Abstract
Sulfolobus acidocaldarius is so far the only hyperthermophilic archaeon in which genetic recombination can be assayed by conjugation and simple selections. Crosses among spontanteous pyr mutants were able to resolve closely spaced chromosomal mutations, identify deletions and rearrangements, and map mutations to a given deletion interval. Frameshift mutations in pyrE exerted polar effects that depressed orotidine-5′-monophosphate decarboxylase activity (encoded by pyrF), whereas base pair substitutions and an 18-bp deletion had no effect.
The hyperthermophilic archaeon Sulfolobus acidocaldarius has a natural mechanism of conjugation and recombination termed marker exchange (ME) (1, 4), which represents a useful genetic capability of this species. Another useful property is the ability to select mutants lacking orotate phosphoribosyltransferase (OPRTase) and orotidine 5′-monophosphate decarboxylase (ODCase) with 5-fluoroorotic acid (FOA) (2). Open reading frames predicted to encode each of these enzymes have been cloned from S. acidocaldarius and sequenced (D. Charlier, personal communication). The present study evaluated a collection of FOA-resistant mutants by ME in order to investigate, for the first time, the genetic properties of homologous recombination in one of the hyperthermophilic archaea.
(A portion of this work has been presented previously [M. Reilly and D. Grogan, Abstr. 98th Gen. Meet. Am. Soc. Microbiol. 1998, abstr. I-63].)
Isolation of mutants.
Independent spontaneous mutants resistant to FOA were selected from a series of liquid cultures as previously described (5). Unstable and leaky mutants were discarded, and the auxotrophs were colony purified on nonselective medium (xylose-tryptone-uracil) (2). Over 300 independent mutants were isolated in this manner; the first was designated MR1, and its corresponding mutant allele was designated pyr-101. Pure cultures were grown in nonselective liquid medium and preserved at −70°C (3).
Assays of recombination.
Due to the large number of strains to be tested, mutants were initially divided, in order of their isolation, into groups of 30 or fewer each. Matings in all pairwise combinations within each group were then performed on plates as follows. Liquid cultures (about 109 cells each) were pelleted, and the cells were resuspended in 0.5 ml of dilution buffer (Sdil) (3). Half of each suspension was spread over the surface of a 10-cm-diameter plate of pyrimidine-free medium. Onto each of the resulting lawns was then spotted an aliquot (5 μl) of each suspension, and the plates were incubated for 6 to 8 days at 75°C. As a result, each mutant in the group was spotted onto itself once and crossed twice with all other members of the same group in the form of a full (i.e., square) matrix. Typical results are shown in Fig. 1. Crosses in which a parental culture yielded many revertants were eliminated from the analysis. Strain pairs yielding no recombinants in these plate tests were retested by matings initiated in liquid suspension and spread on selective plates (1).
FIG. 1.
Assays of ME on plates. Washed cells of one strain were spread over the surface of selective medium, and suspensions of other strains were spotted on top; prototrophic recombinants formed colonies after incubation at 75°C. On the plate shown, 19 of 23 mutant pairs yielded recombinants.
Certain pairs of pyr mutants consistently failed to yield Pyr+ recombinants, but these represented only 7.6% (56 of 741) of the strain pairs tested. We further observed that three mutants (MR54, MR75, and MR103) accounted for over half of the pairs yielding no recombinants in these initial tests. When these three strains were eliminated from the analysis, the empirical probability that a pair of pyr mutants would not yield Pyr+ recombinants in our assays was only about 4% (27 of 677). Since all the pyr mutations are expected to occur within one interval of about 1,240 nucleotides (nt) (Fig. 2), this result suggested that ME was leading to recombination between closely spaced chromosomal markers.
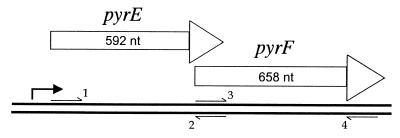
FIG. 2.
