Abstract
In autumn 2022, the Spanish Influenza National Reference Laboratory (NRL) confirmed the detection of influenza A(H5N1) in samples from two asymptomatic workers linked to an outbreak in a poultry farm in Spain. Nasopharyngeal swabs were taken according to a national screening protocol for exposed workers. Absence of symptoms, low viral load and negative serology in both workers suggested environmental contamination. These findings motivated an update of the early detection strategy specifying timing and sampling conditions in asymptomatic exposed persons.
Keywords: highly pathogenic avian influenza, HPAI A(H5N1), Spain, human, asymptomatic, outbreak
The increase in the number of highly pathogenic avian influenza (HPAI) A(H5N1) outbreaks in birds (wild birds, poultry and domestic birds) and the indications of a possible increase in transmissibility in mammals in the 2021/22 northern hemisphere influenza season and at the beginning of the 2022/23 season have generated greater concern about the possible appearance of cases in humans [1]. We report the detection of influenza A(H5N1) in two asymptomatic workers linked to an outbreak of HPAI A(H5N1) in a poultry farm in the autumn of 2022 in Spain. Interpretation of these findings and implications in surveillance strategies are discussed.
Event detection and description
On 20 September 2022, an outbreak of HPAI A(H5N1) was confirmed in a poultry farm in the autonomous region of Castilla-La Mancha, Spain [2]. Two days later, screening for influenza was performed on all 12 exposed workers. A nasopharyngeal swab from one worker, aged ca 20 years who was asymptomatic, was positive for influenza A by RT-PCR performed at the regional reference laboratory on 22 September. The presence of influenza A(H5N1) was confirmed by PCR by the Influenza National Reference Laboratory (NRL) on 27 September. After notification to the World Health Organization (WHO) on 4 October in accordance with International Health Regulation (IHR), a respiratory sample and an additional serum sample taken on 8 October were sent to the WHO Reference Laboratory (WRL) in London, United Kingdom yielding negative results for PCR detection and serology.
In response to the outbreak, culling of all hens at the farm (19,206 were culled and 130,941 died of the infection) was completed by 13 October and a new screening for influenza was conducted in all workers. This screening involved the initial 12 workers and 14 others who were involved in control tasks. A second asymptomatic worker in their late 20s tested positive for influenza A(H5N1) at the NRL. Samples for serology from this second worker were also taken on 19 October and 23 November. They were sent to the WRL and yielded a negative result.
Both workers were involved in egg collection and routine cleaning tasks. They used personal protection equipment, including an FFP2 mask, gloves, boots and an apron. After the outbreak confirmation, they helped in removal of dead hens, cleaning and disinfection of the premises, which ended on 22 October 2022.
The asymptomatic workers stayed in self-isolation, per the national protocol, from the first RT-PCR positive result until a second, negative, sample was obtained (on day 6 and day 9 for Worker 1 and 2, respectively). The other 24 workers remained asymptomatic throughout the monitoring period (up to 10 days after the last day of exposure) and tested negative in both screenings.
The absence of symptoms in both workers together with the laboratory results, which showed a very low viral load and the absence of specific H5 antibodies against the A/H5 virus, suggested that the positive results in the PCR were most likely due to environmental contamination. Of note, all samples (nasopharyngeal swabs) were taken outside the farm at a healthcare centre.
Public health response and control measures
The Practical Operations Manual of the Spanish Ministry of Agriculture, Fisheries and Food establishes the containment measures to be implemented in farms where HPAI outbreaks are detected [3]. Control measures, overseen by the regional official veterinary services, included the on-site culling of the birds, the destruction of contaminated material and the cleaning and disinfection of the facilities.
Passive surveillance of symptoms and RT-PCR testing of exposed workers were implemented in accordance with the national protocol [4]. The protocol indicates the tests should be performed as soon as possible after the confirmation of the outbreak and again 5 days after the last day of exposure. However, as an extraordinary precaution after the first worker tested positive, regional public health authorities decided to perform a second screening on the same day the culling ended, while cleaning and disinfection was still ongoing.
Contact tracing identified one household contact for Worker 1 and two for Worker 2. All three contacts remained asymptomatic during the 10-day follow-up and tested negative by PCR.
