Abstract
Background and objectives: Polypharmacy and chronic kidney disease (CKD) are becoming increasingly common due to an ageing population and the rise of multimorbidity. In line with the therapeutic guidelines, managing CKD and its complications necessitates prescribing multiple medications, which predisposes patients to polypharmacy. The aim of this systematic review and meta-analysis is to describe the prevalence of polypharmacy in patients with CKD and to explore the global trends of factors driving any apparent variability in prevalence estimates.
Methods: PubMed, Scopus, the Cochrane Database of Systematic Reviews (CDSR), and Google Scholar were searched from 1999 to November 2021. Study selection, data extraction, and critical appraisal were conducted by two independent reviewers. The pooled prevalence of polypharmacy was estimated utilizing the random effects model using the default double arcsine transformation.
Results: This review involved 14 studies comprising of 17 201 participants, a significant proportion of which were males (56.12%). The mean age of the review population was 61.96 (SD ± 11.51) years. The overall pooled prevalence of polypharmacy amongst patients with CKD was 69% (95% CI: 49%–86%) (I2 = 100%, p < 0.0001), with a proportionately higher prevalence in North America and Europe as compared to Asia.
Conclusion: The results from this meta-analysis showed a high pooled prevalence estimates of polypharmacy amongst patient cohorts with CKD. The exact interventions that are likely to significantly mitigate its effect remain uncertain and will need exploration by future prospective and systematic studies.
Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/], identifier [CRD42022306572].
Keywords: polypharmacy, chronic kidney disease (CKD), epidemiology, systematic review, meta-analysis
Introduction
Chronic kidney disease (CKD) is a relatively common condition that affects up to 16% of the population globally (Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group, 2013; Gansevoort et al., 2011). It has been associated with adverse health outcomes including cardiovascular disease (CVD) events, poor quality of life, significant morbidity, and CVD or all-cause mortality (Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group, 2013; Couser et al., 2011; James et al., 2010; Yuan et al., 2017; Bansal et al., 2017; Jankowski et al., 2021). CKD is a gradually progressive disease that is linked to a range of complications, such as anemia, bone and mineral disorder, electrolyte imbalance, acid-base abnormalities, hypertension and other CVD, and sexual dysfunction (Widmer et al., 1979; Delmez and Slatopolsky, 1992; Stefanski et al., 1996; Hsu et al., 2002; Bello et al., 2017). Therefore, therapeutic guidelines have been developed to prevent/slow the progression of CKD and to provide therapeutic approaches to manage each clinical manifestation (Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group, 2013). As a consequence of the advanced therapeutics that followed our rising understanding of the pathophysiology, the number of medications taken per patient has substantially increased, which made polypharmacy and its expensive consequences a commonplace across this cohort of patients.
Polypharmacy exhibits a pronounced risk for medication non-adherence, adverse drug events, problematic interactions (drug-drug, drug-food, and pharmacogenetic), emergency department visits, hospitalizations, and sometimes avoidable mortality (Whittaker and Fink, 2017). Consequent upon this, a hefty medical and financial burdens have been imposed on patients, societies, and healthcare systems (Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group, 2013; Jankowski et al., 2021). It is not clear if this polypharmacy is appropriate, as despite the medication burden in CKD patients that could reach more than 30 drugs per patient, the morbidity and mortality rates remain high which raises questions about the effectiveness of these medications in this cohort of patients (Whittaker and Fink, 2017; Schmidt et al., 2019). Additionally, the altered pharmacodynamic and pharmacokinetic parameters in the unique milieu of renal insufficiency further complicates the situation as dosage adjustments and cessation of certain therapies might be required (Parker and Wong, 2019).
Whilst the growing polypharmacy epidemic has garnered attention in the medical community, it is still challenging to address this issue as uncertainty still exist regarding the exact prevalence of polypharmacy in CKD patients as well as the socio-demographic factors that influence its global trends. Understanding the burden of polypharmacy in patients with CKD and identifying vulnerable populations will enable clinicians to develop and implement interventions (e.g., deprescribing) to mitigate polypharmacy and its unfavorable outcomes. Therefore, the aim of this systematic review and meta-analysis is to estimate the prevalence of polypharmacy in CKD patients and to explore the global trends of factors driving any apparent variability in prevalence estimates.
Materials and methods
Registration and methodology reporting
This systematic review and meta-analysis followed the recommendations provided by the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines. The current review has been registered with PROSPERO under the registration number: CRD42022306572. This authorization encompasses patient cohorts with both CKD and chronic liver disease (CLD).
Data sources and search strategy
Searches were undertaken using the following electronic databases and search engines from 1999 to November 2021: PubMed, Scopus, the Cochrane Database of Systematic Reviews (CDSR), and Google Scholar (first 50 pages). The process also included cross-referencing of included papers. The choice of these databases as primary areas of our literature search was based on their validation as repositories of the most current medical literature; in addition to their continued update and renewal.
The following search terms were used: “polypharmacy” [TIAB], AND “kidney” [MeSH] OR “CKD” OR “chronic kidney disease” [MeSH]. We additionally searched grey literature for similar articles that are not captured in the aforementioned databases. The search was limited to “English language” and “Human species” as applicable to each database.
Eligibility criteria
This systematic review and meta-analysis examined studies of patient populations with CKD who had medication counts adjudicated as polypharmacy and reported as such. The main outcome is the pooled estimate of polypharmacy period prevalence amongst these studies. Our review does not involve a new investigational medicinal product (IMP), hence no conceivable need for an intervention and comparator arms.
Studies were considered eligible if they met the following criteria: (1) reported numerical data on the prevalence of polypharmacy; (2) included patients with CKD; (3) included participants of ≥18 years; (4) published in English language. We included studies regardless of what the authors considered to be the threshold for diagnosis of polypharmacy (e.g., ≥5, ≥10 medications, etc.). Case report, case series, and reviews were excluded as were studies that only included patients with polypharmacy (patients who have CKD without polypharmacy were excluded) as this means that the control/denominator is missing which will prevent calculating the prevalence.
