Abstract
Rsd (regulator of sigma D) is an anti-sigma factor for the Escherichia coli RNA polymerase ς70 subunit. The contact site of Rsd on ς70 was analyzed after mapping of the contact-dependent cleavage sites by Rsd-tethered iron-p-bromoacetamidobenzyl EDTA and by analysis of the complex formation between Ala-substituted ς70 and Rsd. Results indicate that the Rsd contact site is located downstream of the promoter −35 recognition helix-turn-helix motif within region 4, overlapping with the regions involved in interaction with both core enzyme and ς70 contact transcription factors.
The survival of bacterial cells in various environments depends on their abilities to sense the external conditions and adapt their internal metabolic systems by turning on and off the expression of specific genes (for reviews, see references 12 and 21). For quick change of the global gene expression pattern in response to sudden environmental changes, bacteria carry modulation systems for the specificity and activity of transcription apparatus. The transcription apparatus of Escherichia coli is composed of the RNA polymerase core enzyme (subunit composition, α2ββ′) with the catalytic activity of RNA polymerization and one of seven species of the ς subunit with the promoter recognition activity (reviewed in references 14, 15, 19, and 22). The major ς subunit, ς70, is responsible for transcription of most genes expressed during steady-state cell growth under laboratory culture conditions. The other six species of the ς subunit are required only during certain growth stages or under specific stress conditions. In agreement with their functional roles, the levels of these alternative ς subunits vary depending on the cell growth conditions (25, 26, 42), and all the ς subunits compete with each other for binding to a fixed amount of the core enzyme (41). In addition to the level control, the activities of at least some E. coli ς subunits are under a control system in which the unused ς subunits are stored in inactive forms by forming complexes with another set of proteins, often designated as anti-ς factors, with the regulatory activity of ς functions (for reviews see references 18 and 21).
Subunit ςF is involved in transcription of the genes needed for flagellum formation and chemotaxis. The flgM gene product is an anti-ςF factor that acts by directly binding to ςF and thereby preventing its interaction with the core RNA polymerase (33). Subunit ςE is a member of the ECF family of ς subunits for transcription of the genes related to extracytoplasmic functions (39) as well as those required for high temperature survival or thermotolerance (9). The ςE activity is regulated by the rseA (regulator of sigma E) gene product or anti-ςE factor, which is associated with the inner membrane and inhibits the activity of ςE by directly interacting with ςE (7, 43). FecI also belongs to the ECF ς family and is involved in transcription activation of the ferric-citrate transport genes (fec) (1). Genetic studies revealed that FecR, an inner membrane protein, negatively regulates the activity of the FecI ς subunit (49). FlgM, RseA, and FecR are classified as members of anti-sigma factors for ςF, ςE, and ςFecI, respectively. A heat shock protein, DnaK, can be an anti-ς factor for the heat shock ςH subunit (18), which is induced following heat shock and is involved in transcription of the genes encoding heat shock proteins, including DnaK, DnaJ, and GrpE (13). After returning from the transient adaptation period to heat shock to steady-state growth at high temperatures, unused ςH becomes stored as DnaJ-DnaK-ςH complexes (38), which are dissociated by the action of GrpE to release ςH for reuse or for degradation by HflB (FtsH) protease (10).
Recently we discovered a novel E. coli protein, referred to as Rsd (regulator of sigma D), which forms a complex with ς70, the major ς70 subunit for growth-related gene transcription, and prevents its function (24). Purified Rsd protein formed complexes in vitro with ς70 but not with other ς subunits and inhibited transcription in vitro by the holoenzyme containing ς70 to various extents, depending on the promoters used (24). Since Rsd is induced in the stationary phase of cell growth, where ς70 is not used, we proposed that Rsd is an anti-ς factor for the major ς70 subunit for storage in the stationary phase. In E. coli mutants lacking the rsd gene, the expression of ς70-dependent genes increases while transient overproduction of Rsd leads to a reduction in ς70-dependent gene expression (23). Based on these results, taken together, we proposed that Rsd is an anti-sigma factor for the ς70 subunit.
