Abstract
Background
Since the beginning of human genetic research, there are very few publications sharing insights of the negative impact of rare genetic skin diseases (RGSD) on patients’ experiences. This systematic review assessed the psychosocial implications of these conditions in terms of daily life experiences, emotional state, self-perception, and Quality of Life (QoL).
Methodology
A systematic review was carried out on albinism, neurofibromatosis type 1 (NF1), birthmarks and inherited ichthyosis. The PubMed, Scopus, PsycArticle, PsychInfo, Psychology and Behavioral Sciences Collection, and SOCindex databases were queried. Inclusion criteria were adult patients with one of these RGSDs. Simple descriptive statistics and qualitative content analysis were conducted to summarize the main results reported by the authors.
Results
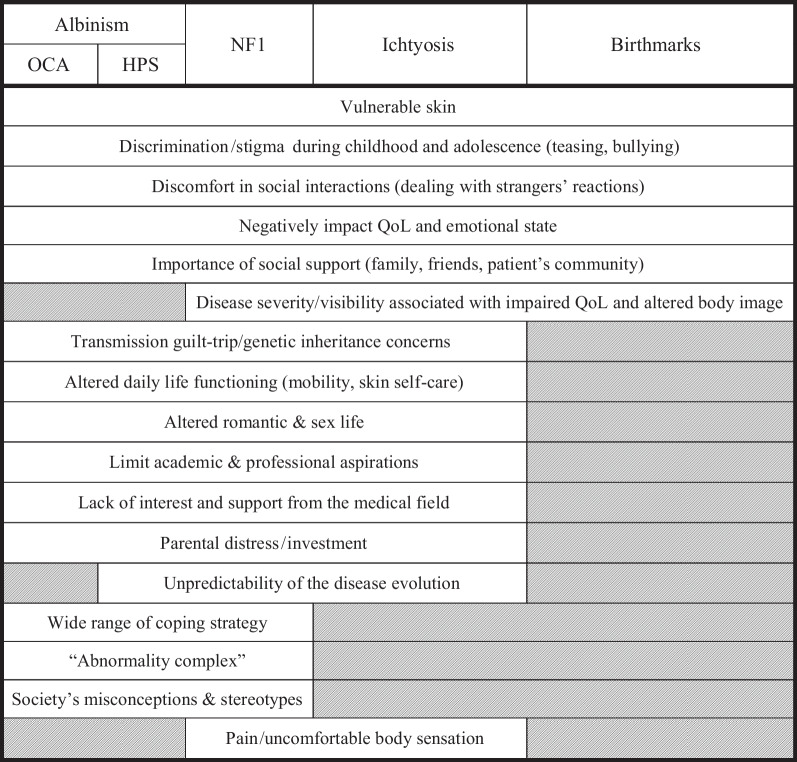
Of the 9987 articles retrieved, 48 articles were included: albinism (16), NF1 (16), inherited ichthyosis (10), birthmarks (6). The majority of the studies on albinism were conducted in Africa. Twenty-seven studies quantitatively assessed diverse psychological parameters: 13 showed a significant impact of the disease on QoL, five on emotional state, two on self-representation and two others on psychiatric comorbidities. Disease severity and visibility were good predictors of QoL (except for albinism). Body image and appearance concerns were also associated with QoL and emotional state. The 19 qualitative studies highlighted recurring themes across each of these diseases: discrimination and stigma during childhood and adolescence, discomfort in social interactions, guilt of transmission, the importance of social support from family and friends, altered daily life functioning, altered romantic and sex life, limited academic and professional aspirations, lack of interest and support from the medical field, and the unpredictability of the evolution of the disease. The only two mixed-method studies in this review were unable to contribute to any inferential analyses but could corroborate some of the qualitative findings.
Conclusion
These results showed that RGSDs have a significant impact on different aspects of patients’ lives. This review has demonstrated that there is a real need for support systems for patients with these diseases. Such systems should be developed to provide them with necessary information and to guide them through an appropriate care pathway.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13023-023-02629-1.
Introduction
Formerly known as “orphan diseases”, rare diseases include a wide range of conditions: physical, sensory, mental, visible, or invisible; are often progressive, sometimes leading to permanent disability; and are most often without cure. In Europe, they are characterized by a prevalence of less than 1/2000 and are mostly of genetic origin (about 80%). There are between 6000 and 8000 rare genetic diseases in the world and, when combined, they affect 6–8% of the population. Extrapolating these data to the European population, between 25 and 30 million people could be affected on the continent [1–3]. In this respect, rare diseases, when grouped together, are no longer truly so and constitute a real health issue that is still not sufficiently studied in the psychosocial field.
As mentioned above, some of these diseases are visible and are responsible for their own set of difficulties. This is especially true for rare genetic skin diseases (RGSDs). Among them, we will focus more specifically on albinism, neurofibromatosis type 1 (NF1), inherited ichthyosis (vulgaris, lamellar, harlequin etc.), and the particular case of congenital birthmarks.1
“Albinism refers to a group of rare congenital diseases globally characterized by poor vision and a variable hypopigmentation phenotype” [4]. Albinism and its many forms have been widely investigated in genetics2 [5–11], but psychosocial research on albinism life experiences is very limited and the results are often not generalizable [12]. In addition, many of the publications on albinism are in the form of essays, testimonies, or appeals [13–18]. On the African continent, people with albinism (PWA) are the target of violent discrimination associated with multiple beliefs and superstitions [19–22]. Nevertheless, several studies have also looked at young PWA’s life experiences in terms of education [23–27], social functioning [28, 29], and self-concept [30]. Moreover, the last decade has seen a significant amount of research emerge in health psychology regarding adults with albinism. [31–45]
“Neurofibromatosis type 1 (NF1) is a clinically heterogeneous, neurocutaneous genetic disorder characterized by café-au-lait spots, iris Lisch nodules, axillary and inguinal freckling, and multiple neurofibromas” [46]. NF1 has already been the subject of numerous studies in psychology, particularly in a child or adolescent's development [47–58] but also in their parents' experiences [59–67]. Moreover, numerous studies have sought to establish a link between neurofibromatosis and atypical developmental profiles such as autism spectrum disorders [68–77] and attention deficit disorders [68, 78–80] in order to explain learning difficulties and social functioning. Other studies have also shown that NF1 has a significant impact on a young person's quality of life (QoL) and on their family functioning [72, 81–86].
Inherited ichthyoses, also known as disorders of keratinisation (DoK), encompass a heterogeneous group of skin diseases that are characterized by extremely dry and rough skin and the presence of an excessive amount of dead skin flakes (squames) that are continuously shed. The severity is variable. The skin is thick and inflammed, sometimes with painful cracks or blisters [87, 88]. Again, over the past 10 years, a number of studies have emerged on the impact of ichthyosis on the QoL of affected adults and children [89–97].
Concerning the particular case of congenital birthmarks, and more specifically here, port-wine stains (PWS or angiomas), these are vascular malformations that affect the capillaries of the skin. They appear as purplish red spots which have an aesthetic impact when they are located on visible areas (face, neck etc.) [98]. They are present from birth and persist throughout life, however they can be treated with pulsed dye lasers to achieve significant lightening [98–100]. Despite the limited amount of research dealing with the experiences of people with PWS, some recent studies have shown a renewed interest in this topic by focusing on the QoL of these patients [101, 102]. The main problem, however, is a significant methodological heterogeneity among these studies.
Despite this growth of research into the psychosocial consequences of rare genetic diseases in the last two decades, data remains very sparse and does not provide a good understanding of how these conditions may be experienced by adult patients. In this context, our objective for this systematic scientific literature review is to investigate the psychosocial implications of an RGSD on daily life, emotional state, self-perception and QoL in adults. Three main questions will guide our research strategy: 1) What is the impact of RGSD on daily life, emotional state (anxiety, depression, well-being), self-perception, and QoL? 2) What are the main predictors of QoL, emotional state (anxiety, depression, well-being) and self-perception of patients with an RGSD? 3) What psychosocial aspects do these RGSDs have in common (i.e., predictors, consequences, functioning)?
Methods and materials
PRISMA [103] and INESSS [104] guidelines were followed throughout the present review.
Eligibility criteria (PICOTS)
Population
Studies including adult (> 18 years) patients with a diagnosis of a genetic skin condition affecting appearance, specifically birthmarks (PWS, congenital melanocytic nevus), albinism (oculocutaneous albinism, Hermansky-Pudlak syndrome), inherited ichthyosis (vulgaris, lamellar, harlequin etc.), and NF1.
Intervention
We only focused on observational studies. All intervention-based studies identified have been excluded. For reasons of overall consistency, we did not include psychosocial studies conducted after a specific treatment (e.g., pulsed dye laser for PWS), which seek to determine the effect of the intervention on patients’ overall outcomes.
Comparison groups
All studies, with or without one or more comparator groups, were included. Thus, all studies that compared RGSD subjects to the general population, chronic health populations or other populations with appearance-altering conditions were considered in this review.
Outcomes
The type of results considered in this review were:
Data that accounted for the impact of genetic skin conditions affecting appearance on QoL, emotional state, or self-perception.
Psychosocial factors that may be associated with QoL, emotional state, or self-perception in patients with genetic skin conditions affecting appearance.
Aspects of the participant's daily life that are particularly affected by their genetic skin condition.
Temporality
This criterion is not binding. Cross-sectional and longitudinal design were both considered with the same interest.
Settings
Studies using qualitative methods (based on semi-structured interviews or focus groups) and quantitative methods (based on numeric scores via questionnaires and scales) have been considered. They could be set as cross-sectional data collection carried out within the healthcare structure (face-to-face interviews, questionnaires completed on site) or remotely (phone interviews, online surveys).
This review has been restricted to English and French language, peer-reviewed, and indexed studies. Conference abstracts, literature reviews, academic dissertations, and case studies have been excluded. We didn’t set any inclusion date because, considering the other criteria we established, we didn’t want to take any risk of omitting interesting results by limiting ourselves to arbitrarily defined publication dates.
Information sources
We queried the following electronic databases: Medline (via PubMed), Scopus, PsycArticle, Psychinfo, Psychology and Behavioral Sciences Collection, and SOCindex (via EBSCO). The search was started in February 2020 and the last update was in May 2022.
Search algorithm
Keywords included successively in all databases were:
| "skin genetic disorders" OR "port-wine-stains" OR "giant congenital melanocytic nevus" OR albinism OR "Hermansky-Pudlak" OR "Chédiak-Higashi" OR "neurofibromatosis type I" OR "Von Recklinghausen" OR Ichthyosis OR "ichthyosis vulgaris" OR "lamellar ichthyosis" OR "ichthyosis bullosa of siemens" OR "X-linked ichthyosis" | 1 |
| AND | |
| Affect* OR Adjustment OR Adaptation OR Acceptance OR Satisfaction OR Happ* OR Optimis* OR Well-being OR Depress* OR Anxi* OR Mood OR Distress OR (Social AND Fuctioning) OR "Quality of life" OR QoL OR Appearance OR Disfigurement OR (Patient AND Satisfaction) OR Factors OR Expect* OR Impact OR (Disease AND Related AND Disability) OR (Quality AND Care) OR Psycho* OR Emotion* OR (Psychological AND Distress) OR (Psychological AND Stress) OR (Psychosocial AND Need) OR (Common AND Mental AND Disorder) OR (Stress AND Disorder*) |
The search strategy outlined above was adapted if (a) too few results were returned, suggesting that some relevant literature may have been missed; or (b) if too many results were found, rendering a meaningful search unfeasible. The first author performed all database searches.
Study selection
Retrieved titles and abstracts (saved on Zotero) were systematically screened to determine possible study eligibility. Any summaries referring to one of the diseases of interest associated with QoL; psychosocial aspects, implications, or consequences; emotional state (anxiety, depression, well-being); and appearance, body image, or self-conception (self-esteem) were included in the full review of the article. Studies were included in the final review when eligibility criteria were confirmed after reading the whole article: adequate population, design, and relevant outcomes. They were excluded when either an irrelevant population (e.g., aged below 18, being family members of PWA, etc.), a study design that relied on a sociological or socio-cultural approach, or irrelevant outcomes (bio-medical parameters, cognitive or intellectual functioning etc.) were present. [see Flowchart—Fig. 1].
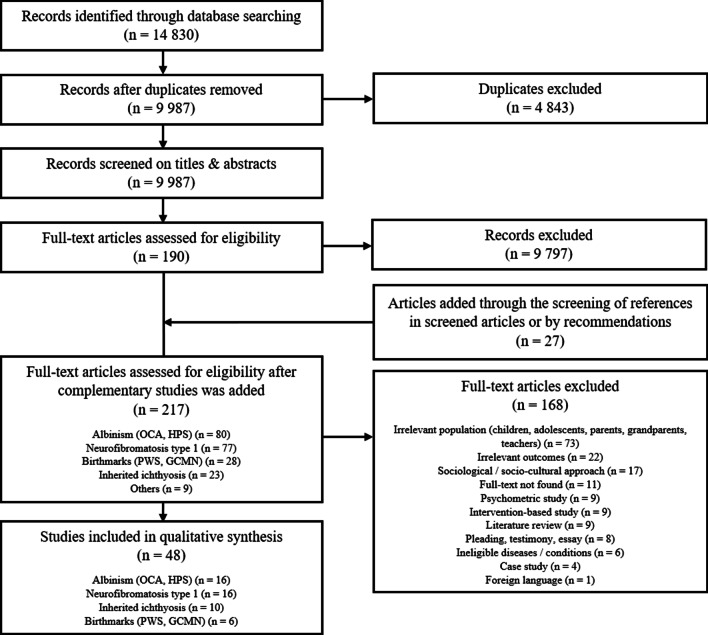
Fig. 1.
Flow diagram of included studies
Data items and collection process
The extraction of information from the selected studies was based on the recommendations of INESSS (2013). For each article, information about design, participant characteristics (sample size, age range, gender, kind of disease, etc.), locations (geographical areas), methods used (control strategy, measurement tool, data analyses), significant outcomes, and limitations had to be systematically noted. These data were manually identified in each study and noted on a spreadsheet. The first author (HF) randomized one third of the articles included for the full-text step and gave those to the second author (NC) in order to realize a dual independent selection, inclusion, and data extraction. In addition to the data mentioned above, each of the two researchers had to state their decision regarding the inclusion of each article, stating their arguments for and against. All articles in which the researchers had doubts about their inclusion were placed in a category "to be discussed collectively". Then, HF and NC pooled their results, and discussed with the other authors in order to validate the studies that had been individually included and to reach a consensus on the uncertain articles.
Summary strategy
Simple descriptive statistics and qualitative content analysis were used. As our studies showed clinical heterogeneity in terms of settings (sample size, kind of diseases, locations), study design, methods (measurement tools, control strategy) and outcomes, it was impractical to run a quantitative analysis for a meta-review. However, we have synthesized the main data from each study in tables constructed from those proposed by the Public Health Agency of Canada (2014) [105], the INESS guidelines (2013) and Calcagni et al. (2019) [106]. An analytical narrative synthesis was conducted to develop an in-depth understanding of the common and specific psychosocial implications of each identified disease (INESSS 2013).
Risk of bias
The Control Guidelines Critical Appraisal Tool Kit (developed by the Public Health Agency of Canada, 2014) [105] was used to independently rate the quality of each quantitative study and to allocate a specific grade (see Appendix). Concerning the qualitative studies, we built our own evaluation grid based on the one developed by the Public Health Agency of Canada (2014) and the work of Santiago-Delefosse (2004) [107] and Stenfors, Kajamaa & Bennett (2020) [108] [see Additional file 1: “Quali study assessment grid”]. Thus, grid selection was adapted, and assessment criteria weighted according to the design of each study. For each design, as some of the scoring criteria are based, in part, on the subjectivity of the first author, the percentages awarded have been transformed into an appreciation corresponding to the interval within which the score is included: High (100–75%), Moderate (74–40%), Low (40–0%). The studies were assessed on the basis of two indicators: one exclusively based on the strength of the study design, the other on the overall article quality (i.e., sampling, internal validity, ethics, etc.). To maximize study inclusions, we did not use a minimum cut-off score as an inclusion criterion.
Results
Study selection
A total of 14,830 references were found, including 7019 through PubMed, 6971 through Scopus, 731 through PsycINFO, 59 through Psychology and Behavioral Sciences Collection, 35 through Socindex, and 15 through PsychArticle databases. All citations were exported to the bibliographic management software Zotero to facilitate the processing of all records. After excluding duplicates (n = 4843), 9987 references were screened (titles and abstracts), which led to the exclusion of 9797 studies. Furthermore, 27 articles were added through the screening of the reviewed studies or by recommendations. Thus, 217 full-text articles were assessed, of which 168 were excluded, leaving 48 studies. (see Fig. 1 for explanations).
Before going any further, it is important to point out that we recorded three recently published literature reviews specifically dedicated to inherited ichthyosis [89] and PWS [109, 110]; they included studies that focused on children and adolescents and also examined psychosocial intervention-based studies. As we cannot include these articles in our current review, we will consider their results in the discussion and see if their findings are consistent with ours.
Overall considerations (all diseases)
A total of 3703 participants were included across all studies. As the present studies are based on two very different methodological frameworks, we will separate the results of qualitative exploratory studies from quantitative cross-sectional studies in the following section (see Table 1).
Table 1.
