Abstract
An epidemiological analysis assessing beverage consumption and risk factors of cardiovascular disease was conducted. Participants were 9–16 years old at enrollment, completed food frequency questionnaires in 1996–2001 and self-reported outcomes in 2010–2014. Exclusion criteria included missing data on relevant variables and covariates, prevalent disease before 2005, and implausible/extreme weight or energy intake. Intakes of orange juice, apple/other fruit juice, sugar-sweetened beverages and diet soda were related to risk of incident hypertension or hyperlipidemia using Cox proportional hazards regression, adjusting for diet, energy intake, age, smoking, physical activity and body mass index. There were 9,043 participants with 618 cases of hypertension and 850 of hyperlipidemia in 17 years mean follow-up. Sugar-sweetened beverage intake but not fruit juice nor diet soda was associated with hypertension (hazard ratio (95% confidence interval): 1.16 (1.03, 1.31)) in males. This study can guide beverage consumption as it relates to early predictors of cardiovascular disease.
Keywords: juice, sugar-sweetened beverage, diet soda, hypertension, hyperlipidemia, adolescent
Introduction
As the leading cause of death in the US, cardiovascular disease is a serious public health threat (Centers for Disease Control and Prevention 2018). Although it disproportionately affects older adults (Mozaffarian et al. 2015), early symptoms such as elevated blood pressure (hypertension) and lipids (hyperlipidemia), both of which are closely associated with the progression of cardiovascular disease (Freiberg et al. 2008; Di Angelantonio et al. 2010; Benjamin et al. 2019), can begin to appear much earlier in age. Therefore, early management of these risk factors is crucial in preventing the onset of cardiovascular events that may otherwise occur years or decades later.
Excessive added sugar intake increases the risk of obesity (Stanhope 2016) and cardiovascular disease (Vos et al. 2017) through caloric imbalance. Additionally, there is evidence suggesting that fructose may be particularly deleterious in the progression of cardiometabolic abnormalities (Stanhope et al. 2015; Muth et al. 2019). Fructose is the monosaccharide most prevalent in high-fructose corn syrup, a sweetener that is common in sugar-sweetened beverages (SSBs), but fructose is also found naturally in food such as fruits. SSBs are the largest source of added sugar in the American diet (U.S. Department of Agriculture and U.S. Department of Health and Human Services 2020), and several studies in adults (Fung et al. 2009; Pacheco et al. 2020) reported an unfavorable association between SSB intake and various forms of cardiovascular disease. Additionally, while 100% fruit juice contains no added sugar, its total carbohydrate content is similar to that of many SSBs, and some researchers have reported that excess fruit juice consumption contributes unfavorably to childhood obesity in young children (Dennison et al. 1999; Libuda et al. 2008; Sonneville et al. 2015; Shefferly et al. 2016). However, studies assessing cardiovascular disease or its biomarkers as the outcome report equivocal findings (Joshipura et al. 1999; Freedman et al. 2001; Park et al. 2004; Hung et al. 2004; Morand et al. 2011). Notably, there may be a differential effect by juice type on the outcome, as the flavonoids (primarily naringenin and hesperidin) and vitamin C in citrus juices are posited to offer additional cardioprotective effects through increased antioxidant activity (Morand et al. 2011; Wang et al. 2011; Ke et al. 2015).
Over 54% of 6–11 year-olds and 58% of 12–19 year-olds consume SSB, making them the largest consumer age group (Martin et al.). Comparatively, 40–44% of adults consume SSB (Martin et al.). Among SSB consumers, 6–11 year-olds drink 14 fl oz. and 12–19 year-olds drink 19 fl oz. daily on average (Martin et al.). Overall, fruit juice intakes in children have declined over the past several years (Bowman et al.), with 28% of 6–11-year-olds and 16% of 12–19-year-olds consuming 100% fruit juice (Martin et al.). Although, despite fewer older children consuming fruit juice, their mean daily intake is higher than 6–11-year-olds’ (12 vs 8 fl oz.) (Martin et al.). Consumption of diet soda in this age group is relatively low (approximately 6% (Bleich et al. 2018)) but is often used as an alternative to SSB or other sweetened beverages to reduce calorie intake and obesity risk (Fitch and Keim 2012). The Dietary Guidelines 2020–2025 recommend limiting 100% fruit juice intake to fewer than 4–10 fl oz. per day (contingent on estimated energy requirements) and added sugar intake to fewer than 10% of one’s total energy intake starting at age 2.
Children are among the largest consumers of sweet beverages such as fruit juice and SSB, yet there is a paucity of longitudinal studies investigating the association between their consumption at an early age and the early development of cardiovascular disease. Given the immense threat cardiovascular disease imposes on public health, it is essential to understand whether early consumption of these beverages is related to progression of cardiovascular disease. Thus, the objective of this study was to determine the association between fruit juice, SSB and diet soda intakes in preadolescent and adolescent years and risk of incident hypertension and hyperlipidemia in young adult years.
Methods
Study population
The Growing Up Today Study (GUTS) is an ongoing longitudinal study of participants who are offspring of Nurses’ Health Study II participants (Bao et al. 2016). Enrollment for GUTS began in 1996 when prospective participants, aged 9–16 years, were invited to complete a survey detailing anthropometric, dietary, physical activity, heath and sociological information. The participant’s mother provided informed consent and completion of the initial survey was considered assent. Enrolled participants were invited to complete follow-up surveys in 1997, 1998, 2001, 2003, 2005, 2007, 2010, 2013 and 2014, when participants were 27–34 years old. Participants were excluded from this study for missing data on relevant dietary variables (n=316), body mass index (BMI, n=255), physical activity (n=10), age (n=5), race/ethnicity (n=104), smoking (n=145), or invalid outcome data (prevalent hypertension or hyperlipidemia prior to 2005 or missing incidence interval, n=383). Participants were also excluded for implausible/extreme BMI z-score (<−3 or >3, n=130) or total energy intake (<500 or >5,000 kcal/day, n=0) (Berkey et al. 2004). Additionally, 6,428 participants were lost to follow-up (did not return questionnaire in 2010, 2013 or 2014). Out of 16,819 participants enrolled at baseline, 9,043 participants (3,264 males and 5,779 females) were included in the final analytical cohort (Figure 1). This study was approved by the Institutional Review Board at Brigham and Women’s Hospital. The return of each questionnaire was considered as informed consent.
Figure 1.
