Abstract
Obstructive sleep apnoea is a substantial clinical and public health problem because it contributes to harmful effects on quality of life, daytime symptoms, road traffic incidents, and cardiometabolic disease. Increasingly, obstructive sleep apnoea is recognised as a heterogeneous disease, and patients have varied susceptibility to long term complications and different responses to treatment. This narrative review summarises the current knowledge of precision medicine in obstructive sleep apnoea, particularly the role of symptom clusters, polysomnogram phenotypes, physiological endotypes, and circulating biomarkers in defining subtypes. In the near future, the prognostic accuracy of these measures in predicting long term complications in obstructive sleep apnoea will likely be improved, together with better matching of treatments to disease subtypes.
Keywords: Sleep medicine specialty, Medicine, Public health, Therapeutics
Introduction
Obstructive sleep apnoea is characterised by recurrent collapse of the upper airway during sleep and is the most common respiratory disorder related to sleep.1 Obstructive sleep apnoea is a major global health problem with substantial economic and social burdens because untreated obstructive sleep apnoea is associated with daytime sleepiness,2 poor sleep quality,3 reduced work productivity, and increased rates of road traffic incidents and occupational injuries.4 Obstructive sleep apnoea also causes multiple adverse physiological and biochemical effects, including hypoxaemia, oxidative stress, inflammation, sympathetic activation, cortical arousal, tachycardia, and vasoconstriction. In some patients, these adverse effects cause end organ damage and long term complications, including premature cardiovascular and cerebrovascular disease such as heart attacks and strokes,5 chronic kidney disease,6 and neurodegeneration.7
Precision medicine, also known as personalised medicine, is an approach tailored to the prevention and treatment of the disease that takes into account variability in genes, the environment, and lifestyle for each person.8 Precision medicine targets treatments to patients who are most likely to benefit from them, in contrast with the traditional one-size-fits-all approach for prevention and care. The goal of precision medicine is to target the right treatments to the right patients at the right time.
Obstructive sleep apnea is being recognised increasingly as a heterogeneous disease, and emerging evidence suggests the potential of using precision medicine approaches in its treatment and management.9 10 11 12 Patients with obstructive sleep apnoea can be classified into different subtypes based on symptoms, physiology, and molecular characteristics. The purpose of this review was to look at the existing body of knowledge on precision medicine in obstructive sleep apnoea, particularly the potential role of symptom clusters, polysomnogram phenotypes, physiological endotypes, and circulating biomarkers, in helping to define these subtypes. We believe that these factors might help us better understand the pathological basis of obstructive sleep apnoea and its complications, improve the prognosis for the long term effects on health, and better match patients to more specific treatments.
Prevalence of obstructive sleep apnoea
According to a comprehensive literature review from 2019, approximately a billion people worldwide have obstructive sleep apnoea, and 425 million adults have moderate-to-severe disease.2
Sources and selection criteria
We searched PubMed and Medline databases for peer reviewed, English language studies of adult obstructive sleep apnoea, published from 1 January 2012 to 31 May 2022, and manually searched the references of selected articles for relevant articles for our narrative review. We used the following medical subject headings search terms in combination with the term “precision medicine”: “pathophysiology,” “cluster analysis,” “polysomnography,” “biomarker/blood,” “classification,” and “treatment.” We also included highly cited papers published before 2012 based on our narrative review of the literature.
Symptom clusters
A symptom cluster is defined as two or more concurrent symptoms that are related to each other.13 Clinical symptom subtypes are associated with differential risks for prevalent and incident cardiovascular and cerebrovascular disease among patients with moderate-to-severe obstructive sleep apnoea. Ye and colleagues first used latent class analysis, an unsupervised cluster analysis, to identify clinical phenotypes of obstructive sleep apnoea based on symptoms and comorbidities.9 With this data driven approach, they originally explored the differences in the clinical presentation of obstructive sleep apnoea by classifying individuals into three groups: disturbed sleep, minimally symptomatic, and excessive daytime sleepiness. The three clusters were not substantially different for apnoea-hypopnoea index, body mass index, or sex. Most individuals in the disturbed sleep and minimally symptomatic clusters reported classic obstructive sleep apnoea symptoms (ie, pauses in breathing at night and loud snoring),1 14 15 but less often than patients in the excessive daytime sleepiness cluster.9
Symptom subtypes of obstructive sleep apnoea were identified, replicated, and validated based on clinical symptoms in a population based sample of patients with obstructive sleep apnoea.10 Particular symptom subtypes were shown to be independent predictors of prevalent cardiovascular and cerebrovascular disease in moderate-to-severe obstructive sleep apnoea, and an increased risk of incident cardiovascular and cerebrovascular disease among patients with obstructive sleep apnoea (excessively sleepy subtype) was found.10 We and others have identified similar symptom subtypes in clinic based cohorts of obstructive sleep apnoea and have found positive associations between these subtypes and future risk of cardiovascular and cerebrovascular disease.16–18 Although the main limitation is the lack of external validation, other cluster analyses on obstructive sleep apnoea have been published.19
In a 2022 study, subpopulations of patients with obstructive sleep apnoea were defined with latent class analysis in a cohort of Hispanic patients based in the community, and an association between symptom clusters of obstructive sleep apnoea and cardiovascular disease was identified20; the insomnia obstructive sleep apnoea phenotype (apnoea-hypopnoea index mean 10.5 events/h (standard deviation 5.2)) was associated with an increased incidence of cardiovascular and cerebrovascular disease (heart failure, hypertension, and diabetes) compared with those with no obstructive sleep apnoea. Other studies have shown a potential increased risk of cardiovascular and cerebrovascular disease in relation to symptoms of insomnia; for example, in the study by Labarca and colleagues, the disturbed sleep cluster had an increased but non-significant hazard ratio for cardiovascular events (2.87, P=0.11).16 Studies have also reported no significant independent relation between these subtypes and incident cardiovascular and cerebrovascular disease,21 however, and more work is needed in this area to better understand the relation between symptoms of obstructive sleep apnoea and the risk of long term effects on health.
