Abstract
Introduction
Colorectal cancer (CRC) is the 3rd most common malignant tumors after breast cancer and lung cancer, accounting for 9.4% of patients. Some patients had distant metastasis at the time of diagnosis without surgery opportunity. It is particularly important to prolong patient survival and improve quality of life.
Patient Concerns
A 73-year-old female was admitted with discomfort over 2 months. Enlarged lymph nodes in the left supraclavicular fossa were observed in chest computed tomography (CT). Enhanced abdominal CT showed thickening of the right colon wall with multiple metastatic lymph nodes in the abdomen. Colonoscopy showed ileocecal mass and pathology showed moderately and poorly differentiated adenocarcinoma. Physical examination showed a 2*2 cm lymph node could be touched in the left supraclavicular fossa. The patient was diagnosed advanced colon cancer by the histopathological examination and imaging findings. Actually, it is hardly to resect radically.
Intervention
Sintilimab combined with XELOX was initiated. Two period of treatment after initial therapy, laparoscopic radical resection of right colon cancer was performed successfully.
Outcomes
After conversion treatment, the enlarged lymph nodes and primary tumor were significantly reduced. The patient was discharged successfully three weeks after surgery. Both specimen and 14 lymph nodes dissected showed no malignancy in pathology. Tumor regression grading (TRG) is 0, which indicate complete regression with no residual tumor cells including lymph nodes. The patient obtained a pathological complete response (pCR).
Lessons
The patient achieved a great therapeutic benefit with the above-mentioned chemotherapy in this case. The case provides a potential reference for pMMR CRC patients treating with immune checkpoint inhibitors (ICIs).
Keywords: immunotherapy, sintilimab, advanced colon cancer, pathological complete response, microsatellite stability, case report
Introduction
Colorectal cancer (CRC) is the 3rd most common malignant tumor type and 2nd major cause of cancer-related deaths all over the world.1–3 Regrettably, about 25–30% of patients with CRC showed distant metastases at the time of diagnosis and lost the chance to resect radically.4 For colon cancer, there are many treatment options, such as surgical resection, systemic chemotherapy, targeted and immune therapies. Undoubtedly, surgery is the main treatment for patients with potentially curable CRC. Recently, some clinical studies have found that some unresectable advanced CRC cases had a chance of radical surgery with chemotherapy and immunotherapy. Here, we report a case of an advanced colon cancer patient who hardly to resect radically. She experienced a pathological complete response (pCR) treated with sintilimab and XELOX chemotherapy
Case Presentation
A 73-year-old female whose medical history and family history are normal was referred to our hospital, complaining of epigastric discomfort over 2 months. Physical examination showed a 2*2 cm lymph node could be touched in the left supraclavicular fossa. Carcinoembryonic antigen (CEA) was 5.9ng/mL. The hemoglobin level was 9.9 g/dL. Laboratory data were normal except for slightly elevated CEA and mild anemia. Gastroscopy showed chronic shallow table gastritis companion rotten. Colonoscopy showed a tumor located between ileum and cecum. Histopathological findings of colonoscopy showed moderately and poorly differentiated adenocarcinoma (Figure 1). Chest computed tomography (CT) showed lymph node enlargement in the left supraclavicular fossa. Enhanced abdominal computed tomography (CT) showed thickening of the right colon wall with multiple metastatic lymph nodes in the abdomen(Figure 2). For the above outcomes, the colon cancer was clinically staged as cT4N3M1, stage IV according to 8th edition of the Union for International Cancer Control (UICC) guidelines.
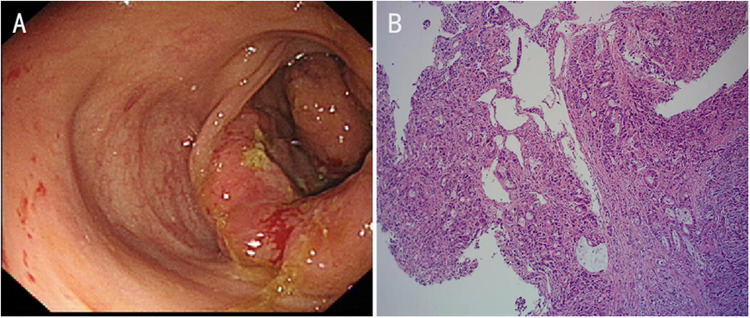
Figure 1.
