Abstract
Steroidal mineralocorticoid-receptor-antagonists (MRAs), such as spironolactone and eplerenone, are guideline-directed therapies in patients with heart failure with reduced ejection fraction or resistant hypertension. However, the associated risk of hyperkalemia and hormonal side effects limit their broad use and downstream cardiorenal protection in high-risk patients with type 2 diabetes mellitus (T2DM) and moderate-to-advanced chronic kidney disease (CKD). The critical unmet need to improve long-term cardiorenal outcomes in such patients with CKD has sparked considerable efforts to the discovery and development of a new class of compounds. Finerenone is a novel, nonsteroidal MRA that has recently received regulatory approval with the indication of cardiorenal protection in patients with CKD associated with T2DM. Two landmark phase 3 clinical trials, FIDELIO-DKD and FIGARO-DKD, demonstrated that among patients with T2DM and a broad spectrum of CKD, finerenone reduced the risk of “hard” cardiovascular and kidney failure outcomes as compared with placebo, with a minimal risk of hyperkalemia. Subgroup analyses of these trials also provided preliminary evidence that the efficacy and safety profile of finerenone was similar and irrespective of background therapy with other guideline-directed therapies, such as sodium-glucose co-transporter type 2 (SGLT-2) inhibitors and glucagone-like peptide 1 receptor agonists. Whether the combination of finerenone with a SGLT-2 inhibitor is more beneficial in patients with T2DM and CKD as compared with either therapy alone is a crucial research question that is currently under investigation in an ongoing clinical trial.
Keywords: blood pressure, cardiorenal outcomes, CKD, finerenone, hypertension, non-steroidal MRAs, type 2 diabetes
STEROIDAL MINERALOCORTICOID-RECEPTOR-ANTAGONISTS―A BRIEF OVERVIEW
The mineralocorticoid receptor (MR) belongs to the group of nuclear hormone receptors and is broadly expressed in several disease-relevant tissues, such as in the heart, kidneys, vasculature, fibroblasts, and immune cells.1 Physiologically, the MR is an important regulator of fluid, electrolyte, and hemodynamic homeostasis. However, in cardiorenal disease states, such as in patients with type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD), there is pathophysiological overactivation of the MR that is not directly related to plasma aldosterone levels.2,3 For example, experimental studies have shown that Rac1, a member of the Rho family GTPases, acts as a potent activator of MR signal transduction both in vitro and in vivo independently from systemic aldosterone status.2,3 Overactivation of the MR promotes inflammation and fibrosis, leading to progression of end-organ damage.4 These deleterious actions extend above and beyond the well-documented retention of sodium and fluid and downstream hypertension.4 Accordingly, pharmacological inhibition of the MR is for long been considered an attractive therapeutic opportunity to down-regulate proinflammatory and profibrotic pathways with the aim to offer cardiorenal protection.4,5
Over the past decades, 2 MR antagonists (MRAs) with steroidal chemical structures were developed. The first one, spironolactone, was discovered in 1957 and originally received regulatory approval for use as a diuretic for the treatment of edematous conditions, primary aldosteronism, and essential hypertension.1 The second steroidal MRA, eplerenone, was developed in 1987 as a more selective version of spironolactone.1 After a prolonged period of hiatus, landmark trials published in late 1990s and thereafter demonstrated the efficacy of add-on therapy with spironolactone and/or eplerenone in lowering the risk of cardiovascular morbidity and mortality among patients with severe heart failure with reduced ejection fraction (HFrEF).6,7 Based on this firm clinical-trial evidence, steroidal MRAs have received the strongest recommendation (Level 1A) in clinical guidelines for the treatment of HFrEF.8 However, these lifesaving therapies remain underused in daily clinical practice, even in patients with an estimated-glomerular-filtration rate (eGFR) ≥45 ml/min/1.73 m2, where their administration is not contraindicated.9 Real-world data from population-based studies indicate that the incidence of hyperkalemia after initiation of spironolactone or eplerenone is high; hyperkalemic events are frequently followed by transient or permanent discontinuation of these therapies.10–12 Therefore, the associated risk of hyperkalemia is a factor that limits the broad use of steroidal MRAs, particularly in high-risk patients with moderate-to-advanced CKD.
Despite the solid body of evidence in HFrEF, data from clinical trials to prove the efficacy of steroidal MRAs in retarding the progression of CKD were sparse.4,13 The efficacy and safety of spironolactone and/or eplerenone when added to background therapy with an angiotensin-converting-enzyme-inhibitor or an angiotensin-receptor-blocker was explored in a 2020 Cochrane meta-analysis of 44 trials involving a total of 5,745 patients with albuminuric CKD.14 Compared with placebo or standard-of-care treatment, add-on administration of steroidal MRAs was associated with potential benefits on intermediate endpoints of cardiorenal diseases, such as albuminuria, eGFR slope, and clinic systolic blood pressure (BP).14 However, these beneficial effects were accompanied by a 2.17-fold increase in the risk of hyperkalemia, a 2.04-fold higher risk of acute kidney injury (AKI), and a 5.14-fold higher incidence of gynecomastia.14 The risks of hyperkalemia and AKI accumulate over time, but none of the trials included in this meta-analysis had a long-term follow-up. Furthermore, none of these trials was adequately powered to detect benefits on clinical outcomes, such as kidney failure, major adverse cardiovascular events, or all-cause death.14 In the absence of long-term clinical-trial data, the benefit/risk ratio of add-on therapy with a steroidal MRA in patients with mild-to-moderate CKD remains uncertain.
The critical unmet need to improve long-term clinical outcomes in patients with cardiorenal diseases has recently led to the discovery and development of a new class of compounds, the nonsteroidal MRAs. These agents have been developed with the rationale to improve the side-effect profile of spironolactone and eplerenone, while maintaining potent and selective blockade of the MR.5 Esaxerenone is a novel, nonsteroidal MRA with a potent BP-lowering action that has been approved for the treatment of essential hypertension in Japan.15 Remission of albuminuria to normal levels with esaxerenone relative to placebo over 52 weeks of treatment was observed in a single-country phase 3 trial enrolling 455 patients with T2DM and high albuminuria.16 The incidence of hyperkalemia was higher with esaxerenone than with placebo, but these events were asymptomatic and easily reversible after dosage reduction or discontinuation of the trial regimen.16 Finerenone is another non-steroidal MRA that has been recently approved by the US Food and Drug Administration (FDA) with the indication of cardiorenal protection in adults with CKD associated with T2DM.17 Heterogeneity in clinical efficacy across nonsteroidal MRAs possibly exists, but definitive conclusions can not be made in the absence of trials to provide direct head-to-head comparisons.
In the following sections of this article, we describe the mechanism of action of finerenone and we explore the safety and efficacy of this agent, as demonstrated in a large phase 3 clinical-trial program involving >13,000 patients with T2DM and a broad spectrum of CKD.
