Highlights
-
•
The lack of precise intraoperative margin assessment tools for bone cancer is an unmet need to reduce local cancer recurrence.
-
•
New emerging technologies should aim to increase precision in detecting tumour cells while also minimally disrupting the workflow during the operation.
-
•
The use of multimodal spectroscopy technology seems promising for intraoperative margin detection.
Keywords: Osteosarcoma; Intra-operative; Margin assessment, relapse
Abstract
Osteosarcoma is the most common malignant tumour of the bone. Complete surgical excision is critical to achieve optimal outcomes and lower recurrence rates. However, accurate assessment of tumour margins remains a challenge and multiple technologies are employed for this purpose. The aim of this study is to highlight current and emerging technologies and their efficacy in detecting clear bone margins intraoperatively, through a systematic review of the literature.
The following databases were searched using the OVID platform: Medline, Embase, Global Health and Google Scholar. Studies were screened using predetermined eligibility criteria. Data was extracted based on study and patient characteristics, modes of detection, and commercial availability, followed by quality assessment.
A total of 17 studies were included. The primary diagnosis varied, with osteosarcoma being reported by 9 studies. Three studies reported relapse, ranging between 17.6%−48%. Twelve studies reported non-invasive imaging as the mode of detection used, while 4 studies reported the use of frozen section. MRI and CT were found to have an accuracy of up to 93 %. Raman spectroscopy was reported to have an accuracy, sensitivity, and specificity of 69%, 58.8% and 83.3% respectively. CT had a sensitivity and specificity up to 83% and 100%, respectively.
In conclusion, there seems to be high potential for the use of multimodal technologies to increase the accuracy of intraoperative margin assessment. Although imaging modalities possess a fair level of accuracy, they carry the risk of radiation exposure, are expensive, and cannot be used in-situ. Future clinical trials are needed to test the effectiveness of these technologies to measure the diagnostic accuracy and overall patient survival.
1. Introduction
Osteosarcoma is the most common primary bone-forming tumour that arises from the malignant transformation of bone building block cells, osteoblasts [1]. The two other types of primary bone tumours are Ewing sarcoma and chondrosarcomas, which are differentiated from osteosarcoma mainly by histological properties and primary localization [2], [3]. The incidence of osteosarcoma is higher in adolescents and young adults (Male: Female ratio of 1.4) and is characterised by a bimodal age distribution with a first main peak at 18 years old and the secondary peak at 60 [2]. While it can affect any bone, it mostly arises at long bone extremities with the most proliferative growth plates such as the distal femur (42%), the tibia (19%) and the humerus (10%), followed by the skull or jaw (8%) and pelvis (8%) [4], [5]. While the exact cause of osteosarcoma remains unknown, there are several common risk factors such as genetic predispositions, race (proportionally higher in indigenous African and African-American males), other bone diseases (e.g., Paget's disease), and increased radiation exposure [6]. Osteosarcoma tumours are highly heterogeneous, with studies showing more than 80-point mutations with over 80 genes involved [2], [7]. They originate mostly from the metaphysis of the long bones causing a large palpable mass with swelling and pain, and as a result, patients usually present with limping, and decreased mobility of the affected limb. The survival rate majorly depends on the metastatic status of the tumour with a 5-year survival rate of 68% for adolescents between ages 15 to 19 [8]. Survival rate significantly drops to around 30% when the tumour metastasizes to the lungs [2]. An Italian study highlighted how patients who did not undergo surgery of recurrence had a 5-year post-relapse survival of 0%, compared to those who do undergo surgery, highlighting the invasive nature of osteosarcoma and the high potential of relapse [9].
2. Diagnosis and treatment
Osteosarcoma is diagnosed through conventional X-ray film and has a “sunburst” pattern of lytic bone lesions, characterised by cortical destruction and a periosteal reaction (Codman triangles) [10].
MRI is used to assess for soft tissue extension and CT scans to assess metastasis, which is often found in the lungs [10]. Osteosarcoma treatment includes a complexity of combinations such as disabling surgery (limb amputation), chemotherapy, radiotherapy, and prolonged rehabilitation. These increase the socio-economic and morbidity burden to patients, the community, and the healthcare system. In fact, there are 17 Disability Adjusted Life Years (DALYs) attributed due to osteosarcoma alone compared to 6.5 for bowel, lung, and breast cancers which makes the treatment for osteosarcoma a public health concern [11].
2.1. The importance of intraoperative margin assessment
Complete surgical excision is a critical aspect of the treatment to ensure optimum outcomes and lower rates of recurrence [4]. Patients who suffer from osteosarcoma recurrence currently face less than 20% survival rate in the long term [10]. Orthopaedic surgeons in oncology face challenging decisions as they assess intraoperative margins of resection; While the main goal is to salvage as much functional tissue as possible, they can often be left with no other option but to resect beyond the margin to decrease the risk of recurrence, which can often lead to amputation. These debilitating procedures affect functionality of the limb, cosmetic appearance, and psychological well-being of patients [10]. Furthermore, after primary surgical treatment, patients face up to a 40% chance of cancer recurrence due to local relapse from residual marginal osteosarcoma cells that were not picked up initially by the surgeon [12], [13]. This requires the need to undergo additional surgeries, chemotherapy, and radiotherapy exposure. It is also worth mentioning the increased costs on the hospital due to longer stays and adverse effects on the patient such as anxiety, increased risk of postoperative infections and poor cosmesis.
The current standard of care to assess intraoperative marginal cells involves examination of the excised tissue by frozen section analysis in the pathology lab [14]. This intraoperative method is labour and resource-intensive, with relatively low sensitivity and specificity [15] and increases surgery time. Additionally, there might be technical challenges with frozen sections due to the amount of fat and the bony aspect of the specimen, which can interfere with the assessment of bone marrow margins [16]. The lack of precise intraoperative margin assessment tools for bone cancer is an unmet need to reduce local cancer recurrence. Sufficient marginal resection is a widely discussed subject and there is no innovative intervention to detect intraoperative margin bone-cancer osteosarcoma cells.