The varied risks of cardiovascular disease seen in these clusters might be relevant when interpreting the results of randomised controlled trials of obstructive sleep apnoea treatment with continuous positive airway pressure. Observational studies have shown a robust reduction in cardiovascular events with continuous positive airway pressure in patients with obstructive sleep apnoea.22 23 By contrast, in large randomised controlled trials, treatment with continuous positive airway pressure did not significantly lower the risk of cardiovascular events in obstructive sleep apnoea. Patients in these randomised controlled trials tended to be non-sleepy, however. For example, in the SAVE (Sleep Apnea Cardiovascular Endpoints), ISAACC (Impact of Sleep Apnea Syndrome in the Evolution of Acute Coronary syndrome. Effect of Intervention with Continuous Positive Airway Pressure), and RICCADSA (Randomised Intervention with Continuous Positive Airway Pressure in Coronary Artery Disease and Obstructive Sleep Apnoea) studies, mean scores on the Epworth sleepiness scale were 7.4,15 5.3,24 and 5.5,25 respectively. The lack of symptoms might have contributed to the low adherence to treatment with continuous positive airway pressure in these trials; furthermore, the selected patients could have had a relatively low risk of cardiovascular and cerebrovascular disease caused by obstructive sleep apnoea itself.26
Physiological endotypes
More precise treatment for obstructive sleep apnoea has been hindered, at least partially, by the lack of an accurate means to assess the pathophysiological mechanisms underlying obstructive sleep apnoea in the clinical setting.27 An endotype of obstructive sleep apnoea represents a subtype that has a distinct physiological mechanism,28 rather than a phenotype which usually refers to the clinical manifestations of the disease.29 The pathogenesis of obstructive sleep apnoea is caused not only by anatomical factors (collapsible or narrow upper airway), but also by potential contributions from non-anatomical factors, such as increased loop gain, reduced pharyngeal muscle responsiveness, and low arousal threshold (physiological endotypes).30 Eckert et al used a three point scale to weigh non-anatomical (muscle responsiveness, arousal threshold, and loop gain) and anatomical (passive critical closing pressure of the upper airway) contributions to obstructive sleep apnoea.31 The study found that 19% of patients with obstructive sleep apnoea had a relatively non-collapsible upper airway similar to many of the control individuals (passive critical closing pressure of the upper airway 22-25 cm H2O) and further highlighted the multifactorial pathophysiology of obstructive sleep apnoea.31
Measuring these physiological characteristics has historically required complex equipment in specialised laboratories, but recent studies suggest that these data might be readily extractable from diagnostic polysomnograms. Sands and colleagues described an automated technique to quantify pharyngeal collapsibility and compensation with diagnostic polysomnographic signals in patients with obstructive sleep apnoea without specialised equipment or interventions.32 Moreover, they extended their approach for estimating arousal threshold and loop gain to evaluate the key traits causing obstructive sleep apnoea, implying that the pathophysiological contributions to the disease can be estimated in the clinical setting.27 33 Finnsson and colleagues reimplemented and validated the methods and algorithms of Sands et al.34 Their new cloud based implementation in Python showed the potential reproducibility and scalability of this approach. These findings could provide access to endophenotyping of obstructive sleep apnoea for more scientists and clinicians, which could then become an integral part of a precision medicine approach to obstructive sleep apnoea.34
Theoretically, treatments could be guided by physiological subtypes. For example, sedatives might reduce the severity of obstructive sleep apnoea in patients who have a low-to-moderate arousal threshold, although this effect is speculative.35 Also, recent data suggest that successful therapeutic responses to currently available treatments for obstructive sleep apnoea might be predicted by these endotypic measurements. Op de Beeck and colleagues used data from diagnostic polysomnography to assess the endotypic mechanisms underlying success versus failure of hypoglossal nerve stimulation (an increasingly common alternative for patients with obstructive sleep apnoea who are intolerant to continuous positive airway pressure).36 Favourable responses to hypoglossal nerve stimulation treatment were independently associated with a higher arousal threshold (odds ratio 6.76, 95% confidence interval 2.44 to 23.3, P=0.001), higher muscle compensation (4.22, 1.70 to 12.55, P=0.004), and a lower loop gain (in milder collapsibility, P=0.003).36
Bamagoos and colleagues performed a secondary endotypic trait analysis of polysomnographic data from patients with obstructive sleep apnoea to find predictors of the efficacy of oral appliances.37 Greater efficacy of oral appliances was associated with particular subgroups of obstructive sleep apnoea (lower loop gain, higher arousal threshold, and lower ventilatory response to arousal), moderate pharyngeal collapsibility, and weaker muscle compensation.37 Op de Beeck and colleagues tested whether endotypes of obstructive sleep apnoea estimated by routine polysomnography could differentiate between responders and non-responders to treatment with mandibular advancement devices.38 Patients who responded to mandibular advancement devices had a significantly lower loop gain (P=0.02), which was calculated from the baseline clinical polysomnogram, consistent with the previous study.37
A secondary data analysis of treatment of obstructive sleep apnoea in patients with acute stroke examined the association between endotypic traits and adherence to continuous positive airway pressure. Decreased arousal threshold and increased pharyngeal muscle compensation were associated with lower adherence to continuous positive airway pressure in patients with stroke, suggesting that these traits might help predict adherence to continuous positive airway pressure.39 Further clarification of specific physiological endotypes in individuals with obstructive sleep apnoea might facilitate the development of new treatments that can be tailored to individual patient needs according to the physiological endotype or endotypes of their disease.
