Abstract
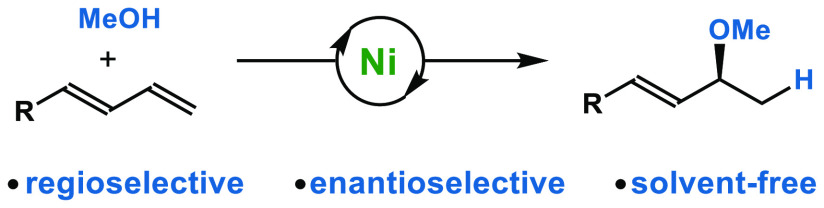
As an advance in hydrofunctionalization, we herein report that alcohols add to 1,3-dienes with high regio- and enantioselectivity. Using Ni-DuPhos, we access enantioenriched allylic ethers. Through the choice of solvent-free conditions, we control the reversibility of C–O bond formation. This work showcases a rare example of methanol as a reagent in asymmetric synthesis.
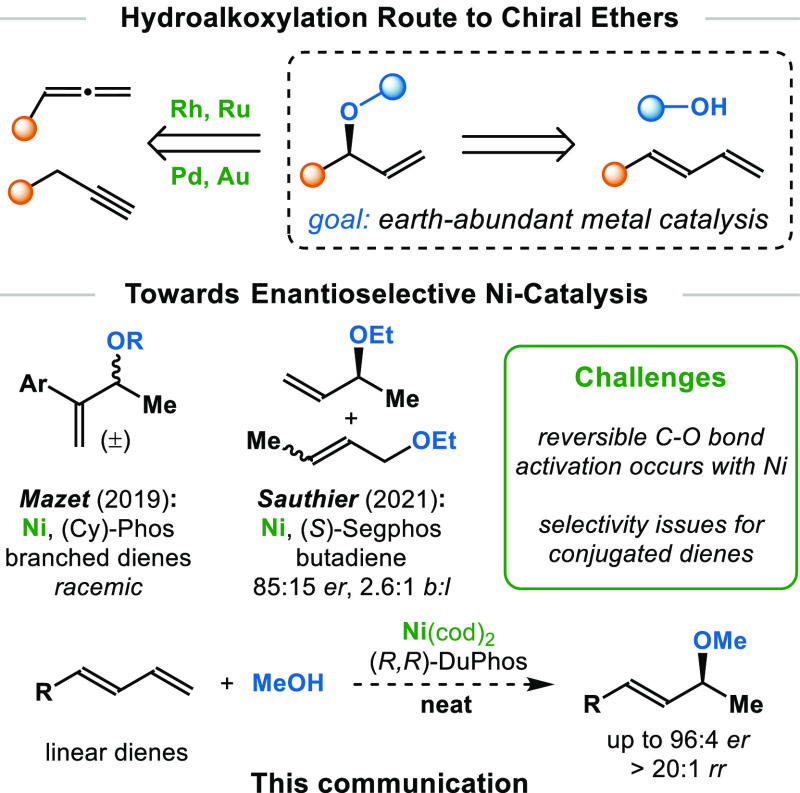
Drawing inspiration from ether-containing pharmaceuticals,1 agrochemicals,2 and natural products,3 chemists strive to identify useful C–O bond-forming methods. Hydrofunctionalization represents an attractive approach to construct C–X bonds from feedstock olefins.4 In contrast to carbon- and nitrogen-based nucleophiles, chalcogen nucleophiles are underdeveloped as coupling partners.5 In most cases, alkynes or allenes have been used as substrates for hydroalkoxylation, with high regioselectivity and enantioselectivity,5a,6 albeit using precious metal catalysts, such as Rh,7 Ru,8 Pd,9 or Au10 (Figure 1). The asymmetric hydroalkoxylation of readily available dienes has attracted attention and warrants further studies, especially using earth-abundant catalysts.11 With Ni-catalysis, Mazet and co-workers demonstrated the promising addition of alcohols to 2-substituted 1,3-dienes to yield racemic allylic ethers (Figure 1).11c By applying a chiral phosphinooxazoline ligand, they achieved an isolated enantioselective example. However, they observed a decreasing enantiomeric ratio during the course of the experiment. Sauthier and co-workers disclosed a Ni-catalyzed enantioselective hydroalkoxylation of butadiene using ethanol; racemization and isomerization were also observed (Figure 1).11b,11d Through our independent investigations, we discovered a complementary and enantioselective Ni-catalyzed hydroalkoxylation of dienes. Petroleum feedstocks and readily available dienes can be transformed into chiral allylic ether building blocks with high regio- and enantiocontrol via Ni-catalysis under solvent-free conditions (Figure 1).12
Figure 1.
Proposal for hydroalkoxylation of 1,3-dienes.
Our laboratory has pursued the hydrofunctionalization of 1,3-dienes, including hydroamination,13 hydrothiolation,14 and hydrophosphinylation.15 In these reports, conjugated dienes could be transformed via metal−π-allyl intermediates to produce the corresponding 1,2- and/or 1,4-addition products. Compared to amines (with nucleophilicity N = 13.2 on the Mayr scale16) and thiols (N = 23.4), alcohols (N = 9.6) present a unique challenge and opportunity due to their lower nucleophilicity.
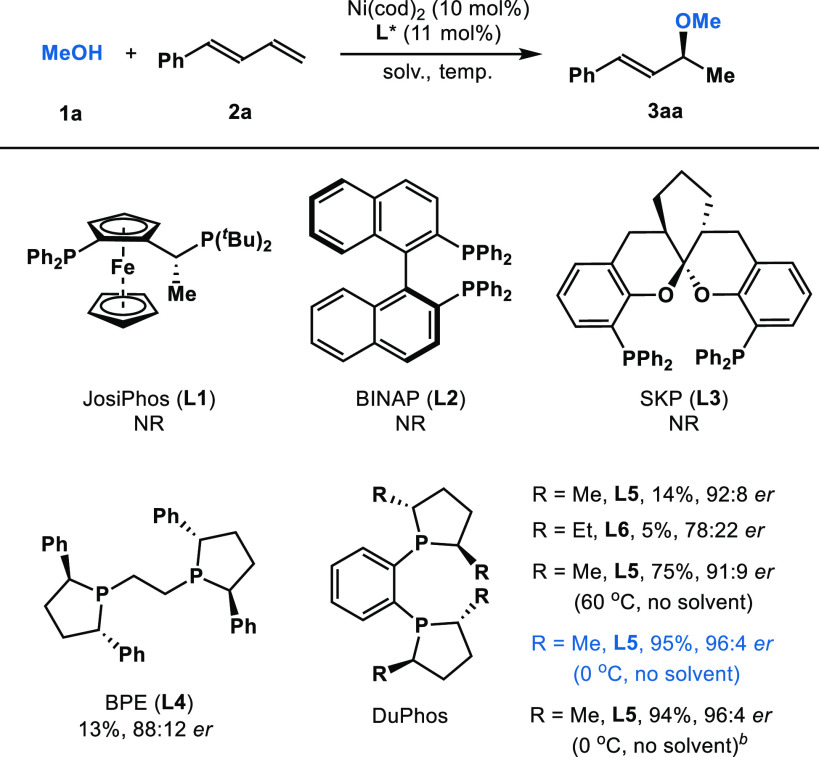
With this challenge in mind, we chose methanol (1a) and 1-phenyl-1,3-butadiene (2a) as the model substrates and surveyed a wide range of metal catalysts. We found that the desired branched allylic ether (3aa) was obtained by using Ni-catalysis with ethereal solvents. We studied the hydroalkoxylation of diene 2a with methanol (1a) using different bidentate phosphine ligands in the presence of Ni(cod)2 (Table 1). With JosiPhos (L1), BINAP (L2), and SKP (L3) ligands, no product formation was detected. Yet, the BPE (L4) and DuPhos families (L5 and L6) afforded promising results. With L5 as the ligand, we obtained excellent regioselectivity for the allylic ether 3aa (>20:1 rr) with 14% yield and 92:8 er by using iPr2O; other ethereal solvents (such as THF or cyclopentyl methyl ether) showed lower reactivity and enantioselectivity. The linear diene 2a showed no reactivity under the conditions previously reported by Mazet.11c However, in accordance with studies by Mazet11c and Sauthier,11b,11d we found that the enantioselectivity decreased dramatically with prolonged reaction times. To our delight, we discovered that this decrease in enantioselectivity over time could be overcome by performing the experiment neat (i.e., without solvent). Under solvent-free conditions, we isolated the enantioenriched ether 3aa in 75% yield and 91:9 er. When the temperature was lowered to 0 °C, the enantioselectivity was increased to 96:4 er with excellent yield (95%, 4 h). Furthermore, the catalyst loading could be decreased to 2.5 mol% (94% yield, 96:4 er, 10 h). This represents a rare example of methanol as a reagent in asymmetric synthesis.17
Table 1. Survey of Ligands and Conditionsa.