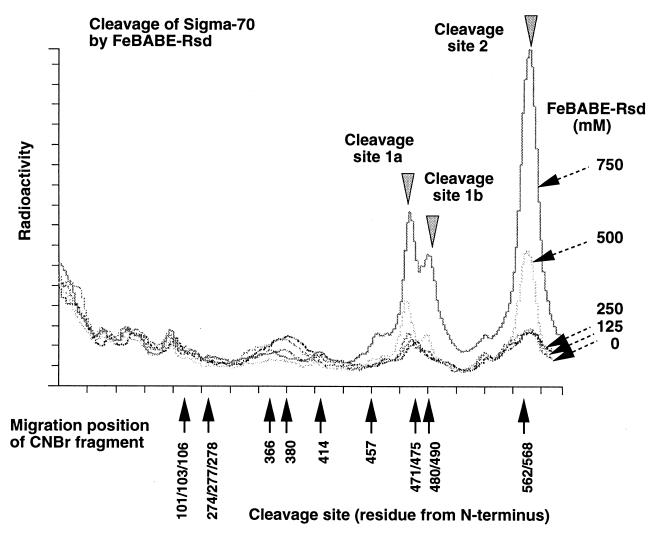
Cleavage sites of ς70 by Rsd-tethered FeBABE.
Previously we estimated the contact site of Rsd on ς70 to be downstream from residue 500, including regions 3.2, 4.1, and 4.2, after analysis of complex formation between Rsd and ς70 fragments (24). For detailed mapping of the contact site of Rsd on the ς70 subunit, we employed the contact-dependent cleavage of target proteins by FeBABE (iron-p-bromoacetamidobenzyl EDTA)-conjugated pairing proteins (6, 20, 22). In this study, FeBABE was tethered to Rsd at all possible Lys residues by using 2-iminothiolane, which links between Lys and FeBABE (48). For detection of the cleavage sites on ς70, a protein kinase tag sequence was added at either its N or C terminus and the tag was phosphorylated using [γ-32P]ATP and protein kinase A. Mixtures of a fixed amount of 32P-labeled ς70 and increasing amounts of FeBABE-tethered Rsd were incubated for 10 min at 37°C to form binary complexes and then were subjected to cleavage reaction by adding ascorbate and H2O2. The reaction mixtures were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by autoradiography. Fig. 1 shows a tracing of the SDS-PAGE pattern of the cleavage products. As size markers, the same 32P-labeled ς70 was treated with CNBr, which induces cleavage at Met residues. Although several nonspecific cleavage products were generated by the addition of H2O2 and ascorbate even in the absence of FeBABE-tethered Rsd, the specific cleavage products increased concomitantly with the increase in Rsd-FeBABE addition. At least three such bands, cleaved at sites 1a, 1b, and 2, were identified, each migrating close to the C-terminal CNBr fragment (471/475–613, 488/490–613, or 562/568–613, respectively). Thus we concluded that the FeBABE tethered on the surface of ς70-bound Rsd approached near the ς70 segment between residues 471 and 568. The cleavage sites 1a and 1b are located within ς70 region 3 while cleavage site 2 is within region 4 (see Fig. 3). The result, however, does not immediately indicate the location of the Rsd contact site on these regions because the spacer length between the BABE-tethered Lys and the BABE-associated Fe is about 18 Å (20, 48).
FIG. 1.
