Abstract
Electrochemical biosensors incorporate a recognition element and an electronic transducer for the highly sensitive detection of analytes in body fluids. Importantly, they can provide rapid readouts and they can be integrated into portable, wearable and implantable devices for point-of-care diagnostics; for example, the personal glucose meter enables at-home assessment of blood glucose levels, greatly improving the management of diabetes. In this Review, we discuss the principles of electrochemical biosensing and the design of electrochemical biosensor devices for health monitoring and disease diagnostics, with a particular focus on device integration into wearable, portable and implantable systems. Finally, we outline the key engineering challenges that need to be addressed to improve sensing accuracy, enable multiplexing and one-step processes, and integrate electrochemical biosensing devices in digital health-care pathways.
Subject terms: Sensors, Diagnostic markers
Electrochemical biosensors can be integrated into wearable, portable and implantable devices for health monitoring and disease diagnosis. This Review discusses the design and integration of different types of electrochemical biosensors for the detection of analytes related to health and disease, and outlines engineering challenges that need to be addressed to enable clinical translation of electrochemical biosensor-based point-of-care devices.
Key points
Electrochemical biosensors are self-contained, analytical devices, in which a biological recognition element is in direct contact with an electrochemcial transduction element to allow the sensitive and specific detection of analytes.
Depending on the design and sensor type, health-related and disease-related biomarkers, such as carbohydrates, proteins, nucleic acids and cells, can be rapidly analysed in different body fluids, including blood, saliva and tears.
Electrochemical biosensors, including amperometric, voltammetric, potentiometric, organic electrochemical transistor, photoelectrochemical and electrochemiluminescent sensors, can be integrated into wearable, portable and implantable devices to enable point-of-care diagnostics and health monitoring.
Commercialization and broad point-of-care applicability of integrated electrochemical biosensors will require improvements in stability, sensitivity, reproducibility, multiplexing, and digitalization and, importantly, low-cost materials and easy fabrication methods.
Introduction
Biosensors have been widely applied in clinical, industrial, environmental and agricultural analyses since Leland Clark Jr introduced the amperometric glucose enzyme electrode in 1962 (ref. 1). According to the definition by the International Union of Pure and Applied Chemistry2, a biosensor is a self-contained, integrated, analytical device, in which a biological recognition element (biochemical receptors, including enzymes, antibodies, antigens, peptides, DNA, aptamers or living cells) is retained in direct spatial contact with a transduction element (such as electrochemical, optical and mechanical transducers). Biosensors were initially developed for point-of-care (POC) testing of biomolecular targets in the hope of extending clinical analysis from specialized laboratories to public settings, including hospitals, non-hospital nursing settings or home settings2. Although various biosensors have been developed for the sensitive and selective detection of a range of disease-related molecules, clinical translation of biosensors remains limited owing to difficulties in integrating and miniaturizing biosensors into portable devices.
Among the different biosensing platforms, electrochemical biosensors, which integrate the biorecognition element in an electrochemical transducer (for example, an electrode or field-effect transistor) are particularly suitable for device integration3–5 (Fig. 1a) because they can be easily miniaturized, batch fabricated and integrated with an electronic acquisition module on a single chip. In addition, electrochemical signals, such as electrical current and potential, can be collected by simple, portable and low-cost peripheral instruments with low power consumption. Moreover, the signal produced through affinity recognition of the target analyte by the biorecognition element can be amplified by physical, chemical or biological strategies, which greatly improves detection sensitivity. As such, electrochemical biosensors hold great promise for the development of POC diagnostic devices. The World Health Organization stipulates that POC biosensors should be affordable, sensitive, specific, user-friendly, rapid, robust, equipment free and deliverable to end users to enable on-site testing and diagnosis in the daily routines of individual patients and consumers. Thus, POC diagnostics are expected to play a key role in revolutionizing the diagnosis and treatment of major global diseases. For example, the electrochemical glucose meter, the most successful commercial POC biosensing device, has been widely used across the globe to help patients with diabetes.
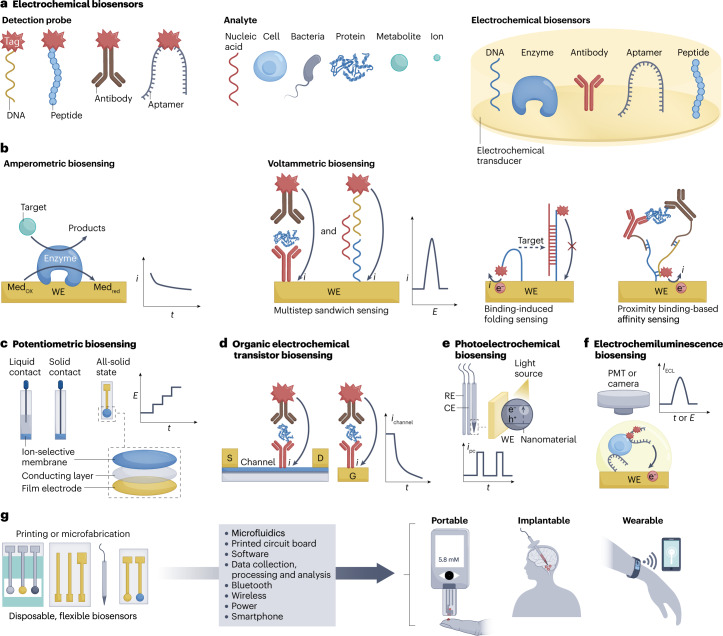
Fig. 1. Electrochemical biosensors.
a, Schematic representation of electrochemical biosensors based on different biochemical receptors and detection probes. b, Amperometric biosensing of metabolite targets based on an enzyme electrode, including the current–time (i–t) curve and the i signal for quantification. Voltammetric biosensing of proteins or nucleic acids using an antibody-modified or nucleic acid-modified electrode through multistep sandwich sensing, one-step binding-induced folding sensing or one-step proximity binding-based affinity sensing, including the current–potential (i–E) curve and i signal for target quantification. c, Ion-selective electrodes with three different structures, including recording of the potential (E) for target quantification. d, Two types of organic electrochemical transistor sensors prepared by immobilizing the recognition element on the channel surface or on the gate electrode (G) for sandwich immunoassays of proteins, including recording of the channel current (ichannel) for target quantification. e, Photoelectrochemistry biosensing based on a three-electrode system and a light source, including recording of the photoelectrode photocurrent (ipc) upon target recognition for quantification. f, Electrochemiluminescence biosensing of cells based on an aptamer-modified electrode through a sandwich-sensing format, including light intensity (IECL) at excited potential by a photomultiplier tube (PMT) or imaging using a camera for target quantification. g, Integration of electrochemical biosensors in portable, wearable and implantable devices. CE, counter electrode; D, drain electrode; Medox, oxidized form of mediator; Medred, reduced form of mediator; RE, reference electrode; S, source electrode; WE, working electrode.
However, the market for electrochemical biosensing devices is currently limited to the detection of some small molecules or ions (Supplementary Table 1), which can be detected directly by electrochemical signals through oxidation, reduction or affinity interactions at the electrode surface. By contrast, the detection of large biomarkers, such as proteins, nucleic acids, bacteria or cells, mainly relies on affinity recognition and, thus, requires multiple steps to produce a detectable signal. Several transduction principles may promote the integration of fully automated electrochemical biosensing devices for affinity biomarkers, including the relation of affinity recognition events with the generation and consumption of glucose6–10, one-step affinity-sensing mechanisms, such as binding-induced folding sensing and proximity binding-based affinity sensing11,12, and integration with automatic fluidic systems such as pump-assisted fluidics, paper-based microfluidics and polydimethylsiloxane (PDMS)-based microfluidics13–15. In addition, owing to the low concentration of disease markers in body fluids, in particular, in the early stages of disease (femtomolar or attomolar level), signal amplification strategies are required to increase detection sensitivity, which can be achieved by implementing nanotechnology-based and biotechnology-based strategies, such as amplification strategies based on nanotags, nanocatalysis, and nanocarriers and assembly-based and polymerase-based DNA amplification strategies16,17.
Efficient health-care management requires electrochemical biosensors to achieve minimally invasive or non-invasive continuous measurement of physiological molecules. Advances in microelectronic engineering, semiconductor precision-machining, flexible and stretchable bioelectronics and wireless communication technologies have spearheaded the integration of electrochemical biosensors in wearable and implantable devices18–21. To achieve long-term detection of molecules in different biofluids (for example, cerebrospinal fluid, interstitial fluid, sweat, saliva, tears and urine), electrochemical biosensors can also be integrated into flexible films, textiles, glasses, teeth and diapers. Furthermore, combining sensors with smartphones and other mobile devices allows continuous monitoring of dynamic physiological processes, and the integration of intelligent or digital processing modules into devices enables the connection of sensors to the Internet of Things and cloud computing for large-scale medical data mining.
In this Review, we discuss key innovations in electrochemical biosensing for preventive and personalized POC diagnostic devices. We discuss the design and integration of amperometric, voltammetric, potentiometric, organic electrochemical transistor (OECT), photoelectrochemical and electrochemiluminescent biosensors (Table 1) for disease diagnosis, health management, cell monitoring and neuroscience. In addition, we examine the fabrication, fluidic manipulation, signal amplification and readout, signal processing algorithms, and result visualization of integrated biosensors.
Table 1.
Electrochemical biosensors
| Sensor type | Main target | Sensing mechanism | Detection performance | Technical challenges | Future directions | Device integration |
|---|---|---|---|---|---|---|
| Amperometric | Metabolites | Enzymatic reaction | Good sensitivity (180 μA cm–2 mmol–1 for glucose detection)26; medium stability (~1 month)57 | Improvement of stability and sensitivity | Robust and highly sensitive sensors for point-of-care diagnostics in different environments, including wearable sweat monitoring | Portable and wearable |
| Voltammetric | Proteins and nucleic acids | Bio-affinity recognition | Good sensitivity and low LOD (10 fM for insulin44, ~1 pg ml–1 for glycoproteins47,48 and ~100 fM for DNA50); easy reuse44,48 | Simplification and automation of multistep affinity reactions | Simple, rapid, cost-effective biosensing systems by designing automatic fluidics or one-step sensing mechanisms | Portable |
| Potentiometric | Electrolytes | Ion-selective penetration | Good stability (more than 1 month) and medium sensitivity (~60 mV per decade)57 | Miniaturization and flexibility of sensors | Stable all-solid-state sensing electrodes | Portable and wearable |
| Organic electrochemical transistor | Small molecules, proteins and nucleic acids | Bio-affinity recognition | Good sensitivity and low LOD (30 nM for glucose58, 10 pM for DNA62 and 1 pg ml–1 for proteins64) | Batch preparation of sensing electrodes | Batch production and portable data processing systems | Portable and wearable |
| Photoelectrochemistry | Bio-affinity recognition | Good sensitivity and low LOD73 (~1 μM for lactate, ~1 fM for DNA and ~1 pg ml–1 for glycoproteins) | Equipped with a light source | Miniaturized, implantable sensing devices for in vivo applications | Portable and implantable | |
| ECL | Bio-affinity recognition | Good sensitivity (LOD of ~3 pM for microRNA83, microimaging of membrane proteins on single cells85 and sensing of dopamine released by a single cell86) | Efficient ECL reactions and probes; equipped with an ECL collection module | Highly sensitive ECL imaging systems for high-throughput detection and single-cell analysis | Portable and implantable |
ECL, electrochemiluminescence; LOD, limit of detection.
Electrochemical sensing of biomarkers
In electrochemical biosensors, the signal is typically triggered by electron or ion transfer on a conductive transducer through a biorecognition process. Signals can involve current (i), potential (E), impedance, conductivity, capacitance and light (I). Among these, impedimetric biosensors are theoretically favoured for POC diagnostics and device integration because they can directly detect biorecognition events by measuring the non-faradaic resistance and capacitance properties of the sensing electrode; however, their practical implementation suffers from non-specific binding of non-target compounds, which leads to low sensitivity and selectivity. In addition, although impedimetric biosensors have been greatly improved during the COVID-19 pandemic, for example, using molecular imprinting technology to fabricate virus-imprinted impedimetric biosensors for sensitive detection of whole virus particles22 or by applying a dielectrophoresis force to improve the detection sensitivity of impedimetric immunosensors fabricated on Au micro-interdigitated electrodes23, their proof-of-concept performance is often only demonstrated using artificial physiological samples instead of clinically relevant samples.
Amperometric and voltammetric biosensors
Amperometric and voltammetric biosensors are operated with a three-electrode system, which contains a biosensor as a working electrode (WE) for target recognition, a counter electrode as the current source, and a reference electrode to apply a stable potential. Current signals are generated by electrochemical reactions on the WE under an applied potential for target quantification. The difference between the two techniques is their applied potential, which is constant for amperometric measurements and variable for voltammetric detection. According to the potential change modes, the latter can be performed with various techniques, including cyclic voltammetry, differential pulse voltammetry, square wave voltammetry and anodic stripping voltammetry.
