Abstract
Aim:
To estimate the number of treatment initiations, averted fatal opioid overdoses and the cost-effectiveness associated with offering buprenorphine–naloxone (buprenorphine) treatment on-site within existing syringe service programs (SSPs) in Massachusetts, USA.
Design, Setting and Participants:
This was a cohort-based mathematical model and cost-effectiveness analysis. We derived model inputs from state and national surveillance data, clinical trials and observational cohort studies. We compared an intervention scenario where 30% of SSP clients initiated buprenorphine treatment on-site at least once annually to a status quo scenario where no buprenorphine was available on-site among community treatment providers in Massachusetts, 2020–30. In individuals with opioid use disorder (OUD) we assumed that 80% of SSP clients had recently injected drugs and that treatment within SSPs would have similar or improved retention compared with standard-of-care buprenorphine programs, but higher rates of active opioid use while in treatment.
Measurements:
Number of treatment initiations (i.e. individuals began treatment on a medication for opioid use disorder or entered medically managed withdrawal), averted fatal opioid overdoses, quality-adjusted life-years (QALYs) and life-time discounted costs from a health sector and a limited societal perspective.
Findings:
The status quo scenario resulted in 23 051 fatal overdoses and 1 511 613 treatment initiations over a 10-year simulation period. An intervention scenario with on-site SSP buprenorphine treatment averted 4797 (−20.8%) fatal opioid overdoses and resulted in 129 359 (+8.6%) additional treatment initiations compared with the status quo. The intervention scenario was the dominating scenario: providing OUD treatment through Massachusetts SSPs cost less (−$3612 per person) with patients accumulating more QALYs (0.2 per person) compared with the status quo scenario.
Conclusions:
Offering buprenorphine treatment on-site within syringe service programs has the potential to decrease fatal overdoses substantially, improve treatment engagement and save on costs.
Keywords: Cost-effectiveness analysis, injection drug use, medications for opioid use disorder, opioid use disorder, simulation modeling, treatment access
INTRODUCTION
In 2020, nearly 69 000 Americans died from an overdose involving an opioid [1]. Medications for opioid use disorder (MOUD) such as methadone or buprenorphine can decrease an individual’s risk of overdose death by more than 50% [2]. Unfortunately, significant barriers to accessing and remaining on treatment exist, particularly for individuals with comorbidities such as mental illness or facing severe socio-economic challenges such as homelessness. Patient-level barriers include health-care access limitations, stigma among health-care providers and sobriety requirements [3]. Only 16% of people with opioid use disorder (OUD) were actively on treatment in 2014, a decrease from 25% in 2010 [4]. Restrictive policies on who can prescribe and who can receive MOUD prevent many individuals who would benefit from accessing treatment. For buprenorphine, these policies include a high threshold for entry and continuation of treatment and, until recently, a required waiver for providers and a cap on their number of patients [5, 6]. In addition, the majority of waivered providers do not prescribe to capacity [7]. New approaches are needed to increase MOUD access and utilization.
Low-threshold buprenorphine treatment seeks to overcome barriers through same-day treatment entry, patient-centered treatment goals, increased flexibility of policies and procedures (e.g. accommodation of missed appointments, non-punitive measures for continued drug use, etc.) and wider availability to those who might benefit [5, 8]. Internationally, low threshold treatment has lowered mortality [9, 10], HIV and hepatitis C incidence [11, 12] and HIV risk behaviors [13]. After France lowered barriers to MOUD by enabling any general practitioner to prescribe buprenorphine without a patient limit, overdose mortality and HIV prevalence significantly decreased [14, 15]. A key component of the low-threshold approach is expanding the type of venues offering MOUD to include non-traditional settings, such as emergency departments and syringe service programs (SSPs) [5]. Despite serving large numbers of individuals with OUD, only 11% of SSPs participating in the North American Syringe Exchange Network provide on-site buprenorphine and/or methadone [16]. This highlights a missed opportunity for treatment initiation, as SSPs are frequented by individuals who are often unable or unwilling to access health-care in traditional settings (e.g. hospitals, clinics) [17]. Pilot programs based out of SSPs have demonstrated successful on-site initiation to buprenorphine treatment. In such programs, retention rates and reductions in opioid use were similar to those seen in traditional health-care settings [18–20]. Massachusetts has 21 SSP programs located across the state, including most major cities. However, the majority have only been open since 2015 and while many have developed relationships with buprenorphine prescribers for same-day access, they do not currently provide low-threshold buprenorphine treatment on-site.
Providing buprenorphine treatment in a safe and trusted space such as an SSP could fill an existing gap in care and potentially decrease overdoses; however, this would require resources and the potential impact of program expansion is unknown. We used a state-transition cohort-based model to estimate the number of overdoses averted, population-level treatment initiations and the cost-effectiveness of offering low-threshold buprenorphine treatment to individuals with opioid use disorder utilizing existing Massachusetts syringe service programs compared to a status quo scenario.
METHODS
Analytical overview
We used the Researching Effective Strategies to Prevent Opioid Death (RESPOND) model, a mathematical model that simulates the natural history of OUD, to compare a hypothetical intervention scenario offering low-threshold buprenorphine treatment within all existing Massachusetts SSPs to a status quo scenario (i.e. no low-threshold buprenorphine treatment in SSPs) over a 10-year time horizon. A 10-year time horizon was selected as policymakers are in need of short- to medium-term targets to reduce overdose deaths, given the current pace and escalation of the opioid epidemic and that a very brief period of time (1–2 years) did not result in stable estimates.
