This randomized clinical trial compares vigorous vs moderate walking exercise to assess the optimal intensity and training duration to maximize improvement in walking capacity among stroke survivors with walking limitations.
Key Points
Question
For walking exercise in patients with chronic stroke, what is the optimal intensity (vigorous vs moderate) and minimum duration (4, 8, or 12 weeks) to maximize immediate improvement in walking capacity?
Findings
In this randomized clinical trial that enrolled 55 stroke survivors, 6-minute walk test gains after 12 weeks of training were 71 m with vigorous training intensity vs 27 m with moderate training intensity, a significant difference. Within the vigorous intensity group, walking capacity significantly increased after each 4-week training block.
Meaning
The findings suggest that optimal dosing for walking exercise for patients with chronic stroke may include at least 12 weeks of training at vigorous intensity.
Abstract
Importance
For walking rehabilitation after stroke, training intensity and duration are critical dosing parameters that lack optimization.
Objective
To assess the optimal training intensity (vigorous vs moderate) and minimum training duration (4, 8, or 12 weeks) needed to maximize immediate improvement in walking capacity in patients with chronic stroke.
Design, Setting, and Participants
This multicenter randomized clinical trial using an intent-to-treat analysis was conducted from January 2019 to April 2022 at rehabilitation and exercise research laboratories. Survivors of a single stroke who were aged 40 to 80 years and had persistent walking limitations 6 months or more after the stroke were enrolled.
Interventions
Participants were randomized 1:1 to high-intensity interval training (HIIT) or moderate-intensity aerobic training (MAT), each involving 45 minutes of walking practice 3 times per week for 12 weeks. The HIIT protocol used repeated 30-second bursts of walking at maximum safe speed, alternated with 30- to 60-second rest periods, targeting a mean aerobic intensity above 60% of the heart rate reserve (HRR). The MAT protocol used continuous walking with speed adjusted to maintain an initial target of 40% of the HRR, progressing up to 60% of the HRR as tolerated.
Main Outcomes and Measures
The main outcome was 6-minute walk test distance. Outcomes were assessed by blinded raters after 4, 8, and 12 weeks of training.
Results
Of 55 participants (mean [SD] age, 63 [10] years; 36 male [65.5%]), 27 were randomized to HIIT and 28 to MAT. The mean (SD) time since stroke was 2.5 (1.3) years, and mean (SD) 6-minute walk test distance at baseline was 239 (132) m. Participants attended 1675 of 1980 planned treatment visits (84.6%) and 197 of 220 planned testing visits (89.5%). No serious adverse events related to study procedures occurred. Groups had similar 6-minute walk test distance changes after 4 weeks (HIIT, 27 m [95% CI, 6-48 m]; MAT, 12 m [95% CI, −9 to 33 m]; mean difference, 15 m [95% CI, −13 to 42 m]; P = .28), but HIIT elicited greater gains after 8 weeks (58 m [95% CI, 39-76 m] vs 29 m [95% CI, 9-48 m]; mean difference, 29 m [95% CI, 5-54 m]; P = .02) and 12 weeks (71 m [95% CI, 49-94 m] vs 27 m [95% CI, 3-50 m]; mean difference, 44 m [95% CI, 14-74 m]; P = .005) of training; HIIT also showed greater improvements than MAT on some secondary measures of gait speed and fatigue.
Conclusions and Relevance
These findings show proof of concept that vigorous training intensity is a critical dosing parameter for walking rehabilitation. In patients with chronic stroke, vigorous walking exercise produced significant and meaningful gains in walking capacity with only 4 weeks of training, but at least 12 weeks were needed to maximize immediate gains.
Trial Registration
ClinicalTrials.gov Identifier: NCT03760016
Introduction
While the majority of stroke survivors eventually regain the ability to walk without physical assistance from another person, most in the chronic phase of recovery still lack sufficient walking capacity (ie, speed and endurance) to resume normal daily activities.1,2 Both the neuromotor impairment and the aerobic deconditioning underlying these limitations in walking capacity can be targeted simultaneously with locomotor exercise (ie, task-specific walking practice at sufficient intensity to challenge the neuromotor and cardiopulmonary systems).3,4,5 With this intervention, training intensity appears to be a critical dosing parameter associated with outcomes.6 However, the optimal intensity for improving walking recovery remains unknown.
Most studies of poststroke locomotor exercise have tested moderate-intensity aerobic training (MAT), typically involving treadmill walking at mean training heart rates (HRs) between 40% and 60% of the heart rate reserve (HRR).7,8 These studies have found that MAT is associated with significantly greater improvements in walking capacity (6-minute walk distance) and other outcomes than lower-intensity walking practice or nonwalking exercise. Consequently, this approach is currently recommended in stroke rehabilitation guidelines.3,4,5
Studies also suggest that a more vigorous training intensity (>60% of HRR vs 40%-60% of HRR) could augment outcomes,6 but a vigorous intensity can be difficult to achieve and sustain for many persons with stroke.9,10,11 Thus, high-intensity interval training (HIIT) has emerged as a promising strategy for poststroke locomotor exercise.6 This method involves bursts of fast walking alternated with recovery periods and is designed to enable higher sustained intensities12 at lower perceived exertion than high-intensity continuous exercise.13 Initial stroke studies indicated that locomotor HIIT is feasible, sufficiently safe for further study, can improve walking capacity and other outcomes,6,14,15,16,17 and has some early signs of potential for eliciting greater improvements than MAT.14,15
However, to our knowledge, no prior studies have been designed to compare outcomes of HIIT and MAT after stroke. In addition, most prior studies of HIIT after stroke14,17,18,19 used short 4-week training durations. Therefore, it was unknown whether a longer 12-week training duration typical of many stroke and cardiac rehabilitation trials could yield better outcomes. Further, poststroke HIIT studies have often focused primarily on improving aerobic fitness among persons with mild stroke or transient ischemic attack who had normal, near-normal, or unreported baseline walking function.15,16 To generate evidence that is generalizable to clinical stroke rehabilitation, it is important to target patients with walking limitations (eg, speed ≤1.0 m/s20).
Therefore, the purpose of this multicenter randomized clinical trial was to assess the optimal training intensity (vigorous vs moderate) and the minimum training duration (4, 8, or 12 weeks) needed to maximize immediate improvement in walking capacity among stroke survivors with chronic walking limitations. We hypothesized that (1) 4 weeks of HIIT would elicit significantly greater improvement in walking capacity compared with 4 weeks of MAT and, (2) compared with 4 or 8 weeks of HIIT, 12 weeks of HIIT would elicit significantly greater improvements in walking capacity and increased benefit compared with MAT.