Study selection
All retrieved studies were exported to EndNote 20® (2021 Clarivate), duplicates were removed, and the remaining papers were imported to Rayyan Qatar Computing Research Institute (QCRI) software. Two independent reviewers (LN and MD) screened the titles, abstracts, and full texts of the records to ascertain eligibility for inclusion. Disagreements between reviews were resolved through consensus discussion with a third reviewer (MK).
Data extraction
Two independent reviewers (LN and MD) initially trialed a sample data collection sheet on five randomly selected studies to determine the robustness of this sheet in abstracting patient data. After which the following variables were extracted from the studies: author, year of publication, study design, site, country, population, age, gender distribution, sample size, proportion of CKD patients with polypharmacy, iteration of polypharmacy definition (where available), and duration of study.
Quality assessment
The risk of bias of the included studies was carried out using the Loney’s criteria. Exhaustive description of this appraisal tool has been done elsewhere (Loney et al., 1998). Briefly, the tool is comprised of eight domains returning a total score of eight for studies with optimal methodological quality. Two reviewers (LN and MD) independently assessed the methodological quality. Disagreements were resolved by consensus or by involving a third reviewer (AA).
Data synthesis
Continuous Variables were presented as means (± standard deviation [SD]) or median (interquartile range [IQR]) as appropriate; whilst categorical variables were presented as numbers (percentages). We quantified the pooled prevalence estimates of polypharmacy (utilizing the random effects model) amongst patients with CKD using the default double arcsine transformation. We did not apply any continuity correction because this transformation does not need one. To ascertain the source of significant heterogeneity amongst the included studies (where this exists), we subsequently carried out a subgroup analyses to examine the effect of age, gender, source of primary data, as well as the risk of bias scores of the reviewed studies on the final point prevalence estimates. We assessed the heterogeneity between studies with I2 statistic and τ2 statistic (Higgins et al., 2003). We considered the I2 thresholds of 25%, 50%, and 75% to represent low, moderate, and high heterogeneity between-study variances, respectively. Where τ2 was reported to be zero, this was indicative of no heterogeneity (Quintana, 2015). We utilized funnel and Doi plots to visualize small-study effect and publication bias (Furuya-Kanamori et al., 2018). We finally carried out sensitivity analysis excluding each study to ascertain its effect on the final point prevalence estimate. All statistical analyses were carried out with Meta XL, version 5.3 (EpiGear International, Queensland, Australia).
Results
Study selection
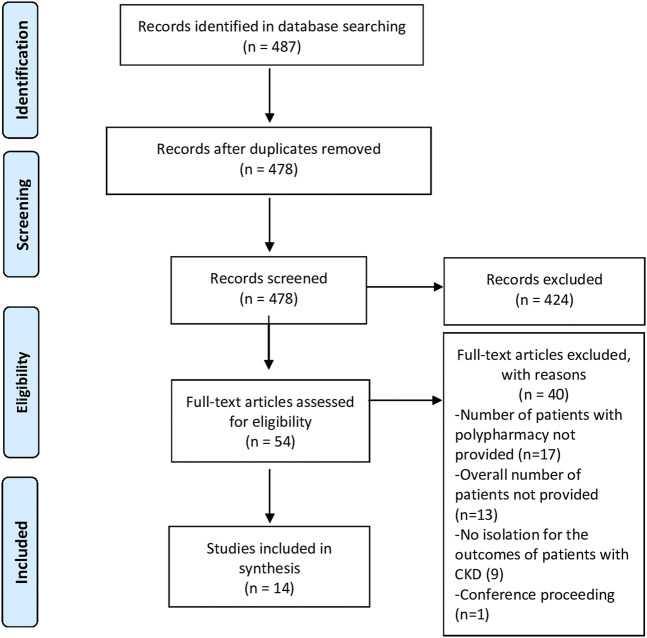
A total of 487 citations were retrieved from the literature search. After duplicate removal (n = 478), the remaining articles were screened by title and abstract. A total of 14 studies were included in the systematic review and meta-analysis (Figure 1). The most common reason for exclusion was the absence of data that allows the estimation of the prevalence of polypharmacy.
FIGURE 1.
PRISMA flow diagram of the study selection process.
Characteristics of included studies
Table 1 summarizes the characteristics of the included studies. The reviewed studies were distributed across four continents with three studies conducted in the USA (Bowling et al., 2014; Sutaria et al., 2016; Hawley et al., 2019), two in Germany (König et al., 2017; Schmidt et al., 2019), and one each in Canada (Battistella et al., 2018), Saudi Arabia (Meraya and Alwhaibi, 2020), Lebanon (Chahine, 2020), Italy (Cojutti et al., 2016), India (Subeesh et al., 2020), Korea (Min et al., 2021), Japan (Kimura et al., 2021), and Ethiopia (Garedow et al., 2019). The last study took place across multiple European countries (Hayward et al., 2020). Of the 14 studies included, six were undertaken in outpatient settings (clinics, community dwelling) (Bowling et al., 2014; König et al., 2017; Battistella et al., 2018; Hawley et al., 2019; Schmidt et al., 2019; Hayward et al., 2020), four in hospitals (Garedow et al., 2019; Chahine, 2020; Subeesh et al., 2020; Kimura et al., 2021), two in multiple settings (Cojutti et al., 2016; Min et al., 2021), and two did not report (Sutaria et al., 2016; Meraya and Alwhaibi, 2020). Most studies were retrospective or prospective cohort studies (n = 6) (Sutaria et al., 2016; Garedow et al., 2019; Hawley et al., 2019; Schmidt et al., 2019; Hayward et al., 2020; Min et al., 2021), followed by cross–sectional observational studies (n = 5) (König et al., 2017; Battistella et al., 2018; Chahine, 2020; Meraya and Alwhaibi, 2020; Subeesh et al., 2020), longitudinal studies (n = 2) (Bowling et al., 2014; Kimura et al., 2021), and point-prevalence study (n = 1) (Cojutti et al., 2016). The follow-up duration ranged from 6 months (Garedow et al., 2019; Chahine, 2020; Subeesh et al., 2020) to 10 years across included studies (Min et al., 2021).