Reaction conditions: 1a (0.1 mmol), 2a (0.3 mmol), Ni(cod)2 (10 mol%), ligand (11 mol%), iPr2O (0.1 mL), 60 °C, 4 h. Isolated yields. Enantiomeric ratio (er) was determined by HPLC.
Using 2.5 mol% Ni(cod)2 and 2.8 mol% L5, 10 h.
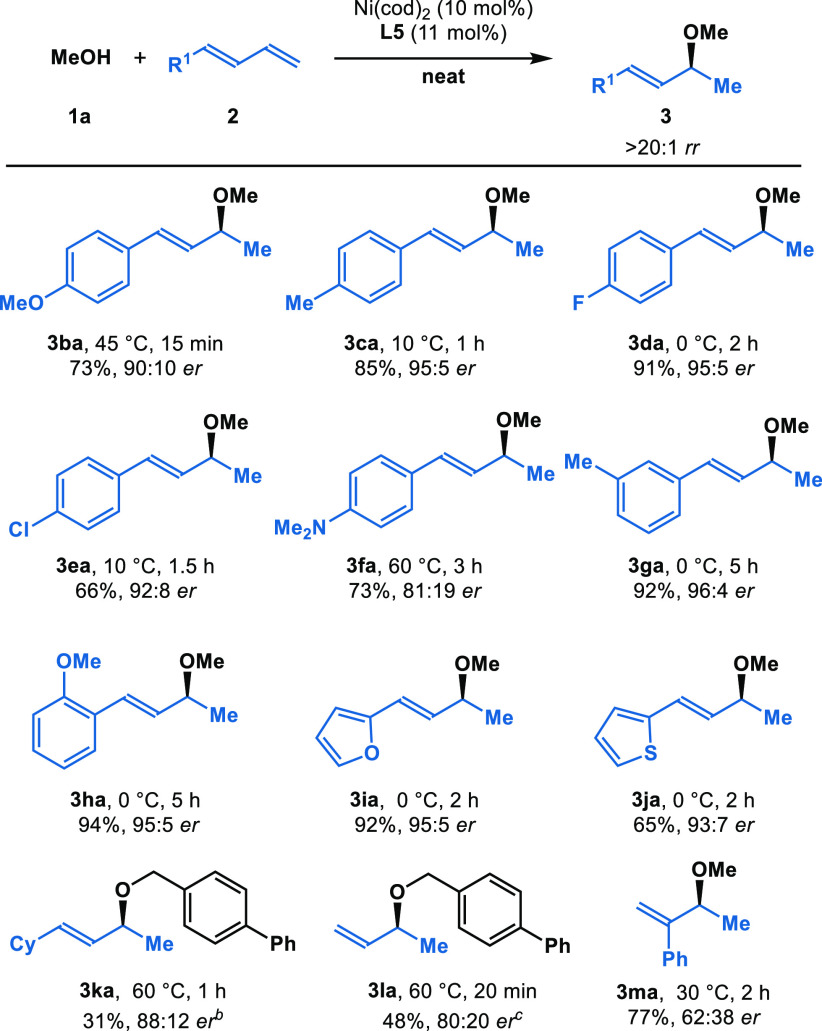
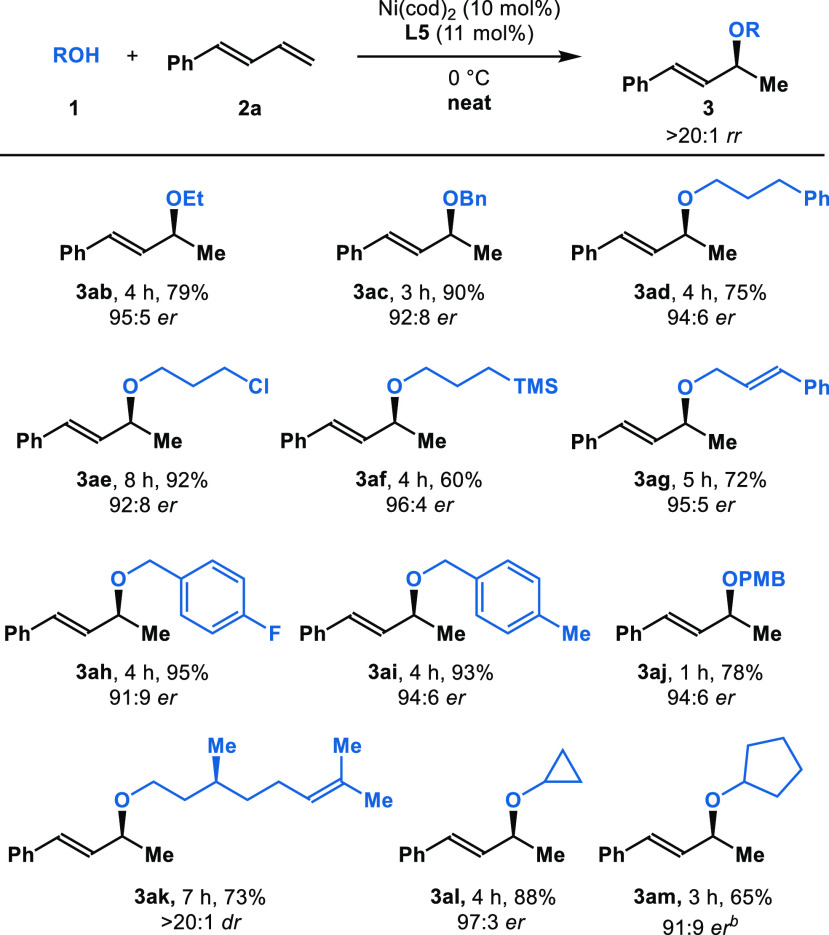
With these conditions in hand, we investigated the hydroalkoxylation of various 1,3-dienes with methanol 1a (Table 2). Products bearing both electron-donating and electron-withdrawing groups on the phenyl ring were obtained with high reactivities and enantioselectivities (3ba–3ha, 66–94% yield, 81:19–96:4 er). This protocol tolerates heterocycle-substituted 1,3-dienes such as 2i (R1 = 2-furyl) and 2j (R1 = 2-thienyl) to afford the corresponding allylic ethers 3ia (92% yield, 95:5 er) and 3ja (65% yield, 93:7 er). In addition, hydroalkoxylation of alkyl-substituted 1,3-diene 2k and feedstock butadiene 2l gave the corresponding products 3ka and 3la in 31% and 48% yields with 88:12 er and 80:20 er, respectively.18 Moreover, addition of methanol (1a) to branched diene 2m provided the allylic ether 3ma in 77% yield with 62:38 er and >20:1 rr. Overall, these results demonstrate the first asymmetric hydroalkoxylation of dienes without erosion of the enantiomeric ratio.
