Abstract
Epithelial ovarian cancer (EOC) is among the top five causes of cancer-related death in women, largely reflecting early, prediagnosis dissemination of malignant cells to the peritoneum. Despite improvements in medical therapies, particularly with the implementation of novel drugs targeting homologous recombination deficiency, the survival rates of patients with EOC remain low. Unlike other neoplasms, EOC remains relatively insensitive to immune checkpoint inhibitors, which is correlated with a tumor microenvironment (TME) characterized by poor infiltration by immune cells and active immunosuppression dominated by immune components with tumor-promoting properties, especially tumor-associated macrophages (TAMs). In recent years, TAMs have attracted interest as potential therapeutic targets by seeking to reverse the immunosuppression in the TME and enhance the clinical efficacy of immunotherapy. Here, we review the key biological features of TAMs that affect tumor progression and their relevance as potential targets for treating EOC. We especially focus on the therapies that might modulate the recruitment, polarization, survival, and functional properties of TAMs in the TME of EOC that can be harnessed to develop superior combinatorial regimens with immunotherapy for the clinical care of patients with EOC.
Keywords: Immunomodulation, Tumor Biomarkers, Immunotherapy, Macrophages, Tumor Microenvironment
Introduction
Ovarian cancer (OC) is the second most common gynecologic cancer in developed countries and the leading cause of gynecologic cancer mortality. Epithelial OC (EOC) accounts for more than 95% of ovarian tumors and high-grade serous ovarian carcinoma (HGSOC) is the most common subtype.1–3 Due to inefficient screening methods for early detection and the absence of specific early warning symptoms, most patients with EOC are diagnosed at an advanced stage, which is characterized by a highly immunosuppressed tumor microenvironment (TME) and distant metastases. The formation of metastatic lesions in HGSOC occurs soon after the primary disease is established and is facilitated by an accumulation of ascites fluid in the peritoneal cavity, which allows the tumor cells to adhere to the omentum and serous membranes lining the peritoneal organs.4
The standard first-line treatment comprises cytoreductive surgery coupled with platinum/taxane doublet-based chemotherapy, which enables complete remission (CR) in the majority of patients. Nonetheless, more than 50% of patients with EOC develop resistance to chemotherapy and eventually experience recurrence.5 6 Over the past decade, several targeted agents have been introduced for the routine clinical management of EOC. These include various poly (ADP-ribose) polymerase inhibitors (PARPi) (eg, niraparib, olaparib, and rucaparib),2 7 which mainly block DNA damage repair (DDR) and DNA replication in cancer cells8 and angiogenesis inhibitors, such as bevacizumab, a monoclonal antibody (mAb) targeting vascular endothelial growth factor (VEGF).9 Homologous recombination DNA-repair pathway defects imposed by germline or somatic BRCA1 or BRCA2 mutations are key determinants of platinum sensitivity in patients with EOC,10 and provide a robust rationale for maintenance therapies based on PARPi.11 As such, maintenance therapy with PARPi has extended progression-free survival (PFS) in patients with advanced EOC that has initially responded to platinum irrespective of HR proficiency.12 However, improved overall survival (OS) is only seen in individuals with BRCA1/2 mutations.7 The combination of olaparib and bevacizumab was recently shown to improve 5-year OS in OC patients with homologous recombination deficiency (HRD) in a phase III PAOLA-1/ENGOT-ov25 trial (NCT02477644). Therefore, developing novel therapeutic strategies alongside with improved understanding of immunocompetent and immunosuppressive components of the TME is of paramount importance to increase the effectiveness of EOC therapy.
Ongoing research into immunotherapeutic strategies, including immune checkpoint inhibitors (ICIs), adoptive cell therapies, and cancer vaccines has changed the field of oncology by putting the host immune response under the spotlight as target for anticancer therapeutic interventions. Notably, ICIs have revolutionized cancer treatment by their enormous success in the clinical management of a wide variety of cancer types.13 However, only a small proportion of patients with EOC respond to ICIs as a stand-alone immunotherapeutic intervention.14 15 The mechanisms of treatment failure in EOC are complex and involve genomic factors, altered metabolism, abnormal neovascularization and, most importantly, robust infiltration of immunosuppressive immune cells into the TME, particularly tumor-associated macrophages (TAMs).16
Here, we review the clinical relevance of TAMs in EOC. We first discuss how the ovarian TME recruits TAMs and modulates their polarization, as well as the mechanisms by which TAMs contribute to the development and progression of EOC. We then summarize the current knowledge on the impact of distinct TAM states and/or TAM-related signatures on the prognosis and response to ICI-based immunotherapy in EOC. Finally, we describe the recent advances of TAM-targeting agents and combinatorial strategies, as well as the rationale for their use in EOC therapy.
Phenotypic and functional diversity of macrophages
Macrophages represent a diverse set of highly plastic mononuclear phagocytic cells which, in response to various microenvironmental stimuli, such as cytokines and chemokines, polarize into distinct phenotypes with specific functionality. Multiple populations of macrophages are known to be present within the same microenvironment and each phenotype has a different combination of expressing receptors and secreting cytokines/chemokines. Their diversity has long been recognized and thus, terms such as the so-called M1- and M2-like phenotypes were introduced to define the possible in vitro polarization extremes.17 18 M1-like macrophages (classically activated, proinflammatory) play a major role in the host defense against infection in the context of TH1 immunity after exposure to proinflammatory cytokines such as interferons (IFNs) and tumor necrosis factor α (TNF-α), toll-like receptor (TLR) ligands, and bacterial products such as lipopolysaccharide. Once activated, they produce proinflammatory cytokines (eg, interleukin (IL)-1α, IL-1β, IL-6, IL-12, IL-23, and TNF-α), generate reactive oxygen species and nitric oxide, and exhibit increased expression of major histocompatibility complex (MHC) class II and costimulatory molecules CD80 and CD86, thereby contributing to the removal of pathogens and tumor cells (table 1).18–21 By contrast, M2-like macrophages (alternatively activated, anti-inflammatory), which are induced by immunoregulatory cytokines (eg, IL-4, IL-10, IL-13, and transforming growth factor β (TGF-β)), glucocorticoids, or colony-stimulating factor 1 (CSF1), mainly support TH2-related tissue repair, remodeling, and tumor promoting processes mediated by IL-10, TGF-β, prostaglandin E2, VEGF, matrix metalloproteinases (MMPs), and arginase 1 (ARG1) secretion (table 1).17 19 22
Table 1.
The characteristics of M1-like versus M2-like macrophages
| M1-like macrophages | M2-like macrophages | |
| Inducers | IFN-γ, TNF-α, TLR ligands, bacterial products (such as LPS) | IL-4, IL-10, IL-13, TGF-β, CSF1, PGE2, glucocorticoids |
| Phenotypic markers | HLA-DR, CD80, CD86 | CD163, CD204, CD206 |
| Secreted/produced molecules | IL-1α, IL-1β, IL-6, IL-12, IL-23, TNF-α, iNOS, ROS | IL-10, TGF-β, PGE2, VEGF, MMPs, ARG1 |
| Metabolism | Highly glycolytic—dependent on the stabilization of HIF-1α; increased generation of lactic acid | Glycolysis is dispensable when OXPHOS is intact |
| ↑PPP flux → ↑NADPH production → generation of NO and ROS; lipid biosynthesis | ↓PPP flux | |
| ↑lipid synthesis → membrane biogenesis, granule formation | ↑FAO | |
| Impaired TCA cycle → accumulation of citrate and succinate → fatty acid synthesis and generation of inflammatory effector molecules—for example, NO | Intact TCA cycle driven by FAO and glutamine catabolism | |
| Impaired OXPHOS and ETC → ↑ROS generation | ↑OXPHOS and mitochondrial biogenesis | |
| ↑iNOS expression → arginine is preferentially catabolized into NO | ↑ARG1 expression → arginine is preferentially catabolized into ornithine and urea → production of polyamines and proline → cell growth and collagen synthesis | |
| Functions | Proinflammatory, pathogen clearance, antitumor properties | Anti-inflammatory, tissue repair and remodeling, protumorigenic properties—immunosuppression, angiogenesis, tumor cell invasion and metastasis |
CSF1, colony-stimulating factor 1; ETC, electron transport chain; FAO, fatty acid oxidation; HIF-1α, hypoxia-inducible factor 1α; HLA-DR, human leukocyte antigen-DR; IFN, interferon; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; MMP, matrix metalloproteinase; NADPH, nicotinamide adenine dinucleotide phosphate; NO, nitric oxide; OXPHOS, oxidative phosphorylation; PGE2, prostaglandin E2; PPP, pentose phosphate pathway; ROS, reactive oxygen species; TCA cycle, tricarboxylic acid cycle; TLR, toll-like receptor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
However, this binary classification is greatly oversimplified because the polarization/activation of macrophages in vivo in the local tissue microenvironment is far more complex process, as they are exposed to M1 and M2 signals of different origins and context. Thus, macrophages found in the TME (ie, TAMs) may display a spectrum of phenotypes with a mixture of proinflammatory and alternatively activated macrophages, coexpressing M1 and M2 gene signatures,18 23 24 or may show expression patterns distinct from the M1/M2 states, such as FABP5- and APOE-expressing macrophages, recently identified in the TME of breast cancer. These two populations bear close transcriptomic similarity to a population of lipid-associated macrophages (LAMs).25 Nevertheless, it seems that during early carcinogenesis, TAMs exhibit a higher degree of similarity to M1-like subtypes and in later stages the majority of tumors, including EOC, recruit macrophages with M2-like phenotypes possessing low tumoricidal activity and high potential to promote immunosuppression, tumor cell invasion, angiogenesis and metastasis.26–31
Given the fact, that most of the results discussed in this review, have been obtained based on the binary M1/M2 classification, a critical view is necessary for proper assessment of the impact of TAMs on the TME and every aspect of tumor growth and progression in EOC.
Role of TAMs in the development and progression of EOC
Recruitment and polarization of TAMs in EOC
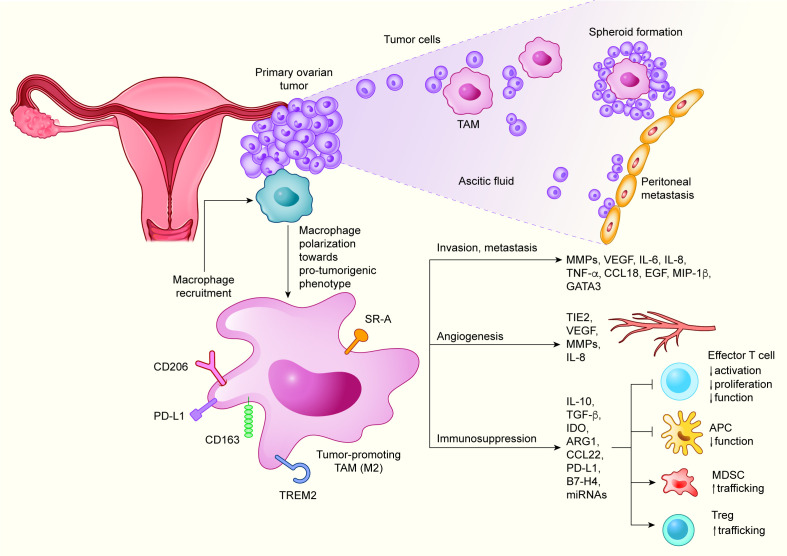
TAMs can originate from tissue-resident macrophages developed from embryonic precursors (eg, fetal yolk sac progenitors), residing in the majority of adult organs, including ovaries, and from monocytes derived from bone-marrow hematopoietic cell progenitors. Compared with physiological conditions, EOC development is characterized by increased monocyte recruitment and/or expansion of tissue-resident macrophages with both populations involved in tumorigenesis.32 33 In EOC, TAMs are the most predominant population of immune cells, constituting up to 39% of total immune cell infiltrate.29 31 34–38 In addition, data from The Cancer Immunome Atlas showed that the majority (51%) of these TAMs display M2-like phenotype.34 TAMs in EOC are not only localized in the TME of primary tumor, but are also abundantly present in ascitic fluid and metastases (eg, omental). Experimental mouse models have demonstrated that TAMs constitute a major cell fraction in intraperitoneal milieu/malignant ascites which play pivotal role in the transcoelomic dissemination of ovarian tumor cells by supporting their survival and invasiveness.39 40 In addition, TAMs are frequently found in the omentum, the most common site of metastases, in areas called milky spots, where they facilitate omental colonization by ovarian tumor cells.41 Various chemokines, cytokines and growth factors have been implicated in the recruitment of suppressive monocytes and macrophages to the ovarian TME, including IL-6, leukemia inhibitory factor, chemokine (C-C motif) ligand 2 (CCL2; also known as monocyte chemoattractant protein 1), CSF1, TNF, CCL22, C-X-C motif chemokine ligand 12 (CXCL12), VEGF, periostin, and semaphorin 4D.26 42–47
The TAM compartment undergoes extensive remodeling of core energy metabolism during tumor progression (table 1).48–50 In hypoxic TMEs, including the TME in EOC, the cells prefer to use glycolytic metabolism. The accumulation of lactic acid in TAMs and other cells present in the TME that use glycolysis results in the differentiation of TAMs into cells characterized by a tumor-promoting phenotype.48 51 52 Because TAM polarization can be altered by lipid metabolism, the expression of 5-lipoxygenase (5-LOX) and upregulation of 5-LOX metabolites in EOC cells are associated with TAM recruitment into hypoxic areas of the ovarian TME in a process mediated by increased MMP-7 expression.53 Moreover, lipidomic analysis of EOC-associated ascites revealed high concentrations of polyunsaturated fatty acids (PUFAs), particularly linoleic acid, which are potent inducers of peroxisome proliferator-activated receptor β/δ in TAMs. Fatty acids accumulate in lipid droplets in TAMs and contribute to their protumorigenic polarization.54 In similar line, results from mouse model of metastatic EOC showed that ovarian tumor cells can promote membrane-cholesterol efflux and depletion of lipid rafts from TAMs. Increased cholesterol efflux promote IL-4-mediated reprogramming of macrophages, including inhibition of IFN-γ-induced gene expression.55 Moreover, the depletion of glutamine, due to its increased consumption by EOC cells, causes upregulation of glutamine synthetase expression in TAMs and promotes their polarization toward an immunosuppressive, proangiogenic, and prometastatic M2-like phenotype.56 57 Overall, it has become clear that TAMs are regulated by the dynamic nature of the TME, which drives changes in their functional phenotypes and distribution as a response to tissue-specific and tumor-specific stimuli. As the tumor grows, the stimuli in the TME alter, eliciting changes in TAM infiltration and polarization.