TABLE 1.
Characteristics of included studies.
| Author, year of publication | Type of study | Age | Gender (male) | Site | Population characteristics | Country | Total number of patients with CKD | Case (CKD with polypharmacy) | Numerical description | Exact definition of polypharmacy in article | Duration |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Battistella3 et al., (2018) | Retrospective cross-sectional study | Mean (±SD) 76.5 (±7.3) | 55.6% | Outpatients | All patients were on hemodialysis. The 6 most common comorbidities were coronary artery disease (62%), diabetes (53%), heart failure (52%), chronic lung disease (42%), arrhythmia (29%), and atrial fibrillation (22%). The median duration of incenter hemodialysis was 3 (1–6) years | Canada | 3094 | 2882 | ≥5 | None | Not reported |
| Bowling et al., (2014) | Population-based longitudinal study | All patients were ≥75 years old | 45.5% | Community dwelling | Black (33.6%). GFR≥60 (73%). GFR 45–59 (17%). GFR <45: 9.4% | United States | 941 | 282 | ≥10 | The concurrent use of ≥10 prescription medications | 5 years |
| Chahine, (2020) | Retrospective cross-sectional study | Mean (±SD) 76.47 (±8.2) | 46.2% | Two teaching hospitals | Patients were admitted for non-renal reasons (88.9%), median length of hospitalization: 8 days, hemodialysis (41.2%), hypertension (94%), coronary heart disease (72.4), diabetes (55.8%) | Lebanon | 199 | 123 | ≥5 | Using ≥5 medications per day | 6 months |
| Cojutti et al., (2016) | Point-prevalence study | Median (IQR) Hospitals: 81 (75–87 Community: 76 (71–82) long-term care facilities (LTCF): 85 (79–89) | Hospitals: 50.6% community: 42.7% LTCF: 29.6% | Hospital (n = 528), community (n = 527), LTCF (n = 527) | ≥5 underlying diseases: hospitals (24.8%), community (33.39%), LTCF (19.35%) Elderly (65–79 years): hospitals (39.6%), community (62.4%), LTCF: (22.9) Very elderly (>79 years): hospital (60.4%), community (37.6%), LTCF (77.1%) | Italy | 1582 | Polypharmacy patients: 1063 Hyperpolypharmacy: 213 | Polypharmacy: ≥5 Hyperpolypharmacy: ≥10 | Polypharmacy: the co-prescription of 5–9 drugs Hyperpolypharmacy: the co-prescription of ≥10 drugs | Not reported |
| Garedow et al., (2019) | Prospective observational study | Mean (±SD) 45.83 (±17.7) | 69.9% | Hospital | Had <5 comorbidities (87.4%), newly diagnosed CKD patients (64.1%), stay in hospital for ≥7 days (77.7%), normal BMI (42.7%), CKD stage II (1.9%), CKD stage III (17.5%), CKD stage IV (15.5%), CKD stage V (65%) | Ethiopia | 103 | 48 | ≥5 | Use of ≥5 medication concomitantly | 6 months |
| Hawley et al., (2019) | Pilot prospective cohort study | Mean (±SD): 73 (±10) | 97% | Outpatient nephrology clinic | Non-dialysis kidney disease (89% stages III-V), hypertension (85%), dyslipidemia (80%), type II diabetes (59%) | United States | 87 | 75 | >10 | Taking > 10 medications | 10 months |
| Hayward et al., (2020) | Prospective cohort study | Mean (±SD) 76.5(±6.7) | 64.2% | Nephrology clinics | The median eGFR was 18.0 mL/min/1.73m2 (IQR 16.0–19.0). All participants had at least one comorbidity in addition to CKD. The most frequent comorbidities were hypertension (84.4%), diabetes (42.4%), and coronary artery disease (27.3%). The mean BMI was 28.5 kg/m2 (±5.4) | Germany, Italy, Netherlands, Poland and the United Kingdom | 1,317 | Polypharmacy: 1194 Hyperpolypharmacy: 564 | Polypharmacy: ≥5 Hyperpolypharmacy: ≥10 | None | 5 years |
| Kimura et al., (2021) | Retrospective longitudinal study | Median (IQR) 66 (58–75) | 56% | Hospital | The median eGFR was 48 mL/minper1.73m2, participants with hypertension (87%), participants with diabetes (49%) | Japan | 1,117 | Polypharmacy: 429 Hyperpolypharmacy: 427 | Polypharmacy: ≥5 Hyperpolypharmacy: ≥10 | Polypharmacy: the regular use of 5–9 per day Hyperpolypharmacy: the regular use of ≥10 medications per day | Not reported |
| König et al., (2017) | Cross-sectional study | Mean (±SD) 68.7 (±3.7) | 48.8% | Community dwelling | Hypertension (77.1%), diabetes (12.4%), obese (18.3%), GFR-CG, mL/min/1.73 m2 77.8 (49.5–122.2) | Germany | 317 | 103 | ≥5 | The use of ≥5 regular drugs, considering prescription and over-the-counter as well as scheduled and as-needed medications | 6 years |
| Meraya and Alwhaibi, (2020) | Cross-sectional study | In all groups (not only CKD) Age (21–39): 6.5% Age (40–49): 12.9% Age (50–64): 37.9% Age (≥65):42.7% | In all groups (not only CKD) 49.3% | Not reported | In all groups (not only CKD): no chronic physical condition (11.8%), 1–2 chronic physical conditions (48.7%), 3–4 chronic physical conditions (30.8%), ≥5 chronic physical conditions (8.7%). Patients having mental conditions (13.8%). Obese (54.6%), overweight (29.8%), underweight/normal (14.1%) | Saudi Arabia | 480 | 354 | ≥6 | Use of ≥6 medication classes | 6 years |