Table 2. Hydroalkoxylation of Various Dienesa.
Reaction conditions: 1a (0.2 mmol), 2 (0.6 mmol), Ni(cod)2 (10 mol%), L5 (11 mol%). Isolated yields. Enantiomeric ratio (er) is determined by HPLC.
iPr2O (2 M) as solvent.
Butadiene (2.0 mmol) in hexane (20%) is used.
Next, we examined the addition of various alcohols 1 to diene 2a (Table 3). We found that a variety of alcohols could be transformed into chiral ethers with good reactivity and selectivity. High reactivities (60–95% yield) and enantioselectivities (91:9–96:4 er) are obtained by using alcohols that bear phenyl, chloro, and trimethylsilyl groups (3ab–3aj). Hydroalkoxylation of diene (2a) with natural product (−)-citronellol (1k) furnishes the desired ether (S,S)-3ak in 73% yield with >20:1 dr. Alcohols such as isopropanol and tert-butanol showed no reactivity. Hydroalkoxylation with secondary alcohols, such as cyclopropanol (1l) and cyclopentanol (1m), provide the corresponding allylic ethers 3al and 3am with high efficiency (88% and 65% yield, respectively) and enantioselectivities (97:3 er and 91:9 er, respectively). In all cases, only one constitutional isomer is obtained.19
Table 3. Hydroalkoxylation with Various Alcoholsa.
Reaction conditions: 1 (0.2 mmol), 2a (0.6 mmol), Ni(cod)2 (10 mol%), L5 (11 mol%), 0 °C. Isolated yields. Enantiomeric ratio (er) is determined by HPLC.
60 °C. PMB = p-methoxybenzyl.
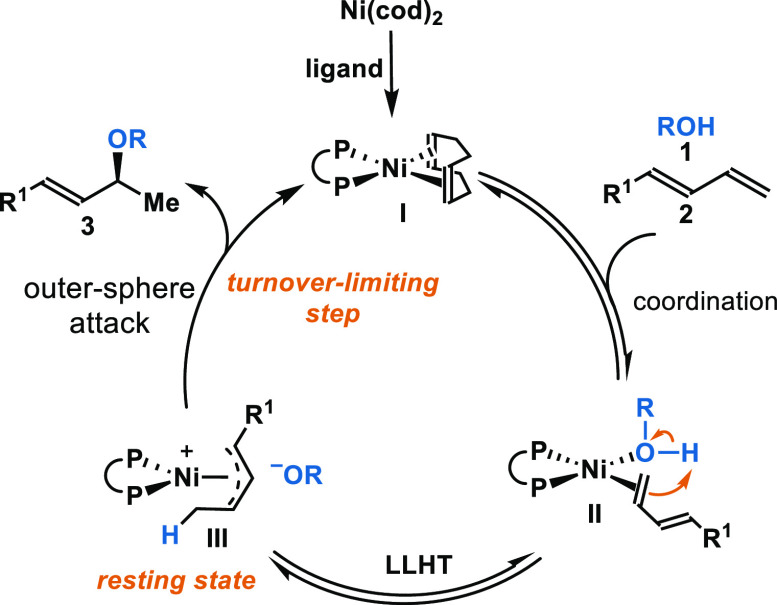
On the basis of literature reports and our own observations, we envision the following mechanistic pathway (Figure 2). Ligand exchange between 1,5-cyclooctadiene (cod) with a bidentate phosphine ligand generates intermediate I. Both alcohol 1 and 1,3-diene 2 bind to Ni via ligand exchange to generate nickel intermediate II. From here, we imagine that the hydrogen atom is transferred directly from alcohol 1 to 1,3-diene 2 through ligand-to-ligand hydrogen transfer (LLHT).12b,12c In accordance with Sauthier and Macgregor’s hydroalkoxylation of butadiene, we propose a cationic allylic intermediate III where the alkoxide is stabilized by hydrogen bonding to the alcohol.11d Intermediate III undergoes outer-sphere nucleophilic attack by the alkoxide at the C3 carbon to provide product 3. Our proposed mechanism fits with the convention of classifying nucleophilic attack on η3-M-π-allyl intermediates for Pd20a and Ni20b allylations. Alkoxides, considered “soft” nucleophiles, would proceed through outer-sphere attack.
Figure 2.
Proposed mechanism via ligand-to-ligand hydrogen transfer (LLHT).
Alternatively, mechanisms involving a Ni–H insertion have been previously proposed for Ni-catalyzed hydrofunctionalization of dienes.12d,12i Inspired by Sauthier’s studies, we investigated both acid and base additives to further probe the Ni–H pathway.11d While no transformation was observed in the presence of acids (e.g., TFA, xylylic acid, TsOH), hydroalkoxylation occurred in the presence of bases (e.g., Et3N, NaOH, tBuONa). The observation of hydroalkoxylation under basic conditions led us to disfavor a Ni–H mechanism. We conducted 1H NMR experiments (at −60 °C) and did not observe Ni–H intermediates. While density functional theory (DFT) studies for this transformation are warranted, LLHT mechanisms have emerged as more energetically favorable for related Ni-catalyzed hydroarylations.12c,21 DFT studies by Zhou,12c Dang,21a and Sakai21b demonstrated that the LLHT pathway was favored across different ligands, including DTBM-Segphos, SpiroAP, and N-heterocyclic carbene, respectively.