The aim of this study is to highlight the current existing and emerging technologies and their efficacy in detecting clear bone margins during intraoperative bone cancer resection.
3. Material and methods
This systematic review protocol was developed and registered in the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD 42021251826 [17]. The Preferred Reporting Items for Systematic Reviews and meta-Analyses (PRISMA) guidelines and flowchart were used for designing this study [18].
3.1. Search strategy
The SPIDER tool (Sample - Phenomenon of Interest - Design - Evaluation - Research type) was adopted and modified to formulate the research question and to establish the eligibility criteria [19]. Search terms were then developed to identify articles from the following databases: Medline, Embase, Global Health and Google Scholar using the OVID platform (Table 1). Key terms that were used in the search were on the relevant technologies, margin assessment intraoperatively for hard tissue tumours and they were combined using Boolean terms (AND/OR). The search was done on articles since inception. Selected articles were then exported to Endnote X9 reference manager software to organise, screen and group articles. After the articles were exported, duplicates were removed manually and electronically.
Table 1.
Medline Database search.
| Number | Search terms | Results |
|---|---|---|
| 1 | (“Medical device*” or Intervention* or Assess* or Detect* or Technolog* or “image device*” or “imaging device*” or probe*).mp. [mp = title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] | 7,007,565 |
| 2 | (Intra?operative or monitor or non?invasive or Residual or Retain* or Remain* or “During surgery” or “Real?time” or Boundar* or Edge or “re?excise”).mp. [mp = title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] | 2,812,795 |
| 3 | (Osteosarcoma or “Ewing* Sarcoma” or “Bone cancer” or “Hard tissue cancer” or “Hard tissue tumo?r” or Chondrosarcoma or “Multiple Myeloma” or “Osteochondroma” or “Giant cell tumo?r” or Chondroma).mp. [mp = title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] | 111,805 |
| 4 | 1 and 2 and 3 | 3833 |
3.2. Study selection
The review included articles published in English, French, Italian, Urdu and Arabic. Studies included were only those conducted intraoperatively on alive human beings with hard tissue tumours. There were no geographic, gender or ethnic background preferences. Study designs such as randomised control studies (RCTs), cohort, case controls, case series and case report were included. All other languages, animal and cadaver studies articles with soft tissue tumours were excluded. Editorials, reviews, and opinions were also excluded.
3.3. Data extraction and analysis
Five reviewers independently extracted the relevant study data from the final pool of included articles and imported the data on a spreadsheet designed a priori on Microsoft Excel 2013 (Microsoft Corporation, Redmond, Washington, USA). Data was extracted based on the study design, primary diagnosis, relapse, technology used, mode of detection, mechanism of action, distinguishing factors, technology class category, commercial availability, and its patent presence. The quality of all included studies was then analysed using the ROBINS-I tool for critical appraisal of the risk of bias (Table 2) [20].
Table 2.
ROBINS-I tool for critical appraisal of the risk of bias in all studies included.
| ROBINS-I Tool | ||||||||
|---|---|---|---|---|---|---|---|---|
| NON-RCTs | Change intervention bias | Classification of Intervention Bias | Measurement Outcome Bias | Missing Data Bias | Reporting Bias | Risk-of-Confounding Bias | Selection Bias | Overall Risk of Bias |
| L.Cannavò et al.[32] | unclear | low | low | low | low | low | low | low |
| Anderson et al.[16] | low | low | high | low | low | unclear | low | low |
| Bajpai et al.[21] | low | low | low | low | low | high | low | low |
| Aszódi et al.[33] | low | low | high | low | high | high | high | high |
| Fayad et al. [22] | low | low | low | low | high | high | high | low |
| Bosma et al.[30] | low | low | high | low | low | high | high | low |
| Boufettal et al. [35] | low | low | low | low | low | low | low | low |
| Cates et al. [24] | low | low | high | high | high | unclear | low | unclear |
| Seong Cho et al. [23] | low | low | low | low | low | unclear | low | low |
| Evrard et al. [31] | low | low | low | low | low | unclear | low | low |
| Fujiwara et al. [28] | low | low | low | low | low | unclear | low | low |
| Hodel et al. [29] | low | low | low | low | low | unclear | low | low |
| S. Shin et al. [34] | low | low | low | low | low | unclear | low | low |
| Malek et al. [25] | low | low | low | low | low | unclear | low | low |
| Meyer et al. [14] | low | low | high | high | low | unclear | high | high |
| Sakamoto et al. [26] | low | low | low | low | low | high | low | low |
| Wong et al. [27] | low | low | high | low | low | low | low | low |
3.4. Data synthesis
The data found will be grouped into mode of detection categories and will be matched to the primary diagnosis found. The technologies behind the operation of these detection modes will be identified and compared with each other through their distinguishing factors. The percentage of cancer relapse amongst the use of these technologies will be found. The accuracy for detection of cancer cells on the margins will be stated.
