Abstract
Introduction
Obstetric anal sphincter injury (OASI) complicates around 5% of deliveries in primiparas. The study objective was to assess the utility of three‐dimensional endoanal ultrasonography (3D‐EAUS) in the diagnosis of OASI.
Material and methods
The present study was designed to mirror screening settings with an unselected cohort of nulliparous women. All enrolled patients underwent clinical examination of the perineum by the caregiver, and 3D‐EAUS was conducted. Post‐processing of ultrasonography volume data was performed by an experienced colorectal surgeon who was blinded to all other data. The sensitivity, specificity, negative predictive value, and positive predictive value of 3D‐EAUS in the diagnosis of OASI was evaluated. The trial is registered at ISCRTN: 18006769.
Results
A total of 680 scans were performed, of which 18.5% were judged as “non‐assessable”, resulting in 554 assessable recordings. Sphincter defects were observed in 12.8% of all assessable recordings on 3D‐EAUS (n = 71). With clinical examination set as the reference standard, ultrasound sensitivity in the diagnosis of OASI was 30.4%, whereas its specificity was 87.9%. The negative predictive value was 96.7% and the positive predictive value was only 9.9%. Comments were left on 175 examinations, of which 74% referred to the management of the examination.
Conclusions
Using 3D‐EAUS in a maternity ward is demanding because staff generally have little experience in endoanal ultrasound, which contributes to difficulties in obtaining good image quality. When 3D‐EAUS is performed to mirror screening settings, it adds no convincing diagnostic power to clinical examination in the diagnosis of OASI.
Keywords: endoanal ultrasound, feasibility, obstetric anal sphincter injury, pelvic floor, postpartum, three‐dimensional endoanal ultrasonography
Acquisition of immediate postpartum optimal three‐dimensional endoanal ultrasound (3D‐EAUS) images presents significant challenges that may affect image quality and interpretation. This article will describe some of these problems and exemplify them in images.
Abbreviations
- OASI
obstetric anal sphincter injury
- 3D‐EAUS
three‐dimensional endoanal ultrasonography
- TPUS
transperineal ultrasound
Key message.
Acquisition of immediate postpartum optimal three‐dimensional endoanal ultrasound images presents significant challenges that may affect image quality and interpretation. This article will describe some of these problems and exemplify them in images.
1. INTRODUCTION
Obstetric anal sphincter injury (OASI) is a common cause of anal incontinence and is associated with urinary incontinence, perineal pain, prolapse, and impaired sexual function. The reported prevalence of OASI by clinical diagnosis ranges widely in different studies. 1 Clinical injury is reported in approximately 5% of primiparous women. 2 Adding ultrasonography to standard clinical examination revealed a 12%–35% prevalence of occult or missed injuries, indicating diagnostic difficulties. 3 , 4 , 5 , 6 The routine in maternity units is an inspection of vagina and perineum and digital rectal palpation performed by the responsible accoucheur. The incidence of OASI is higher in studies where perineal tears are re‐examined by a second person. 7 There is available evidence regarding many aspects of prevention, diagnosis, and management of OASI, but further evaluation of the utility of sonography in the immediate postpartum to diagnose OASI has been suggested . 8 , 9
Three‐dimensional endoanal ultrasonography (3D‐EAUS) is a well‐documented method of evaluating damage to the anal sphincter, 10 and is regarded as the reference standard for the evaluation of anal incontinence. 4 The examination is easily performed and usually well tolerated. Research has indicated a high level of intra–inter observer agreement in the detection of endosonographic sphincter defects. 11 , 12 Experienced examiners have obtained high‐quality interpretable pictures immediately after birth in a limited context. 13 Despite this, 3D‐EAUS is not commonly used in maternity wards and obstetricians are seldom accustomed to performing scans or interpreting recorded images. Few national guidelines mention its use immediately postnatally, even though it is often recommended for follow up. 14
The Swedish Agency for Health Technology Assessment concludes that more injuries to the anal sphincter can be detected and treated if ultrasonography is added to the routine visual and manual examinations currently being performed, and requests research evaluating the diagnostic methods of routine screening of anal sphincter injuries. 8
The aim of the present study was to assess the diagnostic utility of 3D‐EAUS in the postnatal diagnosis of OASI and provide data for its application in clinical practice.
2. MATERIAL AND METHODS
2.1. Study population
This prospective cohort study was conducted in the Department of Obstetrics and Gynecology of Östersund Hospital; this is the only hospital with a delivery department in the county of Jämtland‐Härjedalen, Sweden, and approximately 98% of the women residing in the area give birth at the hospital. Inclusion criteria were all primiparous women who delivered vaginally at more than 34 weeks of gestation between January 2016 and 2018. The exclusion criteria included a history of fecal incontinence or sphincter surgery. The trial is registered at ISCRTN: 18006769.
