Previous studies have established the increased susceptibility of patients with cardiovascular comorbidities to COVID-19 and associated worse outcomes.1 , 2 Heart transplant (HT) recipients often have an increased burden of comorbidities and are also receiving chronic immunosuppressive therapies. Immunosuppression can increase susceptibility to infections but has also been hypothesized to blunt the cytokine storm in COVID-19 patients. Further, recipients of solid organ transplants have been proven to be at greater risk for complications from viral respiratory infections such as seasonal influenza than the general population. However, the effect of COVID-19 on HT recipients remains to be fully elucidated.
We aimed to evaluate the demographic and clinical characteristics, and effect of COVID-19 compared with influenza infection in HT recipients in a large, nationally representative database.
Methods
We queried the National Inpatient Sample from 2019 to 2020 for all adult (≥ 18 years) hospitalizations for COVID-19 or influenza in patients with a history of HT. The primary outcome of interest was in-hospital mortality. Secondary outcomes included respiratory failure, length of stay, and hospitalization costs. A 1:1 propensity score-matched analysis was performed to adjust for possible confounders. STATA 16.0 (StataCorp, College Station, TX) was used for analysis.
Results
We identified 34,930 hospitalizations (18,735 in 2019 and 16,195 in 2020) of patients with a history of HT. There was a total of 535 (1.5%) influenza-related hospitalizations and 1165 (3.3%) COVID-19-associated hospitalizations in patients with a history of HT from 2019 to 2020.
HT patients hospitalized with COVID-19 compared with influenza were more likely to be older (median age 62 vs 55 years; P < 0.001) and had a higher prevalence of comorbidities such as diabetes (53.0% vs 39.3%; P = 0.01), chronic kidney disease (70.5% vs 58.9%; P = 0.04), dementia (3.9% vs 0%; P = 0.04), and obesity (22.2% vs 9.3%; P = 0.004). There was no significant difference in chronic obstructive pulmonary disease among the 2 groups (18.7% vs 17.1%; P = 0.72).
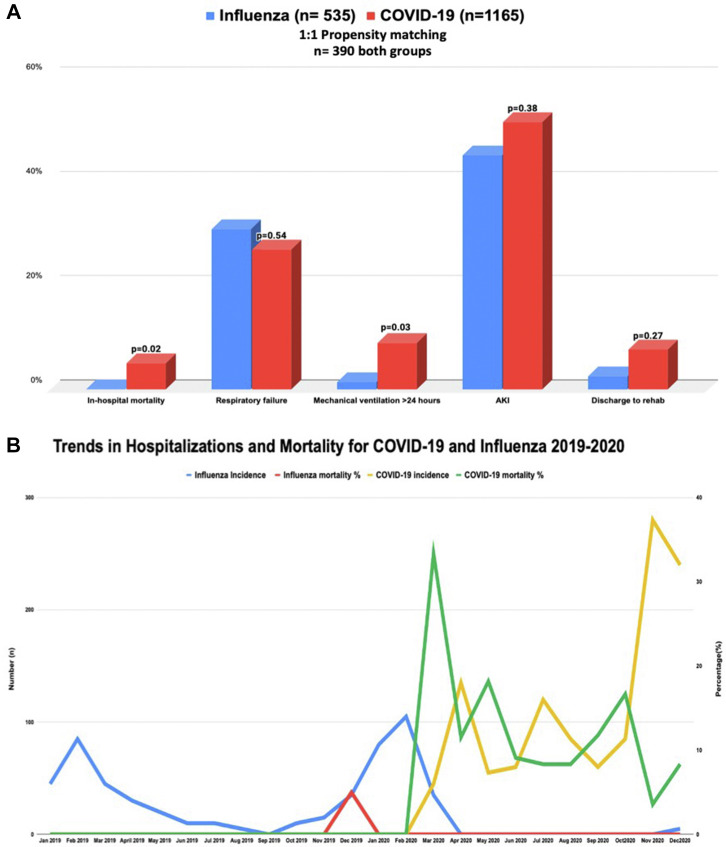
After propensity score-matching, there were 390 hospitalizations in each group. The in-hospital mortality was significantly higher among HT patients with COVID-19 infection compared with influenza infection (20 [5.1%] vs < 10 [0%]; P = 0.02). Respiratory failure requiring intubation > 24 hours was also more common in HT patients with COVID-19 (8.9% vs 1.3%; P = 0.03). Myocarditis was low and comparable in both groups (COVID-19, 5 [0.43%] vs influenza, 0 [0%]; P = 0.50; propensity matching could not be performed because of low number of participants), as was rejection (10 [2.5%] vs 0 [0%]; P = 0.10).