Schematic summary of the pyrE-pyrF region. Relative sizes and positions of the S. acidocaldarius pyrE and pyrF genes are those indicated by nucleotide sequence (D. Charlier, personal communication). This gene arrangement, including the 14-nt overlap, is that found in S. solfataricus (7), despite considerable sequence divergence of the two sets of genes. Heavy black arrow indicates promoter(s) common to both genes; small arrows indicate primers used for PCR (not drawn to scale). Amplification of the combined pyrE-pyrF region used primers 1 and 4. Sequences of primers are given in the text. The 5′ ends of the chromosomal sequences amplified from these primers are positioned relative to the first nucleotide of the pyrE coding sequence (GenBank accession number Y12822) as follows: primer 1, nt 1; primer 2, nt 595; primer 3, nt 581; and primer 4, nt 1261.
The observed failure of certain strains to yield recombinants with a large proportion of the other Foar pyr mutants could not be attributed to clustering of mutations in the pyrE-pyrF interval or to a general deficiency in ME. Most of the partner strains giving no recombinants with MR54, MR75, or MR103 did generate recombinants among themselves, and the three strains formed Pyr+ recombinants when mated with certain other pyrimidine auxotrophs. The three mutants were therefore evaluated for possible deletions. Strain MR54 reverted upon UV irradiation and yielded normal-length amplification products; it was not further characterized. In contrast, neither strain MR75 nor MR103 reverted spontaneously or with UV mutagenesis, despite repeated attempts. These strains yielded no Pyr+ recombinants when crossed to each other, and crosses to 142 other Foar pyr mutants yielded the following results: 27 and 8 of the mutants failed to yield Pyr+ recombinants with only MR75 or only MR103, respectively, whereas 64 of the pyr mutants yielded no recombinants with both. The genetic properties of strains MR75 and MR103 therefore indicated that each contains a deletion or stable rearrangement in the pyrE-pyrF region and that these two mutations (designated pyr-175 and pyr-203, respectively) have substantial overlap.
Molecular characterization of pyr-203 allele.
To investigate the molecular nature of these alleles, we used PCR to amplify the pyrE and pyrF regions of strains MR75 and MR103. Total genomic DNA was extracted from about 2 × 109 cells using a modification of the guanidinium thiocyanate procedure of Pitcher et al. (9). Each PCR mixture consisted of 10 to 20 ng of template DNA, 0.52 pmol of each primer, 0.20 mM deoxynucleoride triphosphate (dNTP) mix, 1 × Taq buffer, and 2.5 U of Taq DNA polymerase in a total volume of 48 μl. The oligodeoxynucleosides used were primer 1 (5′-TTTCATATGGATTTCGTGAAAGCTCTAC-3′), primer 2 (5′-TTTGGATCCCTAGCTTTTTCCAATATTTTTCAC-3′), primer 3 (5′-AAACATATGGAAAAAGCTAGAATAATTTTAG-3′), and primer 4 (5′-AAAATGCATGGATCCTCGCTTAATTGGTTCTTATC-3′). The relative positions of these primers are shown in Fig. 2. Strain MR75 yielded no amplification product with any of the three primer pairs tested. We could not attribute this result to unsuitable PCR conditions, as it was observed in each of several trials using various template preparations and reaction conditions. Furthermore, other primers readily amplified 1.47 kb of the 16S rRNA gene of S. acidocaldarius from MR75 DNA when included in addition to the pyrE or pyrF primer pair (N. Kurosawa, unpublished results). More intensive analysis using other primers indicates that a complex mutational event has occurred in strain MR75 (G. Carver and J. W. Drake, personal communication).
In contrast to these results, all three primer pairs yielded PCR products with chromosomal DNA of strain MR103. However, primers 1 and 2 amplified a smaller segment from MR103 than from the parental strain DG40, implying that pyr-203 is a deletion within the pyrE gene. Sequencing the corresponding PCR product yielded the expected pyrE nucleotide sequence except for 329 consecutive nt missing between positions 126 and 456 of the OPRTase coding region. Sequencing identified no mutations in the pyrF amplification product from strain MR103.
Intragenic recombination at the pyrE locus.
