Abstract
Background
Inflammation is implicated in tumorigenesis and has been reported as an important prognostic factor in cancers. In this study, we aimed to develop and validate a novel inflammation score (IFS) system based on 12 inflammatory markers and explore its impact on intrahepatic cholangiocarcinoma (ICC) survival after hepatectomy.
Methods
Clinical data of 446 ICC patients undergoing surgical treatment were collected from the Primary Liver Cancer Big Data, and then served as a training cohort to establish the IFS. Furthermore, an internal validation cohort including 175 patients was used as internal validation cohort of the IFS. A survival tree analysis was used to divide ICC patients into three groups (low-, median-, and high- IFS-score groups) according to different IFS values. Kaplan-Meier (KM) curves were used to compare the overall survival (OS) and recurrence-free survival (RFS) rates among three different groups. Cox regression analyses were applied to explore the independent risk factors influencing OS and RFS.
Results
In the training cohort, 149 patients were in the low-IFS-score group, 187 in the median-IFS-score group, and 110 in the high-IFS-score group. KM curves showed that the high-IFS-score group had worse OS and RFS rates than those of the low- and median-IFS-score groups (P < 0.001) in both the training and validation cohorts. Moreover, multivariable Cox analyses identified high IFS as an independent risk factor for OS and RFS in the training cohort. The area under the curve values for OS prediction of IFS were 0.703 and 0.664 in the training and validation cohorts, respectively, which were higher than those of the American Joint Committee on Cancer (AJCC) 7th edition TNM stage, AJCC 8th edition TNM stage, and the Child-Pugh score.
Conclusion
Our results revealed the IFS was an independent risk factor for OS and RFS in patients with ICC after hepatectomy and could serve as an effective prognostic prediction system in daily clinical practice.
Keywords: Systemic inflammation, Intrahepatic cholangiocarcinoma, Liver resection, Prognosis
Introduction
Intrahepatic cholangiocarcinoma (ICC) is one of the deadliest malignancies worldwide, which arises from the epithelial cells of intrahepatic bile ducts [1]. ICC has become the second most common primary liver malignancy after hepatocellular carcinoma (HCC) [2]. In recent decades, the incidence of ICC in the United States has been increasing [1, 3]. Although surgical resection is recommended as the first-line treatment for ICC, unfortunately, only 30–40% of patients had surgical opportunities because most patients have been found to present regional or distant metastases at the time of diagnosis [4].
More importantly, even after curative-intent resection, the long-term survival of ICC is still dismal because of the high postoperative recurrence rate [4]. Selection of appropriate patients for surgery is one method to improve survival prognosis. Preoperative evaluation of the resectability of patients with ICC is usually based on BCLC stage, TNM stage, and Child-Pugh grade. Common prognostic indicators, like tumor size, tumor number, vascular invasion, and lymph node metastasis, are often acquired by accurate postoperative pathological data. Consequently, obtaining parameters through preoperative low-cost hematological examination is significant and cost-effective, which can also be used for survival prognosis prediction. Thus, finding an accurate prognostic biomarker is necessary and urgent for surgeons to evaluate prognosis and guide the choice making of treatment before hepatectomy, which provides ICC patients with reasonable individual treatment.
In 1863, Virchow first reported the relationship between inflammation and tumorigenesis [5]. Inflammation has been found to be involved in the onset and progression of diverse diseases. The use of preoperative inflammatory markers to provide individualized survival estimates has been the focus on many reports [6–11]. It has been confirmed that inflammatory markers played an important role in the progression and prognosis of liver cancer [12, 13]. A series of studies have demonstrated that the systemic inflammation score (SIS), the neutrophil-to-lymphocyte ratio (NLR), and the lymphocyte to monocyte ratio (LMR) were all significantly associated with long-term survival in patients with ICC [14]. Nevertheless, there are no studies that combine several inflammation-based indicators to create an integrated prognostic score system for survival prediction in patients with ICC.
In this study, we aimed to develop a novel inflammatory score system that achieves a good survival prediction performance in patients with ICC after surgery using 12 preoperational inflammatory markers. To this end, we used an internal cohort to validate the survival prediction performance of our inflammatory score system at the same time.
Methods
Patients
From January 2010 to December 2013, clinical data of 446 patients with ICC who underwent surgical treatment were collected from the Primary Liver Cancer Big Data in Fujian province [15] and used to form the training cohort. For the internal validation cohort, we collected clinical data of 175 patients with ICC from January 2014 to August 2015 from the same institution. These data were retrospectively reviewed. This study was approved by the Institutional Ethics Committee of the Mengchao Hepatobiliary Hospital. Informed consent was obtained from all patients before surgery in this study.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) good preoperative performance with an ECOG score of 0–2, (2) no records of ICC treatment before surgery, (3) radical resection (R0 resection), (4) no other malignant diseases, (5) Child–Pugh graded A or B7, (6) no evidence of main portal vein, hepatic artery, biliary duct, or inferior vena cava invasion. The exclusion criteria were as follows: (1) incomplete clinicopathological data and (2) loss of follow-up within 30 days.
Liver resection and definitions
All patients underwent routine preoperative laboratory examinations (routine blood tests, platelets, neutrophils, lymphocyte cell count, tumor serum markers, such as carbohydrate antigen 19–9 [CA19–9], carcinoembryonic antigen [CEA], and serum alpha-fetoprotein [AFP]) and imaging examinations (abdominal ultrasound, contrast-enhanced computerized tomography [CT] scan, and/or magnetic resonance imaging [MRI] of the abdomen). The intent of surgery was to completely remove the macroscopic tumors with adequate resection margins. The detailed surgical procedures are described in our previous studies [16].
The diagnosis of ICC through histopathological examination of surgical specimens was based on the WHO criteria [17]. Major hepatectomy was defined as the removal of ≥ 3 Chouinard’s liver segments. Anatomic resection was defined as liver resections based on systematic removal of Couinaud segment(s) containing the tumor together with the tumor-bearing portal vein and corresponding hepatic territory [18]. R0 resection was defined as the absence of macroscopic and microscopic disease at the surgical margin. Microvascular invasion (MVI) was defined as intraparenchymal vascular involvement identified on histological examination [19].
Inflammatory markers
By reviewing previous studies, we selected 15 inflammatory markers for our research. They are listed as follows: NLR (neutrophil-to-lymphocyte ratio), PLR (platelet-to-lymphocyte ratio), LMR (lymphocyte-to-monocyte), GPR (gamma‑glutamyl transpeptidase [γ-GT]-to-platelet ratio), SII (systemic immune-inflammation index, defined as platelet times neutrophil/lymphocyte), PNI (prognostic nutritional index), APRI (aspartate aminotransferase-to-platelet ratio index), ANRI (aspartate aminotransferase-to-neutrophil ratio index), ALRI (aspartate aminotransferase-to-lymphocyte ratio index), NγLR ([neutrophil times γ-GT]/lymphocyte), dNLR (derived neutrophil-to-lymphocyte ratio, defined as neutrophil/[white cell count - neutrophil count]), PMLR (platelet‑monocyte‑lymphocyte ratio), NMLR (neutrophil‑monocyte‑lymphocyte ratio), SIS (inflammation score, defined as combination of preoperative serum CA19-9 and LMR. Grade I, CA19-9Low/LMRHigh; grade II, CA19-9 High/LMRHigh and CA19-9 Low/LMR Low; grade III, CA19-9High/LMR Low) [20], and GAR (globulin-to-albumin ratio).
Follow-up
All patients were followed up regularly after discharge using the protocol described previously [21]. The relapse of ICC was diagnosed using the same criteria as the initial disease diagnosis, and a multidisciplinary approach was used to deal with the recurrence lesions [22]. Overall survival (OS) and recurrence-free survival (RFS) were defined as endpoints. OS was defined as the interval between the date of LR to the date censored, the date of the patient’s death, or last follow-up. RFS was measured from the date of LR to the date of the first ICC recurrence or last follow-up. This study censored the follow-up on August 30, 2020.
Cut-off value and inflammation score (IFS)
In this study, we stratified NLR, PLR, LMR, GPR, SII, PNI, APRI, ANRI, ALRI, NγLR, dNLR, PMLR, NMLR, SIS, and GAR into two groups based on receiver operating characteristic (ROC) curves. The optimal threshold values of the highest Youden index (specificity + specificity − 1) were used as cut-off points in this study [23]. The calculation of IFS for each patient was based on the value of every inflammatory marker and its coefficient of the Cox analysis.
Statistical analysis
Continuous variables were reported as mean ± standard deviation (SD), or median and interquartile range (IQR). The differences were compared by one-way analysis of variance (ANOVA) or Kruskal-Wallis H tests (K-W tests). Categorical variables were reported as frequencies and percentages, and differences in different groups were compared using the Chi-square test. OS and RFS rates were analyzed by the Kaplan-Meier (KM) curves, and the differences were compared using the log-rank test. Cox regression analyses were used to identify independent prognostic factors for OS and RFS. Survival tree analysis was applied to divide patients into three differentiated groups (high-, median-, and low-score groups) with different survival outcomes.