Since the first detection on 27 September and up to 31 December 2022, influenza A-positive samples from all infections in humans in the same administrative area where the farm was located – 198 in total – were subtyped by RT-PCR obtaining negative results for A(H5N1).
Virological investigation
At the NRL, influenza A was first confirmed using a real-time multiplex RT-PCR for influenza A, B and C [5], resulting positive for influenza A with low viral load (based on quantification cycle (Cq) values) of 35.62 and 33.60, respectively. Subtyping of haemagglutinin (HA) of the influenza A virus by real-time RT-PCR identified an A(H5) subtype at low viral load of 35.84 and 34.61 Cq values, respectively.
Whole genome sequences based on universal primers previously described and adapted to NRL [6], were generated from the samples obtained from the two workers and shared through GISAID [7] (Table). To increase the sequencing success given the low viral load, a NovaSeq 6000 System was used to sequence both samples. Sequences from Worker 1 were complete for all segments but for Worker 2, the sequencing of the polymerase genes failed but segments HA, partial NP, NA, MP and NS were obtained (Table).
Table. GISAID accession numbers of sequences from the two farm workers and two reference laying hens affected in the highly pathogenic avian influenza A(H5N1) outbreak, Spain, 20 September–13 October 2022.
| Sample | Isolate name | Isolate ID | PB2 segment ID | PB1 segment ID | PA segment ID | HA segment ID | NP segment ID | NA segment ID | MP segment ID | NS segment ID |
|---|---|---|---|---|---|---|---|---|---|---|
| Worker 1 | A/CastillaLaMancha/3739/2022 | EPI_ISL_15542438 | EPI2197458 | EPI2197459 | EPI2197457 | EPI2197461 | EPI2197455 | EPI2197460 | EPI2352712 | EPI2197456 |
| Worker 2 | A/CastillaLaMancha/3869/2022 | EPI_ISL_16813290 | NP | NP | NP | EPI2352966 | EPI2352962 (partial) | EPI2352965 | EPI2352964 | EPI2352963 |
| Hen 1 | A/laying_hen/Spain/3232–37_22VIR10586–4/2022 | EPI_ISL_15878546 | EPI2220650 | EPI2220651 | EPI2220649 | EPI2220653 | EPI2220646 | EPI2220652 | EPI2220648 | EPI2220647 |
| Hen 2 | A/laying_hen/Spain/3232–35_22VIR10586–3/2022 | EPI_ISL_15878545 | EPI2220642 | EPI2220643 | EPI2220641 | EPI2220645 | EPI2220638 | EPI2220644 | EPI2220640 | EPI2220639 |
NP: not possible.
Virus sequences from samples obtained from the two workers were shared through GISAID [7]. Hen sequences were submitted to the GISAID by the European Reference Laboratory for Avian Influenza.
The analysis of the complete HA gene segment showed that the HPAI H5N1 human viruses belong to clade 2.3.4.4b. Clustering of the two viral genomes from farm workers indicates that they are highly related with those obtained from sequenced laying hen genomes from the same farm. Hen sequences were submitted to the GISAID database by the European Reference laboratory (EURL) for avian influenza (Table). The sequence homology in segments PB2, PB1, PA, NA, MP and NS is 100% between human sequences and those corresponding to laying-hens. The virus from Worker 1 presented two amino acid differences in comparison with the viruses from the laying hens (HA-H289-I (H276I, H3 numbering) and NP-Q327R). The sequence from Worker 2 presented one amino acid difference (HA-I531M (I515M, H3 numbering)) from the hen samples.
Epidemiological context
The HPAI epizootic observed in the 2021/22 season, mainly because of the A(H5N1) subtype, has been the largest recorded to date in Europe [1] and the Americas [8]. Despite this situation, only four additional detections in humans in addition to the two described here have been reported from 2021 [9].