Synthesis of data from reviewed articles, all diseases combined, according to their number, study design, samples’ main characteristics (number of subjects, average size, location), comparator strategies and main outcomes
| N | Design (n) | Sample | Comparator.s (n) | Main outcomes (n) | ||||
|---|---|---|---|---|---|---|---|---|
| Total | M (sd) | Range | Locations (n) | |||||
| Overall | 48 | Quanti (27) | 3265 | 121,89 (66,67) | 26–244 |
Europe (12) North America (7) West Africa (4) South America (1) East Asia (1) Australia (1) |
Database.s extraction.s (15): Non-affected (8) Other disease.s (3) Both (4) Recruited sample (5): Non-affected (3) Other disease.s (2) N/A (9) |
Impaired Quality of Life (global, health related, skin specific) (14) Worse emotional state (anxiety, depression, well-being) (7) Disease severity impact (6) Disease visibility impact (5) Appearance self-awareness / Body image or related attitudes (4) Self-esteem (2) Psychiatric comorbidities (2) |
| Mixed (2) | 23 | 11,5 | 10–23 |
Europe (1) South America (1) |
Non-affected (1) N/A (1) |
Vulnerable skin Discrimination / stigma during childhood and adolescence (teasing, bullying) Discomfort in social interactions (dealing with strangers’ reactions) Transmission guilt-trip / genetic inheritance concerns Importance of social support (family, friends, patient’s community) Altered daily life functioning (mobility, skin self-care) Altered romantic & sex life Limits academic & professional aspirations Lack of interest and support from the medical field Unpredictability of the disease evolution Parental distress / investment |
||
| Quali (19) | 415 | 21,32 (16,15) | 3–62 |
Europe (5) Southern Africa (4) North America (2) Caribbean (2) West Africa (2) Central Africa (1) Middle East (1) East Asia (1) Australia (1) |
Recruited sample (1): Non-affected (1) N/A (18) |
|||
| Albinism | 16 | Quanti (6) | 430 | 75,83 (27,05) | 38–105 |
West Africa (4) Southern Africa (1) South America (1) |
Recruited sample (4): Other disease.s (2) Non-affected (2) N/A (2) |
Worse emotional state (anxiety, depression, well-being) (3) Psychiatric comorbidity (1) Impaired Quality of Life (1) Social support (1) Stigma (1) |
| Quali (10) | 199 | 19,9 (17,18) | 3–62 |
Southern Africa (4) Caribbean (2) West Africa (2) Central Africa (1) East Asia (1) |
Recruited sample (1): Non-affected (1) N/A (9) |
Others’ attitudes / discrimination, stigma (7) Health care gaps (4) Importance of social support (family, community) (4) Superstitions & beliefs (4) Knowledge about albinism (3) |
||
| Neurofibromatosis 1 | 16 | Quanti (8) | 1009 | 126,13 (61,38) | 37–228 |
Europe (5) North America (3) |
Database.s extraction.s (6): Non-affected (4) Other disease.s (0) Both (2) Recruited sample (3): Non-affected (2) Other disease.s (1) N/A (1) |
Impaired Quality of Life (health related, skin specific) (5) Disease visibility (4) Self-esteem (2) Worse emotional state (2) Appearance self-consciousness/Body-image (2) Psychological distress (1) Loneliness (1) Disease severity (1) |
| Mixed (1) | 13 | – | – | South America (1) | Non-affected (1) |
Affected body-image / self-consciousness (6) Affected aspiration, self-esteem & self-confidence (5) Social functioning (5) Unpredictable disease progression (5) Pain (4) Knowledge about NF1 (4) |
||
| Quali (7) | 158 | 22,57 (18,21) | 6–60 |
Europe (3) North America (2) Middle East (1) Australia (1) |
N/A (7) | |||
| Ichthyosis | 10 | Quanti (8) | 1193 (including about 270 subjects < 18 yrs) | 149,13 (71,73) | 26–241 | Europe (5) North America (3) |
Database.s extraction.s (2): Non-affected (1) Other disease.s (0) Both (1) Recruited sample (1): Non-affected (1) N/A (5) |
Impaired Quality of Life (5) Disease severity impact (4) Worse emotional state (2) Family burden (1) |
| Mixed (1) | 10 | – | – | Europe (1) | N/A (1) |
Body discomfort (2) Time-consuming skin care (2) |
||
| Quali (1) | 25 | – | – | Europe (1) | N/A (1) | |||
| Birthmarks | 6 | Quanti (5) | 634 (including about 10 adolescents) | 126,8 (87,46) | 52–244 |
Europe (2) North America (1) East Asia (1) Australia (1) |
Database.s extraction.s (3) Non-affected (1) Other disease.s (1) Both (1) Recruited sample (1): Other disease.s (1) N/A (1) |
Impaired Quality of Life (3) Body image / attitudes (2) Psychiatric & medical comorbidities (1) Social support (1) Disease severity impact (1) Disease visibility impact (1) Worse emotional state (well-being) (1) |
| Quali (1) | 23 | – | – | Europe (1) | N/A (1) | |||
A total of 3265 subjects were included in quantitative protocol (mean = 121.89); by contrast, 415 were included in exploratory qualitative research (mean = 21.32). Only two studies were built on a mixed design and included a total of 23 subjects. Over half of the included studies were led in high income countries (European countries, n = 18; USA, n = 9; Australia, n = 2), except those concerning albinism, of which 3/4 were led in African countries (n = 11). Of the 48 studies selected, 11 also included participants under the age of 18 in their samples (22.92%). For this review, we were only interested in the results concerning adults (which are often compared with the results observed in children and adolescents). It can also be noted that quantitative studies have a greater tendency to mix age groups within their samples than in the qualitative studies we have selected (quan., n = 9 vs. qual., n = 2), and especially in research on ichthyosis (n = 5). In addition, two qualitative studies also included relatives in addition to affected participants. Of the 27 quantitative studies included, 20 used comparators as well as the mixed study, either by recruiting unaffected participants, by creating groups of patients with another disease, or by extracting data from previous research.
Concerning quantitative results, a significant impact of rare skin genetic disorders on QoL was observed in 14 of the 18 studies measuring this variable (global, health-related, and skin-specific form). NF1 and ichthyosis are the most documented diseases in this respect (for each, n = 5), followed by birthmarks (or PWS, n = 3), and finally albinism (n = 1). Furthermore, QoL was often associated with the level of severity and/or visibility of the skin disorder (n = 9, albinism studies excluded). In the same vein, some studies that focused on NF1 and birthmarks have linked body image or appearance self-consciousness with QoL, psychological distress, emotional state, or even self-esteem (n = 4).
On the qualitative side, a wide range of results could be observed. Most of these studies are based on more or less structured individual interviews (n = 16, + 2 including the mixed studies), one was based on focus groups, another on mixed individual interviews and focus groups, and the last one on writing an essay about the participants' condition.
Regarding the quality of the studies, the first observation that can be made concerns the great heterogeneity of the methods and study designs. Among the 19 qualitative studies, 11 were judged to be of high quality, four of moderate quality, and four of low quality. Of the 27 quantitative studies, eight were of high quality, 18 of moderate quality, and only one of low quality. The strength of the designs was globally weak because we did not include any experimental or intervention-based studies (considered as designs that can provide a higher level of evidence). As qualitative studies cannot generalize their findings, all qualitative designs were judged to be low.
Regarding outcomes, even if there are common themes between the four conditions of interest, there are significant specificities, depending on the disease itself, but also on the context in which the subjects live. In the following sections, we will explain these results, disorder by disorder, and finally, propose a synthesis on the lived experiences of patients with RGSDs (common core to specific aspects).
Research on albinism: n = 16
A total of 654 participants were included across albinism-related studies. As mentioned earlier, most of the studies were carried out on the African continent (Nigeria, n = 5; Botswana, n = 2; South Africa, n = 2; Malawi, n = 1; Cameroon, n = 1; Ghana, n = 1) because this genetic condition is more widespread in Africa than in Europe or North America (UE & NA = 1/20,000 vs. AF = 1/5000–15,000) and the psychosocial repercussions it entails are much more serious, as we will see later in this section. The other research were conducted in Taiwan (n = 1), Brazil (n = 1) and Puerto Rico (n = 2). Sixty nine percent of the studies included mostly female participants (6% female-only participants, 63% more than half female participants). The sex ratio is balanced in four qualitative studies [31–33, 111], with men outnumbering women in only one study [34]. Overall, women represent 57.65% of the subjects in all of the samples. The average age could not be calculated because this information was missing in two articles [34, 35]. Two also included participants under 18 years old in their sample [34, 36].
On the quantitative side, six studies were conducted involving 430 subjects (mean = 75.83). On the qualitative side, 10 studies have been included involving 199 subjects (mean = 19.9). Globally, they showed that PWA see albinism as a source of problems and a disadvantage compared to non-affected people (Table 2).
Table 2.
Details of reviewed studies about albinism, according to location, sample, method design and significant outcomes
| Setting | Method | Results | Study quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ref. | Location | N | Design (strength) | Tools | Comparator(s) | IV(s) | DV(s) | Significant outcomes | |
| Anshelevich et al. [37] | Botswana | 27 | Exploratory -qualitative (low) | Semi-structured interviews (TCA) | N/A | N/A | N/A |
Educational environments: health-related barriers to learning; emotional barriers; discriminatory barriers; limited resources vs. positive changes Health care access challenge: availability, accessibility, affordability, adequacy & acceptability Psychosocial impact of stigma & discrimination: social interaction challenges; employment challenges; psychological impacts on emotions & self-concept Myths & superstitions: humanity & soul concerns; contagion of albinism; good & bad luck superstitions |
High |
| Aborisade [41] | Nigeria | 62 | Exploratory -qualitative (low) | Semi-structured interviews (TCA) | N/A | N/A | N/A | Perceived prejudice from family in childhood; disability-specific physical violence; violence severity & impact on family relationship; coping strategies (spiritual consolation/grace of god; taking drugs; confiding in friends; mirror-talking; humor; withdrawal from others/avoidance) | High |
| Tambala-Kaliati et al. [32] | Malawi | 27 | Exploratory -qualitative (low) | In-depth semi-structured interviews; focus group (n = 2) (IPA) | N/A | N/A | N/A | Barriers to education; lack of knowledge about alb; superstitions & beliefs (alb come from mothers; alb as a family/mother curse; supernatural being); economic restraints; stigma & discrimination (humiliation; excluding society; death threats); health care challenges; personal/emotional issues; family issue (abandoned mothers; frightened families; isolating families) | High |
| Chu et al. [43] | Botswana | 50 |
Cross-sectional—quantitative (moderate) |
Questionnaires (OGs) | 99 non-affected subjects | Presence of albinism | Health status, practices & care-access; self-perceptions; beliefs & attitude about alb |
PWA: excellent understanding of the disease (90%) vs. non-PWA (63%) Most PWA felt accepted by friends (88%) and family (94%) PWA: feeling of acceptance by their community↓*; discrimination↑*; impacted by stigma in their social interactions↑ vs. non-affected subjects Almost all PWA believe they deserved extra financial and social support (95%); ½ believe albinism should be considered as a disability |
Moderate |
| Huang et al. [42] | Taïwan | 10 ♀ | Exploratory -qualitative (low) | In-depth semi-structured interviews (CPM) | N/A | N/A | N/A | Discrimination; normality aspirations; sexual & maternal aspects | High |
| Affram et al. [44] | Ghana | 105 | Cross-sectional—quantitative (low) | Questionnaires (PWI-A; ISDL-SS; MSPSS) | N/A | Perceived Social support (PSS); Social stigma (SS) | Subjective well-being (SWB) |
Perceived social support mediates the effect of social stigma on subjective well-being: • SS↑ indirectly → SWB↓***; • SS↑ directly → PSS from friends↓** and significant others↓**; • PSS from significant others↑ → SWB↑* |
Moderate |
| Dapi et al. [38] | Cameroun | 19 | Exploratory –qualitative (low) | Focus groups (n = 3) (TCA) | N/A | N/A | N/A | Discrimination; stigma; injustice; rejection; superstition; associated skin diseases (lack of care & limited resources); knowledge toward alb + + | High |
| Estrada-Hernandez [31] | Puerto-Rico | 8 | Exploratory –qualitative (low) | Semi-structured interviews (TCA) | N/A | N/A | N/A | Knowledge about alb + + ; inappropriate social attitudes; alb boost resiliency; importance of social support; main challenges = visual impairment, sun adapt, lack of independence; need more medical awareness (HPS + +) | Moderate |
| Ojedokun et al. [34] | Nigeria | 75 | Cross-sectional—quantitative (low) | Questionnaires (ESPHWPLWA) | N/A | Social stigma (SS) | Psychological, social and health related well-being |
[Results interpretation irregularities] SS negatively ↔ social well-being* and health well-being* (no information if these are positive/negative correlations) |
Low |
| Christensen et al. [39] | Puerto-Rico | 23 | Exploratory -qualitative (low) | In-depth semi-structured interviews (CPM) | N/A | N/A | N/A | Wandering diagnosis; lack of medical consideration; lack of knowledge (uncertain future); coping = research on HPS, family support, spirituality; burden of being an expert (lead care coordination); HPS community support + + | High |
| Attama et al. [45] | Nigeria | 100 | Cross-sectional—quantitative (moderate) | Questionnaire (GHQ-28); structured interview (MINI) | 100 Leprosy (lep) patients | Socio-demographic; Disease type (Leprosy vs. Albinism) | Psychiatric morbidity; psychiatric diagnosis |
Psychiatric morbidity↑ for: ♂ lep vs. ♂ alb*; married lep vs. married alb*; no or low education lvl lep vs. idem alb***; sales/services & agricultural lep vs. idem alb***; self-employed lep vs. self-employed alb*** Psychiatric morbidity↑ for unmarried alb vs. unmarried lep*** |
High |
| Maia et al. [35] | Brazil | 38 | Cross-sectional—quantitative (moderate) | Questionnaires (WHOQOL-BREF) | 40 non-affected subjects | Presence of albinism | Quality of Life (QoL) | PWA physical QoL is lower than control physical QoL*** | Moderate |
| Phatoli et al. [33] | South Africa | 5 | Exploratory -qualitative (low) | Semi-structured interviews (TCA) | 10 non-affected subjects | N/A | N/A | Myths & stereotypes about alb; discrimination; alb social avoidance | Moderate |
| Ajose et al. [36] | Nigeria | 87 | Cross-sectional—quantitative (moderate) | Dermatologist's clinical assessment & Questionnaire (HADS) | 102 vitiligo patients | Health informations; Disease type (Albinism vs. Vitiligo) | Anxiety; Depression |
Anxiety & depression↑ for: PWA with skin complications vs. without***; PWA without skin complication vs. vitiligo patients**; > 50 years old PWA vs. > 50 years old vitiligo patients** Anxiety↑ for ♀ PWA vs. ♀ vitiligo patients** Depression↓ for married PWA vs. married vitiligo patients* |
High |
| Pooe-Monyemore et al. [40] | South Africa | 15 | Exploratory -qualitative (low) | In-depth semi-structured interviews (CPM) | N/A | N/A | N/A | Importance of self-concept; family role in self-concept; stigma due to appearance, myths & superstitions; role of government, non-governmental organizations, private sectors & media in development of alb recognition | High |
| Ezeilo [111] | Nigeria | 3 | Exploratory -qualitative (low) | Essay writing | N/A | N/A | N/A | Albinism as a demerit: main theme = conspicuous color, sensitive skin, visual impairments, interpersonal problems (romantic) & society's unkind attitude | Low |
All study considered socio-demographic aspects (for example: age; gender; education lvl; socio-economic status; profession; etc.)
IV Independent variable; DV Dependent variable; CPM Colaizzi’s phenomenological approach; TCA Thematic content analysis; PWA People with albinism; HPS Hermansky-Pudlak syndrome; alb Albinism; lep leprosy; lvl level
A ↔ B: A is associated with B; A → B: A predicts B; A↑: A is higher/more important; A↓: A is lower/less important; with: w/; without: w/o; vs.: compared to…
Statistical significance index: p < 0.05*; p < 0.01**; p < 0.001***
Medical considerations
The observations are different depending on the geographical location of the studies. African studies generally report compromised access to healthcare in terms of affordability, accessibility, adequacy, availability, and acceptability [32, 37, 38, 111]. Care access is unevenly distributed over the territories, with rural areas very poorly served, unlike urban areas [32]. However, in Puerto Rico, the observations are of a different order. Firstly, in the early stages, some Hermansky-Pudlak syndrome3 (HPS) patients explained that it took them a long time to find out what they were suffering from due to diagnostic wandering [39]. Subsequently, participants reported that they were often discredited or not taken seriously by doctors or medical professionals [39]. In general, these PWA talk about the burden of being an expert of their own disease toward healthcare providers [31, 39], and express the need for the medical field to be more aware and more supportive of this condition [31]. This last observation inevitably leads us to discuss the relationship that PWA have with sociomedical institutions. Indeed, in some African countries there is no social assistance for PWA with very serious health conditions [38]. Furthermore, the injustices, shortcomings, and difficulties mentioned above are leading PWA to change mentalities and attitudes toward albinism. This need for recognition of albinism in society is illustrated by the fact that they have sought help from government services, non-governmental organizations [37, 38, 40], the private sector, and the media [40].
Educational and professional inclusion
PWA reported different shortcomings related to education and barriers to learning, including vision-related challenges, discrimination, and resource limitations [32, 37, 38]. Moreover, several studies show the extent to which visual impairments, social perceptions, and parental pressure limit academic aspirations, career choice, and employment [31, 32, 37, 38, 41].
Impact on family functioning
Studies showed the important role of the family in the development of self-concept (self-acceptance, self-confidence, self-esteem). First of all, the birth of a child with albinism is often experienced as an upheaval in the parents’ lives that causes fear and distress. However, family functioning drastically differs from one region of the world to another and, as for the rest of our findings, Africa gives us the most detail on these issues.
On the one hand, because of the beliefs and ritual practices that constantly threaten PWA’s lives, families are subjected to an oppressive climate of fear and stress. Parents would then tend to become overprotective of their children, a behavior that is regularly criticized by PWA once they are adults [31, 33, 38–40, 42].
On the other hand, Aborisade (2021) conducted a study on the childhood experience of PWA, whose results testified to a great propensity for intra-familial violence within Nigerian households. Between parents who abandon them and those who mistreat them, young PWA were often the target of physical violence (e.g., one third had physical scars) and psychological abuse from their relatives (e.g., taunting, name-calling, humiliation, confinement/imprisonment) [37, 42]. This violence can be attributed to guilt-ridden family relationships, especially in connection with local superstitions concerning families’ cursed blood or divine punishment. These beliefs tend to incriminate the mother as the only one responsible for the child's fate, placing guilt as the driving force in family interactions.
Perception of the illness and social interactions
Most studies addressed the social functioning of PWA, especially problems related to stigma and discrimination. They explained that stigma associated with albinism may be due to physical appearance (hair and skin colour) [32, 33, 37, 38, 40–43, 111] or to various myths and superstitions (“albinos don't die, they vanish”; “albinos have magical powers”; “albinism is contagious”; “albinism is the mark of a curse”; “albinos' body parts can cure diseases”; “albinos are the result of secret affairs” etc.) [32, 33, 37, 40, 42]. All of these stigmas lead society to reject and be unkind toward PWA, especially in African countries [32, 33, 37, 38, 111]. Meanwhile in Puerto-Rico, society tends to conflate the person with their impairments, giving them the feeling that they are assigned a “disabled identity” [31]. To corroborate these results, two quantitative studies have highlighted the impact of perceived social stigma on the subjective well-being of PWA [34, 44].