Amperometric biosensors are the most popular sensors for the detection of metabolites (for example, glucose, lactate and uric acid). In amperometric biosensors, a target-specific enzyme (for example, glucose oxidase (GOx), lactate oxidase or uricase) is immobilized on the WE to catalyze the oxidation of the target at a constant potential3; for example, glucose meters are typically constructed with amperometric biosensors that use GOx to catalyze the oxidation of glucose by a redox mediator (for example, ferricyanide, ferrocene derivative and transition-metal complexes) (Fig. 1b); alternatively, amperometric glucose sensors can rely on the enzymatic oxidation of glucose with natural oxygen to generate and detect hydrogen peroxide using a mediator such as Prussian blue24. Amperometric biosensors are simple to fabricate, and have high sensitivity and selectivity in target detection, making them suitable for wearable applications. As the concentration of metabolites in non-blood fluids is lower than that in blood (for example, the concentration of sweat glucose (10–200 μM) and tear glucose (0–2 mM) are, respectively, 100-fold and 10-fold lower than that of blood glucose (1–20 mM)), nanomaterials, such as metallic nanoparticles, carbon nanotubes and graphene, can be added to the biosensing interface to facilitate electron transfer to increase the sensitivity and decrease the detection limit25. For example, Au–Pt bimetallic nanocatalysts in combination with nanoporous hydrogels enable GOx immobilization and glucose detection with a sensitivity of 180 μA cm–2 mmol–1 and a detection limit of 0.01 mg dl−1 (0.56 μM), making such a biosensor suitable for integration with a smart contact lens for tear glucose measurement26. In addition, nanomaterials with enzymatic properties (that is, artificial nanoenzymes) can be implemented in amperometric biosensors to avoid denaturation of natural enzymes; for example, using a laser-induced graphene array, co-decorated with Cu2O and Au nanoparticles, a miniaturized, electrochemical, flexible, non-enzymatic biosensor was designed, offering stable sensing signals upon bending back-and-forth 25 times; its integration with a smartphone-based portable station for glucose monitoring has been verified with commercial blood testing devices27.
Metabolites can be detected by enzymatic recognition; by contrast, disease biomarkers, such as proteins and nucleic acids, are detected by affinity recognition, which cannot directly generate electron transfer on the biosensing surface. Thus, an additional electroactive label is needed for target sensing such as enzymes (for example, horseradish peroxidase and alkaline phosphatase), nanomaterials (for example, nanoparticles, nanotubes and quantum dots) and electroactive molecules (for example, ferrocene and methylene blue). These labels are typically detected by cyclic voltammetry, differential pulse voltammetry, square wave voltammetry and anodic stripping voltammetry, and therefore, most affinity sensors are voltammetric biosensors. Such sensors can be fabricated by immobilizing capture biomolecules (for example, antibodies, antigens, aptamers and DNA) on the WE to enable the detection of proteins or nucleic acids using a sandwich assay format (Fig. 1b). Here, sequential incubations with the target, detection molecules and electrochemical nanotags are required3,4, limiting device integration of affinity biosensors. To simplify this operation, automatic fluidic systems can be applied; for example, biosensors fabricated on screen-printed carbon electrodes can be coupled with a flow-injection system to automatically detect multiple protein biomarkers28–30. However, the large size and high cost of this apparatus may limit commercialization.
Microfluidics allows the manipulation of fluids in micrometre-scale channels by integrated fluidic control units such as microvalves, pumps and reactors31. Therefore, multistep liquid processing workflows can be integrated into a single chip for fully automated sample-to-answer analysis32,33. Coupling electrochemical biosensors with microfluidics enables continuous and high-throughput detection of multiple trace analytes in complex samples such as human serum and blood samples. Several electrochemical biosensing devices have been commercialized (for example, Cue Reader from Cue Health, ePlex RP2 from GenMark Diagnostics, Binx io from Binx Health) for chip-based or cartridge-based detection of SARS-CoV-2 nucleic acids, respiratory viral and bacterial organisms, and Chlamydia trachomatis based on the integration of digital microfluidics (such as electrowetting) with amperometric or voltammetric affinity biosensors (Supplementary Table 1). These products enable at-home testing of disease biomarkers but are very expensive. Alternatively, cheaper and highly sensitive electrochemical biosensing systems can be designed by combining amperometric or voltammetric biosensors with paper-based microfluidics with self-pumping ability. These systems can integrate multiplex sensing electrodes for differential pulse voltammetry or square wave voltammetry detection of protein and nucleic acid biomarkers13,15. For example, an origami paper-based aptamer and antibody biosensing chip enables simultaneous detection of C-reactive protein (CRP) and pre-albumin down to the picogram per millilitre level34. By integrating amperometric or voltammetric biosensors with PDMS-based microfluidics, which can be fabricated by high-precision micromachining technologies, including soft lithography, casting, imprinting, injection moulding and laser ablation, the automation, miniaturization and array size of devices can be improved35–37. For example, 16 three-electrode biosensors integrated with a PDMS chip with separate chambers and reservoirs of reagents and samples allow the high-throughput detection of three protein markers of breast cancer38.
Merging biosensors with automatic fluidic systems simplifies detection; however, device integration remains difficult owing to the requirement of pumps and reservoirs. One-step affinity sensors provide a simpler alternative; for example, a binding-induced folding electrochemical biosensor can be fabricated by site-specific modification of a redox-tagged probe DNA on the WE11. The detection of the target then relies on the binding-induced change in rigidity of the probe DNA (Fig. 1b), causing the redox tags to move close or away from the electrode surface, resulting in a respective increase or decrease of the current signal for target biosensing. Such binding-induced folding electrochemical biosensors can achieve sample-in-answer-out sensing of nucleic acids or sensing of some specific proteins using aptamer receptors; however, they suffer from low sensitivity. The signal can be amplified using DNA hybridization strategies39,40; for example, an electrochemical DNA sensor based on target-induced CRISPR–Cas12a cleaving of interfacial single-stranded DNA with methylene blue as the signal tag can detect human papillomavirus 16 (HPV16) and parvovirus B19 (PB19) down to the picomolar level41. The sensitivity of this DNA sensor can be further improved using a hairpin DNA probe42.
Proximity binding-based affinity electrochemical biosensors are particularly suited for protein biomarker detection because they can transfer a protein immunoassay to DNA detection12. In such biosensors, a pair of antibody-DNA affinity probes dually recognizes a target protein, which leads to the formation of proximity ligation products that initiate DNA assembly, causing the ‘on’ or ‘off’ state of the electroactive molecule-tagged probe DNA on the electrode surface (Fig. 1b). A wash-free and separation-free square wave voltammetry biosensor based on a proximity binding-induced ‘on’ state of methylene blue–DNA on the electrode surface allows direct detection of insulin43. By introducing uracils in the DNA sequence, this biosensor can be made reusable, enabling repeated protein quantitation within 3 min (ref. 44). The sensitivity of proximity binding-based affinity electrochemical biosensors can be further improved by DNA amplification strategies; for example, introducing an electrochemical ratiometric readout45,46, nuclease-mediated or DNAzyme-mediated cycle amplification47,48, surface programmatic chain reaction49, or DNA walker amplification50 enables the one-step detection of glycoprotein markers (for example, carcinoembryonic antigen (CEA), prostate-specific antigen (PSA) and thrombin) down to picogram per millilitre or sub-picomolar levels (Supplementary Table 2).
Potentiometric biosensors
Potentiometric biosensors are typically operated with a two-electrode system consisting of a sensing electrode and a reference electrode, allowing direct detection of targets by measuring the potential signal related to the change of surface charge upon target recognition on the sensing electrode. Typically, ion-selective electrodes made of ion-selective membranes and a liquid contact structure are used as potentiometric sensing electrodes (Fig. 1c). Glass membrane ion-selective electrodes (for example, pH electrode), solid membrane ion-selective electrodes (for example, crystalline membrane electrodes for F−, Ag+, Cl− and S2−), and liquid membrane ion-selective electrodes (for example, electrodes based on ionophores (selective host molecules) for H+, K+, Na+, NH4+, Ca2+) are commercially available. Solid and liquid membrane electrodes can further be integrated into clinical analyzers for the detection of blood electrolytes (for example, Na+, K+, Ca2+, H+ and Cl−). Enzymes, nucleic acids and proteins can be detected by integrating the biological element on the ion-selective electrode to catalyze the reaction that forms the ions or by combining the target biorecognition event with an ionic reaction51–54.
Solid-contact ion-selective electrodes, which can be made with solvent polymeric membranes, do not contain internal solutions (Fig. 1c) and benefit from ruggedness (thus, morphological diversity) and easy fabrication, modification and miniaturization. Solid-contact ion-selective electrodes allow protein and nucleic acid analysis through the detection of ions released from nanoparticle-tagged probes; for example, a miniaturized solid-contact Ag ion-selective electrode can detect DNA targets at the femtomolar level in microlitre-volume samples55. In addition, all-solid-state ion-selective electrodes can be made with conducting polymers or nanomaterials to establish a solid contact beneath the ion-selective and reference membranes (Fig. 1c). Such ion-selective electrodes have been implemented in two commercial portable devices for POC detection of electrolytes and blood gases (i-STAT from Abbott and BGA-102 from Wondfo Biotech) (Supplementary Table 1). A paper-based potentiometric biosensor based on an all-solid-state butyrylcholine-sensitive ion-selective electrode and a 3D origami paper-based fluidic system can detect butyrylcholinesterase activity and organophosphate pesticides and, by further integrating a USB-controlled miniaturized electrochemical analyzer, allows the design of a handheld potentiometric device56. All-solid-state ion-selective electrodes can also be integrated into wearable devices for ionic detection in biofluids18–21; for example, a wearable ‘smart wristband’ with Na+ and K+ ion-selective electrodes on a flexible sensing array enables in situ analysis of Na+ and K+ in sweat57.
Organic electrochemical transistor biosensors
OECT biosensors are organic thin-film transistors that consist of gate (G), drain (D) and source (S) electrodes, with an organic semiconductor film between the D and S electrodes. A change in the potential drop or capacitance of the gate–electrolyte or channel–electrolyte interface sensitively changes the channel current. Thus, OECT biosensors can be fabricated by immobilizing the recognition element on the G electrode or on the channel surface (Fig. 1d); here, the specific reactions of the OECT biosensor with the target influence the interface potential, resulting in a channel current response for target quantification.
OECT biosensors benefit from high sensitivity, low cost, flexibility, easy fabrication and low working voltage (<1 V), allowing the detection of both electroactive (for example, dopamine, glucose and epinephrine)58–60 and electro-inactive (for example, cortisol61, DNA62, proteins63,64, bacteria65, cells66 and glycans67–70) molecules or biomacromolecules through electrostatic interactions or affinity binding between targets and the sensing interface71.
OECT biosensors can be easily miniaturized, integrated into devices and designed as arrays because their detection performance does not degrade if their size is reduced at a fixed channel width per length ratio. For example, a ‘lab on a chip’ system based on an OECT biosensor integrated into a flexible microfluidic system allows label-free detection of DNA with a detection limit of 10 pM; here, the microfluidic device is deposited on a flexible substrate that contains a thiolated DNA probe immobilized on the Au gate electrode62. OECT microarrays can also be fabricated by solution processes for high-throughput sensing. The flexibility and robustness of OECT biosensors make them suitable for the non-invasive detection of biomolecules in wearable devices. For example, a fabric OECT biosensor, fabricated by weaving the sensor with cotton yarns, can be embedded in a diaper to monitor glucose in artificial urine, with the sensing signals collected on a mobile phone through Bluetooth72.
Photoelectrochemical biosensors
Photoelectrochemistry studies the effect of light on photoelectrodes or interfacial materials and the conversion of light energy into electrical power. Photoelectrochemical biosensing combines photoelectrochemistry with sensor-based bioanalysis; here, light serves as the excitation source and current as the readout. Photoelectrochemical biosensing systems typically consist of a three-electrode system and a light source (Fig. 1e). Detection is based on the change of photocurrent upon target recognition at the biosensor surface, which induces a charge or energy transfer owing to the photoelectrochemical reaction between an electron donor and acceptor, and a photoactive material on the electrode surface upon light irradiation73.
Photoelectrochemical biosensors combine the advantages of optical and electrochemical assays, in particular, for the detection of disease-related molecules such as glutathione, lactate, DNA, microRNA (miRNA), protein tumour markers and cells73. Light stimuli can be applied contactless rather than through bias voltage, making photoelectrochemical biosensors biocompatible and suitable for in vivo sensing. In addition, separation of the excitation source (light) and detection signal (electricity) and their different energy forms result in low background noise and high sensitivity. Therefore, photoelectrochemical microbiosensors allow in vivo or single-cell analysis74,75; for example, using a fluorescence resonance energy transfer (FRET) process, a photoelectrochemical microbiosensing system can selectively monitor SO2, a potential marker of cerebral ischaemia (reperfusion) and related brain injury, in the brain of living rats76. Here, FRET is implemented based on upconversion nanoparticles (UCNPs) as the energy donor and an organic dye as the energy acceptor. The biosensing interface is then constructed by co-immobilization of the UCNP and dye FRET pair, and CdTe quantum dots on a microelectrode. In the brain of a rat model of cerebral ischaemia-reperfusion and febrile seizure, the presence of SO2 blocks the FRET process and recovers UCNP emission, which, in turn, modulates the photocurrent of the photoactive material, allowing the detection of SO2.