Cleavage of ς70 by Rsd-tethered FeBABE. Purified Rsd was conjugated with FeBABE (Dojin, Kumamoto, Japan) in the presence of 2-iminothiolane according to Traviglia et al. (48), while ς70 with a PK tag at the C terminus was labeled in vitro with 32P with protein kinase A. Mixtures of a fixed amount of 32P-labeled ς70 (final concentration, 250 nM) and the indicated amounts of FeBABE-tethered Rsd were incubated for 30 min at 37°C and then were subjected to contact-dependent protein cleavage reaction by adding ascorbate and H2O2 followed by SDS-PAGE. CNBr-treated 32P-ς70 was run on the same gel as size markers. The gel was exposed to an imaging plate, and the plate was analyzed with the BAS 2000 Image Analyzer (Fuji, Tokyo, Japan). Migration is from left to right. The migration positions of CNBr fragments are indicated at the bottom.
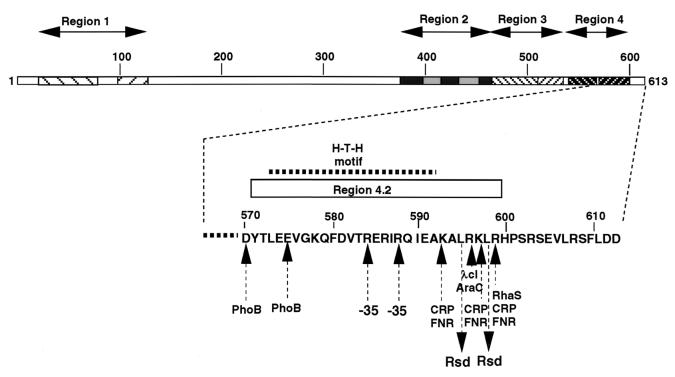
FIG. 3.
The Rsd contact sites on the ς70 subunit. The predicted Rsd contact sites are compared with the known functional sites, including the sites involved in the molecular interaction with class II transcription factors. The HTH motif is involved in the promoter −35 recognition (11, 47). The contact sites for class II transcription factors are located upstream or downstream of this motif. For details, see the text.
The cleavage reaction by Rsd-tethered FeBABE was also performed for all six alternative subunits, ςN, ςS, ςH, ςF, ςE, and ςFecI, but none of them were cleaved even after the addition of excess amounts of Rsd-FeBABE (data not shown). These observations confirm our previous finding that Rsd specifically interacts only with the ς70 subunit and not with the other ς subunits (24).
Rsd-binding activity of Ala-substituted ς70 mutants.
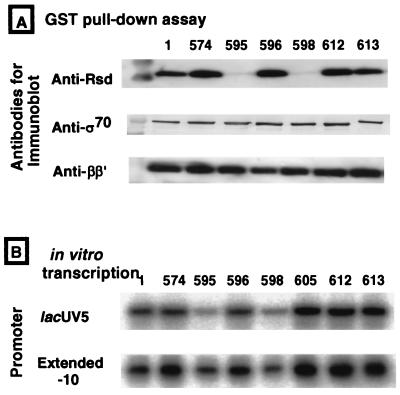
For detailed mapping of the contact site on ς70 with Rsd, we next tested the complex formation in vitro between Rsd and Ala-substituted ς70 subunits. The library of Ala-substituted ς70 was constructed and used for mapping the ς70 contact sites with the core enzyme (46) or with ς70 contact transcription factors CRP and FNR (40). The mutant ς70 subunits with a glutathione S-transferase (GST) tag fused at the N termini were overproduced and were purified to near homogeneity. The GST-tagged ς70 subunits were mixed with purified Rsd, and the complexes formed were recovered using glutathine-conjugated agarose beads. This GST pull-down assay indicated that two ς70 mutants with Ala substitutions at residues 595 and 598 were defective in binding to Rsd (Fig. 2A), indicating that the segment of ς70 including L595 and L598 is involved in molecular interaction with Rsd. The major determinant of core enzyme binding on ς70 is located in region 2.1 (37). L598 in region 4.2 also participates, at least in part, in binding of the core enzyme (46). The corresponding region of ς32 is also involved in core enzyme binding (27). In the case of core enzyme binding, multiple sites on the ς subunits are involved and thus a single mutation is often not so critical for overall functions of the ς subunits. In fact, under the assay conditions employed, the binding of L598A mutant ς70 with the core enzyme is stronger than that with the Rsd protein (Fig. 2A).