Hence these endotypes could help in identifying patients who might have greater improvements in symptoms and more robust responses to specific treatments for obstructive sleep apnoea. Because of the limitations of using only the apnoea-hypopnoea index as an indicator of disease and its severity (see next section), assessment of endotypes of obstructive sleep apnoea should help to better characterise the heterogeneity of the disease.
Advanced polysomnographic metrics
When obstructive sleep apnoea is suspected, patients will often undergo polysomnography in a sleep laboratory, an overnight study where physiological data are continuously collected over eight hours: electroencephalography (EEG), electrocardiography, oxygen saturation, airflow, electromyography, and chest wall and abdominal movements. Based almost solely on the apnoea-hypopnoea index, the number of overnight respiratory events per hour of total sleep, the severity of obstructive sleep apnoea is classified as mild, moderate, or severe.15 Hence little of the polysomnographic data are used in clinical decision making because current practice is to largely reduce data complexity to a simple measurement (apnoea-hypopnoea index).16
As a standard of measurement, metrics aid in the differentiation of disease states and consequently classify the severity of the disease.40 A critique of the apnoea-hypopnoea index over the years has been its inability to accurately predict symptoms, treatment response, and the risk of long term health complications.40 41 This limitation might be partly because the apnoea-hypopnoea index does not capture the different physiological effects of respiratory events within and between individuals because all events are considered equal. Thus substantial interest has been reported in alternative metrics that can be extracted from polysomnograms that might reflect the severity of obstructive sleep apnoea and predict long term health complications better than the apnoea-hypopnoea index.42
New polysomnographic metrics, such as hypoxic burden, heart rate response to arousal, odds ratio product, quantitative EEG related metrics, and respiratory events load (eg, apnoea load), aim to use polysomnography to improve our understanding of sleep and sleep disorders43 44 (table 1). Specifically, hypoxic burden45 46 is calculated by assessing the area under the oximetry desaturation curve coupled with respiratory events. This metric seems to be better in predicting kidney disease,47 stroke,48 cardiovascular and cerebrovascular disease, and mortality than the apnoea-hypopnoea index in cohorts based in the clinic49 and in the community.21 We recognise, however, that there are other ways of measuring the degree of hypoxia in obstructive sleep apnoea. Because desaturation is a key feature of obstructive sleep apnoea and likely drives many of the end organ complications, further work to test these other types of metrics seems warranted.50 51
Table 1.
Polysomnographic metrics associated with outcomes of obstructive sleep apnoea
| Author (year) | Metric | Source | Outcome |
| Azarbarzin 2019,45 Trzepizur 2022,21 Blanchard 2021,48 Jackson 202147 | Hypoxic burden | Oximetry signal | Cardiovascular and cerebrovascular disease, mortality, stroke, kidney disease |
| Azarbarzin 202152 | ΔHR | Pulse (photoplethysmography) | Cardiovascular and cerebrovascular disease, mortality |
| Younes 2015,54 Kim 202155 | Odds ratio product | EEG | Hypertension |
| Lechat 202158 | Delta wave activity | EEG | Mortality |
| Goh 201859 | Respiratory events load | Airflow | Epworth sleepiness scale |
EEG=electroencephalography; ΔHR=heart rate response to respiratory events.
Another metric based on the physiological effects of obstructive sleep apnoea is heart rate response to respiratory events. Heart rate response to respiratory event was a better predictor of cardiovascular and cerebrovascular disease in patients with obstructive sleep apnoea.52 Also, a 2022 study showed that a greater heart rate response to respiratory events before treatment was associated with greater protection of continuous positive airway pressure against adverse cardiovascular outcomes.53
Indices derived from EEG signals based on polysomnography that better reflect the depth of sleep might also be useful to more accurately characterise patients. Based on a spectral analysis of EEG signals with fast Fourier transformation, relative frequency power in an array of frequency ranges (delta, alpha, theta, and beta) can be calculated.54 With proprietary scoring methods, these frequency powers can then be converted to a continuous measurement of sleep depth, the odds ratio product.55 Patterns of odds ratio product across the night could help to classify patients according to symptoms (eg, concomitant insomnia and obstructive sleep apnoea)56 and might help predict adherence to continuous positive airway pressure in severe obstructive sleep apnoea.57
Lechat and colleagues investigated the association between disrupted delta power (calculated by entropy and power spectral analysis of the EEG delta frequency band) and all cause mortality.58 They showed that disrupted delta power (upper and lower thirds of entropy function) during sleep was associated with a 32% increased risk of all cause mortality compared with no fragmentation (mid-third of the entropy distribution function) (hazard ratio 1.32, 95% confidence interval 1.14 to 1.50), after adjusting for confounders.
