Abstract
Three potential regulators of flagellar expression present in the genome sequence of Campylobacter jejuni NCTC 11168, the genes rpoN, flgR, and fliA, which encode the alternative sigma factor ς54, the ς54-associated transcriptional activator FlgR, and the flagellar sigma factor ς28, respectively, were investigated for their role in global regulation of flagellar expression. The three genes were insertionally inactivated in C. jejuni strains NCTC 11168 and NCTC 11828. Electron microscopic studies of the wild-type and mutant strains showed that the rpoN and flgR mutants were nonflagellate and that the fliA mutant had truncated flagella. Immunoblotting experiments with the three mutants confirmed the roles of rpoN, flgR, and fliA in the expression of flagellin.
Campylobacter jejuni is well established as a major cause of food-borne gastroenteritis, associated with rare but serious complications that include Guillain-Barré syndrome (9). This spiral-shaped microaerophilic, gram-negative pathogen bears a single polar flagellum at one or both ends. The flagellum is a well-characterized and distinct pathogenicity factor of the organism. There are about 40 predicted flagellar genes in the C. jejuni genome, but to date, no flagellar regulon or the genes that may control it are known. Organisms such as Salmonella enterica serovar Typhimurium have an intricate well-understood pattern of regulation of expression of their flagellar genes. The principal regulatory genes are the flhDC master operon genes, anti-sigma factor gene flgM, and ς28 gene fliA. However, C. jejuni NCTC 11168 lacks flhDC and flgM, based on gene homologies (14). We therefore propose that the two alternative sigma factors ς54 and ς28 and the NtrC homolog, named FlgR, based on characterization of FlgR in Helicobacter pylori (17), may have a role in controlling flagellar gene expression in C. jejuni. Transcriptional activators of the NtrC family are known to act with ς54, binding to enhancer-like elements upstream of ς54-dependent promoters (7). These regulators are generally members of two-component sensor-regulator systems, the cognate sensor in Escherichia coli for NtrC being NtrB.
Based on the genome sequence (14), C. jejuni has only three predicted sigma factors: ς70, ς54, and ς28. ς70 is encoded by the housekeeping sigma factor gene rpoD (23), while ς54 and ς28 are known to transcribe flagellar genes in C. jejuni as well as Caulobacter crescentus, Pseudomonas aeruginosa, and H. pylori. In C. jejuni, flagellin is encoded by flaA and flaB (5), and expression of the flaB and hook protein flgE genes results from ς54 promoter activity, while expression of flaA results from ς28 promoter activity (5, 11).
In this study, to establish the function and the role of rpoN, flgR, and fliA, the corresponding mutants in C. jejuni were constructed by allelic exchange with genes inactivated by deletion and insertion. The mutants obtained were tested for the presence of flagella and characterized. It was shown that all three affect flagellar expression.
Bacterial strains, plasmids, and growth conditions.
Escherichia coli DH5α was used as the cloning host and grown at 37°C on Luria-Bertani (LB) medium. The plasmid pBluescript [pBS (SK+)] (Stratagene, La Jolla, Calif.), which acts as a suicide vector in C. jejuni, was used in cloning rpoN and flgR. The plasmid pJMK30 containing the kanamycin resistance (Kanr) gene aphA-3 was kindly provided by J. M. Ketley (22). The C. jejuni strains used in the study were NCTC 11168 and NCTC 11828 (also designated 81116) (National Collection of Type Cultures, Colindale, London, United Kingdom). They were grown and maintained on Mueller-Hinton (MH) medium (Oxoid, Basingstoke, United Kingdom) in an atmosphere of 5% (vol/vol) O2, 10% (vol/vol) CO2, and 90% (vol/vol) N2 at 37°C. When necessary, the agar plates were supplemented with ampicillin (100 μg/ml) and/or kanamycin (50 μg/ml).
Cloning of the rpoN, fliA, and flgR genes.