THE MECHANISM OF ACTION OF FINERENONE
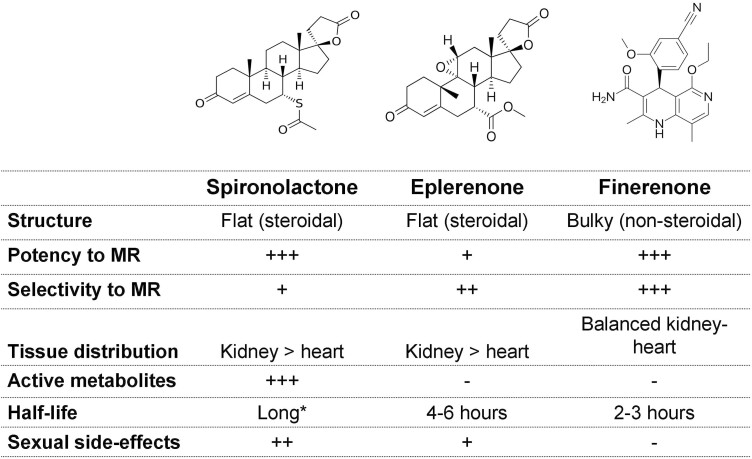
Finerenone is a compound with a nonsteroidal structure that exhibits higher selectivity for the MR over all other steroid hormone receptors and high binding affinity.1 Unlike spironolactone and eplerenone, finerenone binds to the MR as a “bulky” antagonist to inhibit transcriptional cofactor recruitment implicated in the expression of hypertrophic, proinflammatory, and profibrotic genes.18 This unique mechanism of action results in MR inhibition that is at least as potent as with spironolactone and more selective as compared with spironolactone or eplerenone (Figure 1).1
Figure 1.
Key differences in the mechanism of action and pharmacological properties between steroidal mineralocorticoid-receptor-antagonists (MRAs) and finerenone. * Spironolactone is a prodrug with several biologically active metabolites with long plasma half-lives that accumulate over time, particularly in patients with moderate-to-advanced chronic kidney disease.
In preclinical models of kidney injury, the administration of finerenone was associated with less kidney hypertrophy and protection against glomerular, tubulointerstitial, and vascular damage.19–21 These kidney protective effects were mediated through downregulation of renal proinflammatory/profibrotic gene expression in response to kidney injury and were paralleled with a dose-dependent improvement in albuminuria.19–21 In animal models of cardiac fibrosis, finerenone therapy resulted in regression of cardiac hypertrophy, a benefit that was accompanied by a parallel reduction in albuminuria and other markers of inflammation and fibrosis.22–24 These beneficial effects were observed even when finerenone was administered at dosages not inducing significant hemodynamic effects, suggesting that finerenone can possibly protect the heart and the kidney in a BP-independent manner.19–24 Notably, in different rodent models, the antiproliferative, antifibrotic, and anti-inflammatory actions of finerenone were more potent as compared with equinatriuretic doses of eplerenone.1,22,25 The greater end-organ protection with finerenone than with steroidal MRAs may be attributable to the unique binding mode of finerenone to the MR, which leads to more selective cofactor recruitment and distinct target gene regulation.1,22,25
Preclinical studies also provided preliminary evidence that finerenone confers cardiorenal protection with a minimal impact on serum potassium levels, suggesting a more favorable side-effect profile of this novel, nonsteroidal MRA.26 As an example, in an animal model of rapidly progressive glomerulonephritis, BR-4628, a precursor to finerenone, provided substantial suppression of kidney injury mediated through antiinflammatory and antifibrotic actions, without affecting urinary sodium and potassium excretion and without inducing hyperkalemia.27 Several potential mechanisms may contribute to the less hyperkalemic effect of finerenone as compared to steroidal MRAs: (i) the unique mode of action of finerenone and subsequent transcriptional cofactor recruitment28; (ii) its distinct pharmacokinetic properties, such as its short plasma half-live and lack of biologically active metabolites29,30; and (iii) it is equal tissue distribution to the heart and the kidney that contrasts with the predominant renal accumulation of spironolactone and eplerenone.1,31
PHASE 2 CLINICAL TRIALS
A phase 2 clinical trial program was designed to investigate the safety and efficacy of finerenone in >2,000 patients with HFrEF, CKD, and/or T2DM or in patients with CKD and T2DM (Table 1).32–34
Table 1.
Summary of phase 2 clinical trials evaluating the safety and efficacy of finerenone relative to placebo or active-treatment with steroidal MRAs
| Parameter | ARTS34 | ARTS-HF33 | ARTS-DN32 |
|---|---|---|---|
| Year | 2013 | 2016 | 2015 |
| Study population | HFrEF and stage 2 CKD in Part A or stage 3 CKD in Part B | HFrEF and CKD and/or T2DM | T2DM and high or very high albuminuria in patients already treated with an ACEI/ARB |
| N | Part A: 65 patients Part B: 392 patients |
1,066 patients | 823 patients |
| Active-treatment | Part A: finerenone 2.5, 5, or 10 mg/day Part B: finerenone 2.5, 5, or 10 mg/day or 5 mg twice daily |
Finerenone 2.5, 5, 7.5, 10, or 15 mg/day (titrated up to 5, 10, 15, 20, or 20 mg/day, respectively on Day 30) | Finerenone 1.25, 2.5, 5, 7.5, 10, 15, or 20 mg once daily |
| Comparators | Part B: placebo or active treatment with spironolactone (25 or 50 mg/day) | Eplerenone (25 mg every other day, titrated up to 25/day on Day 30 and up to 50 mg/day on Day 60) | Placebo |
| Follow-up | 28 days | 90 days | 90 days |
| Primary outcome | Change in sK during follow-up | Percentage of patients with >30% decline in NT-proBNP during follow-up | Change in UACR |
| Main results | • Significantly smaller increases in sK levels with finerenone than with spironolactone • Finerenone was at least as effective as spironolactone in lowering NT-proBNP levels and albuminuria |
• Finerenone was equally effective with eplerenone in causing >30% reduction in NT-proBNP levels • The prespecified exploratory endpoint of all-cause death, cardiovascular hospitalization, or acute worsening HF was numerically less common with finerenone than with eplerenone |
• Dose-dependent reduction in UACR. • Permanent drug discontinuation due to hyperkalemia not seen with placebo or finerenone 10 mg/day. • Incidence rates of hyperkalemia leading to permanent drug discontinuation: 2.1%, 3.2%, and 1.7% in the finerenone 7.5-, 15-, and 20-mg/day groups, respectively. |
Abbreviations: ACEI, angiotensin-converting-enzyme-inhibitor; ARB, angiotensin-receptor-blocker; CKD, chronic kidney disease; HFrEF, heart failure with reduced ejection fraction; MRAs, mineralocorticoid-receptor-antagonists; NT-proBNP,N-terminal pro-B-type natriuretic peptide; T2DM, diabetes mellitus type; 2UACR, urinary albumin-to-creatinine ratio.