| Min et al., (2021) | Prospective cohort study | Mean (±SD) 53.6 (±12.3) | 60.8% | Multicenter | Mean eGFR was 53.6 mL/min/1.73 m2. The causes of CKD were diabetic nephropathy (25.3%), hypertensive nephropathy (19.6%), glomerulonephritis (30.7%), and other causes (24.5%) | Korea | 1,913 | 518 | ≥6 | None | 10 years |
| Schmidt et al., (2019) | Prospective observational study | Mean (±SD) CKD stage G1 41.8 (±12.9) CKD stage G2: 55.6 (±12.6) CKD stage G3a: 61.3 (±10.4) CKD G3b: 62.6 (±10.6) CKD stage G4/5: 63.5 (±10.1) | CKD stage G1 51.5% CKD stage G2: 54.4% CKD stage G3a: 62% CKD G3b: 61.5% CKD stage G4/5: 62.5% | Multiple outpatient units | CKD stage G3a (33.3%), CKD stage G3b (36.1%). Hypertension, diabetes, CVD and dyslipidemia were the most frequent comorbid conditions showing an increase in prevalence with lower eGFR | Germany | 5,217 | 4,173 | 5 ≥ | The daily use of ≥5 active substances, including the intake of non-oral and OTC medications | 2 years |
| Subeesh et al., (2020) | Cross-sectional study | Mean (±SD) 50.08 (±15.32) | 71.25% | Hospital | All patients had hypertension, diabetes (29.31%), anemia (11%), average comorbidities: 1.7 ± 1.86 | India | 160 | 146 | >5 | None | 6 months |
| Sutaria et al., (2016) | Prospective study | ≥60–69 (27.03%), ≥70–79 (55.76%), ≥80 (73.26) | 43.11% | Not reported | Hypertension (50.74%), diabetes (54.13%), cardiovascular diseases (60.57%) 1,122.46 | United states | 674 | 308 | ≥5 | The use of ≥5 prescription medications per day | 1 year |
Overall, a total of N = 17 201 participants were included in this systematic review and meta-analysis, of which 43.88% were females. Among studies that reported the average age (n = 9), the mean age was 61.96 (SD ± 11.51) years. All the constituent studies had patients with at least one medical condition alongside the CKD, with hypertension being the most frequently reported. Other common co-morbidities were diabetes, coronary artery disease, and other cardiovascular diseases (Table 1). The stage of the CKD amongst the reviewed studies was variable. For instance, Battistella et al. (2018) focused exclusively on patients on hemodialysis (stage 5, glomerular filtration rate (GFR) <15 mL/min), while the majority of patients in Bowling et al. (2014) study and Schmidt et al. (2019) were in stage 2 (GFR >60 mL/min) and stage 3 (GFR 30–60 mL/min), respectively.
Quality of included studies
The overall quality of included studies was moderate to good mainly due to issues related to the sample size calculation and the appropriateness of outcome measures (Table 2).
TABLE 2.
Quality assessment of included studies.
| Author, year of publication | 1. Was the design appropriate for the research question? | 2a. Where setting of study and subjects described in detail? | 2b. Are the subjects comparable to my population of interest? | 3. Was subject sample obtained appropriately? | 4. Was sample size appropriate? | 5. Were objective and appropriate criteria used for measurement of outcomes? | 6. Was outcome measured appropriately? | 7. Are the estimates of prevalence precise? | 8. Was response rate adequate? | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Battistella et al., (2018) | Yes | Yes | Yes | Yes | Yes | Cannot tell | Yes | Yes | Yes | 7 |
| Bowling et al., (2014) | Yes | Yes | Yes | Yes | Yes | Cannot tell | Yes | Yes | Yes | 7 |
| Chahine, (2020) | Yes | Yes | Yes | Yes | No | Cannot tell | Yes | Yes | Yes | 6 |
| Cojutti et al., (2016) | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | 6 |
| Garedow et al., (2019) | Yes | Yes | Yes | Yes | No | Cannot tell | Yes | Yes | Yes | 6 |
| Hawley et al., (2019) | Yes | Yes | Yes | Yes | No | Cannot tell | Yes | Yes | Yes | 6 |
| Hayward et al., (2020) | Yes | Yes | Yes | Yes | Yes | Cannot tell | Yes | Yes | Yes | 7 |
| Kimura et al., (2021) | Yes | Yes | Yes | Yes | Cannot tell | Cannot tell | Yes | Yes | Yes | 6 |
| König et al., (2017) | Yes | Yes | Yes | Yes | Cannot tell | Cannot tell | Yes | Yes | Yes | 6 |
| Meraya and Alwhaibi, (2020) | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | 6 |
| Min et al., (2021) | Yes | Yes | Yes | Yes | No | Cannot tell | Yes | Yes | Yes | 6 |
| Schmidt et al., (2019) | Cannot tell | Yes | Yes | Yes | Yes | Cannot tell | Yes | Yes | Yes | 6 |
| Subeesh et al., (2020) | Yes | Yes | Yes | Yes | Cannot tell | Cannot tell | Yes | Yes | Yes | 6 |
| Sutaria et al., (2016) | Yes | Yes | Yes | Yes | Yes | Cannot tell | Yes | Yes | Yes | 7 |
Prevalence of polypharmacy in patients with CKD
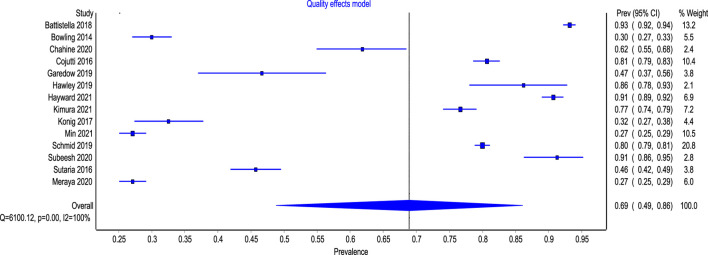
Figure 2 presents the pooled estimates of the included studies. The overall pooled prevalence of polypharmacy amongst patients with CKD was 69% (95% CI: 49%–86%). The overall heterogeneity among the included studies was significant (I2 = 100%, p < 0.0001).
FIGURE 2.