2.2. Study procedures
Participants were clinically examined shortly after giving birth by the midwife or a doctor in eligible cases following current operative routines (including rectal examination). If the responsible midwife or a junior obstetrician was uncertain about the extent of the tear, a second opinion was obtained from a senior obstetrician. The findings were documented in a study protocol and lacerations to the perineum were classified. Whether or not episiotomy had been performed was also recorded. The clinical grades of perineal tears were determined in accordance with international guidelines. 15 Only mediolateral episiotomy was performed. A trained midwife on duty performed 3D‐EAUS immediately after delivery before any perineal or vaginal lacerations were sutured. The person performing the clinical examination was blinded to the 3D‐EAUS results. Doctors and midwives were trained to perform 3D‐EAUS. Training was conducted by the colorectal surgeon and obstetrician responsible for the study. Training comprised a 2‐hour lecture and repeated hands‐on training using a rectal examination trainer as well as assistance in acquiring the right position and level during the first patient examinations. A pilot study of 30 patients was conducted before the study start to ensure that the new routines worked. Follow up consisted of clinical examination and complimentary 3D‐EAUS recording performed by a trained midwife in a specialized outpatient clinic at 3 months postpartum. Patient with OASI were offered additional follow up using questionnaires in a national quality register, 16 which is part of the standard care of OASI in Sweden. When judged necessary, additional care was offered by doctors, physiotherapists, and/or urotherapists.
All 3D‐EAUS recordings were performed using a Flex focus 500 Ultrasound scanner (BK Medical) with an 8838 axial endoscopic probe at 12 MHz. The recordings were standardized by following easy step‐by‐step instructions. The scanner was pre‐set and ready. Digital examination and 3D‐EAUS recordings were performed with the patient in the lithotomy position. All recorded volumes were independently assessed by a colorectal surgeon who specialized in proctology and had substantial experience in 3D‐EAUS. Archived 3D‐EAUS volume data sets were analyzed using proprietary software (BK viewer) on a personal computer by one of the authors (colorectal surgeon), who was blinded to all clinical, demographic, and delivery data. An endosonographic sphincter defect was defined as a discontinuity in the endosonographic image of the internal anal sphincter (IAS, hypoechoic ring) or external anal sphincter (EAS, mixed echoic ring), and/or characterized by the loss of the normal architecture in the appearance of the IAS and/or EAS on ultrasonography. 17 Results were presented as “defect”, “no defect”, or “assessment not possible”. The reviewer also had the option to leave comments on the recorded volume. The intra‐observer agreement in the diagnosis of any sphincter defects (internal and/or external) was based on repeated interpretation of 30 randomly chosen stored 3D volumes. To minimize recall bias, the interpreter was blinded to the repeated interpretation, and a minimum of 3 months was permitted between readings. Background and delivery data were gathered from the patient's medical records.
2.3. Statistical analyses
The sensitivity, specificity, positive predictive value, negative predictive value, and likelihood ratios of immediate postpartum 3D‐EAUS to detect OASI were calculated. Clinical diagnosis of OASI was set as reference standard. The intra‐observer agreement was assessed using κ statistics. Values of κ were defined as: less than 0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and 0.81–0.99, almost perfect agreement. 18 Statistical analyses were performed using SPSS software (version 27; IBM).
2.4. Ethics statement
The study was approved by the local ethics committee in Umeå on August 17, 2015 (Umeå reference Dnr 2015‐183‐31 M). Written consent was obtained from each participant after they had received written and oral information regarding the study. All data were de‐identified before the analyses. Patients could choose to cancel their participation at any stage of the study.
3. RESULTS
A total of 680 women underwent a clinical examination and 3D‐EAUS recording immediately after birth at the delivery ward. Figure 1 shows the inclusion and dropout data. The reasons for missing inclusion (n = 150) were that this was forgotten or missed for some other reason (miscarriage/fetal anomaly, language difficulties) during the fetal scan visit. The demographic data and obstetric characteristics of the study population are presented in Table 1.
FIGURE 1.
