Abstract
Interest in the gut–brain axis and its implications for neurodegenerative diseases, such as Alzheimer’s disease and related dementias, is growing. Microbial imbalances in the gastrointestinal tract, which are associated with impaired cognition, may represent a therapeutic target for lowering dementia risk. Multicomponent lifestyle interventions are a promising dementia risk reduction strategy and most often include diet and exercise, behaviors that are also known to modulate the gut microbiome. A better understanding of the role of the gut microbiome in diet and exercise effects on cognition may help to optimize these lifestyle interventions. The purpose of this review is to summarize findings from diet and exercise interventions that have investigated cognitive changes via effects on the microbiome. We aim to discuss the underlying mechanisms, highlight current gaps in the field, and provide new research directions. There is evidence mainly from rodent studies supporting the notion that microbiota changes mediate the effects of diet and exercise on cognition, with potential mechanisms including end-product metabolites and regulation of local and systemic inflammation. The field lacks whole diet and exercise interventions, especially those involving human participants. It is further limited by heterogeneous rodent models, outcome assessments, and the absence of proper mediation analyses. Trials including older adults with dementia risk factors, factorial designs of diet and exercise, and pre and post measures of microbiota, end-product metabolites, and inflammation would help to elucidate and potentially leverage the role of the microbiome in lowering dementia risk through lifestyle modification.
Keywords: Dementia, Gut–brain axis, Inflammation, Microbiota, SCFAs
Alzheimer’s disease (AD) and related dementias are among the world’s most prevalent and costly medical conditions (1). Over the last 2 decades, the gut microbiome has emerged as an important contributor to human health with implications for neurodegenerative diseases (2,3). Microbial imbalances in the gastrointestinal (GI) tract are associated with impaired cognition, which suggests a potential role of the gut microbiota in the development of dementia, including AD (4). The gut microbiome is modified by factors such as diet and exercise, and targeting the microbiome through lifestyle modification may be a valid strategy for lowering dementia risk (5).
The gut microbiome consists of all microorganisms, bacteria, viruses, protozoa, and fungi, and their cumulative genome within the GI tract (6). The microbiota in the GI tract comprised primarily 4 main phyla: Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria (7). They play an important role in nutrient and mineral absorption, and synthesis of enzymes, vitamins, amino acids, and neurotransmitters. A well-functioning microbial environment also produces metabolites such as short-chain fatty acids (SCFAs; eg, acetate, propionate, and butyrate), which promote epithelial barrier integrity, modulation of the immune system, and protection against pathogens (8). A healthy gut microbiome is generally characterized by a high diversity of bacterial communities and is maintained by a symbiotic relationship between pathogenic and nonpathogenic bacteria, and host-derived factors (eg, mucins, antimicrobial peptides, and immunoglobulins) (9). Gut microbial imbalances, referred to as dysbiosis, are implicated in the development of metabolic, autoimmune, and neurological diseases (7). Dysbiosis can result from aging, poor diet (10), antibiotics, or infections and is associated with increased intestinal permeability and inflammation (11,12). A dysbiotic intestinal environment can negatively affect the brain via the gut–brain axis.
The gut–brain axis refers to communication between the intestinal environment and the brain via neural pathways (vagus nerve), endocrine signaling (hypothalamus–pituitary–adrenal [HPA] axis), and the immune system (cytokines) (13). Microbial dysbiosis leads to increased gut permeability and the release of endotoxins (eg, lipopolysaccharide [LPS]) into the bloodstream triggering a neural immune response (14). The gut communicates its dysbiotic state with the brain via the vagus nerve causing increased HPA axis activity and the release of cortisol and proinflammatory cytokines (15). Chronic high cortisol and circulating proinflammatory cytokines can impair the blood–brain barrier and promote atrophy and neuroinflammation, affecting brain health and cognition (16–18). Microbial dysbiosis is associated with impaired cognition and is observed in individuals with AD (19). A diverse, well-functioning microbial environment is associated with improved learning/memory and behavioral flexibility (4).
As no curative treatment for dementia exists, focus has shifted toward targeting modifiable risk factors for cognitive decline and engaging in healthy lifestyle behaviors. In particular, the synergistic effects of multicomponent lifestyle interventions, including diet and exercise, are becoming increasingly studied. Aside from their effects on cognition, long-term dietary habits are a primary contributor to gut microbiome composition and function, and changes to diet and uptake of exercise can rapidly alter the gut microbiome (20,21). Thus, links between diet and exercise, microbiome health, and cognition are likely; however, very few intervention studies have been conducted to triangulate these relationships, and the results from these studies have yet to be disseminated in a review focusing on cognition. Determining the role of the gut microbiome in diet- and exercise-associated cognitive changes may help to optimize lifestyle interventions aimed at reducing dementia risk. The objective of this review is to discuss the findings from diet and exercise interventions that have investigated changes to both gut microbiota and cognition in order to highlight the current evidence, identify gaps in the literature, and provide future research directions.
Diet, Cognition, and the Microbiome
The Western diet is characterized by foods high in saturated fats and simple sugars, low in fiber, and is associated with cognitive impairment and increased risk of dementia (22,23). Conversely, the Mediterranean (MED) diet, which emphasizes increased intake of nonrefined grains, fruits and vegetables, legumes, nuts, fish, and lower intake of red meat and processed foods, is associated with improved cognition and reduced dementia risk (24). The MED diet benefits the brain by reducing inflammation and oxidative stress (25), promoting neurogenesis, and improving neuronal connectivity (26). Furthermore, contrary to the Western diet, the MED diet is associated with improved gut microbiome composition and diversity and decreased gut permeability and inflammation (27).
The MED diet is high in fermentable dietary fibers and is associated with enhanced abundance of fiber-fermenting bacteria leading to increased SCFAs in the gut and bloodstream (28). In addition to promoting gut health, SCFAs play a role in maintaining the blood–brain barrier (29) and exhibit neuroactive properties such as support of glial cells (30) and modulation of neurotrophic factors (31). Altered SCFA production has been demonstrated in a variety of neuropathologies, including AD (32). Another key feature of the MED diet is omega-3 polyunsaturated fatty acids (PUFAs) found in fatty fish (eicosapentaenoic acid and docosahexaenoic acid) and derived from alpha-linolenic acid found in nuts, seeds, legumes, and green leafy vegetables (33). The intake of omega-3 PUFAs correlates with improved microbiome composition and diversity and increased SCFA-producing bacteria (34). Omega-3 PUFAs are essential in maintaining gut epithelial integrity (35) and act as intra- and intercellular signaling mediators in the GI tract and brain, influencing immune regulation, inflammation, and homeostasis (36). Polyphenols found in plant foods such as fruits, vegetables, herbs, and wine are abundant in the MED diet and promote beneficial bacteria in the gut (37,38). When processed by gut microbiota, they increase the bioavailability of polyphenol-derived metabolites, which protect against neurotoxic injury, suppress inflammation, and promote cognitive functions (39,40).
The majority of diet interventions measuring both microbiome and cognitive outcomes have been conducted in rodents and have investigated individual components of Western and MED diets. One human study has investigated the impact of a MED diet on the gut microbiome and cognition in older adults. The following is a review of the intervention literature.
Diet Interventions Associated With a Western Eating Pattern
High-Fat Diets
Diets high in saturated fat are the most commonly studied interventions associated with a Western eating pattern (Supplementary Table S1). They appear to consistently alter microbiota, but simultaneous cognitive changes are not always observed. Middle-aged male Sprague Dawley rats fed a high-fat diet (HFD; 45% fat by kcal) for 8 weeks presented with changes to gut microbiota but no differences in working memory compared with rats fed a low-fat diet (10% fat by kcal) (41). HFD yielded a greater relative abundance of genera from Firmicutes phylum and lower abundance of genera from Bacteroidetes phylum. Microglial characterization and counts in the cortex, hippocampus, and hypothalamus did not identify differences in neuroinflammation between groups. In adolescent male Sprague Dawley rats, altered microbiota composition (specifically Clostridium sensu stricto genus) and impaired place recognition memory were observed following a 2-week HFD (48% fat by kcal) intervention (42). Bacteria from Lachnospiraceae and Ruminococcace families were correlated with memory. No differences in neuroinflammation or brain-derived neurotrophic factor (BDNF) expression were observed.