In this context, PWA express various difficulties and discomfort in social interactions. Firstly, many participants explained that they had been teased or bullied during childhood, especially in school [31, 32, 37, 38, 42]. Some have even faced traumatic events due to beliefs associated with their condition (aggression, violence, death threats) [32, 38, 41]. In general, studies show that PWA struggle to deal with strangers’ reactions, especially avoidance, scorn, fear, rejection, taunts, humiliation, and in the most extreme cases, aggression [31–33, 37]. Researchers also noted that PWA had difficulties in their romantic and sexual lives, including developing intimate relationships or getting married [31, 32, 37, 111]. Despite these difficulties, subjects reported the desire to reach out to others and initiate friendships [37, 40]. In Puerto Rico for example, the author showed that PWA interacted very well with their peers and made strong friendships [31]. By contrast, some authors also reported that some PWA, overwhelmed by social pressure, preferred to withdraw from social situations to avoid being noticed [41, 111]. Furthermore, the studies showed that social support is the most cited coping strategy of PWA. They identified the importance of having family members, close friends, neighbors, and teachers to rely on, and that made them feel included [31, 33, 37–39, 41, 111]. To support this idea, Affram and his colleagues (2019) showed that social support from significant others partially mediated the effect of social stigma on subjective well-being. Lastly, the studies led in Puerto Rico showed that the participants (mostly Hermansky-Pudlak syndrome) identified the patient’s community as a great source of support, a space where they could share their experiences and feel understood [31, 39].
Impact on physical functioning
Several studies showed the impact of albinism on physical functioning, particularly for visual impairment and skin vulnerable to the sun [31, 32, 37, 38, 111]. To reinforce these findings, a study conducted in Brazil showed that the physical dimension of WHOQOL (World Health Organization Quality of Life) was negatively impacted in PWA [35]. A study conducted in Puerto Rico on subjects with HPS also reported the impact of such a disorder on the circulatory and respiratory systems (e.g., coagulopathy; and in some cases, neutropenia, or pulmonary fibrosis) [39].
Impact on psychological functioning
Finally, at an individual level, participants' perceptions of their disease reflect a good understanding of albinism, which they define as a genetic and inherited condition [31, 33, 36, 43]. However, perceptions of albinism seem to differ according to where the studies were carried out. African PWA were indeed more likely to perceive albinism as a curse [32, 33, 41], while in Puerto-Rico, PWA perceived it more as a source of resilience, an opportunity for self-improvement [31]. Secondly, several studies revealed a major complex that PWA have regarding normality. While some expressed willingness to be seen as normal [33, 42], others reported that they were under social pressure related to the desire to fit society's norms (particularly in relation to performance) [31]. To overcome this “normality complex” problem, some studies emphasized the inexhaustible strength of self-acceptance: a state of mind that develops through education provided by parents [40], or through therapeutic courses [42]. Nevertheless, the symptoms of albinism still have an impact on daily life. For instance, Estrada-Hernandez (2018) highlighted the frustration of PWA not being able to be completely independent in terms of mobility; for example, because of their visual problems, PWA cannot drive a car. The hereditary nature of albinism also raises an important issue: the guilt about the risk of passing on their albinism to their children. Many mothers with albinism have been faced with this dilemma and have had to make choices (i.e. make the decision not to have children, to have a termination of pregnancy, etc.) [42]. Finally, the study conducted among HPS patients reports their uncertainty about the future due to the unpredictability of the disease's evolution [39]. All in all, PWA have learned to develop a wide variety of coping strategies (spiritual consolation/complaining to God, scientific knowledge seeking, social support, humor etc.) [39, 41, 111] related to the development of a “fighting spirit” [31].
In view of all the above, we can detail several psychological consequences observed in these studies, first at the emotional level: sadness, fear, anxiety, anger, resentment, shame, feelings of hopelessness, powerlessness, helplessness, worthlessness, bitterness, loneliness, and frustration [32, 37, 38, 41]; and in terms of thought and behavior patterns: low self-esteem, low self-confidence, self-isolation, low self-efficacy, distrust of others, paranoia, avoidance, denial, guilt, and self-harm or self-reproach [33, 37, 38, 40, 41, 111]. Lastly, a study in Nigeria showed that PWA with severe skin complications tended to be more anxious and depressed than those without. Their results also showed that PWA with severe skin problems were as anxious and depressed as subjects with vitiligo [36].
Research on NF1: n = 16
A total of 1180 participants were included across NF1-related studies. Most studies have been conducted in Europe (Norway, n = 2; England, n = 2; Germany, n = 1; Italy, n = 1; France, n = 1; Sweden, n = 1) and in the USA (n = 5). The other research was led in Australia (n = 1), Brazil (n = 1) and Iran (n = 1). Eighty-one percent of the studies included mostly female participants (6% with female-only participants, 75% with more than half female participants). The sex ratio was balanced in only two qualitative studies [112, 113], with men outnumbering women in only one study [114]. Overall, women represented 66.22% of the participants in all the samples. Among the studies we identified, four of them also included participants under 18 years old [115–118] (see Table 3 for more details).
Table 3.
Details of reviewed studies about neurofibromatosis type 1, according to location, sample, method design and significant outcomes
| Setting | Method | Results | Study quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ref. | Location | N | Design (strength) | Tools | Comparator(s) | IV(s) | DV(s) | Significant outcomes | |
| Foji et al. [121] | Iran | 24 | Exploratory -qualitative (low) | Interview w/ evolving structure (GTM) | N/A | N/A | N/A |
NF1 patients’ life & their response to failure & falling behind in life ‘an unsuccessful struggle to escape’ Environmental conditions: unpleasant appearance due to spots & tumors; inability to have kids; learning disabilities; limitations daily life activities; social rejection and isolation; facing aggression form others; perception of no social support; incurability of NF1 Indivuals’ responses/coping: hiding disease from others; seeking isolation; complaining to God; refusing to receive care; hopelessness; In extreme cases: suicidal ideation, unsuccessful suicide attempts |
High |
| Fjermestad et al. [123] | Norway | 142 | Cross-sectional—quantitative (low) | Questionnaires (HUNT3; OGs) | 46,393 non-affected subjects (extracted from a cohort study) | Biomedical datas; Mental functioning; Health related QoL | Life satisfaction |
HrQoL problems↑ in following domains: life satisfaction, mental health, sleep, pain, gastrointestnal problems, oral health, memory problems, social support (especially women) MLR model → life satisfaction: mental health, sleep, pain, memory problems, social support*** Only mental health was a unique significant predictor*** |
Moderate |
| Jensen et al. [115] | USA | 16 | Exploratory -qualitative (low) | Semi-structured interviews (TCA) | N/A | N/A | N/A | Chronic pain & acute episodes of localized pain; social functioning (limited activity participation, role limitation & relationship impact); mobility difficulties; internalized stigma (more external in youth) | Moderate |
| Rosnau et al. [116] | USA | 49 | Cross-sectional—quantitative (low) | Questionnaires (RSES; OGs) | General population norms (Sinclair et al. 2010) | Biomedical datas; NF1 experiences | Self-esteem (SE); NF1 knowledge |
¾ of the participants had a quite good knowledge about NF1 NF1 SE↓ vs. general pop. norms*** MLR model → SE: learning problems, having friends with NF1, attending a support group & receiving genetic counseling* |
High |
| Bicudo et al. [119] | Brazil | 13 | Cross-sectional mixed—quali-quantitative (moderate) | Hetero-assessment scale (Ablon & Riccardi); questionnaires (WHOQOL-100) & Semi-structured interviews (TCA) | 39 non-affected subjects | NF1 severity & visibility | Quality of Life |
No significant impact of NF1 on QoL Difficulties: pain; concern about the future; shame; discomfort; awkwardness; hiding body; concern about genetic counseling; limited job opportunity & professional life; NF1 as a curiosity for strangers; confusion from strangers with contagious diseases; social prejudices; inappropriate healthcare; lack of information Coping: spirituality/religion/beliefs; relativizing NF1; social support (e.g. family); resection of pNF |
Moderate |
| Crawford et al. [122] | Australia | 60 | Exploratory -qualitative (low) | Interview w/ evolving structure (GTM) | N/A | N/A | N/A | Cosmetic disfigurement as a burden; social discomfort & awkwardness; difficulty in finding partners; lack of awareness and knowledge of NF1 in society; learning and/or attention difficulties in childhood; affected aspirations & self-esteem; genetic inheritance concerns; unpredictable disease progression; pain | High |
| Barke et al. [117] | England | 9 | Exploratory -qualitative (low) | Semi-structured interviews (TCA) | N/A | N/A | N/A | NF1 impacts differs a lot (severity & visibility); social discomfort & awkwardness; social support (family, friends, online support group); adolescence as a period of learning & awareness about NF1; NF1 poorly understood (medical community, medias); unpredictable disease progression | Moderate |
| Hummelvoll & Antonsen [120] | Norway | 15 | Exploratory -qualitative (low) | Semi-structured interviews (TCA +) | N/A | N/A | N/A | Pain (sleep disturbance, fatigue); movement & mobility difficulties; anxio-depressive symptoms; the importance of family background and relations; the role of friendships; low self-confidence; dealing with NF1 visibility; unpredictable disease progression; personal/cognitive aspects mediates the impact of NF1 | High |
| Smith et al. [126] | USA | 127 ♀ | Cross-sectional—quantitative (moderate) | Questionnaires (DAS59; RSES; UCLA Loneliness Scale) | 48 NF2 ♀ + General population & Breast Cancer Survivors’ (BCS) norms (extracted from other studies) | Appearance distress / self-consciousness | Self-esteem (SE); Loneliness |
NF1 women reported more psychosocial reasons for disliking their feature vs. NF2 women* NF1 sexual / bodily self-consciousness↑ vs. general pop norms* and BCS*** NF1 social self-consciousness↑ vs. general pop norms and BCS*** NF1 sexual / bodily self-consciousness↑ ↔ SE↓*** NF1 social self-consciousness of appearance↑ ↔ SE↓***; loneliness↑** |
High |
| Granström et al. [127] | Germany | 228 | Cross-sectional—quantitative (low) | Hetero-assessment scale (Riccardi); Questionnaires (DLQI; Distress Thermometer; FBeK; OGs) | 2047 non-affected subjects + 105 psoriasis subjects (extracted from other studies) | Biomedical datas; NF1 severity & visibility; Body Image | Depressive statement; Psychological distress; Health-related QoL |
NF1 visibility↑ ↔ depressive state↑**, frequency of a lifetime depression diagnosis↑**, psychosocial distress↑***, QoL impairment↑*** and body experience↓*** NF1 patients’ feelings: insecure and uneasy with their own bodies↑***, attractive and self-confident↓*** vs. healthy pop NF1 visibility effect on depressive state was completely mediated by NF1 patients’ body experience***; partially on psychological distress*** and QoL impairment** |
High |
| Dheensa & Williams [112] | England | 6 | Exploratory -qualitative (low) | In-depth semi-structured interviews (IPA) | N/A | N/A | N/A | Lack of information about NF1 (no further explanations; loss faith & trust in medical professions); feeling judged (social self-consciousness aroused; isolation/solitude); social comparisons; variety of coping methods; unpredictable disease progression; some positive appraisal | Low |
| Kodra et al. [125] | Italia | 129 | Cross-sectional—quantitative (low) | Hetero-assessment scale (Ablon) & Questionnaires (SF-36; Skindex-29) | N/A | NF1 visibility | General & skin-disease-specific QoL |
NF1♀ impact on emotions↑** and physical↑*** symptoms vs. for NF1♂ NF1 visibility↑ independently ↔ skin-disease-specific QoL following aspects: emotions↓***, physical symptoms↓* and functioning↓*** |
Moderate |
| Page et al. [124] | USA | 169 | Cross-sectional—quantitative (low) | Hetero-assessment scale (Ablon & Riccardi); Questionnaires (SF-36; Skindex-29) | 154 non-affected U.S. subjects (extracted from another study) | NF1 severity & visibility | General & skin-disease-specific QoL |
NF1 visibility↑ independently → skin-disease-specific QoL following aspects: emotions↓ (♀ especially)**, symptoms↓* & functioning↓*** NF1 severity↑ independently → skin-disease-specific QoL following aspects: symptoms↓*, function↓* NF1 patients general health QoL↓ vs. normative pop* NF1 severity↑ independently → general health QoL↓ (physical function, bodily pain, general health perception, vitality, role emotional, mental health, social functioning, role physical, physical symptoms)** |
Moderate |
| Wolkenstein et al. [118] | France | 128 | Cross-sectional—quantitative (low) | Hetero-assessment scale (Ablon & Riccardi); Questionnaires (SF-36; Skindex-29) | 3656 subjects representative of the French population (extracted from another study) | NF1 severity & visibility | General & skin-disease-specific QoL |
NF1 impact on emotions↑*** & physical symptoms↑** for ♀ vs. ♂ NF1 visibility↑ independently → skin-disease-specific QoL following aspects: emotions↓*, symptoms↓** & functioning↓** Each aspect of NF1 general health QoL↓ vs. normative pop NF1 severity independently → 4 aspects of general health QoL: physical function**, bodily pain*, general health perception**, vitality* NF1 visibility independently → 4 aspects of general health QoL: physical function**, social functioning*, role-emotional*, mental health* |
Moderate |
| Zöller & Rembeck [114] | Sweden | 70 (1978) → 37 (1990) | Longitudinal study—quantitative (12 years follow-up) (moderate) | Medical & psychiatric interviews (CPRS); Questionnaires (KSP; SES) | 27 non-affected subjects | NF1 severity (quantity & location of neurofibromas) | Psychiatric characteristics; Personality profile; Self-concept |
NF1 patients: mood disorders↑ (depression, dysthymia) vs. non-affected subjects*** NF1 patients: sleep↓*; social phobias↑*; worries about trifles↑** vs. non-affected subjects NF1 patients w/o psychiatric diagnosis: self-evaluation & socialization↑*; aggressivity↓***; irritability↓** vs. non-affected subjects No further difference between 1978 & 1990 |
Moderate |
| Ablon [113] | USA | 28 | Exploratory -qualitative (low) | One question-based interviews | N/A | Gender | Life experiences |
♀ ≠ ♂: internalized cosmetic norms & body concerns; parenting prevail over gender; fears of rejection harder for ♀: genetic inheritance concerns; more binding appearance norms harder for ♂: compromised manliness; employability & professional life; represses their feelings; tend to withdraw from social life & avoid romantic situations (unlike women) |
Low |
All study considered socio-demographic aspects (for example: age; gender; education lvl; socio-economic status; profession; etc.)
IV Independent variable; DV Dependent variable; GTM Grounded theory methodology; TCA Thematic content analysis; NF1 Neurofibromatosis type 1; MLR Multiple linear regression; OGs Originals instruments; pop population
A ↔ B: A is associated with B; A → B: A predicts B; A↑: A is higher/more important; A↓: A is lower/less important; with: w/; without: w/o; vs.: compared to…
p < 0.05*; p < 0.01**; p < 0.001***
Concerning the quantitative results, eight studies were conducted involving 1009 participants (m = 126.13). The average age of participants varied from 37 to 51 years (m = 42.65, SD = 4.93). Almost all cross-sectional studies used a control strategy by extracting data from other studies (n = 6) or setting up a group of non-affected subjects (n = 2). Concerning the qualitative results, seven studies have been considered, involving 158 participants (m = 22.57). Only one mixed-method study was incorporated and included 13 participants [119].
Medical considerations
The studies reported a general lack of information about NF1 [112, 119] and NF1 appears to be poorly understood in the medical community, particularly by general practitioners [117]. In these circumstances, the available healthcare is not always appropriate, and the diagnosis is rarely followed by sufficient information and personalized support and counseling that would allow a concrete explanation of what NF1 is [112, 119]. In order to cope with the fear of tumor development, patients are regularly followed-up by a specialist [120]. It should also be noted that resection of neurofibromas is a real health need for many patients [119]. To corroborate these findings, Rosneau et al. (2017) found that subjects’ self-esteem was higher if they received care at a NF clinic or had received genetic counseling.
Impact on family functioning
Family functioning is also greatly impacted by NF1 as it brings worry or guilt about the partner, the children, or future children. NF1 is a “family affair”; considering its inherited genetic nature, NF1 is often a well-known genealogical issue [117]. This familial issue generates a “hereditary anxiety” mentioned in many studies and which is characterized by the fear or guilt that NF1 could be passed to children [115, 119–122]. This fear is the reason why some mothers have decided to never have children [121, 122]. To this extent, many couples have resorted to prenatal tests to assess the risk of the disease’s transferability [121]. Other women, on the other hand, did not feel bound by heredity. However, the birth of an NF1 baby is often experienced as a great upheaval in the family [117] and causes many concerns about the future and the development of the child's NF1 [119, 120]. These latter observations highlight the importance of knowing the genetic background of the family. Indeed, diagnosis in a family without NF1 leads to a lot of misunderstandings, diagnostic wandering, and can be source of conflicts within the family [120].
Perception of the illness and social interactions
NF1 significantly alters social functioning. Several studies showed that many patients have experienced stigma because of their physical appearance, in particular the presence of plexiform neurofibroma (PNf) [115]. Many participants identified adolescence as a time when stigma emerged as a prominent concern [115], particularly because they could be the victim of teasing or bullying [121, 122]. In this context, NF1 patients expressed several difficulties and discomfort in social interactions and several studies showed that participants had difficulties dealing with strangers’ reactions [115, 117, 119, 121, 122]. In most social situations, NF1 patients are seen as a curiosity [115, 117, 119] and in the most extreme cases, they may have been rejected, humiliated, even experienced aggression [121, 122]. In addition, some studies showed that some patients had difficulties forming romantic relationships despite a desire to do so [115, 122]. This kind of difficulty was illustrated in one study by the apprehension that some patients felt about having sexual relations [122]. Moreover, in Iran, researchers have shown that NF1 is a real barrier to marriage [121]. However, there does not seem to be a consensus among these observations, as another study indicated very precisely that NF1 had never been an obstacle to the development of romantic relationships [119]. Considering all these elements, participants deplored being victims of social prejudice and being treated differently than others, even sometimes treated as stupid [112, 119, 121]. In this context, some authors reported that some NF1 patients had a diminished interest in participating in social activities [112, 115, 121]. To compensate for these difficulties, participants knew how to exclusively seek help from their family, close friends, or friends from NF1 patient organizations [117, 119, 120]. To support this point, Rosneau et al. (2017) found that subjects’ self-esteem was higher if they had friends with NF1 or attended an NF1 support group. Nevertheless, some participants preferred online support rather than face-to-face groups [117].
These difficulties can be partially explained by social perceptions of the illness. It should also be noted that, once again, there is a consequent lack of awareness and knowledge about NF1 [119, 122]. In addition, the media convey stereotypes that give a negative and pathologizing image of NF1 (e.g., “Elephant man” disease) [117]. A study also reported some misconceptions that people had about NF1, especially that it was a contagious disease [119]. As a result, to compensate for this lack of knowledge, many patients took the initiative to inform the people around them about NF1 [120].