Colonoscopy showed ileocecal mass (A). Histopathological examination showed moderately and poorly differentiated adenocarcinoma (B).
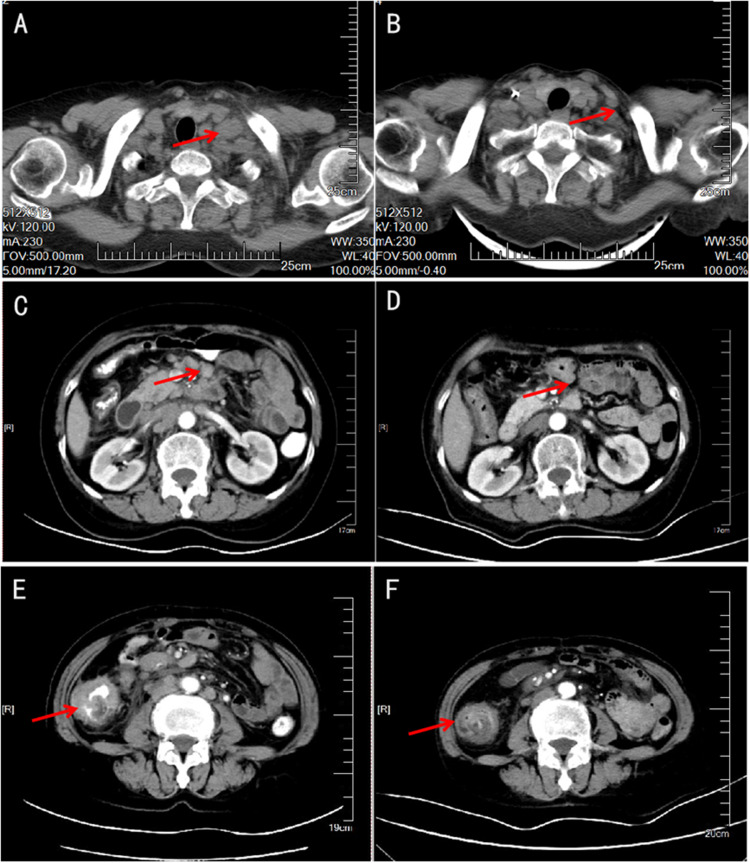
Figure 2.
CT showed lymph node enlargement in the left supraclavicular fossa (A), thickening of the right colon wall with multiple metastatic lymph nodes in the abdomen (C and E). After conversion treatment, the enlarged lymph nodes (B and D) and primary tumor (F) were significantly reduced.
It is difficult to resect radically. After a multidisciplinary team (MDT) discussion, she was treated with sintilimab and XELOX (capecitabine 1250mg/day on day 1–14, oxaliplatin 130mg/m2 and sintilimab 200mg on day 1, q3 weeks).