TAM-mediated immunosuppression in the ovarian TME
TAMs suppress antitumor immune responses in ovarian TME via multiple mechanisms (figure 1). Perhaps the most studied of these mechanisms is the production of cytokines and chemokines, which inhibit the antitumor properties of immunocompetent immune cells, including cytotoxic T cells (CTLs) and antigen-presenting cells (APCs), while supporting the immunosuppressive immune components, such as regulatory T cells (Tregs). For instance, CCL22 produced by TAMs enables the trafficking of Tregs and myeloid-derived suppressor cells, leading to their accumulation in the ovarian TME.58 In a mouse model of lung carcinoma, TAM-derived IL-10 was shown to inhibit the functional capacity of APCs by downregulating the expression of IL-12 and costimulatory molecules.59 M2-like TAMs found in EOC and other solid tumors, such as melanoma, lung carcinoma and multiple myeloma, suppress effector T cells via increased secretion of TGF-β60 and by depleting the amino acids essential for T cell activation, such as tryptophan and L-arginine, reflecting the high expression of the catabolic enzymes indoleamine 2,3-dioxygenase (IDO)61 62 and ARG163 64 by TAMs. Interestingly, exosomes containing ARG1 found in ascites and plasma of EOC patients were found to inhibit the proliferation of CD4+ and CD8+ T cells by distributing this enzyme from tumor cells to APCs in secondary lymphoid organs.65
Figure 1.
Roles of TAMs in the TME of EOC. Ovarian TME drives the polarization of TAMs predominantly toward M2-like phenotype. M2-like TAMs display limited tumoricidal activity and promote immunosuppression, tumor cell invasion and metastasis, and angiogenesis, by producing a variety of cytokines, chemokines, growth factors and other molecules. APC, antigen-presenting cell; ARG1, arginase 1; CCL, chemokine (C-C motif) ligand; EGF, epidermal growth factor; EOC, epithelial ovarian carcinoma; GATA3, GATA binding protein 3; IDO, indoleamine 2,3-dioxygenase; IL, interleukin; MDSC, myeloid-derived suppressor cell; MIP-1β, macrophage inflammatory protein 1β; miRNA, microRNA; MMP, matrix metalloproteinase; PD-L1, programmed death-ligand 1; SR-A, scavenger receptor A; TAM, tumor-associated macrophage; TGF-β, transforming growth factor β; TME, tumor microenvironment; TNF-α, tumor necrosis factor α; Treg, regulatory T cell; TREM2, triggering receptor expressed on myeloid cells 2.
The functional impairment of T cell responses by TAMs can also be mediated by direct cell–cell interactions via immune checkpoint receptors and their ligands. Thus, programmed death-ligand 1 (PD-L1), a ligand of the inhibitory checkpoint receptor PD-1, and the coinhibitory molecule B7-H4, which are expressed at higher levels on TAMs than on EOC cells, induce T cell exhaustion and inactivation of cytotoxic T cell responses.66–68 Recently, it was demonstrated that miRNAs (miR-29a-3p and miR-21–5 p) transferred from TAMs to CD4+ T cells through exosomes synergistically induce imbalance of Treg/TH17 ratio in EOC TME by targeting signal transducer and activator of transcription 3 in CD4+ T cells.69 Taken together, these effects of TAMs on antitumor immune responses strongly foster the immunosuppressive TME in EOC.
Role of TAMs in angiogenesis, invasion and metastasis
Neoangiogenesis, a highly complex process, is crucial for tumor growth, metastasis, and immune evasion. TAMs are emerging as major promoters of angiogenesis in EOC, as in other cancers.26 70 Thus, increased microvessel density and vascular permeability in EOC were correlated with increased intratumoral density of TAMs, Tregs, and TH17 cells.71 In this context, reducing the infiltration of protumorigenic M2-like TAMs and blocking their function by CSF1 receptor (CSF1R) inhibitors, led to the normalization of disrupted peritoneal vasculature and a decrease in ascites volume.72 The tumor foci, ascites, and peripheral blood of EOC patients also contain high frequencies of proangiogenic TAMs characterized by the expression of TIE2, a tyrosine kinase receptor. The proportion of TIE2+ TAMs was positively correlated with increased microvessel density. Supporting this notion, functional studies revealed that angiopoietin-2 (Ang2), a ligand of TIE2, enhanced the recruitment of TIE2+ TAMs in the TME. Recruited TAMs promoted angiogenesis via insulin-like growth factor 1 signaling pathway.73 TAMs are also involved in the regulation of EOC angiogenesis by producing a variety of proangiogenic factors, including VEGF, platelet-derived growth factor, epidermal growth factor (EGF), MMPs, osteopontin, osteonectin, cathepsins, and fibronectin.74 Supporting this notion, high VEGF expression in ovarian primary tumors and peritoneal ascites was associated with poor disease outcome.75 76 In addition to its direct effects on tumor cells, VEGF sustains a permissive environment supporting metastasis of EOC cells by acting on peritoneal ECs to promote angiogenesis and vascular permeability, leading to ascites and cyst formation in EOC xenograft models.77 78
TAMs promote extracellular matrix (ECM) remodeling by secreting MMPs, a process that contributes to the invasion of multiple tumor cell types, including EOC cells.26 79 By producing metastasis-promoting cytokines and chemokines (eg, IL-6, TNF-α, CCL6, and CCL18), TAMs play a central role in the EOC cytokine network supporting the adhesion and invasion of EOC cells.41 80 For instance, TAM-derived TNF-α enhances the invasiveness of ovarian and breast tumor cells by upregulating macrophage migration inhibitory factor, extracellular MMP inducer, and MMP secretion.79 In addition, TAMs found in the omentum and malignant ascites of EOC patients sustain metastasis dissemination by secreting CCL641 and CCL18.81 Interestingly, CCL6 and its human homolog CCL23 promote EOC cell migration into the omentum by activating CCR1 signaling.41 There is also strong evidence that the formation of metastases in EOC is facilitated by inflammation through a mechanism largely mediated by TAMs, presumably involving stromal VEGF production. Thus, depletion of peritoneal TAMs was shown to reduce ascites formation, peritoneal metastasis, and tumor progression.82 Moreover, macrophage inflammatory protein 1β, which is secreted by TAMs via the CCR5-phosphatidylinositol 3-kinase (PI3K) signaling pathway, mediates the upregulation of P-selectin expression on mesothelial cells resulting in an increased rolling under ascites flow and adhesion between EOC cells and mesothelial cells.83
Additional mechanism by which TAMs support malignant cell dissemination and metastasis is mediated by the formation of primary tumor cell-TAM spheroids. During EOC progression, tumor cells detach from the primary tumor and interact with TAMs to survive in the peritoneal fluid as free-floating spheroids. In an EOC mouse model of early planting metastasis, it was observed that 80% of infiltrating macrophages were located in the peritoneal spheroids. These TAMs displayed a predominantly M2-like phenotype providing matrix support and growth factors to tumor cells. In this model TAM-secreted EGF activated the EGF receptor (EGFR) in surrounding tumor cells, thereby supporting their proliferation and protection against anoikis. TAM–tumor cell crosstalk induced autocrine VEGFC–VEGFR3 signaling in neoplastic cells, which further upregulated αMβ2 integrin and intercellular adhesion molecule 1 expression, and thus maintained cell-to-cell contacts and stabilized tumor spheroids.40 Taken together, TAMs employ multiple strategies to promote invasion and metastasis of EOC, representing potential targets for promising EOC therapy.
Clinical relevance of TAMs in EOC
Impact of TAMs on EOC prognosis
TAM polarization has a pronounced effect on survival and the response to therapy in EOC patients. A high M1-like/M2-like TAM ratio is associated with favorable disease outcomes in EOC, whereas a low M1-like/M2-like TAM ratio is correlated with poor OS.38 84–87 M1-like TAMs, defined as CD86+human leukocyte antigen-DR+inducible nitric oxide synthase+ cells, are associated with good prognosis in women with EOC, largely reflecting their ability to promote robust inflammatory responses that limit disease progression.88–90 Conversely, CD163+CD206+CD204+ M2-like TAMs promote disease progression and their frequency increases with tumor stage, ascites volume, lymphatic invasion, and metastasis.31 88 89 M2-like CD68+CD163+ TAMs are highly enriched in the metastatic TME of HGSOC, where they dictate clinically relevant immunosuppression, which is correlated with a poor clinical outcome.35 Interestingly, the poor prognosis of HGSOC patients was recently linked to the presence of CD68+CD163+ TAMs surrounding dying or dead adipocytes within the adipose tissue of the omentum, forming a ‘crown-like structure’.91 In addition, the expression of CD163 in TAMs is correlated with elevated IL-6, IL-10, TGF-β and PUFA levels, which mediate protumor signaling and negatively affect the prognosis in EOC.80 92–94 Similarly, the upregulation of PD-L1 on TAMs is associated with higher disease grade and reduced survival of EOC patients.66 67 95
However, traditionally used histological approaches and flow cytometry rely on a limited set of markers to classify TAMs. Thus, defining TAM-related gene signatures and/or their functions that are involved in tumor progression or regression could be a more sophisticated way to reveal clinically relevant TAM states. For instance, protumorigenic TAM gene signatures encompassing high expression of SLAM7, GNAS, TBX2-AS1, and LYPD6,96 CD163, PCOLCE2, and IL6,97 or CD163, VSIG4 and MS4A798 were correlated with poor prognosis of patients with EOC. Similarly, an analysis of single-cell transcriptomic data in multiple tumor types, including EOC, identified SPP1-expressing TAM state, which upregulates proangiogenic M2-associated genes, to be linked with worse prognosis.23 By contrast, the upregulation of genes linked to IFN signaling99 or other TAM-related genes, including CD3E, IGKV4 and TAP1,98 was associated with favorable clinical outcome in EOC. Intriguingly, a detailed transcriptomic and proteomic analyses of TAMs isolated from EOC patient ascites showed that TAMs found in the patients with predicted short survival selectively expressed/produced protumorigenic growth factors and cytokines (CCL18, KITLG, SEMA6B, S100B) and mediators involved in ECM remodeling (ADAMTS2, CTSB, FBLN5) and angiogenesis (VEGFB), whereas TAM associated with longer survival expressed cytokines linked to effector T cell chemoattraction and activation.100
Association between TAMs and the outcomes of immunotherapy
Over the past decade, various ICIs targeting cytotoxic T-lymphocyte antigen 4 (CTLA-4), PD-1, or PD-L1 have been introduced for the treatment of various cancers.13 However, despite expectations, EOC is one of the few malignancies where ICIs exhibit only modest activity with an objective response rate (ORR) of 8%–9%, and infrequent durable responses.14 15 The basis of ICI therapy failure in EOC involves multiple mechanisms, including low tumor mutational burden (TMB), abnormal neovascularization, altered metabolism, failure to reverse T cell exhaustion, and robust humoral and cellular immunosuppression in the TME.16 The abundance and phenotype of different populations of myeloid cells, particularly TAMs, are critical determinants of the primary and adaptive resistance to ICIs in a broad range of solid tumors.101 102 TAMs confer resistance through direct and indirect effects on T cell effector functions by altering the cytokine/chemokine milieu, as well as by upregulating coinhibitory molecules.101 Interestingly, TAMs were found to limit the efficacy of ICIs in a mouse model of colon adenocarcinoma by capturing anti-PD-1 mAbs from the surface of PD-1+ T cells, a process dependent on the interaction between the antibody Fc-domain glycans and FcγRs on TAMs.103
Considering TMB and PD-L1 expression as not completely reliable predictors of ICI therapy outcome in EOC,104 intensive preclinical and clinical research is now focusing on the identification of better immune biomarkers to integrate into common diagnostic assessments and clinical management of EOC patients. For instance, a density of tumor-infiltrating CD8+ T cells predicts clinical benefit to anti-PD-L1 (avelumab) combined with pegylated liposomal doxorubicin (PLD) in OC patients.105 Similarly, CXCR5+ CD8+ T cells,106 known as follicular cytotoxic T cells, and CXCL13+TIM-3+CD103+ tissue-resident memory CD8+ T cells107 can be linked to better response of EOC patients to anti-PD-1 therapy. Recently, HRD and type I IFN signaling pathways were shown to determine a positive response to combined anti-PD-1 and PARPi therapy in patients with EOC. Further spatial single-cell analyses have revealed prominent interactions between exhausted CD8+ T cells and PD-L1+ TAMs and/or tumor cells as mechanistic determinants of an improved response. Detailed analyses of a sample from one extreme responder revealed that the tumor was enriched with PD-L1highCD163+IBA1+CD11b+ TAMs in a tight cluster with CD8+ T cells. These findings suggest that the interaction between TAMs and exhausted CD8+ T cells might be the most relevant cell–cell interaction in PD-1/PD-L1-mediated immunosuppression.108 In a similar context, M2-like TAMs and a TGF-β signaling pathway signature are correlated with poor responses to ICIs in gynecologic malignancies, including EOC. This may indicate a dynamic interplay between TGF-β and TAMs that suppresses the development of M1-like TAMs and preferentially induces M2-like TAMs thus amplifying the immunosuppression.109
Taken together, these results indicate a crucial impact of TAMs on the final response to immunotherapeutic interventions in EOC. However, the detailed mechanisms by which TAMs affect the outcomes of ICI therapy and the potential predictive signatures require further elucidation. Results of future studies will support the development of clinically viable strategies that modulate the TAM phenotype and functions to enhance the sensitivity of EOC to ICI therapy.