Impact on physical functioning
Firstly, one of the most important symptoms of NF1, but which was not expressed by all patients, was the presence of pain (acute or chronic) and/or uncomfortable body sensation [115, 119, 120]. This physical sensation is usually caused by the development of PNf in patients’ skin. In addition, those who experienced chronic or severe pain were negatively and significantly impacted in health and daily functioning [122]. Other authors showed that pain disturbed sleep and increased fatigue [114, 120, 123]. Some studies also found that NF1 caused movement and mobility impairments (skeletal disorders and tumours, reduced ability to walk, dizziness, vision problems, and sound or photosensitivity) [115, 120, 121]. In this way, several authors showed that the severity of NF1 was especially associated with an impaired QoL [118, 124]. In contrast, one quantitative study found no effect of NF1 on QoL, either in terms of severity or of visibility (Bicudo et al. 2016). This surprising result may be due to the small sample size of this research (n = 13).
Impact on psychological functioning
Firstly, cross-sectional studies showed that NF1 has a negative impact on QoL [118, 123–125], self-esteem [116, 126] and body image (appearance-related concerns) [126, 127]. Many patients also reported that NF1 affected their cognitive abilities during childhood, particularly in terms of learning and attention [117, 120, 122]. Cosmetic disfigurement was globally experienced as a burden that causes emotional distress such as shame [119, 120, 122], discomfort, awkwardness [119], self-consciousness, low self-confidence [122], or self-contempt [120]. NF1 visibility was also associated with an altered QoL [118, 124, 125, 127], depressive state, psychosocial distress, and negative body experience [127]. Smith and colleagues (2013) showed that NF1 patients' self-esteem could be negatively predicted by the degree of awareness they may have regarding their own appearance. To overcome this difficulty, many participants explained that they hide body parts that are particularly affected by NF1 [119–122]. In addition, NF1 seems to affect their autonomy, making them less independent in their personal life [119, 121]. As a result, perceived stigma and altered autonomy impact vocational decision-making and discourage people with NF1 from pursuing their desired professions [115, 121, 122].
The unpredictable evolution of NF1 appeared to be one of the most stressful aspects of the disease (appearance of new pNF or other tumors for example) [112, 117, 119–122]. In light of all these elements, the studies showed that all participants developed a wide range of coping strategies [112, 121]. Among them, we can find two opposing states of mind that act as a “cognitive-coping-posture”: physical hypervigilance vs. existential relativism [120]. The latter can be illustrated as state of acceptance and an awareness of NF1 as an incurable life-long disorder, i.e., as a part of them that cannot be changed [122]. Otherwise, some participants relate to spirituality, religion, or other personal beliefs to help them live with their condition and associated symptoms [119, 121]. For others, seeking information and gaining knowledge about NF1 seemed to be essential in their disease management. In their teenage years, some patients sought to learn more about NF1, to be more aware of it, and became experts on their own condition [117].
To summarize, the experience of NF1 can affect subjects’ mental health. For example, Crawford and his colleagues showed that pain was associated with anxiety, stress, low mood, and depressive symptoms. Thus, subjects have a propensity to develop mood disorders and anxio-depressive symptoms [114, 123], and to have low self-confidence [120] and low self-esteem [122]. Finally, two studies showed that NF1 had more impact on women, particularly on their emotional experiences and physical functioning [118, 124].
Research on inherited ichthyosis: n = 10
A total of 1228 participants were included across ichthyosis-related studies. Most of the studies identified were conducted in Europe (n = 7), especially in France (n = 3) and Sweden (n = 2). Two other studies were carried out in the U.S. More recent studies have been conducted in Wales (n = 1), Italy (n = 1) and Mexico (n = 1). The proportion of adult male and female participants cannot be reported as it is not precisely indicated in two studies [90, 91], but overall, in every study, women are more represented than men. In addition, five studies included participants under 18 years of age, making it difficult to calculate the mean age of the adults included in these 10 studies. Approximately 230 subjects under 18 years old were considered in these samples. Eight studies relied on a quantitative cross-sectional design and gathered 1193 subjects while the two remaining studies used either an exploratory qualitative method [92] or a mixed design [128] (Table 4).
Table 4.
Details of reviewed studies about ichthyosis according to location, sample, method design and significant outcomes
| Setting | Method | Results | Study quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ref. | Location | N | Design | Tools | Comparator(s) | IV(s) | DV(s) | Significant outcomes | |
| Wren et al. [95] | Wales | 371 (54 XLI♂; 83 ♀XLI-carrier; 82 IV; 152 psoriasis) | Cross-sectional—quantitative (moderate) | Questionnaires (CISI, PASI, K10; ASRS; AQ10; BITE; DLQI; FSQ) | 1116–18,700 non-affected subjects, students or outpatients (extractions from other studies) | Condition group; skin disease severity | Mood/neurodevelopmental traits; Skin-disease-specific QoL; stigmatization |
All groups: psychological distress↑ (i.e. depression↑; anxiety↑) vs. non-affected subjects*** All groups: atypical neurodevelopmental trait↑ (i.e. ASD↑; ADHD↑) vs. non-affected subjects* IV ♀: ichthyosis severity↑ ↔ recent adverse mood symptoms↑* Main factors influencing XLI & IV mood: stigma / bullying; embarrassment of social situation; reduced social life (esp.♂-depression); difficulty regulating body temperature (esp.♀-irritability); skin related discomfort & difficulties w/ treating ichthyosis (esp.♀-irritability & ♂-anxiety) |
Moderate |
| Abeni et al. [94] | Italy | 94 (including 52 subjects < 18 yrs) | Cross-sectional—quantitative (low) | Questionnaires (DLQI; FBI) | N/A | Biomedical datas; Ichthyosis severity & symptoms | Skin-disease-specific QoL; Family burden |
QoL↓ ↔ Disease severity↑* (e.g. fissures*, itch**, recurrent infections**, walking problems**) QoL aspects impacted = itch & pain*, embarrassment & self-consciousness*, problems w/ clothing choice*, problems caused by treatment* Family burden↑ ↔ Disease severity↑* (e.g. thick scales*, fissures*, or foul smell*) + harlequin & lamellar ichthyosis** Psychological dimension of Family burden were the most impacted* |
Moderate |
| Cortès et al. [97] | Mexico | 26 (only LI) | Cross-sectional—quantitative (moderate) | Questionnaires (CISI; DBI-II; DLQI) | 26 non-affected subjects | Ichthyosis severity | Depression; Skin-disease-specific QoL |
LI patients’ depression↑ vs. non-affected subjects*** No correlations between ichthyosis severity ↔ depression / QoL |
Moderate |
| Sun et al. [93] | USA | 181 (including 53 subjects < 17 yrs) | Cross-sectional—quantitative (low) | Questionnaires (DLQI; PHQ-9; GAD-7) | N/A | Biomedical datas (e.g. ichthyosis features) | Skin-disease-specific QoL; Depression; Anxiety |
Adults w/ ichthyosis: QoL impairments (95%); depression + (34%); anxiety + (27%) Adults w/ ichthyosis: Leisure impairment ↔ depression↑***; anxiety↑* Adults w/ ichthyosis: Difficulties at work ↔ anxiety↑* |
Moderate |
| Dreyfus et al. [90] | France | 241 (including 70 subjects < 18 yrs) | Cross-sectional—quantitative (low) | Questionnaires (VAS; DLQI) | N/A | Medical care; out-of-pocket expenses; work/school/leisure activities; Ichthyosis severity | Skin-disease-specific QoL |
Medical care: regularly followed by a physician (90%); no feedback about genetic tests (70%) Impact on domestic life: moisturizing creams each day (94%); affects clothing & footwear (71%); additional housework (47%); negative impact on familial and conjugal functioning (25%) Financial burden: care expenses not fully covered (48%); out-of-pocket expenditure (86%) 526€/yr Impairment in outside activities: workplace discrimination (27%), restriction in leisure & sports (e.g. swimming-pool) (35%) |
Moderate |
| Dreyfus et al. [91] | France |
158 (including about 20 subjects < 18 yrs) |
Cross-sectional—quantitative (low) | Questionnaires (VAS; DLQI) | N/A | Ichthyosis severity | Skin-disease-specific QoL |
The most affected QoL areas: symptoms & feelings (86%); daily activities (77%); treatments (62%); work (59%); leisure (55%); personal relationships (45%) QoL↓ ↔ Ichthyosis severity↑*** & ♀* |
Moderate |
| Mazereeuw-Hautier et al. [92] | France | 25 | Exploratory -qualitative (low) | Focus groups (n = 5) (TCA) | N/A | N/A | N/A |
Physical health: pain & impaired mobility, pruritus, smelly skin Daily life: activity or work avoidance, daily cream application Relation to others: dealing w/ other reactions, intimate relations issues, lack of interest from medicine Personal aspects: fear / stress related to future, negative feelings |
High |
| Kamalpour et al. [96] | USA |
235 (including about 70 subjects < 18 yrs) |
Cross-sectional—quantitative (low) | Questionnaires (DLQI; CISI; TimeTx; DermVisits) | N/A | Biomedical datas; Ichthyosis severity | Skin-disease-specific QoL; Resource utilization |
Adults QoL↓ vs. children* + ♀ QoL↓ vs. ♂* QoL↓ ← erythema severity↑**, hyperkeratosis severity↑**, time spent in daily treatment↑**, ichthyosis type**, age↑* (the last one’s really weak…) TimeTx↑ & DermVisits↑ ← disease severity↑*, age↑* (+ family history → DermVisits*) |
Moderate |
| Gånemo et al. [129] | Sweden | 122 | Cross-sectional—quantitative (moderate) | Questionnaires (DLQI; SF-36) | 117 non-affected subjects (extracted from another study) | Biomedical datas (e.g. ichthyosis features) | General & skin-disease-specific QoL |
LI patients QoL↓ vs. XLI patients* Ichthyosis patients QoL↓ on 4 dimensions (…) vs. normative pop.* |
High |
| Gånemo et al. [128] | Sweden | 10 | Cross-sectional mixed—quali-quantitative (low) | Semi-structured interviews (TCA) & questionnaires (NHP-I & II) | N/A | Biomedical datas; Ichtyosis severity | Health-related QoL; Health-related problem in daily life |
Childhood: parents deeply engaged; feeling different from peers; bullying; shyness; gynophobia; professional orientation; thoughts of how to find a partner Adulthood: regular doctor appointment (or naturopath, homeopath); improvement of symptoms; time-consuming skin care; skin discomfort (heat intolerance); genetic inheritance concerns |
Moderate |
All study considered socio-demographic aspects (for example: age; gender; education lvl; socio-economic status; profession; etc.)
IV Independent variable; DV Dependent variable; GTM Grounded theory methodology; TCA Thematic content analysis; XLI X-linked ichthyosis; IV Ichthyosis vulgaris; LI Lamellar ichthyosis; HI Harlequin ichthyosis; MLR Multiple linear regression; ASD Autism spectrum disorders; ADHD Attention-deficit/hyperactivity disorder; pop population
A ↔ B: A is associated with B; A → B: A predicts B; A ← B: A is predicted by B; A↑: A is higher/more important; A↓: A is lower/less important; with: w/; without: w/o; vs.: compared to…
p < 0.05*; p < 0.01**; p < 0.001***
Medical considerations
This disease requires regular follow-up by doctors [128]. Unfortunately, the medical world does not seem to have much interest in patients with ichthyosis [92].
Educational and professional inclusion
Many subjects explained the constraints they have encountered in their professional orientation [128] and shared the difficulties they had at their workplace [90–92]. These difficulties were associated with a higher degree of anxiety [93].
Studies also reported that this skin condition restricted them in certain outdoor activities, such as playing sports or other leisure activities [90, 91, 128].
Impact on family functioning
Some researchers have highlighted the impact of ichthyosis on family functioning. Disease severity was positively associated with family burden, and some types of ichthyoses (associated with more severe symptomatology) were also related to a greater family burden, especially harlequin and lamellar ichthyosis [94, 129]. Here again, the hereditary nature of the disease seems to be a central issue in family functioning [128].
Perception of the illness and social interactions
Many participants reported that they had been teased or bullied by some of their peers in their childhood and adolescence [95, 128]. As they moved into adulthood, several participants expressed discomfort and embarrassment during social interactions, especially when others pointed out or questioned them about their skin lesions [92, 95]. In addition, the skin condition can cause problems in intimate relationships [92] thus impacting the conjugal life of some patients [90].
Impact on physical functioning
We can already note that ichthyosis alters physical functioning. Indeed, ichthyosis causes pain and/or uncomfortable body sensations such as damaged skin (e.g., fissures, itching, pruritus, infections), body odour, or heat intolerance [92, 94, 128]. In addition to this, participants explained that physical symptoms could increase over time [128].
Impact on psychological functioning
Quantitative studies show that ichthyosis has a negative impact on QoL [91, 93, 94, 96, 129] and caused anxiety and depressive states in patients [95, 97].
In those articles, the most studied predictor was self-perceived ichthyosis severity, which was associated with a higher negative impact on QoL [90, 91, 94, 96] and recent adverse mood symptoms [95]. However, Cortès and colleagues found no predictive link between the severity of ichthyosis and QoL. Furthermore, adults seem to have greater QoL impairments due to ichthyosis than children [96].
Some authors have recently identified the main factors influencing mood (in terms of anxiety, depression, and irritability). We can mention for example stigma, bullying, embarrassment in social situations, reduced social life, body hyperthermy, skin discomfort and treatment [95].
This brings us to discuss the impact of ichthyosis on daily life. Subjects reported that they should apply moisturizing cream very regularly to limit skin complications [90–92, 128] but skin care visibly seems to be time consuming for some participants [95], and time spent in daily treatment seems to be associated with a greater impact on QoL [96]. Additionally, ichthyosis symptoms have an impact on participants' mobility (e.g., walking problems), sleep, and clothing [90, 92, 128], making it difficult to do certain activities. Thus, researchers have observed that impairments in leisure activities were associated with a higher anxio-depressive state [93]. Finally, Mazereeuw-Hautier and colleagues (2012) highlighted the stress linked to the unpredictable nature of the disease and its evolution [92].
Research on birthmarks: n = 6
A total of 657 participants with birthmarks were included in the five identified studies. Three studies were led in Europe (n = 3), one in China, another in Australia, and the oldest one in the U.S. Overall, women were more represented than men (70.17%). The mean age could not be calculated because this information was missing in two articles [102, 130], and two studies included some adolescents in their samples [130, 131]. Five studies were based on a quantitative cross-sectional design and included 634 PWS subjects [101, 102, 130–132]. Almost all these studies used a comparison-control strategy, except for one [130]. The sixth study, which is also the oldest, was built on a qualitative exploratory design and included 23 subjects [133]. The only qualitative study showed that several dimensions of the participants' lives were affected by their birthmarks (Table 5).
Table 5.
Details of reviewed studies about birthmarks, according to location, sample, method design and significant outcomes
| Setting | Method | Results | Study quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ref | Location | N | Design | Tools | Comparator(s) | IV(s) | DV(s) | Significant outcomes | |
| Wang et al. [102] | China | 197 | Cross-sectional—quantitative (moderate) | Questionnaire (DLQI) | 196 vitiligo subjects | PWS characteristics | Skin-disease-specific QoL |
PWS patients QoL↓ vs. vitiligo patients (esp. feelings, daily activities, leisure, work/school, treatment)*** QoL↓ ← ♀; hypertrophy↑; size of skin lesion↑* |
High |
| Hagen et al. [101] | USA | 244 | Cross-sectional—quantitative (low) | Questionnaire (Skindex-29; OGs) | 14 other skin conditions extracted from previous studies | Socialization w/ others; medical comorbidities; PWS severity; treatments | Skin-disease-specific QoL |
Anxiety & depression: most reported comorbidities and associated w/ impact on QoL↑*** QoL ↔ comorbid depression**, limited facial mobility**, presence of other skin conditions* Emotional impairments↓ ↔ older patients**, patients from educationnal services*** PWS hypertrophy ↔ emotional↑* & symptomatic↑*** impairments Functional impairments↓ ↔ close friends↑* & social engagements↑* PWS patients: QoL↓ vs. non-affected subjects (but similar to CTCL, rosacea, alopecia & vitiligo) |
Moderate |
| Augustin et al. [132] | Germany | 70 | Cross-sectional—quantitative (low) | Dermatologist's clinical assessment; Questionnaires (SCL-53R; ALLTAG; CSDQ; FKS; OGs) | 1006 non-affected subjects (extracted from another study) | Skin-specific coping; impact of PWS; global QoL limitations | Emotional well-being; body image |
PWS patients: emotional well-being↓ (interpersonal sensitivity***, anxiety**, hostility**, phobic anxiety**, paranoid ideation**); body perception↓ (attractiveness/self-confidence*); QoL↓ (social relationship*) vs. non-affected subjects Body perception & emotional well-being ↔ physical malaise* |
High |
| Ben-Tovim & Walker, [131] | Australia | 52 (including about 5 adolescents) | Cross-sectional—quantitative (moderate) | Questionnaires (BAQ) | 49 rheumatoid arthritis; 23 eczema/psoriasis; 50 type 1 diabetes; 174 non-affected subjects | Body informations (height, weight, BMI) | Body-related attitudes | BVD patients: body-related attitudes impacted (strength/fitness↑*; salience of weight/shape↓*) | Moderate |
| Lanigan & Cotterill, [130] | England | 71 (including about 5 adolescents) | Cross-sectional—quantitative (low) | Questionnaires (GHQ; HADS; OGs) | N/A | PWS characteristics | General health; Emotional state; Attitudes toward their PWS |
No significant impact of PWS on emotional or psychiatric state Social difficulties in PWS patients: dealing with strangers' reactions; need to hide the mark or treat it; impact on self-confidence; feeling different from others; feeling unattractive |
Moderate |
| Malm & Carlberg, [133] | Sweden | 23 | Exploratory -qualitative (low) | Structured interviews | N/A | N/A | N/A |
Patients with Large PWS: dealing with strangers' reactions (emotionally difficult); family as a source of support; childhood's bad experiences; very different coping in social contexts (withdrawall, taunt, humor); mark-hiding techniques (make-up) Patients with Small PWS: mark-hiding techniques (make-up); dealing with strangers' (paranoid anxiety about PWS); exaggerated self-consciousness about the PWS |
Low |
All study considered socio-demographic aspects (for example: age; gender; education lvl; socio-economic status; profession; etc.)