In the ARTS (MR Antagonist Tolerability Study) trial,34 the safety and tolerability of finerenone were compared with placebo and active treatment with spironolactone in 392 patients with HFrEF and moderate CKD. Over 28 days of follow-up, finerenone was associated with significantly smaller mean elevations in serum potassium levels as compared with spironolactone (0.04–0.30 vs. 0.45 mmol/l, respectively) and lower incidences of hyperkalemia (5.3% vs. 12.7%, respectively).34 In addition, the adverse events related to AKI occurred less commonly with finerenone than with spironolactone. With respect to the efficacy, finerenone and spironolactone provoked similar short-term improvement in biomarkers of hemodynamic stress, such as reductions in the levels of B-type natriuretic peptide (BNP), N-terminal proBNP (NT-proBNP) and albuminuria.34
In ARTS-HF (ARTS-Heart Failure) trial,33 different oral doses of finerenone were compared with eplerenone in 1,066 patients with severe HFrEF, CKD, and/or T2DM. The proportion of patients achieving >30% decline in plasma NT-proBNP levels from baseline to day 90 was similar in all dosing regimens of finerenone as compared with eplerenone. Although this phase 2 trial was not originally designed to explore between-group differences in clinical events, the exploratory composite endpoint of all-cause death, cardiovascular hospitalization, or emergency hospital admission due to worsening heart failure occurred less commonly in finerenone-treated patients than in eplerenone-treated patients.33 This was remarkable, given that the trial duration was only 90 days. With respect to safety, the overall incidence of hyperkalemia (defined as serum potassium ≥5.6 mmol/l at any time point) was 4.3%, with a balanced distribution among all treatment arms.33
In ARTS-DN (ARTS-Diabetic Nephropathy) trial,32 different oral doses of finerenone were compared with a placebo in 823 patients with T2DM and high or very high albuminuria, who were receiving background therapy with an angiotensin-converting-enzyme-inhibitor or an angiotensin-receptor-blocker. A dose-dependent reduction in urinary albumin-to-creatinine ratio (UACR) from baseline to day 90 relative to placebo was evident with finerenone (21%–38% in the finerenone 7.5-, 10-, 15-, and 20-mg/day groups vs. placebo).32 The prespecified secondary endpoint of permanent drug discontinuation due to hyperkalemia was not observed in the placebo and finerenone 10-mg/day groups, whereas incidences in the finerenone 7.5-, 15-, and 20-mg/day groups were 2.1%, 3.2%, and 1.7% respectively. In addition, the incidence of prespecified secondary endpoint of ≥30% decline in eGFR as well as incidences of other serious adverse events did not significantly differ between the finerenone and placebo groups.32 The favorable benefit-risk profile of finerenone in ARTS-DN provided the rationale for the design of a large phase 3 clinical-trial program aiming to investigate whether finerenone is superior to placebo in improving “hard” kidney failure and cardiovascular outcomes in patients with T2DM and CKD.
PHASE 3 CLINICAL TRIALS
The phase 3 clinical trial program of finerenone consisted of 2 complementary in nature, double-blind, randomized, placebo-controlled studies: the FIDELIO-DKD (FInerenone in reducing kiDnEy faiLure and dIsease prOgression in Diabetic Kidney Disease) and FIGARO-DKD (FInerenone in reducinG cArdiovascular moRtality and mOrbidity in Diabetic Kidney Disease) (Table 2).35–37 FIDELIO-DKD was designed to investigate the effect of finerenone on a primary composite kidney endpoint, defined as the time to onset of kidney failure, sustained ≥40% decline in eGFR from baseline or death from renal causes.38 FIGARO-DKD aimed to investigate the effect of finerenone on a primary composite cardiovascular endpoint, defined as the time to cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure.39 To improve the ability to explore the kidney protective effect of finerenone, FIDELIO-DKD trial enrolled preferentially patients with T2DM and predominantly advanced CKD with severely increased albuminuria. To ensure a greater kidney failure-free period to explore the cardioprotective effect of finerenone, patients with T2DM and a broader spectrum of CKD with moderately elevated albuminuria were preferentially selected for inclusion in the FIGARO-DKD trial. Notably, the design of both trials prespecified the optimization of background therapy. All the patients were being treated with a renin-angiotensin-system (RAS)-blocker and these therapies had been adjusted before randomization to the maximum tolerated dose according to the manufacturer’s label that did not induce unacceptable side effects.38,39
Table 2.
Summary of phase 3 clinical trials evaluating the safety and efficacy of finerenone on kidney failure and cardiovascular outcomes in patients with type 2 diabetes and chronic kidney disease
| Parameter | FIDELIO-DKD36 | FIGARO-DKD37 | FIDELITY35 (pooled analysis) |
|---|---|---|---|
| Year | 2020 | 2021 | 2022 |
| Patient population | T2DM and predominantly advanced CKD with severely increased albuminuria | T2DM and stage 2–4 CKD with moderately increased albuminuria or stage 1–2 CKD with severely increased albuminuria | T2DM and a broad spectrum of CKD |
| N | 5,674 | 7,352 | 13,026 |
| Intervention | Finerenone (10–20 mg/day) vs. placebo | Finerenone (10–20 mg/day) vs. placebo | Finerenone (10–20 mg/day) vs. placebo |
| Median follow-up (years) | 2.6 | 3.4 | 3.0 |
| Age (years) | 65.6 ± 9.1 | 64.1 ± 9.8 | 64.8 ± 9.5 |
| Male gender (n, %) | 3,983 (70.2%) | 5,105 (69.4%) | 9,088 (69.8%) |
| BMI (kg/m2) | 31.1 ± 6.0 | 31.4 ± 6.0 | 31.3 ± 6.0 |
| HbA1c (%) | 7.7 ± 1.3 | 7.7 ± 1.4 | 7.7 ± 1.4 |
| Serum potassium (mEq/l) | 4.4 ± 0.5 | 4.3 ± 0.4 | 4.35 ± 0.4 |
| eGFR (ml/min/1.73 m2) | 44.3 ± 12.6 | 67.8 ± 21.7 | 57.6 ± 21.7 |
| eGFR categories (n, %) | |||
| ≥60 ml/min/1.73 m2 | 656 (11.6%) | 4,539 (61.7%) | 5,195 (39.9%) |
| ≥45 to <60 ml/min/1.73 m2 | 1,900 (33.5%) | 1,534 (20.9%) | 3,434 (26.4%) |
| ≥25 to <45 ml/min/1.73 m2 | 2,981 (52.5%) | 1,251 (17.0%) | 4,232 (32.5%) |
| <25 ml/min/1.73 m2 | 135 (2.4%) | 27 (0.4%) | 162 (1.2%) |
| Median UACR (mg/g) | 852 (446–1,634) | 308 (108–740) | 515 (198–1,147) |
| UACR categories (n, %) | |||
| <30 mg/g | 23 (0.4%) | 207 (2.8%) | 230 (1.8%) |
| 30–300 mg/g | 685 (12.1%) | 3,414 (46.4%) | 4,099 (31.5%) |
| ≥300 mg/g | 4,963 (87.5%) | 3729 (50.7%) | 8,692 (66.7%) |
| ACEI/ARB use (n,%) | 5,667 (99.8%) | 7,343 (99.9%) | 13,003 (99.8%) |
| HR and 95% CI for key composite endpoints | |||
| Sustained ≥40% decrease in eGFR, kidney failure, or death from renal causes | 0.82 (0.73–0.93) | 0.87 (0.76–1.01) | 0.85 (0.77–0.93) |
| Sustained ≥57% decrease in eGFR, kidney failure, or death from renal causes | 0.76 (0.65–0.90) | 0.77 (0.60–0.99) | 0.77 (0.67–0.88) |
| Nonfatal MI, nonfatal stroke, hospitalization for HF, or cardiovascular death | 0.86 (0.75–0.99) | 0.87 (0.76–0.98) | 0.86 (0.78–0.95) |
Abbreviations: ACEI, angiotensin-converting-enzyme-inhibitor; ARB, angiotensin-receptor-blocker; BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated-glomerular-filtration-rate; HbA1c, glycated hemoglobin A1c; HF, heart failure; HR, hazard ratio; MI, myocardial infarction; T2DM, diabetes mellitus type 2; UACR, urinary albumin-to-creatinine ratio.