Precise molecular definition of the pyr-203 allele provided a basis for investigating more closely the subset of pyrE mutations that lie in this interval. We identified 28 pyr mutants failing to yield recombinants with strain MR103 and then crossed these with each other in all pairwise combinations. Eight percent (30 of 378) of the resulting strain pairs produced no recombinants. Thus, the genetic behavior of mutations confined to a relatively small subinterval of the pyrE-pyrF region of S. acidocaldarius resembled those of our entire set of mutations. To confirm the accuracy of the ME results, we amplified and sequenced the pyrE region of 6 of these 28 mutants. As shown in Fig. 3, all six had mutations within the pyr-203 deletion interval, confirming that conjugational tests can accurately assign chromosomal mutations to such an interval.
FIG. 3.
Sequence analysis of pyrE alleles. The alignment shows DNA sequences of six pyrE alleles mapped to the pyr-203 deletion interval by recombinational criteria; a sixth mutant, MR9, was identical to MR146 and is not shown. Both strands of PCR product from the mutants indicated were sequenced using primers 1 and 2. Nucleotide positions refer to the wild-type (WT) pyrE coding sequence; mutations are shown in boldface type.
Separation required to detect recombination.
The recombination and sequencing data were further analyzed in order to estimate the ability of ME to resolve closely spaced mutations. Two of the six strains sequenced, MR9 and MR146, were found to have the same allele; elimination of MR9 left five mutants that were mated in 10 corresponding pairwise combinations. Only two combinations (MR30 × MR31 and MR146 × MR362) yielded no recombinants. The mutations in strains MR146 and MR362 affect the same base pair, whereas the MR30 and MR31 mutations are separated by 3 bp (Fig. 3). Among the pairs yielding recombinants, strains MR146 and MR362 have mutations only 28 bp away from the MR30 mutation. These results indicate that typical ME tests require separation of more than 3 but less than 28 bp to yield a detectable frequency of recombinants. An independent estimate of the recombinational threshold was also derived from the experimentally measured fraction of 378 crosses among mutations in the pyrE-203 deletion interval that yielded no recombinants. This was based on the fact that the probability that two mutations mapped to an interval L will fall within x nt of each other, P, equals 2x/(L + 2x). Substituting experimentally determined values for P and L (0.08 and 329 bp, respectively) and solving for x yielded 14 bp. This estimate should also be considered an upper limit for the recombinational threshold, since in this set of matings some of the pairs yielding no recombinants result from coincident mutations.
Role of DNA transfer frequency.
In view of reports that transfer of conjugal plasmids among other Sulfolobus spp. is very efficient (10) and our own observations of the high resolving power of S. acidocaldarius matings, we investigated whether frequent transfer of DNA is a basic mechanistic feature of ME in S. acidocaldarius. Because the spontaneous pyr mutations used in this study had been selected in amino acid auxotrophs (his-2 and caa-2 mutants [4, 6]), we were able to determine the relative efficiency of transfer versus recombination by genetic assays. Recombination between the his and caa markers in doubly marked parental strains (his pyr × caa pyr) was selected first by plating the mixtures on minimal medium plus uracil. A number of the resulting recombinant clones were then scored for unselected recombination between the two pyr alleles by spotting onto minimal medium lacking uracil. Controls included the converse enumeration of double recombinants by first selecting Pyr+ followed by scoring growth without amino acids, and by one-step selection of double recombinants (plating directly on minimal medium). In six independent crosses involving four mutant pairs, the frequency of unselected Pyr+ recombinants averaged 0.24 per amino acid prototroph. This value is comparable to frequencies of unselected, nonparental combinations of other markers in S. acidocaldarius (4), and orders of magnitude higher than frequencies of Pyr+ clones selected directly from the same cell mixtures (about 3 × 10−5 per CFU). These results indicate that the recombination phase of ME is much more efficient than the DNA transfer phase (1).
Biochemical consequences of pyrE mutations.