IV Independent variable; DV Dependent variable; GTM Grounded theory methodology; TCA Thematic content analysis; PWS Port-wine-stains; CTCL Cutaneous T-cell lymphoma; BVD Blood vessel disorders; MLR Multiple linear regression; pop Population
A ↔ B: A is associated with B; A → B: A predicts B; A↑: A is higher/more important; A↓: A is lower/less important; with: w/; without: w/o; vs.: compared to…
p < 0.05*; p < 0.01**; p < 0.001***
Social interactions and perception of birthmarks
Many participants reported having difficulties in dealing with people's reactions [130, 133]. It should also be noted that the study conducted by Malm and Carlberg (1988) focused on the differences in the experiences of people with large PWS and small PWS. Participants with large PWS reported having negative childhood experiences with some of their peers [133], making them feel different from other children [130, 132]. In order to overcome these problems, those with birthmarks developed very different ways of coping in social contexts, such as taunts, humor, and avoidance or withdrawal [132, 133]. In addition, subjects identified family and friends as a great source of support [133].
Impact on psychological functioning
All the elements mentioned above have repercussions on the individual and psychological functioning of people with birthmarks. Firstly, these studies showed that, compared to non-affected people, patients with birthmarks have an altered QoL [101, 132], lower emotional well-being [132] and an impaired body image [131, 132]. Hagen and colleagues (2017) observed that the more PWS is severe (tissue hypertrophy, birthmark size), the greater the negative impact on QoL, especially in emotional and symptoms-related domains. However, this result is not consensual, as Lanigan & Cotterill (1989) didn’t find any significant impact of PWS on emotional or psychiatric state. Regarding body image, one study showed that feelings of attractiveness and body-related self-confidence were impacted [132]. Furthermore, body-related attitudes were altered in people with birthmarks, with an acute vigilance to body strength, weight, and shape compared to unaffected people [131]. As a result, studies reported a lack of confidence in people with birthmarks and the fact that they felt unattractive [130, 132]. To that extent, many participants used techniques to hide their birthmark, such as make-up, or sought treatment to reduce the stain: for example, laser therapy [130, 133]. In addition, the authors found that people with small PWS had exaggerated self-consciousness about their PWS compared to those with bigger marks [133]. In this regard, several participants reported having undergone many therapies during their childhood to treat their birthmark [132].
Discussion
The main objectives of this systematic literature review were (1) to explore the impact of RGSD on daily life, emotional state, self-perception, and QoL; (2) to identify the main predictors of these health outcomes; and (3) to identify which characteristics in the psychosocial experience of the subjects are common to each of these various pathologies. We therefore focused on four RGSDs in this review: albinism, NF1, ichthyosis, and birthmarks. Of the 9987 recorded references, 48 were retained for analysis.
Despite deep differences in the biological and physiological mechanisms of the RGSDs we investigated, several common elements have emerged, related to their psychosocial impact. The common core of the main results observed in this review is represented in the following table (Table 6).
Table 6.
Summary table of results observed and common to at least two diseases
Firstly, the results of our investigation show that each RGSD has an impact on patients’ social life. Childhood and especially adolescence are pivotal periods in a person's development, where many changes (physical, cognitive, and social) occur and peers play a predominant role in the social development of the individual, largely influencing their own self-perception and identity construction [134, 135]. Unfortunately, according to the results, this is also the period when subjects are the most exposed to discrimination and stigmatizing experiences. Having a skin disease, a visible difference to others at this stage of life, would therefore favor the construction of a disabled or a stigmatized identity [136] that negatively impacts self-perception and social functioning. To illustrate this point, we can cite the study by Jensen et al. (2018) which showed that adolescence is the period of life when enacted and perceived stigma slides into more internalized stigma.
One of the relevant reviews conducted by Wanitphakdeedecha and colleagues (2021) aimed to explore what impact PWS had on self-stigma. As the authors remind us, self-stigma differs from social stigma in that it is an internalized mechanism in the stigmatized person who is anticipating social rejection. Self-stigma consists of three dimensions: perceived, anticipated, and enacted stigma.4 According to their findings, the negative impact of PWS on their psychosocial condition came mostly from patients’ enacted stigma or experiences with discriminatory acts toward their skin condition. On the other hand, perceived and anticipated stigma varied a lot among different age groups, knowing that anticipated stigma tends to increase with age. One of the most striking findings is that parents of PWS patients are more affected by self-stigma than their children. The psychological burden that this entails can however be regulated by good medical support and parent-health staff communication.
As a result, in all the conditions we have identified, participants expressed discomfort in social interactions, especially when they had to deal with strangers' reactions [37, 89, 95, 115, 117, 119, 121, 122, 130, 133].
However, although these diseases can cause social difficulties, these seemed to be compensated by the support patients receive from family, friends, or other patients involved in the same associations or organizations [31, 33, 37–39, 44, 89, 110, 111, 117, 119, 120, 133]. Indeed, social or family support was shown to be an important factor buffering the negative effects of stress in a wide range of clinical populations, for example, in the case of chronic skin conditions [137, 138] or in more critical health issues such as cancer [139–142]. However, some conditions within a specific context (in this case, albinism in Africa) can induce a very ambivalent family functioning mixing fear, protection, guilt, and violence [41].
Secondly, for each of the RGSDs investigated, patients’ QoL and emotional state were impaired. Very different determinants have been considered, but disease severity and visibility were the variables that researchers most often investigated. Many studies on NF1, ichthyosis, and birthmarks were able to show a link between the visibility or severity of the disorder, and a reduced QoL, depressive or anxious states, and an altered body image. These results echo other research conducted in subjects with chronic skin conditions such as atopic dermatitis [143–145], psoriasis [146–151] or vitiligo [152–156]. Other studies, conducted with "disfigured" patients, also resonate with our results [157]: we can mention craniofacial conditions, such as cleft lip or palate [158, 159], or even people with burn injuries [160, 161]. However, we can assume that there are differences in the impact that these different diseases have on body image. A line of thought is given to us by Ben-Tovim & Walker (1995; PWS and other chronic diseases), who suggest that women with visible diseases from birth or early childhood would be less prone to developing negative body attitudes than those acquiring visible illnesses during adolescence or adulthood. In order to cope with the visible nature of their condition, patients with NF1, ichthyosis, and birthmarks tried to camouflage their body parts that are most affected (clothing, make-up, tattoos) [119, 120, 122, 130, 133, 162]. These body-hiding techniques have shown their benefits on QoL in other visible conditions as vitiligo [163, 164], burn injuries [165, 166], facial skin disorders (acne, atopic dermatitis, psoriasis) [167, 168], infantile hemangiomas [169], and in post-surgical cancer treatment [170–172], which again demonstrates the impact of these conditions on body image [170, 172]. Furthermore, in the case of PWS, having had an early birthmark resorption treatment (e.g., flash-lamp pulsed dye laser) has a positive effect on the subject’s QoL [109]. Although many studies have shown the benefit of cosmetic camouflage in patients with skin disfigurement [173], another research has suggested that hiding any type of stigmatizing identity could have a negative impact on physical and psychological QoL [174]. Thus, the skin is a highly symbolically invested organ [175] and such genetic pathologies, like any visible disease, seem to induce stigmatization and social isolation, which are sources of anxiety, depressive disorders, emotional distress, impairment of QoL, and withdrawal [176].
In a surprising way, albinism is, in this study, the only genetic condition for which body image, and the visibility and severity of disease were not assessed. In PWA, low vision and QoL were the issues that Western researchers focused on most [177, 178]. In sharp contrast with the African continent, studies focused more on issues of discrimination linked to local beliefs and superstitions [19–22]. Many papers reported on the violence suffered by young PWA who were stalked, assaulted, or even killed for their physical attributes (hair, bones, etc.), particularly in Tanzania and Malawi. Furthermore, Africa has a majority dark-skinned population, making albinism all the more visible and therefore stigmatizing in the social environment. As we can see, the geographical and cultural context is a decisive factor in the understanding of this condition; health issues are drastically higher in Africa than in the rest of the world.
Considering this, it can also be noted that albinism, NF1, and ichthyosis are similar on several other issues, including the impact of the disease on daily life [31, 89, 92, 122, 128], on love life and sexuality [31, 37, 92, 110, 111, 115, 122], on academic and professional aspirations [31, 37, 111, 115, 122, 128], and finally on the lack of knowledge and interest from the medical community [39, 92, 112, 119]. Firstly, we can observe that impact on daily life greatly varies depending on the disease and the severity of the associated symptoms. In albinism, low vision seems to restrict patients' mobility as they are not allowed to drive a car [31], whereas in ichthyosis and NF1, unpleasant body sensations and pain disturb patients' sleep and walking [89, 92, 120, 128]. Thus, even though these rare diseases fit into the same taxonomic category, the complexity of their particularities invites us to distinguish them according to the specificities of their psychosocial impact. Mobility issues are also a widely identified difficulty in visual impairments such as glaucoma [179, 180], age-related macular degeneration (AMD) [181, 182], and diabetic retinopathy [183, 184]. Therefore, in terms of daily functioning, albinism is also comparable to ophthalmological conditions. However, there is one aspect on which all of these conditions converge: the importance of taking care of one's skin to avoid discomfort and complications. This long-term preventive treatment seems to play an important role in patients' daily lives.
These three genetic conditions also seemed to have a negative impact on patients' love and sex lives [31, 37, 92, 110, 111, 115, 122]. Studies on other visible conditions have observed similar results such as vitiligo [185], or in the case of severe burns [186]. The skin is an undeniable vector of intimacy and sexuality; when it is altered, body image is disturbed, and concerns about appearance and touch can make intimate relationships difficult.
Albinism and NF1 also share various other problems. Research showed a wide variety of adaptive strategies used by patients: use of repartee and humor in awkward social situations, scientific knowledge seeking, fighting spirit, spirituality, patient community involvement, physical hypervigilance, etc. [31, 32, 39, 111, 112, 120, 121]. These coping strategies are directly related to the fact that patients must constantly adapt themselves to societal norms in order to be seen as “normal”. Some even said they seemed to experience an "abnormality complex" when comparing themselves to others, both in terms of ability and learning [31, 33, 42, 112, 119]. For some people, there is a compelling need to conform, even if it means forcing themselves to pretend that they don’t have a disability (e.g., a child pretending to be able to read on the blackboard when they cannot). This type of behavior requires considerable effort from the person who gradually becomes exhausted and therefore weakens. Other studies revealed similar results in patients with intellectual disabilities [187, 188] and visual impairments [189]. Indeed, depending on the degree to which their impairment is mild or severe, people with physical or sensory impairments might attempt to overcome their difficulties in order to be considered as “normal” [136].
Finally, research has highlighted the distress experienced by some parents of children with NF1 or albinism. In this regard, parental stress and family functioning have been extensively studied in parents of children with developmental or intellectual disabilities [190] and sensory or physical impairments [191]. However, in the context of RGSD, parental distress has been supplemented by another concept. For all these conditions (with the exception of PWS), the prominence of guilt in the transmission of genetic disease is also notable. This notion has already been discussed by Chaumet in 2006, whose study explains that although the genetic disease may strengthen a sense of family belonging, it also makes the parent(s) feel guilty about giving the child a difficult and / or challenging life. Thus, choosing to have a child or not when affected by one of these genetic conditions becomes a real individual and family issue.
Limitations
One of the main limitations of this review is the very attempt to synthesize common findings when the genetic conditions cover a wide range of different symptoms. Even if skin gathers these diseases within the same category, the skin is not affected in the same way in the case of albinism (hypopigmentation, photosensitivity) or NF1 (café-au-lait spots, neurofibromas). Moreover, some of these conditions are divided into subcategories according to the phenotypic or genotypic specificities of each. For example, albinism may be ocular, oculocutaneous, or syndromic, and the related psychosocial issues may vary from one form to another. One of the main differences identified in this review concerns OCA and HPS; syndromic forms of albinism which cause physiological disturbances (coagulopathy, an increased risk of neutropenia, pulmonary fibrosis, or Crohn's disease) that are not found in non-syndromic forms [192]. This aspect of the disease is an additional concern for HPS patients, who are sometimes worried about the unpredictable nature of the disease’s evolution [39].
Secondly, the methodological constraints inherent to the field of rare diseases make the generalization of these results almost impossible. The fact that these genetic diseases are very poorly represented in the general population makes it difficult to recruit voluntary participants. Sample sizes are often too small and suffer from representativeness bias. Even if some of these studies show significant results, the vast majority cannot generalize their observations. This results in great heterogeneity in study designs, sampling, and data collected, making it difficult to harmonize our findings. It should also be noted that the geographical context in which these studies were carried out has a major influence on the results, particularly in the case of albinism, and makes the synthesis of knowledge even more complex.
Perspectives
One of the recurrent themes that we have not detailed above concerns the lack of interest and knowledge of doctors regarding RGSDs [31, 39, 92, 112, 119]. In this respect, it is important to note that rare diseases have never attracted as much interest around the world as they do today. In the last 20 years, medical findings in genetics have grown steadily, have improved diagnostic techniques, and deepened our understanding of how many diseases work. However, there are still large grey areas, and institutional and practitioner priorities remain focused on more common or more severe diseases (cancer, diabetes, cardiovascular diseases, dementia, etc.). As mentioned in our introduction, today 6000 to 8000 rare diseases have been identified in the world, each of them having a very particular functioning, more or less complex depending on the case. It is therefore almost impossible to be an expert on all of these diseases and to know how to take care of these patients. This issue can be illustrated by the fact that, in France, several patients with a rare disease experience significant diagnostic wandering, which can last up to six years after the first symptoms appear [2]. If physicians are in difficulty because they have scarce knowledge of these conditions, would they perhaps express a lack of interest in order to absolve themselves of responsibility for their medical follow-up?
At the medical level, genetic diseases raise a thorny ethical question regarding prenatal diagnosis. When parents know they are carriers of a potentially transmissible genetic anomaly, in particular contexts, they can resort to prenatal diagnosis and even selective termination of pregnancy if the fetus is affected. Clarke (2013) [193] addressed this question in families with Hypohidrotic Ectodermal Dysplasia and showed that opinions were divided among the participants; some were in favor of using these medical techniques, others against. This gap in opinion may then become a central issue for the parental couple who will have to make a crucial decision for their future. These ethical and legal questions have already been raised in the context of trisomy 21, in particular regarding the proximity that certain practices have with eugenics [194–196].
Secondly, in order to optimize healthcare, we could imagine developing a care plan that would begin as soon as the person is diagnosed. In fact, setting up a system with healthcare support time from when the diagnosis is first made would provide patients with important information about their condition, and would allow them to be oriented in the best healthcare pathway. These care systems have already shown their benefits when a serious illness, such as cancer, is diagnosed [197]. With this aim in mind, we can cite a French and a Korean team that have recently developed guidelines to help practitioners in the diagnosis and care of albinism [198] and NF1 [199]. In addition, practitioners could also offer patients the opportunity to participate in therapeutic education (TPE) sessions to learn how to manage their symptoms and develop coping strategies that would be useful in their daily lives. Some hospitals have already started to set up such a programme. For example, another French team has also developed a TPE protocol for children and adolescents with albinism and their parents [200].
This last element clearly highlights the need to offer patients biopsychosocial support based on a systemic approach. In view of the main results of this review, the whole family must be considered in patient follow-up, both at a genetic level (genetic inheritance pattern) and at a psychosocial level (psychoeducation, transmission guilt, coping with stigma). As Chaumet said in 2006, "genetic diseases are family affairs". Moreover, caring for patients with genetic diseases is a complex, time-consuming task that requires a holistic approach toward the patient and interdisciplinary support (links between geneticists, specialists in affected organs [such as the skin], and general practitioners). In order to better understand RGSDs, Bronfenbrenner's ecosystemic model (1979) [201] offers, in our opinion, the most appropriate approach for understanding and supporting such diseases. Bronfenbrenner (1979) maintains that the development of a person must be understood within a complex environmental system in which each system is conceived as a unit communicating with a larger and more organized system. Understanding the functioning of these different systems would therefore allow a better understanding of a patient case and thus promote their well-being. Speaking of a systemic approach, researchers have already proposed a global, cross-cutting framework for health stigma and discrimination. Based on theory, research, and practice, this framework demonstrates its application to a range of health conditions, including leprosy, epilepsy, mental health, cancer, HIV, and obesity/overweight [202]. It would therefore be very interesting to test it in future research on rare skin conditions, or to propose psychosocial interventions based on their findings. Concerning albinism, some French organizations specialized in sensory disabilities already seem to provide interesting interdisciplinary support to young PWA. For example, Assistance Services for Autonomy Acquisition and Schooling (in French: Service d'Aide à l'Acquisition de l'Autonomie et à la Scolarisation) and Early Medical and Social Action Centers (in French: Centres d'Action Médico-Sociale Précoce) work with many actors in order to help families concerned by albinism (or any other ophthalmologic genetic disease); health professionals are increasingly trained in systemic approaches and tend to approach care with transdisciplinarity [203, 204]. It would be judicious to see to what extent related medical, educational, and social establishments (Specialized Education and Home-Care Services; in French: Service d'Éducation Spéciale et de Soins à Domicile) could support families concerned by RGSD.
Finally, it is still essential to keep investigating the experiences of these populations, given the lack of evidence on these health issues in many countries. In order to compensate for sample size issues and to have more robust studies designs, it would be wise to set up longitudinal lifespan studies to see the possible developmental pathways in these diseases more precisely, and which factors would favour one pathway or another. In addition, action research or intervention-based studies could also be considered (prevention in schools, consultation/therapy for parents, etc.) and allow the implementation of direct and beneficial interventions for patients and their families.
Conclusion
Despite the heterogeneity and methodological weaknesses of the studies we have investigated, this review has shed useful light on the psychosocial consequences of RGSDs on affected people and their families. In addition, the care pathway remains complex and tedious, and the professionals involved are few. Although new care systems are slowly being put in place in some institutes, access to adapted care remains limited and unequally distributed across countries. It would therefore be relevant to mobilize public policies in order to give more resources to associations and medico-socio educational centers that already support concerned families. As we have seen above, a systemic approach to the patient, considering all aspects of their life and environment (family, school, work, activities of daily living, etc.) would be the most optimal way to deal with these conditions.
Supplementary Information
Additional file 1: Evaluation grid for qualitative exploratory studies.
Acknowledgements
We would like to thank Victoria Andrews for her help in finalizing the article in English. We would also like to thank Diana Arshakyan for her help in processing some of the article data after the screening phase.