We modeled an open cohort (i.e. population grows to represent migration or development of OUD) representing the population in Massachusetts with OUD from 2020 to 2030. According to the Massachusetts Department of Public Health (MDPH), 78 910 individuals accessed SSPs in Massachusetts during 2019. For our intervention scenario, we estimated the potential reach of an SSP-based intervention by varying annual uptake of buprenorphine treatment among those who access an SSP from 1 to 60%, using age- and sex-stratified information on Massachusetts SSP clientele. For the base case, we assumed that SSP clients would have a 30% annual probability of initiating low-threshold treatment through an SSP (i.e. 30% of those accessing SSP services initiate treatment at least once during a 12-month period). To the best of our knowledge, there is no estimate for the potential uptake of on-site MOUD at SSPs; therefore, we implemented a wide range for this parameter. We then used model-generated estimates to project the cumulative number of opioid overdose fatalities and MOUD treatment initiations in Massachusetts by 2030.
For the cost-effectiveness analysis, we ran a closed cohort simulation to directly compare costs associated with receiving low-threshold buprenorphine through SSPs to a status quo scenario over a life-time horizon from a health sector and a limited societal perspective. We denominate currency in 2019 US dollars [18]. The limited societal perspective includes criminal justice costs associated with OUD, including the costs of policing, court, criminal victimization and correctional housing. Criminal justice costs are a significant contributor to the societal costs of OUD in the United States and have been extensively described [21, 22]. Outcomes from the cost-effectiveness analysis included quality-adjusted life-years (QALYs) and costs. We discounted all costs and benefits by 3% annually [23].
Model overview
The RESPOND model, described in detail elsewhere [24], simulates the natural history of OUD and the delivery of MOUD, as well as entry into inpatient medically managed withdrawal treatment, correctional settings and SSPs.
Epidemiology
The model simulates the clinical progression of OUD by advancing simulated time through a series of discrete, 1-week time steps. In each step, the model employs data-informed probabilities to determine the fraction of the population that experiences various events, such as changes in drug use behaviors, presenting to treatment or experiencing an overdose. When projecting future outcomes within the status quo scenario, we assume that observed trends continue for the rest of the simulation (2020–30).
Opioid use
The model employs four health states to simulate the natural history of OUD: (1) active non-injection use, (2) non-active non-injection use, (3) active injection use and (4) non-active injection use (Fig. 1). Probabilities of transitioning between active to non-active and vice versa reflect the relapsing–remitting nature of OUD (Table 1). The population with active use has a higher risk of overdose, higher competing risks of death, lower quality of life and higher health-care utilization compared to the non-active OUD health state, as informed by the literature (Table 1 and fully described in Supporting information, Data S1). Injection drug use increases the detrimental impacts of active use.
FIGURE 1.
Model structure of the Researching Effective Strategies to Prevent Opioid Death (RESPOND) model. MMT = methadone maintenance treatment
TABLE 1.
Select input parameter values and processes within the Researching Effective Strategies to Prevent Opioid Death (RESPOND) model.a
| Model attributes and processes | Description | Sources |
|---|---|---|
| Cohort characteristics | Simulated population using opioids in Massachusetts | |
| Population size | 394 641 at the start of study period (beginning of 2020); increases by an average of 17 950 per year | MA DPH, NSDUH 2015–2018, Barocas et al. |
| Proportion male | 62.7% male at beginning of study period | MA DPH, NSDUH |
| Age | Mean age: 47 at beginning of model initialization | MA DPH, US Census 2010 |
| Active use | 70.5% of total population actively using at beginning of study period | NSDUH 2015 |
| Injection drug use | 25% of active users and 9% of non-active users are assumed to have a history of injection drug use at model initialization | NSDUH 2013, Hudgins et al. [44], Adams et al. [45] |
| Transitions off treatment | Users can move from non-active to active as well as active to non-active use (bidirectional). These probabilities are estimated using published data | Neaigus et al. [46], Shah et al. [47], Nosyk et al. [48] |
| Background mortality | Based on all-cause mortality data from 2014, stratified by age group and sex. Remains the same for all treatment modalities | NVSS |
| Overdose | Weekly probability of overdose function of age, sex and route of administration; 13–14% of overdoses were fatal. | MA DPH |
| Treatment | The probability of overdose under no treatment was informed by surveillance data. All MOUD decreased the probability of overdose (0.4 hazard for buprenorphrine–naltrexone, 0.75 for methadone, and 0.86 for injectable naltrexone). Overdose not possible within detoxification or a correctional setting. Higher risk of overdose for those in post-treatment | MA DPH, Morgan et al. [25], Sordo et al. [26] |
| Route of administration | Only active users had a probability of overdose. Active injection drug use had a higher probability than active non-injection drug use (varied by age and sex). Empirically observed overdose fatalities from MA PHD were used a target for model calibration | MA DPH |
| Treatment | Simulated the provision of methadone, buprenorphine–naltrexone, and injectable naltrexone | |
| Probability of initiating | Only active users not currently in treatment, detoxification or a correction setting could initiate treatment. Treatment engagement was informed by longitudinal person-level administrative data. At model initialization, 15.5% of total population is assumed to be on MOUD | MA DPH |
| Initiation effect | Upon initiating treatment, we assumed a percentage of active users immediately became non-active (i.e. remission). Methadone: 57%; community-based buprenorphine: 75%; injectable naltrexone: 90%; detoxification and correctional setting: 100% |
CTN-0051 |
| Transitions during treatment | While receiving treatment, users can move from active to non-active use (i.e. remission) or move from inactive to active use (i.e. relapse). These transition probabilities differed by treatment modality, gender and age and were estimated using a multi-state Markov model. Transitions did not occur within detoxification and correctional settings | CTN-051, CTN-027 |
| Retention | While in treatment, there was a weekly probability of discontinuing treatment. This differed by treatment modality | Morgan et al. [4] |
| Post-treatment | Upon cessation of treatment, active users remain active and a set proportion of non-active users become active (i.e. relapse). These differed by treatment modality and overdose risk is increased compared to those in treatment or in no treatment. Individuals have a 25% weekly probability of returning to the ‘no treatment’ block | Bailey et al. [49], calibrated |
| Cost-effectiveness | Impact inventory and description of methods and sources included in the Supporting information | |
| Quality adjusted life years (QALYs) | Health-related quality of life stratified by active versus non-active, injection versus non-injection, gender, age and treatment status | Wittenberg et al. [28], Murphy et al. [36] |
| Health-care utilization | Background and OUD costs (other than costs related to treatment), stratified by age, OUD state and treatment block | CTN |
| Treatment utilization | Pharmaceutical costs derived from the Federal Supply Schedule (FSS) in 2020 dollars. Treatment utilization based on expert opinion and published estimates from NIDA | FSS, NIDA, expert opinion |
| Costs of low-threshold SSP treatment | Pharmaceutical costs equivalent to standard of care buprenorphine, treatment costs identical but without a weekly nurse visit | Expert opinion (buprenorphine provider at a SSP) |
| Overdose | Cost of overdose estimated based on emergency department utilization data, costs of inpatient stays, and assumes the cost of ambulance for each overdose. Cost of a non-fatal overdose was $4557.35, cost of fatal overdose was $885.97. | Jiang et al., Murphy et al., MA DPH |
| Criminal justice involvement | Criminal justice costs, including the cost of policing, court, incarceration, and criminal victimization, stratified by OUD state and treatment block were estimated using CTN-0051 study data. Cost for those incarcerated ($1385 per week) was based on a reported estimate from Department of Corrections and was not stratified | CTN-0051, MA Department of Corrections |
CTN, Clinical Trials Network; FSS, Federal Supply Schedule; MA DPH, Massachusetts Department of Public Health; MOUD, medications for OUD; NIDA, National Institute on Drug Abuse; NSDUH, National Survey on Drug Use and Health; NVSS, National Vital Statistic System; OUD, opioid use disorder; QALY, quality-adjusted life-year; SSP, syringe service programs.