The AJCC 7th TNM stage, AJCC 8th TNM stage, and Child-Pugh stage were compared with the IFS system in testing the performance of prognosis prediction via the time-dependent areas under the ROC (Time-ROC) curve and akaike information criterion (AIC). Statistical analysis was performed using the SPSS® version 25.0 (IBM, Armonk, New York, USA), and R program version 3.2.0 (http://www.r-project.org/). A P-value < 0.05 indicated statistical significance.
Results
The correlation of fifteen inflammatory markers
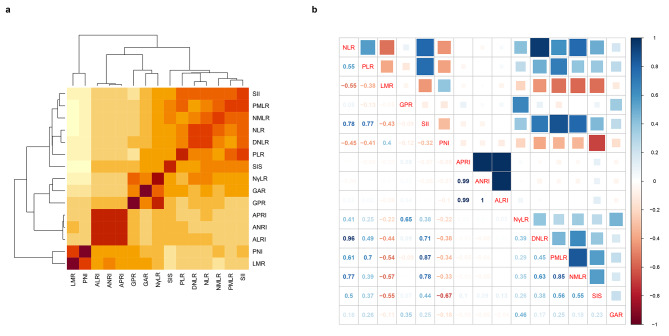
Figure 1a shows the correlation heatmap of 15 inflammatory markers in this study. The darker the yellow, the stronger the correlation (positive or negative correlation). As shown in Fig. 1b, we quantified the correlation of 15 inflammatory markers. We found that the correlation coefficient between the ANRI and ALRI was 1 point. Moreover, we also found that APRI had a strong correlation between ANRI and ALRI, with coefficients of both 0.99. In parallel, the correlations between the NLR and DNLR, SII and PMLR, and PMLR and NMLR were also strong, with coefficients of 0.96, 0.87, and 0.85, respectively.
Fig. 1.
Heatmap of correlation among the fifteen inflammatory markers (a). Correlation plot showing the specific correlation coefficient relationship among the fifteen inflammatory markers (b)
Construction of IFS system
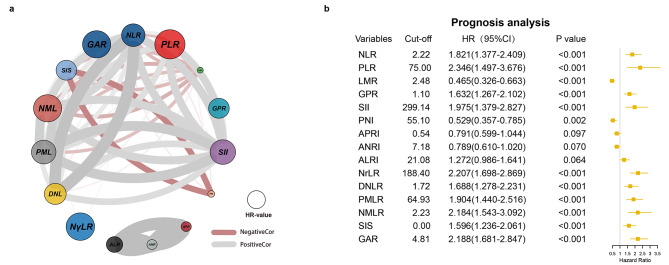
Figure 2a was a circle correlation plot which illustrates the correlation among those inflammatory markers. Figure 2a also shows the influence of these markers on prognosis with their hazard ratio (HR) on OS. In Fig. 2a, link lines in two colors reflected the positive or negative correlation in 15 different inflammatory markers. The correlation coefficient values were positively correlated with the thickness of the lines. Moreover, the size of circles also reflected the HR values, which implied that the larger the circle, the greater the impact on OS.
Fig. 2.
Circle correlation plot showing the relationship among the fifteen inflammatory markers (a). Cut-off values of fifteen inflammatory markers and the univariable Cox prognosis analysis of those markers for OS (b)
Through the ROC curves, we found that the best cut-off values of the 15 inflammatory markers were 2.22, 75.00, 2.48, 1.10, 299.14, 55.10, 0.54, 7.18, 21.08, 188.40, 1.72, 64.93, 2.23, 0.00, and 4.81, respectively (Fig. 2b). After univariate Cox regression analysis, we found that 12 markers were significantly associated with the prognosis of patients with ICC in training cohort. A higher level of NLR (HR = 1.821, 95% confidence interval [CI]: =1.377–2.409), PLR (2.346, 1.497–3.676), GPR (1.632, 1.267–2.102), SII (1.975, 1.379–2.827), NγLR (2.207, 1.698–2.869), dNLR (1.688, 1.278–2.231), PMLR (1.904, 1.440–2.516), NMLR (2.184, 1.543–3.092), SIS (1.596, 1.236–2.061), and GAR (2.188, 1.681–2.847) were risk factors of OS, and a higher level of LMR (0.465, 0.326–0.663) and PNI (0.529, 0.357–0.785) were protective factors of OS. However, APRI, ANRI, and ALRI had no influence on OS after Cox regression analyses. Therefore, we scored every patient using the above 12 positive inflammatory markers, as illustrated in Table 1, according to the marker values and their corresponding coefficients. First, we calculated the median coefficient value of the 12 inflammatory markers for a value of 0.662. Second, we used every marker’s coefficient value to divide 0.662 and then rounded it to the whole number (> 0.5 scored 1 point, < 0.5 scored 0 point) to obtain the inflammatory coefficient. Third, a patient was scored 1 point if his or her inflammatory marker > cut-off value; otherwise, 0 points were scored. Finally, we used inflammatory-coefficient times 0 or 1 for each marker and summed the total points for every patient to form IFS.
Table 1.
Cut-off value and IFS in 15 inflammatory markers
| No. | Variables | Cut-off | HR | Coefficient | Median coefficient† | Inflammatory-coefficient* | Cut-off score†† | IFS |
|---|---|---|---|---|---|---|---|---|
| 1 | NLR | 2.22 | 1.821 | 0.6 | 0.662 | 1 | 0 or 1 | 1 or 0 |
| 2 | PLR | 75 | 2.346 | 0.853 | 0.662 | 1 | 0 or 1 | 1 or 0 |
| 3 | LMR | 2.48 | 0.465 | -0.766 | 0.662 | -1 | 0 or 1 | -1 or 0 |
| 4 | GPR | 1.1 | 1.603 | 0.472 | 0.662 | 1 | 0 or 1 | 1 or 0 |
| 5 | SII | 299.14 | 1.975 | 0.68 | 0.662 | 1 | 0 or 1 | 1 or 0 |
| 6 | PNI | 55.1 | 0.529 | -0.636 | 0.662 | -1 | 0 or 1 | -1 or 0 |
| 7 | APRI | 0.54 | 0.791 | -0.235 | - | - | - | - |
| 8 | ANRI | 7.18 | 0.789 | -0.238 | - | - | - | - |
| 9 | ALRI | 21.08 | 1.272 | 0.241 | - | - | - | - |
| 10 | NrLR | 188.4 | 2.207 | 0.792 | 0.662 | 1 | 0 or 1 | 1 or 0 |
| 11 | dNLR | 1.72 | 1.688 | 0.524 | 0.662 | 1 | 0 or 1 | 1 or 0 |
| 12 | PMLR | 64.93 | 1.904 | 0.644 | 0.662 | 1 | 0 or 1 | 1 or 0 |
| 13 | NMLR | 2.23 | 2.184 | 0.781 | 0.662 | 1 | 0 or 1 | 1 or 0 |
| 14 | SIS | 0 | 1.596 | 0.468 | 0.662 | 1 | 0 or 1 | 1 or 0 |
| 15 | GAR | 4.81 | 2.188 | 0.783 | 0.662 | 1 | 0 or 1 | 1 or 0 |
†† Median coefficient: calculated based on the 12 inflammatory markers (NLR, PLR, LMR, GPR, SII, PNI, NγLR, dNLR, PMLR, NMLR, SIS, and GAR)
*Inflammatory-coefficient: every marker’ s coefficient value is used to divide 0.662 and then be rounded to the whole number (> 0.5 scored 1 point, < 0.5 scored 0 point)
† Cut-off score: if the value of an inflammatory marker over its cut-off value, we score 1 point, otherwise, we score 0 point
IFS: inflammatory-coefficient times cut-off score
Patients were divided into three groups according to IFS
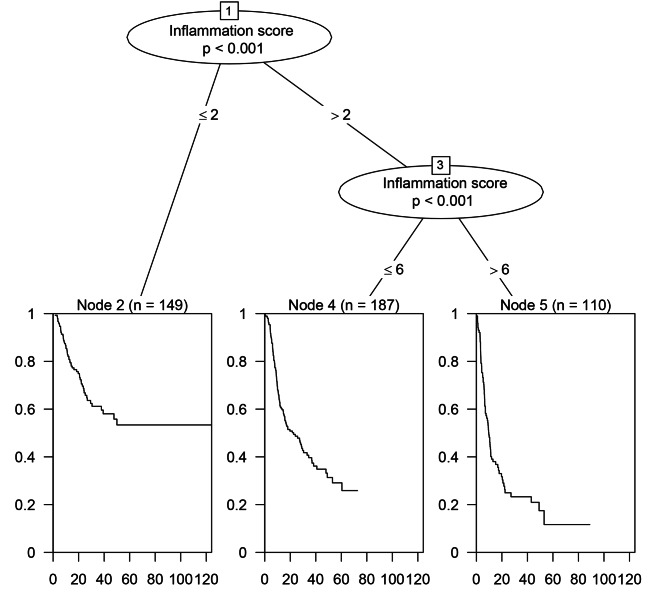
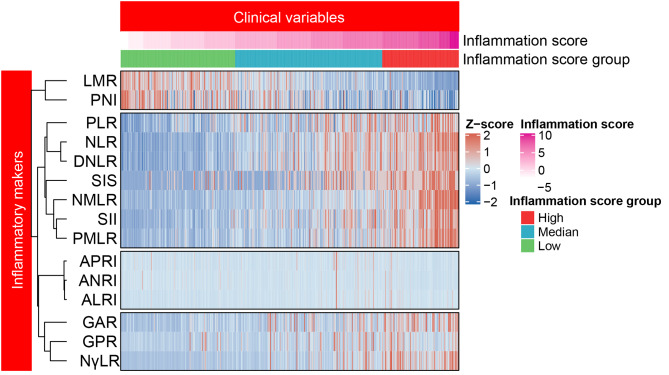
As shown in Fig. 3, the OS survival tree divided the patients in training cohort into three groups with different IFS levels. The best two cut-off values were 2 and 6 points, respectively. As a result, patients with IFS ≤ 2 were assigned to the low-IFS-score group (n = 149), > 6 were assigned to the high-IFS-score group (n = 110), and the remaining patients were assigned to the median-IFS-score group (n = 187). Additionally, Fig. 4 demonstrates the heatmap showing the distribution of the values of the 15 inflammatory markers in the training cohort according to the different IFS values and different IFS groups. On the vertical axis, 15 types of inflammatory markers could be clustered into five categories.
