Abstract
Age-related declines in physical and cognitive function can have tremendous, negative impacts on health span and quality of life. Therefore, we investigated the potential of utilizing a probiotic treatment to target the renin–angiotensin system (RAS) in conjunction with moderate exercise to ameliorate age-related declines in cognitive and physical function in aged rats. Herein we utilized a genetically modified angiotensin (1–7), which activates a “complementary” arm of the RAS through binding Mas (AT7) receptors. This process induces several beneficial physiologic effects, including decreased inflammation and enhanced physical/cognitive function. Thus, in this short research report, we suggest the efficacy of this Ang(1-7) releasing Lactobacillus paracasei (LPA) as either an alternative strategy to exercise, or more likely as an adjuvant to moderate exercise, for the prevention of both physical and cognitive decline especially in female rats.
Keywords: Biobehavioral, Health span, Intervention, Nutraceuticals
A long-standing focus of our group has been the renin–angiotensin system (RAS) and aging. In recent years, numerous pleiotropic effects have been ascribed to the RAS that extend beyond lowering blood pressure (1–8). Classically, the RAS was thought to function primarily by balancing the vasoconstrictive actions of angiotensin II (AngII) binding to the AT1 receptor (AT1R) with the actions of bradykinin—a potent vasodilatory substance. However, in the last 15 years, a complementary RAS axis has been described which functions by binding of the peptide Ang(1–7) to the Mas (AT7) receptor. In contrast to the vasoconstrictive actions of the ANG2/AT1R axis, this angiotensin-converting enzyme 2 (ACE2) axis induces several beneficial physiologic effects ranging from reduced adiposity, improved muscle quality (inflammation, oxidative stress, antiapoptosis), and even enhanced cognitive function due to reduced neuroinflammation.
By way of example, we have reported findings, in both humans and rodents, suggesting that ACE inhibitors may potentiate the effects of exercise on physical function (3,9,10); however, “potentiating” the complementary arm, as opposed to “blocking” the classical arm of the RAS, may provide a more efficacious approach. Toward this end, we developed a novel method of delivering Ang(1–7) orally via a genetically modified probiotic which we refer to as our LPA (Lactobacillus paracasei [LP] modified to express Ang(1–7)). In addition to allowing for oral administration, the LPA has added benefits of easy production, inherent benefits of the bacteria itself (anti-inflammatory properties), and the ability to concomitantly influence intestinal immunity which is highly correlated with improved physiology across multiple organ systems (4,5).
Therefore, the proposed study was designed to evaluate, for the first time, the utility of directly activating the ACE2 axis to improve physical function in late life using our experimentally validated LPA with or without moderate exercise in aged Fisher 344 × Brown Norway (FBN) male and female rats. The primary outcome in this study was exercise tolerance. Secondary outcomes included 2 domains of cognitive performance (anxiety: open field; and memory: object recognition).
Method
Subjects
Eighty-two male and 84 female aged (24 months) FBN rats from the National Institute on Aging colony were used in this study, though only 63 males and 72 females survived to completion (no difference in survival among any group was observed). All rats were individually housed and maintained on a 12-h light and 12-h dark cycle (6:00–18:00) in a specific pathogen-free facility accredited by the American Association for Accreditation of Laboratory Animal Care. Rats were fed a standard rodent chow (18% kcal from fat, no sucrose, 3.1 kcal/g, diet 2018; Harlan Teklad, Madison, WI). All experimental procedures were approved by University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Study Design and Treatment Groups
Rats were randomly assigned to exercise (Ex; n = 31 males, n = 36 females) and sedentary (Sed; n = 32 males, n = 36 females) group; and further into 3 subcategories: LPA (exercise n = 8 males, n = 13 females; sedentary n = 9 males, n = 12 females), probiotic (exercise n = 11 males, n = 12 females; sedentary n = 11 males, n = 11 females), and control (exercise n = 12 males, n = 11 females; sedentary n = 12 males, n = 13 females). Rats were administered 2 × 1011 colony-forming units/kg body weight probiotic or equal volume of buffer via oral gavage 3 times/week for 12 weeks. Optimal dosage was based on our previous experiments (see previous (4,5)).
All rats were treadmill-habituated for 2 weeks prior to exercise training. On the first day of habituation, rats were simply placed on the treadmill for 10 minutes. On the second through fifth day of training, the treadmill was set to 8 cm/s and then increased by 1 cm/s until reaching 12 cm/s (5 minutes). Thereafter, exercise animals were exercised daily (5 days/week) at 12 cm/s for the remainder of the experiment. The total exercise training last 12 weeks. Control sedentary rats were simply exposed to the treadmill for 10 minutes each day.