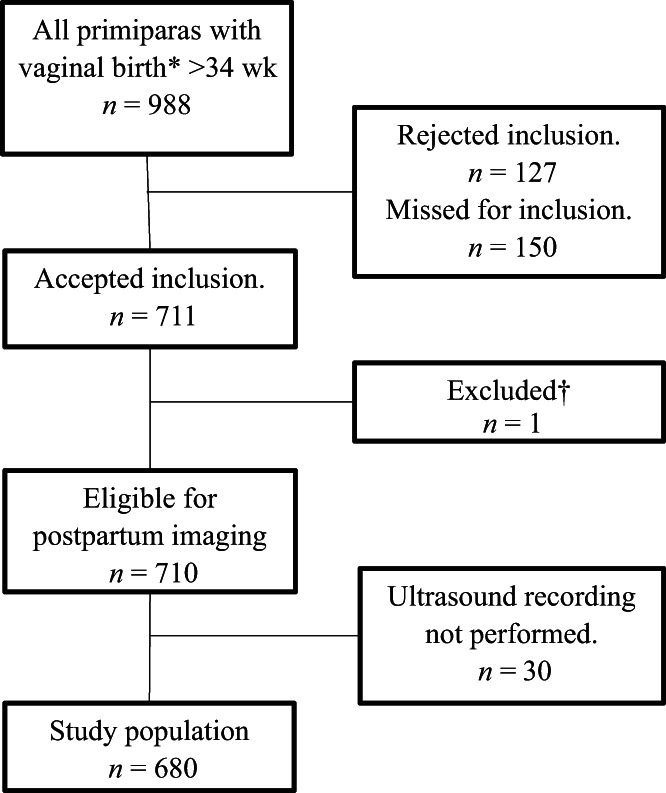
Flow chart of sample selection. *For study period January 2016 to January 2018. †Excluded due to intrauterine fetal loss (n = 1)
TABLE 1.
Demographic and obstetric characteristics of the study population
| Characteristic | |
|---|---|
| Age, y | 28 ± 5 |
| Gestational age, d | 279 ± 10 |
| Body mass index, kg/m2a | 25 ± 4 |
| Active second stage, min | 35 ± 20 |
| Birthweight, g | 3471 ± 472 |
| Head circumference (cm) | 35 ± 2 |
| Apgar ≤7 at 5 min | 16 (0.02) |
| pH: umbilical artery | 7.27 ± 0.1 |
| pH: umbilical vein | 7.33 ± 0.1 |
| Presentation | |
| Occiput anterior | 652 (96) |
| Occiput posterior | 24 (3.5) |
| Breech | 2 |
| Other | 1 |
| Induction of labor | 117 (17) |
| Epidural analgesia | 446 (66) |
| Use of oxytocin | 464 (68) |
| Use of episiotomy | 153 (23) |
| Mode of delivery | |
| Spontaneous vaginal delivery | 605 (89) |
| Operative delivery | 75 (11) |
| Obstetric anal sphincter injury | 34 (5) |
| Second degree tear | 264 (39) |
| First degree tear | 113 (17) |
Note: Data are presented as mean ± SD or as n (%).
Of the 680 performed scans, 18.5% were judged as “non‐assessable”, resulting in 554 assessable recordings. Among these, 23 women had clinically detected OASI (4.2%). Sphincter defect on 3D‐EAUS was observed in 12.8% of assessable recordings. With clinical examination set as a reference, the standard ultrasound sensitivity in the diagnosis of OASI was 30.4%, whereas its specificity was 87.9%. The negative predictive value was 96.7%, but the positive predictive value was only 9.9%. These results yielded a positive likelihood ratio of +2.51 and negative likelihood ratio of −0.79.
In total, sphincter defects were observed in 71 women on 3D‐EAUS immediately after birth. Of these patients, 45 attended the 3‐month follow up, where residual defects were found in only three women, two of whom were clinically diagnosed with OASI at delivery, and one patient that was judged as having a deep second‐degree tear. That tear was sutured by a senior doctor, and the patient was followed up until 3 years after birth. She exhibited no signs of pelvic floor dysfunction.
The intra‐observer agreement for anal sphincter defects on 3D‐EAUS recordings was 0.72. An aggregate of the comments by the reviewer is shown in Figure 2. Aggravating factors of the examination (as understood by the comments left by the reviewer) were described. Comments were left on 175 examinations. Among the comments, 74% referred to examination management. A common performance problem was that the probe went too deeply into the anal canal, thereby making the most distal part only partly visible on the 3D‐EAUS. Examples of immediate postnatal 3D‐EAUS and disturbances to image quality and interpretation are presented in Figures 3, 4, 5, 6.
FIGURE 2.
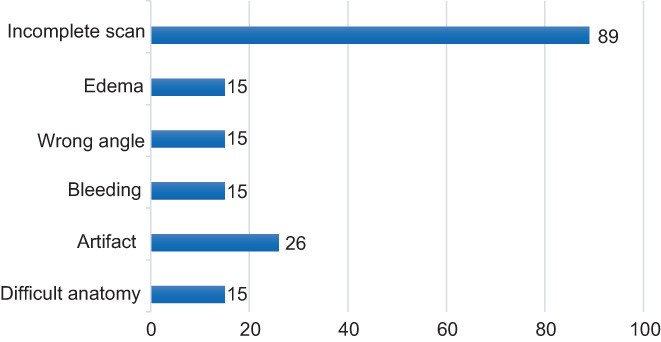
Summary of the reviewer's comments on the three‐dimensional endoanal ultrasound recordings
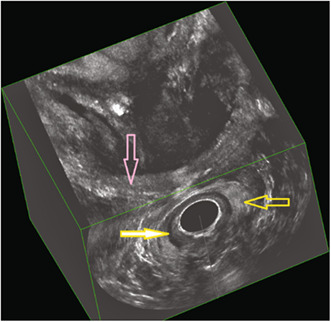
FIGURE 3.
