Abstract
Background
Delta-9-tetrahydrocannabinol (THC) is the primary phytocannabinoid responsible for the psychoactive properties of cannabis and is known to interact with the endocannabinoid system, which is functionally present in the male reproductive system. Since cannabis consumption is the highest among reproductive aged males, the current study aimed to further investigate the effects of THC exposure to phenotypical, physiological, and molecular parameters in sperm. Bull sperm of known fertility were used as a translational model for human sperm and subjected to in vitro treatment with physiologically relevant experimental doses of THC. Sperm parameters, capacitation, apoptosis, and transcript levels were evaluated following treatment.
Results
Motility, morphology, and viability of bovine sperm was unaltered from THC exposure. However, 0.32µM of THC caused an increased proportion of capacitating sperm (p < 0.05) compared to control and vehicle group sperm. Transcriptome analysis revealed that 39 genes were found to be differentially expressed by 0.032µM THC exposure, 196 genes were differentially expressed by 0.32µM THC exposure, and 33 genes were differentially expressed by 3.2µM THC. Secondary analysis reveals pathways involving development, nucleosomes, ribosomes and translation, and cellular metabolism to be significantly enriched.
Conclusion
Phytocannabinoid exposure to sperm may adversely affect sperm function by stimulating premature capacitation. These findings also show for the first time that spermatozoal transcripts may be altered by THC exposure. These results add to previous research demonstrating the molecular effects of cannabinoids on sperm and warrant further research into the effects of cannabis on male fertility.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12860-023-00468-3.
Keywords: Cannabis, THC, Sperm, Capacitation, Transcriptome, Fertility, Motility, Morphology, Apoptosis
Introduction
An estimated 50 million couples are affected by infertility globally, a population that continues to grow [1]. Male factor infertility is suggested to account for 20% of instances of infertility and plays a role in another 30% of cases [2]. Since natural biological causes of infertility cannot account for all cases, environmental and lifestyle variables, including obesity, exposure to endocrine disrupting compounds, cigarette smoking, drug and alcohol use have gained significant attention in fertility studies.
Cannabis is recognized to be the most widely consumed recreational substance globally, with over 6.4 million Canadians and 48.2 million Americans having reported cannabis use in 2019 alone [3, 4]. It is known that men consume more cannabis compared to women and are also more likely to consume higher potency products [3, 5–7]. The overall high prevalence of cannabis use in recent years coincides with legislative changes permitting therapeutic cannabis, such as Nabilone and Dronabinol, which have been prescribed for anti-emetic purposes [8]. Cannabinoids have also been used to alleviate spasticity in multiple sclerosis patients and have been shown to have anti-anxiolytic, analgesic, and anti-epileptic properties [9, 10]. Medicinal cannabis use in the US alone increased over four-fold between 2016 and 2020 based on medicinal cannabis program enrollment [11]. Continual support of legalization and increasing social acceptability of cannabis use raise concerns about how cannabis may impact fertility and reproductive health, especially among reproductive aged men [7].
Delta-9-tetrahydrocannabinol (THC) is the phytocannabinoid responsible for inducing the psychoactive effects of cannabis, including euphoric feelings and cognitive impairment [12]. While delta-9-tetrahydrocannabinol is the primary psychoactive cannabinoid naturally derived from cannabis sativa, several other isoforms of THC have been identified including delta-8-tetrahydrocannabinol which contributes to approximately 10% of THC content in cannabis [13, 14]. Other major isoforms of THC include delta-10-tetrahydrocannabinol and delta-6a(10a)-tetrahydrocannabinol, which are synthetic isomers [15]. Furthermore, levels of THC found in cannabis continues to trend upwards, due to selective breeding of the cannabis plant to cater to consumer preference for higher potency products [16, 17]. The use of THC in the remaining text of this paper will refer to delta-9-tetrahydrocannabinol.
THC can activate cannabinoid receptors 1 and 2 (CB1/2), belonging to the body’s endocannabinoid system (ECS) [18]. The ECS is a pro-homeostatic system of receptors, enzymes, and ligands coined as endocannabinoids (eCBs) [18, 19]. Anandamide (AEA) and 2-arachidonylglycerol (2-AG) are the two endogenously produced cannabinoids, coined as endocannabinoids (eCBs), belonging to this system [18, 20]. The ECS was first identified in the central nervous system, but has since been confirmed to be present in the male and female reproductive systems [21, 22]. Functional cannabinoid receptors and eCBs present in mammalian sperm play a significant role in modulating sperm motility, viability, and capacitation [21, 23, 24]. In this regard, THC behaves as an exocannabinoid, stimulating CB1 and CB2 as a partial agonist [25].
The working and dynamic relationship between the ECS and sperm function has been successfully studied. In humans, eCB levels in seminal fluid were found to be inversely associated with fertility [21]. These results were corroborated by the work of Amoako et al. [26] who reported reduced seminal AEA levels observed among asthenozoospermic and oligoasthenoteratozoospermic men. Furthermore, stimulation of either cannabinoid receptors has been found to negatively impact sperm motility and viability in human sperm and inhibit capacitation and the acrosome reaction in boar sperm [23, 26–28].
Observational studies conducted among cannabis users also show detrimental effects of cannabis on sperm parameters, sperm epigenetics, and molecular and behavioural endpoints in offspring. Most notably, several studies report lower sperm concentrations among cannabis smokers and some suggesting that THC specifically can negatively impact spermatogenesis [29–32]. Cannabis users are also more likely to exhibit abnormal sperm morphology, a traditional indication of sperm quality and fertility [33–36]. Altered methylation patterns have also been observed in sperm from humans and rodents exposed to cannabis [32, 37]. Several of these methylation changes were also identified in the offspring of male mice exposed to cannabis during the preconception period [38]. Paternal cannabis exposure in rodents has also been associated with long-term neurological and behavioural differences in offspring [39, 40].
Despite the number of existing studies regarding the role of the ECS in sperm and observational studies on cannabis users, there have been limited explorations on the direct effect of THC to sperm. Whan et al. [41] explored the effects of THC on fresh human sperm by using concentrations of THC ranging from 0.032µM to 4.8µM, representing levels of THC found after therapeutic use of Marinol, an FDA approved cannabis product, and recreational cannabis use [42]. Both progressive sperm motility and spontaneous acrosome reaction were found to be inhibited by THC in a dose-dependent manner [41].
There have also been limited investigations on changes to sperm transcripts in response to exogenous agents, despite their abundance and evidence of differential gene expression between fertile and infertile sperm [43–45]. In addition, an essential subset of early embryonic transcripts is known to be exclusively derived from sperm [46].
In this study we aimed to evaluate the effects of delta-9-tetrahydrocannabinol (THC), the dominant naturally occurring isomer, on sperm motility and morphology as traditional indicators of sperm quality, in addition to viability and capacitation, which reflect physiological competence [35, 47–49]. Lastly, we explored the potential effects of THC on sperm transcriptome as sperm transcripts are reflective of fertility [43–45]. We hypothesized that THC would adversely affect motility, morphology, and viability, inhibit capacitation, and alter transcript levels. Owing to the constrains of human sperm use, the bovine model was chosen in the present study for its morphological and physiological similarity to human sperm [50, 51].
Results
Effects of THC on motility and morphology
A total of four biological replicates were assessed for both motility and morphology following THC treatment for 6- and 12- hours, representing individual ejaculates from four bulls. These treatment lengths were determined by initial motility and morphology experiments after treatment for various timepoints (Additional File 1, 2).