Input values and sources available in the Supporting information.
Overdose
Only the population with active use has a probability of overdose. There is a risk of overdose in every time step stratified by route of administration (injection versus non-injection), age and sex. Populations with non-injection drug use and those receiving MOUD have lower probabilities of overdose [25, 26]. The proportion with non-fatal overdose continue within the simulation.
Outpatient MOUD treatment
The model simulates the provision of MOUD to the population with active drug use. Upon initiation into MOUD treatment, a proportion shift to non-active use. While receiving MOUD, there is a weekly probability of moving from active to non-active use. There is also a probability of relapse (i.e. non-active to active use), although this is smaller than the probability of remission. The population actively using while within MOUD treatment have a probability of overdose, although this risk is lower than those not in treatment.
Individuals not currently in treatment with active use face a weekly probability of seeking acute opioid detoxification services (detoxification) or of being arrested. When individuals transition into detoxification or corrections, they immediately become non-active users and no transitions in use can occur. Because we assume no active use, overdose probability is zero.
When the simulated population discontinues an MOUD episode, it progresses to a 4-week ‘post-treatment’ state. Immediately upon leaving any of the treatment states, there is a probability of relapse. Within the post-treatment state there is an increased overdose risk for the population actively using, and that risk is temporarily higher than the overdose risk of active users not recently on MOUD [26]. After post-treatment, people move to the ‘no treatment’ health state, where they are eligible to begin treatment, move to detoxification, experience incarceration or remain untreated.
SSP MOUD
Only the population actively using opioids and not currently engaged with treatment has a probability of initiating low-threshold treatment through an SSP. Low-threshold treatment functions identically to the other treatments in the model, in that there is a probability of discontinuing active use upon entry, probabilities of relapse and remission while in treatment and a reduction in overdose risk. We model the following differences between SSP low-threshold and standard treatment: (1) lower probability of stopping active drug use immediately after initiating treatment and (2) lower probability of being lost to follow-up and entering the post-treatment state. The population in SSP treatment can transition to treatment in the community without discontinuing care or become incarcerated.
Data and parameter estimation
The primary data source for RESPOND is the Massachusetts Public Health Data Warehouse (PHD), a longitudinally linked administrative database that includes person-level data for approximately 97% of the Massachusetts population aged 11 and older for the years 2011–15. This database informed the demographic characteristics, rates of treatment-seeking and overdose rates used within RESPOND.
Opioid use and overdose
The scientific literature informed estimates for transitions between opioid use health states for those not currently in treatment (Table 1). Data from the PHD informed age, gender and OUD-status stratified overdose incidence and the proportion of opioid overdoses that are fatal.
Outpatient MOUD treatment
NIDA Clinical Trials Network studies that collected urine toxicology data informed substance use transitions while in treatment. We employed a multistate Markov model that included age and sex as covariates to estimate the weekly transition probability matrix (Table 1). We used real-world claims-based data to estimate retention in buprenorphine treatment [4].
SSP MOUD
We parameterized low-threshold treatment using data from three pilot projects offering buprenorphine within syringe service programs (SSPs) in the United States [18–20], as well as summary data on who accesses syringe services within Massachusetts from the MDPH. Table 2 summarizes differences on patient characteristics and cost between the two treatment modalities.
TABLE 2.
Key parameters for modeling low-threshold buprenorphine treatment on-site at syringe service programs (SSPs) versus standard of care buprenorphine treatment within RESPOND
| Processes | Standard of care buprenorphine treatment | Low-threshold SSP buprenorphine | Source |
|---|---|---|---|
| Patient population | 21% report recent injection drug use | 76% report recent injection drug use | MA DPH |
| Percentage actively using following initiation | 26% | 90% | CTN-051, Hood et al. [19] |
| Percentage actively using while receiving treatment | 28% | 34% | Calibrated, informed by CTN-051, CTN-027, Hood et al. [19], Bachhuber et al. [18] |
| Weekly probability of discontinuing treatment | 3% (17.6% retained at 1 year) | 1.3% (51% retained at year) | Morgan et al. [4], Bachhuber et al. [18], Stancliff et al. [20] |
| Overdose hazard reduction while on treatment | 0.4 | 0.4 | Sordo et al. [26] |
| Percentage experiencing overdose per year while on treatment | 0.10% | 0.28% | Model output resulting from input parameters |
| Costs | |||
| Health-care utilization (i.e. clinical visits) | $65.24/week | $46.03/week | Expert opinion (physicians prescribing buprenorphine in various settings, including an SSP) |
| Pharmaceutical costs (i.e. buprenorphine) | $48.71/week | $48.71/week | Federal Supply Schedule 2020 |
MA DPH = Massachusetts Department of Public Health; CTN = Clinical Trials Network
Because low-threshold buprenorphine treatment typically does not require proof of abstinence to initiate or continue treatment [27], a lower percentage (10%) of the population immediately moved from active to non-active opioid use upon initiating treatment compared to the population receiving buprenorphine within the community (74.5%). However, the percentage of people actively using illicit opioids decreased following the first week of treatment and stabilized at 34%, an estimate informed by data from pilot studies of low-threshold programs [18, 19]. The weekly probability of discontinuing treatment was higher in the standard-of-care buprenorphine compared to the low-threshold program, as informed by published estimates [4, 18, 20].