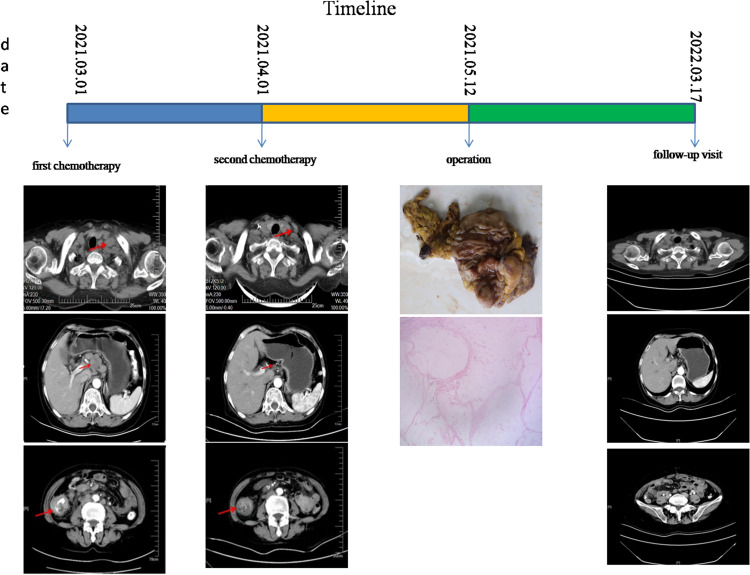
Two period of treatment after initial therapy, enhanced CT showed that the primary tumor (Figure 2) was remarkably reduced. Amazingly, the left supraclavicular fossa lymph node has disappeared completely. According to the outcomes of re-evaluation, it has been resected. Laparoscopic radical resection of right colon cancer was performed successfully on the patient. Specimen examination showed a 4cm×1.5cm bulged mass located ileocecal valve with hard gray texture on section. Histological analysis of the specimen and resected 14 lymph nodes revealed no malignancy (Figure 3). Tumor regression grading (TRG) is 0, which indicated complete regression with no residual tumor cells including lymph nodes. Hence, we can conclude the patient with a pCR (ypT0N0M0). Fortunately, no discomfort occurred during the process of the treatment including myelosuppression, immune pneumonia and other complications. The postoperative course was favorable and was discharged smoothly. The patient underwent capecitabine plus oxaliplatin adjuvant chemotherapy and had no recurrence until now. It showed the patient’s timeline in Figure 4. Written informed consent was obtained from the patient for the publication of any potentially identifiable images or data included in this article.
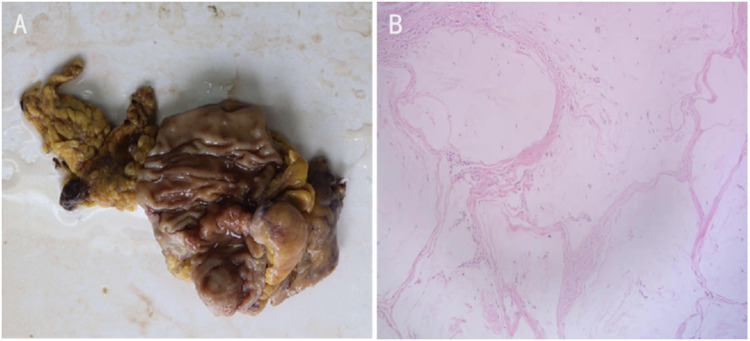
Figure 3.
Specimen examination showed a 4cm×1.5cm bulged mass located ileocecal valve with hard gray texture on section (A). Histological analysis of the specimen and resected 14 lymph nodes revealed no malignancy (B).
Figure 4.
Timeline.
Discussion and Conclusion
About 25–30% of patients with CRC present with metastasis when they are diagnosed.4 Obviously, the opportunity for surgery has been lost. Surgery remains the mainstay of CRC therapy at all sites and the 5-year survival rate for patients at the early stage of CRC undergoing radical surgical resection is above 60%. However, the 5-year survival rate of patients at the advanced stage of CRC undergoing unresectable is less than 10%.5 Therefore, how to gain a chance of surgical resection after therapy is important. Standard treatment options for stage IV CRC are: 1. Surgical resection of primary tumor when bleeding, perforation, or obstruction occurs in the primary tumor; 2. Treatment of isolated metastases occurs in distant organs (liver, lung, ovaries);6–113. Palliative chemotherapy;12–17 4. Biological therapy.18–20 Chinese Society of Clinical Oncology Treatment Guidelines recommend systemic chemotherapy for unresectable colon cancer. Surgery may not be the best option for advanced CRC patients. Many unresectable CRC patients have obtained a chance to resect radically through conversion therapy including chemotherapy, targeted and immune therapies. There were several successful case reports. Suzuki N reported a patient had a complete resection for unresectable CRC with multiple liver metastases via chemotherapy (biweekly CapeOX plus cetuximab) as soon as possible.21 Kazuhiro Watanabe reported 4 (29%) of the 14 locally advanced unresectable colon cancer patients had a complete response for liver metastases, had an R0 resection over six months after chemotherapy with CapeOX.22 Tomonari suetsugu reported an advanced colon cancer female with multiple paraaortic lymph node metastases had a pCR treated with mFOLFOX6 plus cetuximab therapy despite originating from the right colon origin.23