Finally, investigating how the severity of respiratory events would affect outcomes in patients with obstructive sleep apnoea showed that apnoea or hypopnoea load, which considers the length of the event and not only the presence or absence of events, was a better indicator of sleepiness than the apnoea-hypopnoea index alone.59 These new metrics could provide a more nuanced representation of the disease and help predict complications. These metrics need to be validated in other cohorts, however, before they can be used in medical decision making.42
Circulating biomarkers of risk
Biomarkers refer to a broad subcategory of medical signs.60 For this review, however, we will focus on recent data on circulating molecular markers of the risk of cardiovascular disease in obstructive sleep apnoea. Biomarkers are important assessable indicators that can provide complementary information for many diseases, including obstructive sleep apnoea,61 cardiovascular diseases,62 diabetes mellitus,63 hypertension,64 stroke,65 asthma,66 and heart failure.63
Intermittent hypoxia and consequent reoxygenation injury are a hallmark of obstructive sleep apnoea.61 67 Patients with obstructive sleep apnoea have increased circulating biomarkers of inflammation and oxidative stress (eg, adhesion molecules, C reactive protein, and 8-isoprostane).68–70 Therefore, biomarkers reflecting these processes of inflammation and oxidative stress could provide prognostic information on the risk of cardiovascular disease in obstructive sleep apnoea. Identifying a high risk group could have major clinical use because they might represent a target population for more aggressive management of obstructive sleep apnoea and other risk factors.71
In a recent study, multivariate protein measurement was used to quantify 5000 proteins cross sectionally in the plasma of 1391 clinic patients in relation to the presence and severity of obstructive sleep apnoea.72 The effect of treatment with continuous positive airway pressure or positive airway pressure on the proteins identified was also examined to explore potential pathophysiological mechanisms in two separate intervention based cohorts with obstructive sleep apnoea. The study showed that eight proteins (tissue type plasminogen activator, amyloid-like protein 1, plasminogen activator inhibitor 1, secretogranin 3, cystine-rich protein 1, prosurvival protein 1, sex hormone binding globulin, and desmoglein 2) likely predicted the presence and severity of obstructive sleep apnoea; and five protein markers (C reactive protein, tissue type plasminogen activator, plasminogen activator inhibitor 1, tartrate resistant acid phosphatase type 5, and soluble E-selectin) were significantly affected by continuous positive airway pressure or positive airway pressure (P=0.004, P=0.047, P=0.007, P=0.009, P=0.030, respectively).72 These results suggested that the subset of proteins might be promising markers in the management of obstructive sleep apnoea.
Markers of inflammation might also help group patients with obstructive sleep apnoea. In a pilot study of 155 patients with suspected obstructive sleep apnoea, increased levels of C reactive protein were associated with a markedly increased risk of cardiovascular and cerebrovascular disease. Specifically, patients with a C reactive protein concentration >2.38 mg/L had a significantly increased risk of cardiovascular and cerebrovascular disease within eight years of polysomnography (odds ratio 9.72, 95% confidence interval 2.43 to 38.84, P=0.001).73 Also, in a sample of 418 patients with suspected obstructive sleep apnoea, patients with higher levels of intercellular adhesion molecule 1 had a greater risk of cardiovascular events over eight years of follow-up, whereas patients with moderate-to-severe obstructive sleep apnoea with higher levels of E-selectin were more likely to have cardiovascular events.74 A genetic single nucleotide polymorphism that we previously found to be strongly associated with levels of E-selectin (ie, location rs579459 of the ABO gene) was also significantly associated with incident cardiovascular events (P=0.02),75 suggesting that genomic markers might also help with the prediction of risk.76 The limitations of these studies include the relatively small number of events and single centre design; clearly, more work is needed to replicate and expand these preliminary findings. Nevertheless, these results show the potential of inflammatory biomarkers to group potential of inflammatory biomarkers to help risk stratify patients by future cardiovascular risk.
Obstructive sleep apnoea is a common risk factor for hypertension, but in 25-30% of patients with obstructive sleep apnoea who were treated with continuous positive airway pressure (>4 hours/night), a positive effect on their blood pressure was not seen.77 The underlying mechanisms of this variability in response to continuous positive airway pressure are not known. MicroRNAs (miRNAs, a subclass of small single stranded non-coding RNAs) are important in regulating many biological processes and human diseases,78 including obstructive sleep apnoea.79 One study used an 84 miRNA array to discriminate plasma miRNA profiles and predict blood pressure responses to treatment with continuous positive airway pressure in patients with obstructive sleep apnoea and resistant hypertension.80 A cluster of three plasma miRNAs predicted patients with obstructive sleep apnoea with resistant hypertension, whose blood pressure adequately responded to treatment with continuous positive airway pressure. These results need to be replicated in larger cohorts to see if plasma miRNAs might eventually be clinically useful as biomarkers of cardiovascular risk.
A more precise molecular phenotyping of obstructive sleep apnoea with these and other markers could provide a more nuanced representation of the disease. Ultimately, this advanced phenotyping might provide more accurate care, matching precise subtypes to specific treatments. For example, patients with increased levels of biomarkers of oxidative stress or inflammation might have a more robust response to antioxidants or anti-inflammatory drugs, respectively, to reduce the risk of cardiovascular disease.81
Future research directions
Substantial work has been completed regarding personalised care approaches to obstructive sleep apnoea.12 However, substantial limitations to the current state of knowledge exist, and more work is needed before theses approaches can be implemented into clinical practice. Primarily, the use of subtypes of obstructive sleep apnoea defined by endotypes, polysomnographic markers, symptoms, and circulating biomarkers identified in previous studies need to be validated and confirmed in other cohorts that reflect a broader and more diverse patient population (eg, in terms of ethnic group,47 sex,82 and comorbidities83). The prevalence of obstructive sleep apnoea varies substantially by sex,84 and distinct differences exist between men and women in physiology, symptoms, and clinical outcomes of obstructive sleep apnoea.82 Many of the cohorts have included predominantly men, and more studies in women are needed to validate the potential measures discussed in this review. Differences among ethnic groups and other factors might exist; hence more studies that represent a more diverse patient group need to be completed before these measures can be implemented more broadly into clinical practice.
More work is needed to better integrate patients' perspectives and increase participation of patients and caregivers in their clinical care (ie, the participatory component of the P4 approach to personalised medicine).85 Also, because these personalised approaches will put a greater burden on patients (eg, collection of blood and urine samples), on analytical resources and platforms, and on financial costs, studies examining the economic return of this approach (eg, cost effectiveness studies) are also required before large scale investment. The gap between our current conceptual stage and accepted clinical practice is still wide.