Quality of life and costs
Quality-of-life estimates were based on age- and sex-specific utilities related to opioid use and treatment status [28]. Quality-of-life estimates based on of age- and sex-specific utilities related to opioid use and treatment status [27]. Costs comprised three components: (1) costs of health-care services other than addiction care, which were stratified by age, OUD state and treatment state; (2) costs of MOUD, including both pharmaceutical costs and treatment utilization, stratified by medication type; and (3) costs related to overdose. Criminal justice costs were added for the limited societal perspective. Costs for the low-threshold program ($46.03/week for treatment utilization and $48.71/week for pharmaceutical costs) were estimated from data from a New York-based low-threshold treatment SSP program and published cost estimates associated with buprenorphine treatment in an office-based setting (Supporting information, Data S1).
Uncertainty and sensitivity analyses
We conducted sensitivity analyses to identify key drivers of the findings and evaluate the impact of uncertainty in model parameters on major findings. For the deterministic sensitivity analyses, we varied the: (1) percentage of SSP clients actively injecting (decreasing from 80 to 40%), as not all clients of SSPs inject [29]; (2) probability of discontinuing active opioid use upon initiating low-threshold treatment (increasing from 10 to 50%, as informed by transitions for individuals outside SSP treatment settings, [30, 31]); (3) probability of relapsing upon leaving low-threshold treatment (increasing from 60 to 90%, to represent a worst-case scenario where nearly everyone relapses); (4) retention in treatment for low-threshold and standard-of-care buprenorphine (ranging weekly probability of discontinuing treatment from 0.65 to 5.6%, informed by observational cohort studies and claims data [4, 18, 20]); (5) linkage of SSP clients to community-based treatment rather than SSP (a common practice); and (6) shortened time horizons. A set of sensitivity analyses running age cohorts (i.e. everyone in the cohort begins the simulation between ages 20–29 years and remains in the model until death) were run to evaluate changes in cost-effectiveness. For the probabilistic sensitivity analyses, we defined probability density functions around key model parameters (Supporting information, Data S1) and repeated the simulation 1000 times.
The RESPOND model project was reviewed by the Boston University Medical Campus Institutional Review Board and was deemed not to be human subjects research. This analysis was not preregistered and results should be considered exploratory. Recommendations issued by the ISPOR Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist guided writing of this manuscript [32, 33].
RESULTS
Model initialization
We ran the model for an 8-year ‘burn-in’ period, which allows the population to move from baseline parameters into a steady state reflecting state transition parameters and historical trends from 2012 to 2020 (calibration figures in Supporting information, Data S1). At the end of the burn-in (simulated 2020), the population size was 304 641 (Supporting information, Data S1), 70.5% of the total population was within the active use OUD health state, 23% of the cohort with active use were people with injection drug use and 62.7% were male.
Outcome measures
Assuming the current standard of care (i.e. no low-threshold programs housed within Massachusetts SSPs), the model estimated 23 051 [95% uncertainty interval (UI) = 20 578–24 616] overdoses and 1 511 613 (95% UI = 1 366 747–1 560 073) treatment initiations in Massachusetts during the 10-year study period and 3 953 485 person-years of observation with a mean discounted life-time cost of $399 002 (societal perspective) or $250 583 (health-care) per person (Table 3). Assuming the base case scenario (30% annual probability of initiating low-threshold treatment through SSPs), Massachusetts could expect 18 254 (95% UI = 17 228–20 625) overdoses and 1 640 972 (95% UI = 1608339–1 836 835) treatment initiations over 10 years with a mean discounted life-time cost of $395 390 (societal) or $249 814 (health-care) per person. SSP buprenorphine thus emerged as a dominating strategy as it both extended life expectancy and lowered cost. Total savings for Massachusetts during a 10-year period could be as high as $773 million.
TABLE 3.