Abbreviations
- DoK
Disorders of keratinisation
- HPS
Hermansly-Pudlak syndrome
- NF1
Neurofibromatosis type 1
- PNf
Plexiform neurofibroma
- PWA
People with albinism
- PWS
Port-wine-stains
- QoL
Quality of life
- RGSD
Rare genetic skin disease
- TPE
Therapeutic patient education
Author contributions
The first author, HF, was the principal investigator of the review: he defined the initial question, the methodology, collected the essential data, and wrote and revised the manuscript. The last author, BQ, monitored, advised, and revised HF's work throughout the review process. The second author, NC, reviewed one third of the articles selected after screening the titles and abstracts. The third author and medical geneticist, FMP, helped identify the genetic diseases of interest to the review. Finally, all authors held meetings at each stage of the research so that each data was cross-checked, and each review decision was made collectively. All authors read and approved the final manuscript.
Funding
The review work and the writing of this article were financed by the French Rare Diseases Foundation (Fondation des Maladies Rares). The cost of publication was entirely covered by BQ’s professional funds.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
No new human data is used in this review and therefore no ethics approval is applicable.
Consent for publication
Not applicable as only data from the selected articles were used in this paper. The authors declare that they did not have access to any personal data for this review.
Competing interests
The author declares that he has no competing interests.
Footnotes
Other diseases were also considered (primary cutaneous amyloidosis, tuberous sclerosis complex, epidermolysis bullosa, xeroderma pigmentosum) but were not selected for the following reasons: accompanied by too severe or too varied symptoms, no relevant studies, or a review of the literature already published (for more details, see “Method”).
New genes associated with albinism are still being discovered, both in its oculocutaneous form (OCA8) (Pennamen et al. 2021) and in its syndromic forms (HPS11) (Michaud et al. 2021).
Puerto Rico has a large population of people with Hermansky-Pudlak syndrome (HPS): where the global prevalence of HPS is 1–9/1,000,000, in Puerto Rico it drops to 1/1,800 (or almost 556 × larger).
Perceived stigma is an individual’s understanding of how others may think about and act toward an individual with different physical traits. Anticipated stigma refers to expectations of stigma in the future. Enacted stigma denotes discriminatory acts or behaviors experienced by the stigmatized people (Wanitphakdeedecha et al. 2021).
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hugo Fournier, Email: hugo.fournier@u-bordeaux.fr.
Nicolas Calcagni, Email: nicolas.calcagni@chu-bordeaux.fr.
Fanny Morice-Picard, Email: fanny.morice-picard@chu-bordeaux.fr.
Bruno Quintard, Email: bruno.quintard@u-bordeaux.fr.
References
- 1.Boucand MH. Une approche éthique des maladies rares génétiques: Enjeux de reconnaissance et de compétence. Eres. 2018;357.
- 2.Heuyer T, Pavan S, Vicard C. The health and life path of rare disease patients: results of the 2015 French barometer. PROM. 2017;8:97–110. doi: 10.2147/PROM.S131033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kole A, Faurisson F. The voice of 12,000 patients: experiences and expectations of rare disease patients on diagnosis and care in Europe. 2009. http://download2.eurordis.org.s3.amazonaws.com/the-voice-of-rare-disease-patients.pdf. Accessed 1 Nov 2021.
- 4.Montoliu L, Grønskov K, Wei AH, Martínez-García M, Fernández A, Arveiler B, et al. Increasing the complexity: new genes and new types of albinism. Pigment Cell Melanoma Res. 2014;27(1):11–18. doi: 10.1111/pcmr.12167. [DOI] [PubMed] [Google Scholar]
- 5.Grønskov K, Dooley CM, Østergaard E, Kelsh RN, Hansen L, Levesque MP, et al. Mutations in C10orf11, a Melanocyte-differentiation gene, cause autosomal-recessive albinism. Am J Hum Genet. 2013;92(3):415–421. doi: 10.1016/j.ajhg.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei AH, Zang DJ, Zhang Z, Liu XZ, He X, Yang L, et al. Exome sequencing identifies SLC24A5 as a candidate gene for nonsyndromic oculocutaneous albinism. J Invest Dermatol. 2013;133(7):1834–1840. doi: 10.1038/jid.2013.49. [DOI] [PubMed] [Google Scholar]
- 7.Newton JM, Cohen-Barak O, Hagiwara N, Gardner JM, Davisson MT, King RA, et al. Mutations in the human orthologue of the mouse underwhite gene (uw) underlie a new form of oculocutaneous albinism, OCA4. Am J Hum Genet. 2001;69(5):981–988. doi: 10.1086/324340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbosa MDFS, Barrat FJ, Tchernev VT, Nguyen QA, Mishra VS, Colman SD, et al. Identification of mutations in two major mRNA isoforms of the chediak-higashi syndrome gene in human and mouse. Hum Mol Genet. 1997;6(7):1091–1098. doi: 10.1093/hmg/6.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh J, Bailin T, Fukai K, Feng GH, Ho L, Mao J, et al. Positional cloning of a gene for Hermansky-Pudlak syndrome, a disorder of cytoplasmic organelles. Nat Genet. 1996;14(3):300–306. doi: 10.1038/ng1196-300. [DOI] [PubMed] [Google Scholar]
- 10.Boissy RE, Zhao H, Oetting WS, Austin LM, Wildenberg SC, Boissy YL, et al. Mutation in and lack of expression of tyrosinase-related protein-1 (TRP-1) in melanocytes from an individual with brown oculocutaneous albinism: a new subtype of albinism classified as « OCA3 ». Am J Hum Genet. 1996;58(6):1145–1156. [PMC free article] [PubMed] [Google Scholar]
- 11.King RA, Witkop CJ. Hairbulb tyrosinase activity in oculocutaneous albinism. Nature. 1976;263(5572):69–71. doi: 10.1038/263069a0. [DOI] [PubMed] [Google Scholar]
- 12.Estrada-Hernández N, Harper DC. Research on psychological and personal aspects of albinism: a critical review. Rehabil Psychol. 2007;52(3):263–271. doi: 10.1037/0090-5550.52.3.263. [DOI] [Google Scholar]
- 13.Kajiru I, Nyimbi I. The impact of myths, superstition and harmful cultural beliefs against albinism in Tanzania: a human rights perspective. Potchefstroom Electron Law J. 2020;23:1–27. doi: 10.17159/1727-3781/2020/V23I0A8793. [DOI] [Google Scholar]
- 14.Austin CL. Steps toward acceptance: my journey as a woman with albinism and implications for practice. J Vis Impair Blind. 2018;112(6):713–715. doi: 10.1177/0145482X1811200606. [DOI] [Google Scholar]
- 15.Gold ME, He H. A journey of success: an african american woman with oculocutaneous albinism. J Vis Impair Blind. 2018;112(6):772–776. doi: 10.1177/0145482x1811200613. [DOI] [Google Scholar]
- 16.Mswela M. Does albinism fit within the legal definition of disability in the employment context? A comparative analysis of the judicial interpretation of disability under the SA and the US non-discrimination laws. Potchefstroom Electron Law J. 2018;21:1–37. doi: 10.17159/1727-3781/2018/v21i0a1684. [DOI] [Google Scholar]
- 17.Ndlovu I. Writing in and about prison, childhood albinism and human temporality in the book of memory. J Lit Stud. 2018;34(4):33–47. doi: 10.1080/02564718.2018.1538077. [DOI] [Google Scholar]
- 18.Imafidon E. Dealing with the other between the ethical and the moral: albinism on the African continent. Theor Med Bioeth. 2017;38(2):163–177. doi: 10.1007/s11017-017-9403-2. [DOI] [PubMed] [Google Scholar]
- 19.Brocco G. Albinism, stigma, subjectivity and global-local discourses in Tanzania. Anthropol Med. 2016;23(3):229–243. doi: 10.1080/13648470.2016.1184009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brocco G. Labeling albinism: language and discourse surrounding people with albinism in Tanzania. Disabil Soc. 2015;30(8):1143–1157. doi: 10.1080/09687599.2015.1075869. [DOI] [Google Scholar]
- 21.Uromi SM. Violence against persons with albinism and older women: tackling withcraft accusation in Tanzania. Int J Educ Res. 2014;2(6):323–338. [Google Scholar]
- 22.Tanner R. Ideology and the killing of albinos in Tanzania: a study in cultural relativities. Anthropologist. 2010;12(4):229–236. doi: 10.1080/09720073.2010.11891161. [DOI] [Google Scholar]
- 23.Corn AL, Lusk KE. An analysis of parents’ reports on educational services for their children with albinism. J Vis Impair Blind. 2018;112(6):667–682. doi: 10.1177/0145482x1811200603. [DOI] [Google Scholar]
- 24.Corn AL, Lusk KE. Reports from parents about medical and low vision services for their children with albinism: an analysis. J Vis Impair Blind. 2018;112(6):655–666. doi: 10.1177/0145482X1811200602. [DOI] [Google Scholar]
- 25.Lund PM, Gaigher R. A health intervention programme for children with albinism at a special school in South Africa. Health Educ Res. 2002;17(3):365–372. doi: 10.1093/her/17.3.365. [DOI] [PubMed] [Google Scholar]
- 26.Lund PM. Health and education of children with albinism in Zimbabwe. Health Educ Res. 2001;16(1):1–7. doi: 10.1093/her/16.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Ashley JR, Cates DL. Albinism: educational techniques for parents and teachers. Rev Rehab Educ Blind Vis Impair. 1992;24(3):127–132. [Google Scholar]
- 28.De Groot T, Meurs P, Jacquet W. The effect of contact interventions on the stigma of people with albinism in Tanzania. J Vis Impair Blind. 2019;113(5):464–469. doi: 10.1177/0145482X19874188. [DOI] [Google Scholar]
- 29.Vander Kolk CJ, Bright BC. Albinism: a survey of attitudes and behavior. J Vis Impair Blind. 1983;77(2):49–51. doi: 10.1177/0145482x8307700201. [DOI] [Google Scholar]
- 30.Palmer C. Myths, stereotypes and self-perception: the impact of albinism on self-esteem. Br J Vis Impair. 2007;25(2):144–154. doi: 10.1177/0264619607075998. [DOI] [Google Scholar]
- 31.Estrada-Hernandez N. Psychosocial aspects of physical difference: the experiences of eight adults with albinism in Puerto Rico. J Vis Impair Blind. 2018;112(6):701–712. doi: 10.1177/0145482X1811200605. [DOI] [Google Scholar]
- 32.Tambala-Kaliati T, Adomako EB, Frimpong-Manso K. Living with albinism in an African community: exploring the challenges of persons with albinism in Lilongwe District, Malawi. Heliyon. 2021 doi: 10.1016/j.heliyon.2021.e07034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phatoli R, Bila N, Ross E. Being black in a white skin: Beliefs and stereotypes around albinism at a South African university. Afr J Disabil. 2015;4(1):106. doi: 10.4102/ajod.v4i1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ojedokun IM. Effects of stigmatisation on psychosocial and health well-being of people living with albinism in south-west Nigeria. Afr J Soc Work. 2018;8(1):31–38. [Google Scholar]
- 35.Maia M, Volpini BMF, dos Santos GA, Rujula MJP. Quality of life in patients with oculocutaneous albinism. An Bras Dermatol. 2015;90(4):513–517. doi: 10.1590/abd1806-4841.20153498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ajose FOA, Parker RA, Merrall ELC, Adewuya AO, Zachariah MP. Quantification and comparison of psychiatric distress in African patients with albinism and vitiligo: a 5-year prospective study. J Eur Acad Dermatol Venereol. 2014;28(7):925–932. doi: 10.1111/jdv.12216. [DOI] [PubMed] [Google Scholar]
- 37.Anshelevich EE, Mosojane KI, Kenosi L, Nkomazana O, Williams VL. Factors affecting quality of life for people living with albinism in Botswana. Dermatol Clin. 2021;39(1):129–145. doi: 10.1016/j.det.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 38.Dapi LN, Tambe BA, Monebenimp F. Myths surrounding albinism and struggles of persons with albinism to achieve human rights in Yaoundé. Cameroon J Hum Rights Soc Work. 2018;3(1):11–16. doi: 10.1007/s41134-018-0048-5. [DOI] [Google Scholar]
- 39.Christensen S, Wagner L, Coleman MM, Appell D. The lived experience of having a rare medical disorder: Hermansky-Pudlak syndrome. Chronic Illn. 2017;13(1):62–72. doi: 10.1177/1742395316655854. [DOI] [PubMed] [Google Scholar]
- 40.Pooe-Monyemore MBJ, Mavundla TR, Christianson AL. The experience of people with oculocutaneous albinism. Health SA Gesondheid. 2012 doi: 10.4102/hsag.v17i1.592. [DOI] [Google Scholar]
- 41.Aborisade RA. “Why always me?”: Childhood experiences of family violence and prejudicial treatment against people living with albinism in Nigeria. J Fam Viol. 2021;36(8):1081–1094. doi: 10.1007/s10896-021-00264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang M, Chen L, Hung S, Puthussery S. Women’s experiences of living with albinism in Taiwan and perspectives on reproductive decision making: a qualitative study. Disabil Soc. 2020;37(6):916–932. doi: 10.1080/09687599.2020.1867071. [DOI] [Google Scholar]
- 43.Chu B, Maranga A, Mosojane KI, Allen-Taylor L, Ralethaka M, Ngubula JC, et al. Sociodemographic features of a cohort of people living with albinism in Botswana. JAAD Int. 2021;2:153–163. doi: 10.1016/j.jdin.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Affram AA, Teye-Kwadjo E, Gyasi-Gyamerah AA. Influence of social stigma on subjective well-being of persons with albinism in Ghana. J Community Appl Soc Psychol. 2019;29(4):323–335. doi: 10.1002/casp.2403. [DOI] [Google Scholar]
- 45.Attama CM, Uwakwe R, Onyeama GM, Igwe MN. Psychiatric morbidity among subjects with leprosy and albinism in South East Nigeria: a comparative study. Ann Med Health Sci Res. 2015;5(3):197–204. doi: 10.4103/2141-9248.157503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orphanet: the portal for rare diseases and orphan drugs|Neurofibromatosis type 1. (2014). https://www.orpha.net/consor/cgi-bin/OC_Exp.php?Expert=636&lng=EN. Accessed 2 Nov 2021.