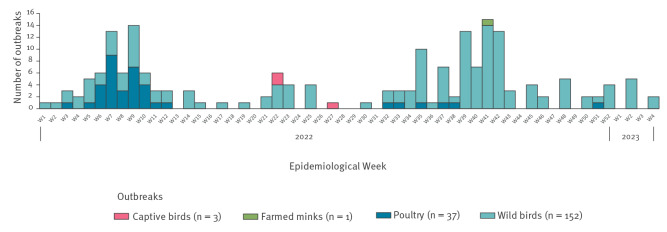
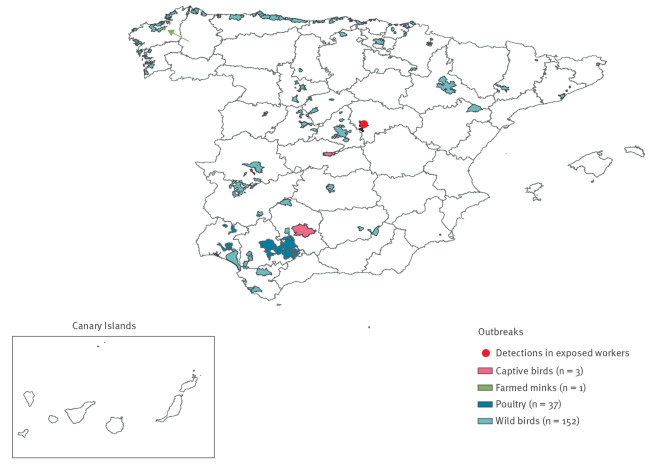
Before the 2021/22 season, there had only been one detection of influenza A(H5N1) in a wild bird in Spain, which occurred in 2006 [10]. The number of HPAI outbreaks in animals in Spain since January 2022 up to week 4 2023 is presented (Figures 1 and 2).
Figure 1.
Highly pathogenic avian influenza A(H5N1) outbreaks in birds and mammals, Spain, week 1 2022−week 4 2023 (n = 193)
Figure 2.
Geographical location of highly pathogenic avian influenza A(H5N1) animal outbreaks (n = 193) and human detections (n = 2) by municipality, Spain, week 1 2022−week 4 2023
The farmed mink outbreak (green) is also denoted by an arrow.
The HPAI epidemiological situation led to the update of the Prevention, Early Detection and Actions against Avian Influenza Protocol on 4 March 2022, in which the screening of all farm workers exposed to outbreaks was incorporated. From this update to week 4 2023, 133 exposed farm workers from 12 premises across Spain (100% of infected farms) were tested for influenza and all were negative, except for the two individuals described here.
Discussion
Although bird to human transmission of HPAI A(H5N1) is considered unusual and person-to-person transmission is very rare, a few human cases and high death rates have been recorded in some countries since 2003 [9]. Furthermore, given their rapid evolution, influenza A viruses might eventually acquire genetic changes that enhance the ability to transmit efficiently between people. Given the changes observed in the pattern of HPAI A(H5N1) during the 2022/23 season in Europe, which extended into the summer months, it is advisable to reinforce the surveillance of possible zoonotic transmission events at the animal–human interface. In addition, using a common approach for early detection of human cases is desired so results are comparable and harmonised between countries.
Testing of asymptomatic exposed persons can be implemented to further strengthen surveillance around HAPI outbreaks in poultry farms, although it raises the possibility of difficult interpretation of equivocal results. Currently, the European Centre for Disease Prevention and Control (ECDC) recommendations and national protocols in most of the European countries do not include PCR screening for asymptomatic HPAI-exposed persons [11-14]. Spain implemented this measure after updating the national protocol in March 2022, which has led to a more comprehensive surveillance of eventual human cases linked to outbreaks in poultry farms. However, these two positive results in asymptomatic workers raised the problem of misclassification because of possible environmental contamination. Moreover, the classification of a confirmed A(H5N1) case according to the ECDC definition [15] obligates notification under IHR. False positives have negative consequences that include social stigma, unnecessary use of pharmaceutical and non-pharmaceutical interventions, economic impact for the food industry or difficulties in risk communication to the public. To minimise the chances of contamination, public health protocols should clearly specify the right conditions and timing for swabbing. As a result of the event described, the Spanish protocol was updated with these details on 3 February 2023, stating that human cases will only be considered confirmed if samples are taken under adequate hygienic conditions, i.e. wearing clean clothes and sampling at a healthcare center rather than on location, and by swabbing 5 and 7 days after the last exposure and avoiding the sampling after the workday [16].
