Abstract
All cells incur DNA damage from exogenous and endogenous sources and possess pathways to detect and repair DNA damage. Post-translational modifications (PTMs), in the past 20 years, have risen to ineluctable importance in the study of the regulation of DNA repair mechanisms. For example, DNA damage response kinases are critical in both the initial sensing of DNA damage as well as in orchestrating downstream activities of DNA repair factors. Mass spectrometry-based proteomics revolutionized the study of the role of PTMs in the DNA damage response and has canonized PTMs as central modulators of nearly all aspects of DNA damage signaling and repair. This review provides a biologist-friendly guide for the mass spectrometry analysis of PTMs in the context of DNA repair and DNA damage responses. We reflect on the current state of proteomics for exploring new mechanisms of PTM-based regulation and outline a roadmap for designing PTM mapping experiments that focus on the DNA repair and DNA damage responses.
1. Introduction
DNA damage arising from internal and external sources is a ubiquitous threat to genome integrity. Un- or improperly repaired DNA lesions result in the accumulation of mutations that often lead to reduced fitness and acute loss of viability. In most multicellular eukaryotes, accumulated mutations from defects in DNA repair promote tumorigenesis, a fact that is underscored by the prevalence of mutations in DNA repair genes among hereditary cancer syndromes [1-5]. The maintenance of genome stability requires the exquisite regulation of the DNA repair machinery. Cells must be able to rapidly sense DNA damage and trigger a plethora of signaling events for not only controlling DNA repair, but also to ensure its proper coordination with other cellular processes such as transcription and the cell cycle [6]. Since many of the key enzymes involved in repair transactions are nucleases, it is imperative that their action is tightly regulated to prevent harmful consequences to genome integrity [7]. Improper execution of repair mechanisms, such as recombination, can result in deleterious outcomes such as gross chromosomal rearrangements, which are hallmarks of cancers [1,8].
Regulation of DNA repair machineries often occur at the level of PTMs [9-13]. DNA repair proteins are decorated with a number of PTMs, including acetylation, phosphorylation, SUMOylation, ubiquitylation, as well as an increasing number of emerging PTMs such as acyl modifications, crotonylation, or propionylation [14-16]. Understanding the regulation of DNA repair, and how its dysregulation leads to disease, requires the comprehensive characterization of the identity, spatio-temporal dynamics, and functional consequences of these PTMs. Figure 1 illustrates some of the most common means by which PTMs alter the targeted repair proteins. Modulation of protein activity, localization and interaction with other proteins are among the most common themes in PTM outcomes. In addition to understanding the effect of a specific PTM event on the targeted protein, it is also critical to understand how multiple PTMs function in a combinatorial mode to expand the possibilities of regulation, as initially illustrated by the histone code [17]. As the study of PTMs evolve, it is becoming increasingly clear that most proteins indeed undergo multiple patterns of complex PTM combinations, yielding distinct proteoforms that are subject to distinct modes of combinatorial control. The ability to disentangle such complexity in PTM patterning and functional outcomes requires powerful analytical methods capable of identifying and quantifying PTMs with high sensitivity. Not surprisingly, proteomic approaches based on mass spectrometry have taken the central stage in PTM analysis.
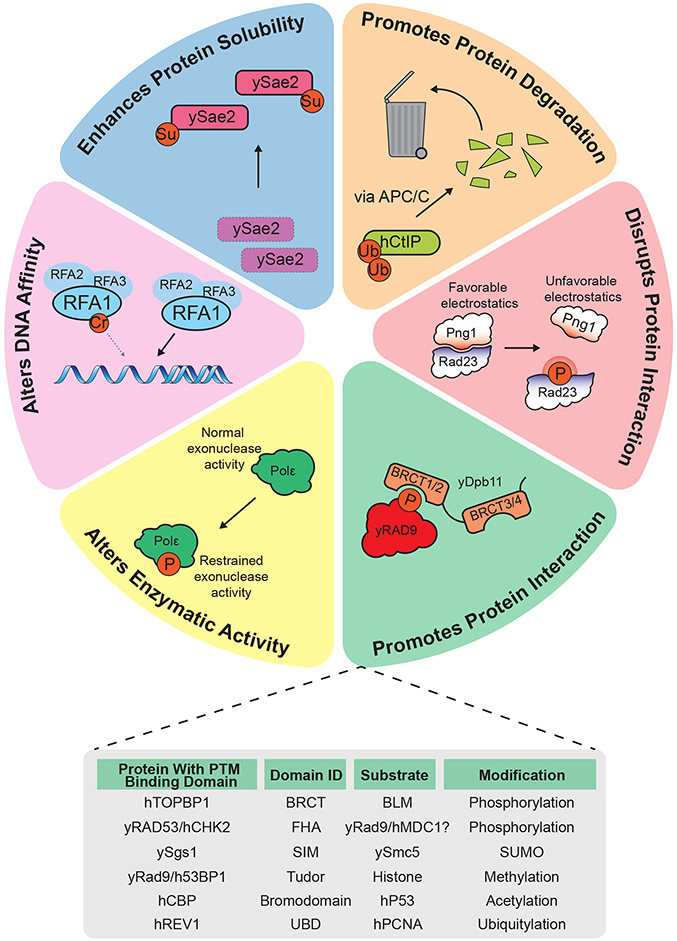
Figure 1: PTMs Expand DNA Repair Protein Functionality.
Left to right: PTMs play important roles in DNA replication, recombination, and repair regulation. Proteins can be targeted for degradation by the proteasome, as in the case of CtIP (h, human) which is degraded in the G1 phase of the cell cycle [228]. Steric or electrostatic clashes may be induced by PTMs which impair protein:protein interactions, such as the Rad23:Png1 interaction in S. cerevisiae which is impaired by phosphorylation of Rad23 [47]. S. cerevisiae Rad9, a checkpoint mediator with important roles in HR regulation, engages with the scaffolding protein Dpb11 through Dpb11’s phosphopeptide-binding BRCT domains, a common theme in PTM mediated protein:protein interactions (see inset table for additional PTM-mediated interactions and binding domains). Modifications can also impair or enhance enzymatic activity, a theme evidenced by the checkpoint-dependent phosphorylation of Polε in S. cerevisiae, which limits exonuclease activity to prevent replication fork degradation [229]. Similarly, DNA binding potential can be altered by modification, as in the case of Rfa1 where its crotonylation limits this protein’s affinity for ssDNA [97]. Finally, some modifications may enhance protein stability or solubility, evidenced by the SUMOylation of Sae2 in S. cerevisiae [59].
In this review we first provide a description of notable PTMs in DNA repair and DNA damage responses. We then provide a biologist-friendly description for how mass spectrometry works and the available methods for studying PTMs. Finally, we provide the reader with a roadmap for experimental design of PTM mapping experiments specifically focused on understanding how PTMs modulate DNA repair outcomes.
2. Notable PTMs in DNA repair
The complexity and fine-tuned regulation of DNA repair is established, in part, by the expanded protein functionality encoded in PTMs. In particular, signaling mechanisms in DNA repair can be quickly activated and subsequently inactivated due to the reversibility of most PTMs which have dedicated writer and eraser enzymes. Kinases and phosphatases fit this classic paradigm—kinases attach phosphate moieties to target substrates to modify their stability, localization, or activity [6], and phosphatases remove phosphate moieties to modulate kinase signaling, often with less specificity than the cognate kinase [6,18]. While phosphorylation is arguably the most well studied PTM in DNA repair, there are many other modifications that have been experimentally linked to the control of DNA damage responses, such as acetylation, PARylation, SUMOylation, and ubiquitylation [19,20]. In this section we will briefly describe some of the notable PTMs, and the role that proteomics has played in elucidating their involvement in DNA repair. While we discuss a few emerging modifications such as crotonylation, the list presented here is not exhaustive, and many experimentally validated PTMs have not been properly explored in the context of DNA repair using quantitative proteomics.
The strength of experimental evidence for the role of a given modification in a DNA repair process can be divided into two main classes. The first class comprises modifications that are induced by genotoxic stress and detected in large-scale datasets. There are hundreds, if not thousands, of residues in proteins documented to undergo some type of PTM, and their exact role in DNA repair is not known, other than that these sites are dependent on DNA damage. This class may include sites for which enzyme dependencies are established. The second class is a much more exclusive group consisting of known modification sites for which a molecular mechanism describing its function in a DNA repair process is known. This class requires genetic evidence of the function of the site in question. For example, phosphorylation of S. cerevisiae Slx4 on serine 486 is critical for this protein’s function as a dampener of the DNA damage response, a function that is validated by the phenotype of a serine-to-alanine mutation imparting a DNA damage checkpoint defect [21,22]. Establishing causal relationships between site-specific mutations that prevent modifications and DNA repair is notoriously difficult, owing in part to the technical difficulty of creating stable mutant cell lines at scale. Additionally, PTMs can co-occur and influence protein function in combinatorial ways that we do not yet fully understand. The generation and validation of site-specific PTM mutants is still a challenging endeavor and seems likely to remain so. Still, there are more than a few examples where a clear molecular mechanism has been successfully elucidated, and continued effort in this area is, in our view, worthwhile.
Phosphorylation
Upon DNA damage, apical kinases Mec1/ATR, Tel1/ATM and DNAPKcs are recruited to sites of damage [23-25] (Note: this review refers to both S. cerevisiae and human proteins. Yeast/Human is the convention we will use). These kinases subsequently phosphorylate a number of downstream targets, including the histone H2A/H2AX [26]; among the downstream targets are DNA damage checkpoint kinases that have their own set of substrates [27-30]. Early on, the study of phosphorylation as it relates to the DNA damage checkpoint in S. cerevisiae focused on a few key apical kinase substrates such as the checkpoint kinase Rad53, as measured by changes in the electrophoretic mobility of Rad53 protein upon phosphorylation [31-33].In the early 2000s, improvements in MS began to allow the analysis of protein phosphorylation from complex samples [34]. By 2007, two studies pioneered the use of MS for the proteome-wide quantitative analysis of protein phosphorylation, comparing cells with normal or impaired DNA damage signaling [30,35]. These studies unraveled the extensive nature of DNA damage phospho-signaling, showing that DNA damage signaling operates as an extensive network of phospho-events instead of a simple linear pathway. Indeed, as data began to accumulate, the full scope of DNA damage-dependent signaling became clear, and phosphoproteomics began to uncover previously unknown substrates of the apical and checkpoint kinases that extended beyond canonical DNA repair substrates. For example, phosphoproteomic screens revealed a number of transcriptional proteins as targets of the apical kinase Mec1 [36]. Likewise, mammalian ATR also targets transcriptional regulators [37], but the functional relevance of many of these targets remains to be fully understood. A particularly surprising finding in phosphoproteomic studies of DNA repair in yeast was that the apical kinase Mec1 (ATR in mammals) has a number of substrates uncoupled from its canonical role in Rad53 or Rad9-mediated checkpoint signaling [36,38]. Indeed, Mec1 activity can be genetically uncoupled from Rad53 activity—cells lacking the checkpoint adaptor Rad9 fail to activate Rad53-dependent signaling even though Mec1 signaling is increased and even target new substrates, such as the helicase Sgs1 [38]. It remains to be seen if a similar uncoupling of ATR signaling from CHK1 downstream signaling exists in mammalian cells, but the available evidence suggests that ATR activity is differentially regulated depending on its activation context; for example, ATR phosphorylation of RPA32 S33 immediately following DNA damage is dependent on the MRN subunit NBS1 but not the 9-1-1 clamp loader RAD17 [39].
Quantitative phosphoproteomics has not only revealed the intricate substrate networks of the DNA damage kinases; it has also facilitated the understanding of how kinases are differentially regulated across DNA lesion types and timescales. For example, Mec1 autophosphorylation at Ser 1964 is critical for DNA damage checkpoint adaptation at extended periods following the response to a single irreparable double strand break in S. cerevisiae [40]. Additional investigation is needed to understand how differential Mec1/ATR autophosphorylation determines kinase signaling outcomes.
While phosphorylation canonically occurs on serine, threonine, or tyrosine residues [41], recent evidence suggests that histidine, aspartate, glutamate, lysine, arginine, and cysteine comprise a significant portion of the mammalian phosphoproteome [42]. The significance of non-canonical phosphorylation in most signaling pathways remains to be tested and represents an especially exciting new avenue of study for the field of DNA repair—see, for example, that phospho-Asp and phospho-Glu sites are enriched for nuclear GO terms [42].
The field of phosphoproteomics has evolved rapidly, as evidenced by the large number of phosphoproteomic datasets deposited to the PRIDE database, outnumbering many other PTM search terms [43], and DNA damage signaling has been one of the foremost beneficiaries of these advancements. Despite the progress, the field of phosphoproteomics faces a few key challenges going forward. While bioinformatic approaches to identify and quantify phosphopeptides have helped ameliorate the problem of the low abundance of many phosphopeptides in data-dependent acquisition modes [44], there are still important limitations in the ability to detect and quantify phosphorylation of extremely low abundance proteins, low-stoichiometry phosphorylation events, or phosphorylation residing in regions that do not yield peptides within the mass range optimal for MS analysis . These limitations are expected to be gradually addressed with advances in mass spectrometer sensitivity and approaches for protein digestion. Furthermore, the translation of phospho-site to phenotype remains a major challenge, and it is one that is not unique to phosphorylation, nor is it easily solvable. Databases that incorporate structural predictions and protein interface data have emerged as attempts to hunt down causative phosphorylation events [45-47]. Still, functional validation of phosphorylation sites is a challenge, and much effort needs to be concentrated on the systemic creation and phenotypic testing of panels of phosphorylation site mutants alone and in combination.
Ubiquitylation and SUMOylation
Ubiquitin is a small protein that can be post-translationally linked to lysine residues of client proteins by E3 ubiquitin ligases. As widespread as its name implies, there are over 600 ubiquitin ligases in human cells [48,49]. While ubiquitin is most notorious as the signal for proteasomal degradation (Fig. 1), its addition to proteins can mediate protein:protein interactions (Fig. 2) and modify enzymatic activity [49-51]. RNF8 and RNF168 are well known E3 ligases in DNA repair [52]. BRCA1 and BARD1, components of the homologous recombination machinery, also possess a potential E3 ligase activity [52]. One well known role for ubiquitin in the DNA damage response is mono-ubiquitination of the replicative sliding clamp, PCNA, which recruits translesion polymerases to replicative polymerase-stalling DNA adducts [53]. Low as well as high throughput mass spectrometry studies have found that ubiquitin regulates several aspects of DNA repair, such as double strand break repair, nucleotide excision repair and the Fanconi anemia pathway.