4. Results
4.1. Database search results
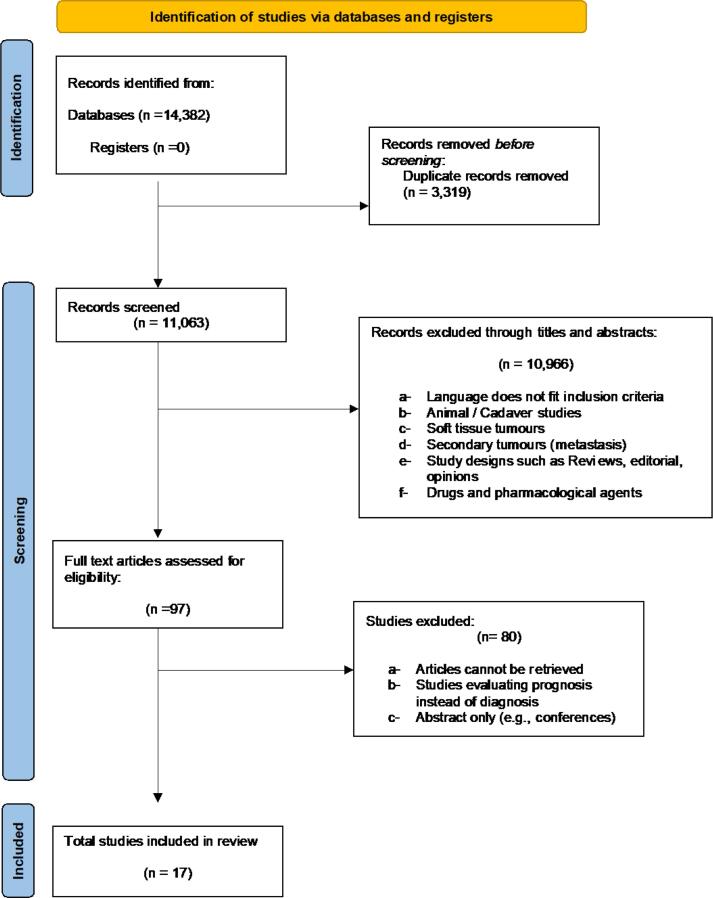
The results of the database search and screening process are detailed in the PRISMA flow diagram (Fig. 1). A total of 14,382 articles were found in the initial database search, with n = 10,441 from Embase, n = 3,833 articles from Medline, and n = 108 from Global Health. Duplicates were removed (n = 3,319), and the remaining 11, 063 articles were screened. An initial title and abstract screening excluded 10, 966 articles following the above-mentioned inclusion and exclusion criteria. Finally, 97 articles were eligible for full-text review. A total of 80 studies were excluded for the following reasons: a) records could not be retrieved, b) records consisted of abstract only, and c) articles were evaluating prognosis instead of diagnosis. Seventeen studies were included in the final systematic review.
Fig. 1.
PRISMA flowchart for results of the literature database search.
4.2. Study and patient Characteristics:
A total of 17 studies were included in this review (Table 3). These studies ranged from the year 1980, to 2020. Eight were retrospective cohort studies, two were prospective studies, one specimen study, and the rest being case controls/series. The number of patients in each study ranged from 5 to 186, with 5 studies reporting over 50 patients. The primary diagnosis for patients varied across the 20 studies. Eight studies reported osteosarcoma as the primary diagnosis [14], [21], [22], [23], [24], [25], [26], [27]. Other bone tumours included chondrosarcoma (reported by 5 studies) [22], [23], [27], [28], [29], pelvic/sacral tumours (2 studies) [30], [31], primary bone tumours such as malignant giant cell tumours, rhabdomyosarcoma, and plasmacytoma (1 study), osteoid osteoma (2 studies), and skull-base tumours (1 study) (Table 3).
Table 3.
Summary of the characteristics of the studies evaluating intraoperative margin assessment.
| Author | Study design | Number of patients | Primary diagnosis (number of patients) | Relapse (%) | Technology | Distinguishing factors | Detection mode |
|---|---|---|---|---|---|---|---|
| L.Cannavò et al.[32] | Retrospective Cohort | 46 | Primary malignant bone tumours | No | MRI + CT | Bone and soft-tissue margins | Non-invasive |
| Anderson et al. [16] | Retrospective Cohort | 142 | Primary bone sarcomas | No | Cryosection + Microscopical analysis | Bone marrow margins | Invasive |
| Bajpai et al. [21] | Prospective Cohort | 31 | Osteosarcoma | No | Dynamic contrast enhanced-magnetic resonance imaging (DCE-MRI) | VEGF and Angiogenesis | Non-invasive |
| Aszodi et al. [33] | Prospective Cohort | 13 | Primary bone sarcomas | No | Cryosection + Microscopical analysis | Histological examination of malignancy | Invasive |
| Fayad et al. [22] | Retrospective Cohort | 13 | Skeletal sarcomas | No | Multivoxel proton magnetic resonance spectroscopic imaging (MRSI) | Markers of malignancy, such as elevated levels of metabolites (choline) that reflect high cell turnover | Non-invasive |
| Bosma et al. [30] | Case control | 70 | Pelvic or Sacral sarcomas | No | CT fluoroscopy / intraop. CT | Bone and soft-tissue surgical margins (Enneking system) | Non-invasive |
| Boufettal et al. [35] | Case series | 5 | Osteoid osteoma | No | Isotopic tracking probe using HMDP-99mTc probe | 99mTc-HMDP tracer fixation on the bone lesion | Minimally invasive |
| Cates et al. [24] | Retrospective cohort | 186 | High-grade osteosarcomas | Yes (20%) | Musculoskeletal Tumor Society (MSTS) + American Joint Committee on Cancer (AJCC) R system + margin distance method |
Pathology reports and operative notes | Margin assessment schemes |
| Seong Cho et al. [23] | Retrospective cohort | 6 | High-grade osteosarcoma, chondrosarcoma, and adamantinoma | No | MR images to navigation-assisted bone tumor surgery | Bone and soft-tissue margins | Non-invasive |
| Evrard et al. [31] | Case-Control | 28 | Primary pelvic bone sarcomas | No | MRI + CT + Patient-specific instruments (PSIs) | Bony margins and soft-tissue margins | Non-invasive |
| Fujiwara et al. [28] | Case control | 50 | Chondrosarcoma | Yes (48%) | Oncology-specific navigation surgery (fused with pre-op CT and MR images) | Bone and soft-tissue margins | Non-invasive |
| Hodel et al. [29] | Retrospective Cohort | 68 | Chondrosarcoma | Yes (17.6%) | CT | Histological grading | Non-invasive |
| Kseniya S. Shin et al. [34] | Specimen study | 16 | Skull base tumours | N/A | Stimulated Raman scattering (SRS) imaging technique with a new pseudo-H&E recolouring methodology | Histological examination | Non-invasive |
| Malek et al. [25] | Case control | 32 | Osteosarcoma | N/A | Diffusion-weighted (DW) imaging and proton magnetic resonance (MR) spectroscopy | MR Spectroscopy- choline containing compounds. DWI- Restriction of water molecules mobility | Non-invasive |
| Meyer et al. [14] | Retrospective Cohort | 113 | High grade osteosarcoma |
Yes ((8.2% (7/85)) |
Frozen section with/without pre-operative MRI | Marrow margins | Non-invasive Pre-operative MRI + Invasive (frozen section) |
| Sakamoto et al. [26] | Specimen study | 5 | Primary bone sarcomas | N/A | Intra-operative specimen MRI |
Marrow margins | Mixed |
| Wong et al. [27] | Retrospective Cohort | 8 | High grade osteosarcoma, parosteal osteosarcoma, Low grade chondrosarcoma | No | MRI + CT + 3D software aided visualization for navigation |
Transition of marrow signal from abnormal to normal in T1-weighted MR images. | Non-invasive |
4.3. Relapse and detection modes:
Relapse of the cancer was reported by four studies ranging between 17.6% − 48% [14], [24], [28], [29]. Ten studies reported non-invasive imaging as the mode of detection used [21], [22], [23], [25], [27], [28], [29], [30], [31], [32], while 4 studies reported the use of frozen section (invasive) [14], [16], [29], [33]. Other methods of detection included Raman scattering (1 study) [34], Spectroscopy (1 study) [22], and margin assessment schemes (1 study) [24].