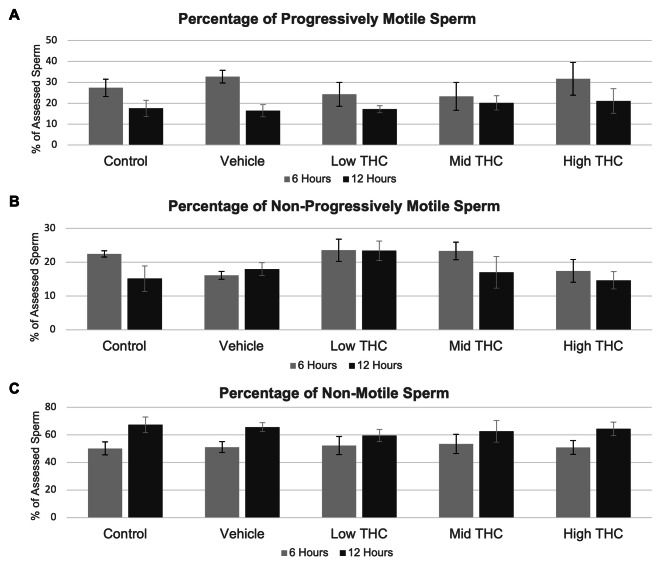
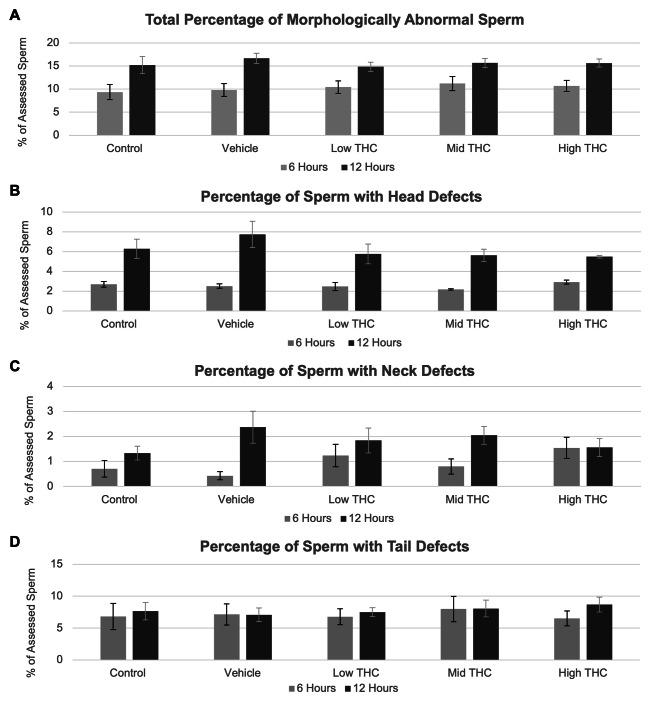
Sperm were categorized based on progressive motility, non-progressive motility, and non-motile. As shown in Fig. 1A-C, THC exposure for 6- or 12-hours at any concentration did not significantly affect motility. THC-treated sperm did not have significantly altered sperm morphology when evaluated as total percentage of cumulative abnormal sperm assessed or as total percentage of assessed sperm with head, neck, or tail defects (Fig. 2). Baseline sperm characteristics of the bulls used in subsequent motility and morphology experiments can be found in Additional File 3.
Fig. 1.
Motility characteristics following THC-treatment. Effect of therapeutic (low THC; 0.032 µM THC) and recreational (mid and high THC; 0.32 µM THC and 3.2 µM THC, respectively) concentrations of THC on (A) percentage progressive motility, (B) percentage non-progressive motility, and (C) percentage non-motile sperm. Motility was determined by manual sperm assessment of a minimum of 100 sperm per replicate after 6 and 12 h of exposure using a Makler counting chamber. Error bars represent average ± SEM, n = 4
Fig. 2.
Morphology characteristics following THC-treatment. Effect of therapeutic (low THC; 0.032 µM THC) and recreational (mid and high THC; 0.32 µM THC and 3.2 µM THC, respectively) concentrations of THC on (A) overall sperm morphology, (B) head defects, (C) neck defects, and (D) tail defects. Morphology was manually determined using a minimum of 200 sperm per group per replicate after 6 and 12 h of treatment. Error bars represent ± SEM, n = 4
Effects of THC on sperm viability
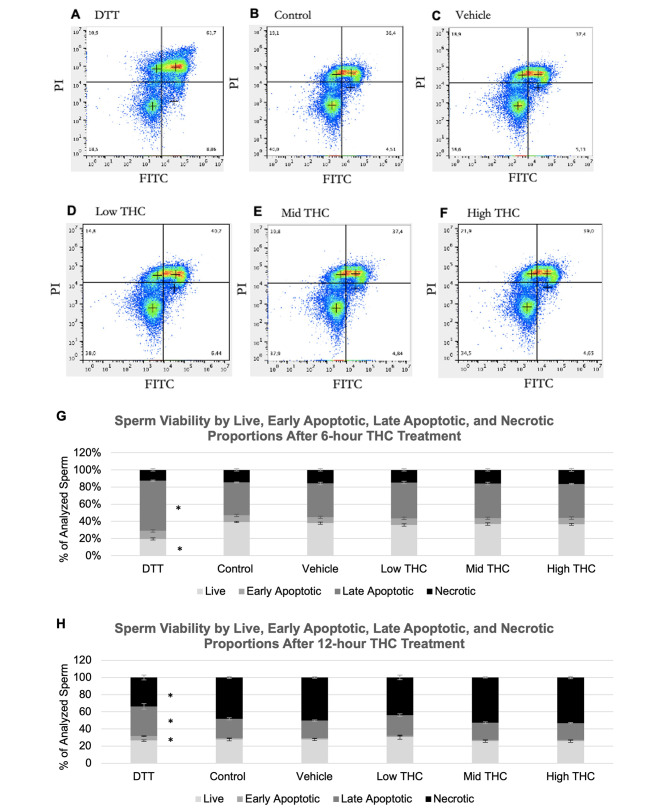
Sperm viability was measured from a total of four biological replicates following THC exposure for 6- and 12-hours, representing sperm from individual ejaculates from four bulls. Flow cytometry density plots indicated different quadrants for sperm subpopulations in each treatment group based on Annexin/PI staining patterns (Fig. 3A-F). In the 6-hour study, DTT-treated group had significantly less live sperm and more late apoptotic sperm compared to the control and vehicle (p < 0.05), whereas THC treatment did not affect viability (Fig. 3G). Similarly, in the 12-hour study, DTT induced early and late apoptosis (p < 0.05) but THC treatment did not alter viability (Fig. 3H).
Fig. 3.
Sperm viability characteristics following THC-treatment. Effects of therapeutic (low THC) and recreational (mid and high THC) concentrations of THC on viability. Representative density plots of sperm in the (A) Dithiothreitriol (DTT) or apoptosis positive control group, (B) control group, (C) vehicle group (0.01% ethanol), (D) low THC group (0.032 µM THC), (E) mid THC group (0.32 µM THC), and (F) high THC group (3.2 µM THC). In each plot, the bottom left quadrant (PI and FITC negative) represent live sperm, the top left quadrant (PI positive, FITC negative) represent necrotic sperm, the top right quadrant (PI positive, FITC positive) represent late apoptotic sperm, and bottom right quadrant (PI negative, FITC positive) represent early apoptotic sperm. Average percentage of live, early apoptotic, late apoptotic, and necrotic proportions of sperm following (G) 6- and (H) 12-hours of treatment. PI, propidium iodide; FITC, Annexin V-FITC. Error bars represent ± SEM, n = 4. Asterisk (*) indicate p < 0.05
Effect of THC on sperm capacitation
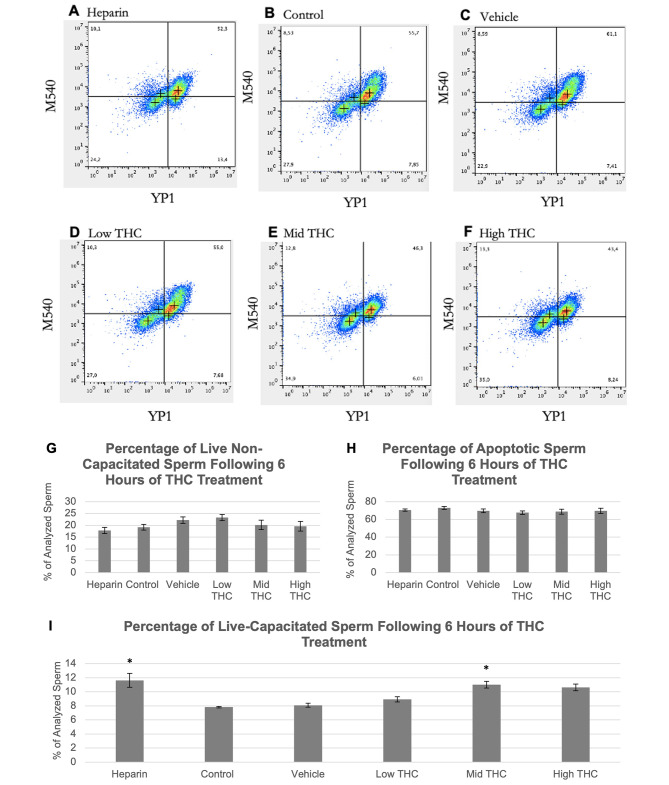
Sperm capacitation was measured from a total of four biological replicates following 6-hours of treatment, representing individual ejaculates from four bulls. Flow cytometry density plots showed the distribution of the sperm populations after incubation with M540/YP1 (Fig. 4A-F). Live/Non-capacitating and apoptotic proportions of sperm were unchanged by THC treatment (Fig. 4G-H), while the Mid-THC treated group exhibited significantly higher proportions of live-capacitating sperm in comparison to the control and vehicle (p < 0.05) (Fig. 4I). Heparin treatment also caused increased capacitation (p < 0.05) (Fig. 4I).