Genes rpoN (Cj0670) (Fig. 1A) and flgR (Cj1024c) (Fig. 1C) were PCR amplified with C. jejuni NCTC 11168 chromosomal DNA as a template. The fliA (Cj0061c) (Fig. 1B) clone (cam148a11) from the C. jejuni genome project was provided by the Sanger Centre and renamed pAJ21. Primer pairs R1-R2 and N1-N2 (Table 1) with 5′ EcoRI restriction sites were designed to amplify the rpoN and flgR genes, respectively. R1 and R2 were 311 bp upstream of the start codon and 276 bp downstream of the stop codon, respectively. N1 and N2 were 298 bp upstream of the start codon and 100 bp downstream of the stop codon, respectively. PCR was performed with 20 ng of genomic DNA in a 50-μl reaction volume containing 20 mM Tris-HCl (pH 8.4), 1 μM forward and reverse primers, 200 μM deoxynucleoside triphosphates (dNTPs), 2 mM MgSO4, and 1 U of Vent DNA polymerase (New England Biolabs). The PCR cycling conditions for rpoN were 96°C for 4 min; 2 cycles of 94°C for 1 min 45 s, 52.4°C for 1 min, and 72°C for 2 min; and finally 28 cycles of 94°C for 1 min 45 s, 61.8°C for 1 min, and 72°C for 2 min. The same sequence was followed for flgR, but the annealing temperatures were 46°C for the first 2 cycles and 57°C for the remaining 28 cycles. The PCR products were purified by using the QIAquick PCR Purification kit (Qiagen), digested with EcoRI, and then cloned into the EcoRI site of pBS (SK+). Clones were selected on ampicillin-containing blue-white plates, screened by restriction analysis, and designated pAJ11 (rpoN) and pAJ31 (flgR). The DNA sequences of the inserts were identical to the genome sequence.
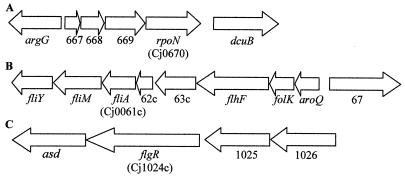
FIG. 1.
Physical and functional map showing the rpoN (A), fliA (B), and flgR (C) genes in C. jejuni.
TABLE 1.
Characteristics of the primers used in this study
| Oligonucleotide | Sequence (5′ to 3′)a | Enzyme | Strand |
|---|---|---|---|
| R1 | GCG (GAATTC) GGAGGTGAAAGAAGGCGTTG | EcoRI | + |
| R2 | GCG (GAATTC) AAACCAAGCGCTATGCCGCC | EcoRI | − |
| F1 | GGCCAGAAAATTACGTAG | + | |
| F2 | GACATCCAATTTGCCAAG | − | |
| N1 | GCG (GAATTC) CTCGAACTTCTTTAGGATTG | EcoRI | + |
| N2 | GCG (GAATTC) CTTTCCACTGGGAAATCAAG | EcoRI | − |
| R1I | GCG (AGATCT) GCTCTTGATGAAGAGGGAGAG | BglII | + |
| R2I | GCG (AGATCT) GCAACCAAGAGCGTAAAGTTT | BglII | − |
| F1I | GCG (AGATCT) GATCTTAAAGAGCGCGATC | BglII | + |
| F2I | GCG (AGATCT) CTTTAGGCGGCTCTTTTAG | BglII | − |
| N1I | GCG (AGATCT)TCCGTTGTACAAAGAGCTTG | BglII | + |
| N2I | GCG (AGATCT) GCAATTTCTAAGGATTTGCG | BglII | − |
The restriction endonuclease site in each oligonucleotide sequence is in parentheses, and the enzyme that cut it is listed.
Inverse PCR mutagenesis (24).
Primer pairs R1I-R2I, F1I-F2I, and N1I-N2I were designed to introduce a unique BglII restriction site and deletions of 953, 488, and 1,015 bp within the cloned rpoN, fliA, and flgR genes, respectively (Table 1). The primers were oriented such that amplification of template DNA extended in opposite directions around the cloning vector, with 20 ng of plasmid DNA as the template. Samples were subjected to 45 cycles of PCR, with denaturation for 1 min at 95°C; annealing for 2 min at 60, 57, and 58°C for the rpoN, fliA, and flgR amplifications, respectively; and extension for 5 min at 72°C. The products were digested with BglII enzyme, purified, ligated, transformed into competent E. coli DH5α cells, and selected for ampicillin resistance. The resulting constructs, pAJ12, pAJ22, and pAJ32, were confirmed by restriction digestion and sequence analysis.