Electrochemiluminescence biosensing and bioimaging
Electrochemiluminescence is an electrochemically triggered energy-relaxation process, in which a luminophore undergoes electron transfer reactions to form excited states that emit light. Electrochemiluminescence biosensing enables the quantitative detection of target molecules through electrochemiluminescence emission signals that are associated with a target biorecognition-induced change in electrochemiluminescence active species. Similar to amperometric and voltammetric biosensors, electrochemiluminescence biosensors also operate with a three-electrode system, in which the WE is modified with the recognition element to serve as the biosensing electrode (Fig. 1f). Owing to the combination of electrochemistry and spectroscopy, electrochemiluminescence biosensing does not require a light source and has negligible background noise, high sensitivity, good reproducibility, and high spatial and temporal control, making it a powerful analytical tool for the detection of a range of disease molecules, including DNA, miRNA, proteins and tumour cells77–79.
A commercialized microbead-based electrochemiluminescence biosensing system (that is, Elecsys 1010/2010/E170, Roche Diagnostics) is used as the gold-standard detection system in hospitals for many glycoprotein tumour markers79; however, this instrument is large and bulky. Alternatively, a portable electrochemiluminescence device, integrating a screen-printed carbon electrode-based electrochemiluminescence biosensor, paper microfluidics and a mobile phone camera, can detect 2-(dibutylamino)-ethanol and NADH80. A portable electrochemiluminescence biosensing system has also been designed for the detection of miRNA-21 by combining a magnetic bead-based switch-on electrochemiluminescence molecular beacon sensing strategy with a portable potentiostat and a mobile phone camera readout81.
Electrochemiluminescence biosensing strategies can be combined with a charge-coupled device camera and a conventional microscope for electrochemiluminescence bioimaging. This system allows simultaneous detection of multiple biomarkers through spatial or potential resolution; for example, a bead-based electrochemiluminescence immunosensing array enables simultaneous detection of three antigens by individually imaging the microbeads located in a microwell array82. Similarly, electrochemiluminescent polymer dots (Pdots), luminol-doped Pdots and diethylamine-coupled Pdots can be exploited for potential-resolved and colour-resolved electrochemiluminescence bioimaging for the high-throughput detection of miRNAs83. Here, luminol-doped Pdots show blue electrochemiluminescence emission at +0.6 V, whereas diethylamine-coupled Pdots show red electrochemiluminescence emission at +1.0 V. On the sensing array, the electrochemiluminescence of two Pdots is initially inhibited by quencher-labelled capture DNAs. After recognition of target miRNAs, the quencher is released through DNA cleaving, and the electrochemiluminescence of Pdots is recovered for target detection. Compared to potential-resolved electrochemiluminescence biosensors, this potential-resolved and colour-resolved bioimaging system prevents interference of the threshold produced by the low potential emitter at high potentials.
Electrochemiluminescence bioimaging is well suited for cell analysis because it can provide both morphological and quantitative information84. Electrochemiluminescence cell bioimaging strategies have been developed for different targets, including small molecules released from cells and membrane proteins on the cell surface. Electrochemiluminescence imaging of membrane proteins is typically achieved by labelling cells with electrochemiluminescence probes through affinity reactions. However, this approach only allows observation of the cell periphery in contact with the electrode or requires membrane permeability treatment. Alternatively, a dual-intramolecular electron transfer strategy can be applied; for example, co-reactant-embedded Pdots with strong electrochemiluminescence emission enable in situ imaging of the membrane protein human epidermal growth factor receptor 2 (HER2) on single living cells85. To quantify detection, the biosensing interface, that is, a Pdot-modified-indium tin oxide (ITO) glass electrode sheet, can be combined with a single-cell-capture microfluidic chip, enabling high-throughput quantification of dopamine secreted by a single cell86.
Device integration
Electrochemical biosensors can be integrated into portable, wearable or implantable devices (Table 1), including microfluidics, printed circuit boards, software, signal processing units, communication units and power units (Fig. 1g). Amperometric biosensors are the most developed and most commonly used sensors for metabolites. Owing to the specific enzyme reaction, they usually exhibit good selectivity. In addition, the enzymatic catalytic signal can be further enhanced by nanomaterials, leading to high sensitivity. Most importantly, these enzyme sensors can be prepared in batches with good reproducibility; however, enzyme activity can be affected by the environment. Thus, robust sensing electrodes are required for work in different environments. Potentiometric biosensors can be integrated for wearable sweat monitoring, in particular, for the detection of electrolytes. Using ion-selective membranes, potentiometric biosensors show good selectivity, reproducibility and stability; however, their sensitivity is low. Alternatively, a flexible, all-solid-state, wearable, ion-selective electrode could achieve continuous sweat monitoring. Voltammetric, OECT, photoelectrochemical and electrochemiluminescent biosensors allow the detection of proteins and nucleic acids, showing good selectivity and high sensitivity. However, such affinity biosensors typically require the specific assembly of bioreceptors on the electrode surface, making their fabrication more complicated than that of enzyme electrodes.
Portable electrochemical biosensing devices
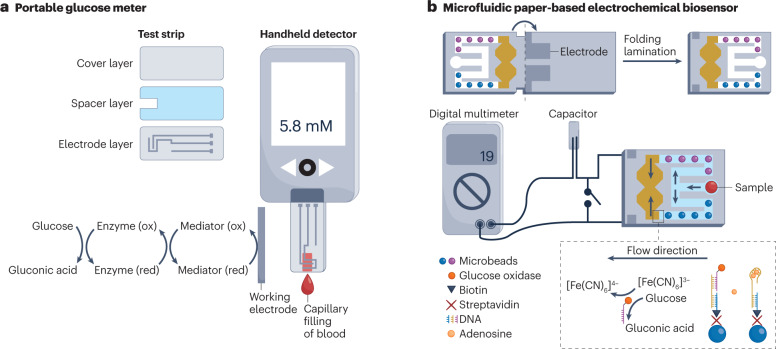
Portable electrochemical sensors have initially been developed for the monitoring of blood glucose levels in patients with diabetes87. The personal glucose meter is a portable electrochemical biosensor that provides rapid quantification of blood glucose levels for personal glycaemic control. The glucose meter, which is typically an amperometric biosensor based on a redox enzyme, consists of a disposable test strip and a pocket-sized handheld electrochemical reader (Fig. 2a). The disposable test strips can be fabricated by printing and cutting at a large scale using low-cost materials such as plastics and conductive pastes; for example, the thin-film electrodes on the test strips can be produced by screen-printing technology, which allows mass production at low cost88. The sensing layer containing the enzyme and the electron mediator is immobilized on the WE for the detection of glucose. Once the blood sample is introduced to the small chamber (electrochemical cell) formed by the spacer layer on the test strip, blood glucose is oxidized by the redox mediator, which is catalyzed by GOx (Fig. 2a). The reduced mediator is then oxidized on the electrode, producing a measurable current signal89, which is converted to glucose concentration by a handheld detector. The personal glucose meter is a result of continuous engineering advances to increase its accuracy, reliability, user-friendliness and affordability90,91 since the first concept of glucose enzyme electrodes was proposed in the 1960s1.
Fig. 2. Portable electrochemical biosensing devices.
a, Portable blood glucose meter consisting of a handheld electrochemical detector and disposable test strips. The test strip contains a bottom electrode layer, an adhesive spacer layer and a hydrophilic cover layer. The blood sample is introduced to the reaction chamber by capillary force. b, A paper-based microfluidic electrochemical biosensor for the detection of adenosine through aptamer-based affinity sensing. In one channel, adenosine is recognized by aptamer-functionalized microbeads (blue), resulting in the release of glucose oxidase-labelled DNA to catalyze the oxidation of glucose, which leads to the conversion of [Fe(CN)6]3− to [Fe(CN)6]4−. In the other channel, the microbeads are not functionalized (purple), allowing quantification of adenosine concentration. The current signal from the discharging of the capacitor is collected by a portable digital multimeter. ox, oxidation; red, reduction. Part b reprinted with permission from ref. 104, Wiley.
The personal glucose meter can also detect metal ions, drugs, organic metabolites, enzymes, proteins, DNA and influenza viruses by relating target recognition events with the generation or consumption of glucose92–97. For example, the personal glucose meter can quantify cocaine, adenosine and uranium in blood through the target-induced release of invertase, from a DNA–invertase conjugate, that catalyzes the conversion of sucrose to glucose92. Moreover, the device can quantitatively detect SARS-CoV-2 antigen in human saliva for COVID-19 screening97; here, antigen-binding events are translated into glucose signals using an aptamer-based competitive mechanism that leads to invertase release to catalyze sucrose hydrolysis. This on-site test can be accomplished within 1 h with a picomolar limit of detection.
For complex samples that require pre-treatment, signal amplification and continuous analysis, electrochemical biosensors can be combined with microfluidic systems98,99, for example, for the detection of SARS-CoV-2 RNA100. In this device, RNA is detected by a reconfigurable enzyme–DNA nanostructure, which comprises DNA strands with inhibitor and inverter sequences that are bound to a Taq DNA polymerase through a cascading molecular circuitry enhancement; here, the biorecognition of target RNA by inverter sequences activates polymerase activity for downstream DNA amplification, labelling and electrochemical detection. The entire assay is automatically completed by a pressure-actuated microfluidic device with embedded sensing electrodes. Electrochemical biosensors can also be integrated with paper microfluidic devices by directly printing electrodes on paper. Paper-based microfluidics is cheap, biodegradable, easy to fabricate and allows pumpless fluidic transport by capillary actions101,102. In addition, paper can be folded (origami) to assemble 3D devices and control fluidic and electrical connectivity for programmed analytical processes103–105; for example, paper with patterned fluidic channels and electrodes can be assembled into a 3D configuration by folding and lamination for the detection of adenosine104 (Fig. 2b). In this device, the adenosine sample is first split into two symmetrical channels. In one channel, adenosine binds an aptamer immobilized on microbeads, which causes the release of GOx-labelled DNA and leads to the conversion of [Fe(CN)6]3− to [Fe(CN)6]4−. In the other channel, the microbeads do not contain the aptamer, leading to different redox concentrations in the cells, allowing the quantitative analysis of adenosine in a portable digital multimeter.
Miniaturized electrochemical analyzers can also be connected to smartphones for powering, processing and storage of data and to display results106. In addition, the test results can be uploaded to mobile health services107. For example, an open-source portable electrochemical detector that can establish wireless communication with a smartphone can be combined with electrochemical biosensors106,108.
Integration into wearable devices
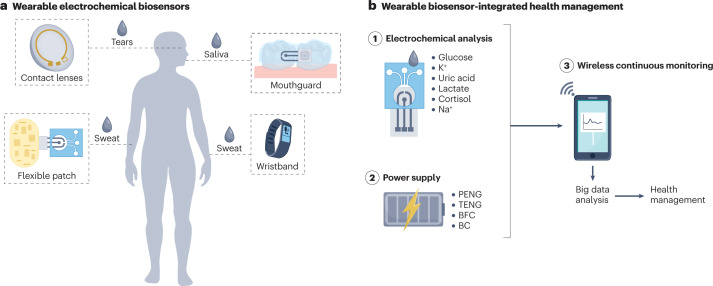
Wearable sensors can be integrated with smartwatches, bracelets and glasses for physiological monitoring, for example, of heart rate, electrocardiogram and electroencephalogram109. Such wearable biosensors also allow non-invasive and continuous monitoring of analytes in body fluids (Fig. 3a), providing invaluable data for diagnostics and health management109–111. For example, the concentrations of glucose in non-blood body fluids, such as sweat and tears, can be converted to their corresponding blood levels through a correlation coefficient obtained from a correlation study between glucose concentration in blood and non-blood biofluids26,112, considering time lags for glucose secretion in different biofluids113–115. Compared to portable electrochemical biosensors, of which some have already been commercialized (Supplementary Table 1), wearable electrochemical biosensors are not yet at the same development stage.
Fig. 3. Integration of electrochemical biosensors in wearable devices.
a, Wearable sensors can be applied to monitor health-related or disease-related analytes in different body fluids, including tears, saliva and sweat. b, Health management can be based on continuous monitoring using wearable devices, including electrochemical biosensors, power supply and wireless communication modules. BC, biocapacitor; BFC, biofuel cell; PENG, piezoelectric nanogenerator; TENG, triboelectric nanogenerator.