Non-invasive imaging modalities involved using Magnetic Resonance Imaging (MRI), Multivoxel proton magnetic resonance spectroscopic imaging (MRSI), Dynamic contrast enhanced-magnetic resonance imaging (DCE-MRI), Computed Tomography (CT), CT-Fluoroscopy. Table 4 refers to the mechanism of action of each of these imaging modalities. Nine studies reported the use of one or several of these non-invasive modalities [14], [22], [23], [26], [27], [29], [30], [31], [32]. MRI, MRSI, CT, and CT-Fluoroscopy distinguished cancerous cells from ordinary cells by detecting bone, marrow, and soft tissue margins. DCE-MRI identified angiogenesis and Vascular Endothelial Growth Factor (VEGF) to identify the presence and growth of blood vessels. Invasive imaging modalities included the use of 2-deoxy-2-[fluorine-18] fluoro- d-glucose Computer Tomography (18F-FDG PET/CT), Isotopic tracking probe using HMDP-99mTc probe, cryo/frozen section, Stimulated Raman Scattering (SRS) Microscopy of histology samples, Intraoperative MRI of resected tissue, and Margin Assessment Schemes under Musculoskeletal Tumor Society (MSTS) and American Joint Committee on Cancer (AJCC) with an R system and margin distance method. Six studies reported the use of at least one invasive methodology [14], [16], [26], [29], [33], [35]. 18F-FDG PET/CT and Isotopic tracking involved intravenous injection of an exogenous reagent/compound. Frozen section, SRS Microscopy, Intraoperative MRI, and Margin Assessment Schemes were performed using resected tissue and biopsies. These methods create pathology reports and histological examination that identify bone, marrow, and soft-tissue margins. Two studies reported the independent use of both invasive and non-invasive modalities in their methods [26], [29].
Table 4.
Mechanism of Action of the technologies mentioned in the studies.
| Technology | Mechanism of action |
|---|---|
| Magnetic Resonance Imaging (MRI) | Production of a magnetic field to force alignment of H protons found in water of tissues |
| Computed Tomography (CT) / CT fluoroscopy | Combination of different X-rays taken from different angles in the body – generation cross section images of the body. A combination with fluoroscopy gives real-time imaging that can be useful in interventional procedures |
| Cryosection + Microscopical analysis | Tissue cut and frozen with the microtome portion of the cryostat and stained for microscopical analysis |
| Dynamic contrast enhanced-magnetic resonance imaging (DCE-MRI) |
Injection of a paramagnetic contrast agent to enhance pattern of tissue – which is seen using MRI |
| Integrated 18F-FDG PET/CT (F-fluorodeoxyglucose positron emission tomography/computed tomography) |
Hybrid imaging approach by the Integration of F-FDG PET and CT. CT data were used for attenuation correction and anatomic localization of PET lesions |
| Multivoxel proton magnetic resonance spectroscopic imaging (MRSI) |
Molecular imaging with MRI – using a map representing signal intensity of metabolites in tissues |
| Isotopic tracking probe using HMDP-99mTc probe | I.V injection of a tracer (HMDP-99mTc) that will be fixed to the lesion – Radio-detection carried out using gamma camera. |
| 3D-multimodality image (3DMMI-based virtual surgical planning) and 3D-printed patient-specific instruments (PSI) |
Assimilating each separate radiographic image into a single 3DMMI revealing all structures in the pelvis |
| Navigation assisted surgery (3D software). By Stryker |
Real-time intraoperative assessments of stability and range of motion |
| Fast simultaneous two-channel stimulated Raman scattering (SRS) imaging technique and a new pseudo-hematoxylin and eosin (H&E) recolouring methodology |
Use of two pulse lasers (pump and Stokes) to excite intrinsic vibrational motions of molecules coherently and detect their unique characteristics on a spectrum |
| Diffusion-weighted (DW) imaging and proton magnetic resonance (MR) | Measures the Brownian motion of water molecules within a voxel of tissue |
| Flat-panel volumetric computed tomography | 8–16-fold higher image resolution than conventional CTs with shorter acquisition time |
Out of the 17 reported studies, 10 reported entire or partial elements of their methods under patent protection. Seven studies reported Class II medical devices under the Food and Drug Administration’s (FDA) device class and regulatory controls, and three studies further reported commercially unavailable methods (Table 5). The different types of technologies used for tumour detection and relapses are presented in Table 6.
Table 5.