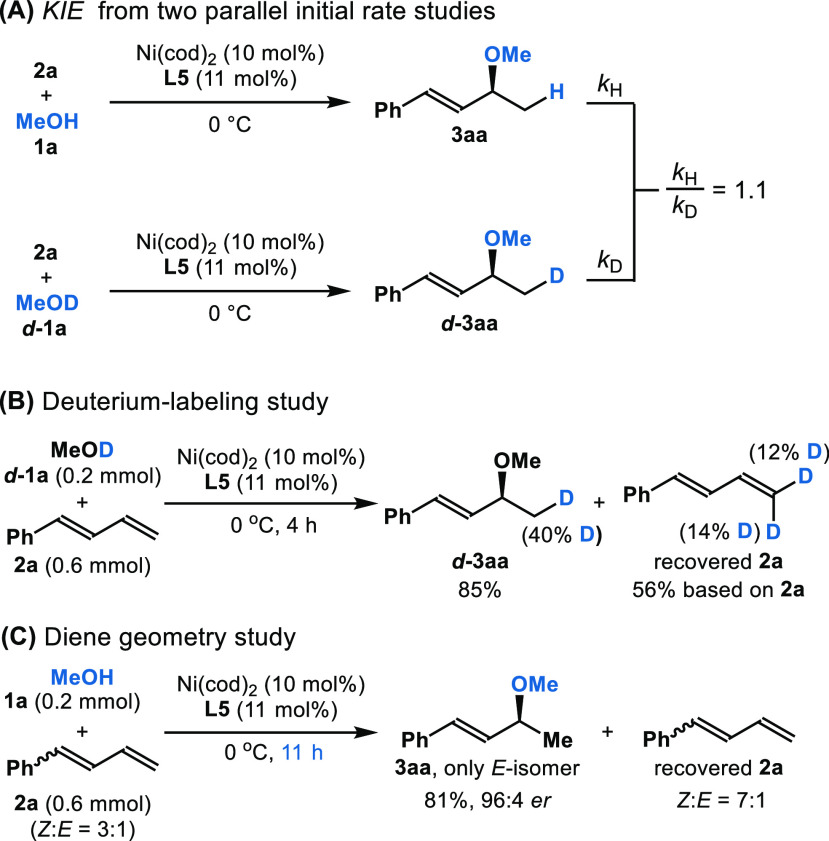
In the rate comparison between methanol (1a) and deuterated methanol (d-1a) under the standard reaction conditions, a secondary (rather than primary) kinetic isotope effect (KIE = 1.1) is observed (Figure 3A). We postulate that outer-sphere nucleophilic attack is the turnover-limiting step. When deuterated methanol is subjected to the standard reaction conditions, deuterium is only incorporated into the terminal position of product d-3aa, and diene with deuterium incorporation is recovered (Figure 3B). The isotopic labeling observed in the recovered diene suggests that LLHT is a reversible step. By using Burés’s variable time normalization analysis (VTNA),22 we studied the kinetic profile and observed a first-order dependence on catalyst and zero-order on diene. Interestingly, the order on alcohol depends on the concentration: inverse order was observed when using a higher concentration of alcohol (2.38 to 5.95 M). However, fractional order was observed when using a lower concentration (1.19 to 2.38 M).23 This result suggests that increasing the concentration of alcohol inhibits the outer-sphere nucleophilic attack, probably due to hydrogen bonding. On basis of these results, we postulate intermediate III as a catalyst resting state. Monitoring of the reaction by 31P NMR shows peaks that are consistent with intermediate III.24 To examine whether such a resting state is detectable, we subjected an authentic catalytic solution to electrospray ionization mass spectrometry (ESI-MS) analysis. At low voltage (Frag = 80 V), we observed a prominent signal with m/z 538.2289, which supports intermediate III but does not rule out the possibility of a Ni-species with OMe associated.24
Figure 3.
Mechanistic studies.
When using the mixture of (Z)- and (E)-2a (3:1, Figure 3C), the (E)-product 3aa was obtained in similar yield (81%), enantioselectivity (96:4 er), and regioselectivity (>20:1 rr), albeit after 11 h. In comparison, the model substrate (E)-2a undergoes complete conversion in 4 h (Table 1, 95% yield, 96:4 er, >20:1 rr). The recovered diene remains enriched in the Z-isomer (7:1), which suggests isomerization is slow compared to alcohol addition.25
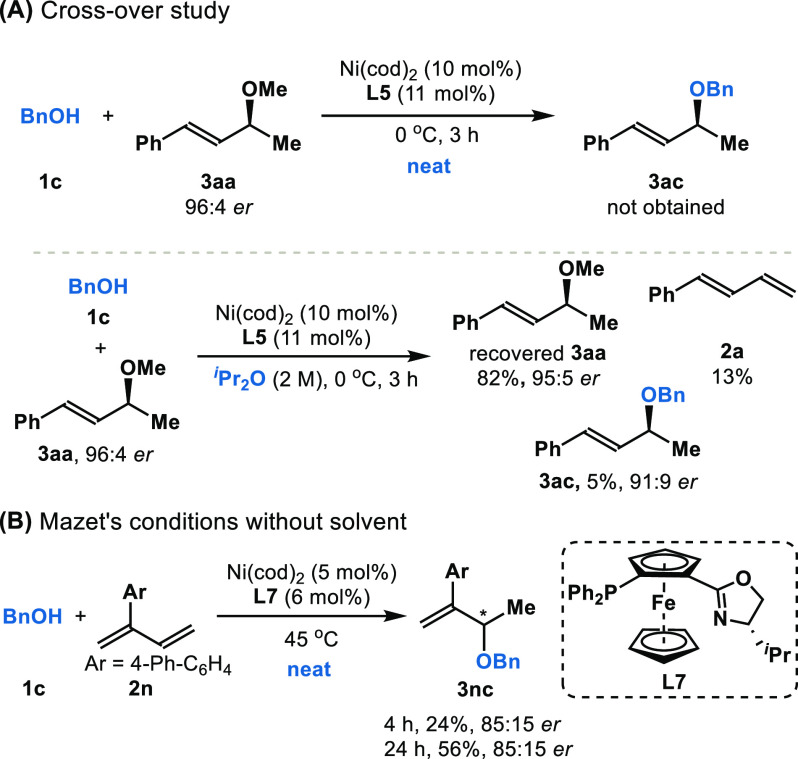
We performed crossover studies to understand the reversibility of C–O bond formation (Figure 4A). When product 3aa was subjected to otherwise standard reaction conditions, in the presence of 1 equiv of benzyl alcohol 1c, no trace of 3ac was detected after 3 h; the er value of recovered starting material 3aa (96:4 er) was constant. However, when a related crossover experiment was performed in the presence of the solvent iPr2O, we observed formation of 3ac (91:9 er) and diene 2a (13% yield). In Sauthier and Macgregor’s study on hydroalkoxylation of butadiene, the overall hydroxylation reactions are computed to be only marginally exergonic with modest barriers. This energetic profile is consistent with a reversible process at an elevated temperature (80 °C).11b,11d We reason that solvent-free conditions enable transformation at lower temperature (0 °C) and thus result in a kinetically controlled process that avoids racemization.
Figure 4.
Reversibility studies.