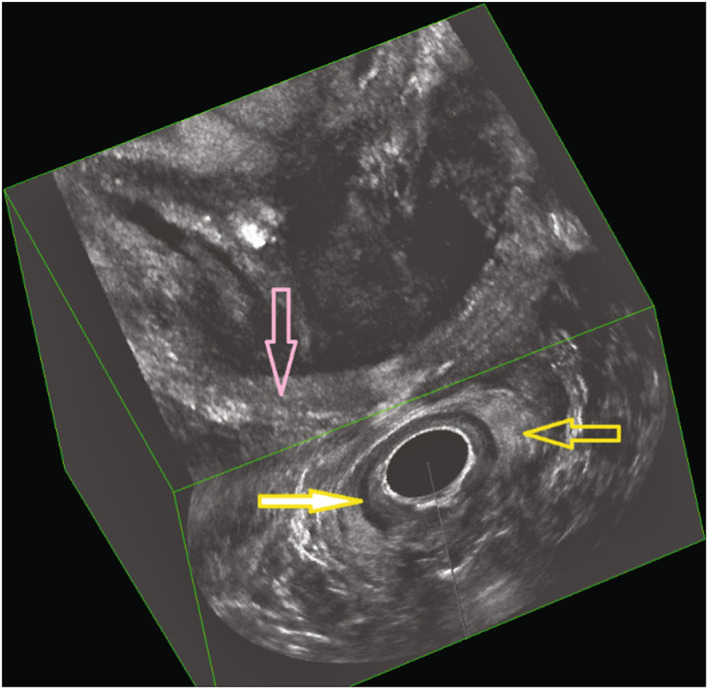
Immediate postpartum three‐dimensional endoanal ultrasonography (deep/intermediate/superficial plane) demonstrating an intact sphincter complex. Yellow arrow, external anal sphincter; yellow filled arrow, internal anal sphincter; pink arrow, puborectalis muscle
FIGURE 4.
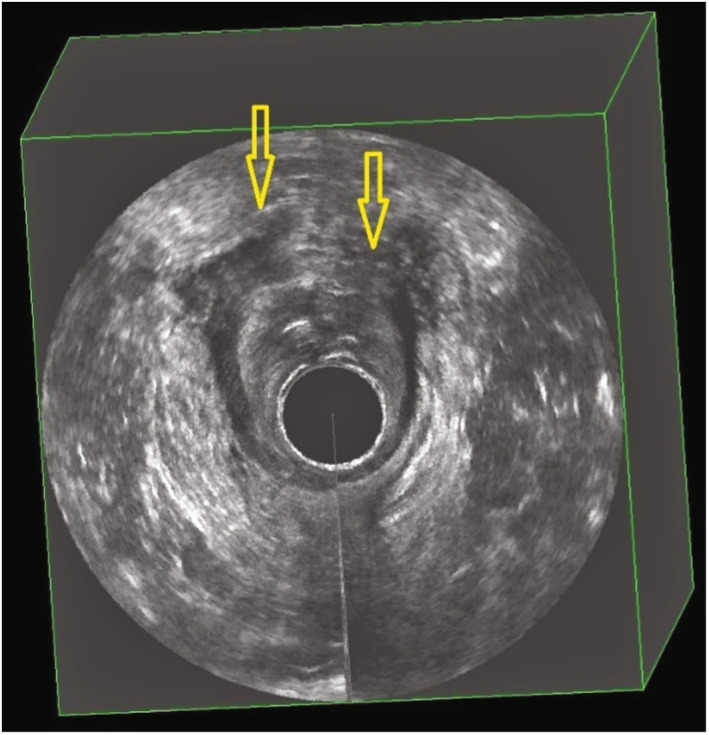
Immediate postpartum three‐dimensional endoanal ultrasonography demonstrating imaging of a total sphincter defect (indicated by yellow arrows)
FIGURE 5.