- 47.Cutting LE, Clements AM, Lightman AD, Yerby-Hammack PD, Denckla MB. Cognitive profile of neurofibromatosis Type 1: rethinking nonverbal learning disabilities. Learn Disabil Res Pract. 2004;19(3):155–165. doi: 10.1111/j.1540-5826.2004.00099.x. [DOI] [Google Scholar]
- 48.Barton B, North K. Social skills of children with neurofibromatosis type l. Dev Med Child Neurol. 2004;46(8):553–563. doi: 10.1017/s0012162204000921. [DOI] [PubMed] [Google Scholar]
- 49.Barton B, North K. The self-concept of children and adolescents with neurofibromatosis type 1. Child Care Health Dev. 2007;33(4):401–408. doi: 10.1111/j.1365-2214.2006.00717.x. [DOI] [PubMed] [Google Scholar]
- 50.Johnson H, Wiggs L, Stores G, Huson SM. Psychological disturbance and sleep disorders in children with neurofibromatosis type 1. Dev Med Child Neurol. 2005;47(4):237–242. doi: 10.1111/j.1469-8749.2005.tb01128.x. [DOI] [PubMed] [Google Scholar]
- 51.Noll RB, Reiter-Purtill J, Moore BD, Schorry EK, Lovell AM, Vannatta K, et al. Social, emotional, and behavioral functioning of children with NF1. Am J Med Genet A. 2007;143A(19):2261–2273. doi: 10.1002/ajmg.a.31923. [DOI] [PubMed] [Google Scholar]
- 52.Krab LC, Oostenbrink R, de Goede-Bolder A, Aarsen FK, Elgersma Y, Moll HA. Health-related quality of life in children with neurofibromatosis type 1: contribution of demographic factors, disease-related factors, and behavior. J Pediatr. 2009;154(3):420–425. doi: 10.1016/j.jpeds.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 53.Huijbregts SCJ, de Sonneville LMJ. Does cognitive impairment explain behavioral and social problems of children with neurofibromatosis type 1? Behav Genet. 2011;41(3):430–436. doi: 10.1007/s10519-010-9430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huijbregts S, Jahja R, De Sonneville L, De Breij S, Swaab-Barneveld H. Social information processing in children and adolescents with neurofibromatosis type 1. Dev Med Child Neurol. 2010;52(7):620–625. doi: 10.1111/j.1469-8749.2010.03639.x. [DOI] [PubMed] [Google Scholar]
- 55.Pasini A, Lo-Castro A, Di Carlo L, Pitzianti M, Siracusano M, Rosa C, et al. Detecting anxiety symptoms in children and youths with neurofibromatosis type I. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(7):869–873. doi: 10.1002/ajmg.b.32095. [DOI] [PubMed] [Google Scholar]
- 56.Lehtonen A, Howie E, Trump D, Huson SM. Behaviour in children with neurofibromatosis type 1: cognition, executive function, attention, emotion, and social competence. Dev Med Child Neurol. 2013;55(2):111–125. doi: 10.1111/j.1469-8749.2012.04399.x. [DOI] [PubMed] [Google Scholar]
- 57.Brei NG, Klein-Tasman BP, Schwarz GN, Casnar CL. Language in young children with neurofibromatosis-1: relations to functional communication, attention, and social functioning. Res Dev Disabil. 2014;35(10):2495–2504. doi: 10.1016/j.ridd.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 58.Holland AA, Stavinoha PL, Swearer SM, Solesbee C, Patel S, Klesse LJ. Rate and frequency of bullying victimization in school-age children with neurofibromatosis type 1 (NF1) Sch Psychol. 2019;34(6):687–694. doi: 10.1037/spq0000333. [DOI] [PubMed] [Google Scholar]
- 59.Sebold CD, Lovell A, Hopkin R, Noll R, Schorry E. Perception of disease severity in adolescents diagnosed with neurofibromatosis type 1. J Adolesc Health. 2004;35(4):297–302. doi: 10.1016/j.jadohealth.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Michaud PA, Suris JC, Viner R. The adolescent with a chronic condition. Part II: healthcare provision. Arch Dis Child. 2004;89(10):943–949. doi: 10.1136/adc.2003.045377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolkenstein P, Rodriguez D, Ferkal S, Gravier H, Buret V, Algans N, et al. Impact of neurofibromatosis 1 upon quality of life in childhood: a cross-sectional study of 79 cases. Br J Dermatol. 2009;160(4):844–848. doi: 10.1111/j.1365-2133.2008.08949.x. [DOI] [PubMed] [Google Scholar]
- 62.Prior J, O’Dell L. ‘Coping quite well with a few difficult bits’: living with disfigurement in early adolescence. J Health Psychol. 2009;14(6):731–740. doi: 10.1177/1359105309338972. [DOI] [PubMed] [Google Scholar]
- 63.Williamson H, Harcourt D, Halliwell E, Frith H, Wallace M. Adolescents’ and parents’ experiences of managing the psychosocial impact of appearance change during cancer treatment. J Pediatr Oncol Nurs. 2010;27(3):168–175. doi: 10.1177/1043454209357923. [DOI] [PubMed] [Google Scholar]
- 64.Esposito M, Marotta R, Roccella M, Gallai B, Parisi L, Lavano SM, et al. Pediatric neurofibromatosis 1 and parental stress: a multicenter study. Neuropsychiatr Dis Treat. 2014;10:141–146. doi: 10.2147/NDT.S55518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin S, Wolters PL, Baldwin A, Roderick MC, Toledo-Tamula MA, Gillespie A, et al. Attitudes about internet support groups among adolescents and young adults with neurofibromatosis type 1 and their parents. J Genet Couns. 2014;23(5):796–804. doi: 10.1007/s10897-014-9688-5. [DOI] [PubMed] [Google Scholar]
- 66.Barke J, Coad J, Harcourt D. Parents’ experiences of caring for a young person with neurofibromatosis type 1 (NF1): a qualitative study. J Community Genet. 2016;7(1):33–39. doi: 10.1007/s12687-015-0247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akre C, Polvinen J, Ullrich NJ, Rich M. Children’s at home: pilot study assessing dedicated social media for parents of adolescents with neurofibromatosis type 1. J Genet Couns. 2018;27(2):505–517. doi: 10.1007/s10897-018-0213-0. [DOI] [PubMed] [Google Scholar]
- 68.Morotti H, Mastel S, Keller K, Barnard RA, Hall T, O’Roak BJ, et al. Autism and attention-deficit/hyperactivity disorders and symptoms in children with neurofibromatosis type 1. Dev Med Child Neurol. 2021;63(2):226–232. doi: 10.1111/dmcn.14558. [DOI] [PubMed] [Google Scholar]
- 69.Payne JM, Walsh KS, Pride NA, Haebich KM, Maier A, Chisholm A, et al. Social skills and autism spectrum disorder symptoms in children with neurofibromatosis type 1: evidence for clinical trial outcomes. Dev Med Child Neurol. 2020;62(7):813–819. doi: 10.1111/dmcn.14517. [DOI] [PubMed] [Google Scholar]
- 70.Eijk S, Mous SE, Dieleman GC, Dierckx B, Rietman AB, de Nijs PFA, et al. Autism spectrum disorder in an unselected cohort of children with neurofibromatosis type 1 (NF1) J Autism Dev Disord. 2018;48(7):2278–2285. doi: 10.1007/s10803-018-3478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bilder DA, Bakian AV, Stevenson DA, Carbone PS, Cunniff C, Goodman AB, et al. Brief report: The prevalence of neurofibromatosis type 1 among children with autism spectrum disorder identified by the Autism and Developmental Disabilities Monitoring Network. J Autism Dev Disord. 2016;46(10):3369–3376. doi: 10.1007/s10803-016-2877-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morris SM, Acosta MT, Garg S, Green J, Huson S, Legius E, et al. Disease burden and symptom structure of autism in neurofibromatosis type 1: a study of the International NF1-ASD Consortium Team (INFACT) JAMA Psychiat. 2016;73(12):1276–1284. doi: 10.1001/jamapsychiatry.2016.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garg S, Heuvelman H, Huson S, Tobin H, Green J. Sex bias in autism spectrum disorder in neurofibromatosis type 1. J Neurodev Disord. 2016 doi: 10.1186/s11689-016-9159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garg S, Plasschaert E, Descheemaeker MJ, Huson S, Borghgraef M, Vogels A, et al. Autism spectrum disorder profile in neurofibromatosis type I. J Autism Dev Disord. 2015;45(6):1649–1657. doi: 10.1007/s10803-014-2321-5. [DOI] [PubMed] [Google Scholar]
- 75.Garg S, Lehtonen A, Huson SM, Emsley R, Trump D, Evans DG, et al. Autism and other psychiatric comorbidity in neurofibromatosis type 1: evidence from a population-based study. Dev Med Child Neurol. 2013;55(2):139–145. doi: 10.1111/dmcn.12043. [DOI] [PubMed] [Google Scholar]
- 76.Plasschaert E, Van Eylen L, Descheemaeker M, Noens I, Legius E, Steyaert J. Executive functioning deficits in children with neurofibromatosis type 1: the influence of intellectual and social functioning. Am J Med Genet B Neuropsychiatr Genet. 2016;171(3):348–362. doi: 10.1002/ajmg.b.32414. [DOI] [PubMed] [Google Scholar]
- 77.Plasschaert E, Descheemaeker MJ, Van Eylen L, Noens I, Steyaert J, Legius E. Prevalence of autism spectrum disorder symptoms in children with neurofibromatosis type 1. Am J Med Genet B Neuropsychiatr Genet. 2015;168(1):72–80. doi: 10.1002/ajmg.b.32280. [DOI] [PubMed] [Google Scholar]
- 78.Koth CW, Cutting LE, Denckla MB. The association of neurofibromatosis type 1 and attention deficit hyperactivity disorder. Child Neuropsychol. 2000;6(3):185–194. doi: 10.1076/chin.6.3.185.3155. [DOI] [PubMed] [Google Scholar]
- 79.Klein-Tasman BP, Janke KM, Luo W, Casnar CL, Hunter SJ, Tonsgard J, et al. Cognitive and psychosocial phenotype of young children with neurofibromatosis-1. J Int Neuropsychol Soc. 2014;20(1):88–98. doi: 10.1017/S1355617713001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rietman AB, van der Vaart T, Plasschaert E, Nicholson BA, Oostenbrink R, Krab LC, et al. Emotional and behavioral problems in children and adolescents with neurofibromatosis type 1. Am J Med Genet B Neuropsychiatr Genet. 2018;177(3):319–328. doi: 10.1002/ajmg.b.32612. [DOI] [PubMed] [Google Scholar]
- 81.Garwood MM, Bernacki JM, Fine KM, Hainsworth KR, Davies WH, Klein-Tasman BP. Physical, cognitive, and psychosocial predictors of functional disability and health-related quality of life in adolescents with neurofibromatosis-1. Pain Res Treat. 2012 doi: 10.1155/2012/975364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Graf A, Landolt MA, Mori AC, Boltshauser E. Quality of life and psychological adjustment in children and adolescents with neurofibromatosis type 1. J Pediatr. 2006;149(3):348–353. doi: 10.1016/j.jpeds.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 83.Oostenbrink R, Spong K, de Goede-Bolder A, Landgraf JM, Raat H, Moll HA. Parental reports of health-related quality of life in young children with neurofibromatosis type 1: influence of condition specific determinants. J Pediatr. 2007;151(2):182–186. doi: 10.1016/j.jpeds.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 84.Reiter-Purtill J, Schorry EK, Lovell AM, Vannatta K, Gerhardt CA, Noll RB. Parental distress, family functioning, and social support in families with and without a child with neurofibromatosis 1. J Pediatr Psychol. 2008;33(4):422–434. doi: 10.1093/jpepsy/jsm077. [DOI] [PubMed] [Google Scholar]
- 85.Wiener L, Battles H, Bedoya SZ, Baldwin A, Widemann BC, Pao M. Identifying symptoms of distress in youth living with neurofibromatosis type 1 (NF1) J Genet Couns. 2018;27(1):115–123. doi: 10.1007/s10897-017-0128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Varni JW, Nutakki K, Swigonski NL. Speech difficulties and patient health communication mediating effects on worry and health-related quality of life in children, adolescents, and young adults with neurofibromatosis type 1. Am J Med Genet Part A. 2019;179A(8):1476–1482. doi: 10.1002/ajmg.a.61197. [DOI] [PubMed] [Google Scholar]
- 87.Marukian NV, Choate KA. Recent advances in understanding ichthyosis pathogenesis. F1000Research. 2016 doi: 10.12688/f1000research.8584.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Association Ichtyose France | L’ichtyose – Présentation. https://www.ichtyose.fr/page/248746-presentation. Accessed 20 May 2021.
- 89.Troiano G, Lazzeri G. A review of quality of life of patients suffering from ichthyosis. J Prev Med Hyg. 2020;61(3):E374–E378. doi: 10.15167/2421-4248/jpmh2020.61.3.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dreyfus I, Pauwels C, Bourrat E, Bursztejn AC, Maruani A, Chiaverini C, Maza A, Mallet S, Bessis D, Barbarot S, Ezzedine K, Vabres P, Mazereeuw-Hautier J. Burden of inherited ichthyosis: a French national survey. Acta Derm Venereol. 2015;95(3):326–328. doi: 10.2340/00015555-1955. [DOI] [PubMed] [Google Scholar]
- 91.Dreyfus I, Bourrat E, Maruani A, Bessis D, Chiavérini C, Vabres P, Ezzedine K, Mazereeuw-Hautier J. Factors associated with impaired quality of life in adult patients suffering from ichthyosis. Acta Derm Venereol. 2014;94(3):344–346. doi: 10.2340/00015555-1710. [DOI] [PubMed] [Google Scholar]
- 92.Mazereeuw-Hautier J, Dreyfus I, Barbarot S, Serrentino L, Bourdon-Lanoy E, Ezzedine K, Maza A, Aujoulat I, Le Rhun A. Factors influencing quality of life in patients with inherited ichthyosis: a qualitative study in adults using focus groups. Br J Dermatol. 2012;166(3):646–648. doi: 10.1111/j.1365-2133.2011.10701.x. [DOI] [PubMed] [Google Scholar]
- 93.Sun Q, Ren I, Zaki T, Maciejewski K, Choate K. Ichthyosis affects mental health in adults and children: a cross-sectional study. J Am Acad Dermatol. 2020;83(3):951–954. doi: 10.1016/j.jaad.2020.01.052. [DOI] [PubMed] [Google Scholar]
- 94.Abeni D, Rotunno R, Diociaiuti A, Giancristoforo S, Bonamonte D, Schepis C, Neri I, Castiglia D, Zambruno G, El Hachem M. A multicenter study on quality of life of the "greater patient" in congenital ichthyoses. Orphanet J Rare Dis. 2021;16(1):440. doi: 10.1186/s13023-021-02085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wren G, Humby T, Thompson A, Davies W. Mood symptoms, neurodevelopmental traits, and their contributory factors in X-linked ichthyosis, ichthyosis vulgaris and psoriasis. Clin Exp Dermatol. 2022;47(6):1097–1108. doi: 10.1111/ced.15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kamalpour L, Gammon B, Chen KH, Veledar E, Pavlis M, Rice ZP, Chen SC. Resource utilization and quality of life associated with congenital ichthyoses. Pediatr Dermatol. 2011;28(5):512–518. doi: 10.1111/j.1525-1470.2011.01432.x. [DOI] [PubMed] [Google Scholar]
- 97.Cortés H, Rojas-Márquez M, Reyes-Hernández OD, Morales-Morfín JC, Guapillo-Vargas MRB, Varela-Cardoso M, Magaña JJ, Leyva-Gómez G, González-Del CM. Increased risk of depression and impairment in quality of life in patients with lamellar ichthyosis. Dermatol Ther. 2021 doi: 10.1111/dth.14628. [DOI] [PubMed] [Google Scholar]
- 98.Aaron D. MSD manual consumer version | port-wine stains. (2022). https://www.msdmanuals.com/home/skin-disorders/noncancerous-skin-growths/port-wine-stains. Accessed 3 Nov 2021.
- 99.Brightman LA, Geronemus RG, Reddy KK. Laser treatment of port-wine stains. Clin Cosmet Investig Dermatol. 2015;8:27–33. doi: 10.2147/CCID.S53118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Katugampola GA, Lanigan SW. Five years’ experience of treating port wine stains with the flashlamp-pumped pulsed dye laser. Br J Dermatol. 1997;137(5):750–754. doi: 10.1046/j.1365-2133.1997.19462061.x. [DOI] [PubMed] [Google Scholar]
- 101.Hagen SL, Grey KR, Korta DZ, Kelly KM. Quality of life in adults with facial port-wine stains. J Am Acad Dermatol. 2017;76(4):695–702. doi: 10.1016/j.jaad.2016.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang J, Zhu YY, Wang ZY, Yao XH, Zhang LF, Lv H, et al. Analysis of quality of life and influencing factors in 197 Chinese patients with port-wine stains. Medicine. 2017 doi: 10.1097/MD.0000000000009446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martin V, Renaud J, Dagenais P. Les normes de production des revues systématiques. Montréal, Quebec: Institut national d’excellence en santé et en services sociaux (INESSS). 2013. http://www.santecom.qc.ca/bibliothequevirtuelle/INESSS/9782550675525.pdf. Accessed 28 Jan 2021.
- 105.Public Health Agency of Canada. Infection prevention and control guidelines critical appraisal tool kit. Ottawa; 2014. https://www.picnet.ca/wp-content/uploads/PHAC-CA-Toolkit-2015-03.pdf. Accessed 9 May 2022.
- 106.Calcagni N, Gana K, Quintard B. A systematic review of complementary and alternative medicine in oncology: psychological and physical effects of manipulative and body-based practices. PLoS ONE. 2019 doi: 10.1371/journal.pone.0223564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Santiago-Delefosse M. Évaluer la qualité des publications: quelles spécificités pour la recherche qualitative? Prat Psychol. 2004;10(3):243–254. doi: 10.1016/j.prps.2004.07.010. [DOI] [Google Scholar]
- 108.Stenfors T, Kajamaa A, Bennett D. How to … assess the quality of qualitative research. Clin Teach. 2020;17(6):596–599. doi: 10.1111/tct.13242. [DOI] [PubMed] [Google Scholar]
- 109.Wanitphakdeedecha R, Ng JNC, Yan C, Manuskiatti W, Sudhipongpracha T, Jantarakolica T. Quality of life and psychological effects of port-wine stain: a review of literature. Clin Cosmet Investig Dermatol. 2021;14:681–690. doi: 10.2147/CCID.S315804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wanitphakdeedecha R, Sudhipongpracha T, Ng J, Yan C, Jantarakolica T. Self-stigma and psychosocial burden of patients with port-wine stain: a systematic review and meta-analysis. J Cosmet Dermatol. 2021;20(7):2203–2210. doi: 10.1111/jocd.14199. [DOI] [PubMed] [Google Scholar]
- 111.Ezeilo B. Psychological aspects of albinism: an exploratory study with Nigerian (IGBO) albino subjects. Soc Sci Med. 1989;29(9):1129–1131. doi: 10.1016/0277-9536(89)90026-9. [DOI] [PubMed] [Google Scholar]
- 112.Dheensa S, Williams GA. ‘I have NF. NF does not have me’: an interpretive phenomenological analysis of coping withneurofibromatosis type 1. Health Psychol Update. 2009;18(1):3–7. doi: 10.53841/bpshpu.2009.18.1.3. [DOI] [Google Scholar]
- 113.Ablon J. Gender response to neurofibromatosis 1. Soc Sci Med. 1996;42(1):99–109. doi: 10.1016/0277-9536(95)00076-3. [DOI] [PubMed] [Google Scholar]
- 114.Zöller ME, Rembeck B. A psychiatric 12-year follow-up of adult patients with neurofibromatosis type 1. J Psychiatr Res. 1999;33(1):63–68. doi: 10.1016/S0022-3956(98)00052-1. [DOI] [PubMed] [Google Scholar]
- 115.Jensen SE, Patel ZS, Listernick R, Charrow J, Lai JS. Lifespan development: symptoms experienced by individuals with neurofibromatosis type 1 associated plexiform neurofibromas from childhood into adulthood. J Clin Psychol Med Settings. 2019;26(3):259–270. doi: 10.1007/s10880-018-9584-5. [DOI] [PubMed] [Google Scholar]
- 116.Rosnau K, Hashmi SS, Northrup H, Slopis J, Noblin S, Ashfaq M. Knowledge and self-esteem of individuals with neurofibromatosis type 1 (NF1) J Genet Couns. 2017;26(3):620–627. doi: 10.1007/s10897-016-0036-9. [DOI] [PubMed] [Google Scholar]
- 117.Barke J, Harcourt D, Coad J. ‘It’s like a bag of pick and mix-you don’t know what you are going to get’: young people’s experience of neurofibromatosis type 1. J Adv Nurs. 2014;70(7):1594–1603. doi: 10.1111/jan.12319. [DOI] [PubMed] [Google Scholar]
- 118.Wolkenstein P, Zeller J, Revuz J, Ecosse E, Leplège A. Quality-of-life impairment in neurofibromatosis type 1: a cross-sectional study of 128 cases. Arch Dermatol. 2001;137(11):1421–1425. doi: 10.1001/archderm.137.11.1421. [DOI] [PubMed] [Google Scholar]
- 119.Bicudo NP, de Menezes Neto BF, da Silva da Avó LR, Germano CMR, Melo DG. Quality of life in adults with neurofibromatosis 1 in Brazil. J Genet Couns. 2016;25(5):1063–1074. doi: 10.1007/s10897-016-9939-8. [DOI] [PubMed] [Google Scholar]
- 120.Hummelvoll G, Antonsen KM. Young adults’ experience of living with neurofibromatosis type 1. J Genet Couns. 2013;22(2):188–199. doi: 10.1007/s10897-012-9527-5. [DOI] [PubMed] [Google Scholar]
- 121.Foji S, Mohammadi E, Sanagoo A, Jouybari L. How do people with neurofibromatosis type 1 (the forgotten victims) live? A grounded theory study. Health Expect. 2022;25(2):659–666. doi: 10.1111/hex.13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Crawford HA, Barton B, Wilson MJ, Berman Y, McKelvey-Martin VJ, Morrison PJ, et al. The impact of neurofibromatosis type 1 on the health and wellbeing of Australian adults. J Genet Couns. 2015;24(6):931–944. doi: 10.1007/s10897-015-9829-5. [DOI] [PubMed] [Google Scholar]
- 123.Fjermestad KW, Nyhus L, Kanavin ØJ, Heiberg A, Hoxmark LB. Health survey of adults with neurofibromatosis 1 compared to population study controls. J Genet Couns. 2018;27(5):1102–1110. doi: 10.1007/s10897-018-0229-5. [DOI] [PubMed] [Google Scholar]
- 124.Page PZ, Page GP, Ecosse E, Korf BR, Leplege A, Wolkenstein P. Impact of neurofibromatosis 1 on quality of life: a cross-sectional study of 176 American cases. Am J Med Genet A. 2006;140A(18):1893–1898. doi: 10.1002/ajmg.a.31422. [DOI] [PubMed] [Google Scholar]
- 125.Kodra Y, Giustini S, Divona L, Porciello R, Calvieri S, Wolkenstein P, et al. Health-related quality of life in patients with neurofibromatosis type 1. DRM. 2009;218(3):215–220. doi: 10.1159/000187594. [DOI] [PubMed] [Google Scholar]
- 126.Smith KB, Wang DL, Plotkin SR, Park ER. Appearance concerns among women with neurofibromatosis: examining sexual/bodily and social self-consciousness. Psychooncology. 2013;22(12):2711–2719. doi: 10.1002/pon.3350. [DOI] [PubMed] [Google Scholar]
- 127.Granström S, Langenbruch A, Augustin M, Mautner VF. Psychological burden in adult neurofibromatosis Type 1 patients: impact of disease visibility on body image. Dermatology. 2012;224(2):160–167. doi: 10.1159/000337548. [DOI] [PubMed] [Google Scholar]
- 128.Gånemo A, Lindholm C, Lindberg M, Sjödén P, Vahlquist A. Quality of life in adults with congenital ichthyosis. J Adv Nurs. 2003;44(4):412–419. doi: 10.1046/j.0309-2402.2003.02820.x. [DOI] [PubMed] [Google Scholar]
- 129.Gånemo A, Sjöden PO, Johansson E, Vahlquist A, Lindberg M. Health-related quality of life among patients with ichthyosis. Eur J Dermatol. 2004;14(1):61–66. [PubMed] [Google Scholar]
- 130.Lanigan SW, Cotterill JA. Psychological disabilities amongst patients with port wine stains. Br J Dermatol. 1989;121(2):209–215. doi: 10.1111/j.1365-2133.1989.tb01800.x. [DOI] [PubMed] [Google Scholar]
- 131.Ben-Tovim DI, Walker MK. Body image, disfigurement and disability. J Psychosom Res. 1995;39(3):283–291. doi: 10.1016/0022-3999(94)00143-S. [DOI] [PubMed] [Google Scholar]
- 132.Augustin M, Zschocke I, Wiek K, Peschen M, Vanscheidt W. Psychosocial stress of patients with port wine stains and expectations of dye laser treatment. Dermatology. 1998;197(4):353–360. doi: 10.1159/000018031. [DOI] [PubMed] [Google Scholar]
- 133.Malm M, Carlberg M. Port-wine stain–a surgical and psychological problem. Ann Plast Surg. 1988;20(6):512–516. doi: 10.1097/00000637-198806000-00002. [DOI] [PubMed] [Google Scholar]
- 134.Claes M. L’expérience adolescente. Bruxelles: P. Mardaga; 1994. [Google Scholar]
- 135.Claes M. Les relations interpersonnelles et le développement à l’adolescence. In: L’univers social des adolescents. Montréal: Presses de l’Université de Montréal; 2018. pp. 13–26.