The activation of C–O bonds under Ni-catalysis in solvent has been investigated both theoretically and experimentally.26 While a number of pathways are possible, we observe that the major isomer of 3ac generated from 3aa has the same configuration as the starting 3aa. The net retention of stereochemistry initially observed could result from an SN2 pathway involving double inversion.26g Alternatively, stereoretentive oxidative additions have also been observed by Watson, Jarvo, and Hong.26a,26c,26d In regards to racemization, Doyle has shown the feasibility of SN1-like pathways.26e Solvent-free conditions prevent reversible C–O bond formation, and this phenomenon may have broader applications. As an example, we investigated Mazet’s conditions for transforming 2n to 3nc; without solvent, we found that racemization did not occur as previously observed when mesitylene was the solvent of choice (Figure 4B).11c
Hydroalkoxylation represents an attractive way to transform dienes into allylic ethers. By using Ni-catalysis, we have achieved the first enantioselective hydroalkoxylation of linear dienes with various alcohols without racemization. The allylation works well with a broad range of alcohols and tolerates different functional groups such as halogens, esters, and silanes. Insights from this study will guide future olefin couplings with chalcogen nucleophiles.
Acknowledgments
We thank the Analysis & Testing Center of Beijing Institute of Technology. We also thank Ryan Le Tourneau and Sumbul Hader for help with preliminary experiments.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.2c12779.
Experimental procedures and spectral data for all new compounds (PDF)
X.-H.Y. thanks the National Natural Science Foundation of China (No. 22201018), Beijing Natural Science Foundation (No. 2222024), and the National Key Research and Development Program of China (No. 2021YFA1401200) for funding. V.M.D. thanks the U.S. National Science Foundation (No. CHE-1956457) and National Institutes of Health (5R35GM127071-05) for funding.
The authors declare no competing financial interest.
Supplementary Material
References
- For select reviews, see:; a Lu Q.; Harmalkar D. S.; Choi Y.; Lee K. An Overview of Saturated Cyclic Ethers: Biological Profiles and Synthetic Strategies. Molecules 2019, 24, 3778. 10.3390/molecules24203778. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Arca H. C.; Mosquera-Giraldo L. I.; Bi V.; Xu D.; Taylor L. S.; Edgar K. J. Pharmaceutical Applications of Cellulose Ethers and Cellulose Ether Esters. Biomacromolecules 2018, 19, 2351–2376. 10.1021/acs.biomac.8b00517. [DOI] [PubMed] [Google Scholar]; c Landelle G.; Panossian A.; Leroux F. R. Trifluoromethyl Ethers and – Thioethers as Tools for Medicinal Chemistry and Drug Discovery. Curr. Top. Med. Chem. 2014, 14, 941–951. 10.2174/1568026614666140202210016. [DOI] [PubMed] [Google Scholar]
- For select review, see:Chen T.; Xiong H.; Yang J.-F.; Zhu X.-L.; Qu R.-Y.; Yang G.-F. Diaryl Ether: A Privileged Scaffold for Drug and Agrochemical Discovery. J. Agric. Food Chem. 2020, 68, 9839–9877. 10.1021/acs.jafc.0c03369. [DOI] [PubMed] [Google Scholar]
- For select reviews, see:; a Yamamoto T.; Saitoh T.; Einaga Y.; Nishiyama S. Anodic Oxidation of Phenols: A Key Step for the Synthesis of Natural Products. Chem. Rec. 2021, 21, 2254–2268. 10.1002/tcr.202100032. [DOI] [PubMed] [Google Scholar]; b Sasaki M.; Fuwa H. Total Synthesis and Complete Structural Assignment of Gambieric Acid A, a Large Polycyclic Ether Marine Natural Product. Chem. Rec. 2014, 14, 678–703. 10.1002/tcr.201402052. [DOI] [PubMed] [Google Scholar]; c Lumbroso A.; Cooke M. L.; Breit B. Catalytic Asymmetric Synthesis of Allylic Alcohols and Derivatives and Their Applications in Organic Synthesis. Angew. Chem., Int. Ed. 2013, 52, 1890–1932. 10.1002/anie.201204579. [DOI] [PubMed] [Google Scholar]
- For select recent reviews, see:; a Rocard L.; Chen D.; Stadler A.; Zhang H.; Gil R.; Bezzenine S.; Hannedouche J. Earth-Abundant 3d Transition Metal Catalysts for Hydroalkoxylation and Hydroamination of Unactivated Alkenes. Catalysts 2021, 11, 674. 10.3390/catal11060674. [DOI] [Google Scholar]; b Guo J.; Cheng Z.; Chen J.; Chen X.; Lu Z. Iron- and Cobalt-Catalyzed Asymmetric Hydrofunctionalization of Alkenes and Alkynes. Acc. Chem. Res. 2021, 54, 2701–2716. 10.1021/acs.accounts.1c00212. [DOI] [PubMed] [Google Scholar]; c Blieck R.; Taillefer M.; Monnier F. Metal-Catalyzed Intermolecular Hydrofunctionalization of Allenes: Easy Access to Allylic Structures via the Selective Formation of C–N, C–C, and C–O Bonds. Chem. Rev. 2020, 120, 13545–13598. 10.1021/acs.chemrev.0c00803. [DOI] [PubMed] [Google Scholar]; d Li G.; Huo X.; Jiang X.; Zhang W. Asymmetric Synthesis of Allylic Compounds via Hydrofunctionalisation and Difunctionalisation of Dienes, Allenes, and Alkynes. Chem. Soc. Rev. 2020, 49, 2060–2118. 10.1039/C9CS00400A. [DOI] [PubMed] [Google Scholar]; e Adamson N. J.; Malcolmson S. J. Catalytic Enantio- and Regioselective Addition of Nucleophiles in the Intermolecular Hydrofunctionalization of 1,3-Dienes. ACS Catal. 2020, 10, 1060–1076. 10.1021/acscatal.9b04712. [DOI] [Google Scholar]; f Chen J.; Lu Z. Asymmetric Hydrofunctionalization of Minimally Functionalized Alkenes via Earth Abundant Transition Metal Catalysis. Org. Chem. Front. 2018, 5, 260–272. 10.1039/C7QO00613F. [DOI] [Google Scholar]