Conclusions
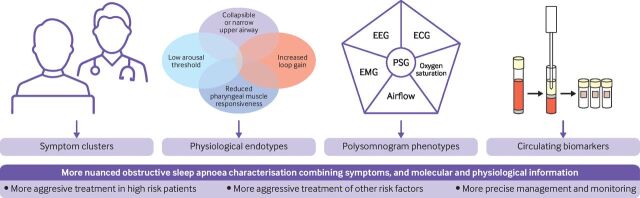
Obstructive sleep apnoea is a heterogeneous disease, and a one-size-fits-all approach to the disease is likely not appropriate. In this review, we have highlighted potential future directions towards a better understanding of the heterogeneity of obstructive sleep apnoea and a precision care approach. Specifically, we have highlighted the emerging role of symptom clusters, physiological endotypes, advanced polysomnographic metrics, and circulating biomarkers in helping to group patients with obstructive sleep apnoea based on risk. We see a future paradigm of care where information from multiple sources is incorporated into statistical models to predict which patients have an increased risk of long term health complications, and to help match patients to more precise treatments (figure 1). This approach should provide a more nuanced representation of the disease for patients and providers, and help in moving research in this area forward.
Figure 1.
Precision care management for obstructive sleep apnoea with symptom clusters, polysomnogram phenotypes, physiological endotypes, and circulating biomarkers. PSG=polysomnography; EEG=electroencephalography; ECG=electrocardiography; EMG=electromyography
Questions for future research.
How can we more precisely assess the underlying differences of patients with obstructive sleep apnoea by using clinical symptoms, physiological endotypes, advanced polysomnographic metrics, and circulating biomarkers?
How can we use more precise phenotyping of patients to help predict the long term effects on health of obstructive sleep apnoea, and match patients to the most appropriate treatment?
How can we accelerate the translation of research to help advance the treatment and diagnosis of obstructive sleep apnoea?
How can we begin to apply a personalised medicine approach to patients with obstructive sleep apnoea in the clinical setting?
Patient involvement.
Patients and the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Footnotes
Contributors: All authors defined the intellectual content, conducted the literature research, acquired the data, and participated in the preparation, editing, and critical review of the manuscript. All authors have given final approval of the manuscript. NA is the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests: We have read and understood the BMJ policy on declaration of interests and declare the following interests: none.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1. Semelka M, Wilson J, Floyd R. Diagnosis and treatment of obstructive sleep apnea in adults. Am Fam Physician 2016;94:355–60. [PubMed] [Google Scholar]
- 2. Chen L, Chapman JL, Yee BJ, et al. Agreement between electronic and paper Epworth sleepiness scale responses in obstructive sleep apnoea: secondary analysis of a randomised controlled trial undertaken in a specialised tertiary care clinic. BMJ Open 2018;8:e019255. 10.1136/bmjopen-2017-019255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heilbrunn ES, Ssentongo P, Chinchilli VM, et al. Sudden death in individuals with obstructive sleep apnoea: a systematic review and meta-analysis. BMJ Open Respir Res 2021;8:e000656. 10.1136/bmjresp-2020-000656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. AlGhanim N, Comondore VR, Fleetham J, et al. The economic impact of obstructive sleep apnea. Lung 2008;186:7–12. 10.1007/s00408-007-9055-5 [DOI] [PubMed] [Google Scholar]
- 5. Golbidi S, Badran M, Ayas N, et al. Cardiovascular consequences of sleep apnea. Lung 2012;190:113–32. 10.1007/s00408-011-9340-1 [DOI] [PubMed] [Google Scholar]
- 6. Abuyassin B, Sharma K, Ayas NT, et al. Obstructive sleep apnea and kidney disease: a potential bidirectional relationship? J Clin Sleep Med 2015;11:915–24. 10.5664/jcsm.4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liguori C, Maestri M, Spanetta M, et al. Sleep-disordered breathing and the risk of Alzheimer's disease. Sleep Med Rev 2021;55:101375. 10.1016/j.smrv.2020.101375 [DOI] [PubMed] [Google Scholar]
- 8. Akhoon N. Precision medicine: a new paradigm in therapeutics. Int J Prev Med 2021;12:12. 10.4103/ijpvm.IJPVM_375_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ye L, Pien GW, Ratcliffe SJ, et al. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J 2014;44:1600–7. 10.1183/09031936.00032314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mazzotti DR, Keenan BT, Lim DC, et al. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med 2019;200:493–506. 10.1164/rccm.201808-1509OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zinchuk AV, Gentry MJ, Concato J, et al. Phenotypes in obstructive sleep apnea: a definition, examples and evolution of approaches. Sleep Med Rev 2017;35:113–23. 10.1016/j.smrv.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carberry JC, Amatoury J, Eckert DJ. Personalized management approach for OSA. Chest 2018;153:744–55. 10.1016/j.chest.2017.06.011 [DOI] [PubMed] [Google Scholar]
- 13. Kim H-J, McGuire DB, Tulman L, et al. Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs 2005;28:270–82. 10.1097/00002820-200507000-00005 [DOI] [PubMed] [Google Scholar]
- 14. Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230–5. 10.1056/NEJM199304293281704 [DOI] [PubMed] [Google Scholar]
- 15. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016;375:919–31. 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 16. Labarca G, Dreyse J, Salas C, et al. A validation study of four different cluster analyses of OSA and the incidence of cardiovascular mortality in a Hispanic population. Chest 2021;160:2266–74. 10.1016/j.chest.2021.06.047 [DOI] [PubMed] [Google Scholar]