In adult male C57BL/6J mice, a 6-week HFD (42% fat by kcal, 30% sucrose) resulted in microbiota shifts (increased Clostridiales, Erysipelotrichales and decreased Bacteroidales), but no differences in object recognition or spatial memory compared with a control chow diet (13% fat by kcal, 3% sucrose) (43). Associations were observed between altered taxa and cognitive flexibility. Following a 16-week HFD (60% fat by kcal), adult male C57BL/6J mice displayed large shifts in gut microbiota (increased relative abundance of taxa from Firmicutes phyla and decreased relative abundance of taxa from Bacteroidetes and Tenericutes phyla), but no differences in contextual or cued memory compared with a low-fat diet (10% fat by kcal) (44). Research utilizing HFD-induced obese male C57BL/6J mice showed that 15 weeks of HFD (55% fat by kcal) led to reduced memory performance, decreases in microbiota richness and diversity (decreased relative abundance of Bacteroidetes and increased abundance of Firmicutes and Protobacteria), and increased colonic, systemic, and hippocampal inflammation compared with mice on a control chow diet (5% fat by kcal) (45,46).
Adolescent male C57BL/6J mice fed a HFD for 9-weeks displayed microbiota shifts and impaired spatial recognition memory compared with controls (47). HFD increased Proteobacteria population, increased fecal and plasma LPS, and suppressed BDNF expression in the hippocampus. Following a 3-week HFD (45% fat by kcal) in adolescent male C57BL/6J mice, no behavioral differences from control were observed in adulthood (10 weeks), but HFD resulted in changes in gene expression related to neuroinflammation and myelination (48).
Inconsistent findings may be due to variations in rodent age, strain, and diet composition. For instance, some studies did not match for caloric value, fiber, sucrose, or protein content (43). In HFD interventions with calorie-matched designs (41,42), HFD groups eat less food overall compared with controls and are less exposed to certain nutrients (eg, sucrose). Other studies substituted fat calories with sucrose in control diets (44,47). Thus, the negative effects of HFD may be offset by negative effects of sucrose.
Rodents fed detrimental diets for prolonged periods will develop various cardiometabolic impairments, making it difficult to directly link diet to microbiota and cognition. To address this, Bruce-Keller et al. (49) used a microbiota transplant approach in which nonobese adult male C57BL/6J mice, maintained on a normal chow diet (13% fat by kcal, 3.7% sucrose by weight), were subjected to microbiota depletion via antibiotics and then were recolonized with microbiota from mice fed either a HFD (60% fat by kcal, 8.9% sucrose by weight) or normal chow diet for 10 weeks. HFD transplantation altered microbiota diversity and composition (mainly shifts of Firmicutes phylum) and led to greater intestinal inflammation and permeability. Memory performance was significantly lower in HFD recipient mice who also showed neuroinflammation and disrupted cerebrovascular homeostasis in the medial prefrontal cortex. Post-transplantation, there were no significant differences in body weight, blood glucose, or hormones/lipids between groups, suggesting that diet-associated microbiome changes influence brain health independent of cardiometabolic changes.
High-Sucrose Diets
Diets high in sucrose appear to consistently affect gut microbiota and cognition. Adult male C57BL/6J mice fed a high-sucrose diet (HSD; 12% fat by kcal, 66% sucrose) for 6 weeks showed greater microbiota shifts and worse spatial memory compared with mice fed a HFD (42% fat by kcal, 30% sucrose) or normal chow diet (13% fat by kcal, 3% sucrose) (43). HSD increased bacteria from Clostridiales and Lactobacilllales orders and decreased bacteria from Bacteroidales order. Altered taxa were related to poorer cognitive flexibility; however, the diets in this study were not nutrient matched, which makes it difficult to determine which nutrients are responsible for differences between groups. In adolescent male Sprague Dawley rats, a 2-week HSD (29% sucrose by kcal) intervention led to differences in microbiota composition (specifically Porphyromonadaceae family) and impaired place recognition memory compared with a control diet (16% sucrose) (42). Bacteria from Lachnospiraceae and Ruminococcace families were correlated with memory, but no differences in neuroinflammation or BDNF expression were observed. An intermittent (2 h/d) HSD (20% fat [lard], 39.6% sucrose) added to a chow diet (12% fat, 65% carbohydrates) led to deficits in social and object recognition memory compared with a chow diet alone in adolescent male Sprague Dawley rats (50). The HSD group displayed increases in bacteria from Lachnospiraceae and Ruminococcace families, and decreased BDNF expression and genes regulating catecholamine metabolism in the prefrontal cortex.
Cafeteria Diets
The cafeteria diet is an experimental rodent diet of unhealthy human foods, which are highly processed and high in saturated fat and sucrose. Compared with a control chow diet (65% kcal by carbohydrates, 22% protein, 13% fat), continuous cafeteria (access to commercially produced cakes, biscuits, and savory foods) and intermittent cafeteria diets (3 days cafeteria, 4 days chow) reduced gut microbiota richness and diversity in adult female Sprague Dawley rats (51). Both diets increased hippocampal cytokine expression, but only the continuous cafeteria diet was associated with impaired short-term memory. Limited access to unhealthy foods may spare cognition, as intermittent caloric restriction has shown to improve cognition in mice (52). In adolescent male C57BL/6JOIaHsd mice, cafeteria diet intake was associated with late-life microbiota composition (48) but did not affect cognition compared with a chow diet. Genes related to neuroinflammation and neurotransmission in adulthood were also affected. In this study, mice were switched back to a normal chow diet following adolescence. Switching to a chow diet after HFD intervention has shown to reverse behavioral effects (53), which may explain the lack of cognitive differences in adulthood.
Diet Interventions Associated With Mediterranean Eating Patterns
Fiber
The foods typical in a MED diet are high in fiber. β-Glucans, soluble fibers found in fungi, yeast, and cereal grains such as oats and barley, have shown to alter microbiota and cognition in several rodent models (Supplementary Table S2). Aβ 1-42-induced adult male C57BL/6J mice supplemented with yeast-derived β-glucan for 4 weeks exhibited memory improvements, which correlated with alterations in beneficial and inflammatory-related microbiota (increased relative abundance of Bacteroidetes and decreased relative abundance of Firmicutes) (54). Increases in hippocampal SCFAs and reductions in neuroinflammation and brain insulin resistance were also observed. In adult male C57Bl/6J mice, 15 weeks of oat-derived β-glucan supplementation (7%) added to a HFD (55% kcal by fat) prevented recognition memory impairments and abrogated microbiome alterations (increased relative abundance of Bacteroides and decreased relative abundance of Proteobacteria) observed with HFD alone (45). Oat-derived β-glucan also countered HFD-induced upregulation of inflammatory cytokines in the hippocampus and decreased endotoxin translocation in the colon. A substudy of these mice found that microbiota changes preceded cognitive changes, and cognitive effects of β-glucan were eliminated in an additional study arm receiving antibiotics in combination with the oat β-glucan diet.