FIG. 2.
Identification of the Rsd contact site on the ς70 subunit. (A) GST-ς70 Ala-substituted mutants were overexpressed and purified to near homogeneity. Each GST-ς70 mutant was mixed with an equal amount of Rsd, and after incubation for 5 min at 37°C, GST-ς70-Rsd complexes were isolated by the GST pull-down assay using glutathione-Sepharose beads. The bead-bound proteins were eluted with 50 mM glutathione and were analyzed by SDS-PAGE. The gel was subjected to immunoblotting against anti-Rsd, anti-ς70, or anti-ββ′ antibodies. (B) Activity of the Ala-substituted mutant ς70 subunits. In vitro transcription was carried out under the standard reaction conditions (28) using 1 pmol each of the GST-ς70 mutants, 1 pmol of the core enzyme, and 1 pmol of either lacUV5 or extended −10 promoter DNA fragment (31, 32).
To analyze the role of these residues in the intrinsic ς70 function of promoting transcription initiation, holoenzymes were reconstituted from each of the Ala-substituted mutant ς70 subunits and the ς-free core enzyme and were used for in vitro transcription. The lacUV5-directed transcription was significantly reduced for only the same two mutants, L595A and L598A, which are required for Rsd interaction (Fig. 2B). Thus, the sites required for Rsd interaction are also critical for expression of the intrinsic ς70 activities, presumably at the step of core enzyme binding (46). The influence of Ala substitution at residues downstream of the −35 recognition helix-turn-helix (HTH) motif of ς70 was also observed in RNA I promoter-directed transcription in vitro (40). The reduction of ς70 activity for Ala-substituted mutant ς70 was, however, not observed when transcription was carried out using the extended −10 promoter (Fig. 2B), which is active in directing transcription even in the absence of −35 promoter -ς70 region 4 interactions (2, 4, 32).
Rsd contact site on the ς70 subunit.
FeBABE cleavage experiments indicate the close location of Rsd near the ς70 segment between residues 471 and 568, including regions 3.1, 3.2, and 4.1 (see Fig. 1), while the mutant studies using an Ala-substituted ς70 library indicate that the Rsd contact site is downstream from region 4.2 (see Fig. 2). Since the reactive Fe3+ is located 18 Å apart from the Lys residue tethered with FeBABE with the use of a 2-iminothiolane linker (20, 48), it is unlikely that the region between 471 and 568 is the direct target of Rsd binding, but instead the Rsd-binding site includes the residues L595 and L598 identified by Ala scanning. The FeEDTA moiety of the FeBABE tethered at this region may be located close to regions 3 and 4.1, where the cleavage sites were identified (see Fig. 1). Thus we conclude that the direct contact site of Rsd is located on region 4 of the ς70 subunit downstream of the HTH motif of region 4.2 (Fig. 3), which is involved in recognition of the promoter −35 sequence (11, 47). The −35 contact is, however, not essential for promoter complex formation when the contact of ς70 region 2 with the promoter −10 sequence alone is strong enough, as in the case of an extended −10 signal (3, 4, 32).
The ς70 contact (or class II) transcription factors support the functional interaction of ς70 with promoters lacking the consensus −35 sequence (19). In these cases, the region upstream or downstream of the −35 contact HTH motif in ς70 region 4 is involved in interaction with class II factors (19, 45). Deletion mutant ς70 lacking region 4 is still functional with the extended −10 promoter, which alone has a high affinity to the ς70 region 2 but is defective in response to CRP (on class II promoters) and PhoB (31). Mutant studies indicate that the contact sites for several class II transcription factors, including λ cI (8, 30), PhoB (29), CRP (40), FNR (40), Ada (35, 36), AraC (17, 40), and RhaS (3) are all located upstream or downstream of the HTH promoter −35 recognition motif (Fig. 3). Transcription activation by λ cI becomes defective in a region 4 mutation of the ς70 subunit (30). At class II CRP-dependent promoters, CRP makes three different contacts, one of which, known as the activating region 3 (AR3), interacts with region 4 of the ς70 subunit (45). The positively charged residues K593, K597, and R599 on ς70 are required for this interaction. Mutations on these residues also affect the ς70 response to FNR (40). Most class I (or α contact) factors activate transcription by stabilizing the closed complex, while class II (or ς70 contact) factors such as λ cI activate the isomerization step of transcription initiation (8).