- For select reviews, see:; a Kennemur J. L.; Maji R.; Scharf M. J.; List B. Catalytic Asymmetric Hydroalkoxylation of C–C Multiple Bonds. Chem. Rev. 2021, 121, 14649–14681. 10.1021/acs.chemrev.1c00620. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Castarlenas R.; Di Giuseppe A.; Pérez-Torrente J. J.; Oro L. A. The Emergence of Transition-Metal-Mediated Hydrothiolation of Unsaturated Carbon–Carbon Bonds: A Mechanistic Outlook. Angew. Chem., Int. Ed. 2013, 52, 211–222. 10.1002/anie.201205468. [DOI] [PubMed] [Google Scholar]; For select enantioselective examples, see:; c Slocumb H. S.; Nie S.; Dong V. M.; Yang X.-H. Enantioselective Selenol-ene Using Rh-Hydride Catalysis. J. Am. Chem. Soc. 2022, 144, 18246–18250. 10.1021/jacs.2c08475. [DOI] [PubMed] [Google Scholar]; d Yang X.; Li X.; Chen P.; Liu G. Palladium(II)-Catalyzed Enantioselective Hydrooxygenation of Unactivated Terminal Alkenes. J. Am. Chem. Soc. 2022, 144, 7972–7977. 10.1021/jacs.2c02753. [DOI] [PubMed] [Google Scholar]; e Han X.; Wang M.; Liang Y.; Zhao Y.; Shi Z. Regio- and Enantioselective Nucleophilic Addition to Gem-Difluoroallenes. Nat. Synth. 2022, 1, 227–234. 10.1038/s44160-021-00023-y. [DOI] [Google Scholar]; f Nie S.; Lu A.; Kuker E. L.; Dong V. M. Enantioselective Hydrothiolation: Diverging Cyclopropenes through Ligand Control. J. Am. Chem. Soc. 2021, 143, 6176–6184. 10.1021/jacs.1c00939. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Li M.-M.; Cheng L.; Xiao L.-J.; Xie J.-H.; Zhou Q.-L. Palladium-Catalyzed Asymmetric Hydrosulfonylation of 1,3-Dienes with Sulfonyl Hydrazides. Angew. Chem., Int. Ed. 2021, 60, 2948–2951. 10.1002/anie.202012485. [DOI] [PubMed] [Google Scholar]; h Hu J.-L.; Bauer F.; Breit B. Ruthenium-Catalyzed Enantioselective Addition of Carboxylic Acids to Allenes. ACS Catal. 2021, 11, 12301–12306. 10.1021/acscatal.1c03306. [DOI] [Google Scholar]; i Zhang Q.; Dong D.; Zi W. Palladium-Catalyzed Regio- and Enantioselective Hydrosulfonylation of 1,3-Dienes with Sulfinic Acids: Scope, Mechanism, and Origin of Selectivity. J. Am. Chem. Soc. 2020, 142, 15860–15869. 10.1021/jacs.0c05976. [DOI] [PubMed] [Google Scholar]; j Schmidt J. P.; Breit B. Rhodium-Catalyzed Cyclization of Terminal and Internal Allenols: An Atom Economic and Highly Stereoselective Access Towards Tetrahydropyrans. Angew. Chem., Int. Ed. 2020, 59, 23485–23490. 10.1002/anie.202009166. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Tsuji N.; Kennemur J. L.; Buyck T.; Lee S.; Prévost S.; Kaib P. S. J.; Bykov D.; Farès C.; List B. Activation of Olefins via Asymmetric Brønsted Acid Catalysis. Science 2018, 359, 1501–1505. 10.1126/science.aaq0445. [DOI] [PubMed] [Google Scholar]; l Pritzius A. B.; Breit B. Asymmetric Rhodium-Catalyzed Addition of Thiols to Allenes: Synthesis of Branched Allylic Thioethers and Sulfones. Angew. Chem., Int. Ed. 2015, 54, 3121–3125. 10.1002/anie.201411402. [DOI] [PubMed] [Google Scholar]; m Koschker P.; Lumbroso A.; Breit B. Enantioselective Synthesis of Branched Allylic Esters via Rhodium-Catalyzed Coupling of Allenes with Carboxylic Acids. J. Am. Chem. Soc. 2011, 133, 20746–20749. 10.1021/ja210149g. [DOI] [PubMed] [Google Scholar]
- For select reviews, see:; a Nanda S. K.; Mallik R. Transition Metal-Catalyzed Hydroalkoxylation of Alkynes: An Overview. Chem. -Eur. J. 2021, 27, 15571–15604. 10.1002/chem.202102194. [DOI] [PubMed] [Google Scholar]; b González-Belman O. F.; Brotons-Rufes A.; Tomasini M.; Falivene L.; Caporaso L.; Jiménez-Halla J. O. C.; Poater A. Towards Dual-Metal Catalyzed Hydroalkoxylation of Alkynes. Catalysts 2021, 11, 704. 10.3390/catal11060704. [DOI] [Google Scholar]
- For select examples, see:; a Liu Z.; Breit B. Rhodium-Catalyzed Enantioselective Intermolecular Hydroalkoxylation of Allenes and Alkynes with Alcohols: Synthesis of Branched Allylic Ethers. Angew. Chem., Int. Ed. 2016, 55, 8440–8443. 10.1002/anie.201603538. [DOI] [PubMed] [Google Scholar]; b Kawamoto T.; Hirabayashi S.; Guo X.-X.; Nishimura T.; Hayashi T. Rhodium-Catalyzed Asymmetric Hydroalkoxylation and Hydrosulfenylation of Diphenylphosphinylallenes. Chem. Commun. 2009, 3528–3530. 10.1039/b900976k. [DOI] [PubMed] [Google Scholar]
- Iio K.; Sachimori S.; Watanabe T.; Fuwa H. Ruthenium-Catalyzed Intramolecular Double Hydroalkoxylation of Internal Alkynes. Org. Lett. 2018, 20, 7851–7855. 10.1021/acs.orglett.8b03368. [DOI] [PubMed] [Google Scholar]
- For select examples, see:; a Jiang L.; Jia T.; Wang M.; Liao J.; Cao P. Pd-Catalyzed Enantioselective Hydroalkoxylation of Alkoxyallenes with Phenol for Construction of Acyclic O, O-Acetals. Org. Lett. 2015, 17, 1070–1073. 10.1021/acs.orglett.5b00146. [DOI] [PubMed] [Google Scholar]; b Patil N. T.; Lutete L. M.; Wu H.; Pahadi N. K.; Gridnev I. D.; Yamamoto Y. Palladium-Catalyzed Intramolecular Asymmetric Hydroamination, Hydroalkoxylation, and Hydrocarbonation of Alkynes. J. Org. Chem. 2006, 71, 4270–4279. 10.1021/jo0603835. [DOI] [PubMed] [Google Scholar]
- For select examples, see:; a Cheng X.; Wang Z.; Quintanilla C. D.; Zhang L. Chiral Bifunctional Phosphine Ligand Enabling Gold-Catalyzed Asymmetric Isomerization of Alkyne to Allene and Asymmetric Synthesis of 2,5-Dihydrofuran. J. Am. Chem. Soc. 2019, 141, 3787–3791. 10.1021/jacs.8b12833. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hamilton G. L.; Kang E. J.; Mba M.; Toste F. D. A Powerful Chiral Counterion Strategy for Asymmetric Transition Metal Catalysis. Science 2007, 317, 496–499. 10.1126/science.1145229. [DOI] [PubMed] [Google Scholar]
- a Fernandes R. A.; Chandra N.; Gangani A. J.; Khatun G. N. Palladium-Catalyzed Regioselective Intermolecular Hydroalkoxylation of 1-Arylbutadienes. J. Org. Chem. 2022, 10.1021/acs.joc.2c01252. [DOI] [PubMed] [Google Scholar]; b Mifleur A.; Suisse I.; Mortreux A.; Sauthier M. Enantioselective Nickel Catalyzed Butadiene Hydroalkoxylation with Ethanol: from Experimental Results to Kinetics Parameters. Catal. Lett. 2021, 151, 27–35. 10.1007/s10562-020-03267-z. [DOI] [Google Scholar]; c Tran G.; Mazet C. Ni-Catalyzed Regioselective Hydroalkoxylation of Branched 1,3-Dienes. Org. Lett. 2019, 21, 9124–9127. 10.1021/acs.orglett.9b03511. [DOI] [PubMed] [Google Scholar]; d Mifleur A.; Merel D. S.; Mortreux A.; Suisse I.; Capet F.; Trivelli X.; Sauthier M.; Macgregor S. A. Deciphering the Mechanism of the Nickel-Catalyzed Hydroalkoxylation Reaction: A Combined Experimental and Computational Study. ACS Catal. 2017, 7, 6915–6923. 