Model estimated costs and outcomes of low-threshold buprenorphine treatment out of syringe service programs (SSPs) in Massachusetts, 2020–30
| Scenario | Total number of fatal opioid overdoses, 2020–30 (N) | Averted fatal overdoses compared to status quo,a % | Additional treatment initiations compared to status quo, % | Cost per person, 2019 US$ Health-care sector perspective | Cost per person, 2019 US$ Limited societal perspective | Total QALY per person | Incremental cost per person | Incremental QALY per person |
|---|---|---|---|---|---|---|---|---|
| Status quo | 23 051 | NA | NA | $250 582 | $399 002 | 9.377 | NA | NA |
| Main analyses (varying annual probability of uptake) | ||||||||
| 1% | 22 876 | 0.8% | 0.3% | $250 554 | $398 867 | 9.385 | $(135) | 0.01 |
| 5% | 22 171 | 3.8% | 1.6% | $250 441 | $398 336 | 9.415 | $(666) | 0.04 |
| 15% | 20 587 | 10.7% | 4.4% | $250 174 | $397 101 | 9.483 | $(1901) | 0.11 |
| 30% (base intervention scenario) | 18 254 | 20.8% | 8.6% | $249 813 | $395 390 | 9.580 | $(3612) | 0.20 |
| 45% | 16 832 | 27.0% | 11.5% | $249 429 | $393 867 | 9.655 | $(5135) | 0.28 |
| 60% | 16 118 | 30.1% | 13.5% | $249 053 | $392 538 | 9.712 | $(6464) | 0.33 |
| Sensitivity analyses | ||||||||
| Assume 40% (rather than 80%) of SSP clients actively inject | 19 757 | 14.3% | 8.5% | $249 487 | $395 344 | 9.541 | $(3658) | 0.16 |
| 50% discontinuation (rather than 10%) upon initiating low-threshold treatment | 18 208 | 21.0% | 8.6% | $249 785 | $395 356 | 9.581 | $(3646) | 0.20 |
| 90% relapse (rather than 83%) upon leaving low-threshold treatment | 18 411 | 20.1% | 9.1% | $249 898 | $395 382 | 9.567 | $(3620) | 0.19 |
| SSP treatment costs doubled ($92.06 versus $46.03 weekly, 30% annual uptake) | 18 254 | 20.8% | 8.6% | $251 580 | $397 156 | 9.580 | $(1846) | 0.20 |
| SSP clients connected to community-based buprenorphine rather than on-site treatment (30% annual uptake) | 20 308 | 11.9% | 17.4% | $250 381 | $397 285 | 9.487 | $(1717) | 0.11 |
| Weekly probability of discontinuing treatment (base: 1.3% for low-threshold and 3% for standard of care) | ||||||||
| 0.65% for low-threshold | 17 761 | 22.9% | 5.5% | $249 758 | $394 491 | 9.608 | $(4511) | 0.23 |
| 16 088 | 19.5% | 8.9% | $249 093 | $384 988 | 9.886 | $6806 | 0.17 | |
| 1.3% (standard of care and low-threshold equivalent)a | ||||||||
| 3% (standard of care and low-threshold equivalent) | 20 007 | 13.2% | 19.8% | $249 866 | $397 827 | 9.500 | $(1175) | 0.12 |
| 5.6% (low-threshold worse than standard of care) | 20 376 | 11.6% | 22.3% | $249 858 | $398 231 | 9.486 | $(771) | 0.11 |
| 2-year time horizona | 3338 | 16.4% | 12.8% | NA | NA | NA | NA | NA |
| 5-year time horizona | 8534 | 19.5% | 9.4% | NA | NA | NA | NA | NA |
Status quo comparisons made to the end of 10-year simulation period, except for the 2- and 5-year time horizon and improved retention for community-based buprenorphine (1.3% probability of discontinuation for both) sensitivities. Values for the 2-year time horizon for the status quo were as follows: 3993 fatal overdoses and 266 499 treatment initiations. Values for the 5-year time horizon for the status quo were as follows: 10603 fatal overdoses and 701 789 treatment initiations. Values for the 1.3% probability of discontinuing treatment for both community-based buprenorphine and low-threshold treatment were as follows: 19804 fatal overdoses and 1 307 903 treatment initiations. NA = not applicable; QALY = quality-adjusted life-year.
The base case averted 4797 (95% UI = −47 to 7387) or 20.8% of total fatal overdoses and resulted in 129 359 (95% UI = 48 266–470 089) or 8.6% additional treatment initiations compared to the status quo scenario. When ranging the annual probability of uptake, the proportion of overdoses averted ranged from 0.8% (with 1% uptake) to 30.1% (with 60% uptake). The proportion of fatal overdoses averted was lower when using a shorter time horizon.
Deterministic sensitivity analyses
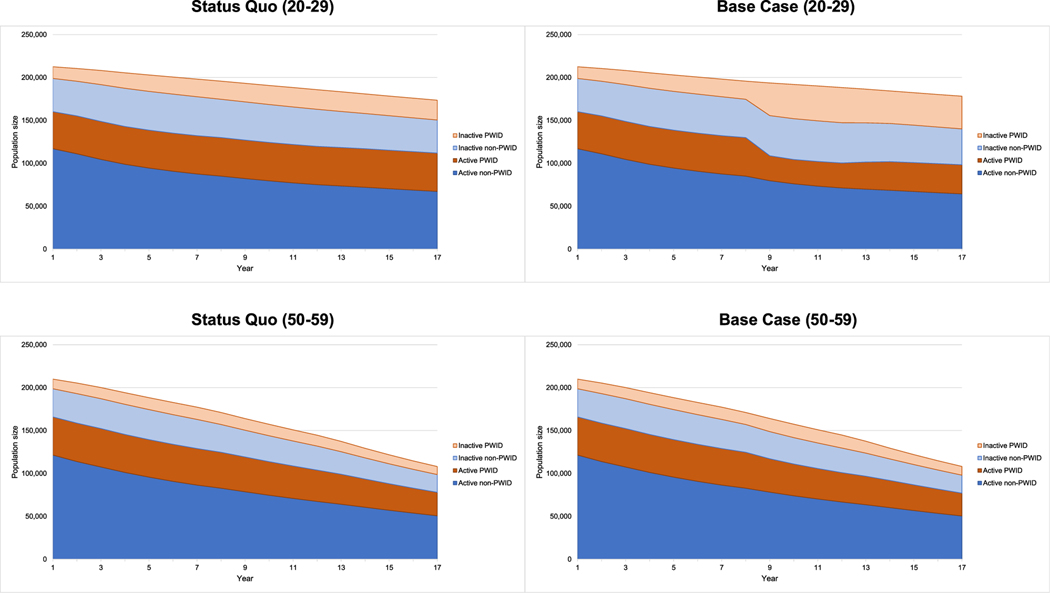
The intervention scenario remained cost saving compared to the status quo in nearly all performed sensitivity analyses (Supporting information, Data S1). One-way deterministic sensitivity analyses demonstrated that cost savings diminished with older age, worse retention within the SSP program, when SSP clients linked to standard-of-care buprenorphine rather than on-site and when standard-of-care buprenorphine retention improved (Fig. 2). Age cohort sensitivities confirmed that the shift from active to non-active drug use among individuals injecting drugs was greater among younger users (Fig. 3), which resulted in greater cost savings.