Forest plot of the prevalence of polypharmacy among patient with CKD.
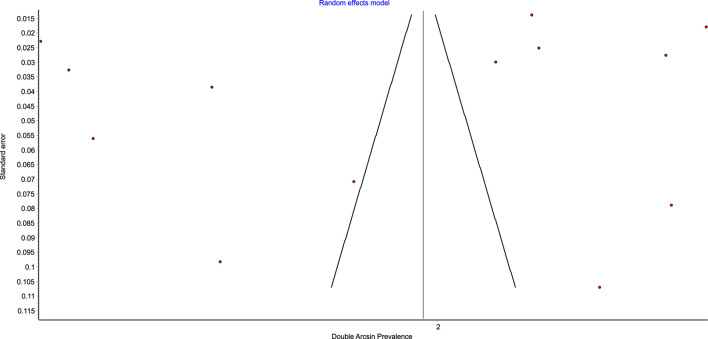
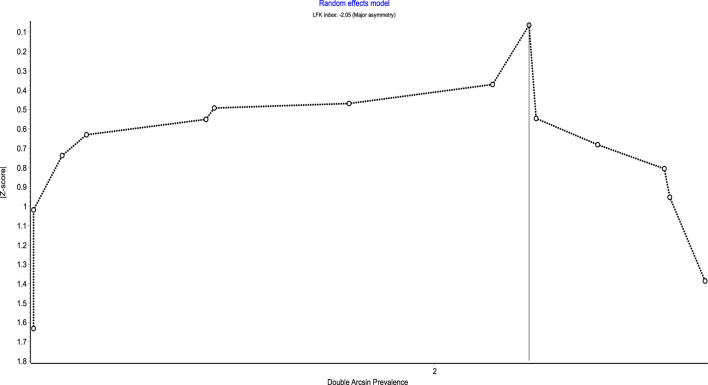
Figures 3, 4 show the comparison adjusted funnel plot and Doi plot of the included studies. The plots demonstrated major (LFK 2.05) asymmetry which indicates major publication bias. This could also be attributed to the substantial between-study heterogeneity and small-study effect. No continuity correction was applied because the double arcsine prevalence transformation does not require one.
FIGURE 3.
Funnel plot of the prevalence of polypharmacy among patient with CKD.
FIGURE 4.
Doi plot of the prevalence of polypharmacy among patient with CKD.
A sensitivity analysis was also performed by excluding one study each time and recalculating the pooled prevalence of polypharmacy for the remaining studies (Table 3). Based on this, the estimated pooled prevalence of polypharmacy in patients with CKD ranged between 61% and 67%.
TABLE 3.
Point estimates of the various included studies following sensitivity analysis.
| Excluded study | Pooled prevalence | LCI 95% | HCI 95% | Cochran Q | P | I2 | I2 LCI 95% | I2 HCI 95% |
|---|---|---|---|---|---|---|---|---|
| Battistella et al. (2018) | 0.61 | 0.45 | 0.77 | 4558.14 | 0.00 | 99.74 | 99.70 | 99.77 |
| Bowling et al. (2014) | 0.67 | 0.50 | 0.82 | 5453.33 | 0.00 | 99.78 | 99.75 | 99.80 |
| Chahine (2020) | 0.65 | 0.47 | 0.80 | 6095.05 | 0.00 | 99.80 | 99.78 | 99.82 |
| Cojutti et al. (2016) | 0.63 | 0.45 | 0.79 | 5979.91 | 0.00 | 99.80 | 99.77 | 99.82 |
| Garedow et al. (2019) | 0.66 | 0.49 | 0.81 | 6077.79 | 0.00 | 99.80 | 99.78 | 99.82 |
| Hawley et al. (2019) | 0.62 | 0.45 | 0.78 | 6085.90 | 0.00 | 99.80 | 99.78 | 99.82 |
| Hayward et al. (2020) | 0.62 | 0.44 | 0.78 | 5667.05 | 0.00 | 99.79 | 99.76 | 99.81 |
| Kimura et al. (2021) | 0.63 | 0.45 | 0.80 | 6067.64 | 0.00 | 99.80 | 99.78 | 99.82 |
| Konig et al. (2017) | 0.67 | 0.50 | 0.82 | 5917.24 | 0.00 | 99.80 | 99.77 | 99.82 |
| Meraya and Alwhaibi (2020) | 0.67 | 0.52 | 0.81 | 4479.19 | 0.00 | 99.73 | 99.70 | 99.76 |
| Min et al. (2021) | 0.67 | 0.52 | 0.81 | 4479.19 | 0.00 | 99.73 | 99.70 | 99.76 |
| Schmid et al. (2019) | 0.63 | 0.44 | 0.81 | 5657.46 | 0.00 | 99.79 | 99.76 | 99.81 |
| Subeesh et al. (2020) | 0.62 | 0.45 | 0.78 | 6048.37 | 0.00 | 99.80 | 99.78 | 99.82 |
| Sutaria et al. (2016) | 0.66 | 0.48 | 0.81 | 5937.59 | 0.00 | 99.80 | 99.77 | 99.82 |
Global trend in the prevalence of polypharmacy in patients with CKD
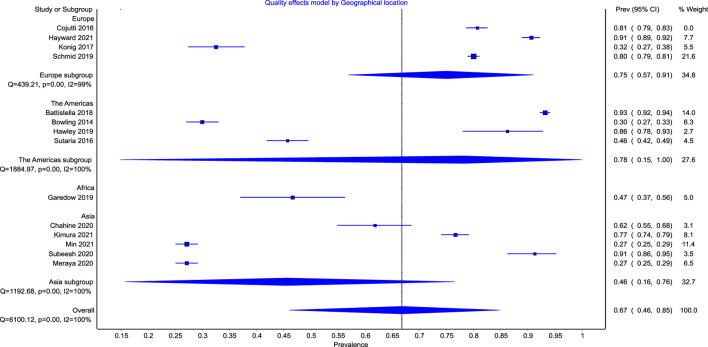
The subgroup meta-regression showed a higher pooled prevalence of polypharmacy of 78% (95% CI: 63%–92%) in Europe and 78% (95% CI: 15%–100%) in North America as compared with 48% (95% CI: 0%–100%) in Asia (Figure 5). The overall heterogeneity was significant for both overall analysis (I2 = 100%, p < 0.0001) and subgroup analyses (I2 = 99%, p < 0.01), (I2 = 100%, p < 0.01), (I2 = 100%, p < 0.01) for the European, North American, and Asian studies, respectively.