Methods
This randomized clinical trial was approved by the University of Cincinnati institutional review board and was performed in rehabilitation and exercise laboratories at the University of Cincinnati, University of Delaware, and University of Kansas Medical Center with participant enrollment from January 2019 to April 2022. Written informed consent was obtained from participants prior to participation. The trial was prospectively registered at ClinicalTrials.gov (NCT03760016); the trial protocol is given in Supplement 1 and is published elsewhere.21 Additional methods, results, and discussion are provided in the eAppendix in Supplement 2. This trial followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Participants
Participants were recruited from the community via outreach to clinicians and support groups, advertisement, existing databases, and health record systems. Screening included a medical history and record review, physical examination, Fugl-Meyer lower limb motor function assessment,22 depression questionnaire,23 10-m walk test,20 treadmill screening test,8 resting electrocardiography (ECG), and a treadmill-graded exercise test with ECG monitoring.8
Inclusion criteria were (1) age of 40 to 80 years at the time of consent, (2) single stroke for which the participant sought treatment 6 months to 5 years prior to consent, (3) walking speed of 1.0 m/s or less,20 (4) ability to walk 10 m over ground with assistive devices as needed and no continuous physical assistance from another person,7 (5) ability to walk at least 3 minutes on a treadmill at 0.13 m/s (0.3 mph) or faster,8 (6) stable cardiovascular condition (American Heart Association class B24), and (7) ability to communicate with investigators, follow a 2-step command, and correctly answer consent comprehension questions. Exclusion criteria were (1) exercise testing uninterpretable for ischemia or arrhythmia,24 (2) evidence of significant arrhythmia or myocardial ischemia on a treadmill ECG-graded exercise test in the absence of a more definitive negative result from clinical testing,24 (3) hospitalization for cardiac or pulmonary disease within the past 3 months, (4) implanted pacemaker or defibrillator; (5) significant ataxia or neglect (National Institutes of Health Stroke Scale item scores >1),25 (6) severe lower limb spasticity (Ashworth Scale scores >2 of 4 for knee flexion, knee extension, or ankle dorsiflexion),26 (7) recent history of illicit drug or alcohol misuse or significant mental illness, (8) major poststroke depression (Patient Health Questionnaire score ≥10)23 in the absence of depression management by a health care professional, (9) currently participating in physical therapy or another interventional study, (10) recent botulinum toxin injection to the paretic lower limb (<3 months previously) or planning to have it in the next 4 months, (11) foot drop or lower limb joint instability in the absence of an adequate stabilizing device (eg, ankle foot orthosis), (12) clinically significant neurologic disorder other than stroke, (13) unable to walk outside the home prior to stroke, (14) other significant medical condition likely to limit improvement or jeopardize safety, (15) pregnancy, and (16) previous exposure to fast treadmill walking in the past year.
Race and ethnicity data were ascertained by self-report and were included in the analysis to describe the study sample. Race categories included American Indian, Asian, Black/African American, and White, and ethnicity categories included Hispanic/Latinx and not Hispanic/Latinx.
Randomization
After screening and baseline testing, eligible participants were randomized to HIIT or MAT in a 1:1 ratio using the web-based REDCap randomization module. Randomization was stratified by site and baseline walking limitation severity (severe, <0.4 m/s; mild or moderate, 0.4-1.0 m/s)17 and used randomly permuted block sizes of 2 or 4. The study statistician (J.C.K.) generated the random allocation sequence and kept it confidential to ensure concealed allocation. When participants arrived for the first training session, the treating therapist or study coordinator initiated the randomization within REDCap to irreversibly assign the participant to a group before revealing the assignment.
Interventions
Target training volume for both groups was 45 minutes 3 times per week for 12 weeks. Participants were not physically assisted with stepping but were guarded and assisted for injury prevention as needed by a physical therapist. During overground training bouts, participants walked back and forth in a corridor. When training on a treadmill, participants wore a fall-protection harness without any weight support and used a handrail for balance support.8 Training HR zones were calculated using the HRR method: (peak HR – resting HR) × percent HRR target + resting HR,24 where peak HR was the highest HR obtained in any previous exercise test and resting HR was the resting HR while standing from the current day. Each session, the training protocol for both groups included a 3-minute warm-up of overground walking at 30% to 40% of the HRR, a 10-minute bout of overground HIIT or MAT, a 20-minute bout of treadmill HIIT or MAT, another 10-minute bout of overground HIIT or MAT, and a 2-minute cool down at 30% to 40% of the HRR.
HIIT and MAT Protocols
The HIIT group used a short-interval HIIT protocol that was specifically developed for locomotor exercise after stroke.14,17,27,28,29 It involved repeated 30-second bursts of walking at maximum safe speed, alternated with 30- to 60-second passive recovery periods (standing or seated rest as tolerated), targeting a mean aerobic intensity above 60% of the HRR. Following current best-practice guidelines for stroke rehabilitation,3,4 the MAT group performed continuous walking practice with speed adjusted to maintain an initial target HR of 40% ± 5% of the HRR, progressing by 5% of the HRR every 2 weeks up to 60% of the HRR as tolerated.8
Adverse Event Monitoring
At each study visit, participants were asked about adverse events (AEs) in general and then were specifically queried about falls, injuries, pain, and fatigue. During exercise testing and training, safety monitoring included HR, blood pressure, and continuous observation for other signs or symptoms of cardiorespiratory insufficiency, worsening neurologic impairments, or orthopedic injury using accepted stopping criteria.24 Adverse events were systematically categorized and graded using Common Terminology Criteria for Adverse Events30 by a centralized physician (O.O.A.) blinded to group randomization.
Outcome Assessment
Outcomes were assessed by blinded raters before randomization (baseline) and after 4, 8, and 12 weeks of training. The primary outcome measure was walking capacity, measured by distance walked during the 6-minute walk test.31 Secondary measures included self-selected and fastest speeds, measured by the 10-m walk test20; self-reported fatigue over the past 7 days, measured by the Patient Reported Outcomes Measurement Information System (PROMIS) Fatigue Scale32; and aerobic capacity, measured by oxygen consumption rate (VO2) at the ventilatory threshold during a treadmill-graded exercise test.33
Statistical Analysis
Statistical analyses followed a prespecified plan,21 used an intent-to-treat approach, and were done with SAS, version 9.4 (SAS Institute Inc). The primary study statistician (J.C.K.) remained blinded to treatment groups until after the primary analysis.
Hypothesis Testing
Primary hypothesis testing used a linear model with the 6-minute walk test as the dependent variable and fixed effects for treatment group, testing time point (baseline, 4 weeks, 8 weeks, and 12 weeks), group-by-time interaction, study site, study site–by-time interaction, baseline walking limitation severity, and baseline walking limitation severity–by-time interaction, with unconstrained covariance between repeated testing time points within the same participant and a significance threshold of 2-sided P < .05. Secondary outcomes were tested using the same model, with false discovery rate correction to the significance threshold to control for multiple testing time points (change at 4 weeks, 8 weeks, and 12 weeks) and the 5 primary or secondary outcome measures. These analyses handled any missing data with the method of maximum likelihood, which assumes that data were missing at random. A sensitivity analysis assessed how much the results depended on the missing at random assumption (eAppendix in Supplement 2).