- 17. Allen AJH, Jen R, Mazzotti DR, et al. Symptom subtypes and risk of incident cardiovascular and cerebrovascular disease in a clinic-based obstructive sleep apnea cohort. J Clin Sleep Med 2022;18:2093–102. 10.5664/jcsm.9986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allen AH, Beaudin AE, Fox N, et al. Symptom subtypes and cognitive function in a clinic-based OSA cohort: a multi-centre Canadian study. Sleep Med 2020;74:92–8. 10.1016/j.sleep.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zinchuk A, Yaggi HK. Phenotypic subtypes of OSA: a challenge and opportunity for precision medicine. Chest 2020;157:403–20. 10.1016/j.chest.2019.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu B, Tarraf W, Wallace DM, et al. Cardiovascular correlates of sleep apnea phenotypes: results from the Hispanic community health study/study of Latinos (HCHS/SOL). PLoS One 2022;17:e0265151. 10.1371/journal.pone.0265151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trzepizur W, Blanchard M, Ganem T, et al. Sleep apnea-specific hypoxic burden, symptom subtypes, and risk of cardiovascular events and all-cause mortality. Am J Respir Crit Care Med 2022;205:108–17. 10.1164/rccm.202105-1274OC [DOI] [PubMed] [Google Scholar]
- 22. Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 2010;182:269–77. 10.1164/rccm.200911-1746OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patil SP, Ayappa IA, Caples SM, et al. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2019;15:335–43. 10.5664/jcsm.7640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sánchez-de-la-Torre M, Sánchez-de-la-Torre A, Bertran S, et al. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. Lancet Respir Med 2020;8:359–67. 10.1016/S2213-2600(19)30271-1 [DOI] [PubMed] [Google Scholar]
- 25. Peker Y, Glantz H, Eulenburg C, et al. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. Am J Respir Crit Care Med 2016;194:613–20. 10.1164/rccm.201601-0088OC [DOI] [PubMed] [Google Scholar]
- 26. Pack AI, Magalang UJ, Singh B, et al. Randomized clinical trials of cardiovascular disease in obstructive sleep apnea: understanding and overcoming bias. Sleep 2021;44. 10.1093/sleep/zsaa229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Terrill PI, Edwards BA, Nemati S, et al. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J 2015;45:408–18. 10.1183/09031936.00062914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Edwards BA, Redline S, Sands SA, et al. More than the sum of the respiratory events: personalized medicine approaches for obstructive sleep apnea. Am J Respir Crit Care Med 2019;200:691–703. 10.1164/rccm.201901-0014TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Light M, Owens RL, Schmickl CN, et al. Precision medicine for obstructive sleep apnea. Sleep Med Clin 2019;14:391–8. 10.1016/j.jsmc.2019.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suzuki M. Obstructive sleep apnea -consideration of its pathogenesis. Auris Nasus Larynx 2022;49:313–21. 10.1016/j.anl.2021.10.007 [DOI] [PubMed] [Google Scholar]
- 31. Eckert DJ, White DP, Jordan AS, et al. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med 2013;188:996–1004. 10.1164/rccm.201303-0448OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sands SA, Edwards BA, Terrill PI, et al. Phenotyping pharyngeal pathophysiology using polysomnography in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2018;197:1187–97. 10.1164/rccm.201707-1435OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sands SA, Terrill PI, Edwards BA, et al. Quantifying the arousal threshold using polysomnography in obstructive sleep apnea. Sleep 2018;41. 10.1093/sleep/zsx183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Finnsson E, Ólafsdóttir GH, Loftsdóttir DL, et al. A scalable method of determining physiological endotypes of sleep apnea from a polysomnographic sleep study. Sleep 2021;44. 10.1093/sleep/zsaa168. [Epub ahead of print: 21 Jan 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jordan AS, O'Donoghue FJ, Cori JM, et al. Physiology of arousal in obstructive sleep apnea and potential impacts for sedative treatment. Am J Respir Crit Care Med 2017;196:814–21. 10.1164/rccm.201612-2511PP [DOI] [PubMed] [Google Scholar]
- 36. Op de Beeck S, Wellman A, Dieltjens M, et al. Endotypic mechanisms of successful hypoglossal nerve stimulation for obstructive sleep apnea. Am J Respir Crit Care Med 2021;203:746–55. 10.1164/rccm.202006-2176OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bamagoos AA, Cistulli PA, Sutherland K, et al. Polysomnographic endotyping to select patients with obstructive sleep apnea for oral appliances. Ann Am Thorac Soc 2019;16:1422–31. 10.1513/AnnalsATS.201903-190OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Op de Beeck S, Dieltjens M, Azarbarzin A, et al. Mandibular advancement device treatment efficacy is associated with polysomnographic endotypes. Ann Am Thorac Soc 2021;18:511–8. 10.1513/AnnalsATS.202003-220OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zinchuk AV, Redeker NS, Chu J-H, et al. Physiological traits and adherence to obstructive sleep apnea treatment in patients with stroke. Am J Respir Crit Care Med 2020;201:1568–72. 10.1164/rccm.201911-2203LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Malhotra A, Ayappa I, Ayas N, et al. Metrics of sleep apnea severity: beyond the apnea-hypopnea index. Sleep 2021;44. 10.1093/sleep/zsab030. [Epub ahead of print: 09 Jul 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vakulin A, D'Rozario A, Kim J-W, et al. Quantitative sleep EEG and polysomnographic predictors of driving simulator performance in obstructive sleep apnea. Clin Neurophysiol 2016;127:1428–35. 10.1016/j.clinph.2015.09.004 [DOI] [PubMed] [Google Scholar]