FIGURE 2.
Incremental cost per person compared to the status quo scenario for selected sensitivity analyses. SSP = syringe service program
FIGURE 3.
Population size over time with active injection drug use, inactive injection drug use, active non-injection drug use and inactive non-injection drug use in status quo compared to intervention scenario for 20–29 year-old cohort compared to the 50–59 cohort. PWID = Persons with injection drug use
Probabilistic sensitivity analyses
A probabilistic sensitivity analysis compared the status quo and the intervention scenario ranging uptake (1–45%) and retention (0.65–5.6% weekly probability of discontinuation) within the low-threshold SSP program (Supporting information, Data S1, Table S24). Within these probabilistic runs, the intervention scenario resulted in an average of 19 158 (95% UI = 17 368–21 060) overdoses and 1 697 507 (95% UI = 1 579 824–1 810 125) treatment initiations over 10 years. On average, there were 3452 (95% UI = −482 to 7248) averted overdoses over 10 years (Fig. 4).
DISCUSSION
Offering low-threshold buprenorphine treatment within all existing Massachusetts SSPs could avert more than a fifth of state-wide fatal overdoses that would otherwise occur and cost less over a life-time compared to not offering treatment at SSPs. A scenario connecting SSP clients to outside buprenorphine providers also provided substantial decreases in fatal overdose but was dominated by an on-site strategy. SSPs in Massachusetts are widely used, with more than 75 000 unique individuals visiting the 21 SSPs in a single calendar year. SSPs are often regarded as a safe place for individuals with active substance use [17], and the perceived lack of stigma could help to engage individuals who may not feel comfortable accessing treatment in other venues. A survey of individuals with opioid use accessing services at a community-based harm reduction agency found that more than half preferred to receive buprenorphine through the harm reduction agency rather than a medical clinic or drug treatment program [17]. The already substantial public health benefits associated with SSPs could be furthered by offering on-site MOUD.
Within our model, offering low-threshold buprenorphine through SSPs costs less than not offering treatment from both a health-care and societal perspective. Because the costs of active opioid use are highest for young people who inject drugs, an intervention which helps shift these individuals from active to non-active opioid use can result in cost-savings even when accounting for additional life-years and treatment costs. For example, a 24-year-old male with active injection drug use within the model is estimated to have an average cost of $38 948 per year from the modified societal perspective. Costs contributing to this estimate include hospitalizations for infective endocarditis (average of $150 671 per hospitalization) or overdose ($4557.35 per non-fatal overdose) [34–37]. If a 24-year-old male then ceases active use in buprenorphine treatment, the average cost per year is reduced to $23 843.56 per year, including cost of treatment. Cost savings diminished with older age and when retention with SSP buprenorphine worsened; however, the intervention remained cost-saving even if only 5% of patients were retained at the end of 1 year. Cost-effectiveness but not cost-savings were achieved when retention within standard-of-care buprenorphine improved. Within the model, standard-of-care buprenorphine has a low rate of retention (34% at 6 months) based on national claims-based data [4]. Improving retention within the standard-of-care buprenorphine decreased the population not currently in treatment, and there was a greater likelihood that individuals would remain in treatment outside the SSP. This suggests that expansion of SSP treatment may not be cost-saving in settings with high levels of OUD treatment access and retention but is likely to remain cost-effective and prevent overdose. It also reveals that one of the main factors driving cost-effectiveness conclusions is the advantage that SSP provides in terms of keeping patients engaged in care.
SSPs are traditionally viewed as venues to decrease the incidence of infectious diseases, such as hepatitis C virus (HCV) and HIV, and previous research has shown that participation within low-threshold treatment programs can decrease HIV-risk related behaviors, including sharing needles and drug equipment [13]. This analysis demonstrates additional potential for these programs to be a critical venue in efforts to stop the syndemic of HIV, HCV and overdose. States with limited access to SSPs and high rates of overdose should consider these additional benefits when considering the opening of SSP locations.
These results suggest that if SSPs were to adopt this model (and state and local governments were to endorse it), they would need to ensure adequate resources including staffing to meet demand. A qualitative study reported that requests for drug treatment was the most frequent referral for SSP clients [38]. However, research is needed into what percentage of people accessing SSPs would initiate treatment, if offered. A 2015 study found that a quarter of syringe exchange participants were not interested in receiving buprenorphine treatment [17]. Ensuring that the barriers to starting treatment are lowered can help to engage these individuals when they decide that treatment might be right for them. A recent survey of Massachusetts SSPs found that half currently have some linkage to a buprenorphine provider and five programs were planning on a program to link to an off-site provider. Loss to follow-up with referrals to outside provider may be a challenge [39, 40]. However, recent changes resulting from the COVID-19 pandemic permit tele-buprenorphine initiation and prescribing which may decrease loss to follow-up and several SSPs in Massachusetts now have on-demand tele-health access to a buprenorphine prescriber [41]. These changes will probably impact treatment uptake as well as retention.
This analysis has limitations. Like all models, it is a simplification of a complex disease process. As such, we do not capture all the elements that impact the risk of overdose (e.g. polysubstance use, fentanyl within a community) or retention in treatment (e.g. poverty level, geographic access to treatment) [42]. Cost-effectiveness studies assume that SSP programs are in steady-state and do not typically include start-up costs. These costs can range from $6400 for a small rural program to $15 200 for a large urban program [43]. We also assumed that costs were limited to the cost of medication and treatment. We did not account for additional costs such as a mobile van or additional staff. While on-site SSP treatment is cost-saving from a societal and healthcare perspective, SSP programs may not be able to afford an on-site program. However, sensitivity analyses that ranged the cost of delivering buprenorphine in SSPs demonstrate a substantial cost cushion, suggesting that dedicated funding should be allocated to off-set the budgetary impact to the SSP to achieve cost-savings for society. Another limitation is the use of clinical trial data to inform transition probabilities. Clinical trials may not be generalizable to all clinical settings. Within the analysis, we assume that MOUD have the same efficacy in the real world as within a clinical trial, conditional on retention in treatment.