The contact site with Rsd is located on the same surface with those of AraC, CRP, FNR, RhaS, and λcI (Fig. 3). Thus, the region downstream from the promoter −35 binding HTH of ς70 seems to be involved in binding the core enzyme, the anti-sigma factor, and a group of class II transcription factors. The anti-ς70 factor Rsd should compete with both the core enzyme and the class II transcription factors in binding with the ς70 subunit. Likewise, the contact site of FlgM, the anti-ςF factor, has been mapped on region 4 of ςF (34). The phage T4 AsiA protein is an anti-sigma factor against the host E. coli ς70 subunit to repress host cell gene transcription (5, 44). The contact site for the AsiA protein on ς70 is located within regions 3 and 4 (16). Thus, all the anti-ς factors so far analyzed seem to interact with the same ς region near the promoter −35 recognition surface.
Acknowledgments
We thank Carol Gross for donating the expression library of Ala-substituted ς70 and for helpful discussion.
This work was supported by Grants-in-Aid from the Ministry of Education, Science, Culture and Sports of Japan and by the CREST fund from the Japan Science and Technology Corporation.
REFERENCES
- 1.Angerer A, Enz S, Ochs M, Braun V. Transcriptional regulation of ferric citrate transport in Escherichia coli K-12. FecI belongs to a new subfamily of ς70-type factors that respond to extracytoplasmic stimuli. Mol Microbiol. 1995;18:163–174. doi: 10.1111/j.1365-2958.1995.mmi_18010163.x. [DOI] [PubMed] [Google Scholar]
- 2.Barne K A, Bown J A, Busby S J, Minchin S D. Region 2.5 of the Escherichia coli RNA polymerase sigma 70 subunit is responsible for the recognition of the ‘extended −10’ motif at promoters. EMBO J. 1997;16:4034–4040. doi: 10.1093/emboj/16.13.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhende P M, Egan S M. Genetic evidence that transcription activation by RhaS involves specific amino acid contacts with sigma 70. J Bacteriol. 2000;182:4959–4969. doi: 10.1128/jb.182.17.4959-4969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bown J A, Owens J T, Meares C F, Fujita N, Ishihama A, Busby S J, Minchin S D. Organization of open complexes at Escherichia coli promoters: location of promoter DNA sites close to region 2.5 of the ς70 subunit of RNA polymerase. J Biol Chem. 1999;274:2263–2270. doi: 10.1074/jbc.274.4.2263. [DOI] [PubMed] [Google Scholar]
- 5.Brody E N, Kassavetis G A, Ouhammouch M, Sanders G M, Tinker R L, Geiduschek E P. Old phage, new insight: two recently recognized mechanisms of transcriptional regulation in bacteriophage T4 development. FEMS Lett. 1995;128:1–8. doi: 10.1111/j.1574-6968.1995.tb07491.x. [DOI] [PubMed] [Google Scholar]
- 6.Datwyler S A, Meares C F. Protein-protein interactions mapped by artificial proteases: where ς factors bind to RNA polymerase. Trends Biochem Sci. 2000;25:408–414. doi: 10.1016/s0968-0004(00)01652-2. [DOI] [PubMed] [Google Scholar]
- 7.De Las Penas A, Connolly L, Gross C A. The ςE-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of ςE. Mol Microbiol. 1997;24:373–385. doi: 10.1046/j.1365-2958.1997.3611718.x. [DOI] [PubMed] [Google Scholar]
- 8.Dove S L, Huang F W, Hochschild A. Mechanism for a transcriptional activation that works at the isomerization step. Proc Natl Acad Sci USA. 2000;97:13215–13220. doi: 10.1073/pnas.97.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson J W, Gross C A. Identification of the ςE subunit of Escherichia coli RNA polymerase: a second alternate ς factor involved in high-temperature gene expression. Genes Dev. 1989;3:1462–1471. doi: 10.1101/gad.3.9.1462. [DOI] [PubMed] [Google Scholar]