Exercise Tolerance
Rats in the sedentary group were reacclimated on the treadmill at 12 cm/s for 10 minutes on the first day. On the second day, both exercise and sedentary rats were placed on the treadmill apparatus at 12 cm/s for 2 minutes, after which the speed increased by 2 cm/s until 16 cm/s max speed. When an animal stopped moving, they were prodded 3 times by the experimenter. If they did not respond by the third prodding, the animal was considered to have reached their limit.
Novel Object Recognition
Test objects were presented in a square arena (51 × 36 cm), walls 32 cm high. The rats’ behavior in the arena was monitored by an overhead video camera and later scored by 2 observers. Rats were given 1 habituation session in which they were allowed to explore the arena, free of objects, for 5 minutes. Two test sessions (sample and testing) were given spaced approximately 24 hours apart. In the sample phase, 2 similar objects were placed in 2 adjacent corners of the arena approximately 10 cm from the edges. The rat was then placed in the arena, facing away from the objects, and was allowed to explore the arena and objects for 5 minutes. During the testing phase, one of the objects from the “sample” phase was replaced, in a random fashion, with a novel object and the rat was allowed to explore the arena and objects for 5 minutes. Exploration of an object was defined as directing the nose less than 2 cm to the object and actively exploring it. A memory index was calculated based on the difference between sample and testing phases. This was defined as ratio of time spent exploring the 2 objects divided by total time spent exploring both objects during ([Object 1 − Object 2]/[Object 1 + Object 2]).
Open-Field Locomotor Activity
Locomotor activity was assessed by placing rats in a 51 cm × 36 cm inch box for 5 minutes. Following a 1-hour habituation to the testing room, rats were placed in the arena. Total distance traveled (within the center) was recorded with Ethovision software (Noldus, Leesburg, VA).
Statistical Analysis
All quantitative data are reported as group means ± 1 standard error of the mean. For all data, the a priori hypothesis that rats receiving Ang(1–7)-expressing probiotic (LPA) would perform significantly better than rats receiving the control treatment was investigated using Dunnett’s multiple comparisons test within each exercise group. To explore other potential interactions, a 2 × 2 multifactorial analysis of variance (ANOVA) was used. For all factorial ANOVAs, a factor with 2 levels (sedentary control [Sed] and exercise [Ex]) and a second factor with 3 levels: (control [Con], probiotic [LP], or Ang(1–7)-expressing probiotic [LPA]) were used. The null hypothesis was rejected at the level of p > .05. All analyses were performed with GraphPad Prism version 9.3.0 for Windows (GraphPad Software, La Jolla, CA).
Results
Exercise Tolerance
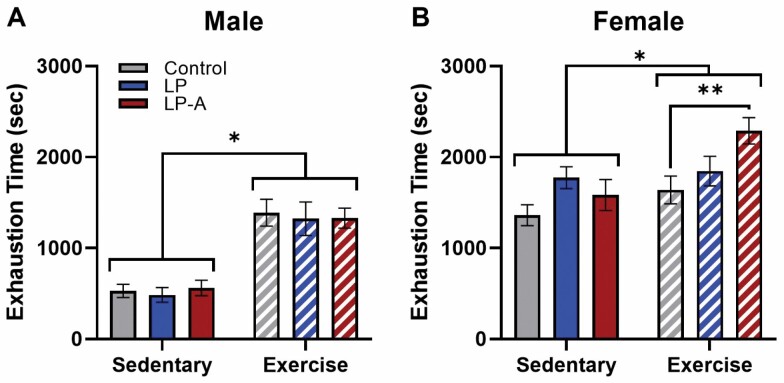
In male rats (Figure 1A), exercise training resulted in significantly greater time to exhaustion (p < .001), though there was no main effect of probiotic (p = .89) nor did the 2 factors significantly interact (p = .93). In female rats (Figure 1B), as hypothesized, the Ex-LPA group did demonstrate a significantly longer time to exhaustion than the Ex-Con group (p = .005), though the Sed-LPA group did not outperform the Sed-Con group (p = .44). Again, exercise training resulted in significantly greater time to exhaustion (p = .005), as did probiotic supplementation (p = .01), though the 2 factors did not significantly interact (p = .10).
Figure 1.