Sample Size
This study was powered to detect a between-group difference of 20 m in 6-minute walk test change34 using the software GLIMMPSE.35 Covariance parameters and MAT group changes were estimated from pilot work.14 Calculations indicated a total target sample size of 40. To account for up to 20% attrition, we initially planned to randomize 50 participants. However, after having to withdraw 4 participants due to a COVID-19 pandemic shutdown, we opted to increase the enrollment target to 55. This decision was made before any analysis of outcome data.
Results
Recruitment and Retention
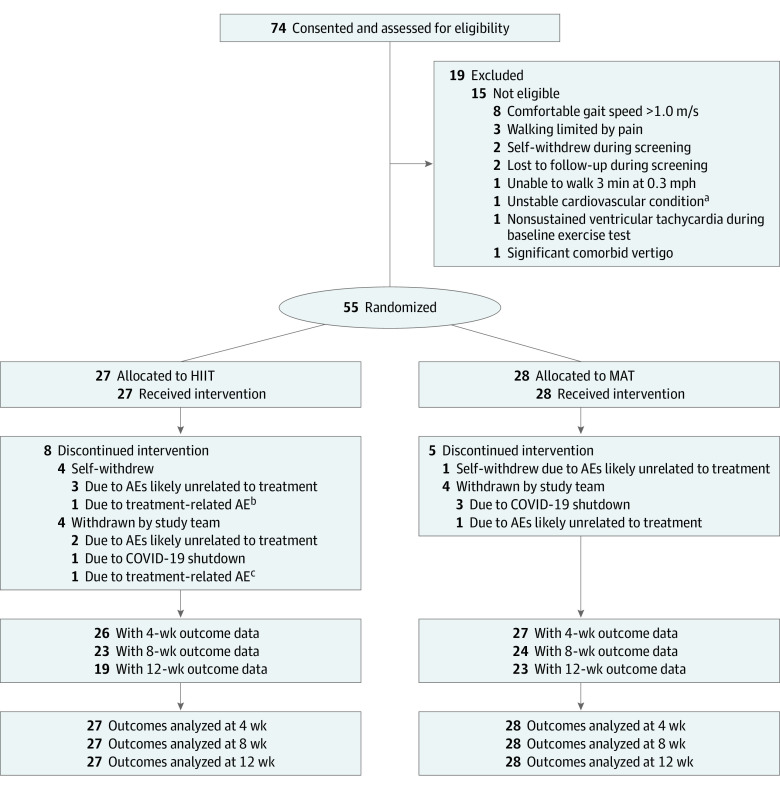
We consented and screened 74 participants to find 55 who were eligible for randomization (Figure 1). The mean [SD] age was 63 [10] years; 19 participants (34.5%) were female, and 36 (65.5%) were male. One participant (1.8%) was American Indian, 3 (5.5%) were Asian, 11 (20.0%) were Black/African American, 1 (1.8%) was Hispanic/Latinx, and 40 (72.7%) were White. The mean (SD) time since stroke was 2.5 (1.3) years. The mean baseline 6-minute walk test distance was 239 (132) m, and the mean baseline self-selected gait speed was 0.63 (0.31) m/s. Twenty-seven participants were randomized to HIIT and 28 to MAT. Baseline characteristics were similar between groups (Table 1). In total, participants attended 1675 of 1980 planned treatment sessions (84.6%; HIIT, 800 of 972 [82.3%]; MAT, 875 of 1008 [86.8%]), with individual participants attending a mean (SD) of 30.5 (8.4) sessions (HIIT, 29.6 [8.3]; MAT, 31.3 [8.5]). Altogether, participants also attended 197 of 220 planned testing sessions (89.5%), leaving 23 of 220 outcome assessments missing (10.5%; HIIT, 13 of 108 [12.0%]; MAT, 10 of 112 [8.9%]).
Figure 1. CONSORT Flow Diagram.
AE indicates adverse event; HIIT, high-intensity interval training; and MAT, moderate-intensity aerobic training.
aPrior myocardial infarction with peri-infarct ischemia on most recent perfusion scan.
bFlare-ups of preexisting back pain.
cRecurring hamstring strain or soreness.
Table 1. Baseline Participant Characteristics.
| Characteristic | Participantsa | |
|---|---|---|
| HIIT (n = 27) | MAT (n = 28) | |
| Age, mean (SD), y | 63.8 (9.9) | 61.5 (9.9) |
| Sex | ||
| Female | 11 (40.7) | 8 (28.6) |
| Male | 16 (59.3) | 20 (71.4) |
| Race | ||
| American Indian | 0 | 1 (3.6) |
| Asian | 0 | 3 (10.7) |
| Black/African American | 4 (14.8) | 7 (25.0) |
| White | 23 (85.2) | 17 (60.7) |
| Hispanic/Latinx ethnicity | 1 (3.7) | 0 |
| Side of paresis | ||
| Left | 14 (51.9) | 14 (50.0) |
| Right | 13 (48.1) | 14 (50.0) |
| Stroke type | ||
| Ischemic | 15 (55.6) | 19 (67.9) |
| Hemorrhagic | 12 (44.4) | 9 (32.1) |
| Stroke chronicity, mean (SD), y | 2.7 (1.4) | 2.2 (1.2) |
| Aphasia present | 7 (25.9) | 7 (25.0) |
| Comorbid medical conditions | ||
| Any | 26 (96.3) | 27 (96.4) |
| History of myocardial infarction | 1 (3.7) | 2 (7.1) |
| Atrial fibrillation | 2 (7.4) | 5 (17.9) |
| History of lightheadedness and/or syncope | 4 (14.8) | 5 (17.9) |
| COPD | 3 (11.1) | 1 (3.6) |
| Diabetes | ||
| Any | 8 (29.6) | 9 (32.1) |
| Insulin dependent | 2 (25.0) | 2 (22.2) |
| Arthritis | 8 (29.6) | 10 (35.7) |
| History of cancer | 4 (14.8) | 3 (10.7) |
| Prescribed medication | ||
| Antihypertensive | ||
| Any | 23 (85.2) | 20 (71.4) |
| β-Blocker | 7 (25.9) | 11 (39.3) |
| Statin | 22 (81.5) | 23 (82.1) |
| Antispasmodic | 11 (40.7) | 12 (42.9) |
| Antidepressant | 20 (74.1) | 13 (46.4) |
| Pain reliever | 11 (40.7) | 11 (39.3) |
| Reported falling in previous 3 mo | 8 (29.6) | 7 (25.0) |
| BMI, mean (SD) | 28.9 (5.3) | 28.7 (4.6) |
| Functional ambulation categoryb | ||
| Mean (SD) | 3.3 (0.6) | 3.4 (0.8) |
| 2 | 2 (7.4) | 5 (17.9) |
| 3 | 14 (51.9) | 8 (28.6) |
| ≥4 | 11 (40.7) | 15 (53.6) |
| Orthotic and/or assistive device use | 18 (66.7) | 19 (67.9) |
| Orthotic device use | 12 (44.4) | 12 (42.9) |
| Solid ankle foot orthosis | 4 (14.8) | 2 (7.1) |
