.1016/j.pec.2015.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bailey SC, O’Conor R, Bojarski EA, et al. Literacy disparities in patient access and health-related use of Internet and mobile technologies. Health Expect 2015;18:3079–87. 10.1111/hex.12294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith SG, O’Conor R, Aitken W, et al. Disparities in registration and use of an online patient portal among older adults: findings from the litcog cohort. J Am Med Inform Assoc 2015;22:888–95. 10.1093/jamia/ocv025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Full KM, Kerr J, Grandner MA, et al. Validation of a physical activity accelerometer device worn on the hip and wrist against polysomnography. Sleep Health 2018;4:209–16. 10.1016/j.sleh.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Constantino DB, Xavier NB, Levandovski R, et al. Relationship between circadian strain, light exposure, and body mass index in rural and urban quilombola communities. Front Physiol 2021;12:773969. 10.3389/fphys.2021.773969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bellone GJ, Plano SA, Cardinali DP, et al. Comparative analysis of actigraphy performance in healthy young subjects. Sleep Sci 2016;9:272–9. 10.1016/j.slsci.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perez-Pozuelo I, Posa M, Spathis D, et al. Detecting sleep outside the clinic using wearable heart rate devices. Sci Rep 2022;12:7956. 10.1038/s41598-022-11792-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marino M, Li Y, Rueschman MN, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep 2013;36:1747–55. 10.5665/sleep.3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep 2012;35:287–302. 10.5665/sleep.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel SR, Weng J, Rueschman M, et al. Reproducibility of a standardized actigraphy scoring algorithm for sleep in a US Hispanic/Latino population. Sleep 2015;38:1497–503. 10.5665/sleep.4998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.George J, Phun YT, Bailey MJ, et al. Development and validation of the medication regimen complexity index. Ann Pharmacother 2004;38:1369–76. 10.1345/aph.1D479 [DOI] [PubMed] [Google Scholar]
- 58.Baker DW, Williams MV, Parker RM, et al. Development of a brief test to measure functional health literacy. Patient Educ Couns 1999;38:33–42. 10.1016/s0738-3991(98)00116-5 [DOI] [PubMed] [Google Scholar]
- 59.Parker RM, Baker DW, Williams MV, et al. The test of functional health literacy in adults: a new instrument for measuring patients’ literacy skills. J Gen Intern Med 1995;10:537–41. 10.1007/BF02640361 [DOI] [PubMed] [Google Scholar]
- 60.Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med 2005;3:514–22. 10.1370/afm.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Curtis LM, Revelle W, Waite K, et al. Development and validation of the comprehensive health activities scale: a new approach to health literacy measurement. J Health Commun 2015;20:157–64. 10.1080/10810730.2014.917744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weintraub S, Dikmen SS, Heaton RK, et al. Cognition assessment using the NIH toolbox. Neurology 2013;80:S54–64. 10.1212/WNL.0b013e3182872ded [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cherry KE, Park DC. Individual difference and contextual variables influence spatial memory in younger and older adults. Psychol Aging 1993;8:517–26. 10.1037//0882-7974.8.4.517 [DOI] [PubMed] [Google Scholar]
- 64.Ekstrom RB, French JW, Harman HH. ETS kit of factor-referenced cognitive tests. Princeton, NJ: Educational Testing Service, 1976. [Google Scholar]
- 65.Kluger A, Ferris SH, Golomb J, et al. Neuropsychological prediction of decline to dementia in nondemented elderly. J Geriatr Psychiatry Neurol 1999;12:168–79. 10.1177/089198879901200402 [DOI] [PubMed] [Google Scholar]
- 66.Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol 1991;13:933–49. 10.1080/01688639108405109 [DOI] [PubMed] [Google Scholar]
- 67.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 68.Marshall GA, Zoller AS, Kelly KE, et al. Everyday cognition scale items that best discriminate between and predict progression from clinically normal to mild cognitive impairment. Curr Alzheimer Res 2014;11:853–61. 10.2174/1567205011666141001120903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ventry IM, Weinstein BE. The hearing handicap inventory for the elderly: a new tool. Ear Hear 1982;3:128–34. 10.1097/00003446-198205000-00006 [DOI] [PubMed] [Google Scholar]
- 70.Salzman SH. The 6-min walk test: clinical and research role, technique, coding, and reimbursement. Chest 2009;135:1345–52. 10.1378/chest.07-1682 [DOI] [PubMed] [Google Scholar]
- 71.Perna FM, Coa K, Troiano RP, et al. Muscular grip strength estimates of the U.S. population from the National health and nutrition examination survey 2011-2012. J Strength Cond Res 2016;30:867–74. 10.1519/JSC.0000000000001104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim M, Liotta EM, Maas MB, et al. Rest-activity rhythm disturbance in liver cirrhosis and association with cognitive impairment. Sleep 2021;44:zsaa288. 10.1093/sleep/zsaa288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morin CM, Belleville G, Bélanger L, et al. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011;34:601–8. 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS™ sleep disturbance and sleep-related impairment item banks. Behav Sleep Med 2011;10:6–24. 10.1080/15402002.2012.636266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chung F, Abdullah HR, Liao P. Stop-bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest 2016;149:631–8. 10.1378/chest.15-0903 [DOI] [PubMed] [Google Scholar]
- 76.Ghotbi N, Pilz LK, Winnebeck EC, et al. The µMCTQ: an ultra-short version of the Munich chronotype questionnaire. J Biol Rhythms 2020;35:98–110. 10.1177/0748730419886986 [DOI] [PubMed] [Google Scholar]
- 77.Baik SH, Fox RS, Mills SD, et al. Reliability and validity of the perceived stress scale-10 in Hispanic Americans with English or Spanish language preference. J Health Psychol 2019;24:628–39. 10.1177/1359105316684938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pearlin LI, Schooler C. The structure of coping. J Health Soc Behav 1978;19:2–21. [PubMed] [Google Scholar]
- 79.Hauser GA, Huber IC, Keller PJ, et al. Evaluation of climacteric symptoms (menopause rating scale). Zentralbl Gynakol 1994;116:16–23. [PubMed] [Google Scholar]
- 80.Potthoff P, Heinemann LA, Schneider HP, et al. The menopause rating scale (MRS II): methodological standardization in the German population. Zentralbl Gynakol 2000;122:280–6. [PubMed] [Google Scholar]
- 81.Tsai C-K, Chen Y-Y, Chou C-H, et al. Female reproductive health and cognitive function. Menopause 2020;27:1357–62. 10.1097/GME.0000000000001630 [DOI] [PubMed] [Google Scholar]
- 82.(CDC) CfDCaP . Behavioral risk factor surveillance system questionnaire. Available: http://www.cdc.gov/brfss/questionnaires/pdf-ques/2013%20BRFSS_English.pdf [Accessed 2 Mar 2015].