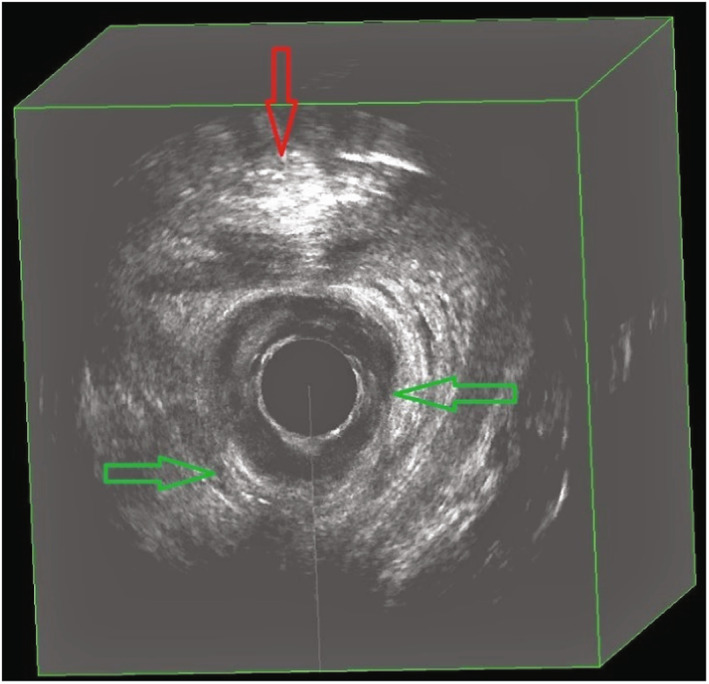
Immediate postpartum three‐dimensional endoanal ultrasonography demonstrating an intact sphincter and showing examples of edema (green arrows) and bleeding (red arrow)
FIGURE 6.
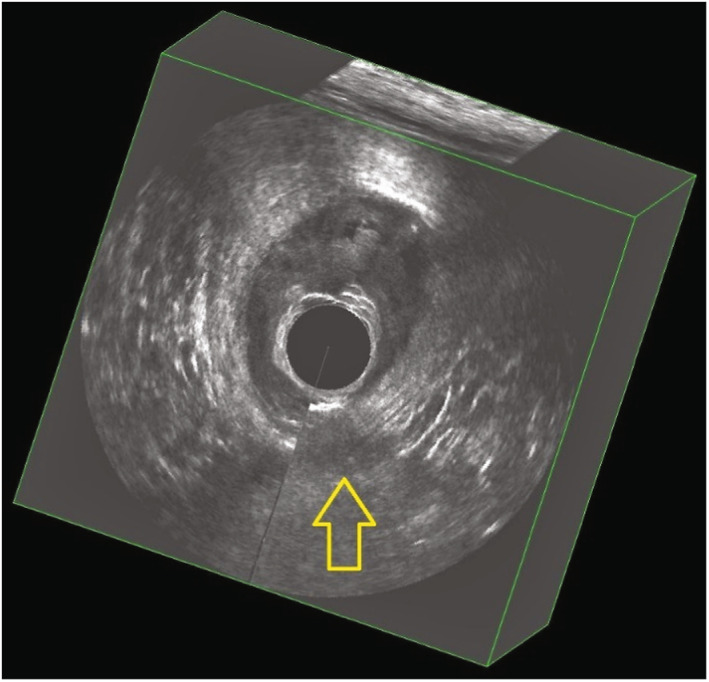
Immediate postpartum three‐dimensional endoanal ultrasonography demonstrating examples of image artifacts created by the patient or clinician moving during recording
4. DISCUSSION
In this setting, where 3D‐EAUS was conducted in primiparous women immediately after birth, it was uncertain whether the procedure improved or added anything to the diagnosis of OASI. Our study has severeral strengths, it was conducted in a real clinical mileu with the original care‐giving team, with a large unselected cohort, and was performed immediately after birth. As midwives are the primary providers of care during labor and childbirth in Sweden and the study was designed to mirror screening settings, we chose to let the midwives perform the acquisition. The aim was to assess only primiparas to minimize influence of previous childbirth‐related pelvic floor dysfunction or scarring. We are unaware of any other study that performed 3D‐EAUS immediately after childbirth, before repair, and irrespective of the grade of perineal tear. As guidelines emphasize systemic examination to assess severity of damage, particularly before suturing, our intent was to test feasibility in this setting.