The median length of stay was significantly longer in HT patients with COVID-19 compared with those with influenza (5 [interquartile range {IQR}, 3-9] vs 3.5 [IQR, 2-5] days; P = 0.01), as was the median cost of hospitalization ($12,620 [IQR, 6561-24,912] vs $8208 [IQR, 5590-16,456]; P = 0.01).
The prevalence of HT patients with concomitant COVID-19 increased through the year in 2020 whereas a decrease in influenza infection in HT recipients was noted (Fig. 1 ). The highest mortality was noted in the initial phase of the pandemic with a significant decrease through the year.
Figure 1.
(A) Propensity score-matched outcomes of COVID-19 vs influenza in heart transplant recipients. (B) Trends in hospitalizations and mortality. Left y axis: number (n); right y axis: percentage (%).
Discussion
To the best our knowledge, this is the first study to directly compare outcomes of COVID-19 and influenza infection in HT recipients.
HT recipients using chronic immunosuppressive medications might have greater viral burden, heightened infectivity, and worse outcomes. Elevated cytokine production and systemic inflammatory state play a critical role in COVID-19 infection whereas the interaction between immunomodulators and the inflammatory state is poorly understood.3 Previous reports have shown a worse mortality with COVID-19 in HT recipients—as high as 20%-30%.3 , 4 Compared with our study, most of these studies were done in the early phases of the pandemic and are comparable to mortality noted in the earlier phase of our study.3 , 4
The American Society of Transplantation recommends annual influenza vaccination for HT recipients because of the higher mortality rate in this vulnerable population. Previous studies have reported worse mortality with COVID-19 compared with influenza in the general population.5 This was also evident in our study of HT recipients, in whom COVID-19 was associated with a significantly higher mortality rate after propensity score-matching for comorbidities.
In our study we used a national database, overcoming the biases with single-center studies, however, there might be misclassification errors and potential confounding. Further, the differential in the vaccination rates of both groups likely also affects outcomes because COVID-19 vaccination was not available in 2020. In summary, among patients hospitalized with a history of HT, COVID-19 was associated with an increased risk of mortality and respiratory failure, and healthcare utilization compared with influenza infection.
Acknowledgments
Funding Sources
None.
Disclosures
Dr Fonarow discloses consulting for Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Eli Lilly, Janssen, Medtronic, Merck, Novartis, and Pfizer. Dr Bhatt discloses the following relationships—Advisory Board: AngioWave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Stasys; Board of Directors: AngioWave (stock options), Boston VA Research Institute, Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock), Society of Cardiovascular Patient Care, and TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Consultant: Broadview Ventures; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical), Novartis, Population Health Research Institute; Rutgers University (for the NIH-funded MINT Trial); Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org" title="http://ACC.org">ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Wiley (steering committee); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women’s Hospital who assigned to Lexicon; neither Dr Bhatt nor Brigham and Women’s Hospital receive any income from this patent); Research Funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, and 89Bio; Royalties: Elsevier (Editor, Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, and Vascular Solutions; Trustee: American College of Cardiology; Unfunded Research: FlowCo, and Takeda. The remaining authors have no conflicts of interest to disclose.
References
- 1.Isath A., Malik A.H., Goel A., et al. Nationwide analysis of the outcomes and mortality of hospitalized COVID-19 patients. Curr Probl Cardiol. 2023;48 doi: 10.1016/j.cpcardiol.2022.101440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satterfield B.A., Bhatt D.L., Gersh B.J. Cardiac involvement in the long-term implications of COVID-19. Nat Rev Cardiol. 2022;19:332–341. doi: 10.1038/s41569-021-00631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alfishawy M., Elbendary A., Mohamed M., et al. COVID-19 mortality in transplant recipients. Int J Organ Transplant Med. 2020;11:145–162. [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed F., Abid M., Maniya T., et al. Incidence and prognosis of COVID-19 amongst heart transplant recipients: a systematic review and meta-analysis. Eur J Prev Cardiol. 2021;29:e224–e226. doi: 10.1093/eurjpc/zwab175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan M.S., Shahid I., Anker S.D., et al. Cardiovascular implications of COVID-19 versus influenza infection: a review. BMC Med. 2020;18:403. doi: 10.1186/s12916-020-01816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]