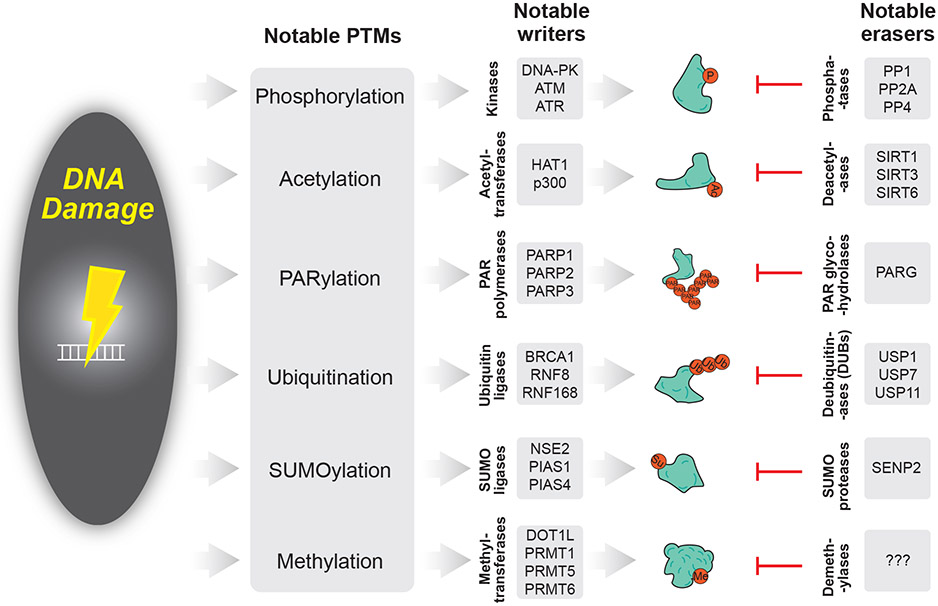
Figure 2: PTMs in the DNA Damage Response.
DNA damage signals PTM writers to confer PTMs on target proteins. Concomitantly, PTM erasers remove cognate PTMs. For example, kinases confer phosphorylation, which is removed by phosphatases. Figure 2 indicates a few notable PTM writers and erasers for each PTM type.
MS has been used for identifying global ubiquitination patterns as well as for the mapping of individual substrates. Ubiquitin proteomics has evaluated global changes in ubiquitination following UV irradiation [54]. Over 300 unique ubiquitination sites were found to be induced upon UV irradiation, suggesting an important role for ubiquitination in the DNA damage response. These data revealed an additional ubiquitination-dependent mechanism for translesion polymerase recruitment via ubiquitination of the PCNA-interacting protein PAF15 [54]. PTM proteomics has also been used to quantify ubiquitination in response to replication fork stalling. Following replication fork stalling, ubiquitination of the ssDNA-binding protein RPA2 promotes both the restart of stalled replication forks and homologous recombination at DSBs that form as a result of stalled forks [55]. Similarly, affinity-purification-based mass spectrometry was used to identify RNF8-dependent ubiquitination sites on the MRN complex subunit NBS1 [56,57]. (This review could be useful for ubi part: PMID: 34950659)
SUMO is an 11kDa protein that, like ubiquitin, is linked to lysine residues on target proteins. Where ubiquitin ligases number in the hundreds, there are roughly a dozen known SUMO ligases. Among these, PIAS1 and PIAS4 appear to be most essential for the mammalian response to double strand breaks [58]. SUMO, like ubiquitin, can modulate protein:protein interactions. Interestingly, SUMO modification has also been found to increase the solubility of HR factors in yeast [59]. Despite the apparent multifunctionality of this modification, the application of proteomics to understand global SUMOylation dynamics lags behind.
SUMO proteomics generally relies on affinity purification of a tagged version of the SUMO protein [60-62]. Whereas ubiquitylation sites can be localized by mass spectrometry due to the characteristic GG modification on lysines following trypsin digestion, identification of SUMOylation sites is more challenging because SUMO-modified peptides are too large to be identified by standard tandem MS approaches. . A creative solution to this problem emerged in yeast, where the Smt3 gene (S. cerevisiae SUMO gene) was mutated so that the resulting SUMO protein contained a lysine instead of an isoleucine near the C terminus (I96K) [63]. This mutation causes SUMO to leave behind the characteristic di-glycine modification on SUMOylated lysine following trypsin digestion [61]. Subsequent iterations of the I96K mutation resulted in SUMO remnants that were distinguishable from the ubiquitin di-GG remnant [64]. This approach was applied to SUMO dynamics during S. cerevisiae meiotic recombination and revealed that in the absence of a key SUMO ligase Aos1, crossover formation is entirely disrupted. A key role for SUMO modification in regulating recombination is supported by additional observations in yeast where SUMOylation of the helicase Sgs1 is critical for its function in homologous recombination [12]. SUMO proteome dynamics have also been evaluated in response to DNA damage using more straightforward affinity purification approaches. Identified targets include several DDR proteins that are SUMOylated following DNA damage such as MDC1, BRCA1 and PARP1 [65]. Interestingly, the demethylase and transcription regulator JARID1C is SUMOylated upon DNA damage, potentially linking SUMOylation and the repression of transcription that occurs in response to double strand breaks [65]. A recent high quality SUMO-interacting protein network identified key components of the NHEJ machinery, indicating that SUMO-dependent interactions are critical in modulating the early response to double strand breaks [66].
Acetylation
Acetyl groups can be linked to primary amines on target proteins by acetyltransferases. The best understood function of acetylation is in gene regulation via the acetylation of histone tails which generally reduces chromatin compaction and promotes gene expression [67]. Indeed, the acetyltransferases linked to DNA repair are bifunctional and also involved in transcription, such as p300 [68]. Canonical gene-regulatory mechanisms based on histone modification have been linked to DNA repair. Histone PTM states have been shown to be modulated upon DNA damage, potentially in part due to crosstalk between PARylation and acetylation, as the PAR Polymerase (PARP) depletes cellular NAD+ pools in the process of synthesizing ADP ribose chains. This in turn ablates sirtuin deacetylase activity, since NAD+ is a necessary cofactor for sirtuin deacetylation activity [69]. Similar to analysis of ubiquitin and SUMO, acetylomics workflows frequently use antibodies against acetylated lysine to enrich for acetylated peptides as well as deacetylase inhibitors such as trichostatin A to reduce PTM loss during sample prep [68,70]. In addition to targeting histones to enable chromatin access to repair factors, acetylases target multiple repair factors, of which some of these modifications, such as acetylation on MDC1, PCNA, RAD51AP1, and TRIM28 are induced upon DNA damage [71,72]. Quantitative SILAC proteomics applied to the post-ionizing radiation (IR) acetylome revealed a burst of de-acetylation immediately following damage induction, possibly suggesting a negative regulatory function in DNA damage response activation [73]. The functional importance of these sites, however, remains to be determined.
PARylation
Poly-ADP-ribosylation (PARylation) is an early DNA damage signal occurring primarily on Glu and Asp residues following formation of DNA lesions, particularly single-strand gaps [74]. PAR Polymerases (PARPs) and PAR Glycohydrolases (PARGs) confer and remove PARylation, respectively [74-76]. PARP1, one of 17 members of the PARP family, is well characterized in the context of the DDR [77], where it contributes to single-strand as well as double-strand break repair. In single-strand break (SSB) repair pathways like base excision repair, PARP1 generates ADP-ribose polymer chains at damage sites to promote recruitment of repair factors such as the scaffolding protein XRCC1 [78,79], which is itself a scaffold for later BER factors. In double strand break (DSB) repair, PARPs are known to influence repair by both non-homologous end joining (NHEJ) and homologous recombination (HR). For example, PARP has been found to promote DNA-PK activity in an in vitro assay [80], suggesting a direct link between PARP and end-joining repair. PARP1 does not directly promote homologous-recombination based repair, a finding that is supported by the synthetic lethality of PARP inhibitors like Olaparib with HR-deficiency [81,82]. Indeed, PARP likely limits HR given the hyper-recombination phenotypes observed in PARP-deficient cell lines [83]. The contribution of PARP1 to multiple DNA repair pathways complicates precise genetic dissection of its function, though this fact makes it an ideal candidate for proteomics-scale studies.
While PARP1 has received much attention, the full scope of DDR-dependent PARylation is understudied, especially considering that the PARP family contains 17 members, at least 5 of which are known to contribute to genome stability [74]. Proteomics studies of PARPs in DNA repair are particularly scarce, in part due to the difficulty of enriching for PARylation as well as the tendency for this modification to occur spontaneously in cell lysates [84-88]. An additional consideration for mass spectrometry studies is that the ADP ribose moiety tends to be quite labile under CID and HCD fragmentation methods, though the advent of electron-transfer dissociation (ETD) has improved the quality of MS/MS spectra for PARylated peptides [89]. Using ETD and EThcD coupled to an affinity purification-based method, a recent screen identified over 7,000 PARylated proteins [89]. Careful dissection of PARP-dependent signaling via quantitative proteomics will be necessary to extend understanding of this widespread and important DDR-related modification beyond the few well-characterized substrates that currently exist. Furthermore, the dynamics of recruitment of DNA repair factors to PAR chains should be investigated by affinity purification mass spectrometry methods. Large datasets could yield genetic insights that will clarify the determinants of whether PARP engages in signaling that promotes either replication fork protection or processing.
Arginine methylation
Arginine side chains can be post-translationally modified with a single or two methyl moieties (MMA), and di-methyl arginine can be further subdivided into symmetric di-methyl arginine (SDMA) and asymmetric di-methyl arginine (ADMA) [90]. Protein arginine methyltransferases (PRMTs) catalyze this modification, with type I enzymes catalyzing ADMA, type II enzymes catalyzing SDMA, and the sole type III enzyme catalyzing MMA. Antibodies specific to either MMA, SDMA, or ADMA are used to purify these modifications for mass spectrometry analysis and an early study in mammalian cells revealed the MRN subunit MRE11 to be modified by ADMA [91]. A more extensive analysis of arginine methylation revealed a general preponderance of nuclear arginine methylase targets with a consensus preference of arginine methylases for glycine at the +1 position [92]. Indeed, arginine methylation of Mre11 was eventually shown to be important for its full exonuclease activity in an in vitro nuclease assay [93], underscoring the utility of proteomics for identifying functionally important protein modifications as well as the importance of arginine methylation in modulating DNA repair.
Acyl modifications
Lysine residue modification by acyl moieties beyond acetylation is an emerging area of PTM study. While, mass spectrometry was used to identify various acyl-chain modifications, such as crotonylation [94], butyrylation, propionylation [95], and malonylation [96], larger-scale studies have been few and far between. Acyl-lysine modification proteomics seems to be an area ripe for study, especially through the lens of the cellular response to DNA damage. Excitingly, a recent crotonylomics analysis in human cells found several crotonylation sites on the ssDNA-binding complex subunit RPA1. Further genetic and biochemical investigation revealed these sites to be DNA-damage inducible and important for RPA1’s ssDNA binding capability as well as for its interaction with several key homologous recombination (HR) factors, such as Rad51 [97]. Crotonylation comprises a small subset of acyl-lysine modifications, and additional work is needed to further establish non-histone acyl-lysine modifications as key mediators of the DNA damage response.
S-acylation, the attachment of acyl- moieties to cysteine residues, is a mechanism for membrane tethering of target proteins due to the hydrophobicity of the long-chain fatty acids that are characteristic of this type of modification [98]. This modification has only recently been appreciated to play a role in DNA repair by localizing factors on the inner nuclear membrane [99]. Enrichment of S-acylation utilizes acyl biotinyl exchange (ABE) [100]: briefly, free cysteines are first blocked with N-ethylmaleimide (NEM); next, palmitoylated cysteines are converted to cysteinyl thiols by hydroxylamine treatment; and finally, cysteinyl thiols are labeled with a thiol-reactive biotin reagent which can then be purified using streptavidin agarose resin. Using this technique, Rif1, a protein involved in DNA replication timing and telomere homeostasis, was identified in a proteomic screen for S-acylation sites in S. cerevisiae [101]. Following the observation that Rif1 foci dynamics are impaired upon deletion of its cognate palmitoyltransferase [102], it was further shown that palmitoylation is required for sequestration of Rif1 at the inner nuclear membrane [99]. While Rif1 is a model example of translating proteomics data into insights about DNA repair biology, it is one of the only DNA repair proteins whose S-acylation has been shown to be functionally important, and this finding is currently limited to S. cerevisiae. Whether Rif1 acylation exists or is functionally important in mammalian cells remains to be tested. Future work is needed to probe the full spectrum of S-acylation dynamics with respect to the DNA damage response, followed by eventual genetic dissection of the functional relevance of these acylation sites.
Histone modifications in DNA repair
Histones comprise a significant fraction of nuclear protein content in eukaryotes and are essential for the packaging of DNA into a cell nucleus. Accordingly, histones play key roles in DNA repair and are some of the first targets of the DNA damage response kinases [103]. Histone proteins are extensively post-translationally modified, particularly pertaining to gene regulation, but these proteins are also highly modified in response to DNA damage. Perhaps the most well-known histone modification in the DNA damage response is the phosphorylation of histone H2A in S. cerevisiae on serine 129 following DNA damage [26], or serine 139 of the H2A variant H2AX in human cells [104]. This modification is conferred primarily by Tel1/ATM early in the DNA damage response (and to a lesser extent by Mec1/ATR and DNA-PKcs) and acts as a critical docking site for DNA damage checkpoint mediators such as Rad9/MDC1 [105]. Importantly, histone phosphorylation beyond H2AX S139 has been extensively mapped, revealing that histones are decorated with numerous phosphorylation sites [103,106,107]. The major challenge going forward is to ascertain how these other sites act alone and in combination to affect cell cycle regulation and DNA damage signaling outcomes, a challenge which will require advanced proteomic tools such as top- and middle-down methods (discussed below).
Histone acetylation is a chromatin mark canonically associated with active transcription. Conversely, histone methylation is generally linked to transcriptional repression [108]. Both of these marks play important roles in the response to DNA damage in yeast and mammalian cells. The checkpoint mediator Rad9 in S. cerevisiae binds to H3-K79me via its Tudor domain, an interaction important for proper activation of the DNA damage checkpoint that is epistatic with the methyltransferase Dot1 [109]. More broadly, chromatin reorganization is a feature of DNA double strand break repair, and it has been proposed that chromatin adopts a heterochromatic state immediately following DNA repair [110]. This heterochromatic state eventually gives way to acetylated histone tails in a Tip60-dependent mechanism which seems to oppose 53BP1 association with chromatin [111]. Such mechanisms, similar in principle to checkpoint dampening mechanisms in budding yeast, are important to restrain persistent activation of the DNA damage checkpoint, promote DNA end resection, and ensure proper repair of DNA lesions [21].