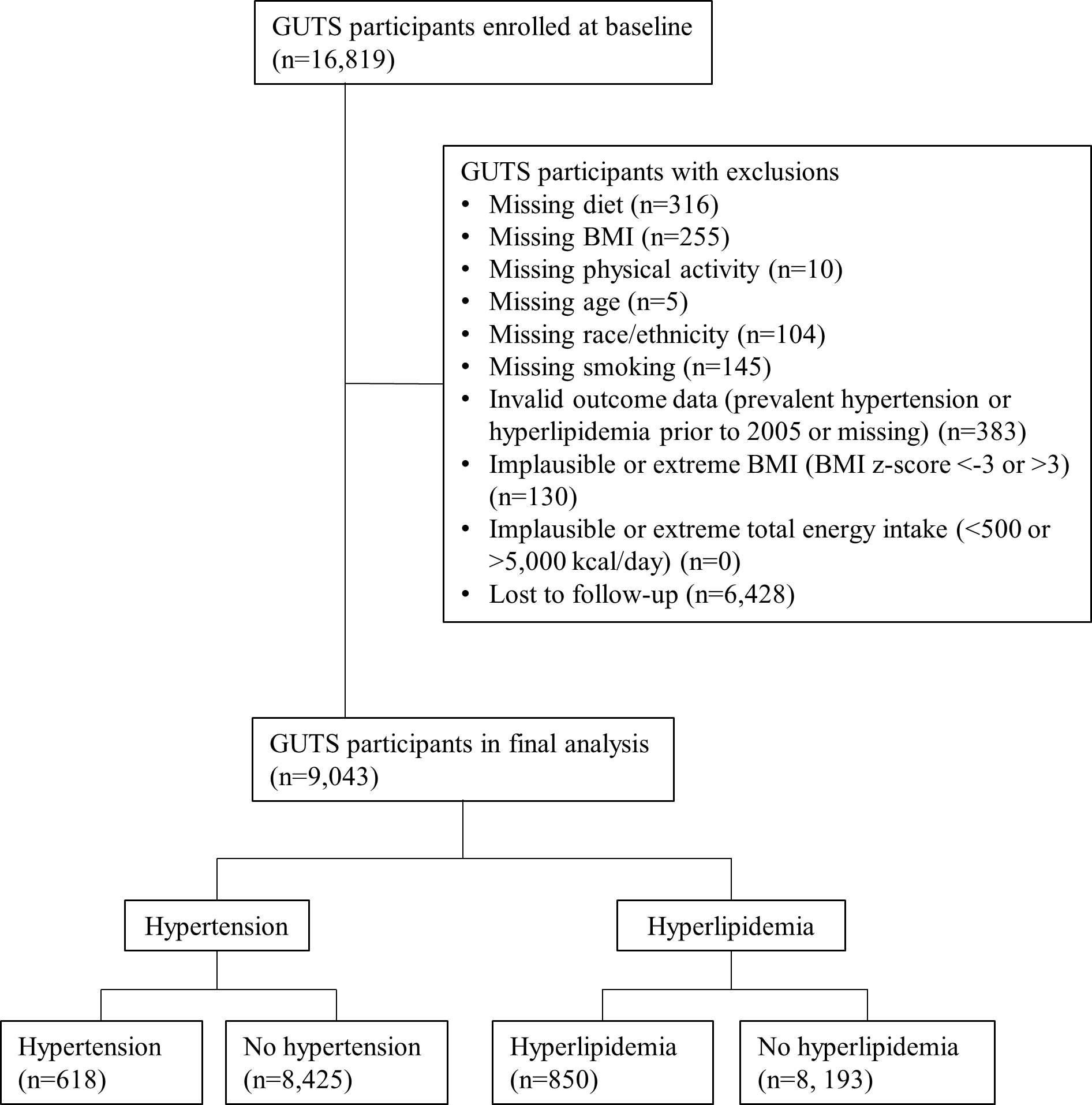
Study flow chart.
Dietary assessment
Semi-quantitative food frequency questionnaires (FFQ) were administered in 1996, 1997, 1998 and 2001. Dietary data were measured using cumulatively updated average intake, wherein all available data are averaged, and the most recent available data is used in the event of missing data (Liao et al. 2011). The FFQ asked participants to quantify their dietary intake over the past 12 months and has been validated against three 24-hour recalls in adolescents with moderate validity (Pearson correlation coefficient = 0.54) (Rockett et al. 1997). Fruit juice was defined as the sum of orange juice and apple juice/other fruit juice. SSB was defined as a sum of soda (not diet) and Hawaiian Punch, lemonade, Koolaid/other non-carbonated fruit drink. Importantly, the appearance of this “other non-carbonated fruit drink” option distinguishes fruit-flavored beverages with added sugar from 100% fruit juice, as is intended for orange and apple/other fruit juices. Sports drinks and tea (hot or iced) were not included in our definition of SSB because the FFQ did not indicate whether or not these were sweetened with sugar. Diet soda intake was estimated using the sole question referring to diet soda intake.
The response options for beverages in the FFQ were categorical and specified portion sizes were “1 can or glass” (SSB and diet soda) or “1 glass” (fruit juice). These categorical intake values were converted to continuous (servings per day) for this study. Response options varied for each food/beverage, and when a range of intakes was specified, the midpoint was used (e.g. “1–3 glasses per month” was considered to be 2 glasses per month and subsequently .067 glasses per day). In cases of other ambiguous response options, a conservative estimate was used. For example, “Never/less than 1 per month” was considered as 0 daily servings, and “≥2 glasses per day” was considered as 3 glasses per day.
Hypertension and hyperlipidemia assessment
The primary outcomes of interest were incidence of hypertension and hyperlipidemia. In 2010, 2013 and 2014, surveys included opportunities to self-report the presence of health conditions along with year of first diagnosis presented as intervals. Specifically, the question asked, “Have you ever been told by a healthcare provider that you have any of the following illnesses?” Participants were instructed to either answer “yes” and follow up by indicating the year of first diagnosis or skip the question to indicate a negative response. In 2010, the response options for the year of first diagnosis included “before 1996,” “1996–1999,” “2000–2004,” “2005–2009,” and “2010+.” In 2013, the response options included, “before 2006,” “2006–2008,” “2009–2011,”and “2012+.” In 2014, the response options included, “before 2009,” “2009–2013,” and “2014+.” The primary outcomes of interest were hypertension and hyperlipidemia. Hypertension was defined by the question, “Hypertension (high blood pressure)” and hyperlipidemia was defined by the questions, “High cholesterol, triglycerides or lipids (2010 and 2013)” and “High cholesterol (2014).” To reduce the potential effect of reverse causation, a 4-year lag time from the end of dietary data assessment was used by excluding participants with prevalent disease prior to 2005.
Covariate assessment
Participants self-reported age (birthday) and race/ethnicity in the 1996 survey, and height, weight, smoking and physical activity in the 1996, 1997, 1998 and 2001 surveys. Instructions were provided for measuring one’s own weight and height. Self-reported height and weight have previously been validated in this age group (Strauss 1999; Goodman et al. 2000; Elgar et al. 2005). These measurements were used to calculate BMI. Using pediatric BMI growth charts from the Centers for Disease Control and Prevention (Kuczmarski et al. 2002), BMI was then normalized to age and sex to determine BMI percentile, BMI z-score and weight status. BMI z-score was used as a covariate since childhood growth is non-linear and therefore BMI must be standardized to age and sex. For smoking status, the last observation was carried forward to fill in 1999 and 2000. Participants were considered to have smoked in the past year if they responded positively to the question, “In the past 12 months, have you smoked a cigarette (1997, 1998, 2001 surveys)” or “Have you ever smoked a cigarette (1996 survey).” Total energy intake was calculated by summing the energy intakes of individual foods and beverages in the FFQ, and averaged across all available FFQs (cumulatively updated average).
Physical activity was calculated using responses from the physical activity questionnaire (1996, 1997, 1998 and 2001) wherein participants indicated the amount of time spent engaged in a wide range of physical activities such as sports, running, weight lifting, manual labor or gardening. The amount of time spent engaged in each activity was multiplied by its metabolic equivalent of task (MET) score and summed. Only activities of at least moderate intensity (MET ≥4) were included in this analysis (Ainsworth et al. 2011).