This study has several strengths, such as the use of Massachusetts-specific data on SSPs and individuals with OUD, parameters informed by studies of existing low-threshold SSP programs and use of clinical trial data to simulate transitions in opioid use. A validated mathematical model, RESPOND, can account for opioid use while in treatment, the remitting–relapsing nature of OUD and movement into correctional settings and detoxification. We performed extensive sensitivity analyses, including probabilistic sensitivities, to test the bounds of the estimated impact.
In conclusion, SSPs represent an underutilized venue for treatment engagement. Offering on-site buprenorphine treatment at SSPs has the potential to significantly decrease fatal overdoses, improve treatment engagement state-wide and save on costs. These considerations should help to guide policy decisions regarding the opening and expansion of SSPs to address the opioid epidemic.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Massachusetts’ opioid overdose education and naloxone distribution (OEND) and harm reduction programs as well as the clients they serve for additional context in interpreting study findings. In addition, we are grateful to Laura de Mondesert, MaryKate Duska, Liisa Randall, Brittni Reilly, Sarah Ruiz and the Bureau of Substance Addiction Services OEND team at the Massachusetts Department of Public Health. This work was supported by the National Institute on Drug Abuse (5R01DA04652703, P30DA040500) and the National Institute of Allergy and Infectious Diseases (T32-AI052074).
Funding information
National Institute of Allergy and Infectious Diseases, Grant/Award Number: T32-AI052074; National Institute on Drug Abuse, Grant/Award Numbers: 5R01DA046527-03, P30DA040500
Footnotes
DECLARATION ON INTERESTS
None.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- 1.National Institute on Drug Abuse Overdose Death Rates 2021. Available at: https://www.drugabuse.gov/drug-topics/trends-statistics/overdose-death-rates (Accessed March 25, 2022).
- 2.Wakeman SE, Larochelle MR, Ameli O, Chaisson CE, McPheeters JT, Crown WH, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3: e1920622-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Health Care for the Homeless Council. Medication-Assisted Treatment: Buprenorphine in the HCH Community. Nashville, TN: National Health Care for the Homeless Council; 2016. [Google Scholar]
- 4.Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J Subst Abuse Treat. 2018;85:90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakubowski A, Fox A. Defining low-threshold buprenorphine treatment. J Addict Med. 2020;14:95–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walley AY, Alperen JK, Cheng DM, Botticelli M, Castro-Donlan C, Samet JH, et al. Office-based management of opioid dependence with buprenorphine: clinical practices and barriers. J Gen Intern Med. 2008;23:1393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huhn AS, Dunn KE. Why aren’t physicians prescribing more buprenorphine? J Subst Abuse Treat. 2017;78:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin SA, Chiodo LM, Bosse JD, Wilson A. The next stage of buprenorphine care for opioid use disorder. Ann Intern Med. 2018; 169:628–35. [DOI] [PubMed] [Google Scholar]
- 9.Nolan S, Hayashi K, Milloy MJ, Kerr T, Dong H, Lima VD, et al. The impact of low-threshold methadone maintenance treatment on mortality in a Canadian setting. Drug Alcohol Depend. 2015;156: 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langendam MW, van Brussel GH, Coutinho RA, van Ameijden EJ. The impact of harm-reduction-based methadone treatment on mortality among heroin users. Am J Public Health. 2001;91:774–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Den Berg C, Smit C, Van Brussel G, Coutinho R, Prins M. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam cohort studies among drug users. Addiction. 2007;102:1454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahamad K, Hayashi K, Nguyen P, Dobrer S, Kerr T, Schütz CG, et al. Effect of low-threshold methadone maintenance therapy for people who inject drugs on HIV incidence in Vancouver, BC, Canada: an observational cohort study. Lancet HIV. 2015;2:e445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millson P, Challacombe L, Villeneuve PJ, Strike CJ, Fischer B, Myers T, et al. Reduction in injection-related HIV risk after 6 months in a low-threshold methadone treatment program. AIDS Educ Prev. 2007;19:124–36. [DOI] [PubMed] [Google Scholar]
- 14.Fatseas M, Auriacombe M. Why buprenorphine is so successful in treating opiate addiction in France. Curr Psychiatry Rep. 2007;9: 358–64. [DOI] [PubMed] [Google Scholar]
- 15.Carrieri MP, Spire B. Harm reduction and control of HIV in IDUs in France. Lancet. 2008;372:448. [DOI] [PubMed] [Google Scholar]
- 16.Behrends CN, Nugent AV, Des Jarlais DC, Frimpong JA, Perlman DC, Schackman BR. Availability of HIV and HCV on-site testing and treatment at syringe service programs in the United States. J Acquir Immune Defic Syndr 2018. 79:e76–8. [DOI] [PMC free article] [PubMed]
- 17.Fox AD, Chamberlain A, Frost T, Cunningham CO. Harm reduction agencies as a potential site for buprenorphine treatment. Subst Abuse. 2015;36:155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bachhuber MA, Thompson C, Prybylowski A, Benitez J, Mazzella S, Barclay D. Description and outcomes of a buprenorphine maintenance treatment program integrated within prevention point Philadelphia, an urban syringe exchange program. Subst Abuse. 2018;39: 167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hood JE, Banta-Green CJ, Duchin JS, Breuner J, Dell W, Finegood B, et al. Engaging an unstably housed population with low-barrier buprenorphine treatment at a syringe services program: lessons learned from Seattle, Washington. Subst Abuse. 2020;41:356–64. [DOI] [PubMed] [Google Scholar]
- 20.Stancliff S, Joseph H, Fong C, Furst T, Comer SD, Roux P. Opioid maintenance treatment as a harm reduction tool for opioid-dependent individuals in New York City: the need to expand access to buprenorphine/naloxone in marginalized populations. J Addict Dis. 2012;31:278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54:901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krebs E, Urada D, Evans E, Huang D, Hser YI, Nosyk B. The costs of crime during and after publicly funded treatment for opioid use disorders: a population-level study for the state of California. Addiction. 2017;112:838–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost Effectiveness in Health and Medicine. 2nd ed. Oxford/New York: Oxford University Press; 2017. [Google Scholar]