Although endoanal ultrasonography is regarded as the most suitable diagnostic imaging modality for OASI, the low positive predictive value when performed in this situation suggests that it is currently unsuitable as a general diagnostic tool immediately after birth. Likelihood ratios also indicated fair odds that a positive or negative test result would be found in a patient with a disease vs without. The technique must be improved further and simplified to be feasible for broader postnatal conditions, possibly even with the development of AI algorithms to interpret recorded volumes. Obviously, results could have been different with 3D‐EUAS performed by well‐trained and well‐experienced physicians or specialized midwives and by real‐time evaluation, but that would not be eligible in most delivery wards on a 24‐hour basis. In addition, 3D‐EAUS is generally unavailable in obstetric wards. It is also more expensive than other ultrasound equipment, and more training may be required to perform the examination correctly. 19 , 20 , 21
There is an ongoing discussion about the underdiagnosis and overdiagnosis of OASI, 3 , 22 and substandard clinical laceration diagnosis can occur. 23 In the present study, 64 of 466 patients, representing 14% of the study population, may have been overdiagnosed. In a study where surgical exploration was performed to verify the 3D‐EAUS diagnosis of OASI, 24% of explored cases were not confirmed. 24 Andrews et al conducted a study where vaginally delivered primiparas were clinically re‐examined and 3D‐EAUS was performed before sutures were inserted. 3 They found only a minimal increase in OASI prevalence (1.2%) from the ultrasonography and concluded that experience in clinical evaluation of perineal tears is essential to diagnose OASI. Corton et al estimated the incidence of anal sphincter defects detected using ultrasonography within 72 hours of birth and almost doubled the prevalence compared with clinical evaluation alone. 4 Notably, rectal examination was not routinely performed in this study. 4 Roper et al examined a cohort of women with grade 3a or 3b injury, who had endoanal ultrasound after birth and found that 11% had a defect greater than the original diagnosis. 25
The significant overlap between the pathological and physiological changes in the postpartum pelvic floor presents many difficulties in interpreting ultrasonography results immediately after birth. Numerous ultrasound modalities and approaches have been experimented with to seek out improved diagnostic accuracy of perineal tears, but most were performed after suturing or during follow up, and not immediately after birth as in our study. Pihl et al used perineal ultrasonography with a vaginal probe in a pilot study to measure anovaginal distance as a way to indicate sphincter injury, but cut‐offs have not been validated. 20 Transperineal ultrasonography (TPUS) has emerged as a procedure that is relatively easy to perform and moreover is widely available in maternity wards. 26 TPUS has good agreement with 3D‐EAUS in investigating residual defects after birth. 27 , 28 Taithongchai et al suggest TPUS or introital ultrasonography as screening modalities for intact sphincter, and only proceed with endoanal ultrasound when defects are found to verify the diagnosis. 29 Gillor et al found fair agreement between TPUS and clinical grading of tears following recent primary OASI repair, and reported both potential clinical and ultrasonography overdiagnosis. 30 Bellussi et al concluded that TPUS performed before suturing is a useful supporting tool for the assessment of the anal sphincter. 26 Hurni et al showed promising results in a smaller study performing immediate postnatal endovaginal ultrasonography in a selected group of patients with deep perineal lacerations. 31 They performed real‐time evaluation and used a pressure test to differentiate EAS from surrounding tissues, which likely augments its feasibility. 31 A reasonable approach in view of current knowledge in postpartum ultrasonography would be to offer TPUS or 3D‐EAUS at about 3 months after vaginal delivery to all high‐risk women, defined by a number of risk factors including for example; previous OASI, macrosomia, prolonged second stage, instrumental delivery, and shoulder dystocia. 6
As with the majority of studies, the design of the current study is subject to limitations. Specificity and sensitivity often vary with prevalence and studies on accuracy tests have intrinsic sources of error such as verification bias and imperfect reference standards. 32 An imperfect reference standard may result in prevalence being underestimated or overestimated, thus affecting test accuracy. The prevalence of OASI during the study period was in line with previous years, suggesting that there was no observer bias. According to the comments left by the reviewer, most issues with the examination were management errors. This is a major limitation of our study as well as of the method itself because it has also contributed to drop out.
Management errors can be due to many reasons, including a lack of examiner skill, obesity, and anatomical variants. The results may have been different if the reviewer both performed and evaluated the examination, by using real‐time evaluation and possibly even a pressure test. This would not be feasible for all patients in a clinical setting but would be possible for a limited selection of patients with deep perineal or vaginal tears and providers could distinguish between second‐degree tears and OASI. 33 Another possibility would have been to use another endoanal probe, the linear 8838 probe used in this study does not provide image in the axial plane in real time, which is important for the correct orientation and evaluation of the landmarks. We could have used the 2052 probe instead, but it was judged as being more difficult to handle for more inexperienced staff and therefore not suitable for screening purposes. The 8838 probe was also chosen because of higher resolution, which proved to make no difference in volume quality but made the recording more sensitive to artifacts caused by movements. However, as the anal canal undergoes marked displacement and distortion during childbirth, the pelvic floor anatomy is significantly altered just after giving birth, 3D‐EAUS recordings of the sphincter complex with adequate quality could be difficult to obtain even in experienced hands. Moreover, as 3D‐EAUS is invasive, this could also affect image quality.
5. CONCLUSION
In summary, we have shown that using 3D‐EAUS in a maternity ward is demanding because staff generally have little experience in endoanal ultrasonography, which contributes to difficulties in obtaining good image quality. Accurate recording and interpretation of immediate postnatal pelvic floor images was complicated by local edema, bleeding, positioning of the woman, and unintended movements.
This does not negate the fact that in perineal clinics, where diverse modalities of imaging are used to attain multicompartment assessment, 3D‐EAUS plays a significant role in evaluating the pelvic floor, particularly the sphincter complex.