Proteomic analysis of histone modifications is somewhat unique compared to the other modified proteins discussed in this review. Whereas bottom-up proteomics (i.e. proteins are digested by enzymatic cleavage into peptides and then analyzed by LC-MS/MS) is most commonly used for proteome-scale studies, analyses of histones often proceeds by top-down (where whole proteins are ionized and fragmented in the mass spectrometer) or middle-down (where large protein fragments are generated by enzymes with rare cleavage sites) methods. Despite the fact that these methods are lower throughput, they provide the distinct advantage of being able to determine combinatorial PTM states. Analysis of combinatorial PTMs is a direction toward which proteomics is inevitably headed, and histone analysis serves as a model system for these types of studies, with top-down methods commonly identifying hundreds of modifications on histone proteins [112].
3. Fundamentals of mass spectrometry-based PTM proteomics
Mass spectrometry got its start well over a century ago in the lab of the famed British physicist, J.J. Thomson [113]. Mass spectrometry remained solely within the purview of physicists until around the 1940s, when Alfred Nier began to champion its applications in biology and chemistry [114]. Notably, these applications still involved the analysis of atomic isotopes and small molecules but did not yet carry over the analysis of larger molecules which were difficult to ionize. Two solutions to the ionization problem for larger molecules came on to the scene in the late 1980s – Matrix Assisted Laser Desorption Ionization (MALDI) and Electrospray Ionization (ESI). In MALDI, the analyte of interest is embedded in a crystalline matrix of dried solvent that has been spotted on a plate. When the matrix containing the analyte is bombarded with a laser, ionized solvent and analyte particles enter the gas phase and are detected by a mass analyzer ([115,116]. In ESI, high voltage is used to create an aerosol wherein analyte particles are contained in small droplets of a volatile solvent (often acetonitrile). These solvent droplets further evaporate as they are dispersed from the ionization source and produce multiply charged, gaseous analyte molecules that are then detected in a mass analyzer [117]. ESI is still the predominant method by which proteins and peptides are ionized, and it sparked the proliferation of biological mass spectrometry into the well-known technique it is today.
Surprisingly, the application of mass spectrometry for the analysis of protein PTMs preceded the application of MALDI and ESI to biological mass spectrometry and started out with small-scale studies of model proteins; for example, the identification of phosphorylation sites in chicken-egg yolk riboflavin-binding protein [118]. Beyond the study of highly abundant purified proteins , however, ESI and MALDI would be required. In the case of phosphorylation, larger-scale datasets began to be reported in the mid-2000s and the field of phosphoproteomics was born [30,34,119-122]. The analysis of other established PTMs follows a similar trajectory, evolving from small-scale analysis of individual, model proteins to proteome-scale datasets. Today, there are hundreds of large-scale datasets now available for nearly any known PTM, and most journals now mandate that proteomics data be made publicly available through digital repositories [30,43,46,123-127].
Bottom-up proteomics
Common MS-based analysis of PTMs rely on the enzymatic cleavage of proteins into peptides, enrichment of PTM-modified peptides, and subsequent MS-analysis of generated peptides leading to the matching of peptide fragmentation spectra to a simulated database [128-130] (Figure 3). Such an approach based on generating peptides used for analysis is referred to as bottom-up proteomics, and contrasts with top-down proteomics where whole proteins are analyzed. The caveat of enzymatic digestion is that the consensus motifs for these enzymes occasionally exclude certain peptides from detection because the peptides are either too large or too small for analysis. Trypsin, with its preference to cleave c-terminally of K and R residues, is the enzyme of choice for bottom-up proteomics. Alternative proteases such as LysC can supplement tryptic data and increase proteome coverage, and appropriate protease selection is a key step in MS workflows [131,132]. Accordingly, in silico digestion has emerged as a potential pre-processing step to aid protease selection. PTMselect, for example, increases the coverage of PTMs observable in proteomic experiments by in silico digestion of protein sequences with different combinations of proteolytic enzymes [133]. The results of this in silico digestion are then used to determine optimal digestion conditions.
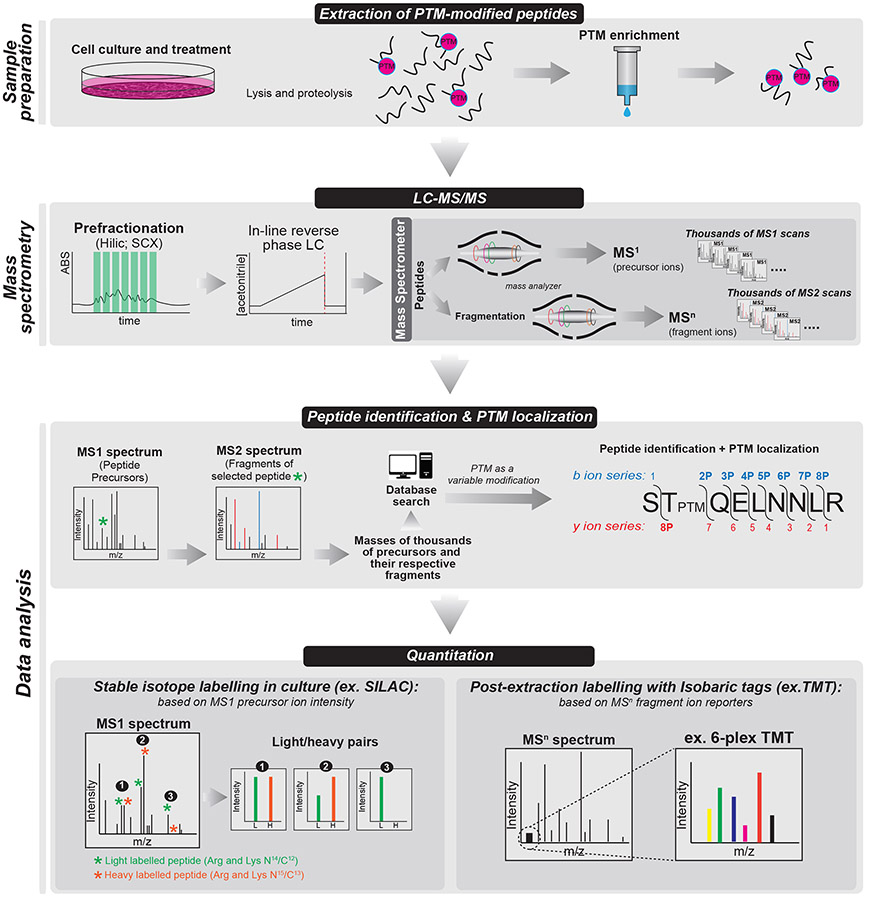
Figure 3: Generalized Workflow for Mass Spectrometry Analysis of PTMs:
Following trypsinization and purification of PTM-modified peptides of interest from cell lysates (A). In (B), peptides are subjected to LC-MS/MS analysis where they are isolated according to their presence in a precursor (MS1) scan and subsequently fragmented to yield an MS2 “fingerprint.” An MS2 spectrum (C) contains multiple b (n-terminal fragment) and y (c-terminal fragment) ions. Depending on its location in the isolated peptide, the mass of the PTM of interest will often be present in b and y ion series, allowing for localization of the PTM of interest on the fragmented peptide. In (C), acquired MS2 spectra are computationally matched to theoretical spectra generated in silico using a search algorithm such as SEQUEST (Eng et al, 1994; Lundgren et al, 2009). In addition, PTMs are included in the search parameters as variable modifications. Often, confidence in PTM localization is scored using any of a number of available tools such as PTMProphet, available as a module within the Trans Proteomic Pipeline [168,230]. Shown in panel (D), quantitation of PTM dynamics between two or more experimental conditions can be achieved either by comparison of MS1 precursor ion intensities between two isotopically labelled biological isolates (SILAC; MS1-based quantitation, left panel) or comparison of MS2-labile reporter ion intensities (TMT; MS2-based quantitation, right panel).
Mid/top-down proteomics
Histone modifications provide an example case for discussing the benefits and basic parameters of middle- and top-down proteomics workflows. Histone modifications are difficult to analyze by bottom-up workflows due to the over-representation of lysine and arginine in these proteins’ primary sequence, which leads to very short tryptic peptides that lack unique sequence-specific information and are difficult to unambiguously map back to a theoretical database. Much effort has been focused on analyzing whole histones (top-down) or large fragments generated by proteases with uncommon cleavage sites such as GluC (middle-down). Intact proteins and large protein fragments are best analyzed under electron-transfer dissociation (ETD) fragmentation [134]. The extent of potential histone proteoforms arising from different combinations of PTMs necessitates prefractionation [135] and requires larger quantities of starting material than most bottom-up methods. That top-down, and to a lesser extent middle-down methods, preserve the various histone proteoforms is important because understanding combinatorial PTM complexity is a major current and future challenge for both solving the histone code and understanding PTMs generally. Top-down mass spectrometry has aided the identification of histone modifications, especially with recent developments in electron transfer dissociation (ETD) and ETD coupled with higher energy collisional dissociation (EThcD), both of which preserve post-translational modifications upon fragmentation [112,136]. Bottom-up proteomics, the focus of this review, is more sensitive and provides site-specific regulatory information, but it is likely that the future of DNA repair PTM proteomics (and PTM proteomics as a whole) will involve combinations of bottom-up proteomics for network-level understanding of signaling events combined with top- and middle-down analyses for identification of combinatorial proteoforms.
4. Strategies for enrichment of PTM-modified peptides
PTMs often exist at sub-stoichiometric levels—a fact that complicates MS analysis, as many PTMs occur below the threshold for detection in protein extracts [137,138]. PTM enrichment, then, is an essential step in PTM proteomics workflows (Fig. 3A, Table 1). Often, the success or failure of PTM analysis by mass spectrometry occurs not at the instrument or experimental design stages, but at the PTM enrichment stage. Efficient enrichment relies on the specificity of reagents to selectively purify PTMs of interest, a requirement that is often bungled by finicky protocols or especially labile modifications. In subsequent sections we describe common methods of PTM enrichment (see Table 1 for commonly used reagents and example publications).
Table 1:
A selection of PTMs and common enrichment strategies.
| PTM | Enrichment Type | Reagent | Advantages | Pitfalls | Publication |
|---|---|---|---|---|---|
| Acetylation | Antibody | Cell Signaling Technology (CST) cat.no. 13416. | Immunoprecipitation easily coupled to MS workflows. | Subset of abundant acetylated proteins can complicate detection of low abundance targets. | [68] |
| Methylation (mono- & di-arginine) | Antibody | CST cat. nos. 12235 (mono-), 13474 (asymmetric di-), or 13563 (symmetric di-). | Specific antibodies to distinguish between mono-, symmetric di-, and asymmetric di-methyl arginine. | Limited number of peer-reviewed studies using referenced kits. Reliant on antibody specificity. | [92] |
| PARylation | Macrodomain | Af1521 macrodomain coupled to resin matrix. | Immunoprecipitation easily coupled to MS workflows. | Possible to detect presence of PARylated peptides but difficult to determine length of PAR chains. | [89] |
| Phosphorylation | Commercial Fe-NTA (1); commercial TiO2(2); homemade Fe-NTA (3) | Thermo Scientific cat. no. A32992 (1). Thermo Scientific cat. no. A32993 (2). | Fast, scalable (1-2). complementary to TiO2 method (1). Complementary to Fe-NTA (2). Easily adapted to small sample quantities in IP-MS (3). | Commercial Fe-NTA or TiO2 are costly compared to homemade resin and are less adaptable to small sample quantities. However, homemade resin often requires optimization. | [36,44,139-141] |
| SUMOylation | His-tag conjugated to SUMO protein/SUMO-interacting motif (SIM) trap | Thermo Scientific cat. no. 88221 for His-tag purification. | SUMO can be tagged with epitope tags (His) to aid purification. | Identification of SUMO sites is difficult, but a trypsin-cleavable version that leaves a di-GG remnant can be engineered. | [61] |
| Ubiquitylation | Antibody against K-ε-GG | CST cat. no. 5562. | di-GG remnant after Trypsin digestion enables easy identification of ubiquitylation sites. | Determination of length/branching of ubiquitin chains challenging by conventional MS workflows. | [142] |
Phosphopeptides within a complex mixture of peptides are generally enriched using either antibody-based immunoprecipitation or immobilized metal affinity chromatography (IMAC) [143,144]. IMAC enrichment strategies take advantage of the affinity of phosphate groups at low pH to positively charged metal ions such as Fe3+[145]. Gallium and titanium have also been used in IMAC column-based approaches, with TiO2 being often used as a complementary approach to Fe3+[146,147]. IMAC and TiO2 chromatography can also be used in tandem to analyze phosphorylated peptides in a process called sequential elution from IMAC (SIMAC) [148]. SIMAC has been shown to greatly increase the ability to identify peptides with multiple phosphorylation events which are typically difficult to observe due to poor ionization. A major benefit of modern IMAC approaches is the broad availability of commercial enrichment kits (Table 1). Less commonly, antibodies recognizing phosphorylated serine, threonine, or tyrosine can be used to enrich phosphorylated substrates at the protein or peptide level [149]. Antibodies can even be raised to recognize phosphorylation at specific consensus motifs such as the S/T-Q motif which is the primary target for the apical DNA damage response kinases ATM, ATR, and DNA-PK [35,150]. A caveat of motif-specific antibodies is that they may display biased preferences for sequence motifs other than the primary consensus being targeted.
The observation of acetylation sites on proteins relies on immunoprecipitation using pan-acetyl lysine antibodies [72,151]. One challenge faced when using this approach is the presence of highly acetylated abundant proteins in cells such as histones, which may occlude the detection of substrates present at low quantities [70]. To combat this, fractionation is often combined with acetyl lysine immunoprecipitation to reduce the complexity of samples and allow less abundant peptides to be observed via MS analysis. Several types of fractionation have been combined with the enrichment of acetylated peptides including subcellular fractionation to enrich proteins from different cellular compartments, strong cation exchange (SCX), and isoelectric focusing [55,72,152]. SCX fractionation prior to acetylation IP has been shown to increase the amount of acetylation sites identified 10-fold relative to an identical experiment performed without fractionation [151]. For phosphopeptides, hydrophilic interaction chromatography (HILIC) has been used to increase phosphoproteome coverage [153].
Ubiquitinated proteins and peptides are typically enriched in proteomic experiments using antibodies recognizing either ubiquitin itself or the di-glycine (GG) motif that is left linked to ubiquitinated lysines after trypsin digestion. The mouse FK2 antibody is a polyclonal antibody that recognizes both mono- any poly-ubiquitinated substrates but not free ubiquitin. This antibody has proven useful not only for the identification of ubiquitinated proteins but also in quantifying changes in the ubiquitination of specific proteins following genomic insult [154,155]. In pursuit of a need to generate more specific antibodies and alternative enrichment methods, UbiSite is an antibody that recognizes a 13-residue remnant left after the digestion of ubiquitin with Lys-C. In principle, UbiSite functions identically to di-GG antibodies except that the remnant recognized is unique to ubiquitin, resulting in a more specific enrichment [156].