In the FIDELIO-DKD trial,36 5,734 patients with T2DM and more advanced CKD (mean eGFR: 44.3 ± 12.6 ml/min/1.73 m2; median UACR: 852 mg/g) were randomized to finerenone or placebo over a median follow-up of 2.6 years. Compared with placebo, finerenone reduced by 18% the occurrence of the primary composite kidney endpoint [hazard ratio (HR): 0.82; 95% confidence interval (CI): 0.73–0.93].36 A prespecified secondary endpoint replicating the primary composite cardiovascular outcome of FIGARO-DKD also occurred less commonly with finerenone than with placebo (HR: 0.86; 95% CI: 0.75–0.99).36 With respect to safety, the overall incidence of adverse events was similar in both groups. As expected, serious hyperkalemia leading to permanent discontinuation of the trial regimen occurred more commonly in finerenone-treated patients than in placebo-treated patients (2.3% and 0.9%, respectively).36
In the FIGARO-DKD trial,37 7,434 patients with T2DM and less advanced CKD (mean eGFR: 67.8 ± 21.7 ml/min/1.73 m2; median UACR: 308 mg/g) were randomly assigned to finerenone or placebo over a medial follow-up of 3.4 years. Compared with placebo, finerenone lowered by 13% the occurrence of the primary composite cardiovascular outcome (HR: 0.87; 95% CI: 0.76–0.98).37 Finerenone did not significantly reduce the occurrence of the prespecified secondary endpoint replicating the primary composite kidney outcome of FIDELIO-DKD. However, finerenone provoked a significant 23% reduction in a key secondary kidney-specific composite endpoint that included a sustained ≥57% decline in eGFR from baseline (HR: 0.77; 95% CI: 0.60–0.99).37 This composite endpoint is more consistent with the definitions of kidney failure outcomes used across clinical trials of CKD in T2DM. Once again, the incidence of hyperkalemia-related adverse events was higher with finerenone than with placebo, but permanent discontinuation of the trial regimen due to hyperkalemia occurred less commonly (1.2% and 0.4% in the finerenone and placebo groups, respectively), despite the longer median follow-up of the FIGARO-DKD trial.37 This can be explained by a better-preserved eGFR in these patients at baseline as compared to FIDELIO-DKD trial.
The FIDELITY (FInerenone in CKD and type 2 diabetes: Combined FIDELIO-DKD and FIGARO-DKD Trial programme analysis) analysis combined data from 13,026 patients (mean eGFR: 57.6 ± 21.7 ml/min/1.73 m2; median UACR: 515 mg/g) enrolled in these 2 complementary trials aiming to provide more robust estimates of the efficacy and safety of finerenone.35 Over a median follow-up of 3.0 years, finerenone reduced by 14% the occurrence of the composite cardiovascular outcome as compared with placebo (HR: 0.86; 95% CI: 0.78–0.95). Although patients with severe HFrEF were a priori excluded from this large phase 3 clinical-trial program, the major driver of the cardiovascular protection afforded by finerenone was a 22% relative risk reduction in the incidence of hospitalization for heart failure (HR: 0.78; 95% CI: 0.66–0.92).35 In addition, finerenone provoked a placebo-subtracted reduction of 23% in the composite kidney outcome (HR: 0.77; 95% CI: 0.67–0.88). Notably, with the exception of time to death from renal causes, all other components of the composite kidney outcome were consistently improved with finerenone as compared with a placebo. The relative risk of a sustained ≥57% decrease in eGFR from baseline was reduced by 30%, end-stage kidney disease by 20%, sustained decrease in eGFR to levels <15 ml/min/1.73 m2 by 19% and kidney failure by 16%.35 Therefore, with the highest power and precision, the FIDELITY analysis demonstrated the efficacy of finerenone in improving “hard” cardiovascular and kidney failure outcomes in a broad spectrum of patients with T2DM and CKD.
On this scientific basis, the nonsteroidal MRA finerenone has recently received regulatory approval for use in daily clinical practice with the indication to reduce the risk of sustained eGFR decline, end-stage kidney disease, cardiovascular death, nonfatal myocardial infarction and heart failure hospitalization in adult patients with CKD associated with T2DM.17 A recent analysis applied the FIDELIO-DKD and FIGARO-DKD enrollment criteria to the publicly available NHANES (National Health and Nutrition Examination Survey) 2009–2018 datasets aiming to estimate the number of US adults who are eligible for treatment with finerenone.40 FIDELIO-DKD criteria applied to approximately 1 million individuals and FIGARO-DKD criteria applied to almost 2 million individuals. Overall, a total of 2.2 million patients in the United States would qualify for finerenone by at least 1 full trial criterion.40
In the FIDELITY analysis, 40% of patients with T2DM would have missed the opportunity to be treated with finerenone, if UACR had not been measured. Screening for microalbuminuria by measuring the levels of UACR, a simple and cost-effective diagnostic test that is commonly omitted in daily clinical practice,41,42 is a crucial step to identify at-risk patients with T2DM and early-stage CKD who are likely to benefit from finerenone. In such patients, treatment with finerenone facilitates the improvement of both cardiovascular and kidney disease burdens.