Exercise tolerance in male and female rats. (A) While there was a significant main effect of exercise in male rats (p < .001), there were no significant effects of probiotic or subgroup interactions. (B) In female rats, there was both a significant main effect of exercise (p < .01) and probiotic group (p = .01). Moreover, there was a significantly longer time to exhaustion in the Ex-LPA group (p = .005). All data are expressed as group means ± 1 standard error of the mean. * indicates p < .05.
Novel Object Recognition
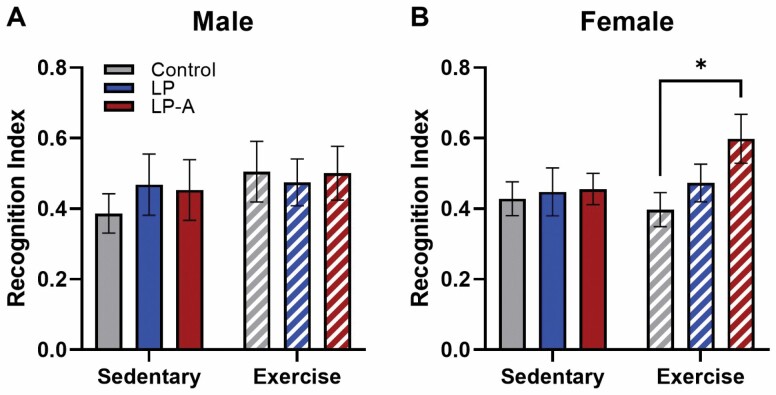
In both male and female rats (Figure 2A and B), there was no impact of exercise or probiotic treatment in any group (all p values > .05). However, only in female rats and as hypothesized, the Ex-LPA group did demonstrate a significantly higher recognition index than did the Ex-Con group (p = .03).
Figure 2.
Recognition index from novel object recognition in male and female rats. (A) There were no differences across any groups in male rats. (B) However, Ex-LPA female rats performed significantly better than Ex-Con female rats (p = .03). All data are expressed as group means ± 1 standard error of the mean. * indicates p < .05.
Open-Field Activity
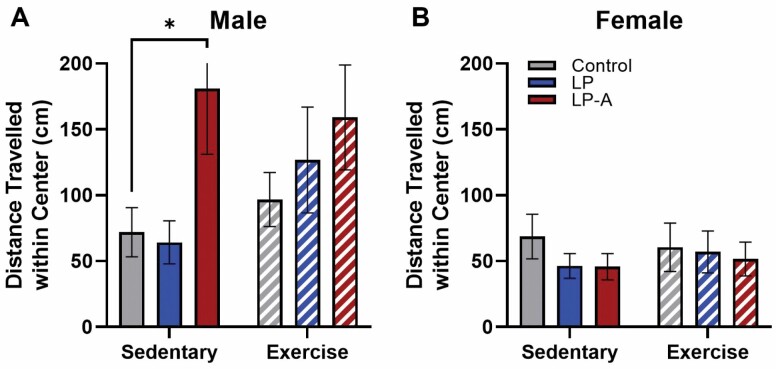
In male rats (Figure 3A), while there was no difference in the distance traveled between LPA and Con rats within the Ex subgroup (p = .31), the LPA rats traveled significantly farther than the Con rats within the Sed subgroup (p = .03). While there was no significant effect of exercise group on distance traveled (p = .40), rats receiving the LPA traveled significantly farther than rats who did not (p = .02), though these 2 factors did not significantly interact (p = .44). No differences across any groups were observed in female rats (all p values > .05; Figure 3B).
Figure 3.
Anxiety-like behaviors in male and female rats. Open field. (A) While male Sed-LPA rats traveled significantly farther than Sed-Con rats (p = .03), there were no other differences either within the Ex subgroup or across groups. (B) There were no differences across any groups or subgroups within the female rats. All data are expressed as group means ± 1 SEM. * indicates p < .05.
Discussion
This is the first preclinical study to investigate a potential translational intervention combining RAS modulation, delivered by a genetically modified probiotic (LPA), and exercise to ameliorate age-related physical and cognitive decline. This study encompassed an ambitious experimental design with 2 exercise and 3 probiotic conditions groups across the 2 sexes. Importantly, incorporating therapeutic interventions that are plausibly translatable to humans, such as this, is a necessary step in the prevention or amelioration of age-related cognitive and physical decline. Our results demonstrate the potential for peripherally targeting interventions to affect cognitive outcomes in aged subjects as well as the ability to utilize a safe, genetically modified probiotic to improve physicality in a sex-dependent manner. Moreover, we demonstrate the effects of multiple interventions (LPA + exercise) can synergistically improve physical performance in aged female rats.