- 24.Linas BP, Savinkina A, Madushani R, Wang J, Yazdi GE, Chatterjee A, et al. Projected estimates of opioid mortality after community-level interventions. JAMA Netw Open. 2021;4:e2037259-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan JR, Schackman BR, Weinstein ZM, Walley AY, Linas BP. Overdose following initiation of naltrexone and buprenorphine medication treatment for opioid use disorder in a United States commercially insured cohort. Drug Alcohol Depend. 2019;200:34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017; 357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kourounis G, Richards BDW, Kyprianou E, Symeonidou E, Malliori M-M, Samartzis L. Opioid substitution therapy: lowering the treatment thresholds. Drug Alcohol Depend. 2016;161:1–8. [DOI] [PubMed] [Google Scholar]
- 28.Wittenberg E, Bray JW, Aden B, Gebremariam A, Nosyk B, Schackman BR. Measuring benefits of opioid misuse treatment for economic evaluation: health-related quality of life of opioid-dependent individuals and their spouses as assessed by a sample of the US population. Addiction. 2016;111:675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox AD, Chamberlain A, Sohler NL, Frost T, Cunningham CO. Illicit buprenorphine use, interest in and access to buprenorphine treatment among syringe exchange participants. J Subst Abuse Treat. 2015;48:112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JD, Nunes EV Jr, Novo P, Bachrach K, Bailey GL, Bhatt S, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X: BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018; 391:309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saxon AJ, Ling W, Hillhouse M, Thomas C, Hasson A, Ang A, et al. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug Alcohol Depend. 2013; 128:71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Eur J Health Econ. 2013; 14:367–72. [DOI] [PubMed] [Google Scholar]
- 33.Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMC Med. 2022;20:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller AC, Polgreen PM. Many opportunities to record, diagnose, or treat injection drug-related infections are missed: a population-based cohort study of inpatient and emergency department settings. Clin Infect Dis. 2019;68:1166–75. [DOI] [PubMed] [Google Scholar]
- 35.Jiang Y, McDonald JV, Koziol J, McCormick M, Viner-Brown S, Alexander-Scott N. Can emergency department, hospital discharge, and death data be used to monitor burden of drug overdose in Rhode Island? J Public Health Manag Pract. 2017;23:499–506. [DOI] [PubMed] [Google Scholar]
- 36.Murphy SM, McCollister KE, Leff JA, Yang X, Jeng PJ, Lee JD, et al. Cost-effectiveness of buprenorphine–naloxone versus extended-release naltrexone to prevent opioid relapse. Ann Intern Med. 2018; 170:90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coffin PO, Sullivan SD. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med. 2013;158: 1–9. [DOI] [PubMed] [Google Scholar]
- 38.Stopka TJ, Hutcheson M, Donahue A. Access to healthcare insurance and healthcare services among syringe exchange program clients in Massachusetts: qualitative findings from health navigators with the iDU (‘I do’) care collaborative. Harm Reduct J. 2017;14:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kidorf M, Disney E, King V, Kolodner K, Beilenson P, Brooner RK. Challenges in motivating treatment enrollment in community syringe exchange participants. J Urban Health. 2005;82:456–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox AD, Sohler NL, Frost T, Lopez C, Cunningham CO. Development and evaluation of a community-based buprenorphine treatment intervention. Harm Reduct J. 2017;14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris M, Johnson S, Mackin S, Saitz R, Walley AY, Taylor JL. Low barrier tele-buprenorphine in the time of COVID-19: a case report. Addict Med. 2020;14:e136–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glick SN, Prohaska SM, LaKosky PA, Juarez AM, Corcorran MA, Des Jarlais DC. The impact of COVID-19 on syringe services programs in the United States. AIDS Behav. 2020;24:2466–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teshale EH, Asher A, Aslam MV, Augustine R, Duncan E, Rose-Wood A, et al. Estimated cost of comprehensive syringe service program in the United States. PLOS ONE. 2019;14: e0216205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hudgins R, McCusker J, Stoddard A. Cocaine use and risky injection and sexual behaviors. Drug Alcohol Depend. 1995;37:7–14. [DOI] [PubMed] [Google Scholar]
- 45.Adams ML, Wejnert C, Finlayson T, Xia M & Paz-Bailey G HIV Infection, risk, prevention, and testing behaviors among persons who inject drugs: National HIV Behavioral Surveillance: injection drug use, 20 US cities, 2015. HIV Surveillance Special Report 18, revised edn. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (Accessed September 30, 2021). [Google Scholar]
- 46.Neaigus A, Gyarmathy VA, Miller M, Frajzyngier VM, Friedman SR, Des Jarlais DC. Transitions to injecting drug use among noninjecting heroin users: social network influence and individual susceptibility. J Acquir Immune Defic Syndr. 2006;41:493–503. [DOI] [PubMed] [Google Scholar]
- 47.Shah NG, Galai N, Celentano DD, Vlahov D, Strathdee SA. Longitudinal predictors of injection cessation and subsequent relapse among a cohort of injection drug users in Baltimore, MD, 1988–2000. Drug Alcohol Depend. 2006;83:147–56. [DOI] [PubMed] [Google Scholar]
- 48.Nosyk B, Li L, Evans E, Huang D, Min J, Kerr T, et al. Characterizing longitudinal health state transitions among heroin, cocaine, and methamphetamine users. Drug Alcohol Depend. 2014;140: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bailey GL, Herman DS, Stein MD. Perceived relapse risk and desire for medication assisted treatment among persons seeking inpatient opiate detoxification. J Subst Abuse Treat. 2013;45: 302–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.