Construction of rpoN, fliA, and flgR mutants in C. jejuni
The 1.4-kbp Kanr gene aphA-3 from pJMK30 was inserted into the BglII site of the inverse PCR clones by selection of kanamycin- and ampicillin-resistant transformants. The Kanr modified constructs of the rpoN, fliA, and flgR genes were designated pAJ13, pAJ23, and pAJ33, respectively. They were verified by restriction analysis and sequencing, and the orientations of the Kanr cassette were determined. Plasmid DNA (1 to 3 μg) was used to electroporate competent cells (19) of C. jejuni strains NCTC 11168 and NCTC 11828. Electroporation of cells at 108 ml−1 was performed with a Bio-Rad Gene Pulser system at 2.5 kV, 200 Ω, and 25 μF with a time constant of 6.0 (20, 22). Kanamycin-resistant mutants were screened and analyzed by PCR. The rpoN, fliA, and flgR mutants in C. jejuni NCTC 11168 were designated CAJ111, CAJ211, and CAJ311, respectively, and the rpoN and fliA mutants in C. jejuni NCTC 11828 were designated CAJ122 and CAJ222, respectively. For confirmation of the C. jejuni mutant, primer pairs R1-R2, F1-F2, and N1-N2 were used to amplify sequences with genomic DNA as the template. Controls were run wherein the template used was that of C. jejuni wild-type, NCTC 11168, and NCTC 11828 chromosomal DNA. Subsequent sequence analysis of the PCR products with the same primer pairs confirmed that the desired recombination events had taken place.
Effect of insertional inactivation of rpoN, fliA, and flgR genes.
Normally, C. jejuni uses a double-crossover mechanism to integrate into its genome the kind of mutagenic constructs used here in allelic replacement. This is supported by the PCR data, because no evidence of the wild-type alleles was seen in the mutant strains, all of which yielded PCR products compatible with the expected sizes after allelic replacement (Fig. 2). Clones of rpoN and flgR from wild-type NCTC 11828 were also amplified and sequenced and were identical to the sequence in NCTC 11168, as was the partial sequence of fliA.
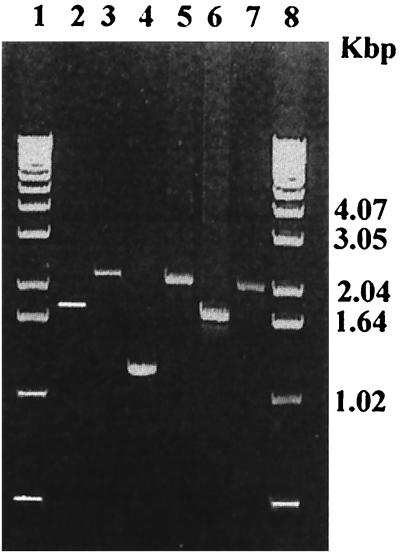
FIG. 2.
PCR confirmation of the rpoN, fliA, and flgR mutants of C. jejuni NCTC 11168. Lanes: 1 and 8, 1-kb DNA marker; 2 and 3, rpoN PCR amplification products in the wild type and mutant, respectively; 4 and 5, fliA PCR products in the wild type and mutant, respectively; 6 and 7, flgR PCR products in the wild type and mutant, respectively.
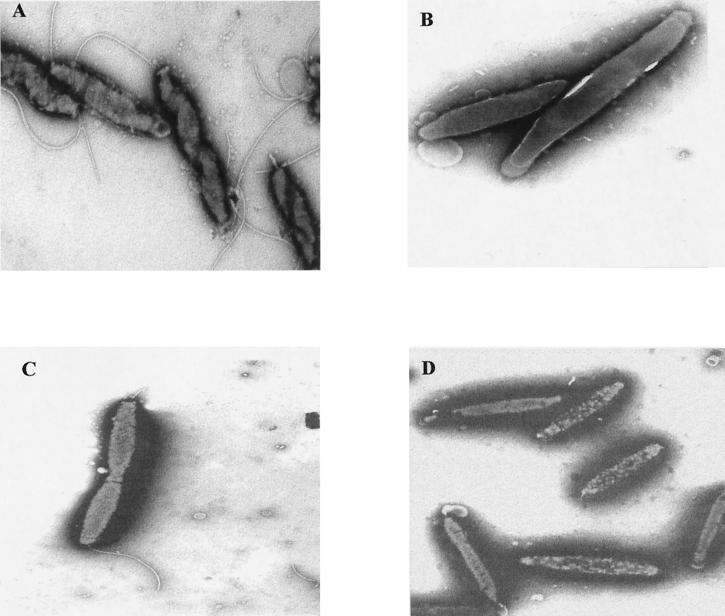
Electron microscopy indicated a complete absence of flagella in the ς54 mutants and FlgR mutants (Fig. 3B and D), in contrast to the wild type (Fig. 3A). In the ς28 mutants, cells with truncated flagella were observed (Fig. 3C). About 20% of ς28 mutant cells had no flagella. Similar results were obtained in strain NCTC 11828.