Fig. 3.
Survival tree analysis of OS in the training cohort and 2 point and 6 point were considered to be two best cut-off values
Fig. 4.
Heatmap showing the distribution of 15 inflammatory markers’ values in the training cohort according to the different IFS values and different IFS groups
Baseline characteristics of the three groups in the training cohort
Table 2 shows the basic clinicopathological characteristics of three groups in the training cohort. As shown in Tables 2 and 149 patients were in the low-IFS-score group (33.4%), 187 patients were in the median-IFS-score group (41.9%), and 110 patients were in the high-IFS-score group (24.7%).
Table 2.
Baseline clinicopathological characteristics of the three groups in the training cohort
| Variables | level | Low IFS score | Median IFS score | High IFS score | P value |
|---|---|---|---|---|---|
| N = 149 | N = 187 | N = 110 | |||
| Age, year (mean ± SD) | 54.36 ± 10.71 | 53.03 ± 10.67 | 55.08 ± 9.18 | 0.220 | |
| Sex (%) | female | 52 (34.9) | 65 (34.8) | 34 (30.9) | 0.753 |
| male | 97 (65.1) | 122 (65.2) | 76 (69.1) | ||
| Hepatolithiasis (%) | no | 135 (90.6) | 172 (92.0) | 99 (90.0) | 0.826 |
| yes | 14 (9.4) | 15 (8.0) | 11 (10.0) | ||
| Diabetes (%) | no | 139 (93.3) | 175 (93.6) | 103 (93.6) | 0.992 |
| yes | 10 (6.7) | 12 (6.4) | 7 (6.4) | ||
| HBsAg (+) (%) | no | 66 (44.3) | 114 (61.0) | 65 (59.1) | 0.006 |
| yes | 83 (55.7) | 73 (39.0) | 45 (40.9) | ||
| Anti-HCV (%) | no | 144 (96.6) | 187 (100.0) | 109 (99.1) | 0.027 |
| yes | 5 (3.4) | 0 (0.0) | 1 (0.9) | ||
| Child-Pugh grade (%) | A | 147 (98.7) | 184 (98.4) | 104 (94.5) | 0.066 |
| B | 2 (1.3) | 3 (1.6) | 6 (5.5) | ||
| TBIL, mg/dL* | 12.60 [9.80, 15.40] | 12.00 [9.20, 16.05] | 12.45 [8.95, 16.32] | 0.563 | |
| ALT, IU/L* | 26.00 [17.00, 39.10] | 25.70 [17.30, 40.70] | 28.20 [17.07, 45.43] | 0.786 | |
| AST, IU/L* | 25.00 [19.00, 32.00] | 27.50 [20.55, 38.80] | 28.10 [20.00, 41.77] | 0.041 | |
| GGT, IU/L* | 45.00 [30.00, 71.00] | 74.00 [47.50, 140.50] | 168.00 [97.75, 275.75] | < 0.001 | |
| HgB, g/L (mean ± SD) | 138.45 ± 16.45 | 133.45 ± 17.73 | 128.15 ± 18.28 | < 0.001 | |
| PT, second* | 11.60 [11.10, 12.20] | 11.60 [11.00, 12.10] | 11.80 [11.20, 12.30] | 0.125 | |
| ALB, g/L (mean ± SD) | 43.69 ± 3.60 | 42.51 ± 3.40 | 40.58 ± 4.03 | < 0.001 | |
| PLT, ×109/L* | 172.00 [132.00, 219.00] | 195.00 [147.00, 240.50] | 219.50 [175.00, 297.50] | < 0.001 | |
| WBC, ×109/L* | 5.54 [4.64, 6.97] | 6.26 [5.21, 7.58] | 7.38 [6.44, 9.34] | < 0.001 | |
| AFP, µg/L* | 3.90 [2.40, 8.50] | 3.50 [2.30, 8.75] | 4.05 [2.42, 12.42] | 0.548 | |
| CEA, µg/L* | 2.30 [1.40, 3.50] | 2.60 [1.50, 5.15] | 3.25 [1.70, 9.38] | 0.004 | |
| CA199, IU/mL* | 25.60 [13.30, 49.40] | 56.90 [14.80, 206.30] | 117.10 [27.62, 1000.00] | < 0.001 | |
| Perioperative transfusion (%) | no | 135 (90.6) | 157 (84.0) | 85 (77.3) | 0.013 |
| yes | 14 (9.4) | 30 (16.0) | 25 (22.7) | ||
| Anatomic resection (%) | no | 32 (21.5) | 27 (14.4) | 22 (20.0) | 0.213 |
| yes | 117 (78.5) | 160 (85.6) | 88 (80.0) | ||
| Major resection (%) | no | 124 (83.2) | 131 (70.1) | 71 (64.5) | 0.002 |
| yes | 25 (16.8) | 56 (29.9) | 39 (35.5) | ||
| Resection margin ≥ 10 mm (%) | yes | 21 (14.1) | 20 (10.7) | 6 (5.5) | 0.081 |
| no | 128 (85.9) | 167 (89.3) | 104 (94.5) | ||
| Cirrhosis (%) | no | 107 (71.8) | 150 (80.2) | 95 (86.4) | 0.015 |
| yes | 42 (28.2) | 37 (19.8) | 15 (13.6) | ||
| Tumor diameter, cm (mean ± SD) | 5.41 ± 2.47 | 7.04 ± 2.81 | 8.08 ± 2.92 | < 0.001 | |
| Multiple tumors (%) | no | 142 (95.3) | 178 (95.2) | 105 (95.5) | 0.994 |
| yes | 7 (4.7) | 9 (4.8) | 5 (4.5) | ||
| Satellite nodules (%) | no | 122 (81.9) | 135 (72.2) | 67 (60.9) | 0.001 |
| yes | 27 (18.1) | 52 (27.8) | 43 (39.1) | ||
| Tumor capsule (%) | no | 133 (89.3) | 171 (91.4) | 106 (96.4) | 0.282 |
| complete | 10 (6.7) | 8 (4.3) | 2 (1.8) | ||
| incomplete | 6 (4.0) | 8 (4.3) | 2 (1.8) | ||
| Tumor differentiation (%) | low | 7 (4.7) | 8 (4.3) | 7 (6.4) | 0.655 |
| median | 140 (94.0) | 178 (95.2) | 103 (93.6) | ||
| high | 2 (1.3) | 1 (0.5) | 0 (0.0) | ||
| Adjacent tissue invasion (%) | no | 145 (97.3) | 177 (94.7) | 98 (89.1) | 0.019 |
| yes | 4 (2.7) | 10 (5.3) | 12 (10.9) | ||
| MVI (%) | no | 141 (94.6) | 163 (87.2) | 94 (85.5) | 0.030 |
| yes | 8 (5.4) | 24 (12.8) | 16 (14.5) | ||
| LNM (%) | no | 140 (94.0) | 158 (84.5) | 78 (70.9) | < 0.001 |
| yes | 9 (6.0) | 29 (15.5) | 32 (29.1) |
Abbreviations: HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; TBIL, total bilirubin; ALB, albumin; ALT, alanine transaminase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase; PT, prothrombin time; PLT, platelet; AFP, alpha-fetoprotein; CEA, carcinoembryonic antigen; CA19-9, carbonhydrateantigen19-9; MVI, microvascular invasion; LNM, lymph node metastasis
* Quantitative variables shown with median and interquartile range (IQR)
When we compared the differences in characteristics, we found that major resection (P = 0.002), perioperative transfusion (P = 0.013), HBsAg (+) (P = 0.006), anti-HCV (P = 0.027), satellite nodules (P = 0.001), cirrhosis (P = 0.015), lymph node metastasis (LNM) (P < 0.001), MVI (P = 0.030), adjacent tissue invasion (P = 0.019), AST (P = 0.041), GGT (P < 0.001), HgB (P < 0.001), ALB (P < 0.001), PLT (P < 0.001), WBC (P < 0.001), CEA (P = 0.004), CA199 (P < 0.001), and tumor diameter (P < 0.001) were significantly different among the three groups.