Like ubiquitin, enrichment of SUMO-modified peptides relies on antibodies raised against the modification. Antibodies recognizing either endogenous SUMO directly or tags added to ectopically expressed SUMO can be used to pull down SUMOylated proteins for MS analysis [157,158]. Determination of specific SUMOylated residues is more difficult since trypsin digestion of SUMOylated proteins yields large remnants (>19 residues) as opposed to ubiquitin. One solution to this challenge involves mutating residues on SUMO itself so that a di-GG or similar remnant is generated upon enzymatic digestion [159,160]. Another approach, Protease-Reliant Identification of SUMO Modification (PRISM), involves chemically blocking all exposed lysines on SUMO and its cognate proteins [62]. The interaction with SUMO and target proteins is then disrupted using the SUMO protease SENP2. This leaves the previously modified residues as the only lysines accessible to trypsin cleavage (arginine residues are still accessible). From this method, the location of cleaved lysines after digestion marks which sites were SUMO modified.
In general, antibody-based enrichment strategies are the current standard operating procedure for enrichment of most forms of PTMs besides phosphorylation. Enrichment of methyl-arginine uses antibodies that can efficiently distinguish between monomethyl arginine, symmetric dimethyl arginine, and asymmetric dimethyl arginine [91]. PARylation enrichment, while not based on an antibody approach per se, uses the Af1521 PAR-binding domain immobilized on a resin support to purify PARylated peptides [89].
Technical challenges in PTM enrichment
A recognized and unavoidable limitation of any affinity purification approach, including those based on IMAC, is the copurification of contaminating peptides not bearing the PTM of interest. In the case of phosphorylation, enrichment is performed under relatively acidic conditions so that Asp and Glu side chains are not negatively charged (as they would be at physiological pH), and thus do not bind to the immobilized iron particles. PTM loss during sample preparation represents another challenge that must be overcome during PTM analysis by mass spectrometry. PTM loss may occur due to the presence of enzymes that facilitate the removal of PTMs or due to loss of the modifications during sample processing by hydrolysis. Sample preparation protocols must be optimized to address these challenges. Lysis buffers in phosphoproteomic experiments are typically supplemented with phosphatase inhibitors [30]. Inhibiting the proteasome in addition to deubiquitinases greatly increases the coverage of ubiquitinated substrates [154]. SUMO enrichment protocols often involve the expression of ectopically expressed or tagged SUMO. In some cases, lysis and enrichment can be performed in denaturing conditions (if using a Histidine tag) thereby eliminating the action of deSUMOylases without need for inhibitors [161].
5. Tandem MS for peptide identification and PTM site localization
Tandem mass spectrometry relies on two (or more) mass analyzers in a single instrument, usually a quadrupole and a higher-resolution analyzer such as the football-shaped orbitrap. Survey MS1 spectra across a broad mass range of 400-2000m/z contain all ionized analytes at a discrete time point in the chromatographic gradient. This mass window is set by the quadrupole. Peptide analytes are then individually isolated by the quadrupole across a very narrow mass range and subjected to fragmentation inside the mass spectrometer by either collision-induced dissociation (CID), higher-energy collisional dissociation (HCD) or electron-transfer dissociation (ETD). Fragment masses are subsequently recorded by the orbitrap and this peptide fingerprint is later matched with a theoretical spectrum to unambiguously identify the peptide of interest [128,162]. Machine parameters must be adjusted to favor the identification of PTM-modified peptides and enhance PTM site localization. For example, when considering the MS/MS fragmentation strategy, higher-energy collisional dissociation (HCD) is used for sequencing of phosphopeptides, as collision-induced dissociation (CID) tends to result in neutral loss of the phosphate moiety and, consequently, poor quality spectra that lack sequence rich information [162,163].
Computational identification of the presence of a PTM is often coupled to localization of the PTM to a specific amino acid residue within the peptide-spectrum match (PSM) based on the presence of the mass of the variable modification within the b and y ion series in the peptide fragmentation spectrum (Fig. 3C). PTM localization has become increasingly sophisticated over the past decade, as determining the exact site of modification is critical for understanding biological significance. PTM scoring algorithms such as PTM score, PhosphoRS, ASCORE, Mascot Delta score, SLIP Score, and PTMProphet assign confidence scores to variable modification sites within PSMs based on information intrinsic to the peptide fragmentation pattern [120,164-168], underscoring the importance of the quality of MS2 (or MS3) spectra.
The inclusion of PTMs as variable modifications increases search space and consequently the amount of computational power required to complete a peptide database search. The increase in search space is exponential and limits the degree to which a given search may account for different PTMs, especially when using quantitation approaches where the mass label must also be included as a variable modification in the database search. Additionally, inclusion of PTMs as variable modifications increases the false positive PSM rate of the database search. Optimizing machine performance to ensure high mass accuracy is therefore of vital importance to ensure MS1 and MS2 mass accuracy parameters minimize false discovery rate (FDR) [169]. To this end, the continued development of faster, higher-resolution mass analyzers has greatly aided PTM analysis [132,170] and will continue to improve the reliability of PTM-MS.
6. MS quantification of PTMs
Effectively quantifying changes in abundance of PTM-modified peptides is essential to understanding how PTMs change in differing biological conditions. Peptide quantification can be assessed through Label Free Quantification (LFQ), metabolic labeling, or chemical labeling [171,172]. Each of these methodologies have unique benefits and drawbacks. Stable isotope labeling with amino acids in cell culture (SILAC), a metabolic labeling strategy, and Tandem Mass Tag (TMT), a chemical labeling strategy, are both effective and widely used for quantitation in large scale mass spectrometry experiments aimed at analyzing PTMs (Fig. 3). SILAC involves incorporating isotopes of amino acids into cells through culture medium. Typically, heavy isotopologues of Lysine and Arginine containing C13 and N14 are used since at least one of these two residues will occur in every tryptic peptide [173,174]. The labeling does not alter chemical properties (such as ionization efficiency and chromatographic elution time), but results in a shift in the mass of peptides containing C13 and N14 relative to those containing normal isotopes, which will be detected in MS1 scans. Signal intensity from each condition can be used to quantify changes in peptide abundance between two experimental conditions (Fig. 3D). In addition to its accuracy and reproducibility, SILAC quantitation is particularly suitable for PTM proteomics since samples are combined and processed together thereby limiting the variation introduced during enrichment steps. As its name suggests, SILAC is limited to cultured cells and cannot be easily applied to other types of biological samples such as animal tissues.
Tandem Mass Tag (TMT), or its early precursor iTRAQ, involves the addition of isobaric tags to peptides [175]. TMT reagents use an NHS ester moiety to target primary amines in peptides [176]. In TMT workflows, peptides from different experimental channels must be labeled in parallel reactions then mixed prior to analysis, which is a potential source of variation that negatively affects accuracy of quantitation. Because tags have equal mass before fragmentation, the same peptide from different channels will appear as a single peak in MS1 (therefore “isobaric”). Upon fragmentation and a subsequent MS2 scan, a reporter ion that differs in mass for each tag can be used for quantification (Fig. 3D). In this way, the signal intensity of each tag reflects the abundance of the labelled peptide in its respective condition [177]. A limitation of this approach is that co-isolation of nearly isobaric contaminating ions in MS1 may alter the ratios of the reporter ions generated in MS2 [172]. Since the levels of most peptides are unchanged between conditions, this contamination has the potential to dampen the observed changes in abundance, a phenomenon known as ratio compression. Ratio compression can be partially reduced using MS3-based instrument methods, though not all mass spectrometers have this capability, and state-of-the-art instruments are costly. Though ratio compression is alleviated in MS3-based approaches made possible by high-end instrumentation, this still comes at the cost of lower sensitivity, as quantification using MS3 is reported to result in a 12% reduction in the number of sites quantified relative to MS2 methods, in part due to increased machine duty cycle time [172,178]. An undeniable advantage of TMT labeling is that it occurs after protein extraction , which renders quantitative mass spectrometry applicable to tissue isolates and other sample types not amenable to metabolic labeling. Additionally, TMT is available in 6-, 10-, 11-, and 16-plex variations allowing many comparisons to be made within a single experiment [171]. Multiplexing can be used to test multiple biological replicates within a single experiment generating statistically significant data with a reduced requirement for machine time. Unfortunately, coupling TMT analyses to analyses of PTMs can be quite costly if labeling is done prior to PTM enrichment; on the other hand, labeling samples after enrichment and then pooling them represents a major source of variation. For the above cited reasons, we view metabolic labeling approaches such as SILAC to be superior in terms of reproducibility and robustness in the analysis of PTMs, though we recognize that in some cases (such as in the analyses of primary tissue isolates), such labeling schemes are not possible. SILAC also introduces fewer sources of experimental variation due to sample handling and is likely to be the method of choice for DNA repair biologists seeking to make an entrée into the field of PTM proteomics. Next, we outline a roadmap for the design and implementation of proteomics experiments by DNA repair biologists.
7. Practical considerations for designing mass spectrometry experiments to study PTMs in DNA repair
We have reviewed a subset of PTMs with demonstrated roles in DNA repair, and we have outlined the basics of mass spectrometry analysis as it pertains to PTM proteomics workflows. In this section we discuss experimental design guidelines for the proteomic study of PTMs in DNA repair. We first highlight pharmacological and genetic methods of perturbing DNA damage signaling. Then we discuss nuclear enrichment and affinity purification as methods to enrich certain cellular fractions or protein complexes involved in DNA repair. Lastly, we describe how to design PTM mapping experiments with quantitative mass spectrometry in mind.
Strategies for DNA damage induction
There are a number of genetic and pharmacological tools in the DNA repair biologist’s toolkit that can be used to perturb DNA damage signaling for quantitative mass spectrometry studies. The first consideration in any DNA damage study should be the type of DNA damaging agent that is used, as DNA lesions are channeled into different signaling pathways depending on the type of lesion. Common genotoxins used in mammalian DNA repair studies include aphidicolin, bleomycin, camptothecin (CPT), hydroxyurea (HU), ionizing radiation (IR), and mitomycin C (MMC) (Table 2). The drug/treatment used will largely depend on the type of DNA damage being studied: for example, IR stimulates a double-strand break repair response while HU induces a replication stress response. Beyond chemical mutagens, genetic tools exist to induce and study DNA damage. A classic example from S. cerevisiae is the HO endonuclease. In budding yeast, the HO endonuclease is a mechanism for mating type switching through recombination at the MAT locus [179]. Under the control of a galactose inducible promoter, this system has been used extensively in the study of DSB repair and DNA end resection kinetics [180]. In mammalian cells, the AsiSI nuclease can be conditionally induced to generate many DSBs genome-wide which can then be monitored by sequencing if desired [181]. Alternatively, site-specific DSBs can be induced with the Cas9 nuclease[182]. Chemically-induced complex DSB ends have slower repair kinetics and are more mutagenic than enzyme-induced clean DSB ends [183-185].These tools can be adapted to mass spectrometry, and many opportunities for interesting proteomics studies exist—for example, PTM mapping of the cellular response to varying numbers of DSBs.
Table 2:
Common genotoxins, their primary effects, and associated DNA lesions.
| Genotoxin | Primary Effect | DNA Lesion | Citation |
|---|---|---|---|
| Hydroxyurea | Nucleotide depletion via ribonucleotide reductase (RNR) inhibition | Stalled replication forks, fork collapse | [187] |
| Cisplatin | DNA interstrand crosslinks | Fork collapse, double strand breaks (DSBs) | [188] |
| Camptothecin | Topoisomerase I inhibition | Replication stress, fork collapse, double strand breaks (DSBs) | [189] |
| Mitomycin C | DNA interstrand crosslinks | Fork collapse, double strand breaks (DSBs) | [190] |
| Phleomycin | IR mimetic | Clustered DNA damage resulting in DSBs | [191] |
| 4-Nitroquinoline oxide | Bulky base adducts | Bulky lesions primarily repaired by nucleotide excision repair (NER) | [192] |
| Methyl methanesulfonate | DNA base alkylation | ssDNA gaps and replication stress (S phase), DSBs breaks at high concentrations | [193] |
| Ionizing radiation | Clustered DNA damage from ionization cascade | DSBs with complex ends requiring nucleolytic processing | [194] |
| Break induction by programmable nuclease | Targeted phosphodiester backbone cleavage by nuclease | DSBs | [181] |
| Normal DNA Replication | DNA replication stress (mild) | Fork stalling, limited local fork collapse | [36, 195] |
Probing physiological DNA damage
The level of genotoxic stress produced in laboratory settings by chemical mutagens is rarely if ever encountered in nature, and if it were, the result would certainly be cell death. Genotoxic chemicals provide a convenient and viable shortcut to studying the mechanisms of DNA repair by saturating cells’ ability to repair the damage and therefore maximizing the outputs of DNA damage signaling. As proteomics becomes increasingly sensitive, the process of DNA repair can be studied in more physiological contexts, and possibly in the absence of exogenous genotoxins. Indeed, meiosis and oncogene-induced replication stress are two physiological processes intimately associated with DNA repair that beg for further proteomic study. Equally important and urgent is the identification of substrates associated with physiological levels of replication stress encountered in normal S phase [36].
Controlling for cell cycle effects
A major hurdle for quantitative proteomics in these physiological cases is the issue of appropriate controls. It may be difficult to disentangle cell-cycle effects in an experiment seeking to identify DNA repair signaling events in unchallenged S phase, for example. One solution we propose here is for DNA repair biologists to first generate an inclusion list of substrates that are reliably induced upon DNA damage, and then look for mild induction of these substrates in unchallenged cells progression through S phase, perhaps using targeted proteomics methods. Another strategy to control for cell cycle effects is to analyze only a specific phase of the cell cycle by synchronization. This approach has long been used in baker’s yeast. Developments in pharmacological inhibition of key cell cycle proteins such as CDC7 in mammalian cells have made this a favored strategy to isolate specific subpopulations of cells [186]. The application of quantitative proteomics to physiological S phase and other instances of endogenous DNA damage will be critical in fully understanding how DNA repair is dysregulated in cancer and other pathologies.