Targeting TAMs for successful immunotherapy of EOC
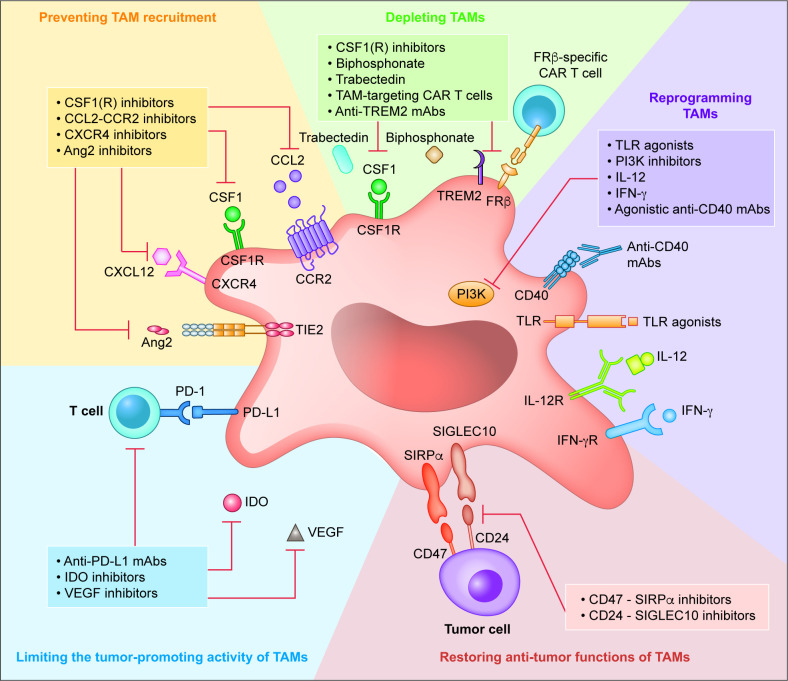
Accumulating preclinical and clinical studies are improving our understanding of the role of TAMs in tumor progression and resistance to various therapies. Thus, TAMs have been intensively explored as potential targets for cancer therapy and/or combined immunotherapy to improve the clinical outcomes of patients with EOC. The most intensively investigated TAM-targeting strategies include: (1) preventing macrophage recruitment to the TME, (2) depleting TAMs/reducing their survival, (3) TAM reprogramming/repolarization, (4) restoring the antitumor functions of TAMs, and (5) limiting the tumor-promoting activity of TAMs (figure 2).
Figure 2.
TAM targets for anticancer therapy. Schematic representation of the most intensively investigated TAM-targeting strategies in EOC. Ang2, angiopoietin-2; CAR, chimeric antigen receptor; CCL2, chemokine (C-C motif) ligand 2; CCR2, chemokine (C-C motif) receptor 2; CSF1, colony-stimulating factor 1; CSF1R, colony-stimulating factor one receptor; CXCL12, C-X-C motif chemokine ligand 10; EOC, epithelial ovarian carcinoma; FRβ, folate receptor β; IDO, indoleamine 2,3-dioxygenase; IFN-γ, interferon γ; IFN-γR, interferon γ receptor; IL-12, interleukin 12; IL-12R, interleukin 12 receptor; mAb, monoclonal antibody; PD-1, programmed cell death 1; PD-L1, programmed death-ligand 1; PI3K, phosphatidylinositol 3-kinase; SIGLEC10, sialic acid-binding Ig-like lectin 10; SIRPα, signal regulatory protein α; TAM, tumor-associated macrophage; TLR, toll-like receptor; TREM2, triggering receptor expressed on myeloid cells 2; VEGF, vascular endothelial growth factor
Preventing TAM recruitment
CSF1–CSF1R inhibitors
Various chemokines, cytokines, and other factors act as chemoattractants to recruit macrophages into the ovarian TME. Thus, regulating chemoattractants is a promising approach for reducing tumor infiltration of TAMs. Overexpression of CSF1 and its receptor CSF1R is associated with poor prognosis of EOC and thus represent attractive therapeutic targets.110 Activation of the CSF1–CSF1R signaling pathway promotes the production and proliferation of macrophage precursors and/or their recruitment and retention within inflamed sites, including tumors.111 Blocking the CSF1–CSF1R axis with mAbs or tyrosine kinase inhibitors can either reduce TAM numbers or alter their tumor-promoting features and thus reduce tumor progression in numerous experimental models (eg, lung carcinoma, glioma and fibrosarcoma).112–114 Administration of GW2580, a CSF1R inhibitor (CSF1Ri), reduced the infiltration of M2-like TAMs, increased the proportion of M1-like TAMs expressing CCR2, IL-12, and IFN-γ, and increased the CD8+/CD4+ T cell ratio accompanied by tumor and ascites regression in a mouse model of advanced EOC.72
Several drugs targeting CSF1 or CSF1R have been or are being tested in clinical trials for treatment of EOC. These drugs include antagonistic mAbs (such as emactuzumab, cabiralizumab and LY3022855) and small-molecule inhibitor, pexidartinib (table 2). Due to their limited efficacy as monotherapy, the current studies are evaluating the combinations with standard of care (SoC) chemotherapy and/or ICIs. In a phase I study, emactuzumab alone or in combination with SoC chemotherapy, specifically reduced immunosuppressive TAMs in patients with advanced solid tumors, including EOC. However, this approach did not elicit clinically relevant antitumor activity.115 Other clinical trials of CSF1R-targeting agents in EOC have used emactuzumab combined with a CD40 agonist (selicrelumab) (NCT02760797),116 LY3022855 combined with anti-PD-L1 mAb (durvalumab) or anti-CTLA-4 mAb (tremelimumab) (NCT02718911),117 and a small-molecule CSF1Ri (pexidartinib, PLX3397) combined with paclitaxel (NCT01525602) or anti-PD-1 mAb (pembrolizumab) (NCT02452424) (table 2). Although, these drugs are well tolerated, the clinical benefits in EOC patients were limited potentially due to indiscriminate ablation of TAMs which might lead to detrimental effects through depletion of proinflammatory TAMs or due to subsequent accumulation of other tumor promoting immune cells such as tumor-associated neutrophils.118 The results of ongoing clinical trials evaluating emactuzumab combined with SoC chemotherapy and bevacizumab in patients with platinum-resistant EOC (NCT02923739), and cabiralizumab combined with an anti-PD-1 mAb (nivolumab) in patients with advanced solid tumors (NCT02526017) are highly anticipated.
Table 2.
Clinical trials of agents depleting TAMs or preventing their recruitment
| Target | Agent | Mechanism of action | Combination partners | Phase | Status | Results | Identifier | Ref. |
| CSF1R | Emactuzumab | antagonistic anti-CSF1R mAb | Monotherapy; paclitaxel | I | Completed | ↓immunosuppressive TAMs; no clinically relevant antitumor activity | NCT01494688 | 115 |
| Paclitaxel+anti-VEGF-A mAb bevacizumab | II | Active | – | NCT02923739 | – | |||
| Agonistic anti-CD40 mAb selicrelumab | I | Completed | ↓CD14dimCD16bright monocytes; ↑activated Ki67+CD8+T cells; limited objective clinical responses | NCT02760797 | 116 | |||
| Cabiralizumab | Antagonistic anti-CSF1R mAb | Anti-PD-1 mAb nivolumab | I | Completed | – | NCT02526017 | – | |
| LY3022855 | Antagonistic anti-CSF1R mAb | Anti-PD-L1 mAb durvalumab; anti-CTLA-4 mAb tremelimumab | I | Completed | Limited clinical activity | NCT02718911 | 117 | |
| Pexidartinib | Inhibitor of CSF1R tyrosine kinase activity | Paclitaxel | I | Completed | – | NCT01525602 | – | |
| (PLX3397) | ||||||||
| Pembrolizumab | I/II | Terminated | Insufficient evidence of clinical efficacy | NCT02452424 | – | |||
| CCL2 | Carlumab (CNTO888) | Anti-CCL2 mAb neutralizing CCL2-induced chemotaxis | Monotherapy | I | Completed | Evidence of antitumor activity | NCT00537368 | 123 |
| SoC chemotherapy | I | Completed | Limited tumor responses | NCT01204996 | 122 | |||
| Ang2 | MEDI3617 | Anti-Ang2 mAb inhibiting Ang2 binding to TIE2 | Monotherapy; paclitaxel; paclitaxel+carboplatin; bevacizumab | I | Completed | Limited clinical activity | NCT01248949 | 124 |
| Trebananib (AMG-386) | Anti-Ang peptibody inhibiting Ang1/2 binding to TIE2 | Paclitaxel+carboplatin | III | Terminated | No improvement in PFS | NCT01493505 | 125 | |
| Pembrolizumab | I | Active | – | NCT03239145 | – | |||
| VEGF/ Ang2 | Vanucizumab (RG7221) | Anti-VEGF/Ang2 bispecific mAb inhibiting neoangiogenesis | Monotherapy; anti-PD-L1 mAb atezolizumab | I | Completed | Reduced tumor vascularity; encouraging antitumor activity | NCT01688206 | 126 |
| TREM2 | PY314 | Anti-TREM2 mAb depleting TREM2+ TAMs through ADCC and/or ADCP | Monotherapy; pembrolizumab | I | Recruiting | – | NCT04691375 | – |
ADCC, antibody-dependent cellular cytotoxicity; ADCP, antibody-dependent cellular phagocytosis; CCL2, C-C motif chemokine ligand 2; CSF1R, colony-stimulating factor one receptor; CTLA-4, cytotoxic T-lymphocyte antigen 4; mAb, monoclonal antibody; PD-1, programmed cell death 1; PD-L1, programmed death-ligand 1; PFS, progression-free survival; SoC, standard of care; TAM, tumor-associated macrophage; TREM2, triggering receptor expressed on myeloid cells 2; VEGF, vascular endothelial growth factor.
CCL2–CCR2 inhibitors
The accumulation of macrophages in tumors is also mediated by the CCL2–CCR2 signaling pathway. CCR2 expressed on circulating inflammatory monocytes binds to CCL2 produced by malignant and stromal cells promoting monocyte differentiation into TAMs on extravasation into the tumor stroma.119 Inhibition of CCL2–CCR2 signaling limits the accumulation of inflammatory monocytes in tumors and delays tumor progression and metastasis in multiple mouse experimental models (eg, breast, prostate and colorectal cancer).119 120 Because EOC cells can release CCL245 and some paclitaxel-resistant EOC cell lines exhibit high CCL2 expression,121 targeting CCL2–CCR2 axis might represent a promising strategy to deplete TAMs in EOC. Two phase I trials have since investigated the effects of an anti-CCL2 mAb (carlumab, CNTO888) as monotherapy or combined with SoC chemotherapy in patients with solid tumors, including advanced EOC. Despite a favorable safety profile, the objective clinical responses were limited (NCT00537368 and NCT01204996)122 123 (table 2).
Ang2–TIE2 inhibitors
Besides chemokines and other soluble factors directly involved in TAM infiltration into the TME, TIE2+ monocytes are recruited into the tumor via the growth factor Ang2, which further enhances the proangiogenic activities of TAMs in EOC.73 However, in clinical studies, a mAb targeting Ang2 (MEDI3617) (NCT01248949)124 or an anti-angiopoietin peptibody (trebananib, AMG-386) (NCT01493505)125 in combination with bevacizumab and/or SoC chemotherapy did not improve the disease outcomes in patients with advanced EOC. Nevertheless, a bispecific mAb targeting both VEGF and Ang2 (vanucizumab, RG7221/RO5520985) reduced tumor vascularity and displayed encouraging anticancer activity in an early phase clinical trial in patients with advanced solid tumors, including EOC (NCT01688206)126 (table 2). These findings provide a rationale for simultaneous targeting of Ang2 and VEGF in EOC.