- 83.Bush K, Kivlahan DR, McDonell MB, et al. The audit alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. ambulatory care quality improvement project (ACQUIP). Alcohol use disorders identification test. Arch Intern Med 1998;158:1789–95. 10.1001/archinte.158.16.1789 [DOI] [PubMed] [Google Scholar]
- 84.Yudko E, Lozhkina O, Fouts A. A comprehensive review of the psychometric properties of the drug abuse screening test. J Subst Abuse Treat 2007;32:189–98. 10.1016/j.jsat.2006.08.002 [DOI] [PubMed] [Google Scholar]
- 85.Dong X, Li S, Sun J, et al. Association of coffee, decaffeinated coffee and caffeine intake from coffee with cognitive performance in older adults: National health and nutrition examination survey (NHANES) 2011-2014. Nutrients 2020;12:840. 10.3390/nu12030840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Woloshin S, Schwartz LM, Tosteson AN, et al. Perceived adequacy of tangible social support and health outcomes in patients with coronary artery disease. J Gen Intern Med 1997;12:613–8. 10.1046/j.1525-1497.1997.07121.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Russell D, Peplau LA, Ferguson ML. Developing a measure of loneliness. J Pers Assess 1978;42:290–4. 10.1207/s15327752jpa4203_11 [DOI] [PubMed] [Google Scholar]
- 88.Cella D, Riley W, Stone A, et al. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol 2010;63:1179–94. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martin M, Park DC. The Martin and Park environmental demands (MPED) questionnaire: psychometric properties of a brief instrument to measure self-reported environmental demands. Aging Clin Exp Res 2003;15:77–82. 10.1007/BF03324483 [DOI] [PubMed] [Google Scholar]
- 90.Topp CW, Østergaard SD, Søndergaard S, et al. The WHO-5 well-being index: a systematic review of the literature. Psychother Psychosom 2015;84:167–76. 10.1159/000376585 [DOI] [PubMed] [Google Scholar]
- 91.Sano M, Zhu CW, Whitehouse PJ, et al. ADCS prevention instrument project: pharmacoeconomics: assessing health-related resource use among healthy elderly. Alzheimer Dis Assoc Disord 2006;20:S191–202. 10.1097/01.wad.0000213875.63171.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Phelan EA, Borson S, Grothaus L, et al. Association of incident dementia with hospitalizations. JAMA 2012;307:165–72. 10.1001/jama.2011.1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Quality AfHRa . AHRQ quality indicators—guide to prevention quality indicators: hospital admission for ambulatory care sensitive conditions. In: Agency for Healthcare Research and Quality. AHRQ Pub. No. 02-R0203. Rockville, MD, 2001. [Google Scholar]
- 94.Katori M, Shi S, Ode KL, et al. The 103,200-arm acceleration dataset in the UK Biobank revealed a landscape of human sleep phenotypes. Proc Natl Acad Sci U S A 2022;119:e2116729119. 10.1073/pnas.2116729119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Plantinga L, Miller E, Stevens L. 159: blood pressure control in CKD by anti-hypertensive medication: nhanes 1999-2006. Am J Kidney Dis 2009;53:B62. 10.1053/j.ajkd.2009.01.180 [DOI] [Google Scholar]
- 96.Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol 2013;177:292–8. 10.1093/aje/kws412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mackinnon DP, Cox MC. Commentary on “mediation analysis and categorical variables: the final frontier” by dawn iacobucci. J Consum Psychol 2012;22:600–2. 10.1016/j.jcps.2012.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Preacher KJ. Advances in mediation analysis: a survey and synthesis of new developments. Annu Rev Psychol 2015;66:825–52. 10.1146/annurev-psych-010814-015258 [DOI] [PubMed] [Google Scholar]
- 99.Fritz MS, Mackinnon DP. Required sample size to detect the mediated effect. Psychol Sci 2007;18:233–9. 10.1111/j.1467-9280.2007.01882.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gelman A. You need 16 times the sample size to estimate an interaction than to estimate a main effect. Available: https://statmodeling.stat.columbia.edu/2018/03/15/need-16-times-sample-size-estimate-interaction-estimate-main-effect/ [Accessed 3 Feb 2020].
- 101.Rabbitt P, Lunn M, Wong D. Death, dropout, and longitudinal measurements of cognitive change in old age. J Gerontol B Psychol Sci Soc Sci 2008;63:271–8. 10.1093/geronb/63.5.p271 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.