Nuclear enrichment maximizes DNA repair PTM coverage
DNA repair mostly occurs in the nucleus, which comprises an estimated 20-50% of cell volume depending on cell type [196]. In cultured mammalian cells, a nuclear fractionation step prior to PTM enrichment for proteomics can dramatically improve coverage as it eliminates contaminating cytoplasmic and other organellar signals. Most protocols use a hypotonic lysis buffer to lyse the plasma membrane, leaving the nucleus intact [197-199]. Following this first step, the intact nuclei can then be lysed in a standard lysis buffer such as radioimmunoprecipitation assay (RIPA) buffer, followed by sonication to shear DNA and prevent its precipitation during subsequent centrifugation. Recently, techniques such as chromatin mass spectrometry (CHROMASS) and isolation of proteins on nascent DNA (iPOND) coupled to mass spectrometry have been employed to analyze specific nuclear milieux ([200,201]). Applying these newer techniques to the analysis of a variety of PTMs in DNA repair is a major challenge and an area of opportunity. In yeast, nuclear fractionation is not recommended due to the relatively lower complexity of the proteome and the difficulties associated with nuclear fractionation in organisms with a cell wall [202]. While nuclear fractionation improves coverage of most DNA repair associated substrates, there are cases when analyzing only the nuclear phosphoproteome would obscure key signaling events, for example the role that DNA repair activities in activating innate immune signaling in the cytoplasm [203,204]. The decision to perform subcellular fractionation should be made on a per-experiment basis, rather than as a universal first step before PTM enrichment.
Assigning enzyme-substrate relationships
Beyond mapping PTM signaling responses according to types of DNA damage, the next step in a proteomic analysis of DNA repair is to map signaling responses according to the enzymes that confer the PTM(s) of interest. Pharmacological inhibition of kinases to determine their contribution to the DNA damage signaling response is one example [205]. To date the field of quantitative phosphoproteomics has focused much attention on the apical DNA damage response kinases—ATM, ATR, and DNA-PK—with relatively few phosphoproteomic datasets covering other potential players in the DNA damage response, such as casein kinase or DYRK kinases [206,207]. This paucity of datasets is a golden opportunity for DNA repair labs interested in expanding the current understanding of kinase signaling in DNA repair. Pharmacological inhibitors of PARPs, some of which are already approved for use as cancer therapies, are readily available. An important consideration when choosing PARP inhibitors is that they vary in their ability to trap PARP on DNA [208]. For emerging modifications where pharmacological inhibitors of the enzymes that confer these modifications are not readily available, genetic ablation by CRISPR knockout, siRNA, or shRNA is the recommended strategy. Indeed, genetic validation of results from pharmacological studies is best practice to ensure that effects observed from the use of chemical inhibitors do not stem from off-target effects of the chemicals themselves. Since druggable proteins belong to evolutionarily-derived families, it is expected that chemical inhibitors will exhibit some binding to closely related proteins. ATR and ATM are both in the PIKK family, of which mTOR is a member–indeed, the ATR inhibitor VE-821 does exhibit some level of mTOR inhibition as an off-target effect [209]. Perturbation of specific DNA repair activities is an orthogonal approach to inhibition of specific enzymes that confer PTMs. For example, DNA repair signaling can be modulated according to the extent of DNA end resection using genetic approaches [38]. Alternatively, chemical inhibitors of resection nucleases can be used to modulate the extent of DNA end resection [210,211], though to date there has been no extensive mapping of PTM signaling according to the extent of resection in mammalian cell lines. In the future, it will be informative to cross-compare PTM mapping experiments by enzyme and DNA repair process dependency, as such a comparison could reveal new modes of signaling that extend beyond the canonical PTM writers in DNA repair.
In vitro and in silico methods of delineating PTM dependencies are an invaluable addition to genetic-proteomic approaches and are useful in assigning PTM events to a particular enzyme as a follow-up to proteomic studies. Enzymatic dependencies for DNA damage signaling events can be inferred by mining PTM proteomics datasets for potential consensus motifs using any of a number of computational tools such as the IceLogo package [212]. If a strong consensus preference appears in the data, this consensus can be searched against known consensus motifs in the literature [213]. This approach is useful in informing future genetic or pharmacological experiments (which potential PTM enzyme to inhibit), but still does not represent direct evidence that a given enzyme is responsible for the PTM of interest. In vitro experiments using purified proteins provide direct evidence of enzyme-substrate relationships, and this approach has been used to great effect to assess the activity of DNA damage response kinases toward putative substrates or motifs [214]. In vitro experiments are quite difficult and low-throughput—it is for this reason that the vast majority of PTMs have not been directly linked to a PTMase in this way. A high-throughput alternative to in vitro experiments is to genetically modify PTMases such that they will use an alternative substrate that results in a traceable chemical modification. Kinases can be mutated so that they will use an ATPγS analog to thiophosphorylate substrate proteins [215]. This approach may not work with all PTMs, and pleiotropic effects of the ATPγS analog-permissive mutation must be carefully controlled for. Direct PTM mapping is an area that should be the focus of intensive efforts going forward, since identifying direct versus indirect effects of PTM enzymes remains a major question in the field.
Quantitative PTM mapping experiments
Now that we have reviewed chemical and genetic strategies to perturb DNA repair as well as methods of enriching for certain subcellular fractions, we now suggest a set of considerations for the design of quantitative mass spectrometry experiments. The first consideration of quantitative proteomics is to ask the following question: “does the experimental design require quantitation?” Surprisingly, the answer to this question is not always a resounding yes. Determining PTM dependencies is greatly aided by quantitative proteomics, but simply mapping the scope of certain PTMs need not involve quantitative methods, a consideration that lowers costs and may improve coverage. In the case of understudied PTMs, such as crotonylation, for which few proteomics datasets even exist, label-free methods represent a logical starting point in a global substrate analysis. However, the ability to assign PTM dependencies (either by PTMase or by cellular insult) strongly relies on quantitative proteomics given the stochastic nature of how ions (especially low abundant ions) are selected in most types of mass spectrometry-based approaches . This section will focus on metabolic labeling approaches such as SILAC, as we consider these methods to result in higher quality quantitation of changes in PTMs. SILAC experiments are designed as binary comparisons, though triple SILAC is an option at the cost of sensitivity [216]. SILAC provides a key advantage in that false positive peptide identifications can be robustly filtered out with a “label-swapping” experiment, that is, running a replicate experiment where the light and heavy channels have been switched [44,217,218]. To illustrate SILAC experimental design with a concrete example, suppose that SILAC mass spectrometry will be used to identify putative substrates of a hypothetical kinase. Four total binary comparisons are needed in order to construct a robust dataset of substrates in response to DNA damage. The first two experiments will define the general cellular response to DNA damage, such as ionizing radiation (IR). In experiment 1, unirradiated cells grown in normal lysine and arginine-containing media are compared to cells grown in media containing 13C15N lysine and arginine isotopologues and acutely subjected to ionizing radiation. In experiment 2, the light channel is subjected to IR and the heavy channel is left unirradiated. The results of experiment 1 and 2 are merged and only phosphopeptides with ratios that invert according to the isotope labelling scheme are considered in the final analysis. In experiment 3 and 4, kinase substrates are identified. In experiment 3, both light and heavy cultures are irradiated, but the heavy culture is treated with a kinase inhibitor; in experiment 4 the scheme is the same, but the light culture is treated with the inhibitor. The results of experiment 3 and 4 are merged and only phosphopeptides with ratios that invert according to the isotope labelling scheme are considered in the final analysis. Then the four datasets can be compared to reveal the subset of IR-induced, kinase-dependent phosphopeptides. This general approach to substrate mapping is applicable to any PTM, and PTM writer dependency can be determined by genetic as well as pharmacological methods.
Achieving maximum PTM coverage is a key consideration of mapping experiments. In the earliest stages this is aided by the inclusion of inhibitors of enzymes that remove PTMs as well as troubleshooting enrichment protocols to minimize the presence of unmodified peptides. In terms of increasing coverage, off-line fractionation is the most important step (Figure 3). Sample complexity is reduced and PTM coverage is dramatically increased at the cost of increased instrument time [153]. Coverage can be further increased by the collection of additional fractions, though this approach will require increasing quantities of starting sample material for best results. At the mass spectrometry analysis stage, it is important to consult the literature and prepare an instrument method that recapitulates prior successful analyses of the PTM of interest, especially for mass spectrometry neophytes. Fragmentation methods and collision energies can be particularly important for certain PTMs, such as PARylation [89]
The final steps in mass spectrometry analyses of DNA repair are data analysis and interpretation. Spectrum files generated by mass spectrometers can be searched using a number of free or paid software packages, such as COMET, MaxQuant, Sorcerer2, or Proteome Discoverer. All of these programs are well-documented with extensive online resources and tutorials to aid the uninitiated. Additionally, these programs are complete with user interfaces that are often absent from other -omics tools. Data interpretation and follow-up can be more difficult, as any proteomic study of DNA repair is likely to unearth a large number of modified sites on client proteins even after filtering by DNA damage and PTM writer dependency. Genetic validation of mass spectrometry results is key to gleaning meaningful insights from large datasets, and this validation could involve the knockdown, overexpression, or generation of point mutants of key targets, followed up by phenotypic testing of these mutants using standard assays in the DNA repair biologist’s toolkit, such as DNA fiber assays, clonogenic survival assays, western blots, or immunofluorescence. Proteomics experiments performed by scientists knowledgeable about the underlying biology of DNA repair have an advantage in follow-up and validation stages.
The utility of focused approaches to map PTMs
Global approaches to map PTMs in DNA repair have been the major focus of this review because these methods generate large-scale unbiased networks of DNA repair signaling events and are excellent for hypothesis generation. A notable limitation of these approaches is that they are often limited in terms of coverage of individual proteins, even with advances in instrumentation and PTM enrichment methods. Sometimes, in-depth analysis of a single protein or protein complex is required, and this is where affinity purification mass spectrometry (AP-MS) is typically used. In AP-MS, individual proteins are purified from cell extracts by immunoprecipitation and then subjected to PTM enrichment prior to proteolytic digestion and mass spectrometry analysis [141,219]. As an alternative to purification of individual proteins, proximity labelling approaches can be used to pull down local protein networks for further analysis. In these protocols, an enzyme is used to link biotin to nearby proteins, which is then enriched from cell extracts with a streptavidin resin [220-222]. These methods can be coupled to PTM enrichment protocols to isolate a single or a small subset of modified proteins with substantially higher coverage than unbiased approaches. These techniques can be especially useful when applied to hits from an unbiased proteomic screen to expand the number of sites identified in a particular protein of interest.
8. Future directions for DNA repair PTM-MS
There are a large number of PTMs that can occur on proteins, often together, and this co-occurrence creates combinatorial complexity [223]. Complicating the full understanding of PTMs in DNA repair is the fact that, while multiple PTMs can occur within a single peptide, mass spectrometry techniques to analyze individual PTMs are not necessarily compatible with the analysis of co-occurring PTMs. For example, the DNA repair helicase Sgs1 is subject to both SUMOylation and phosphorylation, but analyzing both of these modifications in parallel is difficult; likewise, RPA subunits are also subject to multiple modifications [12,47,224]. Progress has been made on this front—a recent report used x-ray irradiation couple to mass spectrometry to characterize complex modifications on a model peptide antibiotic, vancomycin [225]—but the utility and widespread applicability of such approaches to bottom-up data-dependent approaches remains to be seen. Presently, top-down proteomics methods—that is, analysis of whole proteins rather than peptide fragments via mass spectrometry—represent a useful approach for analyzing multiple PTM types [226].
The simultaneous analysis of co-occurring PTMs belies a more widespread hurdle that researchers face when acquiring mass spectrometry data. That is, forging the critical causal linkage between modification sites on proteins of interest and their associated biological function. For example, mass spectrometry analysis might identify several phosphorylation sites within a protein of interest, but mutation of all kinase consensus sites within a given protein domain may be required to elicit a phenotype [11,47]. The coverage issue is partially solvable by digestion of protein extracts with enzymes other than trypsin, though few enzymes are as efficient or as cheap as trypsin and they have not seen widespread adoption in shotgun proteomics workflows [227]. That being said, the use of multiple enzymes approaches 94% sequence coverage of the model protein BSA [227]. As alternative proteases become more widespread in their use, the extent of PTM coverage will increase, yielding new insights into DNA repair biology.
The study of DNA repair has been greatly aided by shotgun proteomics for PTM identification and quantitation. From phosphorylation to SUMOylation, the regulatory repertoire of the DNA damage response has expanded from a few key phospho substrates, monitorable by immunoblotting, to hundreds of proteins with varying PTMs comprising a complex network that promises to keep molecular biologists busy for years to come. Key advancements will include the continued march of instrumentation sensitivity, the analysis of multiple PTMs in parallel, and the adoption of alternative proteases to increase coverage. Coupled with phenotypic analysis of candidate PTM site mutants, these anticipated advances will yield new insights into DNA repair signaling. More broadly, delineating the comprehensive network of PTMs in DNA repair will enable deeper understanding of existing cancer therapeutic strategies that target DNA repair and enable logical design of combination therapies based on synthetic lethalities. In the next decade, the field of proteomics is poised to aid in the discovery of new mechanisms of DNA repair and to translate these findings to the clinic.
FUNDING
This work was supported by grants from the National Institutes of Health to M.B.S. (R35GM141159, R01GM097272, R01GM123018, and R01HD095296; Equipment Supplement R01GM097272-07S1).