Sintilimab is an anti-programmed death 1 (PD-1) antibody. It could block the contact between PD-1 and its ligands, and relieve the immunosuppressive effect to activate T cell function. It could enhance the immune surveillance and killing ability of T cells to tumors, then generate tumor immune response. Yang Y reported sintilimab, pemetrexed and platinum had a constructive role for locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC).24 In addition to lung cancer, sintilimab has also been reported in the treatment of liver cancer. Chen J reported some hepatocellular carcinoma patients treated with sintilimab had a significant improvement in objective response rate and overall survival rate.25
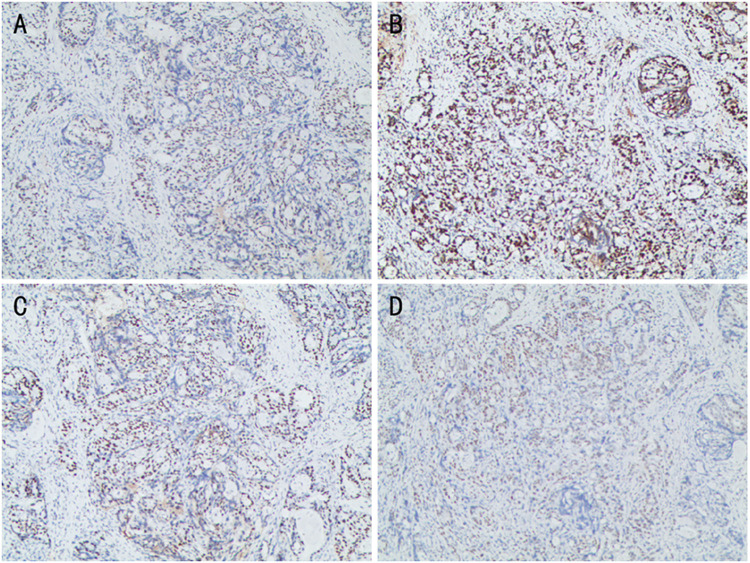
However, sintilimab, was rarely reported in advanced-stage CRC therapies. Gordon SR et al reported programmed cell death protein 1 (PD-1) have shown notable clinical efficacy in patients with colorectal cancer.26 Yaghoubi N reported PD-1/ PD-L1 blockade was a novel treatment for colorectal cancer.27 In this case, Immunohistochemistry showed mismatch-repair-proficient (pMMR) and microsatellite stable (MSS). (Figure 5) The patient achieves a pCR and obtain good clinical outcomes at the most recent follow-up visit. There are some predictive biomarkers for immunotherapy, like mismatch repair-deficient (dMMR), PD-1/Programmed death ligand (PD-L1) expression, tumor mutational burden (TMB), EPHA7-mutant (EPHA7-MUT), tertiary lymphoid structures (TLS) and so on. Wang X reported immunity is positively correlated with TMB after a systematic review of the colon cancer immune microenvironment landscape.28 Maybe, TMB is related to tumor-specific antigen (TSA). TSA can be recognized by anticancer peptides (ACPs) and presented to T cell to activate anti-tumor immunity. Gou Q insisted the binding of PD-L1 to PD-1 inhibits T cell proliferation and activity, leading to tumor immunosuppression and generate tumor immune response.29 Zhang Z reported EPHA7-mutant (EPHA7-MUT) generated an immune response in immune checkpoint inhibitors (ICIs) therapy through the study of 14 EPH genes. As a potential predictive biomarker, EPHA7-MUT maybe predict the ICI-treated patients have a better clinical outcome.30 Vanhersecke L insisted the expression of mature TLS could promote objective response rate and extend overall survival time from the analysis of a large-scale retrospective studies. The mature TLS was a meaningful predictive biomarker in some patients treated with ICIs.31 Some research indicated TP53 co-mutation with KRAS/ATM/EGFR/STK11 had a vital value to predict treatment outcomes to ICIs.32 With the development of the immune mechanism, we could strengthen our understanding of the progress in cancer immunotherapy. More predictive biomarkers for immunotherapy will appear, it will provide a precise and effective treatment plan. Usage of ICIs in CRC is a hot topic. More emphasis on this subject should be given, including how to make an immunologically “cold” tumor to be “hotter”. However, the way how to identify “hotter” tumor is limited. This is just a case report, but it provides an example that some “cold” tumors judged by existent markers, such as MSH are potential “hotter” tumors and can benefit from ICIs. Therefore, more studies should be carried out to find out biology markers, which can identify potential “hotter” CRC.