Similar effects of β-glucan-rich foods have been observed. Male low-density lipoprotein receptor knockout C57Bl/6J mice were fed either a high-fat/cholesterol diet (46% kcal by fat) with or without 0.8% oat fiber (22% β-glucan, 22% insoluble fiber, 20% starch, 5% lipids, 20% protein, 4% ash, 5% water) for 14 weeks (55). Oat fiber ameliorated impairments in spatial learning and memory, and improved microbiota diversity, increasing SCFA-producing microbiota (increased Actinobacteria and decreased Rikenellaceae). Oat fiber also increased the expression of SCFA receptors and tight junction proteins in the distal colon. Barley, a β-glucan-rich food, has shown to ameliorate cognitive impairments and alter gut microbiota in a rodent model of age-related cognitive decline (56). Male 4-week-old senescence-accelerated prone 8 (SAMP8) mice were fed a purified chow diet (AIN-93G) for 4 weeks, and from then until death, fed a mild HFD (27% fat by adding lard) with either barley (7.9% soluble β-glucan) or rice starch components. The barley diet altered microbiota (increased Bacteroides to Firmicutes ratio) and reduced age-associated spatial memory decline compared with the rice diet. Microbiota-accessible carbohydrates (MAC) are supplements rich in a variety of fermentable fibers. In adult male C57Bl/6J mice, MAC added to a HFD prevented dysbiosis (increasing relative abundance of Bacteroidetes, and decreasing abundance of Proteobacteria) and memory impairments observed with HFD alone (46), while reducing endotoxemia and colonic and systemic inflammation. MAC effects on cognition were eliminated when combined with antibiotics in an additional study arm.
Purified fiber supplementation has shown to alter microbiota and cognition in a small sample of healthy young adult females. Participants received either 12.5 g LitesseUltra (>90% ploydextrose polymer [PDX]) or maltodextrin for 4 weeks (57). PDX resulted in modest cognitive improvements and compositional microbiota changes (increased relative abundance of Firmicutes). Minor changes to CD62L receptor expression, a marker of acute stress responsiveness, suggest that PDX benefits the brain by reducing inflammatory status. PDX, however, is a synthetic polymer, not naturally found in foods and may not represent the effects of fiber as part of a MED diet.
Omega-3 Polyunsaturated Fatty Acids
PUFA-enriched diet interventions positively affect gut microbiota, with simultaneous cognitive effects most often observed. In stress-induced adolescent male Wistar rats, a diet enriched in omega-3 PUFAs prevented memory impairments, normalized declines in hippocampal BDNF, and attenuated shifts in microbial composition (increased relative abundance of Ruminococcacea and Lachnospiraceae) compared with a control diet (58). These effects were maintained throughout adulthood, long after the stressful environment was terminated. When provided to pregnant female C57BL/6J mice and their offspring, an omega-3 PUFA diet led to beneficial microbiota development (Bifidobacterium and Lactobacillus), better memory, and dampened HPA axis activity in offspring that persisted until adulthood (59). Behavioral changes were closely associated with alterations in gut microbiota. In contrast, 2 weeks of a PUFA-enriched diet in adolescent male Sprague Dawley rats led to significant differences in microbiota composition (specifically taxa from Ruminococcaceae family), but not object or recognition memory compared with controls (42).
Polyphenols
Sesamol, a polyphenol derived from sesame oil, has shown to alter microbiota while also reducing age-associated impairments in mice (60). Young (2 months old) and middle-aged (12 months) CD-1 male mice on a standard chow diet (AIN-93M) were compared with middle-aged male mice on a chow diet with sesamol (0.1% w/w). Sesamol reduced cognitive impairments observed in aging mice on a chow-only diet and significantly increased microbiota diversity, with beneficial effects seen on aging- and inflammation-associated microbiota. Oxidative stress and neuroinflammation were significantly reduced compared with controls, likely related to alleviated intestinal barrier damage and inhibited gut microbiome-driven LPS entry into the blood.
Whole Diet
The NU-AGE study (new dietary strategies addressing the specific needs of the older adult population for healthy aging in Europe) is the only whole diet intervention in humans that has reported on measures of both gut microbiota and cognition. A sample of 1200 older adults (aged 65–79 years) were randomized to either a MED diet intervention or control diet for 12 months (61). Participants with high MED diet adherence showed significant improvements in global cognition and episodic memory. Gut microbial communities were profiled in a subset of participants (323 on MED diet, 289 controls). There were no significant differences in microbiota diversity between groups; however, across all participants, greater MED diet adherence was associated with increased microbiome diversity, increased SCFAs, and decreased inflammation (62). Bacterial communities enriched by the MED diet were positively associated with memory and visual-spatial abilities.
Exercise, Cognition, and the Microbiome
Most exercise examined in the context of the microbiome describes aerobic exercise. Aerobic exercise throughout the life span is associated with better cognitive function and reduced dementia risk (63). It is proposed to benefit the brain both indirectly, by improving health conditions, and directly, by increasing brain neurotrophic factors, improving cerebrovascular function, and enhancing brain plasticity (64–66). Exercise also increases key antioxidant enzymes, anti-inflammatory cytokines, and antiapoptotic proteins, leading to reduced inflammation (67,68).
Aerobic exercise improves microbial diversity and intestinal barrier permeability in humans (20,69,70). BDNF is elevated by exercise and has shown to regulate GI tight junction proteins, which are crucial for maintaining the epithelial integrity, thereby reducing the translocation of proinflammatory endotoxins (eg, LPS) into circulation (71). Greater exercise and cardiorespiratory fitness are positively associated with SCFA-producing bacteria and fecal SCFA concentrations independent of diet (72,73).
The effects of aerobic exercise on gut health appear to vary by exercise intensity. Moderate-intensity aerobic exercise maintains intestinal blood flow, positively modulating GI motility (74) and reducing inflammation (75). Conversely, long-duration, strenuous aerobic exercise (>60%–70% VO2max) produces an acute stress response, increasing levels of cortisol and epinephrine (76), reducing blood supply to intestinal epithelium (77), and promoting gut permeability and inflammation (78,79). High-intensity interval training, which involves short-duration bursts of strenuous aerobic exercise, has shown to beneficially alter microbiota composition and diversity in mice (80–82) and reduce systemic and adipocyte inflammation in rats (83).
A better understanding of the effects of varying types and intensities of exercise on the gut–brain axis in humans is required. Very few exercise interventions (rodent only) met the criteria for inclusion in this review. The following is a review of the intervention literature.
Aerobic Exercise interventions
Aerobic exercise interventions alter gut microbiota and positively influence brain health in rodents, but evidence in humans is unexplored (Supplementary Table S3). Running wheel exercise for 16 weeks was associated with altered microbiota (increased relative abundance of Firmicutes and decreased relative abundance of Bacteroidetes and Tenericutes) and increased contextual memory compared with controls in adult male C57BL/6J mice (44). Memory performance was associated with bacterial abundances from Ruminococcaceae and Lachnospiraceae families. In a rat model of metabolic syndrome, preoperative treadmill exercise increased microbiome diversity (increased abundance of Firmicutes and decreased abundance of Bacteroidetes) and alleviated postoperative cognitive impairments (84), a common issue in older adult patients with metabolic syndrome. Male high- and low-capacity running rats were randomly assigned to receive preoperative exercise (6 weeks) with surgery (tibia fracture with internal fixation under anesthesia) or sham surgery (anesthesia only), or no exercise with surgery or sham surgery. Preoperative exercise attenuated memory impairments and lowered neuroinflammation in low-capacity running rats postsurgery. In a mouse model of AD (APP/PS1), high-intensity interval treadmill running altered gut microbiota and decreased progression of AD pathology (80) compared with nonexercised controls. Exercise increased the abundance of SCFA-producing bacteria and elevated levels of Lactobacillus reuteri, a vitamin B12 producer. No differences in spatial memory were observed, but the exercise group showed decreases in the size and number of beta-amyloid plaques in the hippocampus.