Depleting TAMs
Beyond mAbs and tyrosine kinase inhibitors targeting CSF1 or CSF1R, TAMs can be directly depleted by pharmacological agents, including bisphosphonates (eg, clodronate and alendronic acid) and trabectedin. Besides their direct antitumor effects,127 these agents also modulate the immune contexture in the TME. For instance, clodronate reduces the numbers of TAMs by inhibiting the secretion of proangiogenic cytokines by ECs in syngeneic murine models of EOC128 and trabectedin has the capacity to promote caspase-8-dependent apoptosis in mononuclear phagocytes.129 However, these pan-macrophage therapeutic approaches may limit critical proinflammatory responses and/or cause systemic toxicities. Thus, it is necessary to develop more specific agents that re-educate the TME by restraining tumor-promoting M2-like TAMs while promoting antitumor M1-like TAMs for clinical testing. Recent technological advances have aided the generation of chimeric antigen receptor (CAR) T cells targeting specific immunosuppressive TAM subsets. For instance, CAR T cells specific for folate receptor β (FRβ) can recognize and lyse FRβ+ M2-like TAMs, as demonstrated in solid tumors, including EOC. Importantly, this approach reprograms the TME by promoting endogenous antitumor T cell-mediated immunity and tumor regression in murine tumor models.130
Recently, transmembrane protein triggering receptor expressed on myeloid cells 2 (TREM2) has emerged as an ideal candidate for promoting M2-like TAM depletion in EOC.131 132 TREM2+ TAMs display enhanced capacity to produce clinically relevant immunosuppressive factors in EOC patients. In addition, TREM2 expression is linked to immune cell exhaustion and resistance to anti-PD-1 therapies in murine models of ovarian, breast and colon cancer.131 132 Consistent with this notion, anti-TREM2 mAbs were shown to deplete TAMs, potentiate the activation of intratumoral CD8+ T cells, and reverse anti-PD-1 therapy resistance in the above-mentioned experimental models.131 132 Based on promising preclinical findings, humanized anti-TREM2 mAb (PY314) is currently being tested in a phase I clinical trial in patients with advanced solid tumors, including EOC (NCT04691375) (table 2).
Reprogramming TAMs
TLR agonists
Reprogramming immunosuppressive TAMs into immunostimulatory TAMs is rapidly emerging as a new therapeutic approach. One such strategy involves activating TLRs expressed by macrophages and other myeloid cells by TLR agonists to trigger potent inflammatory reactions, including the generation of TAMs with M1-like phenotypes, which could protect the host against infections and promote antitumor immunity.133 TLR7 (852A, NCT00319748) and TLR8 (motolimod (VTX-2337), NCT01294293 and NCT01666444) agonists have since been evaluated in clinical trials in patients with solid cancers, including advanced EOC (table 3). TLR agonists demonstrated considerable potency in patients with bladder, superficial basal cell carcinoma, and cervical cancer, which led to their approval by the United States Food and Drug Administration as anticancer agents.134 However, 852A and motolimod displayed limited clinical activity in EOC in two clinical trials (NCT00319748 and NCT01666444, respectively).135 136 Nevertheless, motolimod induced clinically relevant immunomodulation of the TME in patients with injection site reactions (NCT01666444).136 In addition, vidutolimod (CMP-001), a virus-like particle-encapsulated TLR9 agonist, is currently being tested in combination with an anti-PD-L1 mAb (avelumab) in patients with advanced or metastatic solid tumors, including platinum-resistant EOC, in a phase II clinical trial (NCT02554812) (table 3). To improve the pharmacokinetic profiles and reduce the risk of severe side effects, ongoing preclinical studies are focusing on specific and targeted delivery of TLR agonists.137
Table 3.
Clinical trials of TAM reprogrammingagents in EOC
| Target | Agent | Mechanism of action | Combination partners | Phase | Status | Results | Identifier | Ref. |
| TLR7 | 852A | TLR7 agonist inducing the activation of innate immune cell populations | Monotherapy | II | Completed | ↑immune cell activation - ↑CXCL10↑IL-1ra; modest clinical benefit | NCT00319748 | 135 |
| TLR8 | Motolimod | TLR8 agonist inducing the activation of innate immune cell populations | PLD; paclitaxel | I | Completed | – | NCT01294293 | – |
| PLD | II | Completed | Improved OS in patients with ISRs | NCT01666444 | 136 | |||
| TLR9 | Vidutolimod (CMP-001) | VLP-encapsulated TLR9 agonist | Anti-PD-L1 mAb avelumab; avelumab+anti-4-1BB mAb utomilumab; avelumab+anti-OX40 mAb | II | Active | – | NCT02554812 | – |
| PI3Kγ | Eganelisib (IPI-549) | Selective PI3Kγ inhibitor reprogramming key immune suppressive cells | Monotherapy; nivolumab | I | Active | – | NCT02637531 | – |
| Dual adenosine receptor antagonist etrumadenant (AB928)+PLD | I | Completed | – | NCT03719326 | – | |||
| CD40 | Selicrelumab | Agonistic anti-CD40 mAb potentiating APC functions | Paclitaxel+carboplatin | I | Completed | Evidence of antitumor activity with 20% of patients displaying PRs | NCT00607048 | 146 |
| Bevacizumab; bevacizumab+vanucizumab | I | Completed | – | NCT02665416 | – | |||
| CDX-1140 | Agonistic anti-CD40 mAb potentiating APC functions | Monotherapy; gemcitabine+paclitaxel; pembrolizumab or FLT3L | I | Active | – | NCT03329950 | – | |
| Pembrolizumab+bevacizumab | II | Not yet recruiting | – | NCT05231122 | – | |||
| IL-12 | GEN-1 | IL-12 plasmid/lipopolymer complex stimulating the antitumor immune responses | Neoadjuvant paclitaxel+carboplatin | I | Completed | ↑IL-12, IFN-γ; ↑CD8+ T cells and ↓immunosuppression in the TME; preliminary clinical activity | NCT02480374 | 155 |
| Neoadjuvant paclitaxel+carboplatin | II | Recruiting | – | NCT03393884 | – | |||
| HER2 | CT-0508 | CAR macrophages targeting HER2+ tumor cells | Monotherapy | I | Recruiting | – | NCT04660929 | – |
| CD47 | AO-176 | Antagonistic anti-CD47 mAb promoting phagocytosis and direct tumor cell killing | Monotherapy; paclitaxel; pembrolizumab | I/II | Recruiting | – | NCT03834948 | – |
| Magrolimab (Hu5F9-G4) | Antagonistic anti-CD47 mAb promoting phagocytosis and direct tumor cell killing | Monotherapy | I | Completed | PRs in 2 out of 13 OC patients; ↓CA125 | NCT02216409 | 167 | |
| Avelumab | I | Completed | – | NCT03558139 | – | |||
| Anti-EGFR mAb cetuximab | I/II | Completed | ↑TAM infiltration; disease stabilization in some patients | NCT02953782 | 166 | |||
| TTI-622 | Fusion protein blocking CD47 | PLD | I/II | Recruiting | – | NCT05261490 | – | |
| SL-172154 | Fusion protein targeting CD47 on tumor cells and CD40 on APCs | I | Recruiting | – | NCT04406623 | – | ||
| CD47/mesothelin | NI-1801 | Anti-CD47/mesothelin bispecific mAb | Monotherapy | I | Recruiting | – | NCT05403554 | – |
| CD47/PD L1 | PF-07257876 | Anti-CD47/PD-L1 bispecific mAb | Monotherapy | I | Recruiting | – | NCT04881045 | – |
| SIRPα | BI-765063 | antagonistic anti-SIRPα mAb | Anti-PD-1 mAb ezabenlimab | I | Recruiting | – | NCT03990233 | – |
| IDO | Epacadostat | IDO inhibitor restoring the activation of immune cells | Monotherapy | II | Terminated | Lack of evidence of superiority | NCT01685255 | 176 |
| CRS-207 (Listeria-based vaccine expressing human mesothelin); CRS-207+pembrolizumab | I/II | Terminated | Lack of clinical activity | NCT02575807 | – | |||
| Pembrolizumab | II | Terminated | – | NCT03602586 | – | |||
| Monotherapy | I | Active | – | NCT02042430 | – | |||
| Nivolumab; nivolumab+chemotherapy | I/II | Completed | – | NCT02327078 | – |
APC, antigen-presenting cell; CAR, chimeric antigen receptor; CXCL10, C-X-C motif chemokine ligand 10; EGFR, epidermal growth factor receptor; FLT3L, FMS-like tyrosine kinase three ligand; HER2, human epidermal growth factor receptor 2; IDO, indoleamine 2,3-dioxygenase; IFN, interferon; ISR, injection site reaction; mAb, monoclonal antibody; OC, ovarian cancer; OS, overall survival; PD-1, programmed cell death 1; PD-L1, programmed death-ligand 1; PI3Kγ, phosphatidylinositol 3-kinase γ; PLD, pegylated liposomal doxorubicin; PR, partial response; SIRPα, signal regulatory protein α; TAM, tumor-associated macrophage; TLR, toll-like receptor; TME, tumor microenvironment; VLP, virus-like particle.
PI3K inhibitors
Blocking the PI3K signaling pathway represents another therapeutic approach for repolarizing TAMs and inhibiting tumor progression in EOC. Triptolide, an inhibitor of the PI3K–Akt–nuclear factor-κB axis, promoted M1 TAMs polarization and reduced the proliferation, migration, and invasion of a cisplatin-resistant human EOC cell line and lowered the tumor burden in mice.138 However, the effects of triptolide are relatively unspecific due to broad, not only antitumor but also anti-inflammatory and immunosuppressive activities.139 Thus, specific targeting of PI3K pathway could be a better approach to avoid potential side effects. For instance, administration of eganelisib (IPI-549), which selectively inactivates the PI3Kγ isoform expressed by macrophages that is associated with immunosuppression and tumor growth,140 restored the immunostimulatory transcriptional program, resulting in CTL activation. It also synergized with ICI therapy to promote tumor regression and increased survival in murine models of melanoma, breast and colon cancer.140 141 Eganelisib is currently being tested as monotherapy and in combination with nivolumab (MARIO-1, NCT02637531) or in combination with a dual adenosine receptor antagonist (etrumadenant, AB928) plus PLD in patients with advanced solid tumors, including EOC (NCT03719326) (table 3).
CD40 agonists
Among the TAM repolarization strategies, agonistic mAbs targeting the co-stimulatory molecule CD40 have also been a focus of clinical investigation in EOC. CD40 signaling in macrophages and dendritic cells (DCs) stimulates IL-12 production and promotes TH1 cell-dependent immunity, which includes IFN-γ production by lymphocytes and upregulation of MHC class I molecules, features typically associated with antitumor activity.142 In several preclinical models (eg, pancreatic carcinoma), anti-CD40 mAbs potentiated a macrophage-driven shift in the tumor immune landscape, and had considerable anticancer effects as monotherapy143 144 or in combination with other agents, including ICIs.145 Consistent with this notion, the anti-CD40 mAb selicrelumab combined with SoC chemotherapy was well tolerated and a partial response was observed in 20% of patients with advanced solid tumors, including EOC (NCT00607048).146 Other potential therapeutic partners, such as bevacizumab and/or vanucizumab (NCT02665416), are under clinical investigation. In addition, phase I and II trials assessing the therapeutic potential of another anti-CD40 mAb (CDX-1140) with pembrolizumab or chemotherapy (NCT03329950) or with pembrolizumab plus bevacizumab (NCT05231122) in patients with EOC are ongoing (table 3). The results of these studies will provide the first inside into whether anti-CD40 mAb-mediated TAM repolarization can skew the immune landscape in the ovarian TME toward improved responsiveness of ICI therapy. To avoid activating macrophages outside malignant tissue, which might result in immunotherapy-related adverse events, novel agents are currently under development, including bispecific antibodies with conditional activity dependent on binding to a tumor-specific antigen (eg, ABBV-428 targeting human CD40 and mesothelin),147 CAR T cells engineered to constitutively express CD40L,148 or intratumorally delivered oncolytic viruses armed with the CD40L gene (eg, LOAd703, a designed adenovirus armed with trimerized CD40L and 4-1BBL).149
IL-12 and IFN-γ
Polarization of TAMs in the TME is largely impacted by cytokines, including IL-12 and IFN-γ, which typically promote their differentiation toward the M1-like phenotype.89 Thus, on IFN-γ exposure, TAMs purified from EOC ascites retrieved the M1 phenotype, downregulated the secretion of tumor-promoting mediators (eg, CCL18, VEGF and MMP-9), and potentiated the adaptive antitumor immune responses.150 Despite expectations, the success of recombinant IL-12 and IFN-γ in cancer immunotherapy trials was limited, mainly due to their short half-life and toxicity-related side effects. Thus, there has been some progress toward the development of alternative delivery methods using appropriate carriers with the purpose of achieving greater therapeutic outcomes accompanied with reduced toxicity.151 152 Initial phase I trials of an IL-12-plasmid/lipopolymer complex (termed GEN-1) administered intraperitoneally in patients with advanced EOC demonstrated favorable safety profiles.153 154 This complex also showed potential synergy with SoC chemotherapy, in terms of reduced intratumoral immunosuppression with preliminary evidence of clinical activity (NCT02480374).155 GEN-1 is now being tested in a phase 2 clinical trial (OVATION 2, NCT03393884) (table 3). Several other strategies aimed at enhancing local delivery and controlling the release of IFN-γ, including liposomes, biodegradable microspheres, gene therapy or nanoparticles, have been investigated but their success was limited due to pharmacologic and pharmacodynamic obstacles.151
Moreover, recent preclinical studies have focused on the generation of genetically engineered T cells and/or myeloid cells with stable expression of TAM-reprogramming cytokines in the TME. T cells engineered to release IFN-γ and granulocyte-macrophage CSF were shown to activate TAM precursors leading to IL-12 production and tumor rejection in a murine model of OC.156 Similarly, CAR T cells engineered to release inducible IL-12 on CAR engagement in the tumor lesion helped eliminate neoplastic cells, which was accompanied by the accumulation of activated TAMs in a syngeneic mouse model of glioblastoma and melanoma.157 Furthermore, macrophages can be also manipulated to secrete IL-12158 or express specific cell surface receptors. As an example, primary human monocyte-derived macrophages were modified using an adenoviral vector with a CAR targeting human epidermal growth factor receptor 2 (HER2) to recognize and eliminate HER2+ tumor cells. A single infusion of CAR macrophages decreased tumor burden and delayed the progression of solid tumor xenografts, including HER2+ EOC, in mice. Interestingly, it was observed that, regardless of CAR expression, these engineered macrophages displayed a proinflammatory M1 phenotype, which resisted the effects of immunosuppressive cytokines, thus facilitating antitumor immune response.159 This approach is currently being evaluated in a phase I clinical trial in patients with HER2+ solid tumors, including EOC (NCT04660929) (table 3). Given these promising results, genetically engineered macrophages represent an exciting future direction for targeting the immunosuppressive microenvironment in solid tumors. However, the heterogeneity and plasticity of macrophage subsets need to be carefully analyzed to optimize the clinical responses in a tumor-specific manner.