| Articulated or flexible ankle foot orthosis | 7 (25.9) | 7 (25.0) |
| Assistive device use | ||
| Any | 16 (59.3) | 18 (64.3) |
| Single-point cane | 8 (29.6) | 11 (39.3) |
| Narrow-based quad cane | 5 (18.5) | 1 (3.6) |
| Wide-based quad cane | 3 (11.1) | 5 (17.9) |
| Front-wheeled walker | 0 | 1 (3.6) |
| Fugl-Meyer lower limb motor score, mean (SD)c | 23.8 (5.1) | 22.8 (5.1) |
| Self-selected gait speed | ||
| Mean (SD), m/s | 0.65 (0.29) | 0.62 (0.33) |
| Mean (SD), % normative value | 50.5 (23.3) | 47.3 (25.1) |
| <0.4 m/s | 7 (25.9) | 7 (25.0) |
| 6-min Walk test distance | ||
| Mean (SD), m | 248 (136) | 230 (130) |
| Mean (SD), % normative value | 48.5 (26.3) | 44.3 (26.8) |
| Ventilatory threshold VO2 | ||
| Mean (SD), mL/kg/min | 12.1 (3.9) | 11.6 (3.9) |
| Mean (SD), % normative value | 93.1 (24.4) | 82.5 (26.2) |
| Peak VO2 | ||
| Mean (SD), mL/kg/min | 14.3 (4.4) | 14.0 (4.8) |
| Mean (SD), % normative value | 60.4 (15.9) | 55.1 (19.3) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); COPD, chronic obstructive pulmonary disease; HIIT, high-intensity interval training; MAT, moderate-intensity aerobic training; VO2, oxygen consumption rate.
Data are presented as the number (percentage) of participants unless otherwise indicated.
Category 2 indicates dependent, level 1 (requires continuous or intermittent light touch to assist balance or coordination from no more than 1 person during ambulation on level surfaces to prevent falling); category 3, dependent, supervision (can physically ambulate on level surfaces without manual contact of another person but requires standby guarding of no more than 1 person because of poor judgment, questionable cardiac status, or the need for verbal cuing to complete the task); and category ≥4, independent, level surfaces (can ambulate independently on level surfaces).
Range, 0 to 34; higher scores indicate less motor impairment.
Treatment Fidelity and Adverse Events
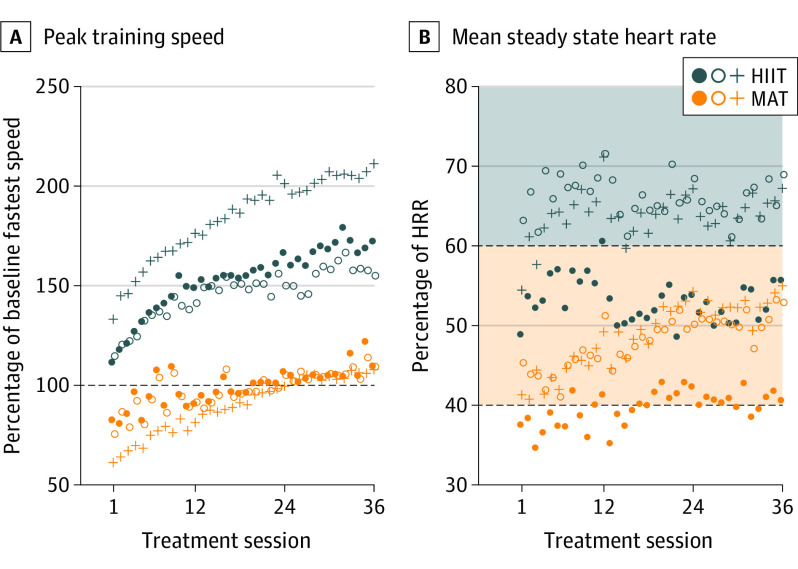
Compared with MAT, HIIT involved significantly higher training speed, HR, blood lactate level, and perceived exertion with significantly lower step count (Figure 2 and eAppendix in Supplement 2). For example, the peak training speed was 161% of the baseline fastest 10-m speed in the HIIT group and 96% in the MAT group. The mean bout steady state HR (compared with true resting HR and baseline peak HR) was 75% of the HRR in the HIIT group and 59% in the MAT group. The mean session step count was 2847 in the HIIT group and 3532 in the MAT group. There were no serious AEs related to study procedures and no significant between-group differences in any AE categories (eAppendix in Supplement 2).
Figure 2. Treatment Intensity.
Values are model estimates for each bout of each session, averaging data across participants. Filled circles indicate overground 1; open circles, overground 2; and crosses, treadmill. A, Speed data are peak values within a bout. B, Steady state mean heart rate (HR) excludes the first 3 minutes of the bout. For treatment monitoring, the percentage of HR reserve (HRR) was relative to the standing resting HR and the most current peak HR, which could increase after sessions 12 and 24. Shading indicates aerobic intensity zones: orange, moderate; gray, vigorous. HIIT indicates high-intensity interval training; MAT, moderate-intensity interval training.
Outcome Changes
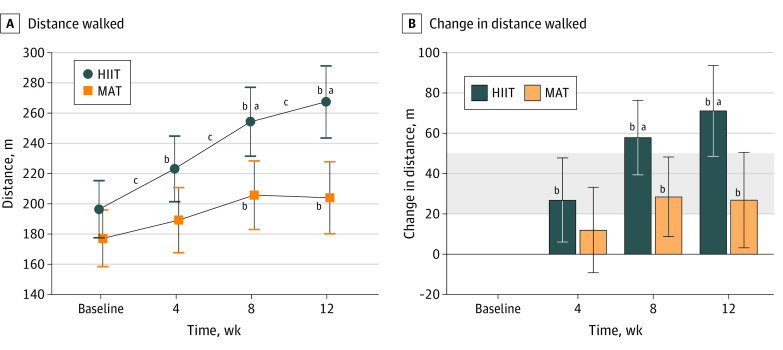
After 4 weeks of training, there was no significant difference in primary outcome measure (6-minute walk test) changes between groups (HIIT: 27 m [95% CI, 6-48 m]; MAT: 12 m [95% CI, –9 to 33 m]; mean difference, 15 m [95% CI, −13 to 42 m]; P = .28). The HIIT group improved significantly more than the MAT group after 8 weeks (58 m [95% CI, 39-76 m] vs 29 m [95% CI, 9-48 m]; mean difference, 29 m [95% CI, 5-54 m]; P = .02) and 12 weeks (71 m [95% CI, 49-94 m] vs 27 m [95% CI, 3-50 m]; mean difference, 44 m [95% CI, 14-74 m]; P = .005) of training (Figure 3 and Table 2).