Synthesis of Findings
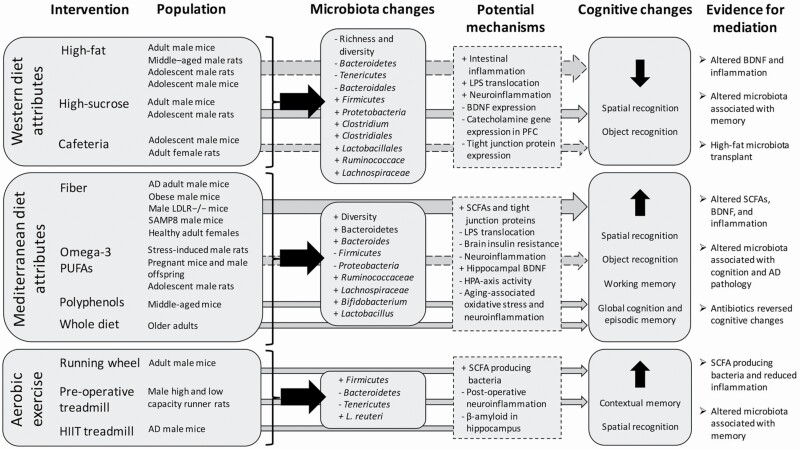
The findings mostly from rodent trials provide evidence for the mediating effect of gut microbiota on diet and exercise effects on cognition (Figure 1). Western-type diets were associated with decreased microbiota richness and diversity (45,51) and poorer spatial and object recognition memory (42,43,46,47,49–51), as well as increased intestinal and neural inflammation (45–47,49,51), and decreased BDNF expression (50). Interventions associated with MED eating patterns often resulted in greater microbiota diversity (46,60,62) and spatial and object recognition memory (54–57). Following a MED diet intervention in older adults, correlations between diet-associated microbiota changes and global cognition were observed (62). Increased SCFAs and tight junction proteins (45,54,55,62), hippocampal BDNF expression (58) and reduced endotoxin translocation (45,46,60), brain insulin resistance (54), and neuroinflammation (45,54,60,62) were identified as potential mediating mechanisms in these studies. Not all diet interventions observed cognitive effects however, and these inconsistencies are likely due to heterogeneity of diets, rodent models, and cognitive assessments. The most promising evidence comes from high-fiber interventions where increased SCFAs and reduced inflammation along with cognitive changes were commonly observed (45,46,54–57).
Figure 1.
Summary of intervention results. Dashed arrows denote mixed evidence for cognitive effects. LPS = lipopolysaccharide; BDNF = brain-derived neurotrophic factor; PFC = prefrontal cortex; AD = Alzheimer’s disease; LDLR−/− = low-density lipoprotein receptor knockout mice; SAMP8 = senescence-accelerated mouse-prone 8; PUFAs = polyunsaturated fatty acids, SCFAs = short-chain fatty acids; HPA axis = hypothalamic–pituitary–adrenal axis, HIIT = high-intensity interval training.
Evidence from a limited number of preclinical exercise trials have shown compositional microbiota changes that were associated with cognition in healthy mice (44) and reduced postoperative cognitive impairment and neuroinflammation in a rat model of metabolic syndrome (84). In AD mice, high-intensity treadmill exercise led to increases in SCFA-producing bacteria and reductions in AD pathology (80), but not cognitive differences. The lack of cognitive effects in AD mice may be related to their advanced disease progression and aligns with the notion that interventions should be applied early in the course of cognitive decline (85).
Supporting the mediating the role of the microbiome are microbiota changes that precede cognitive changes (45), antibiotic elimination of cognitive effects related to diet (45,46), microbiota transplant effects (49), and potential mechanistic links such as SCFA, BDNF, and inflammatory changes. Associations between altered microbiota and cognition were also frequently observed (42–44,51,54,59,62). In particular, bacteria from the Clostridia class and Bacteroidales order, such as Lachnospiraceae, Ruminococcaceae, Coprobacter, and Rikenella, as well as Lactobacillus and Bifidobacterium genera. The field is predominantly dominated by rodent studies, but their findings set the stage for future human trials in this area. Several ongoing human studies are described in the following section.
Ongoing Human Trials
The DGA4ME trial (86) will assess the impact of a 32-week MED diet on cognitive performance and microbiota in females under age 65 (n = 168) with obesity and cardiovascular risk factors. Participants will be randomly assigned to one of the following groups: MED diet to maintain body weight; MED diet to achieve weight loss; typical American diet to achieve weight loss. The 3-group design could give insight on whether the MED diet affects microbiome and cognition independent of adiposity and metabolic changes.
The COMBAT study (87) will investigate the impact of 12 weeks of cranberry intake, rich in polyphenols, on gut microbiota and cognition. Participants aged 55 and older will be allocated to a treatment or control group (n = 30 each). Blood, urine, and fecal samples will be collected to assess diet and microbiome, and all participants will undergo cognitive testing and magnetic resonance imaging (MRI). Secondary analyses of inflammatory and metabolic markers, BDNF levels, and cerebrovascular hemodynamics will be conducted. Strengths of this study include the recruitment of older adults and a comprehensive brain health assessment. The use of a single nutrient intervention, however, only informs us about one attribute of a healthy diet.
The Lifestyle Intervention for Alzheimer’s Disease study (88) will recruit 100 participants with mild cognitive impairment or early AD to participate in a 40-week lifestyle intervention. The intervention will consist of a low-fat vegan diet, aerobic and resistance exercise, stress management, and group support. A waitlist control group will be used, and after 20 weeks, those in the control phase will receive the lifestyle intervention. Cognition and microbiome changes will be assessed at 20 and 40 weeks. The intervention design limits the ability to assess individual lifestyle components and may only be able to inform on the overall combined effects of the intervention.
A study from Sun Yat-sen University in China (89) aims to examine the effects of a combined diet and exercise intervention versus either intervention alone on executive function and intestinal microbiota in 200 undergraduate students. Exercise training will consist of rope skipping (3 cycles of 20 minutes skipping: 10-minute breaks) 3 times per week. The diet intervention consists of 10 hours of restricted eating of a high-fiber diet. Secondary outcomes will include BDNF, CRP, and a variety of inflammatory cytokines. Limitations of this study include the young study sample, lack of a true control group, and the assessment of only one cognitive domain. Additionally, restricted eating does not necessarily reflect a healthy diet, and rope skipping for long durations does not seem appropriate for adults without a moderate fitness level at baseline.
The aforementioned studies speak to the growing interest in the effects of lifestyle modification on the gut–brain axis, but are limited by various sample populations, intervention protocols, and outcome measures. Strengths of these studies include the study of at-risk populations, high-intensity exercise, and multicomponent intervention effects. They also provide a sense of appropriate designs and outcome measures. There are still, however, many gaps in the field that need to be addressed.
Gaps and Recommendations
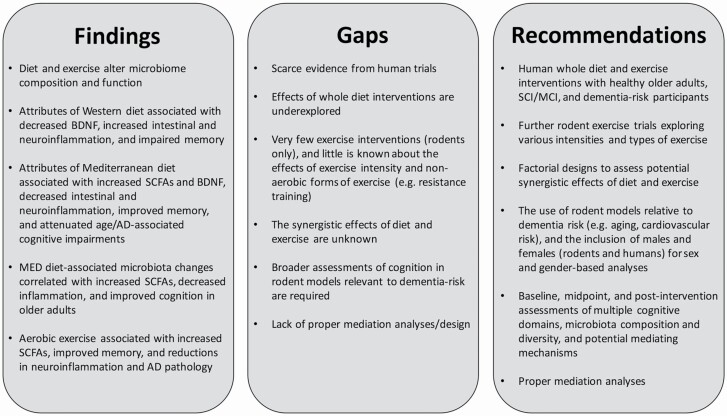
The role of the microbiome in the effects of diet and exercise on cognition is emerging from preclinical trials, but inferences to human physiology, especially in the context of dementia prevention, are uncertain (Figure 2). There is a need for more human trials—especially with individuals experiencing early signs of cognitive impairment and/or presenting with dementia risk factors. A major limitation of rodent research is the narrow selection of cognitive tests. Spatial and object recognition are most always reported due to the frequent use of maze testing and fear conditioning paradigms. Thus, little is known about other cognitive domains, which can be differently impaired in humans experiencing dementia. Human trials provide the opportunity to comprehensively study cognition through neuropsychological assessment and the use of advanced measurement techniques such as MRI. There are numerous studies investigating the effects of diet and exercise on cognition in older adults, but lacking investigation into the microbiome. We encourage researchers working on these studies to collaborate with microbiome scientists, and attempt to include simple, cost-effective measures of microbiota composition, diversity, and function. Fecal samples are an easy, cost-effective collection strategy, and labs can be outsourced to store and/or analyze fecal samples.
Figure 2.
Findings, gaps, and recommendations.