Technical information of the technologies used in the studies.
| Author | Technology | Class category | Commercial availability | Patent for the technology | Accuracy | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| L.Cannavò et al. [32] | MRI + CT | 2 | Yes | N/A | 76% to 83%- Radiologist 68% to 72%- (Orthopaedist) |
– | – |
| Anderson et al. [16] | Frozen section | N/A | Yes | Yes | – | – | – |
| Bajpai et al. [21] | DCE-MRI | 2 | Yes | N/A | – | – | – |
| Aszodi et al. [33] | Frozen section | N/A | Yes | Yes | – | – | – |
| Fayad et al. [22] | MRSI | – | Yes | Yes | – | – | – |
| Bosma et al. [30] | CT fluoroscopy / intraop. CT | 2 | Yes | N/A | – | – | – |
| Boufettal et al. [35] | Isotopic tracking probe using HMDP-99mTc probe | 2 | Yes | Yes | – | – | – |
| Cates et al. [24] | Musculoskeletal Tumor Society (MSTS) + American Joint Committee on Cancer (AJCC) R system + margin distance method |
– | – | – | – | – | – |
| Seong Cho et al. [23] | MR images to navigation-assisted bone tumour surgery | 2 | Yes | Yes | – | – | – |
| Evrard et al.[31] | MRI + CT + Patient-specific instruments (PSIs) | 2 | Yes | Yes | – | – | – |
| Fujiwara et al. [28] | Navigation assisted surgery | – | Yes | Yes | – | – | – |
| Hodel et al. [29] | CT | N/A | Yes | N/A | – | – | – |
| Kseniya S. Shin et al [34] | Stimulated Raman scattering (SRS) imaging technique and a new pseudo-H&E recolouring methodology | – | Yes | Yes | 69% | 58.80% | 83.30% |
| Malek et al. [25] | DW imaging and proton MR spectroscopy | – | 510 (K) clearance by FDA | Not found | 87% (92% FS) | – | – |
| Meyer et al. [14] | Frozen section / MRI | N/A | Yes | Yes | – | – | – |
| Sakamoto et al. [26] | Intra operative specimen MRI |
N/A | Yes | Yes | – | – | – |
| Wong et al. [27] | MRI + CT + 3D software aided visualization for navigation |
N/A | Yes | Yes (software) | – | – | – |
Table 6.
Findings based on the type of bone cancer evaluated in the studies.
| Osteosarcoma | Chondrosarcoma | Ewing Sarcoma | |
|---|---|---|---|
| Technologies used for detection |
|
|
|
| Invasive methods used | Yes | Yes | No |
| Relapse - Technology associated |
2 studies[14], [24] -Margin assessment schemes [24] and Frozen section +/- MRI [14] |
2 studies[28], [29] -CT [29] and Oncology specific navigation surgery [28] |
No |
4.4. Quality assessment
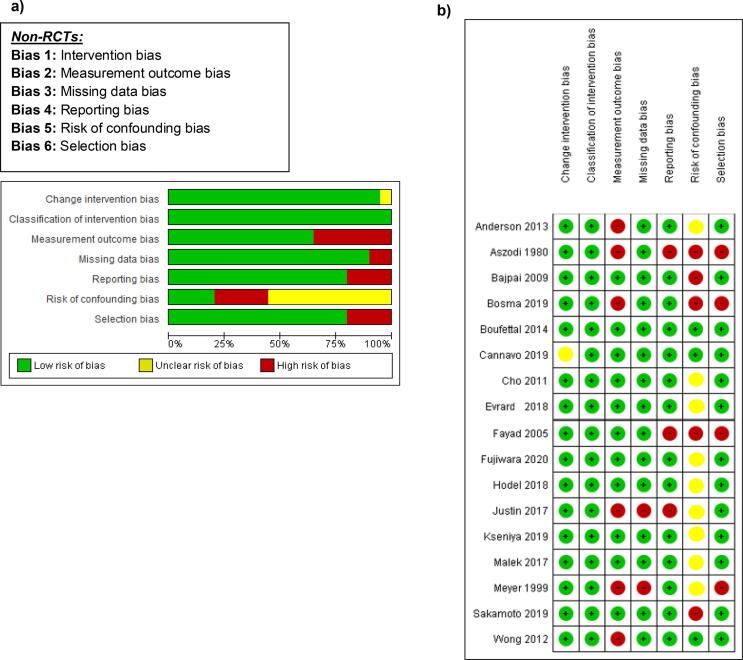
All the studies reported were non-randomized studies. The Cochrane Risk Of Bias In Non-Randomized Studies - of Interventions (ROBINS-I) tool was used to assess and report the risk of bias [20]. All 17 studies reported were measured against Change Intervention Bias, Classification of Intervention Bias, Measurement Outcome Bias, Missing Data Bias, Reporting Bias, Risk-of-Confounding Bias, and Selection Bias. All seven classifications were used to create an Overall Risk of Bias and each classification was determined using a Low, High and Unclear Bias grading measure. Twelve studies were determined to have a low Overall Risk of Bias, three studies were determined to have a high Overall Risk of Bias, and two studies were determined to have intermediate risk of bias. Fig. 2a refers to the risk of bias across all studies. Fig. 2b refers to the risk of bias per study.
Fig. 2.
(a) Cochrane risk of bias graph - assessment of non-randomized studies (b) Cochrane risk of bias summary of all non-randomized studies [14], [16], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35] (Risk of bias graph and summary: review authors' judgements about each risk of bias item presented as percentages across all included studies.).
5. Discussion
This systematic review highlighted the current and emerging technologies for the intraoperative margin assessment of hard tissue/bone tumours. Detection modes of all the technologies used for intraoperative assessment were labelled as invasive, minimally invasive, and non-invasive. Invasive technologies include frozen section since it involves taking a tissue biopsy from the patient and freezing the microtome of the cryostat. Minimally invasive technologies include F-FDG PET/CT and the I.V tracer injection of HMDP-99 m-Tc for radio-detection. Non-invasive technologies include MRI, MR, MRSI, DCE-MRI CT, and Computer 3D-assisted navigation. When considering technologies to evaluate the margins of tumour, an important criterion to consider is the assessment of the local recurrence that can potentially occur if residual tumour cells were left in the tissue. The highest rate of recurrence was observed in the study that used computer-navigation assisted technology, a minimally invasive detection mode [28].