In a prior study, the FOA selection yielded two predominant phenotypic classes of S. acidocaldarius mutants: moderately FOA-resistant auxotrophs (MIC, ≃200 μg/ml), exhibiting decreased OPRTase but normal ODCase levels (low-MIC mutants), and highly FOA-resistant auxotrophs (MIC, ≥1,000 μg/ml), deficient in both OPRTase and ODCase (high-MIC mutants) (2). For mutants isolated in the course of this study, we determined MICs of FOA and similarly found two distinct FOA resistance classes (Table 1). We then characterized representatives of both classes by assaying ODCase, the predicted product of the pyrF gene.
TABLE 1.
Metabolic phenotypes of Foar mutations
| Straina | MIC of FOA (μg/ml) | ODCase activityb
|
|
|---|---|---|---|
| U/g of protein | % of control | ||
| DG6 | 8 | 4.7 ± 2.8 | 86 |
| DG40 | 8 | 6.4 ± 0.2 | 117 |
| DG55 | 8 | 5.3 ± 0.3 | 97 |
| MR30 | ≥1,600 | 1.6 ± 1.5 | 29 |
| MR31 | ≥1,600 | 9.9 ± 0.4 | 181 |
| MR72 | ≥1,600 | 10.4 ± 4.9 | 190 |
| MR103 | ≥1,600 | 0.6 ± 0.4 | 11 |
| MR146 | ≥1,600 | 0.8 ± 0.3 | 15 |
| MR362 | ≥1,600 | 12.7 ± 2.1 | 232 |
| MR367 | 200 | 11.0 ± 2.1 | 201 |
| MR402 | 200 | 14.9 ± 7.6 | 273 |
| MR405 | 200 | 10.4 ± 4.6 | 190 |
DG6 is the wild-type parent; see reference 5 for derivation of other strains.
Values are averages of two independent determinations ± ½ range. The control value is the average for the three Pyr+ strains (5.47 U/g of protein).
Strains MR367, MR402, and MR405 represent the low-MIC mutants described above with regard to both MICs and ODCase levels (Table 1). Conversely, the depressed ODCase levels found in strains MR30, MR103, and MR146 correspond to the biochemical phenotype of the high-MIC class described above. Strains MR31, MR72, and MR362, however, exhibited both high FOA resistance and high ODCase activity (Table 1). This third biochemical phenotype had not been found among the limited number of independent mutants characterized previously (2) and shows that loss of OPRTase activity alone can confer a high level of FOA resistance.
Low ODCase activities among the high-MIC mutants were unexpected because they had been confirmed by sequencing to be pyrE mutants. Comparison of the enzyme levels (Table 1) and sequence data (Fig. 3) revealed a clear pattern, however. All mutations associated with low ODCase activity (represented by strains MR30, MR103, and MR146) are frameshifts within pyrE. Conversely, all sequenced mutations associated with high ODCase activity maintain the pyrE reading frame; strains MR72 and MR362 have distinct missense mutations, whereas strain MR31 bears an 18-bp deletion (Fig. 3).
Three aspects of this last result seem noteworthy. In bacterial operons, polarity due to frameshift and nonsense mutations is attributed to rho-dependent termination of transcription, triggered when ribosomes dissociate and thereby allow the termination factor rho to bind to the unprotected regions of mRNA (11). To our knowledge, no rho homologue has been identified in the genome of any archaeon (G. Olsen, personal communication; S. Bell, personal communication). Thus, if the observed effects of these frameshift mutations indeed reflect transcriptional termination, the role of a protein factor becomes a question of mechanistic and evolutionary interest. Alternatively, the 14-nt overlap of these two genes raises the possibility that efficient translation of pyrF may involve programmed frameshifting of the ribosome (8). In this case, premature termination of translation within the proximal gene would be predicted to preempt translation of the distal gene, because successful frameshifting requires the ribosome to pause near the site of frameshifting (8). In either case, we note that the effect of pyrE frameshift mutations and their apparent abundance among Foar mutants explains the prior observation that spontaneous S. acidocaldarius mutants deficient in both OPRTase and ODCase arise frequently and revert frequently (2). Such mutants were designated pyrF in previous studies (2, 5), but their properties match those of the pyrE mutants MR30, MR103, and MR146.