- 42. Hajipour M, Baumann B, Azarbarzin A, et al. Association of alternative polysomnographic features with patient outcomes in obstructive sleep apnea: a systematic review. J Clin Sleep Med 2022. 10.5664/jcsm.10298. [Epub ahead of print: 15 Sep 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lim DC, Mazzotti DR, Cistulli PA, et al. Reply to Hunasikatti commentary: reinventing polysomnography in the age of precision medicine-Not at cost of discarding the hard data. Sleep Med Rev 2020;54:101373. 10.1016/j.smrv.2020.101373 [DOI] [PubMed] [Google Scholar]
- 44. de Chazal P, Sutherland K, Cistulli PA. Advanced polysomnographic analysis for OSA: a pathway to personalized management? Respirology 2020;25:251–8. 10.1111/resp.13564 [DOI] [PubMed] [Google Scholar]
- 45. Azarbarzin A, Sands SA, Stone KL, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the osteoporotic fractures in men study and the sleep heart health study. Eur Heart J 2019;40:1149–57. 10.1093/eurheartj/ehy624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martinez-Garcia MA, Sánchez-de-la-Torre M, White DP, et al. Hypoxic burden in obstructive sleep apnea: present and future. Arch Bronconeumol 2022;7. 10.1016/j.arbres.2022.08.009 [DOI] [PubMed] [Google Scholar]
- 47. Jackson CL, Umesi C, Gaston SA, et al. Multiple, objectively measured sleep dimensions including hypoxic burden and chronic kidney disease: findings from the multi-ethnic study of atherosclerosis. Thorax 2021;76:704–13. 10.1136/thoraxjnl-2020-214713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Blanchard M, Feuilloy M, Gervès-Pinquié C, et al. Cardiovascular risk and mortality prediction in patients suspected of sleep apnea: a model based on an artificial intelligence system. Physiol Meas 2021;42. 10.1088/1361-6579/ac2a8f. [Epub ahead of print: 29 Oct 2021]. [DOI] [PubMed] [Google Scholar]
- 49. Azarbarzin A, Sands SA, Taranto-Montemurro L, et al. Hypoxic burden captures sleep apnoea-specific nocturnal hypoxaemia. Eur Heart J 2019;40:2989–90. 10.1093/eurheartj/ehz274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sutherland K, Sadr N, Bin YS, et al. Comparative associations of oximetry patterns in obstructive sleep apnea with incident cardiovascular disease. Sleep 2022;45. 10.1093/sleep/zsac179. [Epub ahead of print: 12 Dec 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de Chazal P, Sadr N, Dissanayake H, et al. Predicting cardiovascular outcomes using the respiratory event desaturation transient area derived from overnight sleep studies. Annu Int Conf IEEE Eng Med Biol Soc 2021;2021:5496–9. 10.1109/EMBC46164.2021.9630610 [DOI] [PubMed] [Google Scholar]
- 52. Azarbarzin A, Sands SA, Younes M, et al. The sleep apnea-specific pulse-rate response predicts cardiovascular morbidity and mortality. Am J Respir Crit Care Med 2021;203:1546–55. 10.1164/rccm.202010-3900OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Azarbarzin A, Zinchuk A, Wellman A, et al. Cardiovascular benefit of continuous positive airway pressure in adults with coronary artery disease and obstructive sleep apnea without excessive sleepiness. Am J Respir Crit Care Med 2022;206:767–74. 10.1164/rccm.202111-2608OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Younes M, Ostrowski M, Soiferman M, et al. Odds ratio product of sleep EEG as a continuous measure of sleep state. Sleep 2015;38:641–54. 10.5665/sleep.4588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim JS, Azarbarzin A, Wang R, et al. Association of novel measures of sleep disturbances with blood pressure: the multi-ethnic study of atherosclerosis. Thorax 2020;75:57–63. 10.1136/thoraxjnl-2019-213533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Younes M, Gerardy B, Pack AI, et al. Sleep architecture based on sleep depth and propensity: patterns in different demographics and sleep disorders and association with health outcomes. Sleep 2022;45. 10.1093/sleep/zsac059. [Epub ahead of print: 13 Jun 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Younes MK, Beaudin AE, Raneri JK, et al. Adherence index: sleep depth and nocturnal hypoventilation predict long-term adherence with positive airway pressure therapy in severe obstructive sleep apnea. J Clin Sleep Med 2022;18:1933–44. 10.5664/jcsm.10028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lechat B, Hansen KL, Melaku YA, et al. A novel electroencephalogram-derived measure of disrupted delta wave activity during sleep predicts all-cause mortality risk. Ann Am Thorac Soc 2022;19:649–58. 10.1513/AnnalsATS.202103-315OC [DOI] [PubMed] [Google Scholar]
- 59. Goh JC, Tang J, Cao JX, et al. Apnoeic and hypopnoeic load in obstructive sleep apnoea: correlation with Epworth sleepiness scale. Ann Acad Med Singap 2018;47:216–22. 10.47102/annals-acadmedsg.V47N6p216 [DOI] [PubMed] [Google Scholar]
- 60. Biomarkers Definitions Working Group. . Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89–95. 10.1067/mcp.2001.113989 [DOI] [PubMed] [Google Scholar]
- 61. Badran M, Ayas N, Laher I. Cardiovascular complications of sleep apnea: role of oxidative stress. Oxid Med Cell Longev 2014;2014:1–10. 10.1155/2014/985258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Matsumoto S, Kuroda S, Sano T, et al. Clinical and biomarker profiles and prognosis of elderly patients with coronavirus disease 2019 (COVID-19) with cardiovascular diseases and/or risk factors. Circ J 2021;85:921–8. 10.1253/circj.CJ-21-0160 [DOI] [PubMed] [Google Scholar]