FIGURE 5.
Forest plot of the prevalence of polypharmacy among patient with CKD across continents.
Only one study was identified from Africa (Garedow et al., 2019), therefore estimating the pooled prevalence was not feasible. Similarly, we could not calculate the prevalence in South America, Antarctica, or Australia as no relevant studies were identified from these continents.
Discussion
To the best of our knowledge, this systematic review and meta-analysis represents the first comprehensive synthesis of the prevalence of polypharmacy and the global trends associated with its variability among patients with CKD. We explored the longitudinal data of 17,201 patients from 13 countries in 4 continents. The overall pooled prevalence of polypharmacy amongst patients with CKD was 69% with a proportionately higher prevalence in North America and Europe as compared to Asia. Despite the apparent disparity in the stage of CKD amongst the included studies, our period prevalence estimate provides the first attempt at exploring the burden of this growing therapeutic morbidity in this cohort of patients.
The prevalence estimates reported in this review are substantially higher than the general population including cohorts at high risk of experiencing polypharmacy such as elderly (Veehof et al., 2000), patients with chronic liver disease (Danjuma et al., 2022a), and people living with HIV (Danjuma et al., 2022b). However, the estimates were comparable to other populations that are also known to be more exposed to polypharmacy such as heart failure patients (Beezer et al., 2021).
This remarkably high prevalence is alarming particularly in CKD patients as they represent a more challenging and therapeutically vulnerable population; principally due to the central role of the kidney in drug metabolism (including other aspects of pharmacokinetics and pharmacodynamics) (Laville et al., 2020). This therefore imposes higher risk of adverse drug reactions (ADR) and their sequalae on patients with CKD (Laville et al., 2020).
Additionally, patients included in our review had an average age of 61.96 (SD ± 11.51) years, which is expected as CKD in more prevalent in people older than 60 years old (Aging and Kidney, 2022). This further complicates the situation as there is mounting evidence of the increased risk of mortality in response to increased drug counts, especially in the elderly (Patel et al., 2017; Davies et al., 2022). For instance, the Newcastle 85 + study showed that for each additional medication prescribed in patients aged 85 years and older, there is a 3% associated risk of increased mortality (hazard ratio: 1.03, 95% CI: 1.00–1.06) (Davies et al., 2022).
Our findings underscore the gravity of the rising burden of polypharmacy among patients with CKD and highlight the pressing need to adopt some of the interventions that have been proposed to reduce the burden of polypharmacy in the general population. This includes comprehensive medication reviews, deprescribing algorithms, potentially inappropriate medications (PIM) screening tools (e.g., Beers criteria), and clinical pharmacist-led interventions (Cooper et al., 2015; El‐Awaisi et al., 2022; Isleem et al., 2022). It is noteworthy that these interventions are not widespread and that the research on the effectiveness of such strategies on clinically important outcomes is limited (Cooper et al., 2015). Hence, future research efforts should focus on measuring the effectiveness of these interventions. Moreover, experts should attempt at developing multifaceted theory-based interventions that are tailored to patients with CKD. This approach is expected to yield promising outcomes as other methods of interventions development [i.e., pragmatic approach or ISLAGIATT (It Seemed Like A Good Idea At The Time) principle] were checkered, with some showing unfavorable outcomes or no benefit at all (Michie et al., 2005; Hughes et al., 2016; Steinmo et al., 2016).
Strengths and limitations
The principal strength of this synthesis lies in its novelty at estimating the overall pooled prevalence of polypharmacy among patients with CKD. This will enable clinicians, as well as policy makers, and other stakeholders to more robustly estimate the burden of polypharmacy and more appropriately allocate intervention strategies aimed at mitigating their downstream effects (including bidirectional interactions as well as adverse drug reactions). The review also involved comprehensive searching of three large databases using established methods and was reported following a standardized method. Despite this, there were several limitations. Firstly, only English language publications were included. Secondly, polypharmacy prevalence was not the primary outcome measure in many of the included studies which resulted in the lack of in-depth information relating to it. Finally, significant heterogeneity was noted across the included studies. This could be attributed to the variation in the definition of polypharmacy among included studies. Other factors include the various study design, the geographical disposition of areas where the studies were carried out, and the difference in the mean age of the constituent studies.
Conclusion
The results from this meta-analysis showed a high pooled prevalence estimates of polypharmacy amongst patient cohorts with CKD. The exact interventions that are likely to significantly mitigate its effect remain uncertain and will need exploration by future prospective and systematic studies.