Conclusions
Following the observed increase in the number of HPAI outbreaks, concern about the possible occurrence of human cases has increased. Screening of exposed workers on affected farms is a measure aimed at improving early detection of zoonotic infections. However, the appropriate conditions for carrying out the tests to avoid contamination and the criteria for interpreting the results must be considered. Biosecurity measures, including seasonal influenza vaccination of workers and the use of appropriate PPE, are key to prevent spillover on farms with detected outbreaks.
Ethical statement
The events and measures described were part of routine surveillance and control activities. The workers consented to information being shared as part of a case report in the scientific literature.
Acknowledgements
We gratefully acknowledge all data contributors, i.e., the authors and their originating laboratories responsible for obtaining the specimens, and their submitting laboratories for generating the genetic sequence and metadata and sharing via the GISAID Initiative, on which this research is based. We thank Noelia Reyes, Silvia Moreno, M Jose Casas, Sara Camarero and Mar Molinero, from the National Reference Laboratory for their exceptional technical support. Angel Zaballos, Pilar Jimenez, Mercedes Jimenez from the Genomics department and Isabel Cuesta, Sara Monzon, Sarai Varona from the Bioinformatics department at Instituto de Salud Carlos III who collaborated in the analysis of sequences, Complete whole sequencing has been funded by Instituto de Salud Carlos III through the Project MPY 226/22. We would also like to acknowledge Amparo Larrauri Cámara from the National Center of Epidemiology, Spain, Luis José Romero González and Germán Cáceres Garrido, from the Ministry of Agriculture, Fisheries and Food, Spain, and Prof Nicola Lewis (Director), Dr Rodney Daniels (Deputy Director), Dr Ruth Harvey (Assistant Director), Dr Monica Galiano (Assistant Director), Dr Zheng Xiang, Ms Lynne Whittaker, Ms Christine Carr, Ms Burcu Ermetal, Dr Karen Cross, Ms Aine Rattigan, Ms Alice Lilley, Mr Michael Bennett, Ms Chandrika Halai, Ms Becky Clark, Mr Lorin Adams from the Worldwide Influenza Centre Lab Crick Institute, United Kingdom.
Conflict of interest: None declared.
Authors’ contributions: EA had a role in the public health response at the national level and drafted the first version of the manuscript. IC led the laboratory investigation at the national level and drafted the virological information part of the manuscript. FP and MI-C took part in the human health laboratory investigation at the national level. LGS and MJSM coordinated the alert response at the national level. GS was involved data management at national level. EGV coordinated the Animal Health response at national level. MJRR and ASS led the Animal Health laboratory investigation at national level. ERM led the management of the alert in the Region of Castilla-La Mancha. AGP led the laboratory investigation at regional level. MVGR had a role in the Public Health response at Regional Level. All authors revised the manuscript.
References
- 1. Adlhoch C, Fusaro A, Gonzales JL, Kuiken T, Marangon S, Niqueux É, et al. Avian influenza overview September - December 2022. EFSA J. 2023;21(1):e07786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ministerio de Agricultura, Pesca y Alimentación. Se detecta un segundo foco de influenza aviar de alta patogenicidad (IAAP) H5N1 en una granja de gallinas ponedores en Guadalajara. [A second outbreak of high avian influenza pathogenicity (hpai) h5n1 is detected in a laying hen farm in Guadalajara.] Madrid: Subdirección General de Sanidad e Higiene Animal y Trazabilidad; 2022. Spanish. Available from: https://www.mapa.gob.es/es/ganaderia/temas/sanidad-animal-higiene-ganadera/iaapactualizacionfocos20092022r_tcm30-628242.pdf
- 3.Ministerio de Agrigultura Pesca y Alimentación. Manual práctico de operaciones en la lucha contra la influenza aviar. [Practical manual of operations in the fight against the avian influenza]. Madrid: Subdirección General de Sanidad e Higiene Animal y Trazabilidad; 2022. Spanish. Available from: https://www.mapa.gob.es/es/ganaderia/temas/sanidad-animal-higiene-ganadera/manualiaabril2022_tcm30-437988.pdf