- 63. Spivak YA, Lyulka NO, Potyazhenko MM, et al. Biomarker and echocardiographic characteristics of heart failure in patients having acute myocardial infarction combined with diabetes mellitus of type 2. Wiad Lek 2022;75:759–64. 10.36740/WLek202204102 [DOI] [PubMed] [Google Scholar]
- 64. Sung JH, Lee JE, Lee J-Y. Biomarker differences between controlled and uncontrolled hypertension among US adults: national health and nutrition examination survey 2005-2010. Int J Adv Res Sci Eng Technol 2018;5:6790–7. [PMC free article] [PubMed] [Google Scholar]
- 65. Mojtabavi H, Shaka Z, Momtazmanesh S, et al. Circulating brain-derived neurotrophic factor as a potential biomarker in stroke: a systematic review and meta-analysis. J Transl Med 2022;20:126. 10.1186/s12967-022-03312-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rosenkranz MA, Dean DC, Bendlin BB, et al. Neuroimaging and biomarker evidence of neurodegeneration in asthma. J Allergy Clin Immunol 2022;149:589–98. 10.1016/j.jaci.2021.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ayas NT, Hirsch AAJ, Laher I, et al. New frontiers in obstructive sleep apnoea. Clin Sci 2014;127:209–16. 10.1042/CS20140070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shah A, Mukherjee S, McArdle N, et al. Circulating C-reactive protein levels in patients with suspected obstructive sleep apnea. J Clin Sleep Med 2022;18:993–1001. 10.5664/jcsm.9774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Peres BU, Allen AJH, Kendzerska T, et al. Obstructive sleep apnea severity, body mass index, and circulating levels of cellular adhesion molecules. Lung 2020;198:939–45. 10.1007/s00408-020-00401-x [DOI] [PubMed] [Google Scholar]
- 70. Peres BU, Allen AJH, Shah A, et al. Obstructive sleep apnea and circulating biomarkers of oxidative stress: a cross-sectional study. Antioxidants 2020;9. 10.3390/antiox9060476. [Epub ahead of print: 02 06 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Peres BU, Hirsch Allen AJ, Fox N, et al. Circulating biomarkers to identify cardiometabolic complications in patients with obstructive sleep apnea: a systematic review. Sleep Med Rev 2019;44:48–57. 10.1016/j.smrv.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 72. Cederberg KLJ, Hanif U, Peris Sempere V, et al. Proteomic biomarkers of the apnea hypopnea index and obstructive sleep apnea: insights into the pathophysiology of presence, severity, and treatment response. Int J Mol Sci 2022;23. 10.3390/ijms23147983. [Epub ahead of print: 20 Jul 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ayas NT, Hirsch Allen AJ, Fox N, et al. C-reactive protein levels and the risk of incident cardiovascular and cerebrovascular events in patients with obstructive sleep apnea. Lung 2019;197:459–64. 10.1007/s00408-019-00237-0 [DOI] [PubMed] [Google Scholar]
- 74. Peres BU, Hirsch Allen AJ, Daniele P, et al. Circulating levels of cell adhesion molecules and risk of cardiovascular events in obstructive sleep apnea. PLoS One 2021;16:e0255306. 10.1371/journal.pone.0255306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sandford AJ, Ha A, Ngan DA, et al. Adhesion molecule gene variants and plasma protein levels in patients with suspected obstructive sleep apnea. PLoS One 2019;14:e0210732. 10.1371/journal.pone.0210732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hirsch Allen AJ, Ghafoor AA, Liu Y, et al. The rs579459 ABO gene polymorphism and risk of incident cardiovascular events in obstructive sleep apnea: a pilot study. Sleep Breath 2022. 10.1007/s11325-022-02638-7. [Epub ahead of print: 07 Jun 2022]. [DOI] [PubMed] [Google Scholar]
- 77. Martínez-García M-A, Capote F, Campos-Rodríguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 2013;310:2407–15. 10.1001/jama.2013.281250 [DOI] [PubMed] [Google Scholar]
- 78. O'Brien J, Hayder H, Zayed Y, et al. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol 2018;9:402. 10.3389/fendo.2018.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pinilla L, Barbé F, de Gonzalo-Calvo D. Micrornas to guide medical decision-making in obstructive sleep apnea: a review. Sleep Med Rev 2021;59:101458. 10.1016/j.smrv.2021.101458 [DOI] [PubMed] [Google Scholar]
- 80. Sánchez-de-la-Torre M, Khalyfa A, Sánchez-de-la-Torre A, et al. Precision medicine in patients with resistant hypertension and obstructive sleep apnea: blood pressure response to continuous positive airway pressure treatment. J Am Coll Cardiol 2015;66:1023–32. 10.1016/j.jacc.2015.06.1315 [DOI] [PubMed] [Google Scholar]
- 81. Ayas NT, Foster GE, Shah N, et al. Could adjunctive pharmacology mitigate cardiovascular consequences of obstructive sleep apnea? Am J Respir Crit Care Med 2019;200:551–5. 10.1164/rccm.201811-2097PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wimms A, Woehrle H, Ketheeswaran S, et al. Obstructive sleep apnea in women: specific issues and interventions. Biomed Res Int 2016;2016:1–9. 10.1155/2016/1764837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Labarca G, Dreyse J, Salas C, et al. Risk of mortality among patients with moderate to severe obstructive sleep apnea and diabetes mellitus: results from the SantOSA cohort. Sleep Breath 2021;25:1467–75. 10.1007/s11325-020-02283-y [DOI] [PubMed] [Google Scholar]
- 84. Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev 2017;34:70–81. 10.1016/j.smrv.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 85. Lim DC, Sutherland K, Cistulli PA, et al. P4 medicine approach to obstructive sleep apnoea. Respirology 2017;22:849–60. 10.1111/resp.13063 [DOI] [PMC free article] [PubMed] [Google Scholar]