Relapse of sarcoma was seen in up to 48% of cases where (PET/) CT scan was used to detect cancer cells on hard tissue margins. Comparing this with other studies, this was also seen in similar studies using similar tissue margin detection technologies where cancer recurrence was up to 30% in breast cancer [36], [37], [38]. Leaving residual cancer cells in tissues whether they were hard or soft leads to at least three times increased risk of cancer recurrence compared to those with negative tissue margins [38]. Once cancer reoccurs, it mostly leads to repeated surgeries and this is associated with higher surgical risk including poorer cosmetic outcomes, increased economic and psychological burden along with increased risk of infections and morbidity [39]. Hence, achieving microscopically clear margins is extremely crucial to minimise the risk of local recurrence.
The current practice for margin assessment of tissues in most institutions is performing a frozen section or specimen radiography [37]. Frozen section is amongst the most accurate diagnostic tools for margin detection of residual cells with an accuracy of 83% [15] and a sensitivity of 65–78% [15], [40]. However, it is still associated with some challenges such as being time consuming, leading to increased operation duration, technically challenging, expensive, and requires an experienced pathologist [15], [40].
MRI and CT scanning for marginal assessment in hard tissues showed accuracy of up to 93%. To put this into context with non-hard tumours, this was close to the accuracy found in detecting cancer cells on the margins of excised breast tissues in breast cancer [36]. CT scanning had a sensitivity and specificity up to 83% and 100%, respectively, in marginal detection of hard tissue cancer cells. In breast tissue, however, the sensitivity of CT scanning to detect marginal cancer cells was 56%-83% and specificity between 94.7% − 100% [38], [41]. MRI accuracy, sensitivity, and specificity for margin assessment of breast cancer was 92%, 91% and 93%, but it is not yet endorsed for usage inside the operating room because of its high costs, size, and availability [36]. Diffusion weighted Magnetic Resonance has also shown its way in breast cancer margin assessment with sensitivity and specificity of up to 80% and 84%, respectively [37]. This technology was used with the ClearSight system, and it showed success for assessment of tumours in freshly excised tissue [37]. The main drawbacks of using imaging technologies include the major disruption to workflow, high costs and increased exposure to radiation.
Raman spectroscopy has shown to have an accuracy, sensitivity, and specificity of 69%, 58.8% and 83.3% respectively. When this technology was used for brain cancer cells detection, it was shown to be more accurate than MRI and was able to detect previously undetectable cancer cells with a sensitivity and specificity of 93% and 91% respectively [42]. The use of Raman Spectroscopy is still at its infancy stages for clinical applications, but its use in in vivo models seems promising so far [43]. Raman mainly “reads” the molecular characteristics of tissues and displays the different biomarkers that are pre-programmed through a database on a spectrum in forms of specific peaks. The main advantage of this technology is its capability in being used in situ without disruption to operation workflow and its quick pick up of cancer cells [42].
Research has shown that while intraoperative margin assessment for cancer cells is still a challenge, up to 46% of patients would need a repeat surgery to re-excise residual tumour cells [37]. In situ cancer detection is still a challenge since the interface for interaction between the normal tissue and cancer is hard to visualise [44]. The above-mentioned strategies all involve resection of the pathologic tissue and conducting marginal assessments on the excised tissue while still leaving the normal tissue without assessment. The major drawbacks of these techniques include the disruption of workflow, increased time of operation, high costs and lack of normal tissue marginal assessments. This means that there is still an unmet clinical need for practical and innovative tools to assess margins in situ in real time for an efficient workflow, reduced time and accurate outcomes [44]. Evrard et al., has also recently shown the importance of margin assessment and how using a patient specific instrument for tumour resection with MRI has a synergistic effect in increasing accuracy [45].
The use of multi-modal technologies in one device seems to be the future for increased accuracy. Jermyn et al. showed that using a multimodal spectroscopy that uses Raman spectroscopy, intrinsic fluorescence spectroscopy and diffuse reflectance spectroscopy in situ has maximised cancer cell detection during surgery with accuracy, sensitivity and specificity of 97%, 100% and 93%, respectively [44]. The synergistic effect of these technologies into one innovation have provided maximum cancer detection levels and has the potential to be used for other cancers and tissues.
The limitations that this study encountered included the heterogeneous nature of studies including making it difficult to conduct a meta-analysis of the results due to lack of unified measures of associations across the studies. The studies also had a very wide range in the numbers of patients which might not provide a strong sample size for the power of the studies, thereby affecting the external validity. In addition, not all studies reported the accuracy, sensitivity and specificity of the devices used, making it not possible to determine the effectiveness of these technologies. Finally, the included studies were all observational and there were no randomised control trials which are considered to be of higher quality of evidence for diagnostic studies.
6. Conclusions
In conclusion, intraoperative in situ detection of marginal cancer cells in vivo still remains a challenge with the main aim of reducing residual cancer cells and repeat surgeries. There seems to be high potential for the use of multimodal technologies to increase accuracy for cancer detection intraoperatively. Imaging technologies, although posit decent levels of accuracy, still carry risks of radiation, are expensive, and not used in. It would therefore be interesting to explore alternatives to imaging technologies such as Raman. Finally, future clinical trials are needed to test the effectiveness of these technologies to measure the diagnostic accuracy and overall patient survival.
Funding
No founding was sought for this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Mutsaers A.J., Walkley C.R. Cells of origin in osteosarcoma: mesenchymal stem cells or osteoblast committed cells? Bone. 2014;62:56–63. doi: 10.1016/j.bone.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Brown H.K., Schiavone K., Gouin F., Heymann M.F., Heymann D. Biology of Bone sarcomas and new therapeutic developments. Calcif Tissue Int. 2018;102(2):174–195. doi: 10.1007/s00223-017-0372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AMBOSS. Malignant Bone Tumours - Osteosarcoma 2022 [June 5, 2022]. Available from: https://next.amboss.com/us/article/HQ0Kxf?q=osteosarcoma#Z8ff05dcf1560740b28f49573a0389d8f.