Figure 5.
Immunohistochemistry showed MLH (+) (A), MSH2 (+) (B), MSH6 (+) (C), PMS2 (+) (D). That is mismatch-repair-proficient (pMMR) and microsatellite stable (MSS).
Funding Statement
There is no funding to report.
Abbreviations
CT, computed tomography; pCR, pathological complete response; TRG, Tumor regression grading.
Ethics
We confirm that institutional approval required for publication of these case details and accompanying images have been obtained from the Medical Ethics Committee of Wuhu Second People’s Hospital in China.
Consent for Publication
Written informed consent was obtained from the patient’s for the publication of this case report and any accompanying images.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
- 1.Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325(7):669–685. doi: 10.1001/jama.2021.0106 [DOI] [PubMed] [Google Scholar]
- 2.Malki A, ElRuz RA, Gupta I, Allouch A, Vranic S, Al Moustafa AE. Molecular mechanisms of colon cancer progression and metastasis: recent insights and advancements. Int J Mol Sci. 2020;22(1):130. doi: 10.3390/ijms22010130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johdi NA, Sukor NF. Colorectal cancer immunotherapy: options and strategies. Front Immunol. 2020;11:1624. doi: 10.3389/fimmu.2020.01624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labianca R, Beretta GD, Kildani B, et al. Colon cancer. Crit Rev Oncol Hematol. 2010;74(2):106–133. doi: 10.1016/j.critrevonc.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 5.McQuade RM, Stojanovska V, Bornstein JC, Nurgali K. Colorectal cancer chemotherapy: the evolution of treatment and new approaches. Curr Med Chem. 2017;24(15):1537–1557. doi: 10.2174/0929867324666170111152436 [DOI] [PubMed] [Google Scholar]
- 6.Adam R. The importance of visceral metastasectomy in colorectal cancer. Ann Oncol. 2000;11(Suppl 3):29–36. doi: 10.1093/annonc/11.suppl_3.29 [DOI] [PubMed] [Google Scholar]
- 7.Adson MA, van Heerden JA, Adson MH, Wagner JS. Resection of hepatic metastases from colorectal cancer. Arch Surg. 1984;119:647–651. doi: 10.1001/archsurg.1984.01390180015003 [DOI] [PubMed] [Google Scholar]
- 8.Coppa GF, Eng K, Ranson JH, Gouge TH, Localio SA. Hepatic resection for metastatic colon and rectal cancer. An evaluation of preoperative and postoperative factors. Ann Surg. 1985;202:203–208. doi: 10.1097/00000658-198508000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. doi: 10.1002/bjs.1800771115 [DOI] [PubMed] [Google Scholar]
- 10.Scheele J, Stangl R, Altendorf-Hofmann A, Gall FP. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery. 1991;110:13–29. [PubMed] [Google Scholar]
- 11.Wagman LD, Kemeny MM, Leong L, et al. A prospective, randomized evaluation of the treatment of colorectal cancer metastatic to the liver. J Clin Oncol. 1990;8:1885–1893. doi: 10.1200/JCO.1990.8.11.1885 [DOI] [PubMed] [Google Scholar]
- 12.Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041–1047. doi: 10.1016/S0140-6736(00)02034-1 [DOI] [PubMed] [Google Scholar]
- 13.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938 [DOI] [PubMed] [Google Scholar]
- 14.Giacchetti S, Perpoint B, Zidani R, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136–147. doi: 10.1200/JCO.2000.18.1.136 [DOI] [PubMed] [Google Scholar]
- 15.Goldberg RM, Morton RF, Sargent DJ, et al. N9741: oxaliplatin (oxal) or CPT-11 + 5-fluorouracil (5FU)/leucovorin (LV) or oxal + CPT-11 in advanced colorectal cancer (CRC). Initial toxicity and response data from a GI Intergroup study. Proc ASCO. 2002;21:128a. [Google Scholar]