Thus far, wearable electrochemical biosensors have mainly been explored for glucose monitoring because glucose can be detected in sweat, saliva and tears116–118. Compared to the conventional finger-prick test, wearable glucose analysis allows non-invasive and continuous monitoring, even during sleep, enabling timely feedback for diabetes management. A wearable integrated sensing array allows multiplexed detection of sweat biomarkers, including metabolites and electrolytes (such as glucose, lactate, Na+ and K+); here, signal conditioning, processing and wireless data transmission for in situ sweat analysis are achieved by flexible printed circuit boards57. Such integrated wearable electrochemical biosensors allow non-invasive and dynamic monitoring of the health status at the molecular level, for example, for in situ monitoring of wound healing119, therapeutic drugs, drug abuse120, nutrition121 and the diagnosis of cystic fibrosis114. Wearable electrochemical biosensors can also be incorporated into robots to sense hazardous materials and pathogens for agriculture, security and public health applications122.
Sampling plays an important role in wearable biosensing. The concentration of biochemical analytes in secreted body fluids is affected by various factors, including reabsorption, evaporation, secretion rate, interfering substances and metabolism of the secretion glands109,113,123. Microfluidic devices can be applied for sample collection; for example, sweat can be enriched and transported to a sensor module in a microfluidic device, reducing sweat reabsorption and evaporation, and allowing real-time continuous monitoring. Moreover, a microfluidic sweat sampling device can be designed to collect small volumes of sweat, enabling continuous sweat monitoring at rest by entrapping thermoregulatory-generated sweat in a microfluidic channel124. This design may facilitate wearable sweat sensing platforms that do not require large sweat volumes, for example, during exercise or at high ambient temperatures, making sweat sensing compatible with daily activities. Microfluidic devices with fluidic valves further allow in situ manipulation of collected biofluids, for example, to achieve chrono-sampling of sweat for time-dependent analysis of biomarker variation125. An epidermal microfluidic device with thermo-responsive hydrogel valves enables active control of sweat126, that is, on-demand delivery of sweat to the sensing electrode, thereby eliminating the influence of flow rate variability on the sensor response and allowing scheduled sweat analysis. Although promising for on-body biofluid detection, electrochemical bioassays in this device remain difficult because they require multistep operations for incubation, amplification and washing, limiting its use to monitor protein and nucleic acid biomarkers in sweat. Therefore, innovative fluidic control units are needed to automate multistep bioassays.
A power source is indispensable for continuous electrochemical analysis in wearable devices. Self-powered devices can generate energy from human motion127–129 using a piezoelectric nanogenerator130,131 or a triboelectric nanogenerator132–134 that converts mechanical energy into electrical energy. For example, a self-powered wearable device based on a triboelectric nanogenerator printed on a flexible circuit board enables continuous monitoring of H+ and Na+ in sweat135; here, the output of the power source (~416 mW m–2) can power the multiplexed biosensor and the design allows miniaturization. A triboelectric self-powered sweat sensor based on nanocellulose hydrogels with self-healing ability can monitor ions (Na+, K+, Ca2+) in sweat136. Alternatively, biofuel cells can power wearable biosensors by harvesting energy from redox substances in biological fluids through bioelectrocatalytic reactions128,137,138; for example, using ascorbate in tears as the fuel, a self-powered contact lens can monitor tear glucose levels139. Similarly, a self-powered wireless sensing system based on glucose and lactate biofuel cells can monitor sweat glucose and lactate levels140. If a single power source is insufficient to power the device, a microgrid system incorporating biofuel cells, triboelectric generators and supercapacitors can provide higher power output141.
Long-term wearable electrochemical biosensors can be designed with flexible electrode materials (for example, metals, conductive polymers and low-dimensional materials) that resist mechanical deformation (for example, strain and bending) and that can be self-healing142. In addition, flexible, printed circuit boards that contain full-featured microcontrollers and other components, such as communication modules, can be designed by commercial software, such as the Altium Designer, and fabricated by commercially printed circuit board manufacturers143. Wireless information communication technologies, such as Bluetooth57,144 and near-field communication145,146, have low power consumption and acceptable communication distance, allowing sensing devices to communicate with remote electronic systems such as smartphones, which can analyze, display and store data (Fig. 3b).
However, the performance of wearable biosensors is limited by variations in connectivity and impedances caused by human physical activities that can lead to detection errors. Signal processing and calibration algorithms can be applied to correct for such artefacts147; for example, electrochemical signals that are affected by pH, temperature and flow rate can be calibrated by a multiplexed sensing strategy using lookup tables for real-time and automated calibration57. To reduce signal variation, an accelerometer can further be integrated and the signal can be filtered using short-time fast Fourier transform. More advanced frequency-domain algorithms, such as the wavelet-transform projection, can be employed to decouple motions from the electrochemical measurement148. Furthermore, the relative change in electrochemical signal (for example, Nernstian shift) can be used instead of the absolute signal value to decrease measurement errors149.
Integration into implantable devices
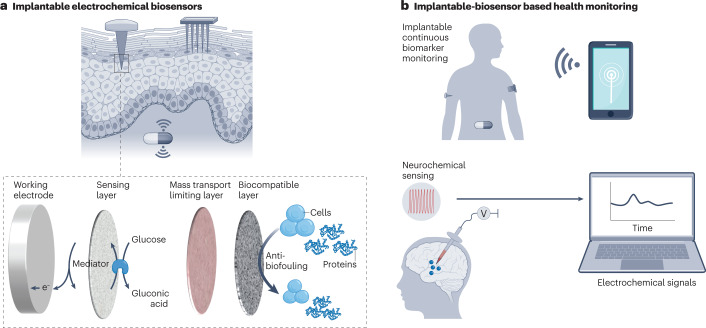
Finger-prick blood tests using portable electrochemical devices are usually highly accurate but require frequent, invasive sample collection150. Wearable electrochemical biosensing is non-invasive but suffers from low analytical accuracy, which is a particular concern in diagnostic applications151,152. Alternatively, implantable electrochemical biosensors combine the high accuracy of invasive finger-prick tests and the long-term monitoring capability of non-invasive wearable analysis153,154. Implantable electrochemical biosensors have been particularly explored for continuous glucose monitoring and in vivo monitoring of biomarkers, such as neurochemicals, in the brain155–158 (Fig. 4).
Fig. 4. Integration of electrochemical biosensors in implantable devices.
a, Microneedle-based implantable electrochemical biosensors for the monitoring of analytes in interstitial fluid. The working electrode is modified with multiple functional layers, including an inner sensing layer consisting of a redox polymer and an enzyme, a mass transport-limiting layer to improve stability, and an outer biocompatible layer to prevent fouling of the sensor. b, Implantable electrochemical biosensors allow continuous glucose monitoring and in vivo detection of neurochemicals in the brain.
In electrochemical biosensors, the detection reaction occurs on the surface of the electrodes and, thus, such sensors can easily be integrated with circuitry and incorporated into a small capsule for implantation159,160. Implantable electrochemical biosensors (for example, subcutaneous or intravascular) can provide dynamic information on glucose levels to guide therapy adjustments157,161–163. Similarly, spatiotemporal electrochemical sensing of neurochemicals, such as dopamine and acetylcholine, in the brain can indicate neuronal activity164–166.
Most implantable electrodes are made of Au, Pt and Ir, which are electrochemically stable and, in principle, biocompatible155,167,168. However, as foreign bodies, implantable devices are subject to biofouling and the foreign body response, compromising their analytical performance169. Therefore, the electrode has to be coated with multiple functional layers, including an inner sensing layer consisting of redox polymer and enzyme, a middle layer to improve stability, and an outer biocompatible layer to prevent fouling of the sensor170,171 (Fig. 4a). For example, NO-releasing polymer coatings can improve the biocompatibility of implantable biosensors (for example, intravascular sensors) because the endogenous gas molecule NO inhibits platelet adhesion and activation, inflammatory responses, and bacterial growth148,172. In addition, implantable devices need to be sterilized; thus, the coating layers need to withstand sterilization treatments such as irradiation173.
The mechanical mismatch between soft tissues and implantable electrodes may lead to inflammatory responses and/or device failure. Therefore, implantable electrodes should be soft and stretchable to seamlessly interface with soft tissues. For example, a soft implantable neurotransmitter sensor can monitor the dynamics of monoamine in the brain and gut of mice174; here, the soft, elastic and thin electrode is fabricated by embedding laser-induced graphene nanofibres in an elastomer matrix, minimizing damage to intestinal tissue and not disturbing the peristaltic movement of the gastrointestinal tract.
Implantable biosensors typically remain in the body for long time periods, which requires an adequate power supply175 with high volumetric energy density (that is, the energy stored per unit of volume) owing to the constraint of the device size152,155. Batteries have high energy densities but require periodic replacement, which may risk infection and additional costs176. Alternatively, implantable electrochemical biosensors could be made self-powered using piezoelectric materials, triboelectric materials or fuel cells156,177–180. In addition, near-field communication may enable wireless power generation and data transmission159,181.
Alternative to implantable devices that typically require surgery, partially implantable electrochemical biosensors have been commercialized (for example, Freestyle Libre from Abbott and G6 CGM system from Dexcom)154,182 (Supplementary Table 1). Such partially implantable biosensors only require subcutaneous insertion of a small probe (for example, a flexible needle) or a probe array, leaving most components, including the power source, readout circuitry and wireless communication modules, on the surface of the skin151,162. For example, minimally invasive biosensors for glucose detection allow continuous glucose monitoring for about 2 weeks and can then be replaced by the patient161,183. However, these glucose biosensors are limited to single analyte analysis and may cause discomfort owing to the long needles (5–11 mm) that need to be inserted to access interstitial fluid. To achieve multiplexed analysis of biomarkers and discomfort-free operation, an integrated microneedle array can be applied that allows continuous monitoring of two analytes (for example, lactate and glucose, or alcohol and glucose) in interstitial fluid184. This device integrates reusable electronics to acquire and wirelessly transmit the electrochemical signals to a smartphone for data analysis and visualization.
Outlook
Electrochemical biosensors are powerful tools to quantitatively analyze biochemical analytes in body fluids, providing digital data of dynamic physiological processes for fundamental research and health-care applications. The integration of electrochemical biosensors in portable, wearable and implantable devices enables decentralized POC detection185–187, which has the potential to revolutionize diagnostics and health management188, particularly in low-resource settings (Box 1). Batch fabrication and integration of disposable, flexible and multi-electrode electrochemical biosensors with different substrates, including plastics, flexible films, textiles and paper, can be achieved by printing (for example, screen28–30, inkjet122, roll-to-roll189 and transfer190 printing) and microfabrication (for example, photolithography57, evaporation124, electron beam evaporation114,119 and laser cutter121); however, engineering challenges remain to be addressed for integrated electrochemical biosensors to make a real impact in POC diagnostics; for example, signal transduction, conditioning (amplification and filtering), processing and wireless transmission need to be improved57; all functional controllers and modules should be integrated on one circuit board; packaging of soft electronics and chipsets needs to be optimized; and microminiaturization, networking and intellectualization of devices needs to be realized191 (Box 2).
Beyond glucose sensing, electrochemical biosensing devices could also allow the POC detection of proteins, nucleic acids, viruses and cells; however, this will require automated multistep and multisolution technology. Digital microfluidics may enable full-automatic on-chip measurements but requires high-precision instruments, limiting its applications in low-resource settings. Therefore, simple, cheap, robust and stable microfluidic systems need to be developed, for example, using paper or hydrophilic and hydrophobic polymers, which can be folded and/or printed into low-cost, disposable devices. Importantly, electrochemical biosensors need to be engineered that achieve one-step biosensing to avoid complex handling processes. In addition, although amperometric, voltammetric, potentiometric and electrochemiluminescent biosensor devices have been commercialized, these are often invasive portable devices rather than non-invasive wearable and implantable devices, in particular, OECT, photoelectrochemical and electrochemiluminescent bioimaging biosensors are still at an early stage. Thus, electrochemical sensors need to be developed according to their specific properties; for example, OECT sensors can be developed for miniaturized wearable devices and photoelectrochemical sensors can be developed for miniaturized composite implantable devices (Table 1).
Smartphones, 5G communication and cloud computing will allow the digitalization of health-related information obtained by integrated electrochemical biosensors. For example, physical sensors connected and/or integrated into smartphones, watches or wristbands allow the daily monitoring of vital signs such as heart rate, electrocardiogram and electroencephalogram. Similarly, electrochemical biosensors can be integrated into wearable devices for the non-invasive monitoring of specific analytes in body fluids related to health management.
Engineering efforts are often dedicated to improving the sensitivity, selectivity and multiplex capability of electrochemical biosensors, making these devices increasingly complex and prone to failure. However, detection sensitivity and selectivity mainly depend on the recognition reaction at the delicate electrolyte–electrode interface, which is affected by a range of factors, such as the friction between electrodes and tissues, and the dynamic change of pH, flow rate and temperature of the body fluid, particularly in wearable devices. Therefore, more robust and maintenance-free electrochemical biosensors need to be designed that allow long-term health monitoring; for example, enzyme-based sensing chemistry can be replaced by nanomaterial-based catalytic sensing chemistry, which is less influenced by environmental conditions such as temperature, pH and ionic strength. In addition, the accuracy and reliability of electrochemical biosensors could be improved by implementing biosensor arrays that enable multiple detections in different environmental conditions. Such arrays can be built using all-solid-state electrodes, which can easily be integrated into printed circuit boards. The convoluted signals measured by the array can then be deconvoluted using algorithms, such as Fourier and wavelet transformation, to achieve simultaneous, multiplex detection. Furthermore, sensing accuracy could be improved by applying techniques commonly used in electrocardiograms, electromyograms and magnetic resonance imaging; for example, compressed sensing, which enables sub-Nyquist processing of sparse signals192,193.