Fig. 4.
Sperm capacitation status following THC-treatment. Effects of therapeutic (low THC) and recreational (mid and high THC) concentrations of THC on capacitation. Representative density plots of sperm in the (A) Heparin or capacitation positive control group, (B) control group, (C) vehicle group (0.01% ethanol), (D) low THC group (0.032 µM THC), (E) mid THC group (0.32 µM THC), and (F) high THC group (3.2 µM THC). In each plot, the bottom left quadrant (Yo-Pro-1 and Merocyanine-540 negative) represent live and non-capacitating sperm, the top left quadrant (Yo-Pro-1 negative and Merocyanine-540 positive) represent live and capacitating sperm, and the two right quadrants (Yo-Pro-1 positive) represent apoptotic sperm. Average percentages of (G) live non-capacitating sperm, (H) apoptotic sperm, and (I) live capacitating sperm. YP1, Yo-Pro-1; M540, Merocyanine-540. Error bars represent ± SEM, n = 4. Asterisk (*) indicate p < 0.05
Effect of THC on transcriptome
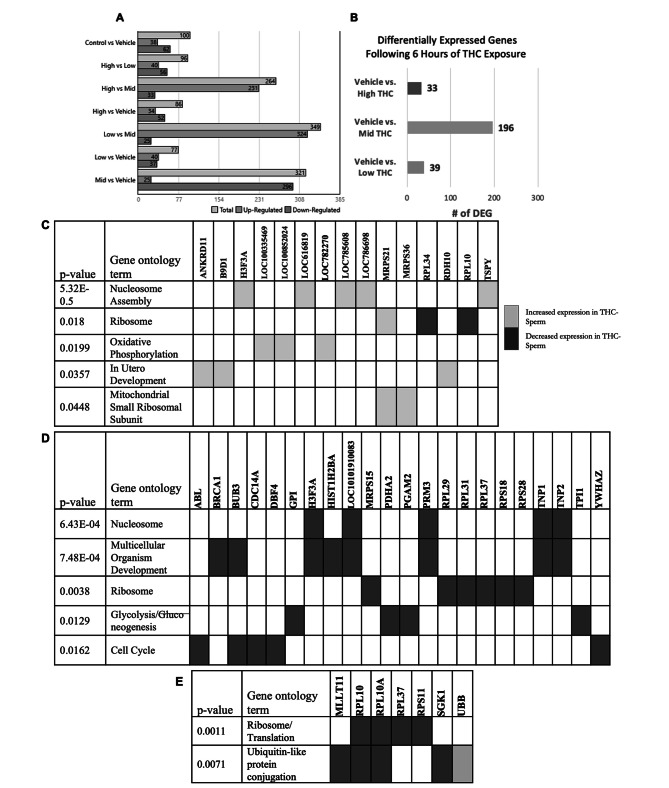
To determine THC effects at the gene expression level, a transcriptome analysis was performed on two biological replicates of sperm. The first biological replicate consisted of RNA from sperm of a single bull, while the second biological replicate consisted of RNA from a pooled sample of sperm originating from three separate bulls (to mitigate individual variation). Differential gene expression analysis revealed 100 DEGs between sperm of the control and vehicle groups, 77 DEGs between the vehicle and low-THC, 321 between the vehicle and Mid-THC, and 86 between the vehicle and high-THC (Fig. 5A). After excluding unknown genes and genes that were differentially expressed between the control and vehicle groups, 39, 196, and 33 DEGs remained between the vehicle and low-THC, mid-THC, and high-THC sperm, respectively (Fig. 5B). There were 7 overlapping DEGs between the Low- and Mid-THC sperm, 14 overlapping between the Mid- and High-THC sperm, and four overlapping between the Low- and High-THC sperm. Three transcripts were detected at lower levels in THC-treated sperm compared to the vehicle-sperm, regardless of THC concentration; ANKRD31 (Ankyrin repeat domain 31), B9D1 (B9 domain 1), and ROPN1L (rhophilin associated tail protein 1-like). Full DEG lists can be found in Supplementary Tables 1–3 in Additional File 4.
Fig. 5.
THC-induced changes to spermatozoal transcripts. Differentially expressed genes (DEGs) and gene ontology (GO) terms in sperm treated with therapeutic (low THC; 0.032 µM) and recreational (mid and high THC; 0.32 µM and 3.2 µM, respectively) concentrations of THC. (A) Total number of DEGs (upregulated and downregulated), (B) Filtered DEG to exclude unknown genes and DEG of the vehicle group, and enriched GO terms in the (C) low-THC DEGs, (D) mid-THC DEGs, and (E) high-THC DEGs. Dark and light boxes indicate decreased and increased expression, respectively, from THC treatment
Secondary analysis of the DEG lists using DAVID 6.8 revealed several enriched gene ontology (GO) groups. Among the DEGs from the low-THC treatment sperm, GO terms relating to nucleosome assembly, ribosomes, oxidative phosphorylation, in utero development, and mitochondrial small ribosomal subunit were enriched (Fig. 5C). Among DEGs from the Mid-THC treated sperm, GO terms relating to the nucleosome, multicellular organism development, ribosomes, glycolysis/gluconeogenesis, and the cell cycle were enriched (Fig. 5D). Finally, GO terms ribosome/translation and ubiquitin-like protein conjugation were enriched based on the DEG list of High-THC treated sperm (Fig. 5E).
Discussion
Sperm function can be influenced by exogenous compounds present in the body through several mechanisms. Previous literature has associated THC use in reproductive aged men with increased spermatid apoptosis, low sperm count, and teratozoospermia [29–34]. The presence of the ECS in the male reproductive tissues and its significant contributions to sperm activity warrant additional investigation into the potential effects of exocannabinoids, including the psychoactive phytocannabinoid THC, on sperm function using an in vitro model.
Regulated levels of eCBs are necessary for the development of sperm as well as mature sperm competence [21, 52, 53]. An existing gradient of eCBs decreasing from seminal plasma to oviductal and follicular fluid is suggested to spatio-temporally regulate sperm motility and capacitation by maintaining a suppressive effect on sperm until they are in closer proximity to the oviduct [54, 55]. Furthermore, stimulation of the cannabinoid receptors has generally been found to be inhibitory towards sperm activity [23, 24, 28].