Exercise Tolerance
While there is a rich preclinical literature studying the impact of high-intensity exercise in older animals, our protocol was quite mild and akin to that used in the Lifestyle Interventions and Independence for Elders (LIFE) study (11). These animals performed mild intensity physical activity 10 min/day for 5 days/week. Thus, it is impressive that we observed a greater than 250% increase in time to exhaustion in our male Ex rats relative to male Con rats. There was no impact of LPA administration. This is perhaps because all males performed quite poorly in this assay; thus, any impact of LPA was masked by the exercise contribution.
The effect of exercise was less impressive in our female rats, which only demonstrated a 105% increase relative to sedentary controls, who performed quite well in this assay. Furthermore, all groups of females, observationally, outperformed males in this assay. Interestingly, this effect was mostly driven by the female exercise LPA group, thus confirming our hypothesis that the combination of exercise + LPA would provide a better outcome than exercise alone. There are no studies in female rats using a moderate exercise protocol like that utilized in the current experiment. Therefore, it is possible that females may tolerate a more intense exercise protocol and demonstrate and even greater exercise effect. However, these results also demonstrate that in females, RAS modulation enhances the impact of exercise in this assay.
Memory
No effects of exercise were observed on object recognition memory in either male or female rats. Rats of this age commonly have impaired object recognition (12) ability, an effect which we replicate here, as no Con or Sed groups spent significantly more time exploring the novel object than the familiar. Interestingly, however, within female rats in the exercise group, only the rats receiving the LPA performed significantly better than Con rats. These data indicate a synergistic effect between exercise and LPA administration, specifically in female rats, but neither exercise nor LPA administration alone was sufficient to produce this effect.
One possible explanation for the lack of exercise effect on object recognition memory is that the exercise was not rigorous enough to affect either body weight or molecular mechanisms of memory enhancement. Aged animals allowed to feed ad libitum throughout their sedentary lives arrive at the facility metabolically morbid, so perhaps combing an exercise paradigm capable of decreasing excessive body fat would be a better adjuvant to LPA. Secondly, our exercise paradigm was designed to promote increased movement, but not confer radical changes in body mass. Previous exercise paradigms that have demonstrated the ability to improve memory through changes in brain-derived neurotrophic factor (BDNF) signaling and/or extracellular vesicles and particle counts included more vigorous output from the animals (13). Thus, in either scenario, obesity may be masking some of the potential benefits of exercise with or without LPA supplementation in males and may explain the requirement of the LPA as an adjuvant to exercise in females. This information is imperative to know, as many older adults will be resistant to highly vigorous exercise paradigms, making potential adjuvant therapies imperative for the restoration or maintenance of physical health in advanced age.
Anxiety-Like Behaviors
In the open-field assay, males in the sedentary LPA group moved around much more in the center arena suggesting they were either less anxious or were more mobile. There was a similar trend in the exercise group that did not reach significance. What is interesting about these findings is that there is evidence in humans that exercising participants often demonstrate lower levels of activity in their “natural” environments given the amount of time they spend exercising in “experimental” environments. However, these data demonstrate that a possible Ang(1–7) effect either increases activity or decreases anxiety in males, primarily in the sedentary condition. Thus, only in males, Ang(1–7) may have an impact on this aspect of cognitive function.
Conclusion
Although the primary purpose of this paper was to assess behavioral measure reflecting physical function and health-span measurement, we have not identified or discussed distinct biological mechanisms by which RAS modulation, exercise, gut health, or the combination thereof influences these outcomes. We are currently conducting studies to address this shortcoming including metagenomics, RNA seq, metabolomics, and targeted pathways in gut, brain, and muscle tissues. Moreover, we were unable to randomize males and females within experimental cohorts because aged female rats were unavailable from the National Institute of Aging (NIA) Rodent Resource Center at study onset. Thus, while we cannot statistically include sex as a variable in our statistical modeling, we do make relative observational comparisons in our discussion. Overall, given the limitations of exercise and the absence of alternative options, this study represents an important step in attempting to identify interventions with the potential for improving physical and overall health-span function among older adults.
Contributor Information
Abbi Hernandez, Department of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Yi Sun, Department of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Anisha Banerjee, Department of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA.