Commercialization and broad applicability of integrated electrochemical biosensors will require concerted efforts in refining sensing techniques and flexible materials and in consolidating electronics, wireless electronics, data processing and data mining.
Box 1 Low-resource considerations.
To achieve point-of-care analysis of health-related molecules in low-resource settings, electrochemical biosensing devices need to be portable, cheap, simple to operate and provide rapid readout and data analysis. In addition, storage and long-term stability should be considered. For example, electrochemical biosensors can be designed as disposable test strips and results can be detected with a handheld reader. The test strips (for example, blood glucose test strip) often have a shelf life of several months at room temperature in dry conditions, allowing transportation and storage without requiring a cold chain. Such a simple design is also compatible with large-scale industrial manufacturing workflows, which lowers the cost. Devices designed as test strips provide accurate and rapid sample-to-answer detection for point-of-care applications without requiring trained personnel. In addition, integration of electrochemical biosensors in smartphones, watches and wristbands enables at-home measurement of biophysiological molecules for health monitoring and disease diagnosis.
Box 2 Translational considerations.
The clinical translation of electrochemical biosensors for point-of-care diagnostic devices requires the establishment of diagnostic criteria for the evaluation of test results in different sample types. For example, diagnostic criteria for glucose tests have been well-established for blood samples; however, diagnostic criteria for other body fluids, such as sweat, saliva and tears, are more difficult to define. In addition, compared with blood samples, these biofluid samples may be affected by sampling location (for example, saliva in different positions in the mouth, sweat from different sweat glands) and by the environment (for example, before and after exercise or water drinking). Therefore, the comparison of test results and validation of test criteria remains challenging. Thus, the translational process of biosensing devices for non-blood samples may differ from that of blood samples, requiring the standardization of body fluid sampling and additional sensing units to monitor the dynamic change in pH, temperature and flow rate of the body fluid for calibration. In addition, commercialization of the blood glucose meter was originally based on blood glucose measurements in hospital settings, outlining the criteria for the design of the device; by contrast, new electrochemical biosensor-based devices intended for other body fluids may not be based on experience in hospital settings but may instead be tested and validated as consumer devices for early health warning and health management in lifestyle and fitness.
The translation of electrochemical biosensors will further depend on their ability to perform full-automatic electrochemical biosensing of affinity analytes. This can be achieved by the integration of test strips with automatic microfluidic systems. However, microfluidic systems are typically fabricated using high-cost materials and microfabrication technologies (for example, soft lithography)125,194–196. Cheap but robust and stable microfluidic systems (for example, paper-based microfluidics) should thus be further developed to promote the application of biosensor devices in health monitoring.
Supplementary information
Acknowledgements
The authors gratefully thank the National Natural Science Foundation of China (21827812, 21890741), the Science and Technology Project of Nanjing City (202110023) and the Independent Research Foundation from the State Key Laboratory of Analytical Chemistry for Life Science (5431ZZXM2006) for start-up supply. The authors are also grateful to Y. Lu in Fasteur Biotechnology for market information, to Q. Yu, Y.C. Chen and L.J. Lei for helpful comments.
Author contributions
J.W., H.L. and H.X.J. arranged the sections of the Review. J.W., W.W.C. and H.X.J. wrote the introduction and the section on electrochemical biosensing of disease biomarkers, and H.L., B.M and H.X.J. wrote the sections on portable electrochemical biosensing devices, integration into wearable devices and integration into implantable devices. All authors discussed the outlook section and display items.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Onur Parlak, Chung Chiun Liu, and Susana Campuzano for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jie Wu, Hong Liu.
Supplementary information
The online version contains supplementary material available at 10.1038/s44222-023-00032-w.
References
- 1.Clark LC, Jr, Lyons C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. NY Acad. Sci. 1962;102:29–45. doi: 10.1111/j.1749-6632.1962.tb13623.x. [DOI] [PubMed] [Google Scholar]
- 2.Lambrianou, A., Demin, S. & Hall, E. A. H. Biosensing for the 21st Century (eds Renneberg, R., & Lisdat, F.) 65–95 (Springer, 2007).
- 3.Labib M, Sargent EH, Kelley SO. Electrochemical methods for the analysis of clinically relevant biomolecules. Chem. Rev. 2016;116:9001–9090. doi: 10.1021/acs.chemrev.6b00220. [DOI] [PubMed] [Google Scholar]
- 4.Wu J, Fu ZF, Yan F, Ju HX. Biomedical and clinical applications of immunoassays and immunosensors for tumor markers. Trends Anal. Chem. 2007;26:679–688. doi: 10.1016/j.trac.2007.05.007. [DOI] [Google Scholar]
- 5.Minteer SD. Advances in electroanalytical chemistry. J. Am. Chem. Soc. 2018;140:2701–2703. doi: 10.1021/jacs.8b00986. [DOI] [PubMed] [Google Scholar]
- 6.Zhang JJ, Xiang Y, Wang M, Basu A, Lu Y. Dose-dependent response of personal glucose meters to nicotinamide coenzymes: applications to point-of-care diagnostics of many non-glucose targets in a single step. Angew. Chem. Int. Ed. 2016;128:742–746. doi: 10.1002/ange.201507563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das A, Cui XK, Chivukula V, Iyer SS. Detection of enzymes, viruses, and bacteria using glucose meters. Anal. Chem. 2018;90:11589–11598. doi: 10.1021/acs.analchem.8b02960. [DOI] [PubMed] [Google Scholar]
- 8.Gong SH, Li JJ, Pan W, Li N, Tang B. Duplex-specific nuclease-assisted CRISPR-Cas12a strategy for microRNA detection using a personal glucose meter. Anal. Chem. 2021;93:10719–10726. doi: 10.1021/acs.analchem.1c02478. [DOI] [PubMed] [Google Scholar]
- 9.Liu R, Hu YS, He Y, Lan T, Zhang JJ. Translating daily COVID-19 screening into a simple glucose test: a proof of concept study. Chem. Sci. 2021;12:9022–9030. doi: 10.1039/D1SC00512J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao YZ, et al. Portable and sensitive detection of non-glucose target by enzyme-encapsulated metal-organic-framework using personal glucose meter. Biosens. Bioelectron. 2022;198:113819. doi: 10.1016/j.bios.2021.113819. [DOI] [PubMed] [Google Scholar]
- 11.Lubin AA, Plaxco KW. Folding-based electrochemical biosensors: the case for responsive nucleic acid architectures. Acc. Chem. Res. 2010;43:496–505. doi: 10.1021/ar900165x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang HQ, Li F, Dever B, Li XF, Le XC. DNA-mediated homogeneous binding assays for nucleic acids and proteins. Chem. Rev. 2013;113:2812–2841. doi: 10.1021/cr300340p. [DOI] [PubMed] [Google Scholar]
- 13.Liu BW, Du D, Hua X, Yu XY, Lin YH. Paper-based electrochemical biosensors: from test strips to paper-based microfluidics. Electroanalysis. 2014;26:1214–1223. doi: 10.1002/elan.201400036. [DOI] [Google Scholar]
- 14.Sassa F, Biswas GC, Suzuki H. Microfabricated electrochemical sensing devices. Lab Chip. 2020;20:1358–1389. doi: 10.1039/C9LC01112A. [DOI] [PubMed] [Google Scholar]
- 15.Noviana E, McCord CP, Clark KM, Jang I, Henry CS. Electrochemical paper-based devices: sensing approaches and progress toward practical applications. Lab Chip. 2020;20:9–34. doi: 10.1039/C9LC00903E. [DOI] [PubMed] [Google Scholar]
- 16.Lei JP, Ju HX. Signal amplification using functional nanomaterials for biosensing. Chem. Soc. Rev. 2012;41:2122–2134. doi: 10.1039/c1cs15274b. [DOI] [PubMed] [Google Scholar]
- 17.Ju HX. Biosensors: signal amplification for highly sensitive bioanalysis based on biosensors or biochips. J. Biochips Tissue Chips. 2012;2:e114. doi: 10.4172/2153-0777.1000e114. [DOI] [Google Scholar]
- 18.Heikenfeld J, et al. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotech. 2019;37:407–419. doi: 10.1038/s41587-019-0040-3. [DOI] [PubMed] [Google Scholar]
- 19.Yu Y, Nyein HYY, Gao W, Javey A. Flexible electrochemical bioelectronics: the rise of in situ bioanalysis. Adv. Mater. 2020;32:1902083. doi: 10.1002/adma.201902083. [DOI] [PubMed] [Google Scholar]
- 20.Xu CH, Yang YR, Gao W. Skin-interfaced sensors in digital medicine: from materials to applications. Matter. 2020;2:1414–1445. doi: 10.1016/j.matt.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song Y, Min JH, Gao W. Wearable and implantable electronics: moving toward precision therapy. ACS Nano. 2019;13:12280–12286. doi: 10.1021/acsnano.9b08323. [DOI] [PubMed] [Google Scholar]
- 22.Hussein HA, et al. SARS-CoV-2-impedimetric biosensor: virus-imprinted chips for early and rapid diagnosis. ACS Sens. 2021;6:4098–4107. doi: 10.1021/acssensors.1c01614. [DOI] [PubMed] [Google Scholar]
- 23.Zeng J, et al. An impedimetric biosensor for COVID-19 serology test and modification of sensor performance via dielectrophoresis force. Biosen. Bioelectron. 2022;213:114476. doi: 10.1016/j.bios.2022.114476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karyakin AA, Gitelmacher OV, Karyakina EE. Prussian blue-based first-generation biosensor: a sensitive amperometric electrode for glucose. Anal. Chem. 1995;67:2419–2423. doi: 10.1021/ac00110a016. [DOI] [Google Scholar]
- 25.Ju, H. X., Zhang, X. J. & Wang, J. NanoBiosensing: Principles, Development and Application 85–102 (Springer, 2011).