With respect to safety and tolerability, hyperkalemia was the most commonly reported side effect associated with the use of finerenone.17 Accordingly, the levels of serum potassium and eGFR should be measured in all patients before the initiation of treatment. Finerenone should not be initiated in patients with an eGFR <25 ml/min/1.73 m2 or when serum potassium is >5.0 mmol/l. During treatment, the levels of serum potassium should be periodically monitored and the dose of finerenone should be appropriately adjusted.17 Clinical-trial experience indicates that the risk of hyperkalemia with finerenone is real, but easily manageable. In the FIDELITY analysis,35 the incidence of the investigator-reported hyperkalemia was 2-fold higher in finerenone-treated patients than in placebo-treated patients (14.0% vs. 6.9%, respectively). However, most of these hyperkalemic events were mild and reversible with transient withdrawal of the trial regimen until the normalization of serum potassium to levels ≤5.0 mmol/l. Serious hyperkalemia-related adverse events with clinical impacts, such as hospitalizations due to serious hyperkalemia, were rare (0.9% and 0.2% in the finerenone and placebo groups, respectively). Permanent discontinuation of the trial regimen due to hyperkalemia was observed in only 110 (1.7%) patients in the finerenone group and 38 (0.6%) patients in the placebo group over a median follow-up of 3 years.35 In addition, hypokalemia occurred less commonly with finerenone than with placebo (1.1% vs. 2.3%, respectively).35 The reduced incidence of hypokalemia with finerenone is noteworthy, because hypokalemia is also associated with an increased risk of adverse clinical outcomes.43
FINERENONE IN PATIENTS WITH HEART FAILURE WITH PRESERVED EJECTION FRACTION
As mentioned above, steroidal mineralocorticoid-receptor-antagonists (MRAs) are strongly recommended by guidelines to reduce the risk of heart failure hospitalization and mortality in patients with HFrEF.8 Although some mechanistic studies have suggested that spironolactone or eplerenone may further exert a favorable effect on left ventricular diastolic function,44 large outcome trials failed to prove a clear cardioprotective benefit of steroidal MRAs in patients with heart failure with mildly reduced ejection fraction (HFmrEF) or preserved ejection fraction (HFpEF).45,46 Accordingly, the clinical use of steroidal MRAs in this specific population is currently restricted to patients with more symptomatic heart failure.
A prespecified subgroup analysis of the FIDELIO-DKD trial provided preliminary data that the effect of finerenone on cardiorenal outcomes in patients with CKD and T2DM was not modified by a baseline history of heart failure.47 Since symptomatic HFrEF was an exclusion criterion, patients reporting a history of heart failure in FIDELIO-DKD (7.7% of the overall population) had either HFmrEF or HFpEF. Over a median follow-up of 2.6 years, the placebo-subtracted reduction in the composite cardiovascular outcome did not differ between patients with vs. without a history of heart failure (HR: 0.73, 95% CI: 0.50–1.06 and HR: 0.90, 95% CI: 0.77–1.04, respectively; Pinteraction = 0.33).47 Similarly, the benefit of finerenone on the composite kidney failure outcome was irrespective of a history of heart failure at baseline (HR: 0.79, 95% CI: 0.52–1.20 and HR: 0.83, 95% CI: 0.73–0.94, respectively; Pinteraction = 0.83).47 Owing to the post hoc nature and limited statistical power of these subgroup analyses, the results are only hypothesis-generating.
More conclusive evidence on the safety and efficacy of finerenone in patients with HFpEF is anticipated from the ongoing FINEARTS-HF (Finerenone Trial to Investigate Efficacy And Safety Superior to Placebo in Patients With Heart Failure) trial.48 In this phase 3 trial, 6,000 patients with heart failure and a documented left ventricular ejection fraction of 40% or greater will be randomly assigned to finerenone (10–40 mg/day) or a matching placebo. The primary composite outcome is defined as the between-group difference in the incidence of cardiovascular death and total heart failure events, including hospitalizations or emergency department visits for decompensated heart failure. The duration of follow-up will be up to 43 months and the completion of the trial is estimated on August 2024.48
EVIDENCE FOR A SYNERGISTIC EFFECT OF FINERENONE WITH OTHER GUIDELINE-DIRECTED THERAPIES
Sodium-glucose co-transporter type 2 (SGLT-2) inhibitors and glucagone-like peptide 1-receptor agonists (GLP1-RAs) are therapeutic agents with cardiorenal protective benefits that are recommended by guidelines for the management of T2DM in patients with CKD.49,50 These agents have a distinct mechanism of action from that of the nonsteroidal MRA finerenone and the question that arises is whether combining finerenone either with a SGLT-2 inhibitor or with a GLP1-RA offers a greater cardiorenal protection as compared with each therapy alone.
Preliminary evidence with respect to the efficacy of combination therapy with finerenone and a SGLT-2 inhibitor were provided by a preclinical model of hypertension-induced end-organ damage.51 In rats, nonsteroidal MR antagonism with finerenone alone and SGLT-2 inhibition with empagliflozin alone protected the heart and the kidney. However, compared with either monotherapy, combination of these two distinct mechanisms of action at low dosages was more effective in reducing the levels of albuminuria and BP as well as in causing regression of cardiac and renal fibrotic lesions, as determined by histopathology.51 These preclinical data suggest that a synergistic effect with the combination of finerenone with a SGLT-2 inhibitor is biologically plausible.
The currently available clinical-trial evidence on the efficacy and safety of combination therapies are limited to prespecified subgroup analyses that were stratified according to the use of SGLT-2 inhibitors or GLP1-RAs at baseline (Table 3).52 Overall, in the FIDELITY pooled analysis,35 877 patients (6.7%) were being treated with a SGLT-2 inhibitor and 944 patients (7.2%) with a GLP1-RA at baseline. The placebo-subtracted relative risk reduction in the composite cardiovascular outcome provoked by finerenone was similar in SGLT-2 inhibitor users and nonusers. Similarly, the cardioprotective benefit of finerenone was at least as large in GLP1-RA users as in nonusers.35 Additional subgroup analyses suggest that the albuminuria-lowering effect of finerenone over the course of the FIDELIO-DKD trial was similar and irrespective of background therapy either with a SGLT-2 inhibitor or with a GLP1-RA.53,54 The safety profile of finerenone over the course of the FIDELIO-DKD trial also remained unmodified by the use of SGLT-2 inhibitors or GLP1-RAs at baseline.53,54 Notably, the incidence of hyperkalemic events appeared to be lower in SGLT-2 inhibitor users than in nonusers, indirect evidence that background therapy with a SGLT-2 inhibitor may mitigate the risk of hyperkalemia associated with the use of finerenone.55
Table 3.