REFERENCES
- [1].Hanahan D, Weinberg RA, Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [DOI] [PubMed] [Google Scholar]
- [2].Hanahan D, Weinberg RA, The hallmarks of cancer. Cell 2000, 100, 57–70. [DOI] [PubMed] [Google Scholar]
- [3].German J, Archibald R, Bloom D, Chromosomal breakage in a rare and probably genetically determined syndrome of man. Science 1965, 148, 506–507. [DOI] [PubMed] [Google Scholar]
- [4].Ellis NA, Groden J, Ye TZ, Straughen J, et al. , The Bloom’s syndrome gene product is homologous to RecQ helicases. Cell 1995, 83, 655–666. [DOI] [PubMed] [Google Scholar]
- [5].Thompson LH, Schild D, Recombinational DNA repair and human disease. Mutat. Res.Fundam. Mol. Mech. Mutagen 2002, 509, 49–78. [DOI] [PubMed] [Google Scholar]
- [6].Lanz MC, Dibitetto D, Smolka MB, DNA damage kinase signaling: checkpoint and repair at 30 years. EMBO J 2019, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ciccia A, Elledge SJ, The DNA Damage Response: Making It Safe to Play with Knives. Molecular Cell 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hadi K, Yao X, Behr JM, Deshpande A, et al. , Distinct Classes of Complex Structural Variation Uncovered across Thousands of Cancer Genome Graphs. Cell 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dion V, Kalck V, Horigome C, Towbin BD, Gasser SM, Increased mobility of double-strand breaks requires Mec1, Rad9 and the homologous recombination machinery. Nat. Cell Biol 2012, 14, 502–509. [DOI] [PubMed] [Google Scholar]
- [10].Flott S, Kwon Y, Pigli YZ, Rice PA, et al. , Regulation of Rad51 function by phosphorylation. EMBO Rep. 2011, 12, 833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ohouo PY, Bastos de Oliveira FM, Almeida BS, Smolka MB, DNA damage signaling recruits the Rtt107-Slx4 scaffolds via Dpb11 to Mediate replication stress response. Mol. Cell 2010, 39, 300–306. [DOI] [PubMed] [Google Scholar]
- [12].Bonner JN, Choi K, Xue X, Torres NP, et al. , Smc5/6 Mediated Sumoylation of the Sgs1-Top3-Rmi1 Complex Promotes Removal of Recombination Intermediates. Cell Rep. 2016, 16, 368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S, RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 2002, 419, 135–141. [DOI] [PubMed] [Google Scholar]
- [14].Deribe YL, Pawson T, Dikic I, Post-translational modifications in signal integration. Nature Structural and Molecular Biology 2010, 17, 666–672. [DOI] [PubMed] [Google Scholar]
- [15].Ochoa D, Jarnuczak AF, Viéitez C, Gehre M, et al. , The functional landscape of the human phosphoproteome. Nat. Biotechnol 2020, 38, 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mann M, Jensen ON, Proteomic analysis of post-translational modifications. Nature Biotechnology 2003, 21, 255–261. [DOI] [PubMed] [Google Scholar]
- [17].Chi P, Allis CD, Wang GG, Covalent histone modifications--miswritten, misinterpreted and mis-erased in human cancers. Nat. Rev. Cancer 2010, 10, 457–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Leroy C, Lee SE, Vaze MB, Ochsenbien F, et al. , PP2C phosphatases Ptc2 and Ptc3 are required for DNA checkpoint inactivation after a double-strand break. Mol. Cell 2003, 11, 827–835. [DOI] [PubMed] [Google Scholar]
- [19].Dantuma NP, Attikum H, Spatiotemporal regulation of posttranslational modifications in the DNA damage response. EMBO J. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bai M, Ti D, Mei Q, Liu J, et al. , The Role of Posttranslational Modifications in DNA Repair. BioMed Research International 2020. [Google Scholar]
- [21].Ohouo PY, Bastos De Oliveira FM, Liu Y, Ma CJ, Smolka MB, DNA-repair scaffolds dampen checkpoint signalling by counteracting the adaptor Rad9. Nature 2013, 493, 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cussiol JR, Jablonowski CM, Yimit A, Brown GW, Smolka MB, Dampening DNA damage checkpoint signalling via coordinated BRCT domain interactions. EMBO J. 2015, 34, 1704–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Maréchal A, Zou L, DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb. Perspect. Biol 2013, 5, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zou L, Elledge SJ, Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 2003, 300, 1542–1548. [DOI] [PubMed] [Google Scholar]
- [25].Deshpande I, Seeber A, Shimada K, Keusch JJ, et al. , Structural Basis of Mec1-Ddc2-RPA Assembly and Activation on Single-Stranded DNA at Sites of Damage. Mol. Cell 2017, 68, 431–445.e5. [DOI] [PubMed] [Google Scholar]
- [26].Downs JA, Lowndes NF, Jackson SP, A role for Saccharomyces cerevisiae histone H2A in DNA repair. Nature 2000, 408, 1001–1004. [DOI] [PubMed] [Google Scholar]
- [27].Harrison JC, Haber JE, Surviving the breakup: The DNA damage checkpoint. Annu. Rev. Genet 2006, 40, 209–235. [DOI] [PubMed] [Google Scholar]
- [28].Desany BA, Alcasabas AA, Bachant JB, Elledge SJ, Recovery from DNA replicational stress is the essential function of the S-phase checkpoint pathway. Genes and Development 1998, 12, 2956–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sweeney FD, Yang F, Chi A, Shabanowitz J, et al. , Saccharomyces cerevisiae Rad9 acts as a Mec1 adaptor to allow Rad53 activation. Curr. Biol 2005, 15, 1364–1375. [DOI] [PubMed] [Google Scholar]
- [30].Smolka MB, Albuquerque CP, Chen SH, Zhou H, Proteome-wide identification of in vivo targets of DNA damage checkpoint kinases. Proc. Natl. Acad. Sci. U. S. A 2007, 104, 10364–10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lee S-J, Schwartz MF, Duong JK, Stern DF, Rad53 Phosphorylation Site Clusters Are Important for Rad53 Regulation and Signaling. Mol. Cell. Biol 2003, 23, 6300–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kondo T, Matsumoto K, Sugimoto K, Role of a Complex Containing Rad17, Mec3, and Ddc1 in the Yeast DNA Damage Checkpoint Pathway. Mol. Cell. Biol 1999, 19, 1136–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pellicioli A, Lucca C, Liberi G, Marini F, et al. , Activation of Rad53 kinase in response to DNA damage and its effect in modulating phosphorylation of the lagging strand DNA polymerase. EMBO J. 1999, 18, 6561–6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ficarro SB, McCleland ML, Stukenberg PT, Burke DJ, et al. , Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat. Biotechnol 2002, 20, 301–305. [DOI] [PubMed] [Google Scholar]
- [35].Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, et al. , ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 2007. [DOI] [PubMed] [Google Scholar]
- [36].BastosdeOliveira FM, Kim D, Cussiol JR, Das J, et al. , Phosphoproteomics Reveals Distinct Modes of Mec1/ATR Signaling during DNA Replication. Mol. Cell 2015, 57, 1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wagner SA, Oehler H, Voigt A, Dalic D, et al. , ATR inhibition rewires cellular signaling networks induced by replication stress. Proteomics 2016, 16, 402–416. [DOI] [PubMed] [Google Scholar]
- [38].Sanford EJ, Comstock WJ, Faça VM, Vega SC, et al. , Phosphoproteomics reveals a distinctive Mec1/ATR signaling response upon DNA end hyper-resection. EMBO J. 2021, 40, e104566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shiotani B, Nguyen HD, Håkansson P, Maréchal A, et al. , Two Distinct Modes of ATR Activation Orchestrated by Rad17 and Nbs1. Cell Rep. 2013, 3, 1651–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Memisoglu G, Lanz MC, Eapen VV, Jordan JM, et al. , Mec1ATR Autophosphorylation and Ddc2ATRIP Phosphorylation Regulates DNA Damage Checkpoint Signaling. Cell Rep. 2019, 28, 1090–1102.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Johnson LN, The regulation of protein phosphorylation. Biochem. Soc. Trans 2009, 37, 627–641. [DOI] [PubMed] [Google Scholar]
- [42].Hardman G, Perkins S, Brownridge PJ, Clarke CJ, et al. , Strong anion exchange-mediated phosphoproteomics reveals extensive human non-canonical phosphorylation. EMBO J. 2019, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Martens L, Hermjakob H, Jones P, Adamsk M, et al. , PRIDE: The proteomics identifications database. Proteomics 2005, 5, 3537–3545. [DOI] [PubMed] [Google Scholar]
- [44].Faca VM, Sanford EJ, Tieu J, Comstock W, et al. , Maximized quantitative phosphoproteomics allows high confidence dissection of the DNA damage signaling network. Sci. Rep 2020, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Floyd BM, Drew K, Marcotte EM, Systematic Identification of Protein Phosphorylation-Mediated Interactions. J. Proteome Res 2021, 20, 1359–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ramasamy P, Turan D, Tichshenko N, Hulstaert N, et al. , Scop3P: A Comprehensive Resource of Human Phosphosites within Their Full Context. J. Proteome Res 2020, 19, 3478–3486. [DOI] [PubMed] [Google Scholar]
- [47].Lanz MC, Yugandhar K, Gupta S, Sanford EJ, et al. , In-depth and 3-dimensional exploration of the budding yeast phosphoproteome. EMBO Rep. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kerscher O, Felberbaum R, Hochstrasser M, Modification of proteins by ubiquitin and ubiquitin-like proteins. Annual Review of Cell and Developmental Biology 2006. [DOI] [PubMed] [Google Scholar]
- [49].Deshaies RJ, Joazeiro CAP, RING domain E3 ubiquitin ligases. Annual Review of Biochemistry 2009. [DOI] [PubMed] [Google Scholar]
- [50].Kulathu Y, Komander D, Atypical ubiquitylation-the unexplored world of polyubiquitin beyond Lys48 and Lys63 linkages. Nature Reviews Molecular Cell Biology 2012. [DOI] [PubMed] [Google Scholar]
- [51].Hurley JH, Lee S, Prag G, Ubiquitin-binding domains. Biochemical Journal 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yu J, Qin B, Lou Z, Ubiquitin and ubiquitin-like molecules in DNA double strand break repair. Cell Biosci. 2020, 10, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chang DJ, Lupardus PJ, Cimprich KA, Monoubiquitination of proliferating cell nuclear antigen induced by stalled replication requires uncoupling of DNA polymerase and mini-chromosome maintenance helicase activities. J. Biol. Chem 2006. [DOI] [PubMed] [Google Scholar]
- [54].Povlsen LK, Beli P, Wagner SA, Poulsen SL, et al. , Systems-wide analysis of ubiquitylation dynamics reveals a key role for PAF15 ubiquitylation in DNA-damage bypass. Nat. Cell Biol 2012. [DOI] [PubMed] [Google Scholar]
- [55].Elia AEH, Wang DC, Willis NA, Boardman AP, et al. , RFWD3-Dependent Ubiquitination of RPA Regulates Repair at Stalled Replication Forks. Mol. Cell 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lu CS, Truong LN, Aslanian A, Shi LZ, et al. , The RING finger protein RNF8 ubiquitinates Nbs1 to promote DNA double-strand break repair by homologous recombination. J. Biol. Chem 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kolas NK, Chapman JR, Nakada S, Ylanko J, et al. , Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Galanty Y, Belotserkovskaya R, Coates J, Polo S, et al. , Mammalian SUMO E3-ligases PIAS1 and PIAS4 promote responses to DNA double-strand breaks. Nature 2009, 462, 935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sarangi P, Steinacher R, Altmannova V, Fu Q, et al. , Sumoylation Influences DNA Break Repair Partly by Increasing the Solubility of a Conserved End Resection Protein. PLoS Genet. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Xiao Z, Chang JG, Hendriks IA, Sigurdsson JO, et al. , System-wide analysis of SUMOylation dynamics in response to replication stress reveals novel small ubiquitin-like modified target proteins and acceptor lysines relevant for genome stability. Mol. Cell. Proteomics 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Bhagwat N, Owens S, Ito M, Boinapalli J, et al. , Sumo is a pervasive regulator of meiosis. Elife 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hendriks IA, D’Souza RC, Chang JG, Mann M, Vertegaal ACO, System-wide identification of wild-type SUMO-2 conjugation sites. Nat. Commun 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wohlschlegel JA, Johnson ES, Reed SI, Yates JR, Improved identification of SUMO attachment sites using C-terminal SUMO mutants and tailored protease digestion strategies. J. Proteome Res 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lamoliatte F, Caron D, Durette C, Mahrouche L, et al. , Large-scale analysis of lysine SUMOylation by SUMO remnant immunoaffinity profiling. Nat. Commun 2014, 5, 5409. [DOI] [PubMed] [Google Scholar]
- [65].Hendriks IA, Treffers LW, Verlaan-deVries M, Olsen JV, Vertegaal ACO, SUMO-2 Orchestrates Chromatin Modifiers in Response to DNA Damage. Cell Rep. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].González-Prieto R, Eifler-Olivi K, Claessens LA, Willemstein E, et al. , Global non-covalent SUMO interaction networks reveal SUMO-dependent stabilization of the non-homologous end joining complex. Cell Rep. 2021, 34, 108691. [DOI] [PubMed] [Google Scholar]
- [67].Shahbazian MD, Grunstein M, Functions of Site-Specific histone acetylation and deacetylation. Annual Review of Biochemistry 2007. [DOI] [PubMed] [Google Scholar]
- [68].Weinert BT, Narita T, Satpathy S, Srinivasan B, et al. , Time-Resolved Analysis Reveals Rapid Dynamics and Broad Scope of the CBP/p300 Acetylome. Cell 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Fang EF, Scheibye-Knudsen M, Brace LE, Kassahun H, et al. , Defective mitophagy in XPA via PARP-1 hyperactivation and NAD +/SIRT1 reduction. Cell 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Guan KL, Yu W, Lin Y, Xiong Y, Zhao S, Generation of acetyllysine antibodies and affinity enrichment of acetylated peptides. Nat. Protoc 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Beli P, Lukashchuk N, Wagner SA, Weinert BT, et al. , Proteomic Investigations Reveal a Role for RNA Processing Factor THRAP3 in the DNA Damage Response. Mol. Cell 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Choudhary C, Kumar C, Gnad F, Nielsen ML, et al. , Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 2009. [DOI] [PubMed] [Google Scholar]
- [73].Bennetzen MV, Lasen DH, Dinant C, Watanabe S, et al. , Acetylation dynamics of human nuclear proteins during the ionizing radiation-induced DNA damage response. Cell Cycle 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Gibson BA, Kraus WL, New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nature Reviews Molecular Cell Biology 2012. [DOI] [PubMed] [Google Scholar]
- [75].Davidovic L, Vodenicharov M, Affar EB, Poirier GG, Importance of poly(adp-ribose) glycohydrolase in the control of poly(adp-ribose) metabolism. Exp. Cell Res 2001. [DOI] [PubMed] [Google Scholar]
- [76].Slade D, Dunstan MS, Barkauskaite E, Weston R, et al. , The structure and catalytic mechanism of a poly(ADP-ribose) glycohydrolase. Nature 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG, PARP inhibition: PARP1 and beyond. Nature Reviews Cancer 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Hoch NC, Hanzlikova H, Rulten SL, Tétreault M, et al. , XRCC1 mutation is associated with PARP1 hyperactivation and cerebellar ataxia. Nature 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Li M, Yu X, Function of BRCA1 in the DNA Damage Response Is Mediated by ADP-Ribosylation. Cancer Cell 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ruscetti T, Lehnert BE, Halbrook J, Le Trong H, et al. , Stimulation of the DNA-dependent protein kinase by poly(ADP-ribose) polymerase. J. Biol. Chem 1998. [DOI] [PubMed] [Google Scholar]