It is postulated that the assessment of anal sphincters after childbirth needs imaging, but whether use of another imaging approach immediately after childbirth is more feasible than the use of 3D‐EAUS remains to be elucidated.
AUTHOR CONTRIBUTION
All authors contributed to the design of the study. MHu and CL recruited the participants. MHu, MHa, and KT analyzed and interpreted the data, and all authors were involved in refining the analysis. MHu and MHa prepared the manuscript. All authors have read and approved the final manuscript and agreed with its submission to Acta Obstetricia et Gynecologica Scandinavica.
FUNDING INFORMATION
This study was funded by grants from the Unit of Research, Education and Development, Region Jämtland‐Härjedalen and VisareNorr, Northern County Councils, Sweden. The funding sources had no impact on the design and execution of the study, nor on the writing of the manuscript.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
ACKNOWLEDGMENTS
We would like to thank Ulla Jamting and Fredrik Bornhov for assistance in collecting data, and Lars Söderström for his contribution to the statistical analysis.
Huber M, Larsson C, Harrysson M, et al. Use of endoanal ultrasound in detecting obstetric anal sphincter injury immediately after birth. Acta Obstet Gynecol Scand. 2023;102:389‐395. doi: 10.1111/aogs.14514
REFERENCES
- 1. Williams AB, Bartram CI, Halligan S, Spencer JA, Nicholls RJ, Kmiot WA. Anal sphincter damage after vaginal delivery using three‐dimensional endosonography. Obstet Gynecol. 2001;97:770‐775. [DOI] [PubMed] [Google Scholar]
- 2. The Swedish medical birth register 1973–2013 . Pregnancies, deliveries and newborn infants, S.O.S.o. Sweden, Editor. 2014, Socialstyrelsen.
- 3. Andrews V, Sultan AH, Thakar R, Jones PW. Occult anal sphincter injuries‐‐myth or reality? BJOG. 2006;113:195‐200. [DOI] [PubMed] [Google Scholar]
- 4. Corton MM, McIntire DD, Twickler DM, Atnip S, Schaffer JI, Leveno KJ. Endoanal ultrasound for detection of sphincter defects following childbirth. Int Urogynecol J. 2013;24:627‐635. [DOI] [PubMed] [Google Scholar]
- 5. Faltin DL, Boulvain M, Irion O, Bretones S, Stan C, Weil A. Diagnosis of anal sphincter tears by postpartum endosonography to predict fecal incontinence. Obstet Gynecol. 2000;95:643‐647. [DOI] [PubMed] [Google Scholar]
- 6. Bellussi F, Dietz HP. Postpartum ultrasound for the diagnosis of obstetrical anal sphincter injury. Am J Obstet Gynecol MFM. 2021;3:100421. [DOI] [PubMed] [Google Scholar]
- 7. Groom KM, Paterson‐Brown S. Can we improve on the diagnosis of third degree tears? Eur J Obstet Gynecol Reprod Biol. 2002;101:19‐21. [DOI] [PubMed] [Google Scholar]
- 8. (SBU) Förlossningsbristningar (National report in Swedish) 2021.
- 9. Seidenari A, Cuicchi D, Youssef A, Oliver EA, Montaguti E, Bellussi F. Obstetric anal sphincter injuries: strategies for prevention, diagnosis, and management. Minerva Obstet Gynecol. 2021;73:74‐81. [DOI] [PubMed] [Google Scholar]
- 10. Sultan AH, Nicholls RJ, Kamm MA, Hudson CN, Beynon J, Bartram CI. Anal endosonography and correlation with in vitro and in vivo anatomy. Br J Surg. 1993;80:508‐511. [DOI] [PubMed] [Google Scholar]
- 11. Starck M, Bohe M, Fortling B, Valentin L. Endosonography of the anal sphincter in women of different ages and parity. Ultrasound Obstet Gynecol. 2005;25:169‐176. [DOI] [PubMed] [Google Scholar]
- 12. Norderval S, Dehli T, Vonen B. Three‐dimensional endoanal ultrasonography: intraobserver and interobserver agreement using scoring systems for classification of anal sphincter defects. Ultrasound Obstet Gynecol. 2009;33:337‐343. [DOI] [PubMed] [Google Scholar]
- 13. Pretlove SJ, Thompson PJ, Guest P, Toozs‐Hobson P, Radley S. Detecting anal sphincter injury: acceptability and feasibility of endoanal ultrasound immediately postpartum. Ultrasound Obstet Gynecol. 2003;22:215‐217. [DOI] [PubMed] [Google Scholar]
- 14. Roper JC, Amber N, Wan OYK, Sultan AH, Thakar R. Review of available national guidelines for obstetric anal sphincter injury. Int Urogynecol J. 2020;31:2247‐2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Socialstyrelsen, International Statistical Classification of Diseases and Related Health Problems ICD‐10 . Swedish version (ICD‐10 SE). 1990.