Conclusions.
S. acidocaldarius is so far the only hyperthermophilic archaeon in which the exchange and recombination of chromosomal markers can be detected and quantified by simple microbiological assays (1, 4). We exploited this fact to perform the first genetic analysis of homologous recombination at extremely high temperature in an archaeon from geothermal environments. Our data indicate that simple mating tests resolve mutations separated by less than 28 bp (and probably less than 14 bp) in the S. acidocaldarius chromosome. ME also enabled large numbers of independent pyr mutations to be characterized. Specifically, a large deletion and a complex rearrangement were first identified among 76 phenotypically similar mutants by their recombinational properties, and other spontaneous mutations were correctly mapped to the deletion interval. This suggests the potential, given suitable selections and tester strains, for convenient mapping of large numbers of new mutations to small intervals of the S. acidocaldarius chromosome. For mutational targets significantly larger than about 500 nt (which includes most individual genes and all groups of genes), this capability would greatly streamline the sequencing of mutants by avoiding the need to individually amplify and sequence from each mutant all the chromosomal regions that could contain the mutation.
Acknowledgments
We thank K. Jacobs, N. Kurosawa, and S. Mallik for valuable technical assistance, J. Drake for communicating unpublished results, and G. Bertani and S. Maloy for thought-provoking comments. The potential relevance of ribosomal frameshifting was suggested by an anonymous reviewer.
This work was supported by grant N00014-94-I-0393 from the Office of Naval Research and grant MCB 9733303 from the National Science Foundation.
REFERENCES
- 1.Ghané F, Grogan D W. Chromosomal marker exchange in the archaeon Sulfolobus acidocaldarius: physiological and cellular aspects. Microbiology (Reading) 1998;144:1649–1657. doi: 10.1099/00221287-144-6-1649. [DOI] [PubMed] [Google Scholar]
- 2.Grogan D W, Gunsalus R P. Sulfolobus acidocaldarius synthesizes UMP via a standard de novo pathway: results of a biochemical-genetic study. J Bacteriol. 1993;175:1500–1507. doi: 10.1128/jb.175.5.1500-1507.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grogan D W. Isolation of Sulfolobus acidocaldarius mutants. In: Robb F T, et al., editors. Archaea: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1995. pp. 125–132. [Google Scholar]
- 4.Grogan D W. Exchange of genetic markers at extremely high temperatures in the archaeon Sulfolobus acidocaldarius. J Bacteriol. 1996;178:3207–3211. doi: 10.1128/jb.178.11.3207-3211.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs K L, Grogan D W. Rates of spontaneous mutation in an archaeon from geothermal environments. J Bacteriol. 1997;179:3298–3303. doi: 10.1128/jb.179.10.3298-3303.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs K L, Grogan D W. Spontaneous mutation in a thermoacidophilic archaeon: evaluation of genetic and physiological factors. Arch Microbiol. 1998;169:81–83. doi: 10.1007/s002030050544. [DOI] [PubMed] [Google Scholar]
- 7.Martusewitsch E, Sensen C, Schleper C. High spontaneous mutation rate in the hyperthermophilic archaeon Sulfolobus solfataricus is mediated by transposable elements. J Bacteriol. 2000;182:2574–2581. doi: 10.1128/jb.182.9.2574-2581.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker J. Errors and alternatives in reading the universal genetic code. Microbiol Rev. 1989;53:273–298. doi: 10.1128/mr.53.3.273-298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitcher D G, Saunders N A, Owen R J. Rapid extraction of bacterial genomic DNA with guanidinium thiocyanate. Lett Appl Microbiol. 1989;8:151–156. [Google Scholar]
- 10.Schleper C, Holz I, Janekovic D, Murphy J, Zillig W. A multicopy plasmid of the extremely thermophilic archaeon Sulfolobus effects its transfer to recipients by mating. J Bacteriol. 1995;177:4417–4426. doi: 10.1128/jb.177.15.4417-4426.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanofsky C, Crawford I P. The tryptophan operon. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1987. p. 1462. [Google Scholar]