Figure 3. Primary Outcome of 6-Minute Walk Test Changes During 12 Weeks of High-intensity Interval Training (HIIT) or Moderate-intensity Aerobic Training (MAT) in Patients With Chronic Stroke.
Values are model estimates; error bars, 95% CIs; and shading, clinically important difference in distance (20-50 m).
aP < .05 for the false discovery rate in between-group differences in change from baseline.
bP < .05 for within-group change from baseline.
cP < .05 for within-group change between consecutive time points.
Table 2. Primary and Secondary Outcome Changes During 12 Weeks of HIIT or MAT Among Patients With Chronic Strokea.
| Outcome, time point | Mean change vs baseline (95% CI) | Mean difference in change between HIIT and MAT (95% CI) | P value | |
|---|---|---|---|---|
| HIIT (n = 27) | MAT (n = 28) | |||
| Primary outcome | ||||
| Walking capacity, 6-min walk distance, m | ||||
| Baseline, mean (SD)b | 196 (98) | 177 (99) | 19 (−31 to 69) | .44 |
| 4 wk | 27 (6 to 48) | 12 (−9 to 33) | 15 (−13 to 42) | .28 |
| 8 wk | 58 (39 to 76) | 29 (9 to 48) | 29 (5 to 54) | .02c |
| 12 wk | 71 (49 to 94) | 27 (3 to 50) | 44 (14 to 74) | .005c |
| Secondary outcomes | ||||
| Self-selected gait speed, 10-m walk test, m/s | ||||
| Baseline, mean (SD)b | 0.52 (0.20) | 0.49 (0.21) | 0.03 (−0.07 to 0.13) | .56 |
| 4 wk | 0.11 (0.06 to 0.15) | 0.02 (−0.02 to 0.07) | 0.08 (0.02 to 0.15) | .009c |
| 8 wk | 0.14 (0.08 to 0.20) | 0.06 (0.00 to 0.12) | 0.08 (0.00 to 0.15) | .04 |
| 12 wk | 0.19 (0.13 to 0.25) | 0.06 (0.00 to 0.12) | 0.13 (0.05 to 0.20) | .003c |
| Fastest gait speed, 10-m walk test, m/s | ||||
| Baseline, mean (SD)b | 0.70 (0.32) | 0.62 (0.32) | 0.08 (−0.08 to 0.24) | .33 |
| 4 wk | 0.22 (0.16 to 0.28) | 0.01 (−0.05 to 0.07) | 0.21 (0.13 to 0.29) | <.001c |
| 8 wk | 0.24 (0.17 to 0.32) | 0.09 (0.01 to 0.17) | 0.15 (0.05 to 0.25) | .003c |
| 12 wk | 0.28 (0.19 to 0.37) | 0.09 (−0.01 to 0.18) | 0.20 (0.08 to 0.32) | .002c |
| PROMIS Fatigue Scale T scored | ||||
| Baseline, mean (SD)b | 52.4 (8.6) | 50.8 (8.7) | 1.7 (−2.7 to 6.0) | .45 |
| 4 wk | −1.7 (−4.0 to 0.6) | 0.0 (−2.3 to 2.3) | −1.7 (−4.8 to 1.3) | .26 |
| 8 wk | −3.0 (−5.5 to −0.5) | 1.0 (−1.6 to 3.6) | −4.0 (−7.3 to −0.7) | .02c |
| 12 wk | −1.1 (−3.7 to 1.5) | −0.1 (−2.7 to 2.5) | −1.0 (−4.4 to 2.5) | .57 |
| VO2, mL/kg/min | ||||
| Baseline, mean (SD)b | 11.5 (4.0) | 10.9 (4.0) | 0.6 (−1.4 to 2.6) | .55 |
| 4 wk | 1.3 (0.4 to 2.2) | 0.4 (−0.5 to 1.3) | 0.9 (−0.4 to 2.1) | .16 |
| 8 wk | 2.0 (0.7 to 3.3) | 1.7 (0.3 to 3.1) | 0.3 (−1.4 to 2.1) | .72 |
| 12 wk | 1.9 (0.4 to 3.4) | 1.4 (−0.2 to 3.0) | 0.5 (−1.5 to 2.6) | .60 |
Abbreviations: HIIT, high-intensity interval training; MAT, moderate-intensity aerobic training; PROMIS, Patient Reported Outcomes Measurement Information System; VO2, oxygen consumption rate.
All models were adjusted for study site, study site–by-time interaction, baseline walking limitation severity, and baseline walking limitation severity–by-time interaction.
Baseline rows show the mean (SD) for each group and the mean difference (95% CI) in baseline values between groups.
Statistically significant after false discovery rate correction across time points and measures (primary and secondary).
Score range, 33.1-77.8; lower scores indicate less fatigue.
Among the secondary outcome measures, both groups exhibited significant increases in self-selected gait speed, fastest gait speed, and ventilatory threshold VO2 at various time points relative to baseline (Table 2), with the HIIT group showing significantly greater increases than the MAT group in self-selected and fastest gait speeds. Only the HIIT group had significantly decreased PROMIS Fatigue Scale T scores compared with the MAT group and only at the 8-week time point (HIIT, −3.0 [95% CI, –5.5 to −0.5]; MAT, 1.0 [95% CI, –1.6 to 3.6]; mean difference, −4.0 [95% CI, −7.3 to −0.7]; P = .02). Given the significant decrease in fatigue scores in the HIIT group at the 8-week time point but not the 12-week time point, we also examined HIIT group PROMIS Fatigue Scale changes between the 8- and 12-week time points and found no significant difference (T-score increase of 1.9; 95% CI, –0.5 to 4.3; P = .12).
Discussion
Results of this multicenter randomized clinical trial give proof of concept that the vigorous training intensity of HIIT is a crucial dosing parameter for locomotor exercise in patients with chronic stroke. Findings also indicated that locomotor HIIT can produce significant and meaningful gains in walking capacity in 4 weeks but that a training duration of at least 12 weeks is needed to maximize immediate gains in walking capacity with this intervention. Our primary hypothesis that HIIT would improve walking capacity significantly more than MAT after 4 weeks of training was not supported. However, the gap between groups continued to widen over time, and HIIT elicited significantly greater improvement in walking capacity than MAT after 8 weeks and 12 weeks of training in the prespecified primary analysis, supporting the secondary hypotheses. Among secondary outcome measures, HIIT also elicited significantly greater improvements than MAT in gait speed (self-selected and fastest at all time points) and fatigue (after 8 weeks of training) after adjustment for multiple comparisons (Table 2). In addition, intensive safety monitoring was successful at identifying expected AEs, and the results suggest that poststroke HIIT is sufficiently safe for further study.