Restoring the antitumor functions of TAMs
The phagocytosis of malignant cells and the presentation of tumor antigens to T cells by APCs, such as macrophages and DCs, are crucial for activating antitumor immunity. Phagocytosis is triggered by ‘eat me’ signals displayed on target non-self or modified-self cells that engage phagocytic receptors on macrophages. Conversely, healthy cells display ‘don’t eat me’ signals (eg, CD47 and CD24), which bind to cognate inhibitory receptors, such as signal regulatory protein-α (SIRPα) and sialic acid-binding Ig-like lectin 10, representing important innate immune checkpoints for preventing autoreactivity.160 These interactions are also employed by malignant cells to escape phagocytosis. Supporting this notion, high expression of CD47 and CD24 in EOC cells is correlated with poor clinical outcomes.161–164 Importantly, blocking these pathways with neutralizing anti-CD47 and anti-CD24 mAbs enhanced non-specific macrophage-mediated phagocytosis, thus inhibiting tumor growth in murine EOC models.163 165 Anti-CD47 mAbs AO-176 (NCT03834948) and magrolimab (Hu5F9-G4) (NCT02216409, NCT03558139 and NCT02953782) and anti-SIRPα mAb (BI-765063) (NCT03990233) have been or are currently being investigated in multiple clinical trials in various tumors, including EOC (table 3). Although antagonistic anti-CD47 mAbs, alone or in combination, showed promising clinical activity,166 167 the ubiquitous expression of CD47 can result in off-target and adverse side effects, particularly platelet aggregation and hemagglutination. Thus, several new strategies have been developed to limit the risk of side effects, such as anti-CD47/tumor antigen or immune checkpoint molecule bispecific antibodies (NI-1801 or PF-07257876, respectively) and CD47-targeting fusion proteins (TTI-622 and SL-172154), which are now under clinical investigation (table 3).
Limiting the tumor-promoting activity of TAMs
IDO inhibitors
An alternative strategy to resolve TAM-mediated immunosuppression involves direct inhibition of their pro-tumoral functions, including the production of IDO, which is implicated in the suppression of CTL proliferation168 169 and linked to peritoneal dissemination and poor survival outcomes in patients with EOC.170 171 Indeed, competitive inhibition of IDO with 1-methyl-D-tryptophan was shown to reactivate immune cells, including CD8+ T and NK cells, and reduced tumor cell dissemination and invasion in preclinical OC models.171 172 Despite promising results in early phase clinical trials,173–175 an IDO inhibitor (epacadostat) failed to demonstrate sufficient clinical activity in patients with EOC in phase II clinical trials (NCT01685255, NCT02575807)176 (table 3). This lack of efficacy might be explained by metabolic adaptation involving a switch from tryptophan catabolism toward the serotonin pathway, resulting in elevated nicotinamide adenine dinucleotide (NAD+) further reducing T cell effector functions. This adaptive resistance might be overcome by combining epacadostat with A2a/A2b purinergic receptor antagonists, as demonstrated in a preclinical IDO-overexpressing experimental EOC model.177
Immune checkpoint (PD-L1) inhibitors
Myelomonocytic cells are part of tumor-extrinsic pathways of primary and adaptive resistance to ICIs by expressing several immunosuppressive molecules, including checkpoint ligands, such as PD-L1, PD-L2.66 178 Besides the indisputable impact of PD-1–PD-L1 blockade on the functional capacity of CTLs,179–181 this approach also affects the survival, proliferation, and activation of human and murine immunostimulatory TAMs leading to TAM-mediated anticancer activity, and reduced tumor growth in vivo.182 Several mAbs targeting PD-L1 (eg, avelumab, atezolizumab and durvalumab) have been tested in numerous clinical studies in patients with EOC. However, the preclinical impact of anti-PD-L1 monotherapy or combination with other ICIs were not confirmed in the early phase clinical trials in patients with advanced EOC.14 183 These findings have prompted subsequent studies to focus on new combinatorial approaches, in which PARPi and anti-angiogenic drugs are the most promising candidates for enhancing the clinical effectiveness of ICIs. PARPi could potentiate ICI activity by multiple mechanisms, which are mainly induced by DNA damage. PARPi-mediated DDR failure promotes the accumulation of double-stranded DNA in cytosol, activating the well-characterized cyclic guanosine monophosphate-adenine monophosphate synthase/stimulator of interferon genes pathway, resulting in the production of type I IFNs and enhanced antitumor immune responses.184 Defects in DDR pathway are also often associated with an increased TMB, which can be further amplified in the presence of HRDs like BRCA1/2 mutations and correlates with tumor-infiltrating lymphocyte (TIL) accumulation in the TME.185 186 In addition, PARPi upregulate PD-L1 expression in tumor cells.187 Durvalumab combined with a PARPi (olaparib) was tested in three phase I/II clinical studies in patients with recurrent EOC. In one study (MEDIOLA), the 12-week disease control rate (DCR) was 81% (NCT02734004)188 and the other studies reported a DCR of 53% (durvalumab plus olaparib or VEGFR1-3 inhibitor (cediranib), NCT02484404),189 and enhanced IFN-γ/TNF-α production, an increase in the numbers of TILs and a DCR of 71% (NCT02484404).190 However, further research is needed to identify potential predictors of the therapeutic response. Moreover, there is evidence that blocking the VEGF–VEGFR pathway is necessary to improve the efficacy of combined anti-PD-L1 and PARPi therapy. In this respect, an ongoing phase III trial (DUO-O, NCT03737643) is investigating the benefit of durvalumab combined with chemotherapy and bevacizumab, followed by durvalumab, bevacizumab, and olaparib in the maintenance setting in patients with newly diagnosed advanced HGSOC after cytoreductive surgery. The results of these trials are urgently awaited.
VEGF–VEGFR inhibitors
The anti-angiogenic drug bevacizumab has now been employed for first-line management of advanced EOC for more than 7 years.9 Based on preclinical findings from experimental models of EOC, bevacizumab can synergize with ICIs.191 Thus, the combination of bevacizumab and nivolumab has been associated with improved ORR (28.9%) and PFS (median 9.4 months) in patients with relapsed EOC.192 However, most patients develop resistance to bevacizumab treatment, which seems to be largely mediated by TAMs.193 194 Consistent with this, bevacizumab-resistant EOC exhibited a restored response after treatment with a TAM-depleting anti-CSF1 mAb in a murine experimental model.195 Specifically, VEGF–VEGFR blockade enhanced tumor hypoxia resulting in chemoattraction of proangiogenic TAM subsets to restore neoangiogenesis.196 This effect underlies the limitations of therapeutically targeting a single mediator, such as VEGF, due to the redundancy within the TME. Thus, combinatorial approaches targeting neoangiogenesis are needed. For instance, blocking the Ang2–TIE2 pathway or simultaneous inhibition of VEGF–VEGFR and Ang2–TIE2 axis by mAbs or other agents have been investigated clinically in patients with advanced EOC, as described above. Using mAbs to target molecules specifically expressed by proangiogenic TAM subsets might represent a more durable therapeutic strategy. This could be achieved by identifying the surface markers selectively expressed on TAM subsets coexpressing VEGF and/or TIE2 to achieve selective depletion without inhibiting anticancer TAM subsets.
Conclusions
EOC is a highly lethal malignancy with limited responses to the current immunotherapy approaches, primarily due to indolent anticancer immunity and robust humoral and cellular immunosuppression.16 102 TAMs constitute the most abundant infiltrating immune cell population in human EOC and ascites, and are associated with disease progression, therapy resistance, and poor clinical outcomes.31 36 89 90 Thus, TAMs represent attractive targets for developing novel anticancer regimens aiming to reverse the strong immunosuppression that exists within the TME.
Current TAM-targeting strategies mainly seek to deplete M2-like TAMs and/or favor their repolarization toward an inflammatory M1-like phenotype. However, the clinical implementation of these approaches has been limited to date, mostly due to the high heterogeneity and plasticity of TAMs.19 133 Because TAMs display high potential for adopting distinct phenotypes and functions in response to the local microenvironment, various TAM states exist in different tumor types and locations within the same tumor.18 To design and develop effective anticancer agents targeting TAMs, it is crucial to expand our knowledge of the biology and behavior of these cells, and to identify markers that can distinguish tumor-promoting TAMs from anticancer TAMs in order to rationally define which TAM states need to be suppressed or enhanced. Moreover, there are several questions regarding the development of TAM-targeting agents that remain to be addressed. First, how does the TAM landscape evolve during cancer progression and in response to SoC therapy and/or immunotherapy? Second, what is the optimal treatment schedule in clinical practice? Most combinatorial regimens are developed based on coadministration paradigms that are not necessarily the most efficient approach in the clinic. Third, what is the best strategy for targeting the key interactions between TAMs and other immune/non-immune cell compartments that promote tumor progression in immunologically ‘indolent’ tumors such as EOC?
In conclusion, better understanding of the temporal and spatial evolution of the TAM compartment in EOC on therapeutic intervention will enhance our insight into this extremely complex immune cell component. In this context, exploiting various functional, epigenetic, and metabolic pathways intrinsic to macrophages to target TAM-mediated immunosuppression and tumor progression in combination with other immunotherapeutic agents might enhance the effectiveness of anticancer therapies, and thus lead to the development of superior combinatorial regimens for clinical care of patients with EOC in the future.
Acknowledgments
This study was sponsored by Sotio Biotech, Prague. The authors thank Nicholas D. Smith for language revisions and editorial support.
Footnotes
Contributors: Concept and design: IT and JF; resources: IT; data curation: DC, RS and JF; writing—original draft preparation: IT, JF; writing—review and editing: DC and RS; visualization: IT.
Funding: This study was sponsored by Sotio Biotech, Prague
Competing interests: RS is minority shareholder of Sotio Biotech a.s. JF and IT are employees of Sotio Biotech.