The majority of studies investigated singular components of Western and MED eating patterns, and the effects of whole diet interventions on microbiota and cognition are underexplored. High-fat and high-sucrose diets are common in North America; thus, there is a lot of research interest in their effects. Furthermore, the frequent use of single nutrient interventions may reflect the desire to discover simple forms of treatment. The general consensus, however, is that the combined attributes of a diet are more important for microbiome composition and cognition than individual components (15). Nutrients that target SCFA-producing bacteria and inflammation appear to have the greatest impact on cognition. Thus, whole diets such as the MED diet, which comprised fruits, vegetables, and healthy fats, are recommended for future studies.
The number of exercise interventions in this field is quite limited compared with dietary interventions. Additional preclinical trials are needed to corroborate the current evidence from a handful of rodent studies and inform the designs of human trials. Little is known about how exercise intensity influences the gut–brain axis, and thus is an important endeavor for future research, as differing intensities have shown to have varying effects on gut health and other physiological outcomes (77–79). Researchers should also consider investigating other types of exercise, such as resistance training. Microbiota transplant and antibiotic treatment designs should also be considered in rodent exercise studies. None of the reviewed exercise interventions measured BDNF, but given that BDNF is elevated by exercise and associated with gut health and cognition, it is recommended that future exercise trials include this as an outcome.
The diet interventions included in this study could warrant their own review entirely; however, it was our intent to present findings from both diet and exercise interventions as there is accruing evidence supporting the synergistic effects of multicomponent lifestyle interventions, and it is of interest whether these are replicated when assessing microbiome and cognitive outcomes (90,91). Diet and exercise-associated microbiome and cognitive changes are accompanied by many of the same physiological changes; thus, it is reasonable to predict that synergistic effects may occur. Factorial designs comparing diet, exercise, and diet combined with exercise are highly encouraged to tease apart these relationships.
The majority of studies reviewed included heterogeneous, mostly male rodent models. Findings from adolescent, young adult, and stress-induced mice may not generalize to at-risk populations, while, conversely, AD rodent models may be too far along in their disease progression. Most studies used healthy adult rodents, a group of interest considering many risk factors for dementia begin early in adult life, and lifestyle behaviors during adulthood are associated with cognition in late life (92). Findings from studies including middle-aged, senescence-accelerated, and cardiometabolic risk rodent models are perhaps the most relevant to dementia prevention and are recommended for future studies. Researchers also tend to use male mice exclusively as they are concerned that estrous cycles in female mice will increase variability; however, these claims have been refuted (93). The underrepresentation of female rodents limits our understanding of female biology and may lead to inadequate treatment for females. Given there are sex and gender differences in cognitive trajectories (94) and lifestyle preferences (95,96), it is important to study the effects of diet and exercise on the gut–brain axis as a function of both sex and gender. Despite these limitations, we believe that for a research area still in its infancy, it is appropriate to consider findings from all studies available that investigate the interplay between diet, exercise, and the gut–brain axis.
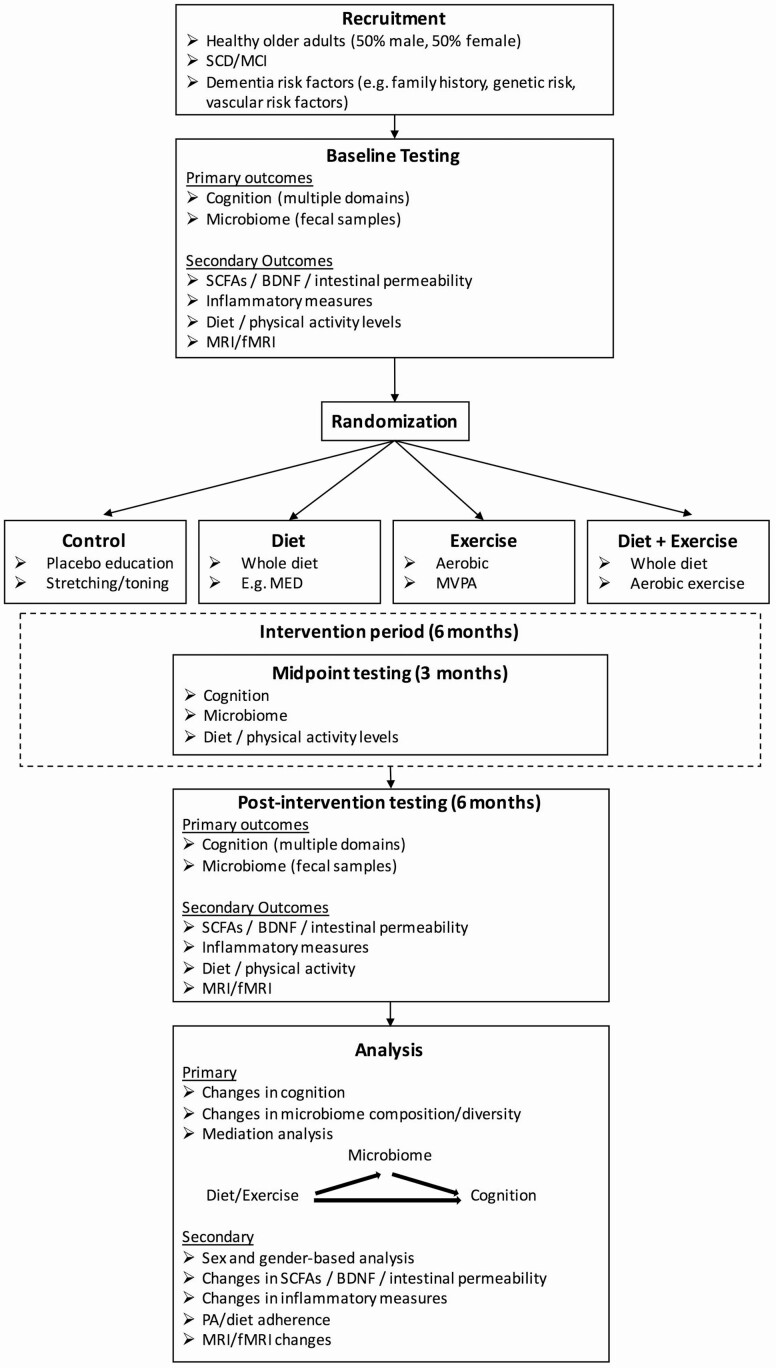
Lastly, a major focus of this review was to infer the mediating role of the microbiome; however, no studies conducted proper mediation analyses. Instead, our conclusions are drawn from evidence of potential mediating mechanisms, correlations between altered microbiota and cognition, and novel designs such as microbiota transplant and antibiotic treatment. Most studies did not assess microbiota and cognition at baseline, a requirement for running proper mediation analyses. Figure 3 provides an example of a trial design for future exercise and diet investigating the mediating role of the gut microbiome on cognitive changes in older adults. In addition to baseline and postintervention assessments, midpoint assessments are useful for identifying whether microbiota changes precede cognitive changes. Measuring potential mediating mechanisms such as SCFAs and tight junction proteins, BDNF, and measures of local, systemic, and neural inflammation are highly recommended.
Figure 3.
Suggested trial design. SCD = subjective cognitive decline; MCI = mild cognitive impairment; SCFAs = short-chain fatty acids; BDNF = brain-derived neurotrophic factor; fMRI = functional magnetic resonance imaging; MED = Mediterranean diet; MVPA = moderate- to vigorous-intensity physical activity.
Conclusion
The intervention literature supports the notion that the gut microbiome, at least in part, mediates diet and exercise effects on cognition. In contrast to Western-style diets, interventions encompassing features of the MED diet, and uptake of exercise were associated with improved microbiota diversity, increased SCFA production, and reduced local and systemic inflammation. The evidence is mainly derived from rodent studies; however, one large MED diet intervention found diet-associated microbiota changes to be correlated with cognitive performance in older adults. Several diet and exercise interventions assessing both microbiome and cognitive outcomes in humans are underway, but are limited by heterogeneous populations and interventions. We encourage the inclusion of baseline and follow-up measures of microbiome composition, diversity, and function in lifestyle interventions aimed at reducing dementia risk in older adults. This effort would help to elucidate the mechanisms by which lifestyle modification affects cognition and may help to develop more targeted dementia prevention strategies.