Survival analysis
The KM curves in Fig. 5a and b showed that significantly different OS and RFS rates were observed among the three groups (both P < 0.0001). The postoperative 1-, 3-, and 5-year OS rates of the low-IFS group (88.6%, 67.7%, and 60.7%, respectively) were significantly higher than those of the median-IFS group (59.9%, 37.1%, and 21.5%, respectively), and the high-IFS group (34.9%, 20.9%, and 9.2%, respectively). Similarly, the postoperative 1-, 3-, and 5-year RFS rates of the low-IFS group (69.9%, 42.9%, and 36.9%, respectively) were significantly higher than those of the median-IFS group (39.8%, 20.2%, and 12.4%, respectively), and the high-IFS group (27.6%, 9.2%, and 3.9%, respectively).
Fig. 5.
KM curves of OS and RFS for patients with ICC in the training cohort (a and b). ROC curves of IFS system (c), and the time-dependent ROC curves for IFS system and other clinical staging systems in the training cohorts (d)
Independent prognostic factors for OS and RFS
The variables listed in Table 2 were included into the Cox regression analysis. All significant covariates (P < 0.050) in the univariate Cox analysis were included in the multivariable Cox analysis. Table 3 shows the results of univariate and multivariate analyses results for OS. We found that anatomic resection (HR = 1.578, 95%CI = 1.085–2.295, P = 0.017), high level of CA19-9 (HR = 1.000, 95%CI = 1.0001–1.001, P = 0.010), satellite nodules (HR = 1.448, 95%CI = 1.095–1.915, P = 0.009), LNM (HR = 1.422, 95%CI = 1.038–1.949, P = 0.028), MVI (HR = 1.014, 95%CI = 1.101–2.291, P = 0.014), and high-IFS group (HR = 2.106, 95%CI = 1.682–2.637, P < 0.001) were independent risk factors for OS.
Table 3.
Univariable and multivariable analyses for OS in the training cohort
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95%CI | P value | HR | 95%CI | P value |
| Age, year | 1.005 | 0.993–1.017 | 0.453 | |||
| Sex, male vs. female | 1.055 | 0.808–1.377 | 0.694 | |||
| Hepatolithiasis, yes vs. no | 1.266 | 0.824–1.945 | 0.282 | |||
| Diabetes, yes vs. no | 0.921 | 0.555–1.530 | 0.751 | |||
| HBsAg (+), yes vs. no | 0.816 | 0.633–1.052 | 0.116 | |||
| Anti-HCV, yes vs. no | 0.749 | 0.239–2.343 | 0.619 | |||
| Child-Pugh grade, B vs. A | 0.446 | 0.143–1.394 | 0.165 | |||
| TBIL, mg/dL | 0.999 | 0.993–1.005 | 0.761 | |||
| ALT, IU/L | 1.001 | 0.999–1.002 | 0.081 | |||
| AST, IU/L | 1.001 | 1.000-1.002 | 0.042 | |||
| GGT, IU/L | 1.001 | 1.000-1.002 | 0.011 | |||
| HgB, g/L | 0.991 | 0.984–0.998 | 0.009 | |||
| PT, second | 1.058 | 0.956–1.172 | 0.274 | |||
| ALB, g/L | 0.950 | 0.92–0.981 | 0.002 | |||
| PLT, ×109/L | 1.002 | 1.000-1.003 | 0.034 | |||
| WBC, ×109/L | 1.075 | 1.014–1.139 | 0.015 | |||
| AFP, µg/L | 1.000 | 0.999–1.001 | 0.615 | |||
| CEA, µg/L | 1.000 | 0.999–1.001 | 0.142 | |||
| CA19-9, IU/mL | 1.001 | 1.000-1.002 | < 0.001 | 1.000 | 1.0001–1.001 | 0.010 |
| Perioperative transfusion, yes vs. no | 1.354 | 0.974–1.883 | 0.071 | |||
| Anatomic resection, yes vs. no | 1.704 | 1.175–2.472 | 0.005 | 1.578 | 1.085–2.295 | 0.017 |
| Major resection, yes vs. no | 1.553 | 1.186–2.033 | 0.001 | |||
| Resection margin < 10 mm, yes vs. no | 1.833 | 1.134–2.963 | 0.012 | |||
| Cirrhosis, yes vs. no | 0.854 | 0.623–1.171 | 0.327 | |||
| Tumor diameter, cm | 1.117 | 1.074–1.162 | < 0.001 | |||
| Multiple tumors, yes vs. no | 0.816 | 0.433–1.538 | 0.530 | |||
| Satellite nodules, yes vs. no | 1.997 | 1.537–2.595 | < 0.001 | 1.448 | 1.095–1.915 | 0.009 |
| Tumor capsule, yes vs. no | 0.620 | 0.421–0.914 | 0.016 | |||
|
Tumor differentiation, high vs. median vs. low |
1.410 | 0.809–2.458 | 0.225 | |||
| Adjacent tissue invasion, yes vs. no | 1.570 | 0.944–2.611 | 0.082 | |||
| MVI, yes vs. no | 1.794 | 1.254–2.566 | 0.001 | 1.587 | 1.101–2.291 | 0.014 |
| LNM, yes vs. no | 2.179 | 1.618–2.935 | < 0.001 | 1.422 | 1.038–1.949 | 0.028 |
| IFS, high vs. median vs. low | 2.320 | 1.955–2.753 | < 0.001 | 2.106 | 1.682–2.637 | < 0.001 |
Abbreviations: HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; TBIL, total bilirubin; ALB, albumin; ALT, alanine transaminase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase; PT, prothrombin time; PLT, platelet; AFP, alpha-fetoprotein; CEA, carcinoembryonic antigen; CA19-9, carbonhydrateantigen19-9; MVI, microvascular invasion; LNM, lymph node metastasis; IFS, inflammation score
Additionally, Table 4 shows the results of univariate and multivariate Cox analyses for RFS. Multivariate analyses identified high level of CA19-9 (HR = 1.001, 95%CI = 1.000-1.008, P = 0.011), larger tumor diameter (HR = 1.059, 95%CI = 1.015–1.105, P = 0.008), satellite nodules (HR = 1.419, 95%CI = 1.098–1.834, P = 0.007), LNM (HR = 1.406, 95%CI = 1.040–1.901, P = 0.027), MVI (HR = 1.664, 95%CI = 1.182–2.342, P = 0.003), and high-IFS group (HR = 1.572, 95%CI = 1.322–1.87, P < 0.001) were independent risk factors for RFS.
Table 4.