Provenance and peer review: Commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Fucikova J, Coosemans A, Orsulic S, et al. Immunological configuration of ovarian carcinoma: features and impact on disease outcome. J Immunother Cancer 2021;9:e002873. 10.1136/jitc-2021-002873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fucikova J, Palova-Jelinkova L, Klapp V, et al. Immunological control of ovarian carcinoma by chemotherapy and targeted anticancer agents. Trends Cancer 2022;8:426–44. 10.1016/j.trecan.2022.01.010 [DOI] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 4.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol 2010;177:1053–64. 10.2353/ajpath.2010.100105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowtell DD, Böhm S, Ahmed AA, et al. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer 2015;15:668–79. 10.1038/nrc4019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cocetta V, Ragazzi E, Montopoli M. Links between cancer metabolism and cisplatin resistance. Int Rev Cell Mol Biol 2020;354:107–64. 10.1016/bs.ircmb.2020.01.005 [DOI] [PubMed] [Google Scholar]
- 7.Poveda A, Floquet A, Ledermann JA, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-ov21): a final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2021;22:620–31. 10.1016/S1470-2045(21)00073-5 [DOI] [PubMed] [Google Scholar]
- 8.Rose M, Burgess JT, O’Byrne K, et al. PARP inhibitors: clinical relevance, mechanisms of action and tumor resistance. Front Cell Dev Biol 2020;8:564601. 10.3389/fcell.2020.564601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011;365:2484–96. 10.1056/NEJMoa1103799 [DOI] [PubMed] [Google Scholar]
- 10.Gillyard T, Davis J. DNA double-strand break repair in cancer: A path to achieving precision medicine. Int Rev Cell Mol Biol 2021;364:111–37. 10.1016/bs.ircmb.2021.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtin NJ, Szabo C. Poly(ADP-ribose) polymerase inhibition: past, present and future. Nat Rev Drug Discov 2020;19:711–36. 10.1038/s41573-020-0076-6 [DOI] [PubMed] [Google Scholar]
- 12.González-Martín A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2019;381:2391–402. 10.1056/NEJMoa1910962 [DOI] [PubMed] [Google Scholar]
- 13.Wilky BA. Immune checkpoint inhibitors: the linchpins of modern immunotherapy. Immunol Rev 2019;290:6–23. 10.1111/imr.12766 [DOI] [PubMed] [Google Scholar]
- 14.Disis ML, Taylor MH, Kelly K, et al. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol 2019;5:393–401. 10.1001/jamaoncol.2018.6258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matulonis UA, Shapira-Frommer R, Santin AD, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol 2019;30:1080–7. 10.1093/annonc/mdz135 [DOI] [PubMed] [Google Scholar]
- 16.Johnson RL, Cummings M, Thangavelu A, et al. Barriers to immunotherapy in ovarian cancer: metabolic, genomic, and immune perturbations in the tumour microenvironment. Cancers (Basel) 2021;13:24. 10.3390/cancers13246231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jayasingam SD, Citartan M, Thang TH, et al. Evaluating the polarization of tumor-associated macrophages into M1 and M2 phenotypes in human cancer tissue: technicalities and challenges in routine clinical practice. Front Oncol 2019;9:1512. 10.3389/fonc.2019.01512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol 2020;15:123–47. 10.1146/annurev-pathmechdis-012418-012718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duan Z, Luo Y. Targeting macrophages in cancer immunotherapy. Signal Transduct Target Ther 2021;6:127. 10.1038/s41392-021-00506-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 2014;6:13. 10.12703/P6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014;41:14–20. 10.1016/j.immuni.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SY, Nair MG. Macrophages in wound healing: activation and plasticity. Immunol Cell Biol 2019;97:258–67. 10.1111/imcb.12236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng S, Li Z, Gao R, et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell 2021;184:792–809. 10.1016/j.cell.2021.01.010 [DOI] [PubMed] [Google Scholar]
- 24.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010;141:39–51. 10.1016/j.cell.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu SZ, Al-Eryani G, Roden DL, et al. A single-cell and spatially resolved atlas of human breast cancers. Nat Genet 2021;53:1334–47. 10.1038/s41588-021-00911-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colvin EK. Tumor-Associated macrophages contribute to tumor progression in ovarian cancer. Front Oncol 2014;4:137. 10.3389/fonc.2014.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong P, Ma L, Liu L, et al. CD86(+)/CD206(+), diametrically polarized tumor-associated macrophages, predict hepatocellular carcinoma patient prognosis. Int J Mol Sci 2016;17:320. 10.3390/ijms17030320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeong H, Hwang I, Kang SH, et al. Tumor-Associated macrophages as potential prognostic biomarkers of invasive breast cancer. J Breast Cancer 2019;22:38–51. 10.4048/jbc.2019.22.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawamura K, Komohara Y, Takaishi K, et al. Detection of M2 macrophages and colony-stimulating factor 1 expression in serous and mucinous ovarian epithelial tumors. Pathol Int 2009;59:300–5. 10.1111/j.1440-1827.2009.02369.x [DOI] [PubMed] [Google Scholar]
- 30.Xu J, Fang Y, Chen K, et al. Single-Cell RNA sequencing reveals the tissue architecture in human high-grade serous ovarian cancer. Clin Cancer Res 2022;28:3590–602. 10.1158/1078-0432.CCR-22-0296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang T, Liu Q, Zhu Y, et al. Lymphocyte and macrophage infiltration in omental metastases indicates poor prognosis in advance stage epithelial ovarian cancer. J Int Med Res 2021;49:03000605211066245. 10.1177/03000605211066245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cotechini T, Atallah A, Grossman A. Tissue-Resident and recruited macrophages in primary tumor and metastatic microenvironments: potential targets in cancer therapy. Cells 2021;10:960. 10.3390/cells10040960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hourani T, Holden JA, Li W, et al. Tumor associated macrophages: origin, recruitment, phenotypic diversity, and targeting. Front Oncol 2021;11:788365. 10.3389/fonc.2021.788365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Arabey AA, Denizli M, Kanlikilicer P, et al. Gata3 as a master regulator for interactions of tumor-associated macrophages with high-grade serous ovarian carcinoma. Cell Signal 2020;68:109539. 10.1016/j.cellsig.2020.109539 [DOI] [PubMed] [Google Scholar]
- 35.Hensler M, Kasikova L, Fiser K, et al. M2-Like macrophages dictate clinically relevant immunosuppression in metastatic ovarian cancer. J Immunother Cancer 2020;8:e000979. 10.1136/jitc-2020-000979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takaishi K, Komohara Y, Tashiro H, et al. Involvement of M2-polarized macrophages in the ascites from advanced epithelial ovarian carcinoma in tumor progression via STAT3 activation. Cancer Sci 2010;101:2128–36. 10.1111/j.1349-7006.2010.01652.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Deavers M, Patenia R, et al. Monocyte/Macrophage and T-cell infiltrates in peritoneum of patients with ovarian cancer or benign pelvic disease. J Transl Med 2006;4:30. 10.1186/1479-5876-4-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan X, Zhang J, Li D, et al. Prognostic significance of tumor-associated macrophages in ovarian cancer: a meta-analysis. Gynecol Oncol 2017;147:181–7. 10.1016/j.ygyno.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 39.Steitz AM, Steffes A, Finkernagel F, et al. Tumor-Associated macrophages promote ovarian cancer cell migration by secreting transforming growth factor beta induced (Tgfbi) and tenascin C. Cell Death Dis 2020;11:249. 10.1038/s41419-020-2438-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin M, Li X, Tan S, et al. Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J Clin Invest 2016;126:4157–73. 10.1172/JCI87252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishnan V, Tallapragada S, Schaar B, et al. Omental macrophages secrete chemokine ligands that promote ovarian cancer colonization of the omentum via CCR1. Commun Biol 2020;3:524. 10.1038/s42003-020-01246-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y, Zhang L, Lv R, et al. Overexpression of Semaphorin4D indicates poor prognosis and prompts monocyte differentiation toward M2 macrophages in epithelial ovarian cancer. Asian Pac J Cancer Prev 2013;14:5883–90. 10.7314/apjcp.2013.14.10.5883 [DOI] [PubMed] [Google Scholar]
- 43.Duluc D, Delneste Y, Tan F, et al. Tumor-Associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood 2007;110:4319–30. 10.1182/blood-2007-02-072587 [DOI] [PubMed] [Google Scholar]
- 44.Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002;23:549–55. 10.1016/s1471-4906(02)02302-5 [DOI] [PubMed] [Google Scholar]
- 45.Negus RP, Stamp GW, Relf MG, et al. The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J Clin Invest 1995;95:2391–6. 10.1172/JCI117933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sawano A, Iwai S, Sakurai Y, et al. Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood 2001;97:785–91. 10.1182/blood.v97.3.785 [DOI] [PubMed] [Google Scholar]
- 47.Tang M, Liu B, Bu X, et al. Cross-Talk between ovarian cancer cells and macrophages through periostin promotes macrophage recruitment. Cancer Sci 2018;109:1309–18. 10.1111/cas.13567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar S, Mittal S, Gupta P, et al. Metabolic reprogramming in tumor-associated macrophages in the ovarian tumor microenvironment. Cancers (Basel) 2022;14:21. 10.3390/cancers14215224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larionova I, Kazakova E, Patysheva M, et al. Transcriptional, epigenetic and metabolic programming of tumor-associated macrophages. Cancers (Basel) 2020;12:1411. 10.3390/cancers12061411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thapa B, Lee K. Metabolic influence on macrophage polarization and pathogenesis. BMB Rep 2019;52:360–72. 10.5483/BMBRep.2019.52.6.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colegio OR, Chu N-Q, Szabo AL, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014;513:559–63. 10.1038/nature13490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cramer T, Yamanishi Y, Clausen BE, et al. Hif-1Alpha is essential for myeloid cell-mediated inflammation. Cell 2003;112:645–57. 10.1016/s0092-8674(03)00154-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wen Z, Liu H, Li M, et al. Increased metabolites of 5-lipoxygenase from hypoxic ovarian cancer cells promote tumor-associated macrophage infiltration. Oncogene 2015;34:1241–52. 10.1038/onc.2014.85 [DOI] [PubMed] [Google Scholar]
- 54.Schumann T, Adhikary T, Wortmann A, et al. Deregulation of PPARβ/δ target genes in tumor-associated macrophages by fatty acid ligands in the ovarian cancer microenvironment. Oncotarget 2015;6:13416–33. 10.18632/oncotarget.3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goossens P, Rodriguez-Vita J, Etzerodt A, et al. Membrane cholesterol efflux drives tumor-associated macrophage reprogramming and tumor progression. Cell Metab 2019;29:1376–89. 10.1016/j.cmet.2019.02.016 [DOI] [PubMed] [Google Scholar]
- 56.De Nola R, Menga A, Castegna A, et al. The crowded crosstalk between cancer cells and stromal microenvironment in gynecological malignancies: biological pathways and therapeutic implication. Int J Mol Sci 2019;20:2401. 10.3390/ijms20102401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palmieri EM, Menga A, Martín-Pérez R, et al. Pharmacologic or genetic targeting of glutamine synthetase skews macrophages toward an M1-like phenotype and inhibits tumor metastasis. Cell Rep 2017;20:1654–66. 10.1016/j.celrep.2017.07.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004;10:942–9. 10.1038/nm1093 [DOI] [PubMed] [Google Scholar]
- 59.Laoui D, Van Overmeire E, Di Conza G, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res 2014;74:24–30. 10.1158/0008-5472.CAN-13-1196 [DOI] [PubMed] [Google Scholar]
- 60.Lecker LSM, Berlato C, Maniati E, et al. Tgfbi production by macrophages contributes to an immunosuppressive microenvironment in ovarian cancer. Cancer Res 2021;81:5706–19. 10.1158/0008-5472.CAN-21-0536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herrera-Rios D, Mughal SS, Teuber-Hanselmann S, et al. Macrophages/Microglia represent the major source of indolamine 2,3-dioxygenase expression in melanoma metastases of the brain. Front Immunol 2020;11:120. 10.3389/fimmu.2020.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan H, Dong M, Liu X, et al. Multiple myeloma cell-derived IL-32γ increases the immunosuppressive function of macrophages by promoting indoleamine 2,3-dioxygenase (IDO) expression. Cancer Lett 2019;446:38–48. 10.1016/j.canlet.2019.01.012 [DOI] [PubMed] [Google Scholar]
- 63.Rodriguez PC, Quiceno DG, Zabaleta J, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res 2004;64:5839–49. 10.1158/0008-5472.CAN-04-0465 [DOI] [PubMed] [Google Scholar]
- 64.Xia H, Li S, Li X, et al. Autophagic adaptation to oxidative stress alters peritoneal residential macrophage survival and ovarian cancer metastasis. JCI Insight 2020;5:e141115. 10.1172/jci.insight.141115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Czystowska-Kuzmicz M, Sosnowska A, Nowis D, et al. Small extracellular vesicles containing arginase-1 suppress T-cell responses and promote tumor growth in ovarian carcinoma. Nat Commun 2019;10:3000. 10.1038/s41467-019-10979-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gottlieb CE, Mills AM, Cross JV, et al. Tumor-associated macrophage expression of PD-L1 in implants of high grade serous ovarian carcinoma: a comparison of matched primary and metastatic tumors. Gynecol Oncol 2017;144:607–12. 10.1016/j.ygyno.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 67.Kryczek I, Wei S, Zhu G, et al. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res 2007;67:8900–5. 10.1158/0008-5472.CAN-07-1866 [DOI] [PubMed] [Google Scholar]
- 68.Sica GL, Choi IH, Zhu G, et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity 2003;18:849–61. 10.1016/s1074-7613(03)00152-3 [DOI] [PubMed] [Google Scholar]
- 69.Zhou J, Li X, Wu X, et al. Exosomes released from tumor-associated macrophages transfer miRNAs that induce a treg/th17 cell imbalance in epithelial ovarian cancer. Cancer Immunol Res 2018;6:1578–92. 10.1158/2326-6066.CIR-17-0479 [DOI] [PubMed] [Google Scholar]
- 70.Lin EY, Pollard JW. Tumor-Associated macrophages press the angiogenic switch in breast cancer. Cancer Res 2007;67:5064–6. 10.1158/0008-5472.CAN-07-0912 [DOI] [PubMed] [Google Scholar]