Within the HIIT group, the mean 6-minute walk test gain after 4 weeks of training was comparable to that found in prior single-site 4-week pilot testing,17 confirming successful multicenter implementation. As hypothesized, gains in walking capacity and other outcomes were even larger after 8 and 12 weeks of HIIT, with no apparent plateau. These findings suggest that most prior poststroke HIIT studies have used insufficient training durations and that HIIT durations of at least 12 weeks should be considered in the future. Outpatient therapy durations longer than 12 weeks (36 sessions) are not likely to be routinely feasible in the current model for clinical rehabilitation due to reimbursement constraints36,37 and adherence issues.38 Thus, a 12-week duration for a clinician-led HIIT program may be an optimal standard to target. Future studies might also consider transitioning to home- or community-based training after clinician-led HIIT to test longer training durations.
Both groups showed significant improvement in aerobic capacity (ventilatory threshold VO2). The lack of significant between-group differences in aerobic capacity gains suggests that the greater improvement in walking capacity with HIIT vs MAT may have been primarily driven by neuromotor rather than cardiopulmonary adaptations. However, it is also possible that this lack of significant between-group differences was due to greater measurement error for VO2 measurement vs other outcomes or confounding effects of gait efficiency changes on VO2 during exercise testing (improved gait efficiency decreases measured VO2).33,39,40,41
The intensity-dependent improvement (decrease) in PROMIS Fatigue Scale T scores after 8 weeks of training (HIIT, −3.0; MAT, 1.0; P = .02) is especially noteworthy because identifying a treatment for poststroke fatigue has been highlighted as a research priority.42 This finding is consistent with prior neurophysiologic evidence showing that poststroke fatigue is associated with lower corticomotor excitability43 and that HIIT is associated with significantly greater acute increases in corticomotor excitability than MAT.29 Interestingly, participants randomized to HIIT reported a substantial decrease in fatigue during a period when they were going to the research sites multiple times per week for training sessions that commonly induced temporary fatigue. One possible explanation for this apparent paradox is that the acute central motor activation effects of poststroke HIIT counterbalanced or exceeded the effects of fatigue.29 It is also possible that the greater longitudinal increases in walking capacity and exercise tolerance with HIIT may have reduced fatigue with everyday activities, especially when coupled with significant reductions in the metabolic cost of gait.
The HIIT group improvements and between-group differences in fatigue (Table 2) were only statistically significant after 8 weeks of training and not after 4 or 12 weeks of training. Yet, all these effect estimates were in the same direction, suggesting that the lack of significance at the 4- and 12-week time points may have been due to insufficient statistical power rather than an exclusive fatigue benefit at only the 8-week time point. Conversely, it is possible that the significant improvement in fatigue observed after 8 weeks of HIIT was due to chance, but such improvement was also observed in a prior 4-week pilot study,17 suggesting otherwise. It is also possible that fatigue decreases begin returning toward baseline values with more extended HIIT durations, but this tendency in the HIIT group between 8 and 12 weeks of training was not statistically significant (fatigue T-score increase, 1.9; 95% CI, –0.5 to 4.3; P = .12). More definitive fatigue assessment in a larger trial with follow-up testing is warranted.
Strengths and Limitations
A strength of this trial is that it successfully recruited participants with a variety of comorbidities, assistive and orthotic device needs, and walking limitations. Further, study participants had a mean (SD) self-selected gait speed of 0.63 (0.31) m/s, which is representative of patients typically seen in outpatient stroke rehabilitation (eg, 0.59 m/s44). Thus, the results should be generally applicable in this setting. Another major strength was our systematic AE data collection with blinded adjudication, which is rare in the rehabilitation literature.
In terms of rigor, this study used randomization, concealed allocation, assessor blinding, and intent-to-treat analysis to minimize risk of bias and only had approximately 10% missing outcome data (HIIT, 12%; MAT, 9%). We also added the rigor of multicenter implementation earlier than usual in the rehabilitation research continuum,45 increasing confidence in the reproducibility and generalizability of the results. In addition, we included more rigorous assessment than many clinical trials about how missing data might influence the results. Further, the comparison group received an active, best-practice treatment (MAT),3,4,5 with mean intensity and volume of walking exercise exceeding previous reports for clinical rehabilitation in outpatient or extended inpatient settings (eg, HRR, 59% vs 24%-47%46,47; steps per session, 3532 vs 501-88648,49; and sessions attended, 31 vs 9-2537,50,51).
A primary limitation of this trial was its relatively modest sample size (N = 55) since it was designed to be an early proof-of-concept study. This meant that the trial was likely underpowered to detect all but very large differences in AEs between HIIT and MAT. The small sample size is also probably why the primary findings were not fully robust to different plausible assumptions about the true values of the missing data (eAppendix in Supplement 2). Thus, a larger trial is needed to provide more definitive evidence to guide clinical practice. The sensitivity analysis also validated the general intuition that larger samples are needed to have more confidence in the results of clinical trials.
Another important limitation was the lack of follow-up testing to assess sustained effects after treatment ended. This also made it more challenging to interpret the results of the PROMIS Fatigue Scale since participants were asked to report on periods in which they were engaged in the study treatment at 4 weeks, 8 weeks, and 12 weeks. We attempted to mitigate this issue by specifically asking participants to answer questions based on their usual activities outside the study, but we are skeptical that this strategy was completely successful.
It is also unclear how well the results might generalize to stroke survivors who need continuous physical assistance from another person to walk (ie, functional ambulation category <2 of 552). Less than 10% of stroke survivors appear to require this level of assistance after inpatient rehabilitation.53 However, this percentage is higher in earlier subacute stroke (eg, 49%-58%).53,54 Thus, future studies seeking to adapt the current training protocols for subacute stroke might consider providing the minimum necessary assistance to enable walking practice for participants who need it.55
Conclusions
In this randomized clinical trial involving locomotor exercise among stroke survivors with chronic walking limitations, vigorous training intensity appeared to be significantly better than moderate intensity for eliciting immediate improvements in walking capacity and other outcomes. With this vigorous-intensity locomotor exercise, 12 weeks of training produced greater gains than shorter durations.