- 136.Goffman E. Stigma: notes on the management of spoiled identity. Englewood Cliffs, N.J.: Prentice-Hall; 1963. [Google Scholar]
- 137.Janowski K, Steuden S, Pietrzak A, Krasowska D, Kaczmarek Ł, Gradus I, et al. Social support and adaptation to the disease in men and women with psoriasis. Arch Dermatol Res. 2012;304(6):421–432. doi: 10.1007/s00403-012-1235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Picardi A, Pasquini P, Cattaruzza MS, Gaetano P, Melchi CF, Baliva G, et al. Stressful life events, social support, attachment security and alexithymia in Vitiligo. Psychother Psychosom. 2003;72(3):150–158. doi: 10.1159/000069731. [DOI] [PubMed] [Google Scholar]
- 139.Fischer GN, Tarquinio C, Dodeler V. 6. Soutien social, santé et maladie. In: Les bases de la psychologie de la santé: Concepts, applications et perspectives. Paris: Dunod; 2020. pp. 173–200.
- 140.Thompson T, Pérez M, Kreuter M, Margenthaler J, Colditz G, Jeffe DB. Perceived social support in African American breast cancer patients: Predictors and effects. Soc Sci Med. 2017;192:134–142. doi: 10.1016/j.socscimed.2017.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stulz A, Boinon D, Dauchy S, Delaloge S, Brédart A. Ajustement psychologique des couples confrontés à un cancer du sein : perceptions des comportements de soutien du conjoint. Bull Cancer. 2014;101(7):690–697. doi: 10.1684/bdc.2014.1951. [DOI] [PubMed] [Google Scholar]
- 142.Zhou ES, Penedo FJ, Bustillo NE, Benedict C, Rasheed M, Lechner S, et al. Longitudinal effects of social support and adaptive coping on the emotional well-being of survivors of localized prostate cancer. J Support Oncol. 2010;8(5):196–201. doi: 10.1016/j.suponc.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cheng BT, Silverberg JI. Depression and psychological distress in US adults with atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123(2):179–185. doi: 10.1016/j.anai.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 144.Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–347. doi: 10.1016/j.anai.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 145.Mikołajczyk J, Rzepa T, Król J, Żaba R. Body image assessment and quality of life in patients with atopic dermatitis. Przegl Dermatol. 2017;104(2):92–102. doi: 10.5114/dr.2017.67383. [DOI] [Google Scholar]
- 146.Nicholas MN, Gooderham M. Psoriasis, depression, and suicidality. Skin Therapy Lett. 2017;22(3):1–4. [PubMed] [Google Scholar]
- 147.Michalek IM, Loring B, John SM. Global report on psoriasis. Geneva, Switzerland: World Health Organization. 2016. https://apps.who.int/iris/handle/10665/204417. Accessed 17 Nov 2021.
- 148.Dowlatshahi EA, Wakkee M, Arends LR, Nijsten T. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134(6):1542–1551. doi: 10.1038/jid.2013.508. [DOI] [PubMed] [Google Scholar]
- 149.Khoury LR, Danielsen PL, Skiveren J. Body image altered by psoriasis. A study based on individual interviews and a model for body image. J Dermatol Treat. 2014;25(1):2–7. doi: 10.3109/09546634.2012.739278. [DOI] [PubMed] [Google Scholar]
- 150.Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146(8):891–895. doi: 10.1001/archdermatol.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kimball AB, Jacobson C, Weiss S, Vreeland MG, Wu Y. The psychosocial burden of psoriasis. Am J Clin Dermatol. 2005;6(6):383–392. doi: 10.2165/00128071-200506060-00005. [DOI] [PubMed] [Google Scholar]
- 152.Nazar I, Kamran F, Masood A. Psychosocial predictors of quality of life in patients with Vitiligo. Pak J Psychol Res. 2021;36:19–36. [Google Scholar]
- 153.Lai YC, Yew YW, Kennedy C, Schwartz RA. Vitiligo and depression: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2017;177(3):708–718. doi: 10.1111/bjd.15199. [DOI] [PubMed] [Google Scholar]
- 154.Amer AAA, Gao XH. Quality of life in patients with vitiligo: an analysis of the dermatology life quality index outcome over the past two decades. Int J Dermatol. 2016;55(6):608–614. doi: 10.1111/ijd.13198. [DOI] [PubMed] [Google Scholar]
- 155.Kostopoulou P, Jouary T, Quintard B, Ezzedine K, Marques S, Boutchnei S, et al. Objective vs. subjective factors in the psychological impact of vitiligo: the experience from a French referral centre. Br J Dermatol. 2009;161(1):128–133. doi: 10.1111/j.1365-2133.2009.09077.x. [DOI] [PubMed] [Google Scholar]
- 156.Ongenae K, Van Geel N, De Schepper S, Naeyaert J. Effect of vitiligo on self-reported health-related quality of life. Br J Dermatol. 2005;152(6):1165–1172. doi: 10.1111/j.1365-2133.2005.06456.x. [DOI] [PubMed] [Google Scholar]
- 157.Rumsey N, Clarke A, White P, Wyn-Williams M, Garlick W. Altered body image: appearance-related concerns of people with visible disfigurement. J Adv Nurs. 2004;48(5):443–453. doi: 10.1111/j.1365-2648.2004.03227.x. [DOI] [PubMed] [Google Scholar]
- 158.Crerand CE, Sarwer DB, Kazak AE, Clarke A, Rumsey N. Body image and quality of life in adolescents with craniofacial conditions. Cleft Palate Craniofac J. 2017;54(1):2–12. doi: 10.1597/15-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stock NM, Feragen KB. Psychological adjustment to cleft lip and/or palate: a narrative review of the literature. Psychol Health. 2016;31(7):777–813. doi: 10.1080/08870446.2016.1143944. [DOI] [PubMed] [Google Scholar]
- 160.Cleary M, Kornhaber R, Thapa DK, West S, Visentin D. A quantitative systematic review assessing the impact of burn injuries on body image. Body Image. 2020;33:47–65. doi: 10.1016/j.bodyim.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 161.van Loey NE, van Beeck EF, Faber BW, van de Schoot R, Bremer M. Health-related quality of life after burns: a prospective multicenter cohort study with 18 months follow-up. J Trauma Acute Care Surg. 2012;72(2):513–520. doi: 10.1097/ta.0b013e3182199072. [DOI] [PubMed] [Google Scholar]
- 162.Salsberg JM, Weinstein M, Shear N, Lee M, Pope E. Impact of cosmetic camouflage on the quality of life of children with skin disease and their families. J Cutan Med Surg. 2016;20(3):211–215. doi: 10.1177/1203475415595175. [DOI] [PubMed] [Google Scholar]
- 163.Tanioka M, Yamamoto Y, Kato M, Miyachi Y. Camouflage for patients with vitiligo vulgaris improved their quality of life. J Cosmet Dermatol. 2010;9(1):72–75. doi: 10.1111/j.1473-2165.2010.00479.x. [DOI] [PubMed] [Google Scholar]
- 164.Ongenae K, Dierckxsens L, Brochez L, van Geel N, Naeyaert JM. Quality of life and stigmatization profile in a cohort of vitiligo patients and effect of the use of camouflage. Dermatology. 2005;210(4):279–285. doi: 10.1159/000084751. [DOI] [PubMed] [Google Scholar]
- 165.Maskell J, Newcombe P, Martin G, Kimble R. Psychological and psychosocial functioning of children with burn scarring using cosmetic camouflage: a multi-centre prospective randomised controlled trial. Burns. 2014;40(1):135–149. doi: 10.1016/j.burns.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 166.Martin G, Swannell S, Mill J, Mott J, Evans J, Frederiksen N, et al. Spray on skin improves psychosocial functioning in pediatric burns patients: a randomized controlled trial. Burns. 2008;34(4):498–504. doi: 10.1016/j.burns.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 167.Peuvrel L, Quéreux G, Brocard A, Saint-Jean M, Vallet C, Mère A, et al. Evaluation of quality of life after a medical corrective make-up lesson in patients with various dermatoses. Dermatology. 2012;224(4):374–380. doi: 10.1159/000339478. [DOI] [PubMed] [Google Scholar]
- 168.Seité S, Deshayes P, Dréno B, Misery L, Reygagne P, Saiag P, et al. Interest of corrective makeup in the management of patients in dermatology. Clin Cosmet Investig Dermatol. 2012;5:123–128. doi: 10.2147/CCID.S33172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Quintard B, Gana K, Constant A, Quintric C, Taïeb A, Léauté-Labrèze C. Social isolation in parents of children with hemangiomas: effects of coping styles and emotional distress. Psychol Health Med. 2013;18(6):698–704. doi: 10.1080/13548506.2013.766351. [DOI] [PubMed] [Google Scholar]
- 170.Chen SC, Huang BS, Lin CY, Fan KH, Chang JTC, Wu SC, et al. Psychosocial effects of a skin camouflage program in female survivors with head and neck cancer: a randomized controlled trial. Psychooncology. 2017;26(9):1376–1383. doi: 10.1002/pon.4308. [DOI] [PubMed] [Google Scholar]
- 171.Nicoletti G, Sasso A, Malovini A, Ponchio L, Scevola S, Faga A, et al. The role of rehabilitative camouflage after cervicofacial reconstructive surgery: a preliminary study. Clin Cosmet Investig Dermatol. 2014;7:43–49. doi: 10.2147/CCID.S55296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Huang S, Liu HE. Effectiveness of cosmetic rehabilitation on the body image of oral cancer patients in Taiwan. Support Care Cancer. 2008;16(9):981–986. doi: 10.1007/s00520-008-0417-1. [DOI] [PubMed] [Google Scholar]
- 173.Kornhaber R, Visentin D, Thapa DK, West S, McKittrick A, Haik J, Cleary M. Cosmetic camouflage improves quality of life among patients with skin disfigurement: a systematic review. Body Image. 2018;27:98–108. doi: 10.1016/j.bodyim.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 174.Quinn DM, Weisz BM, Lawner EK. Impact of active concealment of stigmatized identities on physical and psychological quality of life. Soc Sci Med. 2017;192:14–17. doi: 10.1016/j.socscimed.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 175.Anzieu D. Le moi-peau. Dunod; 1985. 254 p.
- 176.Rumsey N, Harcourt D. Body image and disfigurement: issues and interventions. Body Image. 2004;1(1):83–97. doi: 10.1016/S1740-1445(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 177.Kutzbach BR, Merrill KS, Hogue KM, Downes SJ, Holleschau AM, MacDonald JT, Summers CG. Evaluation of vision-specific quality-of-life in albinism. J AAPOS. 2009;13(2):191–195. doi: 10.1016/j.jaapos.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 178.Morice-Picard F, Taïeb C, Marti A, Gliksohn A, Bennani M, Bodemer C, Ezzedine K. Filière Maladies Rares en Dermatologie: FIMARAD. Burden of albinism: development and validation of a burden assessment tool. Orphanet J Rare Dis. 2018;13(1):162. doi: 10.1186/s13023-018-0894-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ramulu P. Glaucoma and disability: which tasks are affected, and at what stage of disease? Curr Opin Ophthalmol. 2009;20(2):92–98. doi: 10.1097/ICU.0b013e32832401a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Aspinall PA, Johnson ZK, Azuara-Blanco A, Montarzino A, Brice R, Vickers A. Evaluation of quality of life and priorities of patients with glaucoma. Investig Ophthalmol Vis Sci. 2008;49(5):1907–1915. doi: 10.1167/iovs.07-0559. [DOI] [PubMed] [Google Scholar]
- 181.Xu K, Gupta V, Bae S, Sharma S. Metamorphopsia and vision-related quality of life among patients with age-related macular degeneration. Can J Ophthalmol. 2018;53(2):168–172. doi: 10.1016/j.jcjo.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 182.Mitchell J, Bradley C. Quality of life in age-related macular degeneration: a review of the literature. Health Qual Life Outcomes. 2006;4(1):97. doi: 10.1186/1477-7525-4-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Coyne KS, Margolis MK, Kennedy-Martin T, Baker TM, Klein R, Paul MD, et al. The impact of diabetic retinopathy: perspectives from patient focus groups. Fam Pract. 2004;21(4):447–453. doi: 10.1093/fampra/cmh417. [DOI] [PubMed] [Google Scholar]
- 184.Lamoureux EL, Hassell JB, Keeffe JE. The impact of diabetic retinopathy on participation in daily living. Arch Ophthalmol. 2004;122(1):84–88. doi: 10.1001/archopht.122.1.84. [DOI] [PubMed] [Google Scholar]
- 185.Porter JR, Beuf AH, Lerner AB, Nordlund JJ. The effect of vitiligo on sexual relationships. J Am Acad Dermatol. 1990;22:221–222. doi: 10.1016/0190-9622(90)70028-g. [DOI] [PubMed] [Google Scholar]
- 186.Connell KM, Coates R, Wood FM. Sexuality following burn injuries: a preliminary study. J Burn Care Res. 2013;34(5):282–289. doi: 10.1097/BCR.0b013e31827819bf. [DOI] [PubMed] [Google Scholar]
- 187.Monteleone R, Forrester-Jones R. ‘Disability means, um, dysfunctioning people’: a qualitative analysis of the meaning and experience of disability among adults with intellectual disabilities. J Appl Res Intellect Disabil. 2017;30(2):301–315. doi: 10.1111/jar.12240. [DOI] [PubMed] [Google Scholar]
- 188.Ali A, Hassiotis A, Strydom A, King M. Self stigma in people with intellectual disabilities and courtesy stigma in family carers: a systematic review. Res Dev Disabil. 2012;33(6):2122–2140. doi: 10.1016/j.ridd.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 189.Partow S, Cook R, McDonald R. Coping with stigmatization and discrimination related to blindness and low vision. Rehabil Psychol. 2021;66(4):576–588. doi: 10.1037/rep0000391. [DOI] [PubMed] [Google Scholar]
- 190.Dervishaliaj E. Parental stress in families of children with disabilities: a literature review. J Educ Soc Res. 2013;3(7):579–584. doi: 10.5901/JESR.2013.V3N7P579. [DOI] [Google Scholar]
- 191.Meadow-Orlans KP. Parenting with a sensory or physical disability. In: Handbook of parenting: Social conditions and applied parenting. Lawrence Erlbaum Associates Publishers; 2002.
- 192.Gahl W, Huizing M. Orphanet: the portal for rare diseases and orphan drugs | Hermansky Pudlak Syndrome. (2010). https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=en&Expert=79430. Accessed 18 Nov 2021.
- 193.Clarke A. Stigma, self-esteem and reproduction: talking with men about life with hypohidrotic ectodermal dysplasia. Sociology. 2013;47(5):887–905. doi: 10.1177/0038038513493536. [DOI] [Google Scholar]
- 194.Baumann S. Enjeux éthiques posés par le diagnostic anténatal dans le cadre des maladies génétiques à révélation tardive. Caen, France: Université Normandie. 2018. https://tel.archives-ouvertes.fr/tel-02396372/document. Accessed 30 Nov 2022.
- 195.Ganache I, Bélanger S, Fortin JS, Cleret de Langavant G. Les enjeux éthiques soulevés par le dépistage prénatal du syndrome de Down, ou Trisomie 21, au Québec. Quebec: Commissaire à la Santé et au Bien-Être. 2008. https://www.csbe.gouv.qc.ca/fileadmin/www/2008/Trisomie/CSBE_RevueDocumentationSDown_Mai2008.pdf. Accessed 30 Nov 2022.
- 196.Frydman R. Dieu, la médecine et l’embryon. Paris: Odile Jacob; 2003. p. 344. [Google Scholar]
- 197.Pourcel G, Bousquet PJ. ©Étude sur l’annonce du diagnostic de cancer et le ressenti des malades en 2011. Institut National du Cancer (INCa). 2012. https://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Etude-sur-l-annonce-du-diagnostic-de-cancer-et-le-ressenti-des-malades-en-2011. Accessed 17 Dec 2021.
- 198.Moreno-Artero E, Morice-Picard F, Bremond-Gignac D, Drumare-Bouvet I, Duncombe-Poulet C, Leclerc-Mercier S, et al. Management of albinism: French guidelines for diagnosis and care