Supplementary Material
Contributor Information
Noah D Koblinsky, Rotman Research Institute, Baycrest Health Sciences, Toronto, Ontario, Canada.
Krista A Power, School of Nutrition Sciences, University of Ottawa, Ottawa, Ontario, Canada.
Laura Middleton, Department of Kinesiology and Health Sciences, University of Waterloo, Waterloo, Ontario, Canada.
Guylaine Ferland, Montreal Heart Institute Research Centre, Montreal, Quebec, Canada; Departments of Psychology and Psychiatry, University of Toronto, Toronto, Ontario, Canada.
Nicole D Anderson, Rotman Research Institute, Baycrest Health Sciences, Toronto, Ontario, Canada; Department of Psychology, University of Toronto, Toronto, Ontario, Canada; Department of Psychiatry, University of Toronto, Toronto, Ontario, Canada.
Funding
This work was supported by a grant from the Canadian Consortium on Neurodegeneration in Aging (CCNA), which is supported by the Canadian Institutes of Health Research (CIHR) with funding from several partners. The salary of N.D.K. was supported by the grant. The sponsors are not involved in the preparation of the paper.
Conflict of Interest
None declared.
Author Contributions
Everyone who has significantly contributed to this work has been listed as coauthor. N.D.A., G.F., and L.M. participated in the conceptualization of the project. N.D.K. aggregated the data and was the lead writer of the original draft. N.D.A., G.F., K.A.P., and L.M. supported the writing of the original draft. All coauthors were equally involved in reviewing, editing, and approving the final version of this manuscript. N.D.A. was the lead supervisor of the project.
References
- 1. Prince MJ, Wimo A, Guerchet MM, Ali GC, Wu YT, Prina M.. World Alzheimer Report 2015. The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. London, UK: Alzheimer’s Disease International; 2015:1–84. [Google Scholar]
- 2. Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. 2017;595(2):489–503. doi: 10.1113/JP273106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stefano GB, Pilonis N, Ptacek R, Raboch J, Vnukova M, Kream RM. Gut, microbiome, and brain regulatory axis: relevance to neurodegenerative and psychiatric disorders. Cell Mol Neurobiol. 2018;38(6):1197–1206. doi: 10.1007/s10571-018-0589-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davidson GL, Cooke AC, Johnson CN, Quinn JL. The gut microbiome as a driver of individual variation in cognition and functional behaviour. Philos Trans R Soc Lond B Biol Sci. 2018;373(1756):20170286. doi: 10.1098/rstb.2017.0286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gubert C, Kong G, Renoir T, Hannan AJ. Exercise, diet and stress as modulators of gut microbiota: implications for neurodegenerative diseases. Neurobiol Dis. 2020;134:104621. doi: 10.1016/j.nbd.2019.104621 [DOI] [PubMed] [Google Scholar]
- 6. Bäckhed F, Fraser CM, Ringel Y, et al. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe. 2012;12(5):611–622. doi: 10.1016/j.chom.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 7. Belizário JE, Napolitano M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front Microbiol. 2015;6:1050. doi: 10.3389/fmicb.2015.01050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cresci GAM, Izzo K. Chapter 4—gut microbiome. In: Corrigan ML, ed. Adult Short Bowel Syndrome. Academic Press; 2019:45–54. [Google Scholar]
- 9. Rinninella E, Raoul P, Cintoni M, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14. doi: 10.3390/microorganisms7010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martinez KB, Leone V, Chang EB. Western diets, gut dysbiosis, and metabolic diseases: are they linked? Gut Microbes. 2017;8(2):130–142. doi: 10.1080/19490976.2016.1270811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Y, Ning L, Yin Y, et al. Age-related shifts in gut microbiota contribute to cognitive decline in aged rats. Aging (Albany NY). 2020;12(9):7801–7817. doi: 10.18632/aging.103093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stevens BR, Goel R, Seungbum K, et al. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut. 2018;67(8):1555–1557. doi: 10.1136/gutjnl-2017-314759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Westfall S, Lomis N, Kahouli I, Dia SY, Singh SP, Prakash S. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell Mol Life Sci. 2017;74(20):3769–3787. doi: 10.1007/s00018-017-2550-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao J, Bi W, Xiao S, et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci Rep. 2019;9(1):5790. doi: 10.1038/s41598-019-42286-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berding K, Vlckova K, Marx W, et al. Diet and the microbiota–gut–brain axis: sowing the seeds of good mental health. Adv Nutr. 2021;12(4):1239–1285. doi: 10.1093/advances/nmaa181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Souza-Talarico JN, Marin MF, Sindi S, Lupien SJ. Effects of stress hormones on the brain and cognition: evidence from normal to pathological aging. Dement Neuropsychol. 2011;5(1):8–16. doi: 10.1590/S1980-57642011DN05010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gareau MG. Cognitive function and the microbiome. Int Rev Neurobiol. 2016;131:227–246. doi: 10.1016/bs.irn.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 18. Kowalski K, Mulak A. Brain–gut–microbiota axis in Alzheimer’s disease. J Neurogastroenterol Motil. 2019;25(1):48–60. doi: 10.5056/jnm18087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vogt NM, Kerby RL, Dill-McFarland KA, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7(1):13537. doi: 10.1038/s41598-017-13601-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Allen JM, Mailing LJ, Niemiro GM, et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc. 2018;50(4):747–757. doi: 10.1249/MSS.0000000000001495 [DOI] [PubMed] [Google Scholar]
- 21. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Francis H, Stevenson R. The longer-term impacts of Western diet on human cognition and the brain. Appetite. 2013;63:119–128. doi: 10.1016/j.appet.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 23. Parrott MD, Carmichael PH, Laurin D, et al. The association between dietary pattern adherence, cognitive stimulating lifestyle, and cognitive function among older adults from the Quebec Longitudinal Study on Nutrition and Successful Aging. J Gerontol B Psychol Sci Soc Sci. 2021;76(3):444–450. doi: 10.1093/geronb/gbaa178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van den Brink AC, Brouwer-Brolsma EM, Berendsen AAM, van de Rest O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diets are associated with less cognitive decline and a lower risk of Alzheimer’s disease—a review. Adv Nutr. 2019;10(6):1040–1065. doi: 10.1093/advances/nmz054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vauzour D, Camprubi-Robles M, Miquel-Kergoat S, et al. Nutrition for the ageing brain: towards evidence for an optimal diet. Ageing Res Rev. 2017;35:222–240. doi: 10.1016/j.arr.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 26. Gomez P, Tyagi E. Diet and cognition: interplay between cell metabolism and neuronal plasticity. Curr Opin Clin Nutr Metab Care. 2013;16(6):726–733. doi: 10.1097/MCO.0b013e328365aae3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Filippis F, Pellegrini N, Vannini L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65(11):1812–1821. doi: 10.1136/gutjnl-2015-309957 [DOI] [PubMed] [Google Scholar]
- 28. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi: 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood–brain barrier permeability in mice. Sci Transl Med. 2015;6(263):263ra158. doi: 10.1126/scitranslmed.3009759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Erny D, Hrabě de Angelis AL, Prinz M. Communicating systems in the body: how microbiota and microglia cooperate. Immunology. 2017;150(1):7–15. doi: 10.1111/imm.12645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barichello T, Generoso JS, Simões LR, et al. Sodium butyrate prevents memory impairment by re-establishing BDNF and GDNF expression in experimental pneumococcal meningitis. Mol Neurobiol. 2015;52:734–740. doi: 10.1007/s12035-014-8914-3 [DOI] [PubMed] [Google Scholar]