Trial Protocol
eAppendix
eReferences
Data Sharing Statement
References
- 1.Mayo NE, Wood-Dauphinee S, Côté R, Durcan L, Carlton J. Activity, participation, and quality of life 6 months poststroke. Arch Phys Med Rehabil. 2002;83(8):1035-1042. doi: 10.1053/apmr.2002.33984 [DOI] [PubMed] [Google Scholar]
- 2.Rudberg AS, Berge E, Laska AC, et al. Stroke survivors’ priorities for research related to life after stroke. Top Stroke Rehabil. 2021;28(2):153-158. doi: 10.1080/10749357.2020.1789829 [DOI] [PubMed] [Google Scholar]
- 3.Billinger SA, Arena R, Bernhardt J, et al. ; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Clinical Cardiology . Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(8):2532-2553. doi: 10.1161/STR.0000000000000022 [DOI] [PubMed] [Google Scholar]
- 4.Winstein CJ, Stein J, Arena R, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Quality of Care and Outcomes Research . Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(6):e98-e169. doi: 10.1161/STR.0000000000000098 [DOI] [PubMed] [Google Scholar]
- 5.Hornby TG, Reisman DS, Ward IG, et al. ; Locomotor CPG Appraisal Team . Clinical practice guideline to improve locomotor function following chronic stroke, incomplete spinal cord injury, and brain injury. J Neurol Phys Ther. 2020;44(1):49-100. doi: 10.1097/NPT.0000000000000303 [DOI] [PubMed] [Google Scholar]
- 6.Boyne P, Dunning K, Carl D, Gerson M, Khoury J, Kissela B. High-intensity interval training in stroke rehabilitation. Top Stroke Rehabil. 2013;20(4):317-330. doi: 10.1310/tsr2004-317 [DOI] [PubMed] [Google Scholar]
- 7.Ivey FM, Hafer-Macko CE, Macko RF. Task-oriented treadmill exercise training in chronic hemiparetic stroke. J Rehabil Res Dev. 2008;45(2):249-259. doi: 10.1682/JRRD.2007.02.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macko RF, Ivey FM, Forrester LW. Task-oriented aerobic exercise in chronic hemiparetic stroke: training protocols and treatment effects. Top Stroke Rehabil. 2005;12(1):45-57. doi: 10.1310/PJQN-KAN9-TTVY-HYQH [DOI] [PubMed] [Google Scholar]
- 9.Boyne P, Billinger S, MacKay-Lyons M, Barney B, Khoury J, Dunning K. Aerobic exercise prescription in stroke rehabilitation: a web-based survey of US physical therapists. J Neurol Phys Ther. 2017;41(2):119-128. doi: 10.1097/NPT.0000000000000177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds H, Steinfort S, Tillyard J, et al. Feasibility and adherence to moderate intensity cardiovascular fitness training following stroke: a pilot randomized controlled trial. BMC Neurol. 2021;21(1):132-138. doi: 10.1186/s12883-021-02052-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macko RF, Ivey FM, Forrester LW, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke. 2005;36(10):2206-2211. doi: 10.1161/01.STR.0000181076.91805.89 [DOI] [PubMed] [Google Scholar]
- 12.Dupont G, Blondel N, Lensel G, Berthoin S. Critical velocity and time spent at a high level of VO2 for short intermittent runs at supramaximal velocities. Can J Appl Physiol. 2002;27(2):103-115. doi: 10.1139/h02-008 [DOI] [PubMed] [Google Scholar]
- 13.Guiraud T, Nigam A, Juneau M, Meyer P, Gayda M, Bosquet L. Acute responses to high-intensity intermittent exercise in CHD patients. Med Sci Sports Exerc. 2011;43(2):211-217. doi: 10.1249/MSS.0b013e3181ebc5de [DOI] [PubMed] [Google Scholar]
- 14.Boyne P, Dunning K, Carl D, et al. High-intensity interval training and moderate-intensity continuous training in ambulatory chronic stroke: a feasibility study. Phys Ther. 2016;96(10):1533-1544. doi: 10.2522/ptj.20150277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munari D, Pedrinolla A, Smania N, et al. High-intensity treadmill training improves gait ability, VO2 peak and cost of walking in stroke survivors: preliminary results of a pilot randomized controlled trial. Eur J Phys Rehabil Med. 2018;54(3):408-418. doi: 10.23736/S1973-9087.16.04224-6 [DOI] [PubMed] [Google Scholar]
- 16.Gjellesvik TI, Becker F, Tjønna AE, et al. Effects of high-intensity interval training after stroke (the HIIT Stroke Study) on physical and cognitive function: a multicenter randomized controlled trial. Arch Phys Med Rehabil. 2021;102(9):1683-1691. doi: 10.1016/j.apmr.2021.05.008 [DOI] [PubMed] [Google Scholar]
- 17.Boyne P, Doren S, Scholl V, et al. Preliminary outcomes of combined treadmill and overground high-intensity interval training in ambulatory chronic stroke. Front Neurol. 2022;13:812875. doi: 10.3389/fneur.2022.812875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pohl M, Mehrholz J, Ritschel C, Rückriem S. Speed-dependent treadmill training in ambulatory hemiparetic stroke patients: a randomized controlled trial. Stroke. 2002;33(2):553-558. doi: 10.1161/hs0202.102365 [DOI] [PubMed] [Google Scholar]
- 19.Lau KW, Mak MKY. Speed-dependent treadmill training is effective to improve gait and balance performance in patients with sub-acute stroke. J Rehabil Med. 2011;43(8):709-713. doi: 10.2340/16501977-0838 [DOI] [PubMed] [Google Scholar]
- 20.Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97(3):182-189. doi: 10.1016/j.physio.2010.12.004 [DOI] [PubMed] [Google Scholar]
- 21.Miller A, Reisman DS, Billinger SA, et al. Moderate-intensity exercise versus high-intensity interval training to recover walking post-stroke: protocol for a randomized controlled trial. Trials. 2021;22(1):457. doi: 10.1186/s13063-021-05419-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13-31. [PubMed] [Google Scholar]
- 23.Williams LS, Brizendine EJ, Plue L, et al. Performance of the PHQ-9 as a screening tool for depression after stroke. Stroke. 2005;36(3):635-638. doi: 10.1161/01.STR.0000155688.18207.33 [DOI] [PubMed] [Google Scholar]
- 24.American College of Sports Medicine . ACSM's Guidelines for Exercise Testing and Prescription. 9th ed. Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 25.Brott T, Adams HP Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864-870. doi: 10.1161/01.STR.20.7.864 [DOI] [PubMed] [Google Scholar]
- 26.Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner. 1964;192:540-542. [PubMed] [Google Scholar]
- 27.Boyne P, Dunning K, Carl D, Gerson M, Khoury J, Kissela B. Within-session responses to high-intensity interval training in chronic stroke. Med Sci Sports Exerc. 2015;47(3):476-484. doi: 10.1249/MSS.0000000000000427 [DOI] [PubMed] [Google Scholar]
- 28.Boyne P, Scholl V, Doren S, et al. Locomotor training intensity after stroke: effects of interval type and mode. Top Stroke Rehabil. 2020;27(7):483-493. doi: 10.1080/10749357.2020.1728953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyne P, Meyrose C, Westover J, et al. Exercise intensity affects acute neurotrophic and neurophysiological responses poststroke. J Appl Physiol (1985). 2019;126(2):431-443. doi: 10.1152/japplphysiol.00594.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Cancer Institute . Common Terminology Criteria for Adverse Events. Version 4.0. US Dept of Health and Human Services; 2009. NIH publication 09-7473.