- [81].Slade D, PARP and PARG inhibitors in cancer treatment. Genes and Development 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lord C, PARP inhibitors: Synthetic lethality in the clinic. Science 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Morgan WF, Cleaver JE, 3-Aminobenzamide synergistically increases sister-chromatid exchanges in cells exposed to methyl methanesulfonate but not to ultraviolet light. Mutat. Res. Lett 1982. [DOI] [PubMed] [Google Scholar]
- [84].Berger NA, Poly(ADP-ribose) in the cellular response to DNA damage. Radiation Research 1985. [PubMed] [Google Scholar]
- [85].Wang M, Wu W, Wu W, Rosidi B, et al. , PARP-1 and Ku compete for repair of DNA double strand breaks by distinct NHEJ pathways. Nucleic Acids Res. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hochegger H, Dejsuphong D, Fukushima T, Morrison C, et al. , Parp-1 protects homologous recombination from interference by Ku and Ligase IV in vertebrate cells. EMBO J. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ménissier De Murcia J, Niedergang C, Trucco C, Ricoul M, et al. , Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc. Natl. Acad. Sci. U. S. A 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zhang Y, Wang J, Ding M, Yu Y, Site-specific characterization of the Asp-and Glu-ADP-ribosylated proteome. Nat. Methods 2013. [DOI] [PubMed] [Google Scholar]
- [89].Hendriks IA, Larsen SC, Nielsen ML, An advanced strategy for comprehensive profiling of ADP-ribosylation sites using mass spectrometry-based proteomics. Mol. Cell. Proteomics 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Bedford MT, Clarke SG, Protein Arginine Methylation in Mammals: Who, What, and Why. Molecular Cell 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Boisvert FM, Côté J, Boulanger MC, Richard S, A proteomic analysis of arginine-methylated protein complexes. Mol. Cell. Proteomics 2003. [DOI] [PubMed] [Google Scholar]
- [92].Guo A, Gu H, Zhou J, Mulhern D, et al. , Immunoaffinity enrichment and mass spectrometry analysis of protein methylation. Mol. Cell. Proteomics 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Boisvert FM, Déry U, Masson JY, Richard S, Arginine methylation of MRE11 by PRMT1 is required for DNA damage checkpoint control. Genes and Development 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Tan M, Luo H, Lee S, Jin F, et al. , Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Chen Y, Sprung R, Tang Y, Ball H, et al. , Lysine propionylation and butyrylation are novel post-translational modifications in histones. Mol. Cell. Proteomics 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Peng C, Lu Z, Xie Z, Cheng Z, et al. , The first identification of lysine malonylation substrates and its regulatory enzyme. Mol. Cell. Proteomics 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Yu H, Bu C, Liu Y, Gong T, et al. , Global crotonylome reveals CDYL-regulated RPA1 crotonylation in homologous recombination-mediated DNA repair. Science Advances 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Smotrys JE, Linder ME, Palmitoylation of Intracellular Signaling Proteins: Regulation and function. Annual Review of Biochemistry 2004. [DOI] [PubMed] [Google Scholar]
- [99].Fontana GA, Hess D, Reinert JK, Mattarocci S, et al. , Rif1 S-acylation mediates DNA double-strand break repair at the inner nuclear membrane. Nat. Commun 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Drisdel RC, Green WN, Labeling and quantifying sites of protein palmitoylation. Biotechniques 2004. [DOI] [PubMed] [Google Scholar]
- [101].Roth AF, Wan J, Bailey AO, Sun B, et al. , Global Analysis of Protein Palmitoylation in Yeast. Cell 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Park S, Patterson EE, Cobb J, Audhya A, et al. , Palmitoylation controls the dynamics of budding-yeast heterochromatin via the telomere-binding protein Rif1. Proc. Natl. Acad. Sci. U. S. A 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Banerjee T, Chakravarti D, A Peek into the Complex Realm of Histone Phosphorylation. Mol. Cell. Biol 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Rogakou EP, Boon C, Redon C, Bonner WM, Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Stewart GS, Wang B, Bigneli CR, Taylor AMR, Elledge SJ, MDC1 is a mediator of the mammalian DNA damage checkpoint. Nature 2003. [DOI] [PubMed] [Google Scholar]
- [106].Bonenfant D, Coulot M, Towbin H, Schindler P, van Oostrum J, Characterization of histone H2A and H2B variants and their post-translational modifications by mass spectrometry. Mol. Cell. Proteomics 2006. [DOI] [PubMed] [Google Scholar]
- [107].Bonenfant D, Towbin H, Coulot M, Schindler P, et al. , Analysis of dynamic changes in post-translational modifications of human histones during cell cycle by mass spectrometry. Mol. Cell. Proteomics 2007. [DOI] [PubMed] [Google Scholar]
- [108].Lawrence M, Daujat S, Schneider R, Lateral Thinking: How Histone Modifications Regulate Gene Expression. Trends in Genetics 2016. [DOI] [PubMed] [Google Scholar]
- [109].Grenon M, Costelloe T, Jimeno S, O’Shaughnessy A, et al. , Docking onto chromatin via the Saccharomyces cerevisiae Rad9 Tudor domain. Yeast 2007. [DOI] [PubMed] [Google Scholar]
- [110].Dhar S, Gursoy-Yuzugullu O, Parasuram R, Price BD, The tale of a tail: Histone H4 acetylation and the repair of DNA breaks. Philosophical Transactions of the Royal Society B: Biological Sciences 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Tang J, Cho NW, Cui G, Manion EM, et al. , Acetylation limits 53BP1 association with damaged chromatin to promote homologous recombination. Nat. Struct. Mol. Biol 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Liao R, Zheng D, Nie A, Zhou S, et al. , Sensitive and Precise Characterization of Combinatorial Histone Modifications by Selective Derivatization Coupled with RPLC-EThcD-MS/MS. J. Proteome Res 2017. [DOI] [PubMed] [Google Scholar]
- [113].Thomas JM, Thomson JJ: Winner of the Nobel Prize for Physics 1906. Angewandte Chemie - International Edition 2006. [DOI] [PubMed] [Google Scholar]
- [114].Griffiths J, A brief history of mass spectrometry. Analytical Chemistry 2008. [DOI] [PubMed] [Google Scholar]
- [115].Tanaka K, Waki H, Ido Y, Akita S, et al. , Protein and polymer analyses up to m/z 100 000 by laser ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom 1988. [Google Scholar]
- [116].El-Aneed A, Cohen A, Banoub J, Mass spectrometry, review of the basics: Electrospray, MALDI, and commonly used mass analyzers. Applied Spectroscopy Reviews 2009. [Google Scholar]
- [117].Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM, Electrospray ionization for mass spectrometry of large biomolecules. Science 1989. [DOI] [PubMed] [Google Scholar]
- [118].Fenselau C, Heller DN, Miller MS, White HB, Phosphorylation sites in riboflavin-binding protein characterized by fast atom bombardment mass spectrometry. Anal. Biochem 1985, 150, 309–314. [DOI] [PubMed] [Google Scholar]
- [119].Johnson SA, Hunter T, Phosphoproteomics finds its timing. Nature Biotechnology 2004, 22, 1093–1094. [DOI] [PubMed] [Google Scholar]
- [120].Li X, Gerber SA, Rudner AD, Beausoleil SA, et al. , Large-scale phosphorylation analysis of α-factor-arrested Saccharomyces cerevisiae. J. Proteome Res 2007, 6, 1190–1197. [DOI] [PubMed] [Google Scholar]
- [121].Wilson-Grady JT, Villen J, Gygi SP, Phosphoproteome analysis of fission yeast. J. Proteome Res 2008, 7, 1088–1097. [DOI] [PubMed] [Google Scholar]
- [122].Gruhler A, Olsen JV, Mohammed S, Mortensen P, et al. , Quantitative phosphoproteomics applied to the yeast pheromone signaling pathway. Mol. Cell. Proteomics 2005. [DOI] [PubMed] [Google Scholar]
- [123].Cheng H, Deng W, Wang Y, Ren J, et al. , DbPPT: A comprehensive database of protein phosphorylation in plants. Database 2014, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Yu K, Zhang Q, Liu Z, Zhao Q, et al. , QPhos: A database of protein phosphorylation dynamics in humans. Nucleic Acids Res. 2019, 47, D451–D458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Perez-Riverol Y, Csordas A, Bai J, Bernal-Llinares M, et al. , The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Hendriks IA, Vertegaal ACO, A comprehensive compilation of SUMO proteomics. Nat. Rev. Mol. Cell Biol 2016, 17, 581–595. [DOI] [PubMed] [Google Scholar]
- [127].Nguyen VN, Huang KY, Weng JTY, Lai KR, Lee TY, UbiNet: An online resource for exploring the functional associations and regulatory networks of protein ubiquitylation. Database 2016, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Steen H, Mann M, The ABC’s (and XYZ's) of peptide sequencing. Nature Reviews Molecular Cell Biology 2004. [DOI] [PubMed] [Google Scholar]
- [129].Lundgren DH, Martinez H, Wright ME, Han DK, Protein identification using sorcerer 2 and SEQUEST. Current Protocols in Bioinformatics 2009. [DOI] [PubMed] [Google Scholar]
- [130].Eng JK, McCormack AL, Yates JR, An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom 1994. [DOI] [PubMed] [Google Scholar]
- [131].McKittrick E, Gafken PR, Ahmad K, Henikoff S, Histone H3.3 is enriched in covalent modifications associated with active chromatin. Proc. Natl. Acad. Sci. U. S. A 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Zhang K, Yau PM, Chandrasekhar B, New R, et al. , Differentiation between peptides containing acetylated or tri-methylated lysines by mass spectrometry: An application for determining lysine 9 acetylation and methylation of histone H3. Proteomics 2004. [DOI] [PubMed] [Google Scholar]
- [133].Perchey RT, Tonini L, Tosolini M, Fournié JJ, et al. , PTMselect: optimization of protein modifications discovery by mass spectrometry. Sci. Rep 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Tian Z, Tolić N, Zhao R, Moore RJ, et al. , Enhanced top-down characterization of histone post-translational modifications. Genome Biol. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Pesavento JJ, Bullock CR, LeDuc RD, Mizzen CA, Kelleher NL, Combinatorial modification of human histone H4 quantitated by two-dimensional liquid chromatography coupled with top down mass spectrometry. J. Biol. Chem 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Anderson LC, Karch KR, Ugrin SA, Coradin M, et al. , Analyses of histone proteoforms using frontend electron transfer dissociation-enabled orbitrap instruments. Mol. Cell. Proteomics 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Tsai CF, Wang YT, Yen HY, Tsou CC, et al. , Large-scale determination of absolute phosphorylation stoichiometries in human cells by motif-targeting quantitative proteomics. Nat. Commun 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Lim MY, O’Brien J, Paulo JA, Gygi SP, Improved Method for Determining Absolute Phosphorylation Stoichiometry Using Bayesian Statistics and Isobaric Labeling. J. Proteome Res 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Lanz MC, Oberly S, Sanford EJ, Sharma S, et al. , Separable roles for Mec1/ATR in genome maintenance, DNA replication, and checkpoint signaling. Genes and Development 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Ren L, Li C, Shao W, Lin W, et al. , TiO2 with Tandem Fractionation (TAFT): An Approach for Rapid, Deep, Reproducible, and High-Throughput Phosphoproteome Analysis. J. Proteome Res 2018. [DOI] [PubMed] [Google Scholar]
- [141].Sanford EJ, Smolka MB, Fe-nta microcolumn purification of phosphopeptides from immunoprecipitation (ip) eluates for mass spectrometry analysis. Bio-protocol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Udeshi ND, Mani DC, Satpathy S, Fereshetian S, et al. , Rapid and deep-scale ubiquitylation profiling for biology and translational research. Nat. Commun 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Neville DCA, Rozanas CR, Price EM, Gruis DB, et al. , Evidence for phosphorylation of serine 753 in CFTR using a novel metal- ion affinity resin and matrix-assisted laser desorption mass spectrometry. Protein Sci. 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Pinkse MWH, Uitto PM, Hilhorst MJ, Ooms B, Heck AJR, Selective isolation at the femtomole level of phosphopeptides from proteolytic digests using 2D-NanoLC-ESI-MS/MS and titanium oxide precolumns. Anal. Chem 2004. [DOI] [PubMed] [Google Scholar]
- [145].Andersson L, Porath J, Isolation of phosphoproteins by immobilized metal (Fe3+) affinity chromatography. Anal. Biochem 1986. [DOI] [PubMed] [Google Scholar]
- [146].Posewitz MC, Tempst P, Immobilized gallium(III) affinity chromatography of phosphopeptides. Anal. Chem 1999. [DOI] [PubMed] [Google Scholar]
- [147].Bodenmiller B, Mueller LN, Mueller M, Domon B, Aebersold R, Reproducible isolation of distinct, overlapping segments of the phosphoproteome. Nat. Methods 2007, 4, 231–237. [DOI] [PubMed] [Google Scholar]
- [148].Thingholm TE, Jensen ON, Robinson PJ, Larsen MR, in:, Molecular and Cellular Proteomics, 2008. [DOI] [PubMed] [Google Scholar]
- [149].Grønborg M, Kristiansen TZ, Stensballe A, Andersen JS, et al. , A mass spectrometry-based proteomic approach for identification of serine/threonine-phosphorylated proteins by enrichment with phospho-specific antibodies: identification of a novel protein, Frigg, as a protein kinase A substrate. Mol. Cell. Proteomics 2002. [DOI] [PubMed] [Google Scholar]
- [150].Zhang H, Zha X, Tan Y, Hornbeck PV, et al. , Phosphoprotein analysis using antibodies broadly reactive against phosphorylated motifs. J. Biol. Chem 2002. [DOI] [PubMed] [Google Scholar]
- [151].Elia AEH, Boardman AP, Wang DC, Huttlin EL, et al. , Quantitative Proteomic Atlas of Ubiquitination and Acetylation in the DNA Damage Response. Mol. Cell 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Kim SC, Sprung R, Chen Y, Xu Y, et al. , Substrate and Functional Diversity of Lysine Acetylation Revealed by a Proteomics Survey. Mol. Cell 2006. [DOI] [PubMed] [Google Scholar]
- [153].McNulty DE, Annan RS, Hydrophilic interaction chromatography reduces the complexity of the phosphoproteome and improves global phosphopeptide isolation and detection. Mol. Cell. Proteomics 2008, 7, 971–980. [DOI] [PubMed] [Google Scholar]
- [154].Matsumoto M, Hatakeyama S, Oyamada K, Oda Y, et al. , Large-scale analysis of the human ubiquitin-related proteome. Proteomics 2005. [DOI] [PubMed] [Google Scholar]
- [155].Schwertman P, Lagarou A, Dekkers DHW, Raams A, et al. , UV-sensitive syndrome protein UVSSA recruits USP7 to regulate transcription-coupled repair. Nat. Genet 2012. [DOI] [PubMed] [Google Scholar]