- 26.Kim SK, et al. Bimetallic nanocatalysts immobilized in nanoporous hydrogels for long-term robust continuous glucose monitoring of smart contact lens. Adv. Mater. 2022;34:2110536. doi: 10.1002/adma.202110536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang YZ, Han YK, Sun JY, Zhang Y, Han L. Dual nanocatalysts co-decorated three-dimensional, laser-induced graphene hybrid nanomaterials integrated with a smartphone portable electrochemical system for point-of-care non-enzymatic glucose diagnosis. Mater. Today Chem. 2022;24:100895. doi: 10.1016/j.mtchem.2022.100895. [DOI] [Google Scholar]
- 28.Wu J, et al. A disposable electrochemical immunosensor for flow injection immunoassay of carcinoembryonic antigen. Biosens. Bioelectron. 2006;22:102–108. doi: 10.1016/j.bios.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Wu J, Yan F, Tang JH, Zhai C, Ju HX. A disposable multianalyte electrochemical immunosensor array for automated simultaneous determination of tumor markers. Clin. Chem. 2007;53:1495–1502. doi: 10.1373/clinchem.2007.086975. [DOI] [PubMed] [Google Scholar]
- 30.Wu J, et al. Disposable reagentless electrochemical immunosensor array based on a biopolymer/sol-gel membrane for simultaneous measurement of several tumor markers. Clin. Chem. 2008;54:1481–1488. doi: 10.1373/clinchem.2007.102350. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-la-Villa A, Pozo-Ayuso DF, Castano-Alvarez M. Microfluidics and electrochemistry: an emerging tandem for next-generation analytical microsystems. Curr. Opin. Electrochem. 2019;15:175–185. doi: 10.1016/j.coelec.2019.05.014. [DOI] [Google Scholar]
- 32.Kikkeri K, Wu D, Voldman J. A sample-to-answer electrochemical biosensor system for biomarker detection. Lab Chip. 2022;22:100–107. doi: 10.1039/D1LC00910A. [DOI] [PubMed] [Google Scholar]
- 33.Koklu A, et al. Microfluidic integrated organic electrochemical transistor with a nanoporous membrane for amyloid-beta detection. ACS Nano. 2021;15:8130–8141. doi: 10.1021/acsnano.0c09893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun S, et al. Multifunctional self-driven origami paper-based integrated microfluidic chip to detect CRP and PAB in whole blood. Biosens. Bioelectron. 2022;208:114225. doi: 10.1016/j.bios.2022.114225. [DOI] [PubMed] [Google Scholar]
- 35.Feng DZ, et al. DNA tetrahedron-mediated immune-sandwich assay for rapid and sensitive detection of PSA through a microfluidic electrochemical detection system. Microsyst. Nanoeng. 2021;7:33. doi: 10.1038/s41378-021-00258-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee GH, et al. Single microfluidic electrochemical sensor system for simultaneous multi-pulmonary hypertension biomarker analyses. Sci. Rep. 2017;7:7545. doi: 10.1038/s41598-017-06144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pursey JP, Chen Y, Stulz E, Park MK, Kongsuphol P. Microfluidic electrochemical multiplex detection of bladder cancer DNA markers. Sens. Actuators B Chem. 2017;251:34–39. doi: 10.1016/j.snb.2017.05.006. [DOI] [Google Scholar]
- 38.Fragoso A, et al. Integrated microfluidic platform for the electrochemical detection of breast cancer markers in patient serum samples. Lab Chip. 2011;11:625–631. doi: 10.1039/C0LC00398K. [DOI] [PubMed] [Google Scholar]
- 39.Zhao YX, Chen F, Li Q, Wang LH, Fan CH. Isothermal amplification of nucleic acids. Chem. Rev. 2015;115:12491–12545. doi: 10.1021/acs.chemrev.5b00428. [DOI] [PubMed] [Google Scholar]
- 40.Simmel FC, Yurke B, Singh HR. Principles and applications of nucleic acid strand displacement reactions. Chem. Rev. 2019;119:6326–6369. doi: 10.1021/acs.chemrev.8b00580. [DOI] [PubMed] [Google Scholar]
- 41.Dai YF, et al. Exploring the trans-cleavage activity of CRISPR-Cas12a (cpf1) for the development of a universal electrochemical biosensor. Angew. Chem. Int. Ed. 2019;58:17399–17405. doi: 10.1002/anie.201910772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang DC, et al. CRISPR/Cas12a-mediated interfacial cleaving of hairpin DNA reporter for electrochemical nucleic acid sensing. ACS Sens. 2020;5:557–562. doi: 10.1021/acssensors.9b02461. [DOI] [PubMed] [Google Scholar]
- 43.Hu JM, Wang TY, Kim J, Shannon C, Easley CJ. Quantitation of femtomolar protein levels via direct readout with the electrochemical proximity assay. J. Am. Chem. Soc. 2012;134:7066–7072. doi: 10.1021/ja3000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu JM, et al. A reusable electrochemical proximity assay for highly selective, real-time protein quantitation in biological matrices. J. Am. Chem. Soc. 2014;136:8467–8474. doi: 10.1021/ja503679q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren KW, Wu J, Yan F, Ju HX. Ratiometric electrochemical proximity assay for sensitive one-step protein detection. Sci. Rep. 2014;4:4360. doi: 10.1038/srep04360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ren KW, Wu J, Yan F, Zhang Y, Ju HX. Immunoreaction-triggered DNA assembly for one-step sensitive ratiometric electrochemical biosensing of protein biomarker. Biosens. Bioelectron. 2015;66:345–349. doi: 10.1016/j.bios.2014.11.046. [DOI] [PubMed] [Google Scholar]
- 47.Ren KW, Wu J, Zhang Y, Yan F, Ju HX. Proximity hybridization regulated DNA biogate for sensitive electrochemical immunoassay. Anal. Chem. 2014;86:7494–7499. doi: 10.1021/ac5012377. [DOI] [PubMed] [Google Scholar]
- 48.Ren KW, Wu J, Ju HX, Yan F. Target-driven triple-binder assembly of MNAzyme for amplified electrochemical immunosensing of protein biomarker. Anal. Chem. 2015;87:1694–1700. doi: 10.1021/ac504277z. [DOI] [PubMed] [Google Scholar]
- 49.Zhu J, Gan HY, Wu J, Ju HX. Molecular machine powered surface programmatic chain reaction for highly sensitive electrochemical detection of protein. Anal. Chem. 2018;90:5503–5508. doi: 10.1021/acs.analchem.8b01217. [DOI] [PubMed] [Google Scholar]
- 50.Man Y, et al. An anchored monopodial DNA walker triggered by proximity hybridization for amplified amperometric biosensing of nucleic acid and protein. Anal. Chim. Acta. 2020;1107:48–54. doi: 10.1016/j.aca.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 51.Karimi-Maleh H, et al. A critical review on the use of potentiometric based biosensors for biomarkers detection. Biosens. Bioelectron. 2021;184:113252. doi: 10.1016/j.bios.2021.113252. [DOI] [PubMed] [Google Scholar]
- 52.Ding JW, Chen Y, Wang XW, Qin W. Label-free and substrate-free potentiometric aptasensing using polycation-sensitive membrane electrodes. Anal. Chem. 2012;84:2055–2061. doi: 10.1021/ac2024975. [DOI] [PubMed] [Google Scholar]
- 53.Nurlely AM, Heng LY, Tan LL. Potentiometric enzyme biosensor for rapid determination of formaldehyde based on succinimide-functionalized polyacrylate ion-selective membrane. Measurement. 2021;175:109112. doi: 10.1016/j.measurement.2021.109112. [DOI] [Google Scholar]
- 54.Özbek O, Berkel C, Isildak Ö, Isildak I. Potentiometric urea biosensors. Clin. Chim. Acta. 2022;524:154–163. doi: 10.1016/j.cca.2021.11.011. [DOI] [PubMed] [Google Scholar]
- 55.Wu J, et al. Potentiometric detection of DNA hybridization using enzyme-induced metallization and a silver ion selective electrode. Anal. Chem. 2009;81:10007–10012. doi: 10.1021/ac9018507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding JW, Li BW, Chen LX, Qin W. A three-dimensional origami paper-based device for potentiometric biosensing. Angew. Chem. Int. Ed. 2016;55:13033–13037. doi: 10.1002/anie.201606268. [DOI] [PubMed] [Google Scholar]
- 57.Gao W, et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature. 2016;529:509–514. doi: 10.1038/nature16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang H, Yan F, Lin P, Xu JB, Chan HLW. Highly sensitive glucose biosensors based on organic electrochemical transistors using platinum gate electrodes modified with enzyme and nanomaterials. Adv. Funct. Mater. 2011;21:2264–2272. doi: 10.1002/adfm.201002117. [DOI] [Google Scholar]
- 59.Tang H, Lin P, Chan HLW, Yan F. Highly sensitive dopamine biosensors based on organic electrochemical transistors. Biosens. Bioelectron. 2011;26:4559–4563. doi: 10.1016/j.bios.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 60.Mak CH, et al. Highly-sensitive epinephrine sensors based on organic electrochemical transistors with carbon nanomaterial modified gate electrodes. J. Mater. Chem. C. 2015;3:6532–6538. doi: 10.1039/C5TC01100K. [DOI] [Google Scholar]
- 61.Parlak O, Keene ST, Marais A, Curto VF, Salleo A. Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive cortisol sensing. Sci. Adv. 2018;4:eaar2904. doi: 10.1126/sciadv.aar2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin P, Luo XT, Hsing IM, Yan F. Organic electrochemical transistors integrated in flexible microfluidic systems and used for label-free DNA sensing. Adv. Mater. 2011;23:4035–4040. doi: 10.1002/adma.201102017. [DOI] [PubMed] [Google Scholar]
- 63.Fu Y, et al. Highly sensitive detection of protein biomarkers with organic electrochemical transistors. Adv. Mater. 2017;29:1703787. doi: 10.1002/adma.201703787. [DOI] [PubMed] [Google Scholar]
- 64.Kim DJ, et al. Organic electrochemical transistor based immunosensor for prostate specific antigen (PSA) detection using gold nanoparticles for signal amplification. Biosens. Bioelectron. 2010;25:2477–2482. doi: 10.1016/j.bios.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 65.He RX, et al. Detection of bacteria with organic electrochemical transistors. J. Mater. Chem. 2012;22:22072–22076. doi: 10.1039/c2jm33667g. [DOI] [Google Scholar]
- 66.Lin BP, Yan F, Yu JJ, Chan HLW, Yang M. The application of organic electrochemical transistors in cell-based biosensors. Adv. Mater. 2010;22:3655–3660. doi: 10.1002/adma.201000971. [DOI] [PubMed] [Google Scholar]
- 67.Chen LZ, et al. Organic electrochemical transistors for the detection of cell surface glycans. ACS Appl. Mater. Interfaces. 2018;10:18470–18477. doi: 10.1021/acsami.8b01987. [DOI] [PubMed] [Google Scholar]
- 68.Chen LZ, Wang NX, Wu J, Yan F, Ju HX. Organic electrochemical transistor for sensing of sialic acid in serum samples. Anal. Chim. Acta. 2020;1128:231–237. doi: 10.1016/j.aca.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 69.Chen LZ, Wu J, Yan F, Ju HX. A facile strategy for quantitative sensing of glycans on cell surface using organic electrochemical transistors. Biosens. Bioelectron. 2021;175:112878. doi: 10.1016/j.bios.2020.112878. [DOI] [PubMed] [Google Scholar]
- 70.Chen LZ, Wu J, Yan F, Ju HX. Monose-modified organic electrochemical transistors for cell surface glycan analysis via competitive recognition to enzyme-labeled lectin. Mikrochim. Acta. 2021;188:1–7. doi: 10.1007/s00604-021-04918-7. [DOI] [PubMed] [Google Scholar]
- 71.Wang N, Yang A, Fu Y, Li Y, Yan F. Functionalized organic thin film transistors for biosensing. Acc. Chem. Res. 2019;52:277–287. doi: 10.1021/acs.accounts.8b00448. [DOI] [PubMed] [Google Scholar]
- 72.Yang AN, et al. Fabric organic electrochemical transistors for biosensors. Adv. Mater. 2018;30:1800051. doi: 10.1002/adma.201800051. [DOI] [PubMed] [Google Scholar]
- 73.Zang Y, Lei JP, Ju HX. Principles and applications of photoelectrochemical sensing strategies based on biofunctionalized nanostructures. Biosens. Bioelectron. 2017;96:8–16. doi: 10.1016/j.bios.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 74.Ruan YF, et al. An integrated photoelectrochemical nanotool for intracellular drug delivery and evaluation of treatment effect. Angew. Chem. Int. Ed. 2021;133:25966–25969. doi: 10.1002/ange.202111608. [DOI] [PubMed] [Google Scholar]
- 75.Hu FX, Miao JW, Guo CX, Yang HB, Liu B. Real-time photoelectrochemical quantification of hydrogen peroxide produced by living cells. Chem. Eng. J. 2021;407:127203. doi: 10.1016/j.cej.2020.127203. [DOI] [Google Scholar]
- 76.Ye XX, et al. FRET modulated signaling: a versatile strategy to construct photoelectrochemical microsensors for in vivo analysis. Angew. Chem. Int. Ed. 2021;60:11774–11778. doi: 10.1002/anie.202101468. [DOI] [PubMed] [Google Scholar]
- 77.Qi HL, Zhang CX. Electrogenerated chemiluminescence biosensing. Anal. Chem. 2020;92:524–534. doi: 10.1021/acs.analchem.9b03425. [DOI] [PubMed] [Google Scholar]
- 78.Chen YH, Ding ZF. Highly sensitive analysis strategies of microRNAs based on electrochemiluminescence. Curr. Opin. Electrochem. 2022;32:100901. doi: 10.1016/j.coelec.2021.100901. [DOI] [Google Scholar]
- 79.Miao WJ. Electrogenerated chemiluminescence and its biorelated applications. Chem. Rev. 2008;108:2506–2553. doi: 10.1021/cr068083a. [DOI] [PubMed] [Google Scholar]