- 4.Ottaviani G., Jaffe N. The epidemiology of osteosarcoma. Pediatric Adolescent Osteosarcoma. 2009:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 5.Abarrategi A., Tornin J., Martinez-Cruzado L., Hamilton A., Martinez-Campos E., Rodrigo J.P., et al. Osteosarcoma: cells-of-origin, cancer stem cells, and targeted therapies. Stem Cells Internat. 2016;2016 doi: 10.1155/2016/3631764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadykova L.R., Ntekim A.I., Muyangwa-Semenova M., Rutland C.S., Jeyapalan J.N., Blatt N., et al. Epidemiology and risk factors of osteosarcoma. Cancer Investig. 2020;38(5):259–269. doi: 10.1080/07357907.2020.1768401. [DOI] [PubMed] [Google Scholar]
- 7.Bousquet M., Noirot C., Accadbled F., Sales de Gauzy J., Castex M.P., Brousset P., et al. Whole-exome sequencing in osteosarcoma reveals important heterogeneity of genetic alterations. Ann Oncol. 2016;27(4):738–744. doi: 10.1093/annonc/mdw009. [DOI] [PubMed] [Google Scholar]
- 8.Cancer.net. Osteosarcoma - Childhood and Adolescence: Statistics 2022 [Available from: https://www.cancer.net/cancer-types/osteosarcoma-childhood-and-adolescence/statistics.
- 9.Ferrari S., Briccoli A., Mercuri M., Bertoni F., Picci P., Tienghi A., et al. Postrelapse survival in osteosarcoma of the extremities: prognostic factors for long-term survival. J. Clin. Oncol. 2003;21(4):710–715. doi: 10.1200/JCO.2003.03.141. [DOI] [PubMed] [Google Scholar]
- 10.Geller D.S., Gorlick R. Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin. Adv. Hematol. Oncol. 2010;8(10):705–718. [PubMed] [Google Scholar]
- 11.Broadhead M.L., Clark J., Myers D.E., Dass C.R., Choong P.F. The molecular pathogenesis of osteosarcoma: a review. Sarcoma. 2011;2011 doi: 10.1155/2011/959248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimer R., Taminiau A., Cannon S. Surgical outcomes in osteosarcoma. J. Bone Joint Surg. Brit. 2002;84(3):395–400. doi: 10.1302/0301-620x.84b3.12019. [DOI] [PubMed] [Google Scholar]
- 13.Kempf-Bielack B., Bielack S.S., Jürgens H., Branscheid D., Berdel W.E., Exner G.U., et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J. Clin. Oncol. 2005;23(3):559–568. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 14.Meyer M.S., Spanier S.S., Moser M., Scarborough M.T. Evaluating marrow margins for resection of osteosarcoma. A modern approach. Clin. Orthop. Relat. Res. 1999;363:170–175. [PubMed] [Google Scholar]
- 15.Nowikiewicz T., Śrutek E., Głowacka-Mrotek I., Tarkowska M., Żyromska A., Zegarski W. Clinical outcomes of an intraoperative surgical margin assessment using the fresh frozen section method in patients with invasive breast cancer undergoing breast-conserving surgery–a single center analysis. Sci. Rep. 2019;9(1):1–8. doi: 10.1038/s41598-019-49951-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson M.E., Miller P.E., van Nostrand K., Vargas S.O. Frozen section versus gross examination for bone marrow margin assessment during sarcoma resection. Clin Orthop Relat Res. 2014;472(3):836–841. doi: 10.1007/s11999-013-3005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haitham Shoman JA-K, Maryam Ejaz, Kalin Kahla, Sandi Alakhras, Justin Matta, Ahmed Aoude. . Critical assessment of technologies for the intraoperative margin assessment of hard tissue tumors / bone tumors: a systematic review of current and emerging technologies: National Institute for Health Research; 2021 [Available from: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=251826.
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG, Group* P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 19.Cooke A., Smith D., Booth A. Beyond PICO: the SPIDER tool for qualitative evidence synthesis. Qual Health Res. 2012;22(10):1435–1443. doi: 10.1177/1049732312452938. [DOI] [PubMed] [Google Scholar]
- 20.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. bmj. 2016;355. [DOI] [PMC free article] [PubMed]
- 21.Bajpai J., Gamanagatti S., Sharma M.C., Kumar R., Vishnubhatla S., Khan S.A., et al. Noninvasive imaging surrogate of angiogenesis in osteosarcoma. Pediatr. Blood Cancer. 2010;54(4):526–531. doi: 10.1002/pbc.22328. [DOI] [PubMed] [Google Scholar]
- 22.Fayad L.M., Bluemke D.A., McCarthy E.F., Weber K.L., Barker P.B., Jacobs M.A. Musculoskeletal tumors: Use of proton MR spectroscopic imaging for characterization. J. Magn. Reson. Imaging. 2006;23(1):23–28. doi: 10.1002/jmri.20448. [DOI] [PubMed] [Google Scholar]
- 23.Cho H.S., Park I.-H., Jeon I.-H., Kim Y.-G., Han I., Kim H.-S. Direct application of MR images to computer-assisted bone tumor surgery. J. Orthop. Sci. 2011;16(2):190–195. doi: 10.1007/s00776-011-0035-5. [DOI] [PubMed] [Google Scholar]
- 24.Cates J.M.M. Reporting Surgical Resection Margin Status for Osteosarcoma: Comparison of the AJCC, MSTS, and Margin Distance Methods. Am. J. Surg. Pathol. 2017;41(5):633–642. doi: 10.1097/PAS.0000000000000815. [DOI] [PubMed] [Google Scholar]
- 25.Malek M., Kazemi M.A., Saberi S., Hashemi H., Moradi B. Diffusion-Weighted Imaging and Proton Magnetic Resonance Spectroscopy Findings in Osteosarcoma Versus Normal Muscle. 2017;14(4):e13937. [Google Scholar]