- 10.Gamer J, Multhaup G, Tomoyasu T, McCarty J S, Rudiger S, Schönfeld H, Schirra C, Bujard H, Bukau B. A cycle of binding and release of the DnaK, DnaJ and GrpE chaperones regulates activity of the Escherichia coli heat shock transcription factor ς32. EMBO J. 1996;15:607–617. [PMC free article] [PubMed] [Google Scholar]
- 11.Gardella T, Moyle H, Susskind M M. A mutant Escherichia coli ς70 subunit of RNA polymerase with altered promoter specificity. J Mol Biol. 1989;206:579–590. doi: 10.1016/0022-2836(89)90567-6. [DOI] [PubMed] [Google Scholar]
- 12.Gottesman S. Bacterial regulation: global regulatory networks. Annu Rev Genet. 1984;18:415–441. doi: 10.1146/annurev.ge.18.120184.002215. [DOI] [PubMed] [Google Scholar]
- 13.Gross C A. Function and regulation of the heat shock proteins. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1996. pp. 1382–1399. [Google Scholar]
- 14.Gross C A, Lonetto M, Losick R. Bacterial sigma factors. In: Yamamoto K, McKnight S, editors. Transcriptional regulation. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 129–176. [Google Scholar]
- 15.Helmann J D, Chamberlin M J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- 16.Hinton D M, March-Amegadzie R, Gerber J S, Sharma M. Characterization of pre-transcription complexes made at a bacteriophge T4 middle promoter: involvement of the T4 MotA activator and the T4 AsiA protein, a ς70 binding protein, in the formation of the open complex. J Mol Biol. 1996;256:235–248. doi: 10.1006/jmbi.1996.0082. [DOI] [PubMed] [Google Scholar]
- 17.Hu J C, Gross C A. Mutations in the sigma subunit of E. coli RNA polymerase which affect positive control of transcription. Mol Gen Genet. 1985;199:7–13. doi: 10.1007/BF00327502. [DOI] [PubMed] [Google Scholar]
- 18.Hughes K T, Mathee K. The anti-sigma factors. Annu Rev Microbiol. 1998;52:231–286. doi: 10.1146/annurev.micro.52.1.231. [DOI] [PubMed] [Google Scholar]
- 19.Ishihama A. Functional modulation of Escherichia coli RNA polymerase. Annu Rev Microbiol. 2000;54:499–518. doi: 10.1146/annurev.micro.54.1.499. [DOI] [PubMed] [Google Scholar]
- 20.Ishihama A. Molecular anatomy of RNA polymerase using protein-conjugated metal probes with nuclease and protease activities. Chem Commun. 2000;2000:1091–1094. [Google Scholar]
- 21.Ishihama A. Adaptation of gene expression in stationary phase bacteria. Curr Opin Genet Devel. 1997;7:582–588. doi: 10.1016/s0959-437x(97)80003-2. [DOI] [PubMed] [Google Scholar]
- 22.Ishihama A. Promoter selectivity control of Escherichia coli RNA polymerase. In: Eckstein F, Lilley D M J, editors. Mechanisms of transcription. Berlin, Germany: Springer-Verlag; 1997. pp. 53–70. [Google Scholar]
- 23.Jishage M, Ishihama A. Transcriptional organization and in vivo role of the Escherichia coli rsd gene, encoding the regulator of RNA polymerase sigma D. J Bacteriol. 1999;181:3768–3776. doi: 10.1128/jb.181.12.3768-3776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jishage M, Ishihama A. A stationary phase protein in Escherichia coli with binding activity to the major ς subunit of RNA polymerase. Proc Natl Acad Sci USA. 1998;95:4953–4958. doi: 10.1073/pnas.95.9.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jishage M, Ishihama A. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: Intracellular levels of ς70 and ς38. J Bacteriol. 