- 16.Grothey A, Deschler B, Kroening H, et al. Phase III study of bolus 5-fluorouracil (5-FU)/folinic acid (FA) (Mayo) vs weekly high-dose 24 h 5-FU infusion/FA + oxaliplatin (OXA) (FUFOX) in advanced colorectal cancer (ACRC). Proc ASCO. 2002;21:129a. [Google Scholar]
- 17.Kohne CH, Van Cutsem E, Wils J, et al. Irinotecan improves the activity of the AIO regimen in metastatic colorectal cancer: results of EORTC GI Group study 40986. Proc ASCO. 2003;22:254. [Google Scholar]
- 18.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025 [DOI] [PubMed] [Google Scholar]
- 19.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691 [DOI] [PubMed] [Google Scholar]
- 20.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066 [DOI] [PubMed] [Google Scholar]
- 21.Suzuki N, Suzuki N, Tomochika S, et al. A case of conversion surgery for unresectable colorectal liver metastases, following effective combined chemotherapy with molecular targeting agents. Gan to Kagaku Ryoho. 2017;44(12):1272–1274. [PubMed] [Google Scholar]
- 22.Watanabe K, Kawahara H, Enomoto H, et al. Feasibility study of oxaliplatin with oral S-1 or capecitabine as first-line therapy for patients with metastases from colorectal cancer. Anticancer Res. 2013;33:4029–4032. [PubMed] [Google Scholar]
- 23.Suetsugu T, Matsuhashi N, Takahashi T, et al. Pathological complete response to mFOLFOX6 plus cetuximab therapy for unresectable colon cancer with multiple paraaortic lymph node metastases. Mol Clin Oncol. 2018;9(6):587–591. doi: 10.3892/mco.2018.1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Wang Z, Fang J, et al. Efficacy and safety of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous NSCLC: a randomized, double-blind, phase 3 study (oncology program by InnovENT anti-PD-1-11). J Thorac Oncol. 2020;15(10):1636–1646. doi: 10.1016/j.jtho.2020.07.014 [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Hu X, Li Q, et al. Effectiveness and safety of toripalimab, camrelizumab, and sintilimab in a real-world cohort of hepatitis B virus associated hepatocellular carcinoma patients. Ann Transl Med. 2020;8(18):1187. doi: 10.21037/atm-20-6063 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour -associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545(7655):495–499. doi: 10.1038/nature22396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yaghoubi N, Soltani A, Ghazvini K, Hassanian SM, Hashemy SI. PD-1/ PD-L1 blockade as a novel treatment for colorectal cancer. Biomed Pharmacother. 2019;110:312–318. doi: 10.1016/j.biopha.2018.11.105 [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Duanmu J, Fu X, Li T, Jiang Q. Analyzing and validating the prognostic value and mechanism of colon cancer immune microenvironment. J Transl Med. 2020;18(1):324. doi: 10.1186/s12967-020-02491-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gou Q, Dong C, Xu H, et al. PD-L1 degradation pathway and immunotherapy for cancer. Cell Death Dis. 2020;11(11):955. doi: 10.1038/s41419-020-03140-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Z, Wu HX, Lin WH, et al. EPHA7 mutation as a predictive biomarker for immune checkpoint inhibitors in multiple cancers. BMC Med. 2021;19(1):26. doi: 10.1186/s12916-020-01899-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanhersecke L, Brunet M, Guégan JP, et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat Cancer. 2021;2(8):794–802. doi: 10.1038/s43018-021-00232-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun H, Liu SY, Zhou JY, et al. Specific TP53 subtype as biomarker for immune checkpoint inhibitors in lung adenocarcinoma. EBioMedicine. 2020;60:102990. doi: 10.1016/j.ebiom.2020.102990 [DOI] [PMC free article] [PubMed] [Google Scholar]