10.1021/acscatal.7b00616. [DOI] [Google Scholar]; e Mifleur A.; Mortreux A.; Suisse I.; Sauthier M. Synthesis of C4-Chain Glyceryl Ethers via Nickel-Catalyzed Butadiene Hydroalkoxylation Reaction. Mol. Catal. 2017, 427, 25–30. 10.1016/j.molcata.2016.11.022. [DOI] [Google Scholar]; f Mifleur A.; Ledru H.; Lopes A.; Suisse I.; Mortreux A.; Sauthier M. Synthesis of Short-Chain Alkenyl Ethers from Primary and Bio-sourced Alcohols via the Nickel-Catalyzed Hydroalkoxylation Reaction of Butadiene and Derivatives. Adv. Synth. Catal. 2016, 358, 110–121. 10.1002/adsc.201500721. [DOI] [Google Scholar]; g Bigot S.; El Alami M. S. I.; Mifleur A.; Castanet Y.; Suisse I.; Mortreux A.; Sauthier M. Nickel-Catalysed Hydroalkoxylation Reaction of 1,3-Butadiene: Ligand Controlled Selectivity for the Efficient and Atom-Economical Synthesis of Alkylbutenyl Ethers. Chem. - Eur. J. 2013, 19, 9785–9788. 10.1002/chem.201300633. [DOI] [PubMed] [Google Scholar]; h Patrini R.; Lami M.; Marchionna M.; Benvenuti F.; Galletti A. M. R.; Sbrana G. Selective Synthesis of Octadienyl and Butenyl Ethers via Reaction of 1,3-Butadiene with Alcohols Catalyzed by Homogeneous Palladium Complexes. J. Mol. Catal. A: Chem. 1998, 129, 179–189. 10.1016/S1381-1169(97)00155-6. [DOI] [Google Scholar]; i Smutny E. J. Oligomerization and Dimerization of Butadiene Under Homogeneous catalysis. Reaction with Nucleophiles and the Synthesis of 1,3,7-Octatriene. J. Am. Chem. Soc. 1967, 89, 6793–6794. 10.1021/ja01001a089. [DOI] [Google Scholar]
- For a recent review on Ni-catalyzed hydrofunctionalization of alkenes, see:; a Sun X.-Y.; Yao B.-Y.; Xuan B.; Xiao L.-J.; Zhou Q.-L. Recent advances in nickel-catalyzed asymmetric hydrofunctionalization of alkenes. Chem Catal. 2022, 2, 3140–3162. 10.1016/j.checat.2022.10.020. [DOI] [Google Scholar]; For select recent Ni-catalyzed enantioselective hydrofunctionalizations of dienes, see:; b Li J.-F.; Pan D.; Wang H.-R.; Zhang T.; Li Y.; Huang G.; Ye M. Enantioselective C2–H Alkylation of Pyridines with 1,3-Dienes via Ni–Al Bimetallic Catalysis. J. Am. Chem. Soc. 2022, 144, 18810–18816. 10.1021/jacs.2c09306. [DOI] [PubMed] [Google Scholar]; c Cheng L.; Li M.-M.; Li M.-L.; Xiao L.-J.; Xie J.-H.; Zhou Q.-L. Nickel-Catalyzed Regio- and Enantioselective Hydroarylation of 1,3-Dienes with Indoles. CCS Chem. 2022, 4, 2612–2619. 10.31635/ccschem.021.202101472. [DOI] [Google Scholar]; d Long J.; Li Y.; Zhao W.; Yin G. Nickel/Brønsted acid dual-catalyzed regio- and enantioselective hydrophosphinylation of 1,3-dienes: access to chiral allylic phosphine oxides. Chem. Sci. 2022, 13, 1390–1397. 10.1039/D1SC05651D. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Liao L.; Zhang Y.; Wu Z.-W.; Ye Z.-T.; Zhang X.-X.; Chen G.; Yu J.-S. Nickel-catalyzed regio- and enantio-selective Markovnikov hydromonofluoroalkylation of 1,3-dienes. Chem. Sci. 2022, 13, 12519–12526. 10.1039/D2SC03958C. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Shao W.; Besnard C.; Guénée L.; Mazet C. Ni-Catalyzed Regiodivergent and Stereoselective Hydroalkylation of Acyclic Branched Dienes with Unstabilized C(sp3) Nucleophiles. J. Am. Chem. Soc. 2020, 142, 16486–16492. 10.1021/jacs.0c08319. [DOI] [PubMed] [Google Scholar]; g Marcum J. S.; Taylor T. R.; Meek S. J. Enantioselective Synthesis of Functionalized Arenes by Nickel Catalyzed Site-Selective Hydroarylation of 1,3-Dienes with Aryl Boronates. Angew. Chem., Int. Ed. 2020, 59, 14070–14075. 10.1002/anie.202004982. [DOI] [PubMed] [Google Scholar]; h Tran G.; Shao W.; Mazet C. Ni-Catalyzed Enantioselective Intermolecular Hydroamination of Branched 1,3-Dienes Using Primary Aliphatic Amines. J. Am. Chem. Soc. 2019, 141, 14814–14822. 10.1021/jacs.9b07253. [DOI] [PubMed] [Google Scholar]; i Long J.; Wang P.; Wang W.; Li Y.; Yin G. Nickel/Brønsted Acid-Catalyzed Chemo- and Enantioselective Intermolecular Hydroamination of Conjugated Dienes. iScience 2019, 22, 369–379. 10.1016/j.isci.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Lv X.-Y.; Fan C.; Xiao L.-J.; Xie J.-H.; Zhou Q.-L. Ligand-Enabled Ni-Catalyzed Enantioselective Hydroarylation of Styrenes and 1,3-Dienes with Arylboronic Acids. CCS Chem. 2019, 1, 328–334. 10.31635/ccschem.019.20190026. [DOI] [Google Scholar]; k Cheng L.; Li M.-M.; Xiao L.-J.; Xie J.-H.; Zhou Q.-L. Nickel(0)-Catalyzed Hydroalkylation of 1,3-Dienes with Simple Ketones. J. Am. Chem. Soc. 2018, 140, 11627–11630. 10.1021/jacs.8b09346. [DOI] [PubMed] [Google Scholar]
- a Jiu A. Y.; Slocumb H. S.; Yeung C. S.; Yang X.-H.; Dong V. M. Enantioselective Addition of Pyrazoles to Dienes. Angew. Chem., Int. Ed. 2021, 60, 19660–19664. 10.1002/anie.202105679. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Yang X.-H.; Lu A.; Dong V. M. Intermolecular Hydroamination of 1,3-Dienes to Generate Homoallylic Amines. J. Am. Chem. Soc. 2017, 139, 14049–14052. 10.1021/jacs.7b09188. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Yang X.-H.; Dong V. M. Rhodium-Catalyzed Hydrofunctionalization: Enantioselective Coupling of Indolines and 1,3-Dienes. J. Am. Chem. Soc. 2017, 139, 1774–1777. 10.1021/jacs.6b12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Yang X.-H.; Davison R. T.; Nie S.-Z.; Cruz F. A.; McGinnis T. M.; Dong V. M. Catalytic Hydrothiolation: Counterion-Controlled Regioselectivity. J. Am. Chem. Soc. 2019, 141, 3006–3013. 10.1021/jacs.8b11395. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Yang X.-H.; Davison R. T.; Dong V. M. Catalytic Hydrothiolation: Regio- and Enantioselective Coupling of Thiols and Dienes. J. Am. Chem. Soc. 2018, 140, 10443–10446. 10.1021/jacs.8b06957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie S.-Z.; Davison R. T.; Dong V. M. Enantioselective Coupling of Dienes and Phosphine Oxides. J. Am. Chem. Soc. 2018, 140, 16450–16454. 10.1021/jacs.8b11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For Mayr’s Database of Reactivity Parameters, see: https://www.cup.lmu.de/oc/mayr/reaktionsdatenbank/fe/ (accessed 2022-03-05).