We used principal components analysis with 23 food groups contained within the FFQ (excluding beverages) in order to capture dietary patterns in the population, and used these to account for dietary behaviors that correlated with beverage intake patterns. The factor loading coefficient for each food group reflects an estimate of the adherence to the dietary pattern; i.e., the higher the coefficient the greater the intake of that dietary pattern. Factors with an eigenvalue of ≥1.0 were first considered for the analysis (Schmitt 2011) (six factors). Then, those factors that explained >=5.0% variance of intake were kept (four factors).
Statistical analysis
To assess risk of incident hypertension or hyperlipidemia by beverage intake, a Cox proportional hazards regression model was used to fit interval-censored incident hypertension- and hyperlipidemia-related event data (Heller 2011). This model is appropriate for estimating the degree of risk of multiple risk factors, such as the competing beverages fruit juice, SSB and diet soda, simultaneously. Participants were followed up from the baseline questionnaire until the year of first reported diagnosis of hypertension or hyperlipidemia, death, or end of study follow-up (2014), whichever came first. Hypertension and hyperlipidemia were treated as separate outcomes; therefore, in instances where participants reported being diagnosed with both diseases, person-time was calculated independently for each disease. The explanatory variable was cumulatively updated average intake of each beverage. The proportional hazards assumption was checked via martingale residuals. A sensitivity analysis after removing these outliers was carried out and the partial maximum likelihood estimates of the regression coefficients were similar. The covariates included all three beverage types, total energy intake, age, smoking, physical activity, BMI z-score, and diet patterns. An inverse probability weighted approach was used to account for loss-to-follow-up (Mansournia 2016). Physical activity and total energy intake were log-transformed due to non-normal distributions. Results were stratified by outcome and gender. We also conducted a subgroup analysis (a priori) with fruit juice type due to prior literature suggesting a potential benefit of citrus juice (Dennison et al. 1997; Morand et al. 2011; Rampersaud and Valim 2017; Sakaki et al. 2021). Confidence interval (CI) was used to determine statistical significance: an HR value with a 95% CI that does not contain the null value of 1.00 (Attia 2005; du Prel et al. 2009). Statistical analyses were conducted using SAS® software, version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Baseline characteristics
The majority (>96%) of participants were non-Hispanic white (Table 1). The average baseline age was approximately 12 years old and the mean BMI z-score indicated normal weight status. Fewer than 10% of participants reported having smoked a cigarette in their lifetime. The consumption of SSB was the highest among the three beverages, with fruit juice following second. The proportions of orange juice and apple/other fruit juice consumed were relatively similar. A sensitivity test was conducted to compare differences in baseline characteristics of participants who were excluded vs. those who remained in the analysis. Overall, males were more likely to be excluded than females. Excluded participants had a higher prevalence of smoking, consumed more SSB, consumed less orange juice (males only), and engaged in more physical activity.
Table 1.
Baseline characteristics of Growing Up Today Study participants (n=9,043)
| Males (n=3,264) | Females (n=5,779) | |||
|---|---|---|---|---|
|
|
|
|||
| Mean (%) | SD (n) | Mean (%) | SD (n) | |
|
| ||||
| Non-Hispanic white, % | (96.2) | (3139) | (96.2) | (5599) |
| Past-year smoking, % | (9.5) | (311) | (9.2) | (530) |
| Age, y | 11.95 | 1.60 | 12.06 | 1.60 |
| BMIa z-score | 0.24 | 1.01 | 0.10 | 0.98 |
| Fruit juice, servings/dayb | 0.87 | 0.84 | 0.81 | 0.80 |
| Orange juice, servings/dayb | 0.47 | 0.54 | 0.40 | 0.50 |
| Apple/other juice, servings/dayb | 0.41 | 0.52 | 0.41 | 0.51 |
| SSBc, servings/dayd | 1.19 | 1.09 | 0.98 | 1.05 |
| Diet soda, servings/dayd | 0.16 | 0.45 | 0.17 | 0.44 |
| Total energy intake, kcal/day | 2,291 | 704 | 2,047 | 634 |
| Physical activityd, MET-hrs/week | 138.78 | 81.66 | 109.58 | 72.21 |
BMI, body mass index.
Serving size: “1 can or glass.”
SSB, sugar-sweetened beverage.
Serving size: “1 glass.”
Physical activity was calculated by summing the hours per week spent participating in various sports, games and intentional exercise of moderate or vigorous intensity (MET ≥ 4) and multiplying by the MET score. Median (IQR): Males: 124.54 (101.56), Females: 95.48 (87.85).
Principal components analysis of diet patterns
Four distinct diet patterns emerged from the principal components analysis: diets high in plant-based food (factor 1), sweet and salty food (factor 2), meat (factor 3), and starch and dairy (factor 4) (Supplemental Table 1). Together, these four diet patterns explained 40.3% of the variance in total dietary intake. Fruit juice consumption was associated with higher intakes of all four groups. SSB intake was positively associated with intakes of sweet and salty food and meat; and in girls only was negatively associated with starch and dairy intake. Diet soda intake was negatively associated with starch and dairy intake among both sexes; and in girls only was also positively associated with intakes of a plant-based diet, sweet and salty food, and meat.
Beverage intake and risk of hypertension or hyperlipidemia
The results of the Cox proportional hazards regression are displayed in Table 2. The mean ± SD follow-up time among all male participants was 17.3 ± 1.4 years and among all female participants was 17.6 ± 1.7 years. There were 618 hypertension cases and 850 hyperlipidemia cases in total. In males, the risk of incident hypertension increased with SSB intake (hazard ratio (HR) and 95% CI: 1.16 (1.03, 1.31)) after adjusting for diet patterns, total energy intake, age, smoking, physical activity and BMI z-score. Intake of fruit juice was not significantly associated with either hypertension or hyperlipidemia risk. Diet soda intake was positively associated with hypertension in both males and females in the crude model, but was no longer statistically significant after adjusting for covariates. Similarly, diet soda was associated with hyperlipidemia risk in males in the crude model but was not statistically significant in the adjusted model. Additionally, there was no association between fruit juice subgroup (orange juice and apple/other fruit juice) and hypertension or hyperlipidemia risk (Supplemental Table 2).
Table 2.
Cox multivariable regression for hazard ratios (95% confidence interval) of hypertension and hyperlipidemia in relation to beverage intakea in Growing Up Today Study participants (n=9,043, 618 hypertension cases and 850 hyperlipidemia cases)
| Males (n=3,264) | Females (n=5,779) | |
|---|---|---|
|
| ||
| Hypertension | ||
| Crude model | ||
| Fruit juice | 1.07 (0.93, 1.23) | 0.88 (0.73, 1.07) |
| SSB | 1.16 (1.05, 1.28) | 1.20 (1.05, 1.37) |
| Diet soda | 1.46 (1.22, 1.76) | 1.47 (1.20, 1.79) |
| Adjusted modelb | ||
| Fruit juice | 1.12 (0.95, 1.31) | 0.91 (0.74, 1.11) |
| SSB | 1.16 (1.03, 1.31) | 1.10 (0.95, 1.28) |
| Diet soda | 1.21 (0.98, 1.50) | 1.09 (0.87, 1.37) |
|
| ||
| Hyperlipidemia | ||
| Crude model | ||
| Fruit juice | 0.99 (0.85, 1.14) | 0.90 (0.78, 1.05) |
| SSB | 1.14 (1.03, 1.27) | 1.00 (0.90, 1.12) |
| Diet soda | 1.27 (1.00, 1.61) | 1.08 (0.89, 1.31) |
| Adjusted modelb | ||
| Fruit juice | 0.97 (0.82, 1.15) | 0.94 (0.80, 1.11) |
| SSB | 1.10 (0.98, 1.24) | 0.97 (0.85, 1.11) |
| Diet soda | 1.16 (0.90, 1.49) | 1.02 (0.83, 1.24) |
Serving size was defined as “1 can or glass” for fruit juice and “1 glass” for SSB and diet soda daily.