- 26.Sakamoto A., Okamoto T., Ikeguchi R., Matsuda S. MRI examination of resected malignant bone tumor can be an option for assessment of the osseous surgical margin. Br J Radiol. 2019;92(1104):20190518. doi: 10.1259/bjr.20190518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong K.C., Kumta S.M. Joint-preserving tumor resection and reconstruction using image-guided computer navigation. Clin Orthop Relat Res. 2013;471(3):762–773. doi: 10.1007/s11999-012-2536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujiwara T., Kaneuchi Y., Stevenson J., Parry M., Kurisunkal V., Clark R., et al. Navigation-assisted pelvic resections and reconstructions for periacetabular chondrosarcomas. Eur. J. Surg. Oncol. 2021;47(2):416–423. doi: 10.1016/j.ejso.2020.05.025. [DOI] [PubMed] [Google Scholar]
- 29.Hodel S., Laux C., Farei-Campagna J., Götschi T., Bode-Lesniewska B., Müller D.A. The impact of biopsy sampling errors and the quality of surgical margins on local recurrence and survival in chondrosarcoma. Cancer Manag. Res. 2018;10:3765–3771. doi: 10.2147/CMAR.S178768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosma S.E., Cleven A.H.G., Dijkstra P.D.S. Can Navigation Improve the Ability to Achieve Tumor-free Margins in Pelvic and Sacral Primary Bone Sarcoma Resections? A Historically Controlled Study. Clin Orthop Relat Res. 2019;477(7):1548–1559. doi: 10.1097/CORR.0000000000000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evrard R., Schubert T., Paul L., Docquier P.-L. Resection margins obtained with patient-specific instruments for resecting primary pelvic bone sarcomas: A case-control study. Orthop. Traumatol. Surg. Res. 2019;105(4):781–787. doi: 10.1016/j.otsr.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 32.Cannavò L., Albano D., Messina C., Corazza A., Rapisarda S., Pozzi G., et al. Accuracy of CT and MRI to assess resection margins in primary malignant bone tumours having histology as the reference standard. Clin. Radiol. 2019;74(9):736. doi: 10.1016/j.crad.2019.05.022. e13-e21. [DOI] [PubMed] [Google Scholar]
- 33.Aszódi K., Glauber A., Csató Z. The importance of rapid, intraoperative histological diagnosis in the radical surgical treatment of the malignant tumours of the limbs. Arch. Orthop. Trauma. Surg. 1980;96(2):123–130. doi: 10.1007/BF00433291. [DOI] [PubMed] [Google Scholar]
- 34.Shin K.S., Francis A.T., Hill A.H., Laohajaratsang M., Cimino P.J., Latimer C.S., et al. Intraoperative assessment of skull base tumors using stimulated Raman scattering microscopy. Sci. Rep. 2019;9(1):1–12. doi: 10.1038/s41598-019-56932-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boufettal M., Haddam A., Lalya I., El Zanati R., Mahfoud M., El Bardouni A., et al. Place of intraoperative isotopic markers in the management of osteoid osteoma. Pan Afr. Med. J. 2014;19:158. doi: 10.11604/pamj.2014.19.158.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papa M., Allweis T., Karni T., Sandbank J., Konichezky M., Diment J., et al. An intraoperative MRI system for margin assessment in breast conserving surgery: Initial results from a novel technique. J. Surg. Oncol. 2016;114(1):22–26. doi: 10.1002/jso.24246. [DOI] [PubMed] [Google Scholar]
- 37.Thill M., Szwarcfiter I., Kelling K., van Haasteren V., Kolka E., Noelke J., et al. Magnetic resonance imaging system for intraoperative margin assessment for DCIS and invasive breast cancer using the ClearSight™ system in breast-conserving surgery-Results from a postmarketing study. J. Surg. Oncol. 2022;125(3):361–368. doi: 10.1002/jso.26721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiu S.Q., Dorrius M.D., de Jongh S.J., Jansen L., de Vries J., Schröder C.P., et al. Micro-computed tomography (micro-CT) for intraoperative surgical margin assessment of breast cancer: A feasibility study in breast conserving surgery. Eur. J. Surg. Oncol. 2018;44(11):1708–1713. doi: 10.1016/j.ejso.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 39.Houssami N., Morrow M. Margins in breast conservation: A clinician's perspective and what the literature tells us. J. Surg. Oncol. 2014;110(1):2–7. doi: 10.1002/jso.23594. [DOI] [PubMed] [Google Scholar]
- 40.Pleijhuis R.G., Graafland M., de Vries J., Bart J., de Jong J.S., van Dam G.M. Obtaining adequate surgical margins in breast-conserving therapy for patients with early-stage breast cancer: current modalities and future directions. Ann. Surg. Oncol. 2009;16(10):2717–2730. doi: 10.1245/s10434-009-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang R., Coopey S.B., Buckley J.M., Aftreth O.P., Fernandez L.J., Brachtel E.F., et al. A pilot study evaluating shaved cavity margins with micro-computed tomography: A novel method for predicting lumpectomy margin status intraoperatively. Breast J. 2013;19(5):485–489. doi: 10.1111/tbj.12146. [DOI] [PubMed] [Google Scholar]
- 42.Jermyn M., Mok K., Mercier J., Desroches J., Pichette J., Saint-Arnaud K., et al. Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci. Transl. Med. 2015;7(274) doi: 10.1126/scitranslmed.aaa2384. 274ra19-ra19. [DOI] [PubMed] [Google Scholar]
- 43.Choo-Smith L.-P., Edwards H.G.M., Endtz H.P., Kros J.M., Heule F., Barr H., et al. Medical applications of Raman spectroscopy: From proof of principle to clinical implementation. Biopolymers. 2002;67(1):1–9. doi: 10.1002/bip.10064. [DOI] [PubMed] [Google Scholar]
- 44.Jermyn M., Mercier J., Aubertin K., Desroches J., Urmey K., Karamchandiani J., et al. Highly accurate detection of cancer in situ with intraoperative, label-free, multimodal optical spectroscopy. Cancer Res. 2017;77(14):3942–3950. doi: 10.1158/0008-5472.CAN-17-0668. [DOI] [PubMed] [Google Scholar]
- 45.Evrard R., Schubert T., Paul L., Docquier P.-L. Quality of resection margin with patient specific instrument for bone tumor resection. Journal of Bone. Oncology. 2022;100434 doi: 10.1016/j.jbo.2022.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]