In the present study, we studied the effects of physiologically relevant concentrations of THC (0.032µM, 0.32µM, and 3.2µM) representative of plasma levels of THC following therapeutic (low dose) and recreational (mid and high dose) cannabis use [41, 42, 56]. It should be noted that due to high lipophilicity, amount of THC in fatty tissues, such as the epididymis, following repeated THC exposure accumulates at levels up to 80 times higher than amounts in the brain and plasma [57]. As a result, the half-life of THC ranges from 6 min to as long as 22 h [58]. To our knowledge, there has only been one research group to quantify THC levels in the semen of cannabis users, who measured THC in semen 8–10 h following cannabis consumption and reported THC concentrations ranging from 0.87 to 0.97 ng/mL (equivalent to 2.7–3.1nM), below the range of concentrations used in our study [59]. However, since these findings are not likely to represent peak seminal fluid THC levels occurring sooner after consumption, we believe that the use of peak plasma concentration to approximate THC exposure was more appropriate.
Here, we assessed motility and morphology as parameters that have been traditionally used as indicators of sperm quality [35, 47]. We found no significant effects of THC on sperm motility or morphology, contrary to our initial hypothesis and previous literature documenting detrimental effects [23, 31, 41, 60]. It is likely that the lack of morphological changes after THC exposure in this study are a result of the use of mature sperm, whereas previous observations of teratozoospermia among cannabis users reflects the impact of THC during spermatogenesis. Interestingly, biphasic responses to THC in sperm have been recognized, where 2.5nM of methanandamide (Met-AEA), a stable analog of AEA, inhibited motility, but 0.25nM of met-AEA stimulated motility, suggesting that exocannabinoid effects on sperm are concentration-dependent [61].
Although the exact mechanisms are not fully understood, spermatozoal apoptosis involves the release of cytochrome C, caspase activation, and phosphatidylserine (PS) externalization, similar to somatic cell apoptosis [62–64]. Interestingly, the unique compartmentalization of sperm limit the availability cytoplasmic enzymes, which reduce the ability of the sperm to combat oxidative stressors [65]. Stimulation of the ECS is generally regarded to be pro-apoptotic as cannabinoid receptor stimulation causes ceramide accumulation leading to cellular stress responses and activation of the mitochondrial apoptotic pathway [66, 67]. Here, we assessed the effects of THC on sperm viability as a reflection of sperm physiology and potential ECS stimulation. We found no changes to viability resulting from THC exposure, in contrast to our hypothesis. Interestingly, Rossato et al. [23] report that 10µM of AEA, but not 0.1 or 1.0µM, eliminated sperm viability in vitro. Previous reports also implicate levels of THC beyond 30µM THC with the inhibition of mitochondrial membrane potential in sperm, a common indicator of apoptosis [68]. As a result, it is possible that apoptosis is only induced at concentrations substantially higher than the ones used in the present study.
The results of our motility, morphology, and apoptosis experiments may be explained by considering our use of cryopreserved bull sperm as an experimental model. Both extensive washing before and after the cryopreservation and rigorous selection process of bull sperm during commercial sperm cryopreservation leaves only the most robust sperm, which are reported to be more resistant to the effects of exocannabinoids [41]. On the other hand, cryopreservation necessitates removal of round somatic cells and seminal plasma, components which would otherwise be present during THC-sperm interactions and provide possible protective effects [68]. Lastly, the cryopreservation process is known to induce oxidative damage and apoptosis in sperm, which may have limited our potential to observe the effects of THC to viability [69, 70].
To further investigate THC-induced changes in sperm physiology, we examined capacitation, a necessary series of cellular and biochemical changes which allow sperm to propel through the female reproductive tract and prepare to fertilize the ovum [48, 71]. We observed that 6 h of 0.32µM of THC exposure, our mid-THC concentration, significantly increased the proportion of capacitating sperm compared to the sperm in the vehicle group, based on positive M540 and negative YP1 staining. Interstingly, sperm in the same group did not exhibit altered motility, which is typically observed in capacitating sperm through hypermotility. Previous research indicates conflicting evidence regarding the relationship between cannabinoids and capacitation. AEA and THC have been shown to inhibit the acrosome reaction, which is widely accepted as the culminating event of capacitation and is necessary for sperm fertilization competence [23, 41, 61]. On the other hand, 2-AG has been observed to increase the acrosome reaction in human sperm and met-AEA has been found to induce bull sperm capacitation [24, 72]. When intracellular calcium was measured as an indicator of capacitation, Gervasi and collegues [72] report only the two median concentrations of met-AEA elicited a significant change, similarly to the results we observed in the present study.
Our results indicate that THC can impact sperm physiology by promoting membrane disorder characteristic of capacitation. Although capacitation is a crucial aspect of sperm function, premature capacitation may unfavourably affect fertility by stimulating early hypermotility leading to exhaustion of sperm ATP, inability to reach the ovum, and premature death [54]. Moreover, premature acrosome reaction also impairs subsequent fertilization [48].
To our knowledge, this is the first study to explore and report effects of THC to transcripts in mature sperm. Cannabinoid receptor signalling in somatic cells can lead to downstream changes in gene expression [73–75]. However, a lack of transcriptional machinery limits transcriptional activity in sperm [76, 77]. Nonetheless, transcripts in mature sperm are known to be altered from cryopreservation and exposure to endocrine disrupting compounds, suggesting a mechanism for altered transcript levels [78–80].
We report over 200 DEGs in sperm exposed to THC, many relating to development, cellular metabolism, ribosomes and translation, and the nucleosome. It is noteworthy that the secondary gene ontology study performed found enrichment of DEGs relating to in utero development in the low-THC group, where all developmentally related genes had decreased expression, and multicellular organism development mid-THC group, where these genes had increased expression. We also report that all THC-treated sperm, regardless of concentration, shared enrichment in genes relating to ribosomes and translation, largely as a result from the differential expression of mitochondrial ribosomal proteins. Although sperm are generally thought to be translationally silent due to a lack of cytoplasmic ribosomes, Gur & Breitbart [81] described the presence of protein synthesized by mitochondrial ribosomes in mature sperm. Further research suggests that specific transcripts in mature sperm are actively translated by mitochondrial ribosomes during capacitation and the inhibition of mitochondrial translation is detrimental to capacitation, motility, and fertilization [81, 82]. These findings might provide insight on the considerable number of genes found to be decreased in sperm of the mid-THC group, which we also reported to have a significantly increased proportion of capacitating sperm. Since it is unlikely that changes in transcript abundance are due to transcriptional activity for reasons described previously, we speculate that the decrease in transcripts in this treatment group specifically was a result of transcript turnover stemming from increased mitochondrial ribosome activity during capacitation. Moreover, it is possible that the wide variety of transcriptomic changes observed in the presented study is a result of either translation dependent transcript turnover, accumulation, or altered stability. We can even speculate that, given the limited research on the mechanisms of transcript turnover in sperm, transcriptomic changes may be due to the inhibition of transcript degradation or decreased stability, both through mechanisms that have not yet been investigated in sperm. This is a limitation of this study, that warrants further experiments to confirm specific transcripts changes and to clarify the reason of these transcriptional changes.
In conclusion, our findings that THC induces capacitation provide evidence to support the growing body of facts suggesting that cannabinoids impact sperm physiology. To our knowledge, this is the first study to investigate the impacts of cannabinoids on transcripts in mature sperm. We report that THC can induce changes to sperm transcript levels, although additional validation studies should be performed prior to making functional conclusions. This study also utilized physiologically relevant concentrations of THC to mimic natural physiological responses as closely as possible in an in vitro model. Altogether, these findings justify further research on the cellular and molecular impacts of cannabinoids on sperm. In combination with previous work highlighting the detrimental effects of THC on sperm development and function, these results contribute to the growing body of research seeking to address the implications of cannabis use among reproductive aged men, the highest consumers of cannabis.
Materials & methods
Reagents
All media and chemicals were purchased from Sigma Aldrich (Oakville, ON, Canada) unless otherwise specified. THC stock was purchased as Dronabinol (Δ9-THC) from Toronto Research Chemicals (Toronto, ON, Canada).