1995;17:6832–6835. doi: 10.1128/jb.177.23.6832-6835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jishage M, Iwata A, Ueda S, Ishihama A. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular level of four species of sigma subunit under various growth conditions. J Bacteriol. 1996;178:5447–5451. doi: 10.1128/jb.178.18.5447-5451.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joo D M, Nolte A, Calendar R, Zhou Y N, Jin D J. Multiple regions on the Escherichia coli heat shock transcription factor ς32 determine core RNA polymerase binding specificity. J Bacteriol. 1998;180:1095–1102. doi: 10.1128/jb.180.5.1095-1102.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kajitani M, Ishihama A. Determination of the promoter strength in the mixed transcription system: promoters of lactose, tryptophan and ribosomal protein L10 operons from Escherichia coli. Nucleic Acids Res. 1983;11:671–686. doi: 10.1093/nar/11.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S K, Makino K, Amemura A, Nakata A, Shinagawa H. Mutational analysis of the role of the first helix of region 4.2 of the sigma 70 subunit of Escherichia coli RNA polymerase in transcriptional activation by activator protein PhoB. Mol Gen Genet. 1995;248:1–8. doi: 10.1007/BF02456607. [DOI] [PubMed] [Google Scholar]
- 30.Kuldell N, Hochschild A. Amino acid substitutions in the −35 recognition motif of sigma 70 that result in defects in phage lambda repressor-stimulated transcription. J Bacteriol. 1994;176:2991–2998. doi: 10.1128/jb.176.10.2991-2998.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar A, Grimes G, Fujita N, Malloch R A, Hayward R S, Ishihama A. Role of the sigma 70 subunit of Escherichia coli RNA polymerase in transcription. J Mol Biol. 1994;235:405–413. doi: 10.1006/jmbi.1994.1001. [DOI] [PubMed] [Google Scholar]
- 32.Kumar A, Malloch R A, Fujita N, Smillie D A, Ishihama A, Hayward R S. The minus 35-recognition region of Escherichia coli sigma 70 is inessential for initiation of transcription at an “extended minus 10” promoter. J Mol Biol. 1993;232:406–418. doi: 10.1006/jmbi.1993.1400. [DOI] [PubMed] [Google Scholar]
- 33.Kutsukake K. Excretion of the anti-sigma factor through a flagellar substructure couples flagellar assembly in Salmonella typhimurium. Mol Gen Genet. 1994;243:605–612. doi: 10.1007/BF00279569. [DOI] [PubMed] [Google Scholar]
- 34.Kutsukake K, Iino T. Role of the FliA-FlgM regulatory system on the transcriptional control of the flagellar formation in Salmonella typhimurium. J Bacteriol. 1994;176:3598–3605. doi: 10.1128/jb.176.12.3598-3605.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landini P, Busby S J W. The Escherichia coli Ada protein can interact with two distinct determinants in the ς70 subunit of RNA polymerase according to promoter architecture: identification of the target of Ada activation at the alkA promoter. J Bacteriol. 1999;181:1524–1529. doi: 10.1128/jb.181.5.1524-1529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landini P, Bown J A, Volkert M R, Busby S J W. Ada protein-RNA polymerase ς70 subunit interaction and α subunit-promoter DNA interaction are necessary at different steps in transcription initiation at the Escherichia coli ada and aidB promoters. J Biol Chem. 1998;273:13307–13312. doi: 10.1074/jbc.273.21.13307. [DOI] [PubMed] [Google Scholar]