- 32. Zhang L, Wang Y, Xiayu X, et al. Altered gut microbiota in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2017;60:1241–1257. doi: 10.3233/JAD-170020 [DOI] [PubMed] [Google Scholar]
- 33. Galli C, Marangoni F. N-3 fatty acids in the Mediterranean diet. Prostaglandins Leukot Essent Fatty Acids. 2006;75(3):129–133. doi: 10.1016/j.plefa.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 34. Costantini L, Molinari R, Farinon B, Merendino N. Impact of omega-3 fatty acids on the gut microbiota. Int J Mol Sci. 2017;18(12):2645. doi: 10.3390/ijms18122645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chiurchiù V, Maccarrone M. Bioactive lipids and chronic inflammation: managing the fire within. Front Immunol. 2018;9:38. doi: 10.3389/fimmu.2018.0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Russo R, Cristiano C, Avagliano C, et al. Gut-brain axis: role of lipids in the regulation of inflammation, pain and CNS diseases. Curr Med Chem. 2018;25:3930–3952. doi: 10.2174/0929867324666170216113756 [DOI] [PubMed] [Google Scholar]
- 37. Cory H, Passarelli S, Szeto J, Tamez M, Mattei J. The role of polyphenols in human health and food systems: a mini-review. Front Nutr. 2018;5:87. doi: 10.3389/fnut.2018.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Filosa S, Di Meo F, Crispi S. Polyphenols–gut microbiota interplay and brain neuromodulation. Neural Regen Res. 2018;13(12):2055–2059. doi: 10.4103/1673-5374.241429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Figueira I, Garcia G, Pimpao RC, et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci Rep. 2017;7:11456. doi: 10.1038/s41598-017-11512-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spagnuolo C, Napolitano M, Tedesco I, Moccia S, Milito A, Russo GL. Neuroprotective role of natural polyphenols. Curr Top Med Chem. 2016;16(17):1943–1950. doi: 10.2174/1568026616666160204122449 [DOI] [PubMed] [Google Scholar]
- 41. Deshpande NG, Saxena J, Pesaresi TG, et al. High fat diet alters gut microbiota but not spatial working memory in early middle-aged Sprague–Dawley rats. PLoS One. 2019;14(5):e0217553. doi: 10.1371/journal.pone.0217553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beilharz JE, Kaakoush NO, Maniam J, Morris MJ. The effect of short-term exposure to energy-matched diets enriched in fat or sugar on memory, gut microbiota and markers of brain inflammation and plasticity. Brain Behav Immun. 2016;57:304–313. doi: 10.1016/j.bbi.2016.07.151 [DOI] [PubMed] [Google Scholar]
- 43. Magnusson KR, Hauck L, Jeffrey BM, et al. Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience. 2015;300:128–140. doi: 10.1016/j.neuroscience.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 44. Kang SS, Jeraldo PR, Kurti A, et al. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol Neurodegener. 2014;9:36. doi: 10.1186/1750-1326-9-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shi H, Yu Y, Lin D, et al. β-Glucan attenuates cognitive impairment via the gut-brain axis in diet-induced obese mice. Microbiome. 2020;8(1):143. doi: 10.1186/s40168-020-00920-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shi H, Wang Q, Zheng M, et al. Supplement of microbiota-accessible carbohydrates prevents neuroinflammation and cognitive decline by improving the gut microbiota-brain axis in diet-induced obese mice. J Neuroinflammation. 2020;17(1):77. doi: 10.1186/s12974-020-01760-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jeong MY, Jang HM, Kim DH. High-fat diet causes psychiatric disorders in mice by increasing Proteobacteria population. Neurosci Lett. 2019;698:51–57. doi: 10.1016/j.neulet.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 48. Fülling C, Lach G, Bastiaanssen TFS, et al. Adolescent dietary manipulations differentially affect gut microbiota composition and amygdala neuroimmune gene expression in male mice in adulthood. Brain Behav Immun. 2020;87:666–678. doi: 10.1016/j.bbi.2020.02.013 [DOI] [PubMed] [Google Scholar]
- 49. Bruce-Keller AJ, Salbaum JM, Luo M, et al. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry. 2015;77(7):607–615. doi: 10.1016/j.biopsych.2014.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reichelt AC, Loughman A, Bernard A, et al. An intermittent hypercaloric diet alters gut microbiota, prefrontal cortical gene expression and social behaviours in rats. Nutr Neurosci. 2020;23(8):613–627. doi: 10.1080/1028415X.2018.1537169 [DOI] [PubMed] [Google Scholar]
- 51. Leigh SJ, Kaakoush NO, Bertoldo MJ, et al. Intermittent cafeteria diet identifies fecal microbiome changes as a predictor of spatial recognition memory impairment in female rats. Transl Psychiatry. 2020;10:36. doi: 10.1038/s41398-020-0734-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li L, Wang Z, Zuo Z. Chronic intermittent fasting improves cognitive functions and brain structures in mice. PLoS One. 2013;8(6):e66069. doi: 10.1371/journal.pone.0066069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Boitard C, Parkes SL, Cavaroc A, et al. Switching adolescent high-fat diet to adult control diet restores neurocognitive alterations. Front Behav Neurosci. 2016;10:225. doi: 10.3389/fnbeh.2016.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xu M, Mo X, Huang H, et al. Yeast β-glucan alleviates cognitive deficit by regulating gut microbiota and metabolites in Aβ 1-42-induced AD-like mice. Int J Biol Macromol. 2020;161:258–270. doi: 10.1016/j.ijbiomac.2020.05.180 [DOI] [PubMed] [Google Scholar]
- 55. Gao H, Song R, Li Y, Zhang W, et al. Effects of oat fiber intervention on cognitive behavior in LDLR-/- mice modeling atherosclerosis by targeting the microbiome–gut–brain axis. J Agric Food Chem. 2020;68(49):14480–14491. doi: 10.1021/acs.jafc.0c05677 [DOI] [PubMed] [Google Scholar]
- 56. Shimizu C, Wakita Y, Kihara M, Kobayashi N, Tsuchiya Y, Nabeshima T. Association of lifelong intake of barley diet with healthy aging: changes in physical and cognitive functions and intestinal microbiome in senescence-accelerated mouse-prone 8 (SAMP8). Nutrients. 2019;11(8):1770. doi: 10.3390/nu11081770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Berding K, Long-Smith CM, Carbia C, et al. A specific dietary fibre supplementation improves cognitive performance-an exploratory randomised, placebo-controlled, crossover study. Psychopharmacology (Berl). 2021;238(1):149–163. doi: 10.1007/s00213-020-05665-y [DOI] [PubMed] [Google Scholar]
- 58. Provensi G, Schmidt SD, Boehme M, et al. Preventing adolescent stress-induced cognitive and microbiome changes by diet. Proc Natl Acad Sci USA. 2019;116(19):9644–9651. doi: 10.1073/pnas.1820832116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Robertson RC, Seira Oriach C, Murphy K, et al. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav Immun. 2017;59:21–37. doi: 10.1016/j.bbi.2016.07.145 [DOI] [PubMed] [Google Scholar]
- 60. Ren B, Yuan T, Zhang X, et al. Protective effects of sesamol on systemic inflammation and cognitive impairment in aging mice. J Agric Food Chem. 2020;68(10):3099–3111. doi: 10.1021/acs.jafc.9b07598 [DOI] [PubMed] [Google Scholar]
- 61. Marseglia A, Xu W, Fratiglioni L, et al. Effect of the NU-AGE diet on cognitive functioning in older adults: a randomized controlled trial. Front Physiol. 2018;9:349. doi: 10.3389/fphys.2018.00349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ghosh TS, Rampelli S, Jeffery IB, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut. 2020;69(7):1218–1228. doi: 10.1136/gutjnl-2019-319654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Blondell SJ, Hammersley-Mather R, Veerman JL. Does physical activity prevent cognitive decline and dementia? A systematic review and meta-analysis of longitudinal studies. BMC Public Health. 2014;14:510. doi: 10.1186/1471-2458-14-510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lista I, Sorrentino G. Biological mechanisms of physical activity in preventing cognitive decline. Cell Mol Neurobiol. 2010;30(4):493–503. doi: 10.1007/s10571-009-9488-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ma CL, Ma XT, Wang JJ, Liu H, Chen YF, Yang Y. Physical exercise induces hippocampal neurogenesis and prevents cognitive decline. Behav Brain Res. 2017;317:332–339. doi: 10.1016/j.bbr.2016.09.067 [DOI] [PubMed] [Google Scholar]