Acknowledgments
Open Access funding provided by the Qatar National Library.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization: LN and MD. Literature search: LN, AA, MK, and MD. Independent review and risk of bias assessment: LN, AA, MK, and MD. Data curation: AA and MK. Data analysis and synthesis: LN and MD. Initial draft of manuscript: LN and MD. Final manuscript: LN, AA, MK, and MD.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Aging and Kidney (2022). Aging and kidney disease [internet]. New York: National Kidney Foundation. [Google Scholar]
- Bansal N., Katz R., Robinson-Cohen C., Odden M., Dalrymple L., Shlipak M., et al. (2017). Absolute rates of heart failure, coronary heart disease, and stroke in chronic kidney disease: An analysis of 3 community-based cohort studies. JAMA Cardiol. 2 (3), 314–318. 10.1001/jamacardio.2016.4652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella M., Jandoc R., Ng J., McArthur E., Garg A. (2018). A province-wide, cross-sectional study of demographics and medication use of patients in hemodialysis units across ontario. Can. J. Kidney Health Dis. 5, 2054358118760832. 10.1177/2054358118760832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beezer J., Al Hatrushi M., Husband A., Kurdi A., Forsyth P. (2021). Polypharmacy definition and prevalence in heart failure: A systematic review. Heart Fail. Rev. 27 (2), 465–492. 10.1007/s10741-021-10135-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello A., Alrukhaimi M., Ashuntantang G., Basnet S., Rotter R., Douthat W., et al. (2017). Complications of chronic kidney disease: Current state, knowledge gaps, and strategy for action. Kidney Int. Suppl. 7 (2), 122–129. 10.1016/j.kisu.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling C., Booth J., Gutiérrez O., Kurella Tamura M., Huang L., Kilgore M., et al. (2014). Nondisease-specific problems and all-cause mortality among older adults with CKD: The REGARDS study. Clin. J. Am. Soc. Nephrol. 9 (10), 1737–1745. 10.2215/CJN.00880114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine B. (2020). Potentially inappropriate medications prescribing to elderly patients with advanced chronic kidney by using 2019 American Geriatrics Society Beers Criteria. Health Sci. Rep. 3 (4), e214. 10.1002/hsr2.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cojutti P., Arnoldo L., Cattani G., Bruniferro S., Pea F. (2016). Polytherapy and the risk of potentially inappropriate prescriptions (PIPs) among elderly and very elderly patients in three different settings (hospital, community, long-term care facilities) of the friuli venezia giulia region, Italy: Are the very elderl. Pharmacoepidemiol. Drug Saf. 25 (9), 1070–1078. 10.1002/pds.4026 [DOI] [PubMed] [Google Scholar]
- Cooper J., Cadogan C., Patterson S., Kerse N., Bradley M., Ryan C., et al. (2015). Interventions to improve the appropriate use of polypharmacy in older people: A Cochrane systematic review. BMJ Open 5 (12), e009235. 10.1136/bmjopen-2015-009235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couser W., Remuzzi G., Mendis S., Tonelli M. (2011). The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 80 (12), 1258–1270. 10.1038/ki.2011.368 [DOI] [PubMed] [Google Scholar]
- Danjuma M., Adegboye O., Aboughalia A., Soliman N., Almishal R., Abdul H., et al. (2022). Prevalence and global trends of polypharmacy among people living with HIV: A systematic review and meta-analysis. Ther. Adv. Drug Saf. 13, 20420986221080795. 10.1177/20420986221080795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danjuma M., Naseralallah L., Ansari S., Alshibly R., Elhams M., Alshamri M., et al. (2022). Prevalence and global trends of polypharmacy in patients with chronic liver disease: A systematic review and meta-analysis (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies L., Kingston A., Todd A., Hanratty B. (2022). Is polypharmacy associated with mortality in the very old: Findings from the Newcastle 85+ Study. Br. J. Clin. Pharmacol. 88 (6), 2988–2995. 10.1111/bcp.15211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmez J., Slatopolsky E. (1992). Hyperphosphatemia: Its consequences and treatment in patients with chronic renal disease. Am. J. Kidney Dis. 19 (4), 303–317. 10.1016/s0272-6386(12)80446-x [DOI] [PubMed] [Google Scholar]
- El‐Awaisi A., Al‐Shaibi S., Al‐Ansari R., Naseralallah L., Awaisu A. (2022). A systematic review on the impact of pharmacist‐provided services on patients’ health outcomes in Arab countries. J. Clin. Pharm. Ther. 47 (7), 879–896. 10.1111/jcpt.13633 [DOI] [PubMed] [Google Scholar]
- Furuya-Kanamori L., Barendregt J. J., Doi S. A. R. (2018). A new improved graphical and quantitative method for detecting bias in meta-analysis. Int. J. Evidence-Based Healthc. 16 (4), 195–203. 10.1097/XEB.0000000000000141 [DOI] [PubMed] [Google Scholar]
- Gansevoort R., Matsushita K., van der Velde M., Astor B., Woodward M., Levey A., et al. (2011). Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 80 (1), 93–104. 10.1038/ki.2010.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garedow A., Mulisa Bobasa E., Desalegn Wolide A., Kerga Dibaba F., Gashe Fufa F., Idilu Tufa B., et al. (2019). Drug-related problems and associated factors among patients admitted with chronic kidney disease at jimma university medical center, jimma zone, jimma, southwest Ethiopia: A hospital-based prospective observational study. Int. J. Nephrol. 2019, 1504371–1504379. 10.1155/2019/1504371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley C., Triantafylidis L., Paik J. (2019). The missing piece: Clinical pharmacists enhancing the interprofessional nephrology clinic model. J. Am. Pharm. Assoc. 59 (5), 727–735. 10.1016/j.japh.2019.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S., Hole B., Denholm R., Duncan P., Morris J., Fraser S., et al. (2020). International prescribing patterns and polypharmacy in older people with advanced chronic kidney disease: Results from the European quality study. Nephrol. Dial. Transplant. 36 (3), 503–511. 10.1093/ndt/gfaa064 [DOI] [PubMed] [Google Scholar]
- Higgins J. P. T., Thompson S. G., Deeks J. J., Altman D. G. (2003). Measuring inconsistency in meta-analyses. BMJ [Internet] 327 (7414), 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C., McCulloch C., Curhan G. (2002). Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: Results from the third national health and nutrition examination survey. J. Am. Soc. Nephrol. 13 (2), 504–510. 10.1681/ASN.V132504 [DOI] [PubMed] [Google Scholar]