Univariable and multivariable analyses for RFS in the training cohort
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95%CI | P value | HR | 95%CI | P value |
| Age, year | 0.997 | 0.986–1.008 | 0.586 | |||
| Sex, male vs. female | 0.938 | 0.745–1.180 | 0.583 | |||
| Hepatolithiasis, yes vs. no | 0.995 | 0.675–1.467 | 0.980 | |||
| Diabetes, yes vs. no | 0.770 | 0.479–1.240 | 0.283 | |||
| HBsAg (+), yes vs. no | 0.859 | 0.688–1.071 | 0.177 | |||
| Anti-HCV, yes vs. no | 0.313 | 0.078–1.257 | 0.101 | |||
| Child-Pugh grade, B vs. A | 0.754 | 0.374–1.523 | 0.432 | |||
| TBIL, mg/dL | 1.001 | 0.997–1.004 | 0.655 | |||
| ALT, IU/L | 0.999 | 0.996–1.001 | 0.335 | |||
| AST, IU/L | 1.000 | 0.998–1.001 | 0.656 | |||
| GGT, IU/L | 1.000 | 0.999–1.001 | 0.070 | |||
| HgB, g/L | 0.994 | 0.988-1.000 | 0.049 | |||
| PT, second | 1.036 | 0.940–1.141 | 0.479 | |||
| ALB, g/L | 0.986 | 0.959–1.013 | 0.311 | |||
| PLT, ×109/L | 1.002 | 1.000-1.003 | 0.037 | |||
| WBC, ×109/L | 1.064 | 1.010–1.122 | 0.019 | |||
| AFP, µg/L | 1.001 | 0.999–1.002 | 0.151 | |||
| CEA, µg/L | 1.000 | 0.999–1.001 | 0.110 | |||
| CA19-9, IU/mL | 1.001 | 1.0006–1.0012 | < 0.001 | 1.001 | 1.000-1.008 | 0.011 |
| Perioperative transfusion, yes vs. no | 1.152 | 0.857–1.548 | 0.348 | |||
| Anatomic resection, yes vs. no | 1.289 | 0.966–1.720 | 0.085 | |||
| Major resection, yes vs. no | 1.299 | 1.017–1.658 | 0.036 | |||
| Resection margin < 10 mm, yes vs. no | 1.439 | 0.987–2.096 | 0.057 | |||
| Cirrhosis, yes vs. no | 0.788 | 0.596–1.043 | 0.096 | |||
| Tumor diameter, cm | 1.117 | 1.079–1.158 | < 0.001 | 1.059 | 1.015–1.105 | 0.008 |
| Multiple tumors, yes vs. no | 1.039 | 0.637–1.694 | 0.879 | |||
| Satellite nodules, yes vs. no | 1.970 | 1.557–2.494 | < 0.001 | 1.419 | 1.098–1.834 | 0.007 |
| Tumor capsule, yes vs. no | 0.750 | 0.568–0.989 | 0.042 | |||
|
Tumor differentiation, high vs. median vs. low |
1.019 | 0.662–1.569 | 0.93 | |||
| Adjacent tissue invasion, yes vs. no | 1.810 | 1.160–2.825 | 0.009 | |||
| MVI, yes vs. no | 1.955 | 1.400–2.730 | < 0.001 | 1.664 | 1.182–2.342 | 0.003 |
| LNM, yes vs. no | 1.998 | 1.511–2.643 | < 0.001 | 1.406 | 1.040–1.901 | 0.027 |
| IFS, high vs. median vs. low | 1.857 | 1.603–2.153 | < 0.001 | 1.572 | 1.322–1.87 | < 0.001 |
Abbreviations: HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; TBIL, total bilirubin; ALB, albumin; ALT, alanine transaminase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase; PT, prothrombin time; PLT, platelet; AFP, alpha-fetoprotein; CEA, carcinoembryonic antigen; CA19-9, carbonhydrateantigen19-9; MVI, microvascular invasion; LNM, lymph node metastasis; IFS, inflammation score
Comparison of different scoring systems
The ROC curve of IFS demonstrated in Fig. 5c had an AUC value of 0.703 (95%CI = 0.658–0.748), which suggested that the IFS system had a good prognosis predictive performance for OS in the training cohort.
Time-ROC curves were established to compare the performance of the IFS system with the other three scoring systems (Fig. 5d), which showed that the AUC value of the IFS from 0 to 60 months was better than that of the other three scoring systems in the training cohort. The AIC value of the IFS was also obviously lower than others in the training cohort. The comparison results are listed in Table 5, and we found that the survival predictive ability of the IFS was significantly better than that of the Child-Pugh stage (P < 0.0001), AJCC 7th TNM stage (P < 0.0001), and AJCC 8th TNM stage (P < 0.0001).
Table 5.
Comparison of the IFS with other clinical staging systems in the training cohort
| Clinical staging systems | C-index (95%CI) | Change (95%CI) | P value | AIC value |
|---|---|---|---|---|
| IFS | 0.684 (0.654–0.714) | 2646.467 | ||
| AJCC 7th TNM Stage | 0.607 (0.574–0.641) | -0.077 (-0.111 — -0.035) | < 0.0001 | 2701.532 |
| AJCC 8th TNM Stage | 0.614 (0.581–0.648) | -0.070 (-0.106 — -0.027) | 0.0005 | 2697.023 |
| Child-Pugh Stage | 0.505 (0.494–0.516) | -0.179 (-0.212 — -0.144) | < 0.0001 | 2740.608 |
Abbreviation: AJCC: American Joint Committee on Cancer; AIC: Akaike information criterion
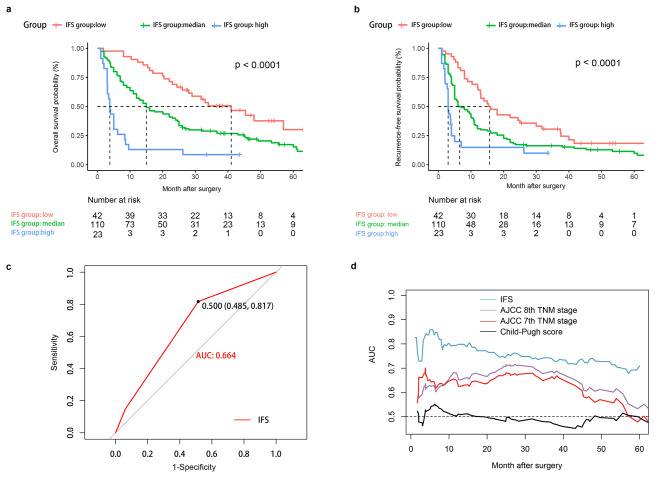
The validation of IFS system
The internal validation cohort consisted of 175 patients, and the basic clinicopathologic characteristics were listed in Table 6. KM curves illustrated that patients in the high-IFS group presented with poorer OS and RFS rates compared with those in the median-IFS and low-IFS groups. The 1-, 3-, and 5-year OS rates were 13.0%, 7.8%, and 0.0% for the high-IFS group, 60.9%, 28.6%, and 16.8% for the median-IFS group, and 90.5%, 51.1%, and 32.3% for the low-IFS group, respectively (P < 0.0001). Moreover, the 1-, 3-, and 5-year RFS rates were 15.5%, 7.8%, and 0.0% for the high-IFS group, 33.6%, 16.3%, and 11.4% for the median-IFS group, and 69.0%, 30.3%, and 17.6% for the low-IFS group (P < 0.0001) (Fig. 6a and b).
Table 6.