- 71.Zhu Q, Wu X, Wang X. Differential distribution of tumor-associated macrophages and treg/th17 cells in the progression of malignant and benign epithelial ovarian tumors. Oncol Lett 2017;13:159–66. 10.3892/ol.2016.5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moughon DL, He H, Schokrpur S, et al. Macrophage blockade using CSF1R inhibitors reverses the vascular leakage underlying malignant ascites in late-stage epithelial ovarian cancer. Cancer Res 2015;75:4742–52. 10.1158/0008-5472.CAN-14-3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang X, Zhu Q, Lin Y, et al. Crosstalk between tems and endothelial cells modulates angiogenesis and metastasis via IGF1-IGF1R signalling in epithelial ovarian cancer. Br J Cancer 2017;117:1371–82. 10.1038/bjc.2017.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yousefzadeh Y, Hallaj S, Baghi Moornani M, et al. Tumor associated macrophages in the molecular pathogenesis of ovarian cancer. Int Immunopharmacol 2020;84:106471. 10.1016/j.intimp.2020.106471 [DOI] [PubMed] [Google Scholar]
- 75.Bekes I, Friedl TWP, Köhler T, et al. Does VEGF facilitate local tumor growth and spread into the abdominal cavity by suppressing endothelial cell adhesion, thus increasing vascular peritoneal permeability followed by ascites production in ovarian cancer? Mol Cancer 2016;15:13. 10.1186/s12943-016-0497-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen W, Li H-L, Liu L, et al. Expression levels of PTEN, HIF-1α, and VEGF as prognostic factors in ovarian cancer. Eur Rev Med Pharmacol Sci 2017;21:2596–603. [PubMed] [Google Scholar]
- 77.Byrne AT, Ross L, Holash J, et al. Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clin Cancer Res 2003;9:5721–8. [PubMed] [Google Scholar]
- 78.Duyndam MCA, Hilhorst MCGW, Schlüper HMM, et al. Vascular endothelial growth factor-165 overexpression stimulates angiogenesis and induces cyst formation and macrophage infiltration in human ovarian cancer xenografts. Am J Pathol 2002;160:537–48. 10.1016/s0002-9440(10)64873-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hagemann T, Wilson J, Kulbe H, et al. Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J Immunol 2005;175:1197–205. 10.4049/jimmunol.175.2.1197 [DOI] [PubMed] [Google Scholar]
- 80.Worzfeld T, Pogge von Strandmann E, Huber M, et al. The unique molecular and cellular microenvironment of ovarian cancer. Front Oncol 2017;7:24. 10.3389/fonc.2017.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lane D, Matte I, Laplante C, et al. Ccl18 from ascites promotes ovarian cancer cell migration through proline-rich tyrosine kinase 2 signaling. Mol Cancer 2016;15:58. 10.1186/s12943-016-0542-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robinson-Smith TM, Isaacsohn I, Mercer CA, et al. Macrophages mediate inflammation-enhanced metastasis of ovarian tumors in mice. Cancer Res 2007;67:5708–16. 10.1158/0008-5472.CAN-06-4375 [DOI] [PubMed] [Google Scholar]
- 83.Carroll MJ, Fogg KC, Patel HA, et al. Alternatively-activated macrophages upregulate mesothelial expression of P-selectin to enhance adhesion of ovarian cancer cells. Cancer Res 2018;78:3560–73. 10.1158/0008-5472.CAN-17-3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.He Y, Zhang M, Wu X, et al. High MUC2 expression in ovarian cancer is inversely associated with the M1/M2 ratio of tumor-associated macrophages and patient survival time. PLoS One 2013;8:e79769. 10.1371/journal.pone.0079769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Le Page C, Marineau A, Bonza PK, et al. BTN3A2 expression in epithelial ovarian cancer is associated with higher tumor infiltrating T cells and a better prognosis. PLoS One 2012;7:e38541. 10.1371/journal.pone.0038541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Macciò A, Gramignano G, Cherchi MC, et al. Role of M1-polarized tumor-associated macrophages in the prognosis of advanced ovarian cancer patients. Sci Rep 2020;10:6096. 10.1038/s41598-020-63276-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reinartz S, Finkernagel F, Adhikary T, et al. A transcriptome-based global map of signaling pathways in the ovarian cancer microenvironment associated with clinical outcome. Genome Biol 2016;17:108. 10.1186/s13059-016-0956-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gupta V, Yull F, Khabele D. Bipolar tumor-associated macrophages in ovarian cancer as targets for therapy. Cancers (Basel) 2018;10:366. 10.3390/cancers10100366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang Y, Yang Y, Yang J, et al. Tumor microenvironment in ovarian cancer: function and therapeutic strategy. Front Cell Dev Biol 2020;8:758. 10.3389/fcell.2020.00758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang M, He Y, Sun X, et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res 2014;7:19. 10.1186/1757-2215-7-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liang Y-L, Lin C-N, Tsai H-F, et al. Omental macrophagic “ crown-like structures ” are associated with poor prognosis in advanced-stage serous ovarian cancer. Curr Oncol 2021;28:4234–46. 10.3390/curroncol28050359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lane D, Matte I, Rancourt C, et al. Prognostic significance of IL-6 and IL-8 ascites levels in ovarian cancer patients. BMC Cancer 2011;11:210. 10.1186/1471-2407-11-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reinartz S, Schumann T, Finkernagel F, et al. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: correlation of CD163 expression, cytokine levels and early relapse. Int J Cancer 2014;134:32–42. 10.1002/ijc.28335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yanaihara N, Anglesio MS, Ochiai K, et al. Cytokine gene expression signature in ovarian clear cell carcinoma. Int J Oncol 2012;41:1094–100. 10.3892/ijo.2012.1533 [DOI] [PubMed] [Google Scholar]
- 95.Qu Q-X, Huang Q, Shen Y, et al. The increase of circulating PD-L1-expressing CD68 (+) macrophage in ovarian cancer. Tumour Biol 2016;37:5031–7. 10.1007/s13277-015-4066-y [DOI] [PubMed] [Google Scholar]
- 96.Liu C, Zhang Y, Li X, et al. Ovarian cancer-specific dysregulated genes with prognostic significance: scrna-seq with bulk RNA-seq data and experimental validation. Ann N Y Acad Sci 2022;1512:154–73. 10.1111/nyas.14748 [DOI] [PubMed] [Google Scholar]
- 97.Finkernagel F, Reinartz S, Lieber S, et al. The transcriptional signature of human ovarian carcinoma macrophages is associated with extracellular matrix reorganization. Oncotarget 2016;7:75339–52. 10.18632/oncotarget.12180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tan Q, Liu H, Xu J, et al. Integrated analysis of tumor-associated macrophage infiltration and prognosis in ovarian cancer. Aging (Albany NY) 2021;13:23210–32. 10.18632/aging.203613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Adhikary T, Wortmann A, Finkernagel F, et al. Interferon signaling in ascites-associated macrophages is linked to a favorable clinical outcome in a subgroup of ovarian carcinoma patients. BMC Genomics 2017;18:243. 10.1186/s12864-017-3630-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Worzfeld T, Finkernagel F, Reinartz S, et al. Proteotranscriptomics reveal signaling networks in the ovarian cancer microenvironment. Mol Cell Proteomics 2018;17:270–89. 10.1074/mcp.RA117.000400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer 2018;118:9–16. 10.1038/bjc.2017.434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weissleder R, Pittet MJ. The expanding landscape of inflammatory cells affecting cancer therapy. Nat Biomed Eng 2020;4:489–98. 10.1038/s41551-020-0524-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arlauckas SP, Garris CS, Kohler RH, et al. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med 2017;9:eaal3604. 10.1126/scitranslmed.aal3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu Y, Zuo F, Wang H, et al. The current landscape of predictive and prognostic biomarkers for immune checkpoint blockade in ovarian cancer. Front Immunol 2022;13:1045957. 10.3389/fimmu.2022.1045957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pujade-Lauraine E, Fujiwara K, Ledermann JA, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (javelin ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol 2021;22:1034–46. 10.1016/S1470-2045(21)00216-3 [DOI] [PubMed] [Google Scholar]
- 106.Yang M, Lu J, Zhang G, et al. Cxcl13 shapes immunoactive tumor microenvironment and enhances the efficacy of PD-1 checkpoint blockade in high-grade serous ovarian cancer. J Immunother Cancer 2021;9:e001136. 10.1136/jitc-2020-001136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vlaming M, Bilemjian V, Freile JÁ, et al. Tumor infiltrating CD8/CD103/TIM-3-expressing lymphocytes in epithelial ovarian cancer co-express CXCL13 and associate with improved survival. Front Immunol 2022;13:1031746. 10.3389/fimmu.2022.1031746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Färkkilä A, Gulhan DC, Casado J, et al. Immunogenomic profiling determines responses to combined PARP and PD-1 inhibition in ovarian cancer. Nat Commun 2020;11:1459. 10.1038/s41467-020-15315-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ni Y, Soliman A, Joehlin-Price A, et al. High TGF-β signature predicts immunotherapy resistance in gynecologic cancer patients treated with immune checkpoint inhibition. NPJ Precis Oncol 2021;5:101. 10.1038/s41698-021-00242-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chambers SK, Kacinski BM, Ivins CM, et al. Overexpression of epithelial macrophage colony-stimulating factor (CSF-1) and CSF-1 receptor: a poor prognostic factor in epithelial ovarian cancer, contrasted with a protective effect of stromal CSF-1. Clin Cancer Res 1997;3:999–1007. [PubMed] [Google Scholar]
- 111.Stanley ER, Chitu V. Csf-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol 2014;6:a021857. 10.1101/cshperspect.a021857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Akkari L, Bowman RL, Tessier J, et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci Transl Med 2020;12:eaaw7843. 10.1126/scitranslmed.aaw7843 [DOI] [PubMed] [Google Scholar]
- 113.Pfirschke C, Zilionis R, Engblom C, et al. Macrophage-Targeted therapy unlocks antitumoral cross-talk between ifnγ-secreting lymphocytes and IL12-producing dendritic cells. Cancer Immunol Res 2022;10:40–55. 10.1158/2326-6066.CIR-21-0326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ries CH, Cannarile MA, Hoves S, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 2014;25:846–59. 10.1016/j.ccr.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 115.Gomez-Roca CA, Italiano A, Le Tourneau C, et al. Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages. Ann Oncol 2019;30:1381–92. 10.1093/annonc/mdz163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Machiels J-P, Gomez-Roca C, Michot J-M, et al. Phase Ib study of anti-CSF-1R antibody emactuzumab in combination with CD40 agonist selicrelumab in advanced solid tumor patients. J Immunother Cancer 2020;8:e001153. 10.1136/jitc-2020-001153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Falchook GS, Peeters M, Rottey S, et al. A phase 1a/1b trial of CSF-1R inhibitor LY3022855 in combination with durvalumab or tremelimumab in patients with advanced solid tumors. Invest New Drugs 2021;39:1284–97. 10.1007/s10637-021-01088-4 [DOI] [PubMed] [Google Scholar]
- 118.Cannarile MA, Weisser M, Jacob W, et al. Colony-Stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer 2017;5:53. 10.1186/s40425-017-0257-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lim SY, Yuzhalin AE, Gordon-Weeks AN, et al. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget 2016;7:28697–710. 10.18632/oncotarget.7376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qian B-Z, Li J, Zhang H, et al. Ccl2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011;475:222–5. 10.1038/nature10138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moisan F, Francisco EB, Brozovic A, et al. Enhancement of paclitaxel and carboplatin therapies by CCL2 blockade in ovarian cancers. Mol Oncol 2014;8:1231–9. 10.1016/j.molonc.2014.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brana I, Calles A, LoRusso PM, et al. Carlumab, an anti-C-C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open-label, multicenter phase 1b study. Target Oncol 2015;10:111–23. 10.1007/s11523-014-0320-2 [DOI] [PubMed] [Google Scholar]
- 123.Sandhu SK, Papadopoulos K, Fong PC, et al. A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), A human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer Chemother Pharmacol 2013;71:1041–50. 10.1007/s00280-013-2099-8 [DOI] [PubMed] [Google Scholar]
- 124.Hyman DM, Rizvi N, Natale R, et al. Phase I study of MEDI3617, a selective angiopoietin-2 inhibitor alone and combined with carboplatin/paclitaxel, paclitaxel, or bevacizumab for advanced solid tumors. Clin Cancer Res 2018;24:2749–57. 10.1158/1078-0432.CCR-17-1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vergote I, Scambia G, O’Malley DM, et al. Trebananib or placebo plus carboplatin and paclitaxel as first-line treatment for advanced ovarian cancer (TRINOVA-3/ENGOT-ov2/GOG-3001): a randomised, double-blind, phase 3 trial. Lancet Oncol 2019;20:862–76. 10.1016/S1470-2045(19)30178-0 [DOI] [PubMed] [Google Scholar]
- 126.Hidalgo M, Martinez-Garcia M, Le Tourneau C, et al. First-In-Human phase I study of single-agent vanucizumab, a first-in-class bispecific anti-angiopoietin-2/anti-VEGF-A antibody, in adult patients with advanced solid tumors. Clin Cancer Res 2018;24:1536–45. 10.1158/1078-0432.CCR-17-1588 [DOI] [PubMed] [Google Scholar]
- 127.Kobayashi Y, Kashima H, Rahmanto YS, et al. Drug repositioning of mevalonate pathway inhibitors as antitumor agents for ovarian cancer. Oncotarget 2017;8:72147–56. 10.18632/oncotarget.20046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Reusser NM, Dalton HJ, Pradeep S, et al. Clodronate inhibits tumor angiogenesis in mouse models of ovarian cancer. Cancer Biol Ther 2014;15:1061–7. 10.4161/cbt.29184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Germano G, Frapolli R, Belgiovine C, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 2013;23:249–62. 10.1016/j.ccr.2013.01.008 [DOI] [PubMed] [Google Scholar]
- 130.Rodriguez-Garcia A, Lynn RC, Poussin M, et al. Car-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat Commun 2021;12:877. 10.1038/s41467-021-20893-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Binnewies M, Pollack JL, Rudolph J, et al. Targeting TREM2 on tumor-associated macrophages enhances immunotherapy. Cell Rep 2021;37:109844. 10.1016/j.celrep.2021.109844 [DOI] [PubMed] [Google Scholar]
- 132.Molgora M, Esaulova E, Vermi W, et al. TREM2 modulation remodels the tumor myeloid landscape enhancing anti-PD-1 immunotherapy. Cell 2020;182:886–900. 10.1016/j.cell.2020.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pittet MJ, Michielin O, Migliorini D. Clinical relevance of tumour-associated macrophages. Nat Rev Clin Oncol 2022;19:402–21. 10.1038/s41571-022-00620-6 [DOI] [PubMed] [Google Scholar]