Semen preparation & treatment
Cryopreserved bull semen from bulls with known fertility were acquired from Semex (Guelph, ON, Canada). For each bull, 200uL of cryopreserved semen containing approximately 50 million sperm was thawed and washed using a discontinuous Percoll gradient described by Nguyen et al. [80]. The washed sperm was then split into one of five treatment groups containing 1mL of media each; HEPES/Sperm TALP supplemented with 0.3% bovine serum albumin (Control), 0.01% of ethanol diluted in the control group media (Vehicle), 3.2µM of THC diluted in the control group media (High-THC), 0.32 µM of THC made by 10-times serial dilution of High-THC media (Mid-THC), and 0.032µM of THC made by 10-times serial dilution of Mid-THC media (Low-THC). THC concentrations used in this study were meant to reflect plasma concentrations of THC following therapeutic and recreational cannabis use as described by Misner et al. [56]. Sperm were incubated at 38.5 °C with 5% CO2 until assessment.
Effects of THC on motility and morphology
Sperm were retrieved after incubation for 6 and 12 h in the aforementioned conditions and centrifuged for 7 min at 2,500 RPM. Supernatant was removed and sperm pellet was resuspended in 50uL of warmed HEPES/Sperm TALP. For morphological assessment, 10uL of the resulting sperm suspension was spread onto a warmed glass slide using the pipette method [83] and air dried before being fixed overnight in a 3:1 methanol:acetic acid solution. Slides were then washed with water, stained with a Giemsa staining solution for 15 min, washed again, and evaluated under a Leica DMIRB microscope (Leica Microsystems; Richmond Hill, ON, Canada). Morphology of a minimum of 200 sperm per treatment group per biological replicate was manually assessed as previously described by Nguyen et al. [80].
For motility assessments, 10uL of sperm from resuspended pellet was placed onto a prewarmed Makler counting chamber and 90 s video recordings (25 frames per second) containing a minimum of 6 fields generated using the EPView camera and software (Olympus Life Sciences; Richmond Hill, ON, Canada). Recordings were used to manually evaluate the motility of a minimum of 100 sperm per treatment group per biological replicate as described by Nguyen et al. [80]. Both motility and morphology assessments were conducted by a single user and under blinded conditions.
Effects of THC on sperm apoptosis and capacitation
Apoptosis was evaluated using the Annexin V-FITC Apoptosis Staining/Detection Kit consisting of binding buffer, propidium iodide (PI), and annexin V-FITC (FITC) (Abcam, Cambridge, MA, USA). Sperm were treated for 6 and 12 h and washed as previously described before being resuspended in 180uL of warmed binding buffer. A sixth treatment group containing 0.7µM of dithiotretriol (DTT) added 45 min before the end of the treatment period was used as a positive control for apoptosis. Three additional groups consisting of no stain, only FITC, and only PI controls required for flow cytometry compensation were included. In a dark room, 5uL of PI and FITC were added to each sample and incubated for 15 min before being assessed using the BD C6 Accuri Flow Cytometer (BD Biosciences, Mississauga, ON, Canada). FITC labels phosphatidylserine residues which accumulate on the outer leaflet of the lipid bilayer under apoptotic conditions while PI, a DNA dye, enters the cell upon membrane compromise during necrosis [84, 85]. FITC negative/PI negative staining represents the live sperm population, FITC positive/PI negative staining indicates early apoptosis, FITC positive/PI positive indicates late apoptosis, and FITC negative/PI positive indicates necrosis [86].
To assess capacitation, sperm were incubated for 6 h before being washed and resuspended in 180uL of warmed HEPES/Sperm TALP. Merocyanine-540 (M540) and Yo-Pro-1 (YP1) diluted in DMSO were added to sperm suspensions to achieve final staining concentrations of 50nM and 1.5µM, respectively. An additional group containing 10uL of 2U/mL heparin, a glycoaminoglycan known to induce capacitation in bovine sperm, was used as a positive control group [87, 88]. Three additional groups consisting of no stain, only M540, and only YP1 controls required for flow cytometry compensation were included. Samples were incubated in the dark before being assessed by flow cytometry. M540 intercalates into cell membranes with increasing membrane disorder while YP1, a DNA binding dye, enters early apoptotic cells with permeable membranes [89, 90]. Events that are YP1 positive indicate an apoptotic sperm, M540 positive/YP1 negative events represent live and capacitating sperm, and M540 negative/YP1 negative events represent the live and non-capacitating sperm [91].
Flow cytometry from both apoptosis and capacitation experiments were analysed using FlowJo v10 (BD Biosciences) using a minimum of 25,000 events per sample after gating to remove debris. Quadrants were determined based on no stain controls.
Transcriptome analysis
Washed sperm from 400uL of cryopreserved semen were placed in 4mL of each of the treatment groups described previously for 6 h. Treated sperm pellets from two biological replicates were snap frozen and stored at -80 °C until total RNA extraction, using the Qiagen miRNeasy Micro RNA extraction kit according to manufacturer’s instructions (Qiagen, Toronto, ON, Canada). RNA samples from two biological replicates of bull sperm underwent a transcriptome analysis using a GeneChip Bovine Gene 1.0 ST Microarray (Thermo Fisher Scientific; Whitby, ON, Canada) at the David Braley Research Institute in Hamilton, Ontario, Canada. One biological replicate is representative of a single bull, while the second biological replicate consists of sperm pooled from three different bulls to mitigate individual variability.
Results of the transcriptome analysis were evaluated using Transcriptome Analysis Console (Thermo Fisher Scientific). Differentially expressed genes (DEG) were considered to be statistically significance if the fold change was greater than ± 2 and had a p-value of less than 0.05. The Database for Annotation, Visualization and Integrated Discovery (DAVID 6.8) was used to perform a secondary gene ontology analysis.
Statistics
Statistical analysis was performed on GraphPad Prism 6 using data from at least 3 biological replicates. All data sets were subjected to the Kolmogorov-Smirnov test of normality. Normally distributed data sets were analyzed using one-way analysis of variance (ANOVA) and non-normally distributed data sets were analyzed using the Kruskal–Wallis test. Tukey’s post-hoc test was used to determine statistical significance between the treatment groups.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Monica Antenos, Elizabeth St. John, Allison MacKay, and all members of the Reproductive Health and Biotechnology Lab in the Department of Biomedical Sciences at the University of Guelph for their technical assistance and resourceful scientific discussion.
Authors’ Contribution
VBT wrote the main manuscript and performed all data analysis. VBT and OSD carried out motility and morphology assessments. JG performed capacitation experiments. VBT, LAF, MSN, and JYK conceptualized experiments. Final manuscript was read and approved by all authors.