- 31.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 32.Cella D, Lai JS, Jensen SE, et al. PROMIS Fatigue Item Bank had clinical validity across diverse chronic conditions. J Clin Epidemiol. 2016;73:128-134. doi: 10.1016/j.jclinepi.2015.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyne P, Reisman D, Brian M, et al. Ventilatory threshold may be a more specific measure of aerobic capacity than peak oxygen consumption rate in persons with stroke. Top Stroke Rehabil. 2017;24(2):149-157. doi: 10.1080/10749357.2016.1209831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743-749. doi: 10.1111/j.1532-5415.2006.00701.x [DOI] [PubMed] [Google Scholar]
- 35.Guo Y, Logan HL, Glueck DH, Muller KE. Selecting a sample size for studies with repeated measures. BMC Med Res Methodol. 2013;13:100. doi: 10.1186/1471-2288-13-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwakkel G. Impact of intensity of practice after stroke: issues for consideration. Disabil Rehabil. 2006;28(13-14):823-830. doi: 10.1080/09638280500534861 [DOI] [PubMed] [Google Scholar]
- 37.Duncan PW, Sullivan KJ, Behrman AL, et al. ; LEAPS Investigative Team . Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med. 2011;364(21):2026-2036. doi: 10.1056/NEJMoa1010790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiedemann A, Sherrington C, Dean CM, et al. Predictors of adherence to a structured exercise program and physical activity participation in community dwellers after stroke. Stroke Res Treat. 2012;2012:136525. doi: 10.1155/2012/136525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marzolini S, Oh P, McIlroy W, Brooks D. The feasibility of cardiopulmonary exercise testing for prescribing exercise to people after stroke. Stroke. 2012;43(4):1075-1081. doi: 10.1161/STROKEAHA.111.635128 [DOI] [PubMed] [Google Scholar]
- 40.Marzolini S, Oh P, McIlroy W, Brooks D. The effects of an aerobic and resistance exercise training program on cognition following stroke. Neurorehabil Neural Repair. 2013;27(5):392-402. doi: 10.1177/1545968312465192 [DOI] [PubMed] [Google Scholar]
- 41.Holleran CL, Rodriguez KS, Echauz A, Leech KA, Hornby TG. Potential contributions of training intensity on locomotor performance in individuals with chronic stroke. J Neurol Phys Ther. 2015;39(2):95-102. doi: 10.1097/NPT.0000000000000077 [DOI] [PubMed] [Google Scholar]
- 42.Saunders DH, Greig CA, Mead GE. Physical activity and exercise after stroke: review of multiple meaningful benefits. Stroke. 2014;45(12):3742-3747. doi: 10.1161/STROKEAHA.114.004311 [DOI] [PubMed] [Google Scholar]
- 43.Kuppuswamy A, Clark EV, Turner IF, Rothwell JC, Ward NS. Post-stroke fatigue: a deficit in corticomotor excitability? Brain. 2015;138(pt 1):136-148. doi: 10.1093/brain/awu306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lohse K, Bland MD, Lang CE. Quantifying change during outpatient stroke rehabilitation: a retrospective regression analysis. Arch Phys Med Rehabil. 2016;97(9):1423-1430.e1. doi: 10.1016/j.apmr.2016.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dobkin BH. Progressive staging of pilot studies to improve phase III trials for motor interventions. Neurorehabil Neural Repair. 2009;23(3):197-206. doi: 10.1177/1545968309331863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koopman ADM, Eken MM, van Bezeij T, Valent LJM, Houdijk H. Does clinical rehabilitation impose sufficient cardiorespiratory strain to improve aerobic fitness? J Rehabil Med. 2013;45(1):92-98. doi: 10.2340/16501977-1072 [DOI] [PubMed] [Google Scholar]
- 47.Kuys S, Brauer S, Ada L. Routine physiotherapy does not induce a cardiorespiratory training effect post-stroke, regardless of walking ability. Physiother Res Int. 2006;11(4):219-227. doi: 10.1002/pri.344 [DOI] [PubMed] [Google Scholar]
- 48.Lang CE, Macdonald JR, Reisman DS, et al. Observation of amounts of movement practice provided during stroke rehabilitation. Arch Phys Med Rehabil. 2009;90(10):1692-1698. doi: 10.1016/j.apmr.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore JL, Roth EJ, Killian C, Hornby TG. Locomotor training improves daily stepping activity and gait efficiency in individuals poststroke who have reached a “plateau” in recovery. Stroke. 2010;41(1):129-135. doi: 10.1161/STROKEAHA.109.563247 [DOI] [PubMed] [Google Scholar]
- 50.Dobkin BH. Clinical practice: rehabilitation after stroke. N Engl J Med. 2005;352(16):1677-1684. doi: 10.1056/NEJMcp043511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duncan P, Studenski S, Richards L, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. 2003;34(9):2173-2180. doi: 10.1161/01.STR.0000083699.95351.F2 [DOI] [PubMed] [Google Scholar]
- 52.Mehrholz J, Wagner K, Rutte K, Meissner D, Pohl M. Predictive validity and responsiveness of the functional ambulation category in hemiparetic patients after stroke. Arch Phys Med Rehabil. 2007;88(10):1314-1319. doi: 10.1016/j.apmr.2007.06.764 [DOI] [PubMed] [Google Scholar]
- 53.Hill K, Ellis P, Bernhardt J, Maggs P, Hull S. Balance and mobility outcomes for stroke patients: a comprehensive audit. Aust J Physiother. 1997;43(3):173-180. doi: 10.1016/S0004-9514(14)60408-6 [DOI] [PubMed] [Google Scholar]
- 54.Louie DR, Simpson LA, Mortenson WB, Field TS, Yao J, Eng JJ. Prevalence of walking limitation after acute stroke and its impact on discharge to home. Phys Ther. 2022;102(1):pzab246. doi: 10.1093/ptj/pzab246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holleran CL, Straube DD, Kinnaird CR, Leddy AL, Hornby TG. Feasibility and potential efficacy of high-intensity stepping training in variable contexts in subacute and chronic stroke. Neurorehabil Neural Repair. 2014;28(7):643-651. doi: 10.1177/1545968314521001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix
eReferences
Data Sharing Statement