Baseline clinicopathological characteristics of the three groups in the internal validation cohort
| Variables | level | Low IFS score | Median IFS score | High IFS score | P value |
|---|---|---|---|---|---|
| N = 42 | N = 110 | N = 23 | |||
| Age, year (mean ± SD) | 58.12 ± 9.78 | 56.56 ± 10.68 | 58.87 ± 10.80 | 0.521 | |
| Sex (%) | female | 14 (33.3) | 34 (30.9) | 6 (26.1) | 0.833 |
| male | 28 (66.7) | 76 (69.1) | 17 (73.9) | ||
| Hepatolithiasis (%) | no | 42 (100.0) | 106 (96.4) | 22 (95.7) | 0.436 |
| yes | 0 (0.0) | 4 (3.6) | 1 (4.3) | ||
| Diabetes (%) | no | 38 (90.5) | 101 (91.8) | 22 (95.7) | 0.758 |
| yes | 4 (9.5) | 9 (8.2) | 1 (4.3) | ||
| HBsAg (+) (%) | no | 22 (52.4) | 67 (60.9) | 15 (65.2) | 0.526 |
| yes | 20 (47.6) | 43 (39.1) | 8 (34.8) | ||
| Anti-HCV (%) | no | 42 (100.0) | 110 (100.0) | 22 (95.7) | 0.036 |
| yes | 0 (0.0) | 0 (0.0) | 1 (4.3) | ||
| Child-Pugh grade (%) | A | 37 (88.1) | 104 (94.5) | 17 (73.9) | 0.027 |
| B | 2 (4.8) | 4 (3.6) | 4 (17.4) | ||
| TBIL, mg/dL* | 12.45 [10.03, 16.17] | 11.90 [9.83, 14.35] | 10.30 [8.85, 12.25] | 0.153 | |
| ALT, IU/L* | 18.50 [14.00, 25.90] | 21.90 [14.00, 31.08] | 28.00 [15.75, 46.00] | 0.151 | |
| AST, IU/L* | 22.15 [17.00, 27.88] | 22.95 [17.25, 31.75] | 31.80 [23.05, 42.00] | 0.012 | |
| GGT, IU/L* | 49.00 [28.25, 86.25] | 73.50 [38.25, 154.75] | 92.00 [55.00, 199.00] | 0.079 | |
| HgB, g/L (mean ± SD) | 136.05 ± 15.16 | 134.75 ± 13.86 | 124.65 ± 18.64 | 0.007 | |
| PT, second* | 11.55 (1.17) | 11.65 (0.90) | 12.22 (1.19) | 0.027 | |
| ALB, g/L (mean ± SD) | 42.69 ± 5.27 | 42.36 ± 3.37 | 40.00 ± 3.78 | 0.021 | |
| PLT, ×109/L* | 162.00 [133.50, 199.00] | 186.50 [146.25, 242.50] | 185.00 [136.50, 223.00] | 0.054 | |
| WBC, ×109/L* | 5.98 [4.66, 6.82] | 6.08 [5.03, 7.46] | 6.13 [5.04, 8.27] | 0.428 | |
| AFP, µg/L* | 3.50 [1.90, 6.05] | 3.05 [2.12, 5.70] | 6.60 [2.40, 21.00] | 0.208 | |
| CEA, µg/L* | 2.30 [1.40, 3.65] | 2.90 [2.00, 6.10] | 3.30 [1.55, 4.70] | 0.024 | |
| CA199, IU/mL* | 36.45 [18.35, 124.85] | 69.75 [24.20, 670.12] | 45.20 [16.65, 171.60] | 0.104 | |
| Perioperative transfusion (%) | no | 37 (88.1) | 96 (87.3) | 19 (82.6) | 0.804 |
| yes | 5 (11.9) | 14 (12.7) | 4 (17.4) | ||
| Anatomic resection (%) | no | 30 (71.4) | 71 (64.5) | 13 (56.5) | 0.472 |
| yes | 12 (28.6) | 39 (35.5) | 10 (43.5) | ||
| Major resection (%) | no | 30 (71.4) | 74 (67.3) | 18 (78.3) | 0.559 |
| yes | 12 (28.6) | 36 (32.7) | 5 (21.7) | ||
| Cirrhosis (%) | no | 31 (73.8) | 89 (80.9) | 15 (65.2) | 0.223 |
| yes | 11 (26.2) | 21 (19.1) | 8 (34.8) | ||
| Tumor diameter, cm (mean ± SD) | 5.55 ± 3.54 | 6.58 ± 3.60 | 8.02 ± 3.76 | 0.031 | |
| Multiple tumors (%) | no | 34 (81.0) | 70 (63.6) | 18 (78.3) | 0.073 |
| yes | 8 (19.0) | 40 (36.4) | 5 (21.7) | ||
| Satellite nodules (%) | no | 27 (64.3) | 58 (52.7) | 16 (69.6) | 0.203 |
| yes | 15 (35.7) | 52 (47.3) | 7 (30.4) | ||
| Tumor capsule (%) | no | 40 (95.2) | 100 (90.9) | 21 (91.3) | 0.747 |
| complete | 1 (2.4) | 7 (6.4) | 2 (8.7) | ||
| incomplete | 1 (2.4) | 3 (2.7) | 0 (0.0) | ||
| Tumor differentiation (%) | low | 3 (7.1) | 9 (8.2) | 3 (13.0) | 0.466 |
| median | 37 (88.1) | 96 (87.3) | 17 (73.9) | ||
| high | 2 (4.8) | 5 (4.5) | 3 (13.0) | ||
| Adjacent tissue invasion (%) | no | 40 (95.2) | 105 (95.5) | 20 (87.0) | 0.267 |
| yes | 2 (4.8) | 5 (4.5) | 3 (13.0) | ||
| MVI (%) | no | 35 (83.3) | 88 (80.0) | 21 (91.3) | 0.425 |
| yes | 7 (16.7) | 22 (20.0) | 2 (8.7) | ||
| LNM (%) | no | 40 (95.2) | 96 (87.3) | 19 (82.6) | 0.242 |
| yes | 2 (4.8) | 14 (12.7) | 4 (17.4) |
Abbreviations: HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; TBIL, total bilirubin; ALB, albumin; ALT, alanine transaminase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase; PT, prothrombin time; PLT, platelet; AFP, alpha-fetoprotein; CEA, carcinoembryonic antigen; CA19-9, carbonhydrateantigen19-9; MVI, microvascular invasion; LNM, lymph node metastasis
* Quantitative variables shown with median and interquartile range (IQR)
Fig. 6.
KM curves of OS and RFS for patients with ICC in the internal validation cohort (a and b). ROC curves of IFS system (c), and the time-dependent ROC curves for IFS system and other clinical staging systems in the internal validation cohort (d)
The ROC curve of IFS in the internal validation cohort was shown in Fig. 6c, and the AUC value was 0.664 (95%CI = 0.569–0.760). Moreover, we also compared the prognosis predictive performance of several scoring systems through time–ROC curves, which showed that the survival predictive ability of IFS was still better than that of the Child-Pugh stage, AJCC 7th TNM stage, and AJCC 8th TNM stage (Fig. 6d).
Discussion
In this study, we developed a new inflammatory score system, abbreviated as the IFS system based on the weight of different inflammatory markers impact on OS in the training cohort, to make a prognostic prediction for ICC after surgery. When patients were divided into high-, median-, and low-IFS groups according to different IFS levels, we found that patients in the high-IFS group had worse OS and RFS rates than those in the median- and low-IFS groups, and the same outcomes were observed in the internal validation cohort. In addition, we found that a high IFS score was an independent risk factor for OS and RFS after Cox regression analyses in the training cohort. More importantly, the IFS score had a good performance of prognostic prediction in ICC with an AUC value of 0.703 and 0.664 in the training and internal validation cohorts, respectively, which were better than those of other scoring systems. In summary, our newly constructed IFS system is a good prognostic predictor for ICC, and a high IFS is significantly associated with worse OS and RFS in patients after hepatectomy.
Extensive studies have revealed that inflammatory responses play decisive roles in tumor development and progression [24]. Over time, an increasing number of inflammation-related indicators have been constructed to serve as predictors of survival after therapies in many tumors. SIS, PNI, NLR, PLR, and Glasgow prognostic score (GPS) are all markers that have been proven to have good prognostic predictive abilities in malignant liver disease [25, 26]. However, most studies used only one or two inflammatory markers in their studies to predict survival in patients with ICC. Inconsistent with these findings, we are the first to use 12 common inflammatory markers as an integrated indicator (known as IFS) to predict survival for patients with ICC. Additionally, the establishment of IFS is based on the weight of impact of every inflammatory marker on OS so that we could guarantee that the scoring system is more reasonable and comprehensive.
In our study, we included 15 inflammatory markers and finally selected 12 markers, including NLR, PLR, LMR, GPR, SII, PNI, NγLR, dNLR, PMLR, NMLR, SIS, and GAR, that were associated with survival. We then used them to build an IFS system. Those markers are a series of scoring systems that can reflect the inflammation-related characteristics of ICC. Previous studies have found that about 25% of cancers are associated with chronic inflammation, which is caused by infections or inflammatory conditions, such as hepatitis infection or prostatitis. Moreover, the existence of inflammatory cells or mediators not only participates in promotion of angiogenesis, extracellular matrix restructuring, and pre-metastatic niche formation, but also can induce some non-epidemiological characteristics in tumors [27]. Detailed exploration of the forms of cancer-related inflammation is a useful approach to discover important prognostic markers for malignant tumors in daily clinical practice. Regulating the systemic inflammatory response might become an important therapeutic target for the tumor itself in future.
Recently, some studies have demonstrated the prognostic utility of NLR, PLR, and LMR in patients with ICC after surgery [26, 28, 29]. In our study, we found that elevated NLR and PLR were negative predictors of OS in the training cohort, while low LMR was a protective factor of OS, which was consistent with the aforementioned studies. In addition, PNI and γ‑glutamyl transferase-associated enzymes, such as GPR and GAR, have been reported to be associated with the prognosis of ICC [30–32]. Our study also revealed that GPA, GAR, and PNI were parameters that were significantly associated with the prognosis of ICC in the training cohort. As we know, a high level of GGT usually reflects the disease severity of bile tract disarrangement, and low PNI levels can reflect a patient’s nutritional status in ICC.
In 2019, Wang et al. reported that in early recurrent HCC, only the SII (P < 0.001) was an independent predictor for post-recurrence survival in multivariate analyses [33]. Another study also found that increased SII was associated with decreased survival (P < 0.01) in ICC [8]. The SII score is based on lymphocytes, neutrophils, and platelets. Known studies have demonstrated that neutrophils and platelets can promote tumor cell proliferation and metastasis [34, 35]. Lymphocytes can lead to cell apoptosis and suppress cancer cell proliferation, migration, and invasion [36]. All the above studies supported our findings that SII was a risk factor for ICC survival after surgery.
Li et al. found that in 724 patients with HCC undergoing curative resection, increased NγLR is an independent risk factor for OS and progression-free survival [37]. The univariate Cox analysis result in our study illustrated that NγLR could significantly predict worse survival in patients with ICC, so, it was included into the IFS system. Additionally, the dNLR is composed of only the WBC and neutrophil counts. Previous studies have found that elevated dNLR is a negative factor of survival in HCC, pancreatic cancer, and colorectal cancer [38–41]. However, several studies also concluded that dNLR was not an independent risk factor for survival in renal cell carcinoma [42] and gastric cancer [43]. In our study, we found that a high dNLR level, which was a part of the IFS system, was a risk factor for OS in ICC.