Funding
This project is supported by the General Purpose Account (# 072049) of LAF through the Department of Biomedical Sciences, University of Guelph and the Canadian Graduate Scholarship (Master’s, NSERC) of VBT.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, Regional, and global Trends in Infertility Prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9:e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfeifer S, Butts S, Dumesic D, et al. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril. 2015;103:e18–e25. doi: 10.1016/j.fertnstert.2014.12.103. [DOI] [PubMed] [Google Scholar]
- 3.Canadian Alcohol and Drugs Survey (CADS). : summary of results for 2019 - Canada.ca. https://www.canada.ca/en/health-canada/services/canadian-alcohol-drugs-survey/2019-summary.html#a4. Accessed 19 May 2022
- 4.Substance Abuse and Mental Health Services Administration. (2020) Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health. Rockville, MD
- 5.Greaves L, Hemsing N. Sex and gender interactions on the Use and Impact of recreational Cannabis. Int J Environ Res Public Health 2020. 2020;17:509. doi: 10.3390/ijerph17020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemsing N, Greaves L. Gender norms, roles and relations and Cannabis-use patterns: a scoping review. Int J Environ Res Public Health 2020. 2020;17:947. doi: 10.3390/ijerph17030947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canadian Cannabis Survey 2021: Summary - Canada.ca. https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/research-data/canadian-cannabis-survey-2021-summary.html. Accessed 19 May 2022
- 8.Amin MR, Ali DW. Pharmacology of Medical Cannabis. Adv Exp Med Biol. 2019;1162:151–65. doi: 10.1007/978-3-030-21737-2_8. [DOI] [PubMed] [Google Scholar]
- 9.Wade DT, Collin C, Stott C, Duncombe P. Meta-analysis of the efficacy and safety of Sativex (nabiximols), on spasticity in people with multiple sclerosis. Mult Scler. 2010;16:707–14. doi: 10.1177/1352458510367462. [DOI] [PubMed] [Google Scholar]
- 10.Maroon J, Bost J. Review of the neurological benefits of phytocannabinoids. Surg Neurol Int. 2018 doi: 10.4103/SNI.SNI_45_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boehnke KF, Dean O, Haffajee RL, Hosanagar A. 2022. U.S. Trends in Registration for Medical Cannabis and Reasons for Use From 2016 to 2020. [DOI] [PMC free article] [PubMed]
- 12.Maccarrone M, Rapino C, Francavilla F, Barbonetti A. (2020) Cannabinoid signalling and effects of cannabis on the male reproductive system. Nature Reviews Urology 2020 18:1 18:19–32 [DOI] [PubMed]
- 13.Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of Hashish. J Am Chem Soc. 1964;86:1646–7. doi: 10.1021/ja01062a046. [DOI] [Google Scholar]
- 14.Hively RL, Mosher WA, Hoffmann FW. Isolation of trans-∆6-Tetrahydrocannabinol from Marijuana. J Am Chem Soc. 1966;88:1832–3. doi: 10.1021/ja00960a056. [DOI] [PubMed] [Google Scholar]
- 15.la Maida N, di Giorgi A, Pichini S, Busardò FP, Huestis MA. Recent challenges and trends in forensic analysis: ∆9-THC isomers pharmacology, toxicology and analysis. J Pharm Biomed Anal. 2022;220:114987. doi: 10.1016/j.jpba.2022.114987. [DOI] [PubMed] [Google Scholar]
- 16.ElSohly MA, Chandra S, Radwan M, Majumdar CG, Church JC. A Comprehensive Review of Cannabis Potency in the United States in the last decade. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:603–6. doi: 10.1016/j.bpsc.2020.12.016. [DOI] [PubMed] [Google Scholar]
- 17.ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in Cannabis Potency over the last 2 decades (1995–2014): analysis of Current Data in the United States. Biol Psychiatry. 2016;79:613–9. doi: 10.1016/j.biopsych.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pertwee RG, Howlett AC, Abood ME, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meccariello R, Battista N, Bradshaw HB, Wang H. (2014) Updates in Reproduction Coming from the Endocannabinoid System. Int J Endocrinol 2014:412354 [DOI] [PMC free article] [PubMed]
- 20.Devane WA, Hanuš L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–9. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 21.Lewis SEM, Rapino C, di Tommaso M, Pucci M, Battista N, Paro R, Simon L, Lutton D, Maccarrone M. Differences in the endocannabinoid system of sperm from fertile and infertile men. PLoS ONE. 2012 doi: 10.1371/JOURNAL.PONE.0047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pertwee RG. Endocannabinoids and their pharmacological actions. Handb Exp Pharmacol. 2015;231:1–37. doi: 10.1007/978-3-319-20825-1_1. [DOI] [PubMed] [Google Scholar]
- 23.Rossato M, Popa FI, Ferigo M, Clari G, Foresta C. Human sperm express cannabinoid receptor Cb1, the activation of which inhibits motility, acrosome reaction, and mitochondrial function. J Clin Endocrinol Metab. 2005;90:984–91. doi: 10.1210/jc.2004-1287. [DOI] [PubMed] [Google Scholar]
- 24.Francou MM, Girela JL, de Juan A, Ten J, Bernabeu R, de Juan J. Human sperm motility, capacitation and acrosome reaction are impaired by 2-arachidonoylglycerol endocannabinoid. Histol Histopathol. 2017;32:1351–8. doi: 10.14670/HH-11-911. [DOI] [PubMed] [Google Scholar]
- 25.Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol. 2005;168:1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- 26.Amoako AA, Marczylo TH, Marczylo EL, Elson J, Willets JM, Taylor AH, Konje JC. Anandamide modulates human sperm motility: implications for men with asthenozoospermia and oligoasthenoteratozoospermia. Hum Reprod. 2013;28:2058–66. doi: 10.1093/humrep/det232. [DOI] [PubMed] [Google Scholar]
- 27.Maccarrone M, Wenger T. Effects of cannabinoids on hypothalamic and reproductive function. Handb Exp Pharmacol. 2005;168:555–71. doi: 10.1007/3-540-26573-2_18. [DOI] [PubMed] [Google Scholar]
- 28.Agirregoitia E, Carracedo A, Subirán N, Valdivia A, Agirregoitia N, Peralta L, Velasco G, Irazusta J. The CB2 cannabinoid receptor regulates human sperm cell motility. Fertil Steril. 2010;93:1378–87. doi: 10.1016/j.fertnstert.2009.01.153. [DOI] [PubMed] [Google Scholar]
- 29.Kolodny RC, Masters WH, Kolodner RM, Toro G. Depression of plasma testosterone levels after chronic intensive marihuana use. N Engl J Med. 1974;290:872–4. doi: 10.1056/NEJM197404182901602. [DOI] [PubMed] [Google Scholar]
- 30.Banerjee A, Singh A, Srivastava P, Turner H, Krishna A. Effects of chronic bhang (cannabis) administration on the reproductive system of male mice. Birth Defects Res B Dev Reprod Toxicol. 2011;92:195–205. doi: 10.1002/bdrb.20295. [DOI] [PubMed] [Google Scholar]
- 31.Gundersen TD, Jørgensen N, Andersson AM, Bang AK, Nordkap L, Skakkebæk NE, Priskorn L, Juul A, Jensen TK. Association between Use of Marijuana and Male Reproductive Hormones and Semen Quality: a study among 1,215 healthy Young Men. Am J Epidemiol. 2015;182:473–81. doi: 10.1093/aje/kwv135. [DOI] [PubMed] [Google Scholar]
- 32.Murphy SK, Itchon-Ramos N, Visco Z, et al. Cannabinoid exposure and altered DNA methylation in rat and human sperm. Epigenetics. 2018;13:1208–21. doi: 10.1080/15592294.2018.1554521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pacey AA, Povey AC, Clyma JA, McNamee R, Moore HD, Baillie H, Cherry NM. Modifiable and non-modifiable risk factors for poor sperm morphology. Hum Reprod. 2014;29:1629–36. doi: 10.1093/humrep/deu116. [DOI] [PubMed] [Google Scholar]
- 34.Carroll K, Pottinger AM, Wynter S, DaCosta V. Marijuana use and its influence on sperm morphology and motility: identified risk for fertility among jamaican men. Andrology. 2020;8:136–42. doi: 10.1111/andr.12670. [DOI] [PubMed] [Google Scholar]
- 35.Menkveld R, Holleboom CAG, Rhemrev JPT. Measurement and significance of sperm morphology. Asian J Androl. 2011;13:59. doi: 10.1038/aja.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.García-Vázquez F, Gadea J, Matás C, Holt W. Importance of sperm morphology during sperm transport and fertilization in mammals. Asian J Androl. 2016;18:844–50. doi: 10.4103/1008-682X.186880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schrott R, Acharya K, Itchon-Ramos N, Hawkey AB, Pippen E, Mitchell JT, Kollins SH, Levin ED, Murphy SK. Cannabis use is associated with potentially heritable widespread changes in autism candidate gene DLGAP2 DNA methylation in sperm. Epigenetics. 2020;15:161–73. doi: 10.1080/15592294.2019.1656158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schrott R, Modliszewski JL, Hawkey AB, Grenier C, Holloway Z, Evans J, Pippen E, Corcoran DL, Levin ED, Murphy SK. Sperm DNA methylation alterations from cannabis extract exposure are evident in offspring. Epigenetics Chromatin. 2022;15:1–15. doi: 10.1186/s13072-022-00466-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slotkin TA, Skavicus S, Levin ED, Seidler FJ. Paternal ∆9-Tetrahydrocannabinol exposure prior to mating elicits deficits in Cholinergic synaptic function in the offspring. Toxicol Sci. 2020;174:210–7. doi: 10.1093/toxsci/kfaa004. [DOI] [PubMed] [Google Scholar]
- 40.Holloway ZR, Hawkey AB, Pippin E, White H, Wells C, Kenou B, Rezvani AH, Murphy SK, Levin ED. Paternal factors in neurodevelopmental toxicology: THC exposure of male rats causes long-lasting neurobehavioral effects in their offspring. Neurotoxicology. 2020;78:57–63. doi: 10.1016/j.neuro.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Whan LB, West MCL, Mcclure N, Lewis SEM. Effects of delta-9-tetrahydrocannabinol, the primary psychoactive cannabinoid in marijuana, on human sperm function in vitro. Fertil Steril. 2006;85:653–60. doi: 10.1016/j.fertnstert.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 42.FDA. (2004) MARINOL ® (Dronabinol).