YouFeng Yang, Department of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Amrisha Verma, Department of Ophthalmology, University of Florida, Gainesville, Florida, USA.
Qiuhong Li, Department of Ophthalmology, University of Florida, Gainesville, Florida, USA.
Liliana Baptista, Department of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA; Research Center in Physical Activity, Health and Leisure, Faculty of Sports, University of Porto, Porto, Portugal.
Thomas W Buford, Department of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA; Birmingham/Atlanta Geriatric Research, Education, and Clinical Center, Birmingham VA Medical Center, Birmingham, Alabama, USA.
Christy S Carter, Department of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Funding
This work was funded by a grant from the National Institutes of Health/National Institute on Aging to T.W.B. and C.S.C. (R01AG054538) and A.H. (1K99AG078402-01). The work was also supported by the UAB Nathan Shock Center (P30AG50886).
Conflict of Interest
None declared.
Author Contributions
Y.S. and A.H. contributed equally to this work and wrote the methods and results. T.W.B. and C.S.C. are both corresponding authors and contributed equally to the writing and interpretation of the data. Q.L. and A.V. performed biological analyses and interpretation. Y.Y. and A.B. collected and interpreted the behavioral data. L.B. contributing to analyze the data and interpret them. C.S.C. current position is at The National Institute on Aging, Division of Aging Biology, Bethesda, MD.
References
- 1. Baptista LC, Jaeger BC, Anton SD, et al. . Multimodal intervention to improve functional status in hypertensive older adults: a pilot randomized controlled trial. J Clin Med. 2019;8(2):E196. doi: 10.3390/jcm8020196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anton SD, Woods AJ, Ashizawa T, et al. . Successful aging: advancing the science of physical independence in older adults. Ageing Res Rev. 2015;24(pt B):304–327. doi: 10.1016/j.arr.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buford TW, Miller ME, Church TS, et al. . Antihypertensive use and the effect of a physical activity intervention in the prevention of major mobility disability among older adults: the LIFE study. J Gerontol A Biol Sci Med Sci. 2016;71(7):974–981. doi: 10.1093/gerona/glv222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buford TW, Sun Y, Roberts LM, et al. . Angiotensin (1–7) delivered orally via probiotic, but not subcutaneously, benefits the gut-brain axis in older rats. GeroScience. 2020. doi: 10.1007/s11357-020-00196-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carter CS, Morgan D, Verma A, et al. . Therapeutic delivery of Ang(1-7) via genetically modified probiotic: a Dosing Study. J Gerontol A Biol Sci Med Sci. 2020;75(7):1299–1303. doi: 10.1093/gerona/glz222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harper SA, Baptista LC, Roberts LM, et al. . Angiotensin converting enzyme inhibitors combined with exercise for hypertensive seniors (the ACES trial): study protocol of a randomized controlled trial. Front Med. 2020;6:327. doi: 10.3389/fmed.2019.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marzetti E, Calvani R, DuPree J, et al. . Late-life enalapril administration induces nitric oxide-dependent and independent metabolic adaptations in the rat skeletal muscle. Age. 2013;35(4):1061–1075. doi: 10.1007/s11357-012-9428-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simon CB, Lee-McMullen B, Phelan D, Gilkes J, Carter CS, Buford TW. The renin–angiotensin system and prevention of age-related functional decline: where are we now? Age. 2015;37(1):1307–1321. doi: 10.1007/s11357-015-9753-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carter CS, Marzetti E, Leeuwenburgh C, et al. . Usefulness of preclinical models for assessing the efficacy of late-life interventions for sarcopenia. J Gerontol A Biol Sci Med Sci. 2012;67A(1):17–27. doi: 10.1093/gerona/glr042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buford TW, Manini TM, Hsu F-C, et al. . Angiotensin-converting enzyme inhibitor use by older adults is associated with greater functional responses to exercise. J Am Geriatr Soc. 2012;60(7):1244–1252. doi: 10.1111/j.1532-5415.2012.04045.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pahor M, Guralnik JM, Ambrosius WT, et al. . Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi: 10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burke SN, Wallace JL, Nematollahi S, Uprety AR, Barnes CA. Pattern separation deficits may contribute to age-associated recognition impairments. Behav Neurosci. 2010;124(5):559–573. doi: 10.1037/a0020893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martin B, Ji S, Maudsley S, Mattson MP. “Control” laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci USA. 2010;107(14):6127–6133. doi: 10.1073/pnas.0912955107 [DOI] [PMC free article] [PubMed] [Google Scholar]