Effect of finerenone on the composite cardiovascular outcome according to use of SGLT-2 inhibitors or GLP1-RAs as background therapy
| (A) Subgroup analyses stratified by the SGLT-2 inhibitor use at baseline | ||||||
|---|---|---|---|---|---|---|
| Trial | Subgroup | Finerenone | Placebo | HR, (95% CI) | ||
| Events (n/N) | Per 100 patient-yrs | Events (n/N) | Per 100 patient-yrs | |||
| FIDELIO-DKD36 | Without a SGLT-2 inhibitor | 352/2,709 | 5.12 | 405/2,706 | 5.99 | 0.85, (0.74–0.98) |
| With a SGLT-2 inhibitor | 15/124 | 4.9 | 15/135 | 4.44 | 1.12, (0.55–2.30) | |
| FIGARO-DKD37 | Without a SGLT-2 inhibitor | 434/3,372 | 4.01 | 482/3,362 | 4.50 | 0.89, (0.78–1.01) |
| With a SGLT-2 inhibitor | 24/314 | 2.37 | 37/304 | 3.95 | 0.49, (0.28–0.86) | |
| FIDELITY35 | Without a SGLT-2 inhibitor | 786/6,081 | 4.44 | 887/6,068 | 5.08 | 0.87, (0.77–0.96) |
| With a SGLT-2 inhibitor | 39/438 | 2.95 | 52/439 | 4.08 | 0.63. (0.40-<1.00) | |
| (B) Subgroup analyses stratified by the GLP1-RA use at baseline | ||||||
| FIDELIO-DKD36 | Without a GLP1-RA | 340/2,644 | 5.07 | 392/2,636 | 5.98 | 0.85, (0.73–0.98) |
| With a GLP1-RA | 27/189 | 5.54 | 28/205 | 5.20 | 1.02, (0.60–1.74) | |
| FIGARO-DKD37 | Without a GLP1-RA | 427/3,378 | 3.96 | 483/3,424 | 4.44 | 0.89, (0.78–1.01) |
| With a GLP1-RA | 31/308 | 2.97 | 36/242 | 4.69 | 0.62, (0.38–1.03) | |
| FIDELITY35 | Without a GLP1-RA | 767/6,062 | 4.38 | 875/6,060 | 5.02 | 0.87, (0.79–0.96) |
| With a GLP1-RA | 58/497 | 3.79 | 64/447 | 4.90 | 0.79, (0.52–1.11) | |
Abbreviations: CI, confidence interval; GLP1-RA, glucagone-like peptide type 1 receptor agonist; HR, hazard ratio; SGLT-2, sodium-glucose co-transporter type 2.
These preliminary data provided the rationale for the design of the CONFIDENCE (COmbinatioN effect of FInerenone anD EmpaglifloziN in participants with CKD and type 2 diabetes using an UACR Endpoint study),56 a double-blind, randomized, 3-armed, parallel-group, phase 2 trial that will explore the superiority of dual therapy with finerenone and a SGLT-2 inhibitor as compared to either therapy alone. In this trial, 807 patients with T2DM, stage 2–3 CKD and a UACR ranging from ≥300 to <5,000 mg/g will be randomly assigned to receive the combination of finerenone (10–20 mg/day) plus empagliflozin (10 mg/day), or empagliflozin (10 mg/day) alone or finerenone (10–20 mg/day) alone for 6 months.56 The primary efficacy endpoint is defined as the between-group differences in the change from baseline in UACR. Secondary endpoints, such as between-group differences in the change of eGFR and incidences of hyperkalemia, will further elucidate the safety of combination therapy.56 A short-term treatment-induced reduction in albuminuria is shown to be closely associated with a long-term improvement in kidney failure and cardiovascular outcomes.57 Therefore, if CONFIDENCE trial proves that combination therapy results in an additive albuminuria-lowering effect, this evidence will provide a strong scientific basis to use finerenone together with a SGLT-2 inhibitor with the aim to offer the greatest cardiorenal protection in patients with CKD associated with T2DM.
Contributor Information
Panagiotis I Georgianos, Section of Nephrology and Hypertension, 1st Department of Medicine, AHEPA Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Rajiv Agarwal, Division of Nephrology, Department of Medicine, Indiana University School of Medicine and Richard L. Roudebush Veterans Administration Medical Center, Indianapolis, IN, USA.
FUNDING
R.A. is supported by the National Heart Lung and Blood Institute (grant R01 HL126903).
CONFLICTS OF INTEREST
R.A. reports personal fees and nonfinancial support from Bayer Healthcare Pharmaceuticals, Akebia Therapeutics, Boehringer Ingelheim, Eli Lilly, Relypsa, Vifor Pharma, Lexicon, and Reata; is a member of data safety monitoring committees for Vertex and Chinook and a member of steering committees of randomized trials for Akebia Therapeutics, Bayer, and Reata; has served as an associate editor of the American Journal of Nephrology and Nephrology Dialysis and Transplantation and has been an author for UpToDate, and has received research grants from the National Institutes of Health and the US Veterans Administration.
P.I.G. has nothing to disclose.
REFERENCES
- 1. Agarwal R, Kolkhof P, Bakris G, Bauersachs J, Haller H, Wada T, Zannad F.. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J 2021; 42:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, Miyoshi J, Takai Y, Fujita T.. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med 2008; 14:1370–6. [DOI] [PubMed] [Google Scholar]
- 3. Shibata S, Ishizawa K, Uchida S.. Mineralocorticoid receptor as a therapeutic target in chronic kidney disease and hypertension. Hypertens Res 2017; 40:221–225. [DOI] [PubMed] [Google Scholar]
- 4. Georgianos PI, Agarwal R.. Mineralocorticoid receptor antagonism in chronic kidney disease. Kidney Int Rep 2021; 6:2281–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agarwal R, Anker SD, Bakris G, Filippatos G, Pitt B, Rossing P, Ruilope L, Gebel M, Kolkhof P, Nowack C, Joseph A; FIDELIO-DKD and FIGARO-DKD Investigators. Investigating new treatment opportunities for patients with chronic kidney disease in type 2 diabetes: the role of finerenone. Nephrol Dial Transplant 2022; 37:1014–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J.. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med 1999; 341:709–17. [DOI] [PubMed] [Google Scholar]
- 7. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–21. [DOI] [PubMed] [Google Scholar]
- 8. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18:891–975. [DOI] [PubMed] [Google Scholar]
- 9. Savarese G, Carrero JJ, Pitt B, Anker SD, Rosano GMC, Dahlstrom U, Lund LH.. Factors associated with underuse of mineralocorticoid receptor antagonists in heart failure with reduced ejection fraction: an analysis of 11 215 patients from the Swedish Heart Failure Registry. Eur J Heart Fail 2018; 20:1326–1334. [DOI] [PubMed] [Google Scholar]
- 10. Leon SJ, Whitlock R, Rigatto C, Komenda P, Bohm C, Sucha E, Bota SE, Tuna M, Collister D, Sood M, Tangri N.. Hyperkalemia-related discontinuation of renin-angiotensin-aldosterone system inhibitors and clinical outcomes in CKD: a population-based cohort study. Am J Kidney Dis 2022; 80:164–173.e1. [DOI] [PubMed] [Google Scholar]
- 11. Wetmore JB, Yan H, Horne L, Peng Y, Gilbertson DT.. Risk of hyperkalemia from renin-angiotensin-aldosterone system inhibitors and factors associated with treatment discontinuities in a real-world population. Nephrol Dial Transplant 2021; 36:826–839. [DOI] [PubMed] [Google Scholar]