FIG. 3.
Transmission electron microscopy of the wild-type C. jejuni strain NCTC 11168 (A) compared to the rpoN (B), fliA (C), and flgR (D) mutants. Transmission electron microscopy was performed by scraping cells from plates grown overnight on MH agar at 37°C for 24 h under microaerophilic conditions. Cells were suspended in 50 μl of 1.5% (wt/vol) sodium phosphotungstate (pH 7.0), and a small drop of the suspension was applied to Formvar-coated copper grids. Excess suspension was removed with the edge of a filter paper, and negatively stained cells were visualized in a JEOL 1200EX 80-kV transmission electron microscope.
Overnight C. jejuni cultures of NCTC 11168 and the rpoN, fliA, and flgR mutants were harvested from MH agar plates and suspended in MH broth to an optical density at 600 nm (OD600) of 3.0. The samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12% [wt/vol] acrylamide) by the method of Laemmli (10) with broad-range SDS-PAGE markers (Bio-Rad). Gels were either stained with Coomassie brilliant blue or immunoblotted with a monoclonal antibody that recognizes conserved epitopes of the flaA and flaB flagellin genes of C. jejuni.
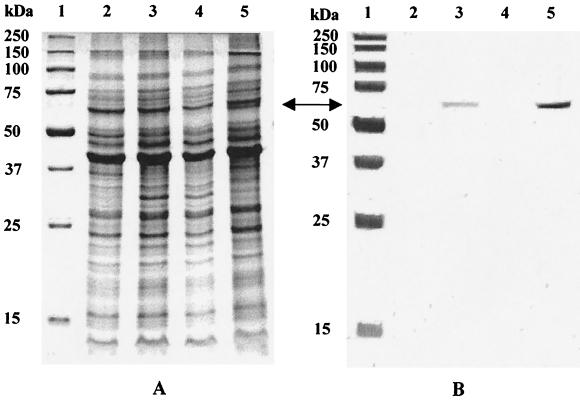
SDS-PAGE of the flagellar mutants compared to that of the wild type showed differences in the protein pattern (Fig. 4A). Immunoblot analysis of the ς54 and FlgR mutants showed the absence of the flagellar antigen (Fig. 4B). The ς28 mutant displayed a faint band indicating partial flagellin gene expression. Identical results were obtained for strain 11828 (data not shown).
FIG. 4.
SDS-PAGE and Western blot analysis of the rpoN, fliA, and flgR mutants compared to the wild-type C. jejuni NCTC 11168. In panel A, lane 1 contains a broad-range protein marker, and in panel B, lane 1 contains a prestained broad-range protein marker. In both panels A and B, lane 2 shows the ς54 mutant, lane 3 shows the ς28 mutant, lane 4 shows the FlgR mutant, and lane 5 shows wild-type strain NCTC 11168. Arrows show the location of the flagellin band.
Sequence analysis of the FlgR transcriptional activator.
BLAST analysis (1) identified the FlgR homolog as a response regulator in C. jejuni that shares significant identity with FlgR or NtrC homologs in other organisms. The alignment by the GCG multiple sequence analysis program PILEUP (4) of the FlgR homolog in C jejuni, along with those of the FlgR of H. pylori (17), FlbD of C. crescentus (15), FleR of P. aeruginosa (16), NtrC of E. coli (12), and FleQ of P. aeruginosa (2), is shown in Fig. 5. FlgR, like NtrC, comprises three domains: an amino-terminal receiver domain, a central activator domain, and a carboxy-terminal DNA binding domain. To activate transcription, NtrC must be phosphorylated on an aspartate residue in its N-terminal receiver domain (18). The FlgR homolog of C. jejuni contains all of the domains required by a response regulator. The N-terminal domain of 130 residues contains residues corresponding to Asp-11, Asp-12, and Asp-54 of E. coli NtrC, which form an acid pocket, and Lys-104, which along with Asp-54 forms a salt bridge (6). In this context, it is dissimilar to the transcriptional regulators FleQ in P. aeruginosa, in which Asp-54 and Lys-104 are absent, and FlbD in C. crescentus, which lacks Asp-11 and Asp-12. The presence of all four residues in C. jejuni suggests that the FlgR homolog is likely to be phosphorylated at Asp-54 by a kinase, as generally observed in two-component regulators. The central activator domain of 240 residues is strongly conserved and includes the ς54 ATP binding domains A and B. The highly conserved C-terminal DNA binding domain possesses a helix-turn-helix motif that is essential for regulators of the NtrC subfamily. No typical coupled sensor protein like NtrB in E. coli and FleS in P. aeruginosa (12, 16) has been identified in C. jejuni. However, the C. jejuni gene product of Cj0793, an uncoupled two-component sensor, shows sequence homology with the gene product of HP244, a cognate sensor for FlgR in H. pylori (3).