Adjusted for diet patterns, total energy intake (log-transformed), age, smoking, physical activity (log-transformed) and BMI z-score.
SSB, sugar-sweetened beverage.
Discussion
In this longitudinal cohort analysis, 9,043 children and adolescents without hypertension or hyperlipidemia at baseline were followed for a maximum of 18 years, into early adulthood, in order to evaluate the relationship between early adolescent beverage intake and risk of incident hypertension and hyperlipidemia in early adulthood. Among males only, intakes of SSB and diet soda were determined to be risk factors for incident hypertension, and fruit juice consumption was not associated with risk of either disease.
A recent meta-analysis (Kim and Je 2016) of six longitudinal cohort studies of adults similarly determined that SSB intake was associated with risk of incident hypertension, and with a magnitude of risk comparable to that in our study (pooled relative risk comparing highest to lowest categories of intake: 1.12 (95% CI: 1.07, 1.17)). Fructose, a sugar available in free form or as a disaccharide in the form of sucrose or high-fructose corn syrup, may underlie hypertension pathology. Fructose induces depletion of phosphate in the liver (Lustig 2010), increasing uric acid production (Choi et al. 2010), and is correlated with high serum uric acid (Nguyen et al. 2009). Uric acid is involved in the pathology of hypertension as it inhibits endothelial nitric oxide synthase production—reducing nitric oxide synthesis—induces renal inflammation and arteriosclerosis, and activates the renin-angiotensin-aldosterone system (Feig et al. 2006; Lustig 2010). Through these mechanisms, it is posited that hyperuricemia is pathogenically involved in the development of early onset hypertension (Feig 2012; Mallat et al. 2016), and in children and adolescents it predicts 89% of essential hypertension cases (Feig and Johnson 2003). Indeed, adolescents consuming SSBs tend to have higher serum uric acid (Shih et al. 2020), likely explaining the elevated blood pressure. Also, we cannot entirely rule out the possibility that residual confounding regarding undesirable dietary or lifestyle behaviors that are correlated with SSB consumption, such as poor diet quality (Doherty et al. 2021), persisted. However, we attempted to account for these factors by adjusting for key potential confounders including total energy intake, dietary patterns, smoking, physical activity and BMI.
The observation that hypertension risk was elevated only among males and not females was surprising given that the aforementioned mechanism regarding fructose and hypertension is gender-independent. However, gender-based differences in hypertension risk factors and prevalence has been documented (Everett and Zajacova 2015; Choi et al. 2017). The overall prevalence of hypertension is higher among men than women (Cutler et al. 2008; Everett and Zajacova 2015; Choi et al. 2017), owing to both biological and behavioral reasons. First, males tend to have higher blood pressure sensitivity to angiotensin II, an inducer of hypertension (Tatchum-Talom et al. 2005; Ji et al. 2010). Second, estrogen is postulated to function as a protector against hypertension, as evidenced by elevated blood pressure in women with low estrogen such as those with Turner syndrome or experiencing menopause, the latter holding true even when adjusting for age (Nathwani et al. 2000; Pal and Santoro 2002). Furthermore, there are differences in the utilization of health care resources by gender that lead to disparities in health. In general, young men are less likely to seek routine clinical care (e.g. annual check-ups with a primary care physician), where screening for hypertension would occur (Williams 2003). The lack of awareness of being hypertensive or at high risk thus removes the impetus to adjust dietary and lifestyle behaviors that would otherwise lower hypertension risk. Comparatively, young women may have a higher likelihood of health screening if they are routinely visiting a gynecologist (Schmittdiel et al. 2011). Gender norms, such as the paradigm that men may be considered less masculine or weak if they seek medical care, likely play a role in the disparity in health care utilization (Jeffries and Grogan 2012). Together, these factors may explain the discrepancy in hypertension risk for SSB consumption between genders.
Interestingly, SSB intake was not associated with risk of hyperlipidemia, despite a well-supported connection between added sugar and cardiovascular disease risk (Vos et al. 2017). It is hypothesized that excess fructose and glucose both contribute to dyslipidemia. Glucose induces insulin secretion which subsequently increases fatty acid synthesis in adipose tissue (Geissler and Powers 2011). Fructose, on the other hand, is metabolically fated for the liver where it undergoes de novo lipogenesis and converts to short chain fatty acids (Parks et al. 2008). Our results are consistent with a similar, although smaller and of shorter duration, prospective cohort study. Ambrosini et al. (Ambrosini et al. 2013) reported an unfavorable association between change in SSB intake from 14 to 17 years old and triglyceride and HDL concentrations; however, these associations became attenuated and non-significant after adjusting for dietary patterns.
Overall, fruit juice was not associated with risk of either hypertension or hyperlipidemia. This is significant given the similarities in total sugar content with SSB and that 100% fruit juice even has a slightly higher ratio of fructose-to-glucose than does SSB (Walker et al. 2014). Several studies support a neutral or even favorable impact of fruit juice intake on biomarkers of cardiovascular disease (Reid et al. 2010; Morand et al. 2011; Foroudi et al. 2014; Rangel-Huerta et al. 2015; Schar et al. 2015; Ponce et al. 2019). A potential explanation is that antioxidant flavonoids such as hesperidin and naringenin counter deleterious effects induced by free sugar. Hesperidin, a flavanone abundant in citrus fruit, has been postulated to be a chief agent in promoting cardiovascular health due to its function as a superoxide anion scavenger and its ability to reduce hydrogen peroxide production and subsequently lipid peroxidation. However, the evidence regarding its utility in improving blood pressure or an abnormal lipid profile is mixed (Morand et al. 2011; Schar et al. 2015; Mohammadi et al. 2019; Valls et al. 2021). Therefore, it is likely that healthier dietary patterns and lifestyle factors associated with fruit juice consumption (Wang et al. 2012; Yang et al. 2013; Sakaki et al. 2019) attenuate the risk of hypertension or hyperlipidemia. In the subgroup analysis, apple/other fruit juice but not orange juice was positively related to hyperlipidemia, contrary to our initial hypothesis. Whether this finding is spurious or reflective of an actual protective effect is unclear, though given the relative lack of beneficial flavanones and vitamin C in non-citrus juice it is likely the former. Future studies are needed to elucidate the clinical roles of these citrus juice bioactive components on cardiovascular disease risk factors in young adults.