- 37.Lesley S A, Burgess R R. Characterization of the Escherichia coli transcription factor ς70: localization of a region involved in the interaction with core RNA polymerase. Biochemistry. 1989;28:7728–7734. doi: 10.1021/bi00445a031. [DOI] [PubMed] [Google Scholar]
- 38.Libereck K, Wall D, Georgopoulos C. The DnaJ chaperone catalytically activates the DnaK chaperone to preferentially bind the ς32 heat shock transcriptional regulator. Proc Natl Acad Sci USA. 1995;92:6224–6228. doi: 10.1073/pnas.92.14.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lonetto M A, Brown K L, Rudd K E, Buttner M J. Analysis of the Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial RNA polymerase ς factors involved in the regulation of extracytoplasmic functions. Proc Natl Acad Sci USA. 1994;91:7573–7577. doi: 10.1073/pnas.91.16.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lonetto M A, Rhodius V, Lamberg K, Kiley P, Busby S, Gross C. Identification of a contact site for different transcription activators in region 4 of the Escherichia coli RNA polymerase ς70 subunit. J Mol Biol. 1998;284:1353–1365. doi: 10.1006/jmbi.1998.2268. [DOI] [PubMed] [Google Scholar]
- 41.Maeda H, Fujita N, Ishihama A. Sigma competition: comparison of binding affinity to the core RNA polymerase among seven E. coli sigma subunits. Nucleic Acids Res. 2000;28:3497–3503. doi: 10.1093/nar/28.18.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maeda H, Jishage M, Nomura T, Fujita N, Ishihama A. Promoter selectivity of the RNA polymerase holoenzyme containing the extracytoplasmic function (ECF) sigma subunit ςE or ςFecI. J Bacteriol. 2000;182:1181–1184. doi: 10.1128/jb.182.4.1181-1184.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Missiakas D, Raina S, Georgopoulos C. Modulation of the Escherichia coli ςE (RpoE) heat-shock transcription-factor activity by the RseA, RseB, and RseC proteins. Mol Microbiol. 1996;24:355–371. doi: 10.1046/j.1365-2958.1997.3601713.x. [DOI] [PubMed] [Google Scholar]
- 44.Orsini G, Ouhammouch M, Lecaer J P, Brody E N. The asiA gene of bacteriophage T4 codes for the anti-sigma 70 protein. J Bacteriol. 1993;175:85–93. doi: 10.1128/jb.175.1.85-93.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhodius V A, Busby S J. Interactions between activating region 3 of the Escherichia coli cyclic AMP receptor protein and region 4 of the RNA polymerase ς70 subunit: application of suppression genetics. J Mol Biol. 2000;299:311–324. doi: 10.1006/jmbi.2000.3737. [DOI] [PubMed] [Google Scholar]
- 46.Sharp M M, Chan C L, Lu C Z, Marr M T, Nechaev S, Merritt E W, Severinov K, Roberts J W, Gross C A. The interface of ς with core RNA polymerase is extensive, conserved, and functionally specialized. Genes Dev. 1999;13:3015–3026. doi: 10.1101/gad.13.22.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siegele D A, Hu J C, Walter W A, Gross C A. Altered promoter recognition by mutant forms of the ς70 subunit of Escherichia coli RNA polymerase. J Mol Biol. 1989;206:591–603. doi: 10.1016/0022-2836(89)90568-8. [DOI] [PubMed] [Google Scholar]
- 48.Traviglia S L, Datwyler S A, Yan D, Ishihama A, Meares C F. Targeted protein footprinting: where different transcription factors bind to RNA polymerase. Biochemistry. 1999;38:15774–15778. doi: 10.1021/bi9917232. [DOI] [PubMed] [Google Scholar]
- 49.Van Hove B, Staudenmaier H, Braun V. Novel two-component transmembrane transcription control: regulation of iron dicitrate transport in Escherichia coli K-12. J Bacteriol. 1990;172:6749–6758. doi: 10.1128/jb.172.12.6749-6758.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]