FIG. 5.
Multiple alignment of FlgR of C. jejuni (FlgRCj) with homologous transcriptional regulators: FlgR of H. pylori (FlgRHp), FlbD of C. crescentus (FlbDCc), FleR and FleQ of P. aeruginosa (FleQPa and FleRPa), and NtrC of E. coli (NtrCEc). Conserved residues (CON) are indicated wherever four or more sequences have the same residue. A, B, and C indicate the conserved aspartic acid residues corresponding to positions 11, 12, and 54, respectively. D corresponds to the conserved lysine residue at position 104. I and II are the two ATP binding domains, and III indicates the helix-turn-helix DNA binding motif.
Role of rpoN, fliA, and flgR in flagellar expression.
Inactivation of the rpoN and flgR regulatory genes abolished flagellar function completely, indicating a global regulatory role of FlgR as a transcriptional activator of ς54-dependent flagellar genes in C. jejuni. Inactivation of the fliA gene had a less detrimental effect on the flagella of C. jejuni. Studies with the flagellin genes flaA and flaB (the former with a ς28 promoter and the latter with a ς54 promoter) showed that the flaB mutants have nearly full-length flagella with wild-type motility, while flaA mutants have severely truncated flagella (13, 21) and exhibit little or no motility. The flagellar filament of C. jejuni comprises a major protein, FlaA, and a minor protein, FlaB, that may not be expressed by wild-type cells under standard conditions (8). The complete absence of flagellin expression in the rpoN and flgR mutants suggests that neither the flaB gene possessing a ς54 promoter nor the flaA gene with a ς28 promoter is transcribed. According to the model proposed by Spohn and Scarlato (17), FlgR represses flaA gene expression in H. pylori, and deletion of flgR caused upregulation of flaA transcript levels. Upregulation of FlaA in the flgR mutant was not observed in C. jejuni, a close relative of H. pylori, suggesting there is a different mode of flagellar regulation in C. jejuni. This also suggests lack of negative regulatory control by FlgR as a transcriptional factor on the flaA gene.
No clear hierarchy of flagellar gene expression can be deduced from the phenotypes of mutants we have described, although it appears that expression of ς28-dependent FlaA is repressed when early genes controlled by ς54 and/or FlgR are not expressed in C. jejuni. Beier and Frank (3) suggested that flagellin degradation may explain this apparent loss of flagellin expression in aflagellate mutants in H. pylori, but no evidence of this has been presented.
Control of flagellin expression in C. jejuni involves the alternative sigma factor genes rpoN and fliA and transcriptional regulator flgR. Other transcriptional regulators that may be involved in the flagellar regulon need to be identified. Understanding the regulatory pathway of flagellar synthesis will provide insights into the pathogenesis of C. jejuni as well as enhanced understanding of underlying features of control of gene expression in this organism.