- 66. MacIntosh BJ, Crane DE, Sage MD, et al. Impact of a single bout of aerobic exercise on regional brain perfusion and activation responses in healthy young adults. PLoS One. 2014;9(1):e85163. doi: 10.1371/journal.pone.0085163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hoffman-Goetz L, Pervaiz N, Packer N, Guan J. Freewheel training decreases pro- and increases anti-inflammatory cytokine expression in mouse intestinal lymphocytes. Brain Behav Immun. 2010;24(7):1105–1115. doi: 10.1016/j.bbi.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 68. Packer N, Hoffman-Goetz L. Exercise training reduces inflammatory mediators in the intestinal tract of healthy older adult mice. Can J Aging. 2012;31(2):161–171. doi: 10.1017/S0714980812000104 [DOI] [PubMed] [Google Scholar]
- 69. Morita E, Yokoyama H, Imai D, et al. Aerobic exercise training with brisk walking increases intestinal bacteroides in healthy elderly women. Nutrients. 2019;11(4):868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Taniguchi H, Tanisawa K, Sun X, et al. Effects of short-term endurance exercise on gut microbiota in elderly men. Physiol Rep. 2018;6(23):e13935. doi: 10.14814/phy2.13935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li C, Cai YY, Yan ZX. Brain-derived neurotrophic factor preserves intestinal mucosal barrier function and alters gut microbiota in mice. Kaohsiung J Med Sci. 2018;34(3):134–141. doi: 10.1016/j.kjms.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. doi: 10.1186/s40168-016-0189-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mitchell CM, Davy BM, Hulver MW, Eilson AP, Bennet BJ, Davy KP. Does exercise alter gut microbial composition? A systematic review. Med Sci Sports Exerc. 2019;51(1):160–167. doi: 10.1249/MSS.0000000000001760 [DOI] [PubMed] [Google Scholar]
- 74. Oettlé GJ. Effect of moderate exercise on bowel habit. Gut. 1991;32(8):941–944. doi: 10.1136/gut.32.8.941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Scheffer DDL, Latini A. Exercise-induced immune system response: anti-inflammatory status on peripheral and central organs. Biochim Biophys Acta Mol Basis Dis. 2020;1866(10):165823. doi: 10.1016/j.bbadis.2020.165823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Clark A, Mach N. Exercise-induced stress behavior, gut–microbiota–brain axis and diet: a systematic review for athletes. J Int Soc Sports Nutr. 2016;13:43. doi: 10.1186/s12970-016-0155-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Qamar MI, Read AE. Effects of exercise on mesenteric blood flow in man. Gut. 1987;28(5):583–587. doi: 10.1136/gut.28.5.583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cerqueira E, Marinho DA, Neiva HP, Lourenço O. Inflammatory effects of high and moderate intensity exercise—a systematic review. Front Physiol. 2020;10:1550. doi: 10.3389/fphys.2019.01550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lamprecht M, Frauwallner A. Exercise, intestinal barrier dysfunction and probiotic supplementation. Med Sport Sci. 2012;59:47–56. doi: 10.1159/000342169 [DOI] [PubMed] [Google Scholar]
- 80. Abraham D, Feher J, Scuderi GL, et al. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: role of microbiome. Exp Gerontol. 2019;115:122–131. doi: 10.1016/j.exger.2018.12.005 [DOI] [PubMed] [Google Scholar]
- 81. Denou E, Marcinko K, Surette MG, Steinberg GR, Schertzer JD. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. Am J Physiol Endocrinol Metab. 2016;310(11):E982–E993. doi: 10.1152/ajpendo.00537.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang G, Zhou H, Zhang L, et al. Effects of high-intensity interval training on gut microbiota profiles in 12 months’ old ICR mice. J Physiol Biochem. 2020;76(4):539–548. doi: 10.1007/s13105-020-00758-w [DOI] [PubMed] [Google Scholar]
- 83. Maillard F, Vazeille E, Sauvanet P, et al. High-intensity interval training promotes total and visceral fat mass loss in obese Zucker rats without modulating gut microbiota. PLoS One. 2019;14(4):e0214660. doi: 10.1371/journal.pone.0214660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Feng X, Uchida Y, Koch L, et al. Exercise prevents enhanced postoperative neuroinflammation and cognitive decline and rectifies the gut microbiome in a rat model of metabolic syndrome. Front Immunol. 2017;8:1768. doi: 10.3389/fimmu.2017.01768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Barnett JH, Lewis L, Blackwell AD, Taylor M. Early intervention in Alzheimer’s disease: a health economic study of the effects of diagnostic timing. BMC Neurol. 2014;14:101. doi: 10.1186/1471-2377-14-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Metabolic and Bio-behavioural Effects of Following Recommendations in the Dietary Guidelines for Americans (DGA4ME). ClinicalTrials.gov identifier: NCT04293224. Updated January 31, 2022. Accessed February 22, 2022. https://clinicaltrials.gov/ct2/show/NCT04293224. [Google Scholar]
- 87. The Impact of Cranberries on the Microbiome and the Brain in Healthy Ageing Study (COMBAT). ClinicalTrials.gov identifier: NCT03679533. https://clinicaltrials.gov/ct2/show/NCT03679533. Updated September 30, 2020. Accessed February 22, 2022. [Google Scholar]
- 88. Can Lifestyle Changes Reverse Early-Stage Alzheimer’s Disease. ClinicalTrials.gov identifier: NCT04606420. https://clinicaltrials.gov/ct2/show/NCT04606420. Updated October 28, 2020. Accessed February 22, 2022. [Google Scholar]
- 89. Effects of Diet and Exercise Interventions on Cardiometabolic Risk Markers, Executive Function and Intestinal Flora. ClinicalTrials.gov identifier: NCT04834687. https://clinicaltrials.gov/ct2/show/NCT04834687. Updated September 22, 2021. Accessed February 22, 2022. [Google Scholar]
- 90. Schneider N, Yvon C. A review of multidomain interventions to support healthy cognitive ageing. J Nutr Health Aging. 2013;17(3):252–257. doi: 10.1007/s12603-012-0402-8 [DOI] [PubMed] [Google Scholar]
- 91. Toman J, Klímová B, Vališ M. Multidomain lifestyle intervention strategies for the delay of cognitive impairment in healthy aging. Nutrients. 2018;10(10):1560. doi: 10.3390/nu10101560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. LaPlume AA, McKetton L, Anderson ND, Troyer AK. Sex differences and modifiable dementia risk factors synergistically influence memory over the adult lifespan. Alzheimers Dement (Amst). 2022;14(1):e12301. doi: 10.1002/dad2.12301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Fritz AK, Amrein I, Wolfer DP. Similar reliability and equivalent performance of female and male mice in the open field and water-maze place navigation task. Am J Med Genet C Semin Med Genet. 2017;175(3):380–391. doi: 10.1002/ajmg.c.31565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ferretti MT, Martinkova J, Biskup E, et al. Sex and gender differences in Alzheimer’s disease: current challenges and implications for clinical practice: position paper of the Dementia and Cognitive Disorders Panel of the European Academy of Neurology. Eur J Neurol. 2020;27(6):928–943. doi: 10.1111/ene.14174 [DOI] [PubMed] [Google Scholar]
- 95. Masella R, Malorni W. Gender-related differences in dietary habits. Clin Manage Issue. 2017;11(2):59–62. doi: 10.7175/cmi.v11i2.1313 [DOI] [Google Scholar]
- 96. van Uffelen JG, Khan A, Burton NW. Gender differences in physical activity motivators and context preferences: a population-based study in people in their sixties. BMC Pub Health. 2017;17(1):624. doi: 10.1186/s12889-017-4540-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.