- 80.Delaney JL, Hogan CF, Tian J, Shen W. Electrogenerated chemiluminescence detection in paper-based microfluidic sensors. Anal. Chem. 2011;83:1300–1306. doi: 10.1021/ac102392t. [DOI] [PubMed] [Google Scholar]
- 81.Kerr E, et al. Amplification-free electrochemiluminescence molecular beacon-based microRNA sensing using a mobile phone for detection. Sens. Actuators B Chem. 2021;330:129261. doi: 10.1016/j.snb.2020.129261. [DOI] [Google Scholar]
- 82.Deiss F, et al. Multiplexed sandwich immunoassays using electrochemiluminescence imaging resolved at the single bead level. J. Am. Chem. Soc. 2009;131:6088–6089. doi: 10.1021/ja901876z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang NN, Chen LZ, Chen WW, Ju HX. Potential-and color-resolved electrochemiluminescence of polymer dots for array imaging of multiplex microRNAs. Anal. Chem. 2021;93:5327–5333. doi: 10.1021/acs.analchem.1c00620. [DOI] [PubMed] [Google Scholar]
- 84.Ma C, Cao Y, Gou XD, Zhu JJ. Recent progress in electrochemiluminescence sensing and imaging. Anal. Chem. 2020;92:431–454. doi: 10.1021/acs.analchem.9b04947. [DOI] [PubMed] [Google Scholar]
- 85.Wang NN, et al. Dual intramolecular electron transfer for in situ coreactant‐embedded electrochemiluminescence microimaging of membrane protein. Angew. Chem. Int. Ed. 2021;60:197–201. doi: 10.1002/anie.202011176. [DOI] [PubMed] [Google Scholar]
- 86.Wang NN, et al. Confined electrochemiluminescence imaging microarray for high-throughput biosensing of single cell-released dopamine. Biosens. Bioelectron. 2022;201:113959. doi: 10.1016/j.bios.2021.113959. [DOI] [PubMed] [Google Scholar]
- 87.Teymourian H, Barfidokht A, Wang J. Electrochemical glucose sensors in diabetes management: an updated review (2010-2020) Chem. Soc. Rev. 2020;49:7671–7709. doi: 10.1039/D0CS00304B. [DOI] [PubMed] [Google Scholar]
- 88.Nagata R, Yokoyama K, Clark SA, Karube I. A glucose sensor fabricated by the screen printing technique. Biosens. Bioelectron. 1995;10:261–267. doi: 10.1016/0956-5663(95)96845-P. [DOI] [PubMed] [Google Scholar]
- 89.Wang J. Electrochemical glucose biosensors. Chem. Rev. 2008;108:814–825. doi: 10.1021/cr068123a. [DOI] [PubMed] [Google Scholar]
- 90.Tonyushkina K, Nichols JH. Glucose meters: a review of technical challenges to obtaining accurate results. J. Diabetes Sci. Technol. 2009;3:971–980. doi: 10.1177/193229680900300446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Karon BS, Boyd JC, Klee GG. Glucose meter performance criteria for tight glycemic control estimated by simulation modeling. Clin. Chem. 2010;56:1091–1097. doi: 10.1373/clinchem.2010.145367. [DOI] [PubMed] [Google Scholar]
- 92.Xiang Y, Lu Y. Using personal glucose meters and functional DNA sensors to quantify a variety of analytical targets. Nat. Chem. 2011;3:697–703. doi: 10.1038/nchem.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang J, Lu Y. Biocomputing for portable, resettable, and quantitative point-of-care diagnostics: making the glucose meter a logic-gate responsive device for measuring many clinically relevant targets. Angew. Chem. Int. Ed. 2018;57:9702–9706. doi: 10.1002/anie.201804292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Amalfitano E, et al. A glucose meter interface for point-of-care gene circuit-based diagnostics. Nat. Commun. 2021;12:724. doi: 10.1038/s41467-020-20639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang X, et al. Electrochemical assay to detect influenza viruses and measure drug susceptibility. Angew. Chem. Int. Ed. 2015;54:5929–5932. doi: 10.1002/anie.201412164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ahn JK, Kim HY, Park KS, Park HG. A personal glucose meter for label-free and washing-free biomolecular detection. Anal. Chem. 2018;90:11340–11343. doi: 10.1021/acs.analchem.8b02014. [DOI] [PubMed] [Google Scholar]
- 97.Singh NK, et al. Hitting the diagnostic sweet spot: point-of-care SARS-CoV-2 salivary antigen testing with an off-the-shelf glucometer. Biosens. Bioelectron. 2021;180:113111. doi: 10.1016/j.bios.2021.113111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rackus DG, Shamsi MH, Wheeler AR. Electrochemistry, biosensors and microfluidics: a convergence of fields. Chem. Soc. Rev. 2015;44:5320–5340. doi: 10.1039/C4CS00369A. [DOI] [PubMed] [Google Scholar]
- 99.de Campos RPS, et al. “Plug-n-Play” sensing with digital microfluidics. Anal. Chem. 2019;91:2506–2515. doi: 10.1021/acs.analchem.8b05375. [DOI] [PubMed] [Google Scholar]
- 100.Zhao H, et al. Accessible detection of SARS-CoV-2 through molecular nanostructures and automated microfluidics. Biosens. Bioelectron. 2021;194:113629. doi: 10.1016/j.bios.2021.113629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gong MM, Sinton D. Turning the page: advancing paper-based microfluidics for broad diagnostic application. Chem. Rev. 2017;117:8447–8480. doi: 10.1021/acs.chemrev.7b00024. [DOI] [PubMed] [Google Scholar]
- 102.Dungchai W, Chailapakul O, Henry CS. Electrochemical detection for paper-based microfluidics. Anal. Chem. 2009;81:5821–5826. doi: 10.1021/ac9007573. [DOI] [PubMed] [Google Scholar]
- 103.Liu H, Crooks RM. Three-dimensional paper microfluidic devices assembled using the principles of origami. J. Am. Chem. Soc. 2011;133:17564–17566. doi: 10.1021/ja2071779. [DOI] [PubMed] [Google Scholar]
- 104.Liu H, Xiang Y, Lu Y, Crooks RM. Aptamer-based origami paper analytical device for electrochemical detection of adenosine. Angew. Chem. Int. Ed. 2012;51:6925–6928. doi: 10.1002/anie.201202929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen CA, et al. An electricity- and instrument-free infectious disease sensor based on a 3D origami paper-based analytical device. Lab Chip. 2021;21:1908–1915. doi: 10.1039/D1LC00079A. [DOI] [PubMed] [Google Scholar]
- 106.Su J, et al. Smartphone-based electrochemical biosensors for directly detecting serum-derived exosomes and monitoring their secretion. Anal. Chem. 2022;94:3235–3244. doi: 10.1021/acs.analchem.1c04910. [DOI] [PubMed] [Google Scholar]
- 107.Guo JH. Smartphone-powered electrochemical biosensing dongle for emerging medical IoTs application. IEEE T. Ind. Inform. 2018;14:2592–2597. doi: 10.1109/TII.2017.2777145. [DOI] [Google Scholar]
- 108.Ainla A, et al. Open-source potentiostat for wireless electrochemical detection with smartphones. Anal. Chem. 2018;90:6240–6246. doi: 10.1021/acs.analchem.8b00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim J, Campbell AS, de Avila BE, Wang J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019;37:389–406. doi: 10.1038/s41587-019-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bariya M, Nyein HYY, Javey A. Wearable sweat sensors. Nat. Electron. 2018;1:160–171. doi: 10.1038/s41928-018-0043-y. [DOI] [Google Scholar]
- 111.Park J, et al. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci. Adv. 2018;4:eaap9841. doi: 10.1126/sciadv.aap9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moyer J, Wilson D, Finkelshtein I, Wong B, Potts R. Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol. Ther. 2012;14:398–402. doi: 10.1089/dia.2011.0262. [DOI] [PubMed] [Google Scholar]
- 113.Baker LB. Physiology of sweat gland function: the roles of sweating and sweat composition in human health. Temperature. 2019;6:211–259. doi: 10.1080/23328940.2019.1632145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Emaminejad S, et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl Acad. Sci. USA. 2017;114:4625–4630. doi: 10.1073/pnas.1701740114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sonner Z, et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics. 2015;9:031301. doi: 10.1063/1.4921039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu Y, Jiang K, Chen D, Shen GZ. Wearable sweat monitoring system with integrated micro-supercapacitors. Nano Energy. 2019;58:624–632. doi: 10.1016/j.nanoen.2019.01.084. [DOI] [Google Scholar]
- 117.Arakawa T, et al. A wearable cellulose acetate-coated mouthguard biosensor for in vivo salivary glucose measurement. Anal. Chem. 2020;92:12201–12207. doi: 10.1021/acs.analchem.0c01201. [DOI] [PubMed] [Google Scholar]
- 118.Khalil OS. Noninvasive photonic-crystal material for sensing glucose in tears. Clin. Chem. 2004;50:2236–2237. doi: 10.1373/clinchem.2004.042978. [DOI] [PubMed] [Google Scholar]
- 119.Gao Y, et al. A flexible multiplexed immunosensor for point-of-care in situ wound monitoring. Sci. Adv. 2021;7:eabg9614. doi: 10.1126/sciadv.abg9614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Teymourian H, et al. Wearable electrochemical sensors for the monitoring and screening of drugs. ACS Sens. 2020;5:2679–2700. doi: 10.1021/acssensors.0c01318. [DOI] [PubMed] [Google Scholar]
- 121.Wang MQ, et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 2022;6:1225–1235. doi: 10.1038/s41551-022-00916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yu Y, et al. All-printed soft human-machine interface for robotic physicochemical sensing. Sci. Robot. 2022;7:eabn0495. doi: 10.1126/scirobotics.abn0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Quinton PM. Sweating and its disorders. Annu. Rev. Med. 1983;34:429–452. doi: 10.1146/annurev.me.34.020183.002241. [DOI] [PubMed] [Google Scholar]
- 124.Nyein HYY, et al. A wearable patch for continuous analysis of thermoregulatory sweat at rest. Nat. Commun. 2021;12:1823. doi: 10.1038/s41467-021-22109-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Choi J, Kang D, Han S, Kim SB, Rogers JA. Thin, soft, skin-mounted microfluidic networks with capillary bursting valves for chrono-sampling of sweat. Adv. Healthc. Mater. 2017;6:1601355. doi: 10.1002/adhm.201601355. [DOI] [PubMed] [Google Scholar]
- 126.Lin H, et al. A programmable epidermal microfluidic valving system for wearable biofluid management and contextual biomarker analysis. Nat. Commun. 2020;11:4405. doi: 10.1038/s41467-020-18238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Manjakkal L, Yin L, Nathan A, Wang J, Dahiya R. Energy autonomous sweat-based wearable systems. Adv. Mater. 2021;33:2100899. doi: 10.1002/adma.202100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chong YW, Ismail W, Ko K, Lee CY. Energy harvesting for wearable devices: a review. IEEE Sens. J. 2019;19:9047–9062. doi: 10.1109/JSEN.2019.2925638. [DOI] [Google Scholar]
- 129.Lou Z, Wang LL, Shen GZ. Recent advances in smart wearable sensing systems. Adv. Mater. Technol. 2018;3:1800444. doi: 10.1002/admt.201800444. [DOI] [Google Scholar]
- 130.Yang P, et al. Monitoring the degree of comfort of shoes in-motion using triboelectric pressure sensors with an ultrawide detection range. ACS Nano. 2022;16:4654–4665. doi: 10.1021/acsnano.1c11321. [DOI] [PubMed] [Google Scholar]
- 131.Yang JH, et al. Effect of garment design on piezoelectricity harvesting from joint movement. Smart Mater. Struct. 2016;25:035012. doi: 10.1088/0964-1726/25/3/035012. [DOI] [Google Scholar]
- 132.Fu K, Zhou J, Wu HU, Su ZQ. Fibrous self-powered sensor with high stretchability for physiological information monitoring. Nano Energy. 2021;88:106258. doi: 10.1016/j.nanoen.2021.106258. [DOI] [Google Scholar]
- 133.Hao Y, et al. Self-rebound cambered triboelectric nanogenerator array for self-powered sensing in kinematic analytics. ACS Nano. 2022;16:1271–1279. doi: 10.1021/acsnano.1c09096. [DOI] [PubMed] [Google Scholar]
- 134.Luo XX, et al. Tribovoltaic nanogenerators based on mxene-silicon heterojunctions for highly stable self-powered speed, displacement, tension, oscillation angle, and vibration sensors. Adv. Funct. Mater. 2022;32:2113149. doi: 10.1002/adfm.202113149. [DOI] [Google Scholar]
- 135.Song Y, et al. Wireless battery-free wearable sweat sensor powered by human motion. Sci. Adv. 2020;6:eaay9842. doi: 10.1126/sciadv.aay9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Qin Y, et al. Stretchable triboelectric self-powered sweat sensor fabricated from self-healing nanocellulose hydrogels. Adv. Funct. Mater. 2022;32:2201846. doi: 10.1002/adfm.202201846. [DOI] [Google Scholar]
- 137.Kim J, et al. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 2015;74:1061–1068. doi: 10.1016/j.bios.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jia W, Valdes-Ramirez G, Bandodkar AJ, Windmiller JR, Wang J. Epidermal biofuel cells: energy harvesting from human perspiration. Angew. Chem. Int. Ed. 2013;52:7233–7236. doi: 10.1002/anie.201302922. [DOI] [PubMed] [Google Scholar]
- 139.Falk M, Andoralov V, Silow M, Toscano MD, Shleev S. Miniature biofuel cell as a potential power source for glucose-sensing contact lenses. Anal. Chem. 2013;85:6342–6348. doi: 10.1021/ac4006793. [DOI] [PubMed] [Google Scholar]