In 2018, Liao et al. found that NMLR and PMLR were risk factors for OS and RFS in patients with HCC [44], which was consistent with ours in patients with ICC. More importantly, a previous study from China also found that NMLR and PMLR were associated with OS and RFS in gastric cancer [45]. NMLR and PMLR are inflammatory markers involved in various immune cells, which might reflect the synergistic effects or complex interaction between different immune cells in the tumor milieu. In 2019, another study from China found that the SIS, which is based on preoperative serum CA19-9 and LMR, was a powerful prognostic biomarker in ICC. In their study, Zhang et al. found that SIS was an independent risk factor for OS and TTR. Moreover, high SIS was significantly associated with aggressive tumor behavior, such as high TNM stage, large tumor size, multiple tumors, and LNM [20]. In our study, we found that SIS was a risk factor for OS in the training cohort, and IFS was an independent risk factor for OS and RFS in ICC.
Our study had several limitations. First, inflammatory marker such as C-reactive protein (CRP) was not included in this analysis because preoperative CRP is not a routine test in our institution, so we lack the data to complete the relevant statistical analysis. CRP is considered to have a higher sensitivity, but a poorer specificity, and CRP values are susceptible to many factors. Therefore, we believe that CRP is not suitable for being an excellent inflammatory marker parameter to predict the prognosis of patients with ICC. Second, collinearity exists on some inflammatory markers, which is a complex problem to avoid, especially including too many variates. In this study, we combined all the inflammatory markers for a single indicator (IFS) according to their prognostic weights to assess the role of inflammatory markers systematically and comprehensively. In this manner, the effect of covariance can be reduced to a minimum level. Third, we only used tumor differentiation instead of the histological type (mass-forming, periductal-infiltrating, intraductal-growing), which is a shortcoming of this study. Finally, it was a retrospective study. Further validation from more external cohorts is needed.
Conclusion
Collectively, the IFS established based on 12 common inflammatory markers in our study is a potentially powerful prognostic predictor in patients with ICC after curative resection. IFS is an independent risk factor for OS and RFS in ICC and has a more accurate predictive ability than the AJCC 7th edition, AJCC 8th edition, and Child-Pugh score, which underlines the necessity of considering IFS as an important prognostic factor for ICC in daily clinical practice.
Acknowledgements
Not applicable.
Abbreviations
- IFS
Inflammation score
- ICC
Intrahepatic cholangiocarcinoma
- KM
Kaplan-Meier
- OS
Overall survival
- RFS
Recurrence-free survival
- AUC
Area under the curve
- AIC
Akaike information criterion
- AJCC
American Joint Committee on Cancer
- HCC
Hepatocellular carcinoma
- SIS
Systemic inflammation score
- NLR
Neutrophil-to-lymphocyte ratio
- LMR
Lymphocyte to monocyte ratio
- ECOG
Eastern Cooperative Oncology Group
- CA19-9
Carbohydrate antigen 19–9
- CEA
Carcinoembryonic antigen
- AFP
Alpha-fetoprotein
- CT
Computerized tomography
- MRI
Magnetic resonance imaging
- MVI
Microvascular invasion
- NLR
Neutrophil-to-lymphocyte ratio
- PLR
Platelet-to-lymphocyte ratio
- LMR
Lymphocyte-to-monocyte
- GPR
Gamma‑glutamyl transpeptidase-to-platelet ratio
- SII
Systemic immune-inflammation index
- PNI
Prognostic nutritional index
- APRI
Aspartate aminotransferase-to-platelet ratio index
- ANRI
Aspartate aminotransferase-to-neutrophil ratio index
- ALRI
Aspartate aminotransferase-to-lymphocyte ratio index
- NγLR
Neutrophil times γ-GT]/lymphocyte
- dNLR
Derived neutrophil-to-lymphocyte ratio
- PMLR
Platelet‑monocyte‑lymphocyte ratio
- NMLR
Neutrophil‑monocyte‑lymphocyte ratio
- SIS
Inflammation score
- GAR
Globulin-to-albumin ratio
- CRP
C-reactive protein
- LNM
lymph node metastasis
- ROC
Receiver operating characteristic
Authors’ Contribution
Jun Fu, Qinjunjie Chen and Zisen Lai contributed to the study concept and design and drafted the manuscript. Kongying Lin and Yuzhen Gao analyzed the data, Guoxu Fang and Zongren Ding provided assistance with data collection. Yongyi Zeng contributed to manuscript revision. All the co-authors approved the final version of the manuscript before submission.
Funding
This study was supported by Science and Technology project of Fuzhou (Grant number: 2019-S-88).
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing Interests
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
The study protocol was performed in accordance with the guidelines outlined in the Declaration of Helsinki. The Institutional Review Board of Mengchao Hepatobiliary Hospital of Fujian Medical University approved the study (ID: 2018-048-01). The patients/participants provided their written informed consent.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jun Fu, Qinjunjie Chen and Zisen Lai contribute equally to this work.
References
- 1.Rahnemai-Azar AA, Weisbrod A, Dillhoff M, Schmidt C, Pawlik TM. Intrahepatic cholangiocarcinoma: molecular markers for diagnosis and prognosis. Surg Oncol. 2017;26(2):125–37. doi: 10.1016/j.suronc.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Van Dyke AL, Shiels MS, Jones GS, Pfeiffer RM, Petrick JL, Beebe-Dimmer JL, Koshiol J. Biliary tract cancer incidence and trends in the United States by demographic group, 1999–2013. Cancer. 2019;125(9):1489–98. doi: 10.1002/cncr.31942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60(6):1268–89. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 6.Chen Q, Li F, Zhong C, Zou Y, Li Z, Gao Y, Zou Q, Xia Y, Wang K, Shen F. Inflammation score system using preoperative inflammatory markers to Predict Prognosis for Hepatocellular Carcinoma after Hepatectomy: a Cohort Study. J Cancer. 2020;11(17):4947–56. doi: 10.7150/jca.45274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang T, Liu Z, Zhao X, Mao Z, Bai L. A novel prognostic score model based on combining systemic and hepatic inflammation markers in the prognosis of HBV-associated hepatocellular carcinoma patients. Artif Cells Nanomed Biotechnol. 2019;47(1):2246–55. doi: 10.1080/21691401.2019.1573174. [DOI] [PubMed] [Google Scholar]
- 8.Sellers CM, Uhlig J, Ludwig JM, Stein SM, Kim HS. Inflammatory markers in intrahepatic cholangiocarcinoma: Effects of advanced liver disease. Cancer Med. 2019;8(13):5916–29. doi: 10.1002/cam4.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe A, Harimoto N, Araki K, Kubo N, Igarashi T, Tsukagoshi M, Ishii N, Yamanaka T, Yoshizumi T, Shirabe K. Absolute Neutrophil Count predicts postoperative prognosis in Mass-forming Intrahepatic Cholangiocarcinoma. Anticancer Res. 2019;39(2):941–7. doi: 10.21873/anticanres.13197. [DOI] [PubMed] [Google Scholar]
- 10.Nie D, Gong H, Mao X, Li Z. Systemic immune-inflammation index predicts prognosis in patients with epithelial ovarian cancer: a retrospective study. Gynecol Oncol. 2019;152(2):259–64. doi: 10.1016/j.ygyno.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Shang X, Ren P, Gong L, Ahmed A, Ma Z, Ma R, Wu X, Xiao X, Jiang H, et al. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol. 2019;234(2):1794–802. doi: 10.1002/jcp.27052. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Tarocchi M, Polvani S, Marroncini G, Galli A. Molecular mechanism of hepatitis B virus-induced hepatocarcinogenesis. World J Gastroenterol. 2014;20(33):11630–40. doi: 10.3748/wjg.v20.i33.11630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakao Y, Yamashita YI, Arima K, Miyata T, Itoyama R, Yusa T, Umezaki N, Yamao T, Nakagawa S, Okabe H, et al. Clinical usefulness of Perioperative C-reactive Protein/Albumin ratio in patients with Intrahepatic Cholangiocarcinoma: a retrospective single institutional study. Anticancer Res. 2019;39(5):2641–6. doi: 10.21873/anticanres.13388. [DOI] [PubMed] [Google Scholar]