- 4.Ministerio de Sanidad. Prevención, detección precoz y actuaciones frente a la gripe aviar. [Prevention, early detection and actions against avian influenza]. Madrid: Gobierno de Espana; 2022. Spanish. Available from: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/docs/20220304_Vigilancia_prevencion_gripe_aviar.pdf
- 5. Garcia-Garcia ML, Calvo C, Ruiz S, Pozo F, Del Pozo V, Remedios L, et al. Role of viral coinfections in asthma development. PLoS One. 2017;12(12):e0189083. 10.1371/journal.pone.0189083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou B, Donnelly ME, Scholes DT, St George K, Hatta M, Kawaoka Y, et al. Single-reaction genomic amplification accelerates sequencing and vaccine production for classical and Swine origin human influenza a viruses. J Virol. 2009;83(19):10309-13. 10.1128/JVI.01109-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elbe S, Buckland-Merrett G. Data, disease and diplomacy: GISAID’s innovative contribution to global health. Glob Chall. 2017;1(1):33-46. 10.1002/gch2.1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Organisation for Animal Health (WOAH). High Pathogenicity Avian Influenza (HPAI)-Situation Report. Paris: WOAH; 2022. Available from: https://www.woah.org/app/uploads/2022/10/hpai-situation-report-20221020.pdf
- 9.World Health Organization (WHO). Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003-2021. Geneva: WHO; 2021. Available from: https://www.who.int/publications/m/item/cumulative-number-of-confirmed-human-cases-for-avian-influenza-a(h5n1)-reported-to-who-2003-2021-15-april-2021
- 10.CIDRAP News. Spain reports first avian flu case in birds. Minneapolis: University of Minnesota; 7 Jul 2006. Available from: https://www.cidrap.umn.edu/avian-influenza-bird-flu/spain-reports-first-avian-flu-case-birds
- 11.European Center for Disease Prevention and Control (ECDC). Testing and detection of zoonotic influenza virus infections in humans in the EU/EEA, and occupational safety and health measures for those exposed at work. Stockholm: ECDC; 2022. Available from https://www.ecdc.europa.eu/en/publications-data/zoonotic-influenza-virus-infections-humans-testing-and-detection
- 12.Haut Conseil de la Sante Publique. Avis relatif à la prévention de la transmission à l’homme des virus influenza porcins et aviaires. [Statement on the prevention of transmission of swine and avian influenza viruses to humans]. Paris: Haut Conseil de la Sante Publique; 10 Dec 2021. French. Available from: https://www.hcsp.fr/Explore.cgi/AvisRapportsDomaine?clefr=1142
- 13.Terveyden ja hyvinvoinnin laitos (THL). Toimenpideohje ihmisen lintuinfluenssatartuntojen torjumiseksi. [Guidelines for the control of avian influenza infections in humans]. Helsinki: THL. [Accessed: 10 Oct 2022]. Finnish. Available from: https://thl.fi/fi/web/infektiotaudit-ja-rokotukset/taudit-ja-torjunta/taudit-ja-taudinaiheuttajat-a-o/lintuinfluenssa/toimenpideohje-ihmisen-lintuinfluenssatartuntojen-torjumiseksi
- 14.Health Protection Surveillance Centre (HPSC). Guidance on public health actions to be taken on notification of Avian Influenza (AI) in poultry and wild birds in Ireland. Dublin: HPSC; 2017. Available from: https://www.hpsc.ie/a-z/respiratory/influenza/pandemicinfluenza/guidance/pandemicinfluenzapreparednessforireland/supplement11tochapter11part1/2017%20Supplement%2011Part%201.pdf
- 15.European Centre for Disease Prevention and Control (ECDC). EU case definitions. Stockholm: ECDC. [Accessed: 23 Dec 2022]. Available from: https://www.ecdc.europa.eu/en/all-topics/eu-case-definitions
- 16.Ministerio de Sanidad. Prevención, detección precoz y control de gripe aviar en personas expuestas a focos en aves y visones [Prevention, early detection and control of avian influenza in persons exposed to outbreaks in birds and mink]. Madrid: Gobierno de Espana; 2023. Spanish. Available from: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/docs/20230203_Vigilancia_prevencion_gripe_aviar.pdf