Acknowledgments
A. Jagannathan was supported by the Darwin Trust of Edinburgh. We are grateful to the Sanger Centre for providing the genome sequence of C. jejuni NCTC 11168 prior to publication. We thank P. Whittle and the staff of the Electron Microscopy Unit, University of Birmingham, for assistance with electron microscopy and photography.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Arora S K, Ritchings B W, Almira E C, Lory S, Ramphal R. A transcriptional activator, FleQ, regulates mucin adhesion and flagellar gene expression in Pseudomonas aeruginosa in a cascade manner. J Bacteriol. 1997;179:5574–5581. doi: 10.1128/jb.179.17.5574-5581.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beier D, Frank R. Molecular characterization of two-component systems of Helicobacter pylori. J Bacteriol. 2000;182:2068–2076. doi: 10.1128/jb.182.8.2068-2076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerry P, Alm R A, Power M E, Logan S M, Trust T J. Role of two flagellin genes in Campylobacter motility. J Bacteriol. 1991;173:4757–4764. doi: 10.1128/jb.173.15.4757-4764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hazelbauer G L H, Berg H C, Matsumura P. Bacterial motility and signal transduction. Cell. 1993;73:15–22. doi: 10.1016/0092-8674(93)90156-k. [DOI] [PubMed] [Google Scholar]
- 7.Helmann J D. Alternative sigma factors and the regulation of flagellar gene expression. Mol Microbiol. 1991;5:2875–2882. doi: 10.1111/j.1365-2958.1991.tb01847.x. [DOI] [PubMed] [Google Scholar]
- 8.Ketley J M. Pathogenesis of enteric infection by Campylobacter. Microbiology. 1997;143:5–21. doi: 10.1099/00221287-143-1-5. [DOI] [PubMed] [Google Scholar]
- 9.Kuroki S, Saida T, Nukina M, Haruta T, Yoshioka M, Kobayashi Y, Nakanishi H. Campylobacter jejuni strains from patients with Guillain-Barré syndrome belong mostly to Penner serogroup 19 and contain beta N-acetylglucosamine residues. Ann Neurol. 1993;33:243–247. doi: 10.1002/ana.410330304. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 11.Lüneberg E, Glenn-Calvo E, Hartmann M, Bär W, Frosch M. The central, surface-exposed region of the flagellar hook protein FlgE of Campylobacter jejuni shows hypervariability among strains. J Bacteriol. 1998;180:3711–3714. doi: 10.1128/jb.180.14.3711-3714.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miranda-Rios J, Sanchez-Pescador R, Urdea M, Covarrubias A A. The complete nucleotide sequence of the glnALG operon of Escherichia coli K12. Nucleic Acids Res. 1987;15:2757–2770. doi: 10.1093/nar/15.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nuijten P J M, van Asten F J A M, Gaastra W, van der Zeijst B A M. Structural and functional analysis of two Campylobacter jejuni flagella genes. J Biol Chem. 1990;265:17798–17804. [PubMed] [Google Scholar]
- 14.Parkhill J, Wren B W, Mungall K, Ketley J M, Churcher C, Basham D, Chillingworth T, Davies R M, Feltwell T, Holroyd S, Jagels K, Karlyshev A V, Moule S, Pallen M J, Penn C W, Quail M A, Rajandream M-A, Rutherford K M, van Vliet A H M, Whitehead S, Barrell B G. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 15.Ramakrishna G, Newton A. FlbD of Caulobacter crescentus is a homologue of the NtrC (NRI) protein and activates ς54-dependent flagellar gene promoters. Proc Natl Acad Sci USA. 1990;87:2369–2373. doi: 10.1073/pnas.87.6.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritchings B W, Almira E C, Lory S, Ramphal R. Cloning and phenotypic characterization of fleS and fleR, new response regulators of Pseudomonas aeruginosa which regulate motility and adhesion to mucin. Infect Immun. 1995;63:4868–4876. doi: 10.1128/iai.63.12.4868-4876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spohn G, Scarlato V. Motility of Helicobacter pylori is coordinately regulated by the transcriptional activator FlgR, an NtrC homologue. J Bacteriol. 1999;181:593–599. doi: 10.1128/jb.181.2.593-599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stock B J, Ninfa A J, Stock A M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Vliet A H M, Wood A C, Henderson J, Wooldridge K G, Ketley J M. Genetic manipulation of enteric Campylobacter species. Methods Microbiol. 1998;27:407–419. [Google Scholar]
- 20.van Vliet A H M, Wooldridge K G, Ketley J M. Iron-responsive gene regulation in Campylobacter jejuni fur mutant. J Bacteriol. 1998;180:5291–5298. doi: 10.1128/jb.180.20.5291-5298.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wassenaar T M, Bleumink-Pluym N M, van der Zeijst B A M. Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J. 1991;10:2055–2061. doi: 10.1002/j.1460-2075.1991.tb07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wassenaar T M, Fry B N, van der Zeijst B A M. Genetic manipulation of Campylobacter: evaluation of natural transformation and electro-transformation. Gene. 1993;132:131–135. doi: 10.1016/0378-1119(93)90525-8. [DOI] [PubMed] [Google Scholar]
- 23.Wosten M S M M, Boeve M, Gaastra W, van der Zeijst B A M. Cloning and characterization of the gene encoding the primary ς-factor of Campylobacter jejuni. FEMS Microbiol Lett. 1998;162:97–103. doi: 10.1111/j.1574-6968.1998.tb12984.x. [DOI] [PubMed] [Google Scholar]
- 24.Wren B W, Henderson J, Ketley J M. A PCR-based strategy for the rapid construction of defined bacterial deletion mutants. BioTechniques. 1994;16:994–996. [PubMed] [Google Scholar]