- 15.Fu J, Ding Z, Chen Q, Lin K, Liu H, Gao Y, Zeng Y, Li H, Shen F, Liu J. Distinct impacts of pre-operative antiviral treatment on post-operative outcomes of HBV-related Hepatocellular Carcinoma: a Landmark Analysis. J Cancer. 2021;12(1):170–80. doi: 10.7150/jca.47125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, Wan X, Liu G, Wu D, Shi L, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31(9):1188–95. doi: 10.1200/JCO.2012.41.5984. [DOI] [PubMed] [Google Scholar]
- 17.de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Clary BM, Aldrighetti L, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29(23):3140–5. doi: 10.1200/JCO.2011.35.6519. [DOI] [PubMed] [Google Scholar]
- 18.Cucchetti A, Cescon M, Ercolani G, Bigonzi E, Torzilli G, Pinna AD. A comprehensive meta-regression analysis on outcome of anatomic resection versus nonanatomic resection for hepatocellular carcinoma. Ann Surg Oncol. 2012;19(12):3697–705. doi: 10.1245/s10434-012-2450-z. [DOI] [PubMed] [Google Scholar]
- 19.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–9. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Shi SM, Yang H, Yang LX, Wang Z, Li XD, Yin D, Shi YH, Cao Y, Dai Z, et al. Systemic inflammation score predicts survival in patients with intrahepatic cholangiocarcinoma undergoing curative resection. J Cancer. 2019;10(2):494–503. doi: 10.7150/jca.26890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Q, Li F, Gao Y, Xue H, Li Z, Zou Q, Xia Y, Wang K, Shen F. Developing a selection-aided model to screen cirrhotic intrahepatic cholangiocarcinoma for Hepatectomy. J Cancer. 2020;11(19):5623–34. doi: 10.7150/jca.46587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang K, Liu J, Yan ZL, Li J, Shi LH, Cong WM, Xia Y, Zou QF, Xi T, Shen F, et al. Overexpression of aspartyl-(asparaginyl)-beta-hydroxylase in hepatocellular carcinoma is associated with worse surgical outcome. Hepatology. 2010;52(1):164–73. doi: 10.1002/hep.23650. [DOI] [PubMed] [Google Scholar]
- 23.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–5. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang PY, Wang CC, Lin CC, Lu SN, Wang JH, Hung CH, Kee KM, Chen CH, Chen KD, Hu TH et al. Predictive Effects of Inflammatory Scores in Patients with BCLC 0-A Hepatocellular Carcinoma after Hepatectomy.J Clin Med2019, 8(10). [DOI] [PMC free article] [PubMed]
- 26.Lin J, Fang T, Zhu M, Xu X, Zhang J, Zheng S, Jing C, Zhang M, Liu B, Zhang B. Comparative performance of inflammation-based prognostic scores in patients operated for intrahepatic cholangiocarcinoma. Cancer Manag Res. 2019;11:9107–19. doi: 10.2147/CMAR.S198959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaczynski J, Hansson G, Wallerstedt S. Incidence, etiologic aspects and clinicopathologic features in intrahepatic cholangiocellular carcinoma–a study of 51 cases from a low-endemicity area. Acta Oncol. 1998;37(1):77–83. doi: 10.1080/028418698423212. [DOI] [PubMed] [Google Scholar]
- 28.Chen Q, Yang LX, Li XD, Yin D, Shi SM, Chen EB, Yu L, Zhou ZJ, Zhou SL, Shi YH, et al. The elevated preoperative neutrophil-to-lymphocyte ratio predicts poor prognosis in intrahepatic cholangiocarcinoma patients undergoing hepatectomy. Tumor Biol. 2015;36(7):5283–9. doi: 10.1007/s13277-015-3188-6. [DOI] [PubMed] [Google Scholar]
- 29.Gu FM, Gao Q, Shi GM, Zhang X, Wang J, Jiang JH, Wang XY, Shi YH, Ding ZB, Fan J, et al. Intratumoral IL-17(+) cells and neutrophils show strong prognostic significance in intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2012;19(8):2506–14. doi: 10.1245/s10434-012-2268-8. [DOI] [PubMed] [Google Scholar]
- 30.Jing CY, Fu YP, Shen HJ, Zheng SS, Lin JJ, Yi Y, Huang JL, Xu X, Zhang J, Zhou J, et al. Albumin to gamma-glutamyltransferase ratio as a prognostic indicator in intrahepatic cholangiocarcinoma after curative resection. Oncotarget. 2017;8(8):13293–303. doi: 10.18632/oncotarget.14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang BG, Ge RL, Sun LL, Zong M, Wei GT, Zhang YJ. Clinical parameters predicting survival duration after hepatectomy for intrahepatic cholangiocarcinoma. Can J Gastroenterol. 2011;25(11):603–8. doi: 10.1155/2011/917097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang C, Wang H, Ning Z, Xu L, Zhuang L, Wang P, Meng Z. Serum liver enzymes serve as prognostic factors in patients with intrahepatic cholangiocarcinoma. Onco Targets Ther. 2017;10:1441–9. doi: 10.2147/OTT.S124161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C, He W, Yuan Y, Zhang Y, Li K, Zou R, Liao Y, Liu W, Yang Z, Zuo D, et al. Comparison of the prognostic value of inflammation-based scores in early recurrent hepatocellular carcinoma after hepatectomy. Liver Int. 2020;40(1):229–39. doi: 10.1111/liv.14281. [DOI] [PubMed] [Google Scholar]
- 34.Mollinedo F. Neutrophil degranulation, plasticity, and Cancer Metastasis. Trends Immunol. 2019;40(3):228–42. doi: 10.1016/j.it.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 35.van der Windt DJ, Sud V, Zhang H, Varley PR, Goswami J, Yazdani HO, Tohme S, Loughran P, O’Doherty RM, Minervini MI, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology. 2018;68(4):1347–60. doi: 10.1002/hep.29914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Liao Y, Suo L, Zhu P, Chen X, Dang W, Liao M, Qin L, Liao W. A novel prognostic index-neutrophil times gamma-glutamyl transpeptidase to lymphocyte ratio (NgammaLR) predicts outcome for patients with hepatocellular carcinoma. Sci Rep. 2017;7(1):9229. doi: 10.1038/s41598-017-09696-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu C, Li L, Lu WS, Du H, Yan LN, Wen TF, Wei WR, Jiang L, Xu MQ. A novel combined systemic inflammation-based score can predict survival of intermediate-to-advanced hepatocellular carcinoma patients undergoing transarterial chemoembolization. BMC Cancer. 2018;18(1):216. doi: 10.1186/s12885-018-4121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wood G, Grenader T, Nash S, Adams R, Kaplan R, Fisher D, Maughan T, Bridgewater J. Derived neutrophil to lymphocyte ratio as a prognostic factor in patients with advanced colorectal cancer according to RAS and BRAF status: a post-hoc analysis of the MRC COIN study. Anticancer Drugs. 2017;28(5):546–50. doi: 10.1097/CAD.0000000000000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou D, Liang J, Xu LI, He F, Zhou Z, Zhang Y, Chen M. Derived neutrophil to lymphocyte ratio predicts prognosis for patients with HBV-associated hepatocellular carcinoma following transarterial chemoembolization. Oncol Lett. 2016;11(5):2987–94. doi: 10.3892/ol.2016.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu JX, Li A, Zhou LY, Liu XF, Wei ZH, Wang XZ, Ying HQ. Significance of combined preoperative serum alb and dNLR for diagnosis of pancreatic cancer. Future Oncol. 2018;14(3):229–39. doi: 10.2217/fon-2017-0339. [DOI] [PubMed] [Google Scholar]
- 42.Hu H, Yao X, Xie X, Wu X, Zheng C, Xia W, Ma S. Prognostic value of preoperative NLR, dNLR, PLR and CRP in surgical renal cell carcinoma patients. World J Urol. 2017;35(2):261–70. doi: 10.1007/s00345-016-1864-9. [DOI] [PubMed] [Google Scholar]
- 43.Dirican A, Ekinci N, Avci A, Akyol M, Alacacioglu A, Kucukzeybek Y, Somali I, Erten C, Demir L, Can A, et al. The effects of hematological parameters and tumor-infiltrating lymphocytes on prognosis in patients with gastric cancer. Cancer Biomark. 2013;13(1):11–20. doi: 10.3233/CBM-130331. [DOI] [PubMed] [Google Scholar]
- 44.Liao R, Peng C, Li M, Li DW, Jiang N, Li PZ, Ding X, Wu Q, Du CY, Gong JP. Comparison and validation of the prognostic value of preoperative systemic immune cells in hepatocellular carcinoma after curative hepatectomy. Cancer Med. 2018;7(4):1170–82. doi: 10.1002/cam4.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xue W, Xu X, Tan Y, Qian Y, Wang H, Wang Y, Xu Y, Zhu X, Jiang P, Ding W. Evaluating and validating the predictive ability of preoperative systemic inflammatory/immune cells in gastric cancer following R0 resection. Oncol Lett. 2019;18(5):5205–14. doi: 10.3892/ol.2019.10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.