- 12. Georgianos PI, Agarwal Rajiv.. Revisiting RAAS blockade in CKD with newer potassium-binding drugs. Kidney Int 2018; 93:325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bolignano D, Palmer SC, Navaneethan SD, Strippoli GF.. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev 2014; 4:CD007004. [DOI] [PubMed] [Google Scholar]
- 14. Chung EY, Ruospo M, Natale P, Bolignano D, Navaneethan SD, Palmer SC, Strippoli GF.. Aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev 2020; 10:CD007004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duggan S. Esaxerenone: first global approval. Drugs 2019; 79:477–481. [DOI] [PubMed] [Google Scholar]
- 16. Ito S, Kashihara N, Shikata K, Nangaku M, Wada T, Okuda Y, Sawanobori T.. Esaxerenone (CS-3150) in patients with type 2 diabetes and microalbuminuria (ESAX-DN): phase 3 randomized controlled clinical trial. Clin J Am Soc Nephrol 2020; 15:1715–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frampton JE. Finerenone: first approval. Drugs 2021; 81:1787–1794. [DOI] [PubMed] [Google Scholar]
- 18. Gerisch M, Heinig R, Engelen A, Lang D, Kolkhof P, Radtke M, Platzek J, Lovis K, Rohde G, Schwarz T.. Biotransformation of finerenone, a novel nonsteroidal mineralocorticoid receptor antagonist, in dogs, rats, and humans, in vivo and in vitro. Drug Metab Dispos 2018; 46:1546–1555. [DOI] [PubMed] [Google Scholar]
- 19. Barrera-Chimal J, Estrela GR, Lechner SM, Giraud S, El MS, Kaaki S, Kolkhof P, Hauet T, Jaisser F.. The myeloid mineralocorticoid receptor controls inflammatory and fibrotic responses after renal injury via macrophage interleukin-4 receptor signaling. Kidney Int 2018; 93:1344–1355. [DOI] [PubMed] [Google Scholar]
- 20. Gonzalez-Blazquez R, Somoza B, Gil-Ortega M, Martin RM, Ramiro-Cortijo D, Vega-Martin E, Schulz A, Ruilope LM, Kolkhof P, Kreutz R, Fernandez-Alfonso MS.. Finerenone attenuates endothelial dysfunction and albuminuria in a chronic kidney disease model by a reduction in oxidative stress. Front Pharmacol 2018; 9:1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lattenist L, Lechner SM, Messaoudi S, Le MA, El MS, Prince S, Bobadilla NA, Kolkhof P, Jaisser F, Barrera-Chimal J.. Nonsteroidal mineralocorticoid receptor antagonist finerenone protects against acute kidney injury-mediated chronic kidney disease: role of oxidative stress. Hypertension 2017; 69:870–878. [DOI] [PubMed] [Google Scholar]
- 22. Grune J, Benz V, Brix S, Salatzki J, Blumrich A, Hoft B, Klopfleisch R, Foryst-Ludwig A, Kolkhof P, Kintscher U.. Steroidal and nonsteroidal mineralocorticoid receptor antagonists cause differential cardiac gene expression in pressure overload-induced cardiac hypertrophy. J Cardiovasc Pharmacol 2016; 67:402–11. [DOI] [PubMed] [Google Scholar]
- 23. Gueret A, Harouki N, Favre J, Galmiche G, Nicol L, Henry JP, Besnier M, Thuillez C, Richard V, Kolkhof P, Mulder P, Jaisser F, Ouvrard-Pascaud A.. Vascular smooth muscle mineralocorticoid receptor contributes to coronary and left ventricular dysfunction after myocardial infarction. Hypertension 2016; 67:717–23. [DOI] [PubMed] [Google Scholar]
- 24. Kolkhof P, Delbeck M, Kretschmer A, Steinke W, Hartmann E, Barfacker L, Eitner F, Albrecht-Kupper B, Schafer S.. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol 2014; 64:69–78. [DOI] [PubMed] [Google Scholar]
- 25. Grune J, Beyhoff N, Smeir E, Chudek R, Blumrich A, Ban Z, Brix S, Betz IR, Schupp M, Foryst-Ludwig A, Klopfleisch R, Stawowy P, Houtman R, Kolkhof P, Kintscher U.. Selective mineralocorticoid receptor cofactor modulation as molecular basis for finerenone’s antifibrotic activity. Hypertension 2018; 71:599–608. [DOI] [PubMed] [Google Scholar]
- 26. Orena S, Maurer TS, She L, Eudy R, Bernardo V, Dash D, Loria P, Banker ME, Tugnait M, Okerberg CV, Qian J, Boustany-Kari CM.. PF-03882845, a non-steroidal mineralocorticoid receptor antagonist, prevents renal injury with reduced risk of hyperkalemia in an animal model of nephropathy. Front Pharmacol 2013; 4:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma FY, Han Y, Nikolic-Paterson DJ, Kolkhof P, Tesch GH.. Suppression of rapidly progressive mouse glomerulonephritis with the non-steroidal mineralocorticoid receptor antagonist BR-4628. PLoS One 2015; 10:e0145666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Amazit L, Le BF, Kolkhof P, Lamribet K, Viengchareun S, Fay MR, Khan JA, Hillisch A, Lombes M, Rafestin-Oblin ME, Fagart J.. Finerenone impedes aldosterone-dependent nuclear import of the mineralocorticoid receptor and prevents genomic recruitment of steroid receptor coactivator-1. J Biol Chem 2015; 290:21876–21889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heinig R, Kimmeskamp-Kirschbaum N, Halabi A, Lentini S.. Pharmacokinetics of the novel nonsteroidal mineralocorticoid receptor antagonist finerenone (BAY 94–8862) in individuals with renal impairment. Clin Pharmacol Drug Dev 2016; 5:488–501. [DOI] [PubMed] [Google Scholar]
- 30. Heinig R, Lambelet M, Nagelschmitz J, Alatrach A, Halabi A: Pharmacokinetics of the novel nonsteroidal mineralocorticoid receptor antagonist finerenone (bay 94–8862) in individuals with mild or moderate hepatic impairment. Eur J Drug Metab Pharmacokinet 2019; 44:619–628. [DOI] [PubMed] [Google Scholar]
- 31. Heinig R, Gerisch M, Engelen A, Nagelschmitz J, Loewen S.. Pharmacokinetics of the novel, selective, non-steroidal mineralocorticoid receptor antagonist finerenone in healthy volunteers: results from an absolute bioavailability study and drug-drug interaction studies in vitro and in vivo. Eur J Drug Metab Pharmacokinet 2018; 43:715–727. [DOI] [PubMed] [Google Scholar]
- 32. Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack C, Kolkhof P, Joseph A, Pieper A, Kimmeskamp-Kirschbaum N, Ruilope LM; Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy (ARTS-DN) Study Group. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA 2015; 314:884–94. [DOI] [PubMed] [Google Scholar]
- 33. Filippatos G, Anker SD, Bohm M, Gheorghiade M, Kober L, Krum H, Maggioni AP, Ponikowski P, Voors AA, Zannad F, Kim SY, Nowack C, Palombo G, Kolkhof P, Kimmeskamp-Kirschbaum N, Pieper A, Pitt B.. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J 2016; 37:2105–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, Nowack C, Kolkhof P, Kim SY, Zannad F.. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94–8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J 2013; 34:2453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, Kolkhof P, Nowack C, Gebel M, Ruilope LM, Bakris GL; FIDELIO-DKD and FIGARO-DKD investigators. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J 2022; 43:474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, Filippatos G; FIDELIO-DKD Investigators. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020; 383:2219–2229. [DOI] [PubMed] [Google Scholar]