- 43.Fagerlind M, Stålhammar H, Olsson B, Klinga-Levan K. Expression of miRNAs in Bull Spermatozoa correlates with Fertility Rates. Reprod Domest Anim. 2015;50:587–94. doi: 10.1111/rda.12531. [DOI] [PubMed] [Google Scholar]
- 44.Lambard S, Galeraud-Denis I, Martin G, Levy R, Chocat A, Carreau S. Analysis and significance of mRNA in human ejaculated sperm from normozoospermic donors: relationship to sperm motility and capacitation. Mol Hum Reprod. 2004;10:535–41. doi: 10.1093/molehr/gah064. [DOI] [PubMed] [Google Scholar]
- 45.Li C, Zhou X. Gene transcripts in spermatozoa: markers of male infertility. Clin Chim Acta. 2012;413:1035–8. doi: 10.1016/j.cca.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Fang P, Zeng P, Wang Z, et al. Estimated diversity of messenger RNAs in each murine spermatozoa and their potential function during early zygotic development. Biol Reprod. 2014;90:94–5. doi: 10.1095/biolreprod.114.117788. [DOI] [PubMed] [Google Scholar]
- 47.Dcunha R, Hussein RS, Ananda H, Kumari S, Adiga SK, Kannan N, Zhao Y, Kalthur G. (2020) Current Insights and Latest Updates in Sperm Motility and Associated Applications in Assisted Reproduction. Reproductive Sciences 2020 29:1 29:7–25 [DOI] [PMC free article] [PubMed]
- 48.Yanagimachi R. Mammalian fertilization. The Physiology of Reproduction; 1994.
- 49.Foroozan-Broojeni S, Tavalaee M, Lockshin RA, Zakeri Z, Abbasi H, Nasr-Esfahani MH. (2019) Comparison of main molecular markers involved in autophagy and apoptosis pathways between spermatozoa of infertile men with varicocele and fertile individuals. Andrologia. 10.1111/AND.13177 [DOI] [PubMed]
- 50.Miller MR, Mansell SA, Meyers SA, Lishko P. Flagellar ion channels of sperm: similarities and differences between species. Cell Calcium. 2015;58:105–13. doi: 10.1016/j.ceca.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 51.Gracia Gervasi M, Rapanelli M, Laura Ribeiro M, Farina M, Billi S, María Franchi A, Perez Martinez S. The endocannabinoid system in bull sperm and bovine oviductal epithelium: role of anandamide in sperm-oviduct interaction. Reproduction. 2009;137:403–14. doi: 10.1530/REP-08-0204. [DOI] [PubMed] [Google Scholar]
- 52.Grimaldi P, Orlando P, di Siena S, Lolicato F, Petrosino S, Bisogno T, Geremia R, de Petrocellis L, di Marzo V. The endocannabinoid system and pivotal role of the CB2 receptor in mouse spermatogenesis. Proc Natl Acad Sci U S A. 2009;106:11131–6. doi: 10.1073/pnas.0812789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aquila S, Guido C, Santoro A, Perrotta I, Laezza C, Bifulco M, Sebastiano A. Human sperm anatomy: ultrastructural localization of the Cannabinoid1 receptor and a potential role of Anandamide in sperm survival and acrosome reaction. Anat Record: Adv Integr Anat Evolutionary Biology. 2010;293:298–309. doi: 10.1002/ar.21042. [DOI] [PubMed] [Google Scholar]
- 54.Bernabò N, Palestini P, Chiarini M, Maccarrone M, Mattioli M, Barboni B. Endocannabinoid-binding CB1 and TRPV1 receptors as modulators of sperm capacitation. Commun Integr Biol. 2012;5:68–70. doi: 10.4161/cib.18118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schuel H, Burkman LJ, Lippes J, Crickard K, Forester E, Piomelli D, Giuffrida A. N-Acylethanolamines in human reproductive fluids. Chem Phys Lipids. 2002;121:211–27. doi: 10.1016/S0009-3084(02)00158-5. [DOI] [PubMed] [Google Scholar]
- 56.Misner MJ, Taborek A, Dufour J, Sharifi L, Khokhar JY, Favetta LA. (2021) Effects of Delta-9 Tetrahydrocannabinol (THC) on Oocyte Competence and Early Embryonic Development. Frontiers in toxicology. 10.3389/FTOX.2021.647918 [DOI] [PMC free article] [PubMed]
- 57.Nahas GG, Frick HC, Lattimer JK, Latour C, Harvey D. Pharmacokinetics of THC in brain and testis, male gametotoxicity and premature apoptosis of spermatozoa. Hum Psychopharmacol. 2002;17:103–13. doi: 10.1002/hup.369. [DOI] [PubMed] [Google Scholar]
- 58.Heuberger JAAC, Guan Z, Oyetayo OO, Klumpers L, Morrison PD, Beumer TL, van Gerven JMA, Cohen AF, Freijer J. Population Pharmacokinetic Model of THC integrates oral, intravenous, and Pulmonary Dosing and characterizes short- and long-term pharmacokinetics. Clin Pharmacokinet. 2015;54:209–19. doi: 10.1007/s40262-014-0195-5. [DOI] [PubMed] [Google Scholar]
- 59.Lee MS, Lanes A, Ginsburg ES, Fox JH. Delta-9 THC can be detected and quantified in the semen of men who are chronic users of inhaled cannabis. J Assist Reprod Genet. 2020;37:1497–504. doi: 10.1007/s10815-020-01762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nassan FL, Arvizu M, Mínguez-Alarcón L, Williams PL, Attaman J, Petrozza J, Hauser R, Chavarro J, Ford JB, Keller MG. Marijuana smoking and markers of testicular function among men from a fertility centre. Hum Reprod. 2019;34:715–23. doi: 10.1093/humrep/dez002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schuel H, Burkman LJ, Lippes J, Crickard K, Mahony MC, Giuffrida A, Picone RP, Makriyannis A. Evidence that anandamide-signaling regulates human sperm functions required for fertilization. Mol Reprod Dev. 2002;63:376–87. doi: 10.1002/mrd.90021. [DOI] [PubMed] [Google Scholar]
- 62.Paasch U, Grunewald S, Agarwal A, Glandera HJ. Activation pattern of caspases in human spermatozoa. Fertil Steril. 2004;81:802–9. doi: 10.1016/j.fertnstert.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 63.Aitken RJ, Koppers AJ. Apoptosis and DNA damage in human spermatozoa. Asian J Androl. 2011;13:36. doi: 10.1038/aja.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koppers AJ, Mitchell LA, Wang P, Lin M, Aitken RJ. Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem J. 2011;436:687–98. doi: 10.1042/BJ20110114. [DOI] [PubMed] [Google Scholar]
- 65.Castellini C, D’andrea S, Cordeschi G, Totaro M, Parisi A, di Emidio G, Tatone C, Francavilla S, Barbonetti A. (2021) Pathophysiology of Mitochondrial Dysfunction in Human Spermatozoa: Focus on Energetic Metabolism, Oxidative Stress and Apoptosis. Antioxidants 2021, Vol 10, Page 695 10:695 [DOI] [PMC free article] [PubMed]
- 66.Raisova M, Bektas M, Wieder T, Daniel P, Eberle J, Orfanos CE, Geilen CC. Resistance to CD95/Fas-induced and ceramide-mediated apoptosis of human melanoma cells is caused by a defective mitochondrial cytochrome c release. FEBS Lett. 2000;473:27–32. doi: 10.1016/S0014-5793(00)01491-5. [DOI] [PubMed] [Google Scholar]