Given diet soda’s absence of free sugar and calories it was expected that its consumption would not increase hypertension risk. However, similar to SSB’s mild’s association with hyperlipidemia risk, the lower bound of the 95% confidence interval for diet soda’s risk of hypertension barely crosses over the non-significant threshold after full adjustment. The relatively low prevalence of diet soda consumption in this cohort may contribute to the lack of statistical significance when there may in fact be some correlation. This finding is complicated by the conflicting reports in other studies. Low-calorie sweeteners such as steviosides, aspartame and sucralose have been shown to reduce blood pressure in hypertensive rats (Kiritsy and Maher 1986; Hsu et al. 2002) and healthy humans (Pham et al. 2018), purportedly via prostaglandin-mediated vasodilation (Melis and Sainati 1991) or inhibition of calcium influx (Lee et al. 2001). Yet, observational studies evaluating diet soda’s association with blood pressure offer varying and oppositional results (Cohen et al. 2012; Maersk et al. 2012; Cheungpasitporn et al. 2015; Kim and Je 2016). People who are already at elevated risk of incident hypertension or hyperlipidemia (such as obese individuals) may turn to consuming diet soda as a substitute for added sugar in order to reduce caloric or sugar intake. This phenomenon is known reverse causation, in which the relationship between exposure and outcome is distorted and seemingly reversed (Willett 2012). In the US, obese people are more likely to consume low-calorie sweeteners than their non-obese counterparts (Sylvetsky et al. 2017), which independently elevates the risk of hypertension (Rahmouni et al. 2005). Given that diet soda is commonly used as a sugar-free substitute for SSB, it is possible that diet soda consumers exhibit similar dietary and lifestyle characteristics to SSB consumers that predispose them to hypertension. In any case, we attempted to account for the possibility of reverse causation by using a 4-year gap between measurements of exposure and outcome.
This study had several notable strengths. First, using principal components analysis to determine diet patterns allowed us to independently evaluate the associations between the intake of the beverages and the diseases. As diet is a critical modifiable factor in disease progression, it was essential to account for its potential confounding effects. Second, the study design was longitudinal with a follow-up of 18 years and included a 4-year lag time between the collection of dietary data and disease assessment. This is an ideal structure for an epidemiologic investigation of the prospective nature between diet and disease as it allows sufficient time for the development of chronic conditions and reduces the potential impact of reverse causality. However, there are limitations that deserve consideration. As the disease data were collected via self-report, there is the possibility of reporting bias. Participants may have erroneously under-reported the presence of disease, whether voluntarily or due to a lack of knowledge of their own disease status. Yet, self-reported data on cardiovascular disease is likely to result in underestimation (Oksanen et al. 2010) which would err the current analysis on the conservative side. It is also important to consider that hypertension and hyperlipidemia are early risk factors of cardiovascular disease and not in and of themselves diseases of the heart. While assessing the predictors of risk factors is beneficial in ultimately preventing or deterring from the onset of cardiovascular disease, we were not able to make causal inferences between beverage intake and heart disease itself (such as myocardial infarction or stroke). Furthermore, the principal components analysis for diet factors required a subjective assessment of food groups. While many items on the FFQ neatly fit into one of four categories, certain items with unspecified ingredients such as “mixed dish” or “dressing” did not objectively fit into a distinct food group. For these items, assumptions had to be made regarding standard ingredients that aligned with the food group to which they belonged. Also, the GUTS cohort was not designed to be nationally representative which potentially limits generalization, especially racially/ethnically given the relative dearth of racial diversity. Finally, nearly 46% of participants did not follow up or were excluded from this analysis due to missing data which may have led to survivorship bias, particularly due to the higher prevalence of smoking among those excluded.
In conclusion, SSB intake in preadolescence/adolescence were positively associated with incident hypertension risk in early adulthood among males, while fruit juice and diet soda were not associated with either hypertension or hyperlipidemia risk. Parents, clinicians, health advisors and children can utilize this data to make informed decisions regarding their beverage intake as it pertains to cardiovascular health. Future studies should investigate these relationships in other populations, particularly in ethnic minority groups, and evaluate the gender-based differences.
Supplementary Material
Sources of support:
The Florida Department of Citrus, an executive agency of the state of Florida, provided funding for this project to Ock K. Chun, Contract Doc #17–16. Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School provided the data of the Growing Up Today Study (GUTS) for this study (life-course and CVD/lung disease infrastructure, grant #U01 HL145386). No party played a role in the design, implementation, analysis or interpretation of the study. JEH and JEC were supported by the National Institutes of Health (U01 HL145386).
Footnotes
Conflicts of interest: The authors report no conflict of interest
Contributor Information
Junichi R. Sakaki, Department of Nutritional Sciences, University of Connecticut, 27 Manter Rd., Unit 4017, Storrs, CT 06269.
Simiao Gao, Department of Statistics, University of Connecticut, 215 Glenbrook Rd., U-4120, Storrs, CT, 06269.
Kyungho Ha, Department of Food Science and Nutrition, Jeju National University, Jeju, South Korea.
Jorge E. Chavarro, Department of Nutrition and Epidemiology, Harvard T.H. Chan School of Public Health, 677 Huntington Ave., Boston, MA.; Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA.
Ming-Hui Chen, Department of Statistics, University of Connecticut, 215 Glenbrook Rd., U-4120, Storrs, CT, 06269.
Qi Sun, Department of Nutrition and Epidemiology, Harvard T.H. Chan School of Public Health, 677 Huntington Ave., Boston, MA.; Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA.
Jaime E. Hart, Department of Environmental Health, Harvard T.H. Chan School of Public Health, 401 Park Drive, 3rd Fl West, Boston, MA 02215.; Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA;.
Ock K. Chun, Department of Nutritional Sciences, University of Connecticut, 27 Manter Rd., Unit 4017, Storrs, CT 06269.
Data availability:
The data that support the findings of this study are available from the corresponding author, OKC, upon reasonable request.
References
- Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Tudor-locke C, Greer JL, Vezina J, Whitt-glover MC, Leon AS. 2011. Second update of codes and MET values. Med Sci Sport Exerc. 43(8):1575–1581. 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- Ambrosini GL, Oddy WH, Huang RC, Mori TA, Beilin LJ, Jebb SA. 2013. Prospective associations between sugar-sweetened beverage intakes and cardiometabolic risk factors in adolescents. Am J Clin Nutr. 98(2):327–334. 10.3945/ajcn.112.051383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray K, Thompson A, Wood A, Lewington S, Sattar N, Packard C, et al. 2010. Major Lipids, Apolipoproteins, and Risk of Vascular Disease. Yearb Vasc Surg. 2010(18):42–44. 10.1016/s0749-4041(10)79320-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia A 2005. Why should researchers report the confidence interval in modern research? Middle East Fertil Soc J. 10(1):78–81. [Google Scholar]
- Bao Y, Bertoia ML, Lenart EB, Stampfer MJ, Willett WC, Speizer FE, Chavarro JE. 2016. Origin, methods, and evolution of the three nurses’ health studies. Am J Public Health. 106(9):1573–1581. 10.2105/AJPH.2016.303338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. 2019. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. [place unknown]. 10.1161/CIR.0000000000000659 [DOI] [PubMed]
- Berkey CS, Rockett HRH, Field AE, Gillman MW, Colditz GA. 2004. Sugar-added beverages and adolescent weight change. Obes Res. 12(5):778–788. 10.1038/oby.2004.94 [DOI] [PubMed] [Google Scholar]
- Bleich SN, Vercammen KA, Koma JW, Li Z. 2018. Trends in Beverage Consumption Among Children and Adults, 2003–2014. Obesity. 26(2):432–441. 10.1002/oby.22056 [DOI] [PubMed] [Google Scholar]
- Bowman S, Clemens J, Friday J, Anand J. Changes in the total fruit and fruit juice intakes of individuals: WWEIA, NHANES 2005–2006 to 2017–2018. Food Surveys Research Group. Dietary Data Brief No. 41. [Google Scholar]
- Centers for Disease Control and Prevention. 2018. Underlying Cause of Death, 1999–2018. CDC Wonder Online Database; Atlanta, GA: Centers Dis Control Prev. [Google Scholar]
- Cheungpasitporn W, Thongprayoon C, Edmonds PJ, Srivali N, Ungprasert P, Kittanamongkolchai W, Erickson SB. 2015. Sugar and artificially sweetened soda consumption linked to hypertension: A systematic review and meta-analysis. Clin Exp Hypertens. 37(7):587–593. 10.3109/10641963.2015.1026044 [DOI] [PubMed] [Google Scholar]
- Choi HK, Willett W, Curhan G. 2010. Fructose-rich beverages and risk of gout in women. JAMA - J Am Med Assoc. 304(20):2270–2278. 10.1001/jama.2010.1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HM, Kim HC, Kang DR. 2017. Sex differences in hypertension prevalence and control: Analysis of the 2010–2014 Korea national health and nutrition examination survey. PLoS One. 12(5):1–12. 10.1371/journal.pone.0178334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Curhan G, Forman J. 2012. Association of sweetened beverage intake with incident hypertension. J Gen Intern Med. 27(9):1127–1134. 10.1007/s11606-012-2069-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. 2008. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertens (Dallas, Tex 1979). 52(5):818–827. 10.1161/HYPERTENSIONAHA.108.113357 [DOI] [PubMed] [Google Scholar]
- Dennison BA, Rockwell HL, Baker SL. 1997. Excess fruit juice consumption by preschool-aged children is associated with short stature and obesity. Pediatrics. 99(1):15–22. [PubMed] [Google Scholar]
- Dennison BA, Rockwell HL, Nichols MJ, Jenkins P. 1999. Children’s growth parameters vary by type of fruit juice consumed. J Am Coll Nutr. 18(4):346–352. 10.1080/07315724.1999.10718874 [DOI] [PubMed] [Google Scholar]
- Doherty AM, Lacko AM, Popkin BM. 2021. Sugar-sweetened beverage (SSB) consumption is associated with lower quality of the non-SSB diet in US adolescents and young adults. Am J Clin Nutr [Internet]. 113(3):657–664. 10.1093/ajcn/nqaa342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgar FJ, Roberts C, Tudor-Smith C, Moore L. 2005. Validity of self-reported height and weight and predictors of bias in adolescents. J Adolesc Heal. 37(5):371–375. 10.1016/j.jadohealth.2004.07.014 [DOI] [PubMed] [Google Scholar]
- Everett B, Zajacova A. 2015. Gender differences in hypertension among young adults. Biodemography Soc Biol2. 61(1):1–17. 10.1080/19485565.2014.929488.Gender [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig DI. 2012. Hyperuricemia and Hypertension. Adv Chronic Kidney Dis [Internet]. 19(6):377–385. 10.1053/j.ackd.2012.05.009 [DOI] [PubMed] [Google Scholar]
- Feig DI, Johnson RJ. 2003. Hyperuricemia in childhood primary hypertension. Hypertension. 42(3):247–252. 10.1161/01.HYP.0000085858.66548.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig DI, Mazzali M, Kang DH, Nakagawa T, Price K, Kannelis J, Johnson RJ. 2006. Serum uric acid: A risk factor and a target for treatment? J Am Soc Nephrol. 17(SUPPL. 2):69–73. 10.1681/ASN.2005121331 [DOI] [PubMed] [Google Scholar]
- Fitch C, Keim KS. 2012. Position of the Academy of Nutrition and Dietetics: Use of Nutritive and Nonnutritive Sweeteners. J Acad Nutr Diet. 112(5):739–758. 10.1016/j.jand.2012.03.009 [DOI] [PubMed] [Google Scholar]
- Foroudi S, Potter AS, Stamatikos A, Patil BS, Deyhim F. 2014. Drinking orange juice increases total antioxidant status and decreases lipid peroxidation in adults. J Med Food. 17:312–7. 10.1089/jmf.2013.0034 [DOI] [PubMed] [Google Scholar]
- Freedman JE, Parker C 3rd, Li L, Perlman JA, Frei B, Ivanov V, Deak LR, Iafrati MD, Folts JD. 2001. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation. 103(23):2792–2798. 10.1161/01.cir.103.23.2792 [DOI] [PubMed] [Google Scholar]
- Freiberg J, Tybjaerg-Hansen A, Jansen J, Nordestgaard B. 2008. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA [Internet]. 300(2142–2152). 10.1001/jama.2008.621 [DOI] [PubMed] [Google Scholar]
- Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. 2009. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 89(4):1037–1042. 10.3945/ajcn.2008.27140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler C, Powers H. 2011. Human Nutrition. 12th ed. Philadelphia, PA: Churchill Livingstone Elsevier. [Google Scholar]
- Goodman E, Hinden BR, Khandelwal S. 2000. Accuracy of teen and parental reports of obesity and body mass index. Pediatrics. 106(1):52–58. 10.1542/peds.106.1.52 [DOI] [PubMed] [Google Scholar]
- Heller G 2011. Proportional hazards regression with interval censored data using an inverse probability weight. Lifetime Data Anal. 17(3):373–385. 10.1007/s10985-010-9191-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y, Liu J, Kao P, Lee C, Chen Y, Hsieh M, Chan P. 2002. Antihypertensive effect of stevioside in different strains of hypertensive rats. Chinese Med J. 65:1–6. [PubMed] [Google Scholar]
- Hung H-C, Joshipura KJ, Jiang R, Hu FB, Hunter D, Smith-Warner SA, Colditz GA, Rosner B, Spiegelman D, Willett WC. 2004. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 96(21):1577–1584. 10.1093/jnci/djh296 [DOI] [PubMed] [Google Scholar]
- Jeffries M, Grogan S. 2012. “Oh, I’m just, you know, a little bit weak because I’m going to the doctor’s”: young men’s talk of self-referral to primary healthcare services. Psychol Health. 27(8):898–915. 10.1080/08870446.2011.631542 [DOI] [PubMed] [Google Scholar]