- 134.Pahlavanneshan S, Sayadmanesh A, Ebrahimiyan H, et al. Toll-like receptor-based strategies for cancer immunotherapy. J Immunol Res 2021;2021:9912188. 10.1155/2021/9912188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Geller MA, Cooley S, Argenta PA, et al. Toll-like receptor-7 agonist administered subcutaneously in a prolonged dosing schedule in heavily pretreated recurrent breast, ovarian, and cervix cancers. Cancer Immunol Immunother 2010;59:1877–84. 10.1007/s00262-010-0914-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Monk BJ, Brady MF, Aghajanian C, et al. A phase 2, randomized, double-blind, placebo- controlled study of chemo-immunotherapy combination using motolimod with pegylated liposomal doxorubicin in recurrent or persistent ovarian cancer: A gynecologic oncology group partners study. Ann Oncol 2017;28:996–1004. 10.1093/annonc/mdx049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kang Y, Flores L, Ngai HW, et al. Large, anionic liposomes enable targeted intraperitoneal delivery of a TLR 7/8 agonist to repolarize ovarian tumors’ microenvironment. Bioconjug Chem 2021;32:1581–92. 10.1021/acs.bioconjchem.1c00139 [DOI] [PubMed] [Google Scholar]
- 138.Le F, Yang L, Han Y, et al. TPL inhibits the invasion and migration of drug-resistant ovarian cancer by targeting the PI3K/AKT/NF-κb-signaling pathway to inhibit the polarization of M2 tams. Front Oncol 2021;11:704001. 10.3389/fonc.2021.704001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Meng C, Zhu H, Song H, et al. Targets and molecular mechanisms of triptolide in cancer therapy. Chin J Cancer Res 2014;26:622–6. 10.3978/j.issn.1000-9604.2014.09.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kaneda MM, Messer KS, Ralainirina N, et al. PI3Kγ is a molecular switch that controls immune suppression. Nature 2016;539:437–42. 10.1038/nature19834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.De Henau O, Rausch M, Winkler D, et al. Overcoming resistance to checkpoint blockade therapy by targeting pi3kγ in myeloid cells. Nature 2016;539:443–7. 10.1038/nature20554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Suttles J, Stout RD. Macrophage CD40 signaling: a pivotal regulator of disease protection and pathogenesis. Semin Immunol 2009;21:257–64. 10.1016/j.smim.2009.05.011 [DOI] [PubMed] [Google Scholar]
- 143.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011;331:1612–6. 10.1126/science.1198443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kashyap AS, Schmittnaegel M, Rigamonti N, et al. Optimized antiangiogenic reprogramming of the tumor microenvironment potentiates CD40 immunotherapy. Proc Natl Acad Sci U S A 2020;117:541–51. 10.1073/pnas.1902145116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Garris CS, Arlauckas SP, Kohler RH, et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-γ and IL-12. Immunity 2018;49:1148–61. 10.1016/j.immuni.2018.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vonderheide RH, Burg JM, Mick R, et al. Phase I study of the CD40 agonist antibody CP-870,893 combined with carboplatin and paclitaxel in patients with advanced solid tumors. Oncoimmunology 2013;2:e23033. 10.4161/onci.23033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ye S, Cohen D, Belmar NA, et al. A bispecific molecule targeting CD40 and tumor antigen mesothelin enhances tumor-specific immunity. Cancer Immunol Res 2019;7:1864–75. 10.1158/2326-6066.CIR-18-0805 [DOI] [PubMed] [Google Scholar]
- 148.Kuhn NF, Purdon TJ, van Leeuwen DG, et al. Cd40 ligand-modified chimeric antigen receptor T cells enhance antitumor function by eliciting an endogenous antitumor response. Cancer Cell 2019;35:473–88. 10.1016/j.ccell.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Eriksson E, Milenova I, Wenthe J, et al. Shaping the tumor stroma and sparking immune activation by CD40 and 4-1BB signaling induced by an armed oncolytic virus. Clin Cancer Res 2017;23:5846–57. 10.1158/1078-0432.CCR-17-0285 [DOI] [PubMed] [Google Scholar]
- 150.Duluc D, Corvaisier M, Blanchard S, et al. Interferon-Gamma reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. Int J Cancer 2009;125:367–73. 10.1002/ijc.24401 [DOI] [PubMed] [Google Scholar]
- 151.Castro F, Cardoso AP, Gonçalves RM, et al. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front Immunol 2018;9:847. 10.3389/fimmu.2018.00847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sun Y. Therapeutic effect of recombinant plasmid-encoded human interleukin-12 in tumor-bearing mice. Mol Med Rep 2012;6:645–50. 10.3892/mmr.2012.973 [DOI] [PubMed] [Google Scholar]
- 153.Anwer K, Barnes MN, Fewell J, et al. Phase-I clinical trial of IL-12 plasmid/lipopolymer complexes for the treatment of recurrent ovarian cancer. Gene Ther 2010;17:360–9. 10.1038/gt.2009.159 [DOI] [PubMed] [Google Scholar]
- 154.Anwer K, Kelly FJ, Chu C, et al. Phase I trial of a formulated IL-12 plasmid in combination with carboplatin and docetaxel chemotherapy in the treatment of platinum-sensitive recurrent ovarian cancer. Gynecol Oncol 2013;131:169–73. 10.1016/j.ygyno.2013.07.081 [DOI] [PubMed] [Google Scholar]
- 155.Thaker PH, Bradley WH, Leath CA, et al. GEN-1 in combination with neoadjuvant chemotherapy for patients with advanced epithelial ovarian cancer: A phase I dose-escalation study. Clin Cancer Res 2021;27:5536–45. 10.1158/1078-0432.CCR-21-0360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Spear P, Barber A, Rynda-Apple A, et al. Chimeric antigen receptor T cells shape myeloid cell function within the tumor microenvironment through IFN-γ and GM-CSF. J Immunol 2012;188:6389–98. 10.4049/jimmunol.1103019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chmielewski M, Kopecky C, Hombach AA, et al. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res 2011;71:5697–706. 10.1158/0008-5472.CAN-11-0103 [DOI] [PubMed] [Google Scholar]
- 158.Brempelis KJ, Cowan CM, Kreuser SA, et al. Genetically engineered macrophages persist in solid tumors and locally deliver therapeutic proteins to activate immune responses. J Immunother Cancer 2020;8:e001356. 10.1136/jitc-2020-001356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Klichinsky M, Ruella M, Shestova O, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol 2020;38:947–53. 10.1038/s41587-020-0462-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol 2013;5:a008748. 10.1101/cshperspect.a008748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brightwell RM, Grzankowski KS, Lele S, et al. The CD47 “ do’n’t eat me signal” is highly expressed in human ovarian cancer. Gynecol Oncol 2016;143:393–7. 10.1016/j.ygyno.2016.08.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kristiansen G, Denkert C, Schlüns K, et al. Cd24 is expressed in ovarian cancer and is a new independent prognostic marker of patient survival. Am J Pathol 2002;161:1215–21. 10.1016/S0002-9440(10)64398-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu R, Wei H, Gao P, et al. Cd47 promotes ovarian cancer progression by inhibiting macrophage phagocytosis. Oncotarget 2017;8:39021–32. 10.18632/oncotarget.16547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nakamura K, Terai Y, Tanabe A, et al. Cd24 expression is a marker for predicting clinical outcome and regulates the epithelial-mesenchymal transition in ovarian cancer via both the Akt and ERK pathways. Oncol Rep 2017;37:3189–200. 10.3892/or.2017.5583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Barkal AA, Brewer RE, Markovic M, et al. Cd24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 2019;572:392–6. 10.1038/s41586-019-1456-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Osborn G, Stavraka C, Adams R, et al. Macrophages in ovarian cancer and their interactions with monoclonal antibody therapies. Clin Exp Immunol 2022;209:4–21. 10.1093/cei/uxab020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sikic BI, Lakhani N, Patnaik A, et al. First-in-human, first-in-class phase I trial of the anti-CD47 antibody hu5f9-G4 in patients with advanced cancers. J Clin Oncol 2019;37:946–53. 10.1200/JCO.18.02018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mbongue JC, Nicholas DA, Torrez TW, et al. The role of indoleamine 2, 3-dioxygenase in immune suppression and autoimmunity. Vaccines (Basel) 2015;3:703–29. 10.3390/vaccines3030703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Uyttenhove C, Pilotte L, Théate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 2003;9:1269–74. 10.1038/nm934 [DOI] [PubMed] [Google Scholar]
- 170.Okamoto A, Nikaido T, Ochiai K, et al. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res 2005;11:6030–9. 10.1158/1078-0432.CCR-04-2671 [DOI] [PubMed] [Google Scholar]
- 171.Tanizaki Y, Kobayashi A, Toujima S, et al. Indoleamine 2,3-dioxygenase promotes peritoneal metastasis of ovarian cancer by inducing an immunosuppressive environment. Cancer Sci 2014;105:966–73. 10.1111/cas.12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ma H, Qin Q, Mi J, et al. 1-MT inhibits the invasion of CBP-resistant ovarian cancer cells via down-regulating IDO expression and re-activating immune cells function. BMC Pharmacol Toxicol 2020;21:67. 10.1186/s40360-020-00439-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Komiya T, Huang CH. Updates in the clinical development of epacadostat and other indoleamine 2,3-dioxygenase 1 inhibitors (IDO1) for human cancers. Front Oncol 2018;8:423. 10.3389/fonc.2018.00423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mitchell TC, Hamid O, Smith DC, et al. Epacadostat plus pembrolizumab in patients with advanced solid tumors: phase I results from a multicenter, open-label phase I/II trial (ECHO-202/KEYNOTE-037). J Clin Oncol 2018;36:3223–30. 10.1200/JCO.2018.78.9602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nayak-Kapoor A, Hao Z, Sadek R, et al. Phase Ia study of the indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor navoximod (GDC-0919) in patients with recurrent advanced solid tumors. J Immunother Cancer 2018;6:61. 10.1186/s40425-018-0351-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kristeleit R, Davidenko I, Shirinkin V, et al. A randomised, open-label, phase 2 study of the IDO1 inhibitor epacadostat (INCB024360) versus tamoxifen as therapy for biochemically recurrent (CA-125 relapse)-only epithelial ovarian cancer, primary peritoneal carcinoma, or fallopian tube cancer. Gynecol Oncol 2017;146:484–90. 10.1016/j.ygyno.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 177.Odunsi K, Qian F, Lugade AA, et al. Metabolic adaptation of ovarian tumors in patients treated with an IDO1 inhibitor constrains antitumor immune responses. Sci Transl Med 2022;14:eabg8402. 10.1126/scitranslmed.abg8402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 2007;104:3360–5. 10.1073/pnas.0611533104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Topalian SL, Hodi FS, Brahmer JR, et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol 2019;5:1411–20. 10.1001/jamaoncol.2019.2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tseng SY, Otsuji M, Gorski K, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med 2001;193:839–46. 10.1084/jem.193.7.839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zou W, Wolchok JD, Chen L. Pd-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med 2016;8:328rv4. 10.1126/scitranslmed.aad7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hartley GP, Chow L, Ammons DT, et al. Programmed cell death ligand 1 (PD-L1) signaling regulates macrophage proliferation and activation. Cancer Immunol Res 2018;6:1260–73. 10.1158/2326-6066.CIR-17-0537 [DOI] [PubMed] [Google Scholar]
- 183.Liu JF, Gordon M, Veneris J, et al. Safety, clinical activity and biomarker assessments of atezolizumab from a phase I study in advanced/recurrent ovarian and uterine cancers. Gynecol Oncol 2019;154:314–22. 10.1016/j.ygyno.2019.05.021 [DOI] [PubMed] [Google Scholar]
- 184.Lee EK, Konstantinopoulos PA. Parp inhibition and immune modulation: scientific rationale and perspectives for the treatment of gynecologic cancers. Ther Adv Med Oncol 2020;12:1758835920944116. 10.1177/1758835920944116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li A, Yi M, Qin S, et al. Prospects for combining immune checkpoint blockade with PARP inhibition. J Hematol Oncol 2019;12:98. 10.1186/s13045-019-0784-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pilié PG, Gay CM, Byers LA, et al. Parp inhibitors: extending benefit beyond BRCA-mutant cancers. Clin Cancer Res 2019;25:3759–71. 10.1158/1078-0432.CCR-18-0968 [DOI] [PubMed] [Google Scholar]
- 187.Jiao S, Xia W, Yamaguchi H, et al. Parp inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res 2017;23:3711–20. 10.1158/1078-0432.CCR-16-3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Domchek SM, Postel-Vinay S, Im S-A, et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study. Lancet Oncol 2020;21:1155–64. 10.1016/S1470-2045(20)30324-7 [DOI] [PubMed] [Google Scholar]
- 189.Lee J-M, Cimino-Mathews A, Peer CJ, et al. Safety and clinical activity of the programmed death-ligand 1 inhibitor durvalumab in combination with poly (ADP-ribose) polymerase inhibitor olaparib or vascular endothelial growth factor receptor 1-3 inhibitor cediranib in women’s cancers: a dose-escalation, phase I study. J Clin Oncol 2017;35:2193–202. 10.1200/JCO.2016.72.1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lampert EJ, Zimmer A, Padget M, et al. Combination of PARP inhibitor olaparib, and PD-L1 inhibitor durvalumab, in recurrent ovarian cancer: a proof-of-concept phase II study. Clin Cancer Res 2020;26:4268–79. 10.1158/1078-0432.CCR-20-0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shrimali RK, Yu Z, Theoret MR, et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res 2010;70:6171–80. 10.1158/0008-5472.CAN-10-0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liu JF, Herold C, Gray KP, et al. Assessment of combined nivolumab and bevacizumab in relapsed ovarian cancer: A phase 2 clinical trial. JAMA Oncol 2019;5:1731–8. 10.1001/jamaoncol.2019.3343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Giornelli GH. Management of relapsed ovarian cancer: a review. Springerplus 2016;5:1197. 10.1186/s40064-016-2660-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Haibe Y, Kreidieh M, El Hajj H, et al. Resistance mechanisms to anti-angiogenic therapies in cancer. Front Oncol 2020;10:221. 10.3389/fonc.2020.00221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lyons YA, Pradeep S, Wu SY, et al. Macrophage depletion through colony stimulating factor 1 receptor pathway blockade overcomes adaptive resistance to anti-VEGF therapy. Oncotarget 2017;8:96496–505. 10.18632/oncotarget.20410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rivera LB, Meyronet D, Hervieu V, et al. Intratumoral myeloid cells regulate responsiveness and resistance to antiangiogenic therapy. Cell Rep 2015;11:577–91. 10.1016/j.celrep.2015.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]