- 67.von Haefen C, Wieder T, Gillissen B, Stärck L, Graupner V, Dörken B, Daniel PT. (2002) Ceramide induces mitochondrial activation and apoptosis via a Bax-dependent pathway in human carcinoma cells. Oncogene 2002 21:25 21:4009–4019 [DOI] [PubMed]
- 68.Badawy ZS, Chohan KR, Whyte DA, Penefsky HS, Brown OM, Souid AK. Cannabinoids inhibit the respiration of human sperm. Fertil Steril. 2009;91:2471–6. doi: 10.1016/j.fertnstert.2008.03.075. [DOI] [PubMed] [Google Scholar]
- 69.Ahmad L, Jalali S, Shami SA, Akram Z, Batool S, Kalsoom O. (2010) Effects of Cryopreservation on Sperm DNA Integrity in Normospermic and Four Categories of Infertile Males. http://dx.doi.org/103109/19396360903428352 56:74–83 [DOI] [PubMed]
- 70.Tatone C, di Emidio G, Vento M, Ciriminna R, Artini PG. Cryopreservation and oxidative stress in reproductive cells. Gynecol Endocrinol. 2010;26:563–7. doi: 10.3109/09513591003686395. [DOI] [PubMed] [Google Scholar]
- 71.Visconti PE. Understanding the molecular basis of sperm capacitation through kinase design. Proc Natl Acad Sci U S A. 2009;106:667–8. doi: 10.1073/pnas.0811895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gervasi MG, Osycka-Salut C, Caballero J, Vazquez-Levin M, Pereyra E, Billi S, Franchi A, Perez-Martinez S. Anandamide Capacitates Bull Spermatozoa through CB1 and TRPV1 activation. PLoS ONE. 2011;6:e16993. doi: 10.1371/journal.pone.0016993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karaliota S, Siafaka-Kapadai A, Gontinou C, Psarra K, Mavri-Vavayanni M. Anandamide increases the differentiation of rat adipocytes and causes PPARγ and CB1 receptor upregulation. Obesity. 2009;17:1830–8. doi: 10.1038/oby.2009.177. [DOI] [PubMed] [Google Scholar]
- 74.Díaz-Alonso J, Aguado T, de Salas-Quiroga A, Ortega Z, Guzmán M, Galve-Roperh I. CB1 Cannabinoid receptor-dependent activation of mTORC1/Pax6 signaling drives Tbr2 expression and basal progenitor expansion in the developing mouse cortex. Cereb Cortex. 2015;25:2395–408. doi: 10.1093/cercor/bhu039. [DOI] [PubMed] [Google Scholar]
- 75.Chiarlone A, Börner C, Martín-Gómez L, et al. MicroRNA let-7d is a target of cannabinoid CB1 receptor and controls cannabinoid signaling. Neuropharmacology. 2016;108:345–52. doi: 10.1016/j.neuropharm.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 76.Kierszenbaum AL, Tres LL. Structural and transcriptional features of the mouse spermatid genome. J Cell Biol. 1975;65:258–70. doi: 10.1083/jcb.65.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grunewald S, Paasch U, Glander HJ, Anderegg U. Mature human spermatozoa do not transcribe novel RNA. Andrologia. 2005;37:69–71. doi: 10.1111/j.1439-0272.2005.00656.x. [DOI] [PubMed] [Google Scholar]
- 78.Chen X, Wang Y, Zhu H, Hao H, Zhao X, Qin T, Wang D. Comparative transcript profiling of gene expression of fresh and frozen-thawed bull sperm. Theriogenology. 2015;83:504–11. doi: 10.1016/j.theriogenology.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 79.Qin Z, Wang W, Ali MA, Wang Y, Zhang Y, Zhang M, Zhou G, Yang J, dong, Zeng C. (2021) Transcriptome-wide m6A profiling reveals mRNA post-transcriptional modification of boar sperm during cryopreservation. BMC Genomics 2021 22:1 22:1–15 [DOI] [PMC free article] [PubMed]
- 80.Nguyen M, Sabry R, Davis OS, Favetta LA. (2022) Effects of BPA, BPS, and BPF on Oxidative Stress and Antioxidant Enzyme Expression in Bovine Oocytes and Spermatozoa. Genes 2022, Vol 13, Page 142 13:142 [DOI] [PMC free article] [PubMed]
- 81.Gur Y, Breitbart H. Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes Dev. 2006;20:411–6. doi: 10.1101/gad.367606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rajamanickam GD, Kastelic JP, Thundathil JC. Content of testis-specific isoform of Na/K-ATPase (ATP1A4) is increased during bovine sperm capacitation through translation in mitochondrial ribosomes. Cell Tissue Res. 2017;368:187–200. doi: 10.1007/s00441-016-2514-7. [DOI] [PubMed] [Google Scholar]
- 83.(2010) WHO laboratory manual for the Examination and processing of human semen FIFTH EDITION.
- 84.Koopman G, Reutelingsperger CPM, Kuijten GAM, Keehnen RMJ, Pals ST, van Oers MHJ. Annexin V for Flow Cytometric detection of Phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–20. doi: 10.1182/blood.V84.5.1415.bloodjournal8451415. [DOI] [PubMed] [Google Scholar]
- 85.Garner DL, Johnson LA. Viability Assessment of mammalian sperm using SYBR-14 and Propidium Iodide. Biol Reprod. 1995;53:276–84. doi: 10.1095/biolreprod53.2.276. [DOI] [PubMed] [Google Scholar]
- 86.Villegas J, Schulz M, Soto L, Sanchez R. Bacteria induce expression of apoptosis in human spermatozoa. Apoptosis. 2005;10:105–10. doi: 10.1007/s10495-005-6065-8. [DOI] [PubMed] [Google Scholar]
- 87.Parrish JJ. Bovine in vitro fertilization: in vitro oocyte maturation and sperm capacitation with heparin. Theriogenology. 2014;81:67–73. doi: 10.1016/j.theriogenology.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rodríguez-Villamil P, Mentz D, Ongaratto FL, Aguiar LH, Rodrigues JL, Bertolini M, Moura AA. (2020) Assessment of binder of sperm protein 1 (BSP1) and heparin effects on in vitro capacitation and fertilization of bovine ejaculated and epididymal sperm. Zygote. 10.1017/S0967199420000374 [DOI] [PubMed]
- 89.Idziorek T, Estaquier J, de Bels F, Ameisen JC. YOPRO-1 permits cytofluorometric analysis of programmed cell death (apoptosis) without interfering with cell viability. J Immunol Methods. 1995;185:249–58. doi: 10.1016/0022-1759(95)00172-7. [DOI] [PubMed] [Google Scholar]
- 90.Hallap T, Nagy S, Jaakma Ü, Johannisson A, Rodriguez-Martinez H. Usefulness of a triple fluorochrome combination merocyanine 540/Yo-Pro 1/Hoechst 33342 in assessing membrane stability of viable frozen-thawed spermatozoa from Estonian Holstein AI bulls. Theriogenology. 2006;65:1122–36. doi: 10.1016/j.theriogenology.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 91.Steckler D, Stout TAE, Durandt C, Nöthling JO. Validation of merocyanine 540 staining as a technique for assessing capacitation-related membrane destabilization of fresh dog sperm. Theriogenology. 2015;83:1451–60. doi: 10.1016/j.theriogenology.2015.01.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Boehnke KF, Dean O, Haffajee RL, Hosanagar A. 2022. U.S. Trends in Registration for Medical Cannabis and Reasons for Use From 2016 to 2020. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.