- Ji H, Zheng W, Wu X, Liu J, Ecelbarger CM, Watkins R, Arnold AP, Sandberg K. 2010. Sex chromosome effects unmasked in angiotensin II-induced hypertension. Hypertens (Dallas, Tex 1979). 55(5):1275–1282. 10.1161/HYPERTENSIONAHA.109.144949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshipura KJ, Ascherio A, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Hennekens CH, Spiegelman D, Willett WC. 1999. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA. 282(13):1233–1239. 10.1001/jama.282.13.1233 [DOI] [PubMed] [Google Scholar]
- Ke JY, Kliewer KL, Hamad EM, Cole RM, Powell KA, Andridge RR, Straka SR, Yee LD, Belury MA. 2015. The flavonoid, naringenin, decreases adipose tissue mass and attenuates ovariectomy-associated metabolic disturbances in mice. Nutr Metab. 12(1):1–10. 10.1186/1743-7075-12-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Je Y. 2016. Prospective association of sugar-sweetened and artificially sweetened beverage intake with risk of hypertension. Arch Cardiovasc Dis [Internet]. 109(4):242–253. 10.1016/j.acvd.2015.10.005 [DOI] [PubMed] [Google Scholar]
- Kiritsy P, Maher T. 1986. Acute effects of aspartame on systolic blood pressure in spontaneously hypertensive rats. J Neural Trasm. 66:121–8. [DOI] [PubMed] [Google Scholar]
- Kuczmarski R, Ogden C, Guo S, Al. E. 2002. 2000 CDC growth charts for the United States: methods and development. Vital Heal Stat 11. 246:1–190. [PubMed] [Google Scholar]
- Lee CN, Wong KL, Liu JC, Chen YJ, Cheng JT, Chan P. 2001. Inhibitory effect of stevioside on calcium influx to produce antihypertension. Planta Med. 67(9):796–799. 10.1055/s-2001-18841 [DOI] [PubMed] [Google Scholar]
- Liao X, Zucker DM, Li Y, Spiegelman D. 2011. Survival Analysis with Error-Prone Time-Varying Covariates: A Risk Set Calibration Approach. Biometrics. 67(1):50–58. 10.1111/j.1541-0420.2010.01423.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libuda L, Alexy U, Sichert-Hellert W, Stehle P, Karaolis-Danckert N, Buyken AE, Kersting M. 2008. Pattern of beverage consumption and long-term association with body-weight status in German adolescents - Results from the DONALD study. Br J Nutr. 99(6):1370–1379. 10.1017/S0007114507862362 [DOI] [PubMed] [Google Scholar]
- Lustig RH. 2010. Fructose: metabolic, hedonic, and societal parallels with ethanol. J Am Diet Assoc [Internet]. 110(9):1307–1321. 10.1016/j.jada.2010.06.008 [DOI] [PubMed] [Google Scholar]
- Maersk M, Belza A, Stødkilde-Jørgensen H, Ringgaard S, Chabanova E, Thomsen H, Pedersen SB, Astrup A, Richelsen B. 2012. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: A 6-mo randomized intervention study. Am J Clin Nutr. 95(2):283–289. 10.3945/ajcn.111.022533 [DOI] [PubMed] [Google Scholar]
- Mallat SG, Al Kattar S, Tanios BY, Jurjus A. 2016. Hyperuricemia, Hypertension, and Chronic Kidney Disease: an Emerging Association. Curr Hypertens Rep [Internet]. 18(10):74. 10.1007/s11906-016-0684-z [DOI] [PubMed] [Google Scholar]
- Mansournia M 2016. Inverse probability weighting. BMJ. 352:i189. [DOI] [PubMed] [Google Scholar]
- Martin C, Clemens J, Moshfegh A. Beverage Choices among Children: What We Eat in America, NHANES 2017–2018. Food Surveys Research Group Data Brief No. 32.
- Melis M, Sainati A. 1991. Participation of prostaglandins in the effect of stevioside on rat renal function and arterial pressure. Braz J Med Biol Res. 24:1269–76. [PubMed] [Google Scholar]
- Mishra G, Ball K, Arbuckle J, Crawford D. 2002. Dietary patterns of Australian adults and their association with socioeconomic status: Results from the 1995 National Nutrition Survey. Eur J Clin Nutr. 56(7):687–693. 10.1038/sj.ejcn.1601391 [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Ramezani-Jolfaie N, Lorzadeh E, Khoshbakht Y, Salehi-Abargouei A. 2019. Hesperidin, a major flavonoid in orange juice, might not affect lipid profile and blood pressure: A systematic review and meta-analysis of randomized controlled clinical trials. Phyther Res. 33(3):534–545. 10.1002/ptr.6264 [DOI] [PubMed] [Google Scholar]
- Morand C, Dubray C, Milenkovic D, Lioger D, Martin JF, Scalbert A, Mazur A. 2011. Hesperidin contributes to the vascular protective effects of orange juice: A randomized crossover study in healthy volunteers. Am J Clin Nutr. 93(1):73–80. 10.3945/ajcn.110.004945 [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin E, Go A, Arnett D, Blaha M, Cushman M, de Ferranti S, Després J-P, Fullerton H, Howard V, et al. 2015. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 131:e29–e322. [DOI] [PubMed] [Google Scholar]
- Muth ND, Dietz WH, Magge SN, Johnson RK, Bolling CF, Armstrong SC, Haemer MA, Muth ND, Rausch JC, Rogers VW. 2019. Public policies to reduce sugary drink consumption in children and adolescents. Pediatrics. 143(4). 10.1542/peds.2019-0282 [DOI] [PubMed] [Google Scholar]
- Nathwani NC, Unwin R, Brook CG, Hindmarsh PC. 2000. Blood pressure and Turner syndrome. Clin Endocrinol (Oxf). 52(3):363–370. 10.1046/j.1365-2265.2000.00960.x [DOI] [PubMed] [Google Scholar]
- Nguyen S, Choi HK, Lustig RH, Hsu C yuan. 2009. Sugar-Sweetened Beverages, Serum Uric Acid, and Blood Pressure in Adolescents. J Pediatr. 154(6):807–813. 10.1016/j.jpeds.2009.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen T, Kivimäki M, Pentti J, Virtanen M, Klaukka T, Vahtera J. 2010. Self-Report as an Indicator of Incident Disease. Ann Epidemiol. 20(7):547–554. 10.1016/j.annepidem.2010.03.017 [DOI] [PubMed] [Google Scholar]
- Pacheco LS, Lacey JVJ, Martinez ME, Lemus H, Araneta MRG, Sears DD, Talavera GA, Anderson CAM. 2020. Sugar-Sweetened Beverage Intake and Cardiovascular Disease Risk in the California Teachers Study. J Am Heart Assoc. 9(10):e014883. 10.1161/JAHA.119.014883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal L, Santoro N. 2002. Premature ovarian failure (POF): discordance between somatic and reproductive aging. Ageing Res Rev. 1(3):413–423. 10.1016/s1568-1637(02)00009-0 [DOI] [PubMed] [Google Scholar]
- Park YK, Kim J-S, Kang M-H. 2004. Concord grape juice supplementation reduces blood pressure in Korean hypertensive men: double-blind, placebo controlled intervention trial. Biofactors. 22(1–4):145–147. 10.1002/biof.5520220128 [DOI] [PubMed] [Google Scholar]
- Parks EJ, Skokan LE, Timlin MT, Dingfelder CS. 2008. Dietary sugars stimulate fatty acid synthesis in adults. J Nutr. 138(6):1039–1046. 10.1093/jn/138.6.1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham HT, Stevens JE, Rigda RS, Phillips LK, Wu T, Hausken T, Soenen S, Visvanathan R, Rayner CK, Horowitz M, Jones KL. 2018. Effects of intraduodenal administration of the artificial sweetener sucralose on blood pressure and superior mesenteric artery blood flow in healthy older subjects. Am J Clin Nutr. 108(1):156–162. 10.1093/ajcn/nqy060 [DOI] [PubMed] [Google Scholar]
- Ponce O, Benassi R, Cesar T. 2019. Orange juice associated with a balanced diet mitigated risk factors of metabolic syndrome: A randomized controlled trial. J Nutr Intermed Metab [Internet]. 17(October):100101. 10.1016/j.jnim.2019.100101 [DOI] [Google Scholar]
- du Prel J-B, Hommel G, Röhrig B, Blettner M. 2009. Confidence interval or p-value?: part 4 of a series on evaluation of scientific publications. Dtsch Arztebl Int [Internet]. 106(19):335–339. 10.3238/arztebl.2009.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni K, Correira M, Haynes W, Mark A. 2005. Obesity-Associated Hypertension: New Insights Into Mechanisms. Hypertension. 45:9–14. [DOI] [PubMed] [Google Scholar]