- a Holmes M.; Nguyen K. D.; Schwartz L. A.; Luong T.; Krische M. J. Enantioselective Formation of CF3-Bearing All-Carbon Quaternary Stereocenters via C-H Functionalization of Methanol: Iridium Catalyzed Allene Hydrohydroxymethylation. J. Am. Chem. Soc. 2017, 139, 8114–8117. 10.1021/jacs.7b04374. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Nguyen K. D.; Herkommer D.; Krische M. J. Enantioselective Formation of All-Carbon Quaternary Centers via C-H Functionalization of Methanol: Iridium-Catalyzed Diene Hydrohydroxymethylation. J. Am. Chem. Soc. 2016, 138, 14210–14213. 10.1021/jacs.6b09333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MeOH was replaced with aromatic substituted alcohols to ensure UV–vis detection for er determination.
- Absolute configuration of related products assigned by analogy to 3ac. The absolute configuration of 3ac was assigned as S by comparison to optical rotation with previous reported literature.Feng B.; Cheng H.-G.; Chen J.-R.; Deng Q.-H.; Lu L.-Q.; Xiao W.-J. Palladium/Sulfoxide–Phosphine-Catalyzed Highly Enantioselective Allylic Etherification and Amination. Chem. Commun. 2014, 50, 9550–9553. 10.1039/C4CC03920C. [DOI] [PubMed] [Google Scholar]
- a Trost B. M.; Van Vranken D. L. Asymmetric Transition Metal-Catalyzed Allylic Alkylations. Chem. Rev. 1996, 96, 395–422. 10.1021/cr9409804. [DOI] [PubMed] [Google Scholar]; b Sha S.-C.; Jiang H.; Mao J.; Bellomo A.; Jeong S. A.; Walsh P. J. Nickel-Catalyzed Allylic Alkylation with Diarylmethane Pronucleophiles: Reaction Development and Mechanistic Insights. Angew. Chem., Int. Ed. 2016, 55, 1070–1074. 10.1002/anie.201507494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Cheng Q.; Dang Y. Mechanistic Studies of Nickel-Catalyzed Hydroarylation of Styrenes. Org. Lett. 2020, 22, 8998–9003. 10.1021/acs.orglett.0c03395. [DOI] [PubMed] [Google Scholar]; b Tang S.; Eisenstein O.; Nakao Y.; Sakaki S. Aromatic C–H σ-Bond Activation by Ni0, Pd0, and Pt0 Alkene Complexes: Concerted Oxidative Addition to Metal vs Ligand-to-Ligand H Transfer Mechanism. Organometallics 2017, 36, 2761–2771. 10.1021/acs.organomet.7b00256. [DOI] [Google Scholar]
- a Nielsen C. D.-T.; Burés J. Visual Kinetic Analysis. Chem. Sci. 2019, 10, 348–353. 10.1039/C8SC04698K. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Burés J. Variable Time Normalization Analysis: General Graphical Elucidation of Reaction Orders from Concentration Profiles. Angew. Chem., Int. Ed. 2016, 55, 16084–16087. 10.1002/anie.201609757. [DOI] [PubMed] [Google Scholar]
- We thank one of the reviewers for constructive suggestions regarding the kinetic experiments: “The author must perform VTNA where the relative stoichiometry is decreased and not only increased as indicated in the SI (from 0.2 to 0.4 mmol). They find a completely different order in ROH. Overall, the role/nature of the solvent (presence vs absence; reversible process vs irreversible process) might also be intimately connected with the nature of the H-bonding network.”
- See SI for details.
- We propose (Z)- to (E)-isomerization via a familiar σ-π-σ process; please see the SI for figure and details.
- a Chen P.-P.; Lucas E. L.; Greene M. A.; Zhang S.-Q.; Tollefson E. J.; Erickson L. W.; Taylor B. L. H.; Jarvo E. R.; Hong X. A Unified Explanation for Chemoselectivity and Stereospecificity of Ni-Catalyzed Kumada and Cross-Electrophile Coupling Reactions of Benzylic Ethers: A Combined Computational and Experimental Study. J. Am. Chem. Soc. 2019, 141, 5835–5855. 10.1021/jacs.9b00097. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhang H. J.; Gu Q.; You S. L. Recent Advances in Ni-Catalyzed Allylic Substitution Reactions. Chin. J. Org. Chem. 2019, 39, 15–27. 10.6023/cjoc201809037. [DOI] [Google Scholar]; c Zhang S.-Q.; Taylor B. L. H.; Ji C. L.; Gao Y.; Harris M. R.; Hanna L. E.; Jarvo E. R.; Houk K. N.; Hong X. Mechanism and Origins of Ligand-Controlled Stereoselectivity of Ni-Catalyzed Suzuki–Miyaura Coupling with Benzylic Esters: A Computational Study. J. Am. Chem. Soc. 2017, 139, 12994–13005. 10.1021/jacs.7b04973. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Zhou Q.; Srinivas H. D.; Zhang S.; Watson M. P. Accessing Both Retention and Inversion Pathways in Stereospecific, Nickel-Catalyzed Miyaura Borylations of Allylic Pivalates. J. Am. Chem. Soc. 2016, 138, 11989–11995. 10.1021/jacs.6b07396. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Sylvester K. T.; Wu K.; Doyle A. G. Mechanistic Investigation of the Nickel-Catalyzed Suzuki Reaction of N, O-Acetals: Evidence for Boronic Acid Assisted Oxidative Addition and an Iminium Activation Pathway. J. Am. Chem. Soc. 2012, 134, 16967–16970. 10.1021/ja3079362. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Evans P. A.; Nelson J. D. Conservation of Absolute Configuration in the Acyclic Rhodium-Catalyzed Allylic Alkylation Reaction: Evidence for an Enyl (σ + π) Organorhodium Intermediate. J. Am. Chem. Soc. 1998, 120, 5581–5582. 10.1021/ja980030q. [DOI] [Google Scholar]; g Faller J. W.; Linebarrier D. Reversal of Stereochemical Path in Allylic Alkylations Promoted by Palladium and Molybdenum Complexes. Organometallics 1988, 7, 1670–1672. 10.1021/om00097a040. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.