- [156].Akimov V, Barrio-Hernandez I, Hansen SVF, Hallenborg P, et al. , Ubisite approach for comprehensive mapping of lysine and n-terminal ubiquitination sites. Nat. Struct. Mol. Biol 2018. [DOI] [PubMed] [Google Scholar]
- [157].Tammsalu T, Matic I, Jaffray EG, Ibrahim AFM, et al. , Proteome-wide identification of SUMO2 modification sites. Sci. Signal 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Becker J, Barysch SV, Karaca S, Dittner C, et al. , Detecting endogenous SUMO targets in mammalian cells and tissues. Nat. Struct. Mol. Biol 2013. [DOI] [PubMed] [Google Scholar]
- [159].Hendriks IA, D’Souza RCJ, Yang B, Verlaan-De Vries M, et al. , Uncovering global SUMOylation signaling networks in a site-specific manner. Nat. Struct. Mol. Biol 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Hendriks IA, Lyon D, Su D, Skotte NH, et al. , Site-specific characterization of endogenous SUMOylation across species and organs. Nat. Commun 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Filosa G, Barabino SML, Bachi A, Proteomics strategies to identify sumo targets and acceptor sites: A survey of RNA-Binding proteins SUMO ylation. NeuroMolecular Medicine 2013. [DOI] [PubMed] [Google Scholar]
- [162].Diedrich JK, Pinto AFM, Yates JR, Energy dependence of HCD on peptide fragmentation: Stepped collisional energy finds the sweet spot. J. Am. Soc. Mass Spectrom 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Cui L, Yapici I, Borhan B, Reid GE, Quantification of competing H3PO4 Versus HPO 3 + H2O neutral losses from regioselective 18O-labeled phosphopeptides. J. Am. Soc. Mass Spectrom 2014. [DOI] [PubMed] [Google Scholar]
- [164].Olsen JV, Blagoev B, Gnad F, Macek B, et al. , Global, In Vivo, and Site-Specific Phosphorylation Dynamics in Signaling Networks. Cell 2006. [DOI] [PubMed] [Google Scholar]
- [165].Taus T, Köcher T, Pichler P, Paschke C, et al. , Universal and confident phosphorylation site localization using phosphoRS. J. Proteome Res 2011. [DOI] [PubMed] [Google Scholar]
- [166].Savitski MM, Lemeer S, Boesche M, Lang M, et al. , Confident phosphorylation site localization using the mascot delta score. Mol. Cell. Proteomics 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Baker PR, Trinidad JC, Chalkley RJ, Modification site localization scoring integrated into a search engine. Mol. Cell. Proteomics 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Shteynberg DD, Deutsch EW, Campbell DS, Hoopmann MR, et al. , PTMProphet: Fast and Accurate Mass Modification Localization for the Trans-Proteomic Pipeline. J. Proteome Res 2019, 18, 4262–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Liu T, Belov ME, Jaitly N, Qian WJ, Smith RD, Accurate mass measurements in proteomics. Chemical Reviews 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Olsen JV, de Godoy LMF, Li G, Macek B, et al. , Parts per million mass accuracy on an orbitrap mass spectrometer via lock mass injection into a C-trap. Mol. Cell. Proteomics 2005. [DOI] [PubMed] [Google Scholar]
- [171].Stepath M, Zülch B, Maghnouj A, Schork K, et al. , Systematic Comparison of Label-Free, SILAC, and TMT Techniques to Study Early Adaption toward Inhibition of EGFR Signaling in the Colorectal Cancer Cell Line DiFi. J. Proteome Res 2020. [DOI] [PubMed] [Google Scholar]
- [172].Hogrebe A, Von Stechow L, Bekker-Jensen DB, Weinert BT, et al. , Benchmarking common quantification strategies for large-scale phosphoproteomics. Nat. Commun 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Olsen JV, Ong SE, Mann M, Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Mol. Cell. Proteomics 2004. [DOI] [PubMed] [Google Scholar]
- [174].Chen X, Wei S, Ji Y, Guo X, Yang F, Quantitative proteomics using SILAC: Principles, applications, and developments. Proteomics 2015, 15, 3175–3192. [DOI] [PubMed] [Google Scholar]
- [175].Wiese S, Reidegeld KA, Meyer HE, Warscheid B, Protein labeling by iTRAQ: A new tool for quantitative mass spectrometry in proteome research. Proteomics 2007. [DOI] [PubMed] [Google Scholar]
- [176].Thompson A, Schäfer J, Kuhn K, Kienle S, et al. , Tandem mass tags: A novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal. Chem 2003. [DOI] [PubMed] [Google Scholar]
- [177].Zhang L, Elias JE, in:, Methods in Molecular Biology, vol. 1550, 2017, pp. 185–198. [DOI] [PubMed] [Google Scholar]
- [178].Ting L, Rad R, Gygi SP, Haas W, MS3 eliminates ratio distortion in isobaric multiplexed quantitative proteomics. Nat. Methods 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Lee C-S, Haber JE, Mating-type Gene Switching in Saccharomyces cerevisiae. Microbiology Spectrum 2015. [DOI] [PubMed] [Google Scholar]
- [180].Zhu Z, Chung WH, Shim EY, Lee SE, Ira G, Sgs1 Helicase and Two Nucleases Dna2 and Exo1 Resect DNA Double-Strand Break Ends. Cell 2008, 134, 981–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Canela A, Sridharan S, Sciascia N, Tubbs A, et al. , DNA Breaks and End Resection Measured Genome-wide by End Sequencing. Mol. Cell 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Dibitetto D, La Monica M, Ferrari M, Marini F, Pellicioli A, Formation and nucleolytic processing of Cas9-induced DNA breaks in human cells quantified by droplet digital PCR. DNA Repair 2018. [DOI] [PubMed] [Google Scholar]
- [183].Symington LS, Gautier J, Double-strand break end resection and repair pathway choice. Annu. Rev. Genet 2011. [DOI] [PubMed] [Google Scholar]
- [184].Ceccaldi R, Rondinelli B, D’Andrea AD, Repair Pathway Choices and Consequences at the Double-Strand Break. Trends in Cell Biology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Schipler A, Iliakis G, DNA double-strand-break complexity levels and their possible contributions to the probability for error-prone processing and repair pathway choice. Nucleic Acids Res. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Montagnoli A, Valsasina B, Croci V, Menichincheri M, et al. , A Cdc7 kinase inhibitor restricts initiation of DNA replication and has antitumor activity. Nat. Chem. Biol 2008, 4, 357–365. [DOI] [PubMed] [Google Scholar]
- [187].Singh A, Xu Y-J, The Cell Killing Mechanisms of Hydroxyurea. Genes 2016, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Dasari S, Tchounwou PB, Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol 2014, 740, 364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Mei C, Lei L, Tan L-M, Xu X-J, et al. , The role of single strand break repair pathways in cellular responses to camptothecin induced DNA damage. Biomed. Pharmacother 2020, 125, 109875. [DOI] [PubMed] [Google Scholar]
- [190].Mladenov E, Tsaneva I, Anachkova B, Activation of the S phase DNA damage checkpoint by mitomycin C. J. Cell. Physiol 2007, 211, 468–476. [DOI] [PubMed] [Google Scholar]
- [191].Sleigh MJ, The mechanism of DNA breakage by phleomycin in vitro. Nucleic Acids Res. 1976, 3, 891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Ishikawa T, Ide F, Kodama K, Takayama S, Unscheduled DNA synthesis in human bronchial epithelium treated with various chemical carcinogens in vitro. J. Natl. Cancer Inst 1984, 73, 101–106. [PubMed] [Google Scholar]
- [193].Lundin C, North M, Erixon K, Walters K, et al. , Methyl methanesulfonate (MMS) produces heat-labile DNA damage but no detectable in vivo DNA double-strand breaks. Nucleic Acids Res. 2005, 33, 3799–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Mavragani IV, Nikitaki Z, Kalospyros SA, Georgakilas AG, Ionizing Radiation and Complex DNA Damage: From Prediction to Detection Challenges and Biological Significance. Cancers 2019, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Koundrioukoff S, Carignon S, Técher H, Letessier A, et al. , Stepwise activation of the ATR signaling pathway upon increasing replication stress impacts fragile site integrity. PLoS Genet. 2013, 9, e1003643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Moore MJ, Sebastian JA, Kolios MC, Determination of cell nucleus-to-cytoplasmic ratio using imaging flow cytometry and a combined ultrasound and photoacoustic technique: a comparison study. J. Biomed. Opt 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Luo Y, Hara T, Ishido Y, Yoshihara A, et al. , Rapid preparation of high-purity nuclear proteins from a small number of cultured cells for use in electrophoretic mobility shift assays. BMC Immunol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Senichkin VV, Prokhorova EA, Zhivotovsky B, Kopeina GS, Simple and efficient protocol for subcellular fractionation of normal and apoptotic cells. Cells 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Masuda T, Sugiyama N, Tomita M, Ohtsuki S, Ishihama Y, Mass Spectrometry-Compatible Subcellular Fractionation for Proteomics. J. Proteome Res 2020. [DOI] [PubMed] [Google Scholar]
- [200].Räschle M, Smeenk G, Hansen RK, Temu T, et al. , Proteomics reveals dynamic assembly of Repair complexes during bypass of DNA cross-links. Science 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Sirbu BM, McDonald WH, Dungrawala H, Badu-Nkansah A, et al. , Identification of proteins at active, stalled, and collapsed replication forks using isolation of proteins on nascent DNA (iPOND) coupled with mass spectrometry. J. Biol. Chem 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Rieder SE, Emr SD, Isolation of Subcellular Fractions from the Yeast Saccharomyces cerevisiae. Curr. Protoc. Cell Biol 2000. [DOI] [PubMed] [Google Scholar]
- [203].Härtlova A, Erttmann SF, Raffi FAM, Schmalz AM, et al. , DNA Damage Primes the Type I Interferon System via the Cytosolic DNA Sensor STING to Promote Anti-Microbial Innate Immunity. Immunity 2015. [DOI] [PubMed] [Google Scholar]
- [204].Guan J, Lu C, Jin Q, Lu H, et al. , MLH1 Deficiency-Triggered DNA Hyperexcision by Exonuclease 1 Activates the cGAS-STING Pathway. Cancer Cell 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Schlam-Babayov S, Bensimon A, Harel M, Geiger T, et al. , Phosphoproteomics reveals novel modes of function and inter-relationships among PIKKs in response to genotoxic stress. EMBO J. 2021, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Yoshida S, Yoshida K, Multiple functions of DYRK2 in cancer and tissue development. FEBS Letters 2019. [DOI] [PubMed] [Google Scholar]
- [207].Greer YE, Gao B, Yang Y, Nussenzweig A, Rubin JS, Lack of casein kinase 1 delta promotes genomic instability - The accumulation of DNA damage and down-regulation of checkpoint kinase 1. PLoS One 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Pommier Y, O’Connor MJ, De Bono J, Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Science Translational Medicine 2016. [DOI] [PubMed] [Google Scholar]
- [209].Šalovská B, Janečková H, Fabrik I, Karlíková R, et al. , Radio-sensitizing effects of VE-821 and beyond: Distinct phosphoproteomic and metabolomic changes after ATR inhibition in irradiated MOLT-4 cells. PLoS One 2018, 13, e0199349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Shibata A, Moiani D, Arvai AS, Perry J, et al. , DNA Double-Strand Break Repair Pathway Choice Is Directed by Distinct MRE11 Nuclease Activities. Mol. Cell 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Kumar S, Peng X, Daley J, Yang L, et al. , Inhibition of DNA2 nuclease as a therapeutic strategy targeting replication stress in cancer cells. Oncogenesis 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Maddelein D, Colaert N, Buchanan I, Hulstaert N, et al. , The iceLogo web server and SOAP service for determining protein consensus sequences. Nucleic Acids Res. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Blasius M, Forment JV, Thakkar N, Wagner SA, et al. , A phosphoproteomic screen identifies substrates of the checkpoint kinase Chk1. Genome Biol. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Thada V, Cortez D, Common motifs in ETAA1 and TOPBP1 required for ATR kinase activation. J. Biol. Chem 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Hertz NT, Wang BT, Allen JJ, Zhang C, et al. , Chemical Genetic Approach for Kinase-Substrate Mapping by Covalent Capture of Thiophosphopeptides and Analysis by Mass Spectrometry. Curr. Protoc. Chem. Biol 2010, 2, 15–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Hilger M, Mann M, Triple SILAC to determine stimulus specific interactions in the Wnt pathway. J. Proteome Res 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, et al. , Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics 2002, 1, 376–386. [DOI] [PubMed] [Google Scholar]
- [218].Ong SE, Mann M, A practical recipe for stable isotope labeling by amino acids in cell culture (SILAC). Nat. Protoc 2007, 1, 2650–2660. [DOI] [PubMed] [Google Scholar]
- [219].Baile MG, Guiney EL, Sanford EJ, MacGurn JA, et al. , Activity of a ubiquitin ligase adaptor is regulated by disordered insertions in its arrestin domain. Mol. Biol. Cell 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Kido K, Yamanaka S, Nakano S, Motani K, et al. , Airid, a novel proximity biotinylation enzyme, for analysis of protein–protein interactions. Elife 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Lobingier BT, Hüttenhain R, Eichel K, Miller KB, et al. , An Approach to Spatiotemporally Resolve Protein Interaction Networks in Living Cells. Cell 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Roux KJ, Kim DI, Raida M, Burke B, A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Aebersold R, Agar JN, Amster IJ, Baker MS, et al. , How many human proteoforms are there? Nature Chemical Biology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Dhingra N, Wei L, Zhao X, Replication protein A (RPA) sumoylation positively influences the DNA damage checkpoint response in yeast. J. Biol. Chem 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Abdelmouleh M, Lalande M, El Feghaly J, Vizcaino V, et al. , Mass Spectral Signatures of Complex Post-Translational Modifications in Proteins: A Proof-of-Principle Based on X-ray Irradiated Vancomycin. J. Am. Soc. Mass Spectrom 2020. [DOI] [PubMed] [Google Scholar]
- [226].Schaffer LV, Millikin RJ, Miller RM, Anderson LC, et al. , Identification and Quantification of Proteoforms by Mass Spectrometry. Proteomics 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Giansanti P, Tsiatsiani L, Low TY, Heck AJR, Six alternative proteases for mass spectrometry-based proteomics beyond trypsin. Nat. Protoc 2016. [DOI] [PubMed] [Google Scholar]
- [228].Schwertman P, Bekker-Jensen S, Mailand N, Regulation of DNA double-strand break repair by ubiquitin and ubiquitin-like modifiers. Nature Reviews Molecular Cell Biology 2016. [DOI] [PubMed] [Google Scholar]
- [229].Pellicanò G, Al Mamun M, Jurado-Santiago D, Villa-Hernández S, et al. , Checkpoint-mediated DNA polymerase ε exonuclease activity curbing counteracts resection-driven fork collapse. Mol. Cell 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Deutsch EW, Mendoza L, Shteynberg D, Farrah T, et al. , A guided tour of the Trans-Proteomic Pipeline. Proteomics 2010, 10, 1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]