- Rampersaud GC, Valim MF. 2017. 100% citrus juice: Nutritional contribution, dietary benefits, and association with anthropometric measures. Crit Rev Food Sci Nutr. 57(1):129–140. 10.1080/10408398.2013.862611 [DOI] [PubMed] [Google Scholar]
- Rangel-Huerta OD, Aguilera CM, Martin MV., Soto MJ, Rico MC, Vallejo F, Tomas-Barberan F, Perez-de-la-Cruz AJ, Gil A, Mesa MD. 2015. Normal or high polyphenol concentration in orange juice affects antioxidant activity, blood pressure, and body weight in obese or overweight adults. J Nutr. 145(8):1808–1816. 10.3945/jn.115.213660 [DOI] [PubMed] [Google Scholar]
- Reid M, Hammersley R, Duffy M. 2010. Effects of sucrose drinks on macronutrient intake, body weight, and mood state in overweight women over 4 weeks. Appetite [Internet]. 55(1):130–136. 10.1016/j.appet.2010.05.001 [DOI] [PubMed] [Google Scholar]
- Rockett HRH, Breitenbach M, Frazier AL, Witschi J, Wolf AM, Field AE, Colditz GA. 1997. Validation of a youth/adolescent food frequency questionnaire. Prev Med (Baltim). 26(6):808–816. 10.1006/pmed.1997.0200 [DOI] [PubMed] [Google Scholar]
- Sakaki JR, Chavarro JE, Chen M-H, Hart JE. 2021. Associations between fruit juice and milk consumption and change in BMI in a large prospective cohort of U.S. adolescents and preadolescents. Pediatr Obes.:e12781. 10.1111/ijpo.12781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki JR, Melough MM, Li J, Tamimi RM, Chavarro JE, Chen M-H, Chun OK. 2019. Associations between 100% Orange Juice Consumption and Dietary, Lifestyle and Anthropometric Characteristics in a Cross-Sectional Study of U.S. Children and Adolescents. Nutrients. 11:2687. 10.3390/nu11112687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schar MY, Curtis PJ, Hazim S, Ostertag LM, Kay CD, Potter JF, Cassidy A. 2015. Orange juice-derived flavanone and phenolic metabolites do not acutely affect cardiovascular risk biomarkers: A randomized, placebo-controlled, crossover trial in men at moderate risk of cardiovascular disease1–5. Am J Clin Nutr. 101(5):931–938. 10.3945/ajcn.114.104364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt TA. 2011. Current methodological considerations in exploratory and confirmatory factor analysis. J Psychoeduc Assess. 29(4):304–321. 10.1177/0734282911406653 [DOI] [Google Scholar]
- Schmittdiel J, Selby JV, Swain B, Daugherty SL, Leong TK, Ho M, Margolis KL, O’Connor P, Magid DJ, Bibbins-Domingo K. 2011. Missed opportunities in cardiovascular disease prevention?: low rates of hypertension recognition for women at medicine and obstetrics-gynecology clinics. Hypertens (Dallas, Tex 1979). 57(4):717–722. 10.1161/HYPERTENSIONAHA.110.168195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefferly A, Scharf RJ, Deboer MD. 2016. Longitudinal evaluation of 100% fruit juice consumption on BMI status in 2–5-year-old children. Pediatr Obes. 11(3):221–227. 10.1111/ijpo.12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YH, Chang HY, Wu HC, Stanaway FF, Pan WH. 2020. High sugar-sweetened beverage intake frequency is associated with smoking, irregular meal intake and higher serum uric acid in Taiwanese adolescents. J Nutr Sci. 9:1–10. 10.1017/jns.2020.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneville KR, Long MW, Rifas-Shiman SL, Kleinman K, Gillman MW, Taveras EM. 2015. Juice and water intake in infancy and later beverage intake and adiposity: Could juice be a gateway drink? Obesity. 23(1):170–176. 10.1002/oby.20927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhope KL. 2016. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit Rev Clin Lab Sci [Internet]. 53(1):52–67. 10.3109/10408363.2015.1084990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhope KL, Medici V, Bremer AA, Lee V, Lam HD, Nunez MV., Chen GX, Keim NL, Havel PJ 2015. A dose-response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am J Clin Nutr. 101(6):1144–1154. 10.3945/ajcn.114.100461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R 1999. Comparison of measured and self-reported weight and height in a cross-sectional sample of young adolescents. Int J Obes. 23(8):904–908. 10.1038/sj.ijo.0800971 [DOI] [PubMed] [Google Scholar]
- Sylvetsky AC, Jin Y, Mathieu K, DiPietro L, Rother KI, Talegawkar SA. 2017. Low-Calorie Sweeteners: Disturbing the Energy Balance Equation in Adolescents? Obesity. 25(12):2049–2054. 10.1002/oby.22005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatchum-Talom R, Eyster KM, Martin DS. 2005. Sexual dimorphism in angiotensin II-induced hypertension and vascular alterations. Can J Physiol Pharmacol. 83(5):413–422. 10.1139/y05-012 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. 2020. Dietary Guidelines for Americans, 2020–2025 [Internet]. 9th ed. [place unknown]. DietaryGuidelines.gov [Google Scholar]
- Valls RM, Pedret A, Calderón-Pérez L, Llauradó E, Pla-Pagà L, Companys J, Moragas A, Martín-Luján F, Ortega Y, Giralt M, et al. 2021. Effects of hesperidin in orange juice on blood and pulse pressures in mildly hypertensive individuals: a randomized controlled trial (Citrus study). Eur J Nutr. 60(3):1277–1288. 10.1007/s00394-020-02279-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos MB, Kaar JL, Welsh JA, Van Horn L V, Feig DI, Anderson CAM, Patel MJ, Cruz Munos J, Krebs NF, Xanthakos SA, Johnson RK. 2017. Added Sugars and Cardiovascular Disease Risk in Children: A Scientific Statement From the American Heart Association. Circulation [Internet]. 135(19):e1017–e1034. 10.1161/CIR.0000000000000439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RW, Dumke KA, Goran MI. 2014. Fructose content in popular beverages made with and without high-fructose corn syrup. Nutrition [Internet]. 30(7–8):928–935. 10.1016/j.nut.2014.04.003 [DOI] [PubMed] [Google Scholar]
- Wang X, Hasegawa J, Kitamura Y, Wang Z, Matsuda A, Shinoda W, Miura N, Kimura K. 2011. Effects of hesperidin on the progression of hypercholesterolemia and fatty liver induced by high-cholesterol diet in rats. J Pharmacol Sci. 117(3):129–138. 10.1254/jphs.11097fp [DOI] [PubMed] [Google Scholar]
- Wang Y, Lloyd B, Yang M, Davis CG, Lee SG, Lee W, Chung SJ, Chun OK. 2012. Impact of orange juice consumption on macronutrient and energy intakes and body composition in the US population. Public Health Nutr. 15(12):2220–2227. 10.1017/S1368980012000742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett W 2012. Nutritional Epidemiology. 3rd ed. Oxford: Oxford University Press. 10.1093/acprof:oso/9780199754038.001.0001 [DOI] [Google Scholar]
- Williams DR. 2003. The health of men: structured inequalities and opportunities. Am J Public Health [Internet]. 93(5):724–731. 10.2105/ajph.93.5.724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Lee SG, Wang Y, Lloyd B, Chung SJ, Song WO, Chun OK. 2013. Orange juice, a marker of diet quality, contributes to essential micronutrient and antioxidant intakes in the united states population. J Nutr Educ Behav. 45(4):340–348. 10.1016/j.jneb.2012.07.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, OKC, upon reasonable request.