- 140.Huang XC, et al. Epidermal self-powered sweat sensors for glucose and lactate monitoring. Bio-Des. Manuf. 2022;5:201–209. doi: 10.1007/s42242-021-00156-1. [DOI] [Google Scholar]
- 141.Yin L, et al. A self-sustainable wearable multi-modular E-textile bioenergy microgrid system. Nat. Commun. 2021;12:1542. doi: 10.1038/s41467-021-21701-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen Z, Xi J, Huang W, Yuen MMF. Stretchable conductive elastomer for wireless wearable communication applications. Sci. Rep. 2017;7:10958. doi: 10.1038/s41598-017-11392-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Takei K, et al. Nanowire active-matrix circuitry for low-voltage macroscale artificial skin. Nat. Mater. 2010;9:821–826. doi: 10.1038/nmat2835. [DOI] [PubMed] [Google Scholar]
- 144.Nyein HYY, et al. A wearable microfluidic sensing patch for dynamic sweat secretion analysis. ACS Sens. 2018;3:944–952. doi: 10.1021/acssensors.7b00961. [DOI] [PubMed] [Google Scholar]
- 145.Rose DP, et al. Adhesive RFID sensor patch for monitoring of sweat electrolytes. IEEE Trans. Biomed. Eng. 2015;62:1457–1465. doi: 10.1109/TBME.2014.2369991. [DOI] [PubMed] [Google Scholar]
- 146.Keum DH, et al. Wireless smart contact lens for diabetic diagnosis and therapy. Sci. Adv. 2020;6:eaba3252. doi: 10.1126/sciadv.aba3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lukocius R, Vaitkunas M, Virbalis JA, Dosinas A, Vegys A. Physiological parameters monitoring system for occupational safety. Elektron. Elektrotech. 2014;20:57–60. doi: 10.5755/j01.eee.20.5.7100. [DOI] [Google Scholar]
- 148.Lee K, et al. Mechano-acoustic sensing of physiological processes and body motions via a soft wireless device placed at the suprasternal notch. Nat. Biomed. Eng. 2020;4:148–158. doi: 10.1038/s41551-019-0480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bujes-Garrido J, Arcos-Martínez MJ. Development of a wearable electrochemical sensor for voltammetric determination of chloride ions. Sens. Actuators B Chem. 2017;240:224–228. doi: 10.1016/j.snb.2016.08.119. [DOI] [Google Scholar]
- 150.Vaddiraju S, Burgess DJ, Tomazos I, Jain FC, Papadimitrakopoulos F. Technologies for continuous glucose monitoring: current problems and future promises. J. Diabetes Sci. Technol. 2010;4:1540–1562. doi: 10.1177/193229681000400632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang J, et al. Wearable glucose monitoring and implantable drug delivery systems for diabetes management. Adv. Healthc. Mater. 2021;10:2100194. doi: 10.1002/adhm.202100194. [DOI] [PubMed] [Google Scholar]
- 152.Rodrigues D, et al. Skin-integrated wearable systems and implantable biosensors: a comprehensive review. Biosensors. 2020;10:79. doi: 10.3390/bios10070079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bobrowski T, Schuhmann W. Long-term implantable glucose biosensors. Curr. Opin. Electrochem. 2018;10:112–119. doi: 10.1016/j.coelec.2018.05.004. [DOI] [Google Scholar]
- 154.Li P, et al. From diagnosis to treatment: recent advances in patient-friendly biosensors and implantable devices. ACS Nano. 2021;15:1960–2004. doi: 10.1021/acsnano.0c06688. [DOI] [PubMed] [Google Scholar]
- 155.Dalrymple AN. Implanted devices: the importance of both electrochemical performance and biological acceptance. Neural Regen. Res. 2021;16:1188–1189. doi: 10.4103/1673-5374.300342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kotanen CN, Moussy FG, Carrara S, Guiseppi-Elie A. Implantable enzyme amperometric biosensors. Biosens. Bioelectron. 2012;35:14–26. doi: 10.1016/j.bios.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 157.Li CM, Dong H, Cao X, Luong JH, Zhang X. Implantable electrochemical sensors for biomedical and clinical applications: progress, problems, and future possibilities. Curr. Med. Chem. 2007;14:937–951. doi: 10.2174/092986707780362970. [DOI] [PubMed] [Google Scholar]
- 158.Wagner J, Tennen H, Wolpert H. Continuous glucose monitoring: a review for behavioral researchers. Psychosom. Med. 2012;74:356–365. doi: 10.1097/PSY.0b013e31825769ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu J, et al. Implantable platinum nanotree microelectrode with a battery-free electrochemical patch for peritoneal carcinomatosis monitoring. Biosens. Bioelectron. 2021;185:113265. doi: 10.1016/j.bios.2021.113265. [DOI] [PubMed] [Google Scholar]
- 160.Singh P, et al. Biomedical perspective of electrochemical nanobiosensor. Nanomicro Lett. 2016;8:193–203. doi: 10.1007/s40820-015-0077-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McGarraugh G. The chemistry of commercial continuous glucose monitors. Diabetes Technol. Ther. 2009;11:S17–S24. doi: 10.1089/dia.2008.0133. [DOI] [PubMed] [Google Scholar]
- 162.Gross TM, et al. Performance evaluation of the MiniMed continuous glucose monitoring system during patient home use. Diabetes Technol. Ther. 2000;2:49–56. doi: 10.1089/152091500316737. [DOI] [PubMed] [Google Scholar]
- 163.Lee H, Hong YJ, Baik S, Hyeon T, Kim DH. Enzyme-based glucose sensor: from invasive to wearable device. Adv. Healthc. Mater. 2018;7:1701150. doi: 10.1002/adhm.201701150. [DOI] [PubMed] [Google Scholar]
- 164.Clark JJ, et al. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat. Methods. 2010;7:126–129. doi: 10.1038/nmeth.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tavakolian-Ardakani Z, Hosu O, Cristea C, Mazloum-Ardakani M, Marrazza G. Latest trends in electrochemical sensors for neurotransmitters: a review. Sensors. 2019;19:2037. doi: 10.3390/s19092037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Azzouz A, et al. Nanomaterial-based electrochemical sensors for the detection of neurochemicals in biological matrices. Trends Anal. Chem. 2019;110:15–34. doi: 10.1016/j.trac.2018.08.002. [DOI] [Google Scholar]
- 167.Lei LJ, et al. Nonenzymatic electrochemical sensor for wearable interstitial fluid glucose monitoring. Electroanalysis. 2022;34:415–422. doi: 10.1002/elan.202060601. [DOI] [Google Scholar]
- 168.Seaton BT, Heien ML. Biocompatible reference electrodes to enhance chronic electrochemical signal fidelity in vivo. Anal. Bioanal. Chem. 2021;413:6689–6701. doi: 10.1007/s00216-021-03640-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang DH, et al. Dealing with the foreign-body response to implanted biomaterials: strategies and applications of new materials. Adv. Funct. Mater. 2021;31:2007226. doi: 10.1002/adfm.202007226. [DOI] [Google Scholar]
- 170.Wickramasinghe Y, Yang Y, Spencer SA. Current problems and potential techniques in in vivo glucose monitoring. J. Fluoresc. 2004;14:513–520. doi: 10.1023/B:JOFL.0000039339.36839.19. [DOI] [PubMed] [Google Scholar]
- 171.Nichols SP, Koh A, Storm WL, Shin JH, Schoenfisch MH. Biocompatible materials for continuous glucose monitoring devices. Chem. Rev. 2013;113:2528–2549. doi: 10.1021/cr300387j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Malone-Povolny MJ, Merricks EP, Wimsey LE, Nichols TC, Schoenfisch MH. Long-term accurate continuous glucose biosensors via extended nitric oxide release. ACS Sens. 2019;4:3257–3264. doi: 10.1021/acssensors.9b01779. [DOI] [PubMed] [Google Scholar]
- 173.Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chem. Soc. Rev. 2006;35:780–789. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- 174.Li JX, et al. A tissue-like neurotransmitter sensor for the brain and gut. Nature. 2022;606:94–101. doi: 10.1038/s41586-022-04615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ben Amar A, Kouki AB, Cao H. Power approaches for implantable medical devices. Sensors. 2015;15:28889–28914. doi: 10.3390/s151128889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rebelo R, Barbosa AI, Correlo VM, Reis RL. An outlook on implantable biosensors for personalized medicine. Engineering. 2021;7:1696–1699. doi: 10.1016/j.eng.2021.08.010. [DOI] [Google Scholar]
- 177.Yang Y, Wei XJ, Liu J. Suitability of a thermoelectric power generator for implantable medical electronic devices. J. Phys. D Appl. Phys. 2007;40:5790–5800. doi: 10.1088/0022-3727/40/18/042. [DOI] [Google Scholar]
- 178.Hwang GT, et al. Self-powered cardiac pacemaker enabled by flexible single crystalline PMN-PT piezoelectric energy harvester. Adv. Mater. 2014;26:4880–4887. doi: 10.1002/adma.201400562. [DOI] [PubMed] [Google Scholar]
- 179.Zheng Q, et al. In vivo powering of pacemaker by breathing-driven implanted triboelectric nanogenerator. Adv. Mater. 2014;26:5851–5856. doi: 10.1002/adma.201402064. [DOI] [PubMed] [Google Scholar]
- 180.Simons P, Schenk SA, Gysel MA, Olbrich LF, Rupp JLM. A ceramic-electrolyte glucose fuel cell for implantable electronics. Adv. Mater. 2022;34:2109075. doi: 10.1002/adma.202109075. [DOI] [PubMed] [Google Scholar]
- 181.Scholten K, Meng E. A review of implantable biosensors for closed-loop glucose control and other drug delivery applications. Int. J. Pharm. 2018;544:319–334. doi: 10.1016/j.ijpharm.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 182.Green RA, Lovell NH, Wallace GG, Poole-Warren LA. Conducting polymers for neural interfaces: challenges in developing an effective long-term implant. Biomaterials. 2008;29:3393–3399. doi: 10.1016/j.biomaterials.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 183.Kutner N, Kunduru KR, Rizik L, Farah S. Recent advances for improving functionality, biocompatibility, and longevity of implantable medical devices and deliverable drug delivery systems. Adv. Funct. Mater. 2021;31:2010929. doi: 10.1002/adfm.202010929. [DOI] [Google Scholar]
- 184.Tehrani F, et al. An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid. Nat. Biomed. Eng. 2022;6:1214–1224. doi: 10.1038/s41551-022-00887-1. [DOI] [PubMed] [Google Scholar]
- 185.Hsieh K, Ferguson BS, Eisenstein M, Plaxco KW, Soh HT. Integrated electrochemical microsystems for genetic detection of pathogens at the point of care. Acc. Chem. Res. 2015;48:911–920. doi: 10.1021/ar500456w. [DOI] [PubMed] [Google Scholar]
- 186.Zhang J, Lan T, Lu Y. Translating in vitro diagnostics from centralized laboratories to point-of-care locations using commercially-available handheld meters. Trends Anal. Chem. 2020;124:115782. doi: 10.1016/j.trac.2019.115782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Loncaric C, Tang YT, Ho C, Parameswaran MA, Yu HZ. A USB-based electrochemical biosensor prototype for point-of-care diagnosis. Sens. Actuators B Chem. 2012;161:908–913. doi: 10.1016/j.snb.2011.11.061. [DOI] [Google Scholar]
- 188.Tu JB, Torrente-Rodriguez RM, Wang MQ, Gao W. The era of digital health: a review of portable and wearable affinity biosensors. Adv. Funct. Mater. 2020;30:1906713. doi: 10.1002/adfm.201906713. [DOI] [Google Scholar]
- 189.Bariya M, et al. Roll-to-roll gravure printed electrochemical sensors for wearable and medical devices. ACS Nano. 2018;12:6978–6987. doi: 10.1021/acsnano.8b02505. [DOI] [PubMed] [Google Scholar]
- 190.Tian L, et al. Large-area MRI-compatible epidermal electronic interfaces for prosthetic control and cognitive monitoring. Nat. Biomed. Eng. 2019;3:194–205. doi: 10.1038/s41551-019-0347-x. [DOI] [PubMed] [Google Scholar]
- 191.Gordon WJ, Stern AD. Challenges and opportunities in software-driven medical devices. Nat. Biomed. Eng. 2019;3:493–497. doi: 10.1038/s41551-019-0426-z. [DOI] [PubMed] [Google Scholar]
- 192.Liu H, Zhao C. Wearable electrochemical sensors for noninvasive monitoring of health-a perspective. Curr. Opin. Electrochem. 2020;23:42–46. doi: 10.1016/j.coelec.2020.03.008. [DOI] [Google Scholar]
- 193.Dixon AM, Allstot EG, Gangopadhyay D, Allstot DJ. Compressed sensing system considerations for ECG and EMG wireless biosensors. IEEE Trans. Biomed. Circuits Syst. 2012;6:156–166. doi: 10.1109/TBCAS.2012.2193668. [DOI] [PubMed] [Google Scholar]
- 194.Koh A, et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl Med. 2016;8:366ra165. doi: 10.1126/scitranslmed.aaf2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kamei K, et al. 3D printing of soft lithography mold for rapid production of polydimethylsiloxane-based microfluidic devices for cell stimulation with concentration gradients. Biomed. Microdevices. 2015;17:36. doi: 10.1007/s10544-015-9928-y. [DOI] [PubMed] [Google Scholar]
- 196.Martin A, et al. Epidermal microfluidic electrochemical detection system: enhanced sweat sampling and metabolite detection. ACS Sens. 2017;2:1860–1868. doi: 10.1021/acssensors.7b00729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.