- 16. Barnmorskeförbundet SS. Utbildningsprogram för ökad kunskap inom svensk förlossningsvård om förlossningars påverkan på bäckenbottens strukturer. avilable at backenbottenutbildning.se.
- 17. Frudinger A, Halligan S, Bartram CI, Spencer J, Kamm MA, Winter R. Assessment of the predictive value of a bowel symptom questionnaire in identifying perianal and anal sphincter trauma after vaginal delivery. Dis Colon Rectum. 2003;46:742‐747. [DOI] [PubMed] [Google Scholar]
- 18. Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37:360‐363. [PubMed] [Google Scholar]
- 19. Guzman Rojas RA, Shek KL, Langer SM, Dietz HP. Prevalence of anal sphincter injury in primiparous women. Ultrasound Obstet Gynecol. 2013;42:461‐466. [DOI] [PubMed] [Google Scholar]
- 20. Pihl S, Blomberg M, Uustal E. Internal anal sphincter injury in the immediate postpartum period; prevalence, risk factors and diagnostic methods in the Swedish perineal laceration registry. Eur J Obstet Gynecol Reprod Biol. 2019;245:1‐6. [DOI] [PubMed] [Google Scholar]
- 21. Abdool Z, Sultan AH, Thakar R. Ultrasound imaging of the anal sphincter complex: a review. Br J Radiol. 2012;85:865‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sioutis D, Thakar R, Sultan AH. Overdiagnosis and rising rate of obstetric anal sphincter injuries (OASIS): time for reappraisal. Ultrasound Obstet Gynecol. 2017;50:642‐647. [DOI] [PubMed] [Google Scholar]
- 23. Diko S, Sheeder J, Guiahi M, et al. Identification of obstetric anal sphincter injuries (OASIs) and other lacerations: a national survey of nurse‐midwives. Int Urogynecol J. 2021;32:1745‐1753. [DOI] [PubMed] [Google Scholar]
- 24. Faltin DL, Boulvain M, Floris LA, Irion O. Diagnosis of anal sphincter tears to prevent fecal incontinence: a randomized controlled trial. Obstet Gynecol. 2005;106:6‐13. [DOI] [PubMed] [Google Scholar]
- 25. Roper JC, Thakar R, Sultan AH. Under‐classified obstetric anal sphincter injuries. Int Urogynecol J. 2022;33:1473‐1479. [DOI] [PubMed] [Google Scholar]
- 26. Bellussi F, Montaguti E, Youssef A, Salsi G, Ghi T, Pilu G. Dynamic 2‐dimensional transperineal ultrasound evaluation in labor room as a screening tool for anal sphincter injuries and anal incontinence in primiparous women. Am J Obstet Gynecol MFM. 2019;1:100037. [DOI] [PubMed] [Google Scholar]
- 27. Ros C, Martínez‐Franco E, Wozniak MM, et al. Postpartum two‐ and three‐dimensional ultrasound evaluation of anal sphincter complex in women with obstetric anal sphincter injury. Ultrasound Obstet Gynecol. 2017;49:508‐514. [DOI] [PubMed] [Google Scholar]
- 28. Stuart A, Ignell C, Orno AK. Comparison of transperineal and endoanal ultrasound in detecting residual obstetric anal sphincter injury. Acta Obstet Gynecol Scand. 2019;98:1624‐1631. [DOI] [PubMed] [Google Scholar]
- 29. Taithongchai A, van Gruting IMA, Volløyhaug I, Arendsen LP, Sultan AH, Thakar R. Comparing the diagnostic accuracy of 3 ultrasound modalities for diagnosing obstetric anal sphincter injuries. Am J Obstet Gynecol. 2019;221:134 e1‐134 e9. [DOI] [PubMed] [Google Scholar]
- 30. Gillor M, Shek KL, Dietz HP. How comparable is clinical grading of obstetric anal sphincter injury with that determined by four‐dimensional translabial ultrasound? Ultrasound Obstet Gynecol. 2020;56:618‐623. [DOI] [PubMed] [Google Scholar]
- 31. Hurni Y, Maes E, Avau F, et al. Immediate postpartum assessment of the anal sphincter by endovaginal ultrasound: a new technical approach. Int Urogynecol J. 2021;33:1689‐1692. [DOI] [PubMed] [Google Scholar]
- 32. Leeflang MM, Rutjes AWS, Reitsma JB, Hooft L, Bossuyt PMM. Variation of a test's sensitivity and specificity with disease prevalence. CMAJ. 2013;185:E537‐E544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leombroni M, Buca D, Liberati M, et al. Post‐partum pelvic floor dysfunction assessed on 3D rotational ultrasound: a prospective study on women with first‐ and second‐degree perineal tears and episiotomy. J Matern Fetal Neonatal Med. 2021;34:445‐455. [DOI] [PubMed] [Google Scholar]