- 37. Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, Joseph A, Kolkhof P, Nowack C, Schloemer P, Ruilope LM; FIGARO-DKD Investigators. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021; 385:2252–2263. [DOI] [PubMed] [Google Scholar]
- 38. Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Nowack C, Kolkhof P, Ferreira AC, Schloemer P, Filippatos G; on behalf of the FIDELIO-DKD study investigators. Design and baseline characteristics of the finerenone in reducing kidney failure and disease progression in diabetic kidney disease trial. Am J Nephrol 2019; 50:333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ruilope LM, Agarwal R, Anker SD, Bakris GL, Filippatos G, Nowack C, Kolkhof P, Joseph A, Mentenich N, Pitt B; FIGARO-DKD study investigators. Design and baseline characteristics of the finerenone in reducing cardiovascular mortality and morbidity in diabetic kidney disease trial. Am J Nephrol 2019; 50:345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chiu N, Aggarwal R, Bakris GL, Pitt B, Bhatt DL.. Generalizability of FIGARO-DKD and FIDELIO-DKD trial criteria to the US population eligible for finerenone. J Am Heart Assoc 2022; 11:e025079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Folkerts K, Petruski-Ivleva N, Comerford E, Blankenburg M, Evers T, Gay A, Fried L, Kovesdy CP.. Adherence to chronic kidney disease screening guidelines among patients with type 2 diabetes in a US administrative claims database. Mayo Clin Proc 2021; 96:975–986. [DOI] [PubMed] [Google Scholar]
- 42. Shin JI, Chang AR, Grams ME, Coresh J, Ballew SH, Surapaneni A, Matsushita K, Bilo HJG, Carrero JJ, Chodick G, Daratha KB, Jassal SK, Nadkarni GN, Nelson RG, Nowak C, Stempniewicz N, Sumida K, Traynor JP, Woodward M, Sang Y, Gansevoort RT; CKD Prognosis Consortium. Albuminuria testing in hypertension and diabetes: an individual-participant data meta-analysis in a global consortium. Hypertension 2021; 78:1042–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sfairopoulos D, Arseniou A, Korantzopoulos P.. Serum potassium and heart failure: association, causation, and clinical implications. Heart Fail Rev 2021; 26:479–486. [DOI] [PubMed] [Google Scholar]
- 44. Pandey A, Garg S, Matulevicius SA, Shah AM, Garg J, Drazner MH, Amin A, Berry JD, Marwick TH, Marso SP, de Lemos JA, Kumbhani DJ.. Effect of mineralocorticoid receptor antagonists on cardiac structure and function in patients with diastolic dysfunction and heart failure with preserved ejection fraction: a meta-analysis and systematic review. J Am Heart Assoc 2015; 4:e002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O’Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B.. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015; 131:34–42. [DOI] [PubMed] [Google Scholar]
- 46. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370:1383–92. [DOI] [PubMed] [Google Scholar]
- 47. Filippatos G, Pitt B, Agarwal R, Farmakis D, Ruilope LM, Rossing P, Bauersachs J, Mentz RJ, Kolkhof P, Scott C, Joseph A, Bakris GL, Anker SD; FIDELIO-DKD Investigators. Finerenone in patients with chronic kidney disease and type 2 diabetes with and without heart failure: a prespecified subgroup analysis of the FIDELIO-DKD trial. Eur J Heart Fail 2022; 24:996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. ClinicalTrials.gov. Study to Evaluate the Efficacy (Effect on Disease) and Safety of Finerenone on morbidity (Events Indicating DiseaseWorsening) and Mortality (Death Rate) in Participants with Heart Failure and Left Ventricular Ejection Fraction (Proportion of Blood Expelled Per Heart Stroke) Greater or Equal to 40% (FINEARTS-HF). https://clinicaltrials.gov/ct2/show/NCT04435626?cond=finerenone&draw=3&rank=14. Accessed on 10 August 2022.
- 49. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020; 98:S1–S115. [DOI] [PubMed] [Google Scholar]
- 50. Georgianos PI, Vaios V, Roumeliotis S, Leivaditis K, Eleftheriadis T, Liakopoulos V.. Evidence for cardiorenal protection with SGLT-2 inhibitors and GLP-1 receptor agonists in patients with diabetic kidney disease. J Pers Med 2022; 12:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kolkhof P, Hartmann E, Freyberger A, Pavkovic M, Mathar I, Sandner P, Droebner K, Joseph A, Huser J, Eitner F.. Effects of finerenone combined with empagliflozin in a model of hypertension-induced end-organ damage. Am J Nephrol 2021; 52:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Agarwal R, Rifkin B.. Moderating effects in randomized trials-interpreting the P Value, confidence intervals, and hazard ratios. Kidney Int Rep 2022; 7:371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rossing P, Filippatos G, Agarwal R, Anker SD, Pitt B, Ruilope LM, Chan JCN, Kooy A, McCafferty K, Schernthaner G, Wanner C, Joseph A, Scheerer MF, Scott C, Bakris GL; FIDELIO-DKD Investigators. Finerenone in predominantly advanced CKD and type 2 diabetes with or without sodium-glucose cotransporter-2 inhibitor therapy. Kidney Int Rep 2022; 7:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rossing P, Agarwal R, Anker SD, Filippatos G, Pitt B, Ruilope LM, Amod A, Marre M, Joseph A, Lage A, Scott C, Bakris GL; FIDELIO-DKD Investigators. Efficacy and safety of finerenone in patients with chronic kidney disease and type 2 diabetes by GLP-1RA treatment: a subgroup analysis from the FIDELIO-DKD trial. Diabetes Obes Metab 2022; 24:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Agarwal R, Joseph A, Anker SD, Filippatos G, Rossing P, Ruilope LM, Pitt B, Kolkhof P, Scott C, Lawatscheck R, Wilson DJ, Bakris GL; FIDELIO-DKD Investigators. Hyperkalemia risk with finerenone: results from the FIDELIO-DKD trial. J Am Soc Nephrol 2022; 33:225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Green JB, Mottl AK, Bakris G, Heerspink HJL, Mann JFE, McGill JB, Nangaku M, Rossing P, Scott C, Gay A, Agarwal R.. Design of the COmbinatioN effect of FInerenone anD EmpaglifloziN in participants with chronic kidney disease and type 2 diabetes using an UACR Endpoint study (CONFIDENCE). Nephrol Dial Transplant 2022. In Press. doi: 10.1093/ndt/gfac198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Heerspink HJL, Greene T, Tighiouart H, Gansevoort RT, Coresh J, Simon AL, Chan TM, Hou FF, Lewis JB, Locatelli F, Praga M, Schena FP, Levey AS, Inker LA; Chronic Kidney Disease Epidemiology Collaboration. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol 2019; 7:128–139. [DOI] [PubMed] [Google Scholar]