- Hughes C. M., Cadogan C. A., Ryan C. A. (2016). Development of a pharmacy practice intervention: Lessons from the literature. Int. J. Clin. Pharm. 38 (3), 601–606. 10.1007/s11096-015-0180-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isleem N., Shoshaa S., AbuGhalyoun A., Khatib M., Naseralallah L., Ibn‐Mas'ud Danjuma M., et al. (2022). Critical care tele‐pharmacy services during covid ‐19 pandemic: A qualitative exploration of healthcare practitioners' perceptions. J. Clin. Pharm. Ther. 47, 1591–1599. 10.1111/jcpt.13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James M., Hemmelgarn B., Tonelli M. (2010). Early recognition and prevention of chronic kidney disease. Lancet 375 (9722), 1296–1309. 10.1016/S0140-6736(09)62004-3 [DOI] [PubMed] [Google Scholar]
- Jankowski J., Floege J., Fliser D., Böhm M., Marx N. (2021). Cardiovascular disease in chronic kidney disease: Pathophysiological insights and therapeutic options. Circulation 143 (11), 1157–1172. 10.1161/CIRCULATIONAHA.120.050686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group (2013). KGIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 3 (1), 1–150. [DOI] [PubMed] [Google Scholar]
- Kimura H., Tanaka K., Saito H., Iwasaki T., Oda A., Watanabe S., et al. (2021). Association of polypharmacy with kidney disease progression in adults with CKD. Clin. J. Am. Soc. Nephrol. 16 (12), 1797–1804. 10.2215/CJN.03940321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König M., Gollasch M., Demuth I., Steinhagen-Thiessen E. (2017). Prevalence of impaired kidney function in the German elderly: Results from the berlin aging study II (BASE-II). Gerontology 63 (3), 201–209. 10.1159/000454831 [DOI] [PubMed] [Google Scholar]
- Laville S., Gras-Champel V., Moragny J., Metzger M., Jacquelinet C., Combe C., et al. (2020). Adverse drug reactions in patients with CKD. Clin. J. Am. Soc. Nephrol. 15 (8), 1090–1102. 10.2215/CJN.01030120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loney P. L., Chambers L. W., Bennett K. J., Roberts J. G., Stratford P. W. (1998). Critical appraisal of the health research literature: Prevalence or incidence of a health problem. Chronic Dis. Can. 19 (4), 170–176. [PubMed] [Google Scholar]
- Meraya A., Alwhaibi M. (2020). Health related quality of life and healthcare utilization among adults with diabetes and kidney and eye complications in the United States. Health Qual. Life Outcomes 18 (1), 85. 10.1186/s12955-020-01336-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie S., Johnston M., Abraham C., Lawton R., Parker D., Walker A., et al. (2005). Making psychological theory useful for implementing evidence based practice: A consensus approach. Qual. Saf. Health Care 14 (1), 26–33. 10.1136/qshc.2004.011155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H., Sung S., Chung W., Kim Y., Chae D., Ahn C., et al. (2021). Polypharmacy and the progression of chronic kidney disease: Korean cohort study for outcome in patients with chronic kidney disease. Kidney Blood Press. Res. 46 (4), 460–468. 10.1159/000516029 [DOI] [PubMed] [Google Scholar]
- Parker K., Wong J. (2019). Is polypharmacy an increasing burden in chronic kidney disease? The German experience. Clin. Kidney J. 12 (5), 659–662. 10.1093/ckj/sfz072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P., Hayward K., Rudra R., Horsfall L., Hossain F., Williams S., et al. (2017). Multimorbidity and polypharmacy in diabetic patients with NAFLD: Implications for disease severity and management. Medicine 96 (26), e6761. 10.1097/MD.0000000000006761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana D. S. (2015). From pre-registration to publication: A non-technical primer for conducting a meta-analysis to synthesize correlational data. Front. Psychol. 6, 1549. 10.3389/fpsyg.2015.01549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt I., Hübner S., Nadal J., Titze S., Schmid M., Bärthlein B., et al. (2019). Patterns of medication use and the burden of polypharmacy in patients with chronic kidney disease: The German chronic kidney disease study. Clin. Kidney J. 12 (5), 663–672. 10.1093/ckj/sfz046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanski A., Schmidt K., Waldherr R., Ritz E. (1996). Early increase in blood pressure and diastolic left ventricular malfunction in patients with glomerulonephritis. Kidney Int. 50 (4), 1321–1326. 10.1038/ki.1996.444 [DOI] [PubMed] [Google Scholar]
- Steinmo S. H., Michie S., Fuller C., Stanley S., Stapleton C., Stone S. P. (2016). Bridging the gap between pragmatic intervention design and theory: Using behavioural science tools to modify an existing quality improvement programme to implement “sepsis six”. Implement. Sci. 11 (1), 14. 10.1186/s13012-016-0376-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subeesh V., Abraham R., Satya Sai M., Koonisetty K. (2020). Evaluation of prescribing practices and drug-related problems in chronic kidney disease patients: A cross-sectional study. Perspect. Clin. Res. 11 (2), 70–74. 10.4103/picr.PICR_110_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutaria A., Liu L., Ahmed Z. (2016). Multiple medication (polypharmacy) and chronic kidney disease in patients aged 60 and older: A pharmacoepidemiologic perspective. Ther. Adv. Cardiovasc. Dis. 10 (4), 242–250. 10.1177/1753944716634579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veehof L., Stewart R., Haaijer-Ruskamp F., Jong B. (2000). The development of polypharmacy. A longitudinal study. Fam. Pract. 17 (3), 261–267. 10.1093/fampra/17.3.261 [DOI] [PubMed] [Google Scholar]
- Whittaker C., Fink J. (2017). Deprescribing in CKD: The proof is in the process. Am. J. Kidney Dis. 70 (5), 596–598. 10.1053/j.ajkd.2017.05.025 [DOI] [PubMed] [Google Scholar]
- Widmer B., Gerhardt R. E., Harrington J. T., Cohen J. J. (1979). Serum electrolyte and acid base composition. The influence of graded degrees of chronic renal failure. Archives Intern. Med. 139 (10), 1099–1102. 10.1001/archinte.139.10.1099 [DOI] [PubMed] [Google Scholar]
- Yuan J., Zou X., Han S., Cheng H., Wang L., Wang J., et al. (2017). Prevalence and risk factors for cardiovascular disease among chronic kidney disease patients: Results from the Chinese cohort study of chronic kidney disease (C-stride). BMC Nephrol. 18 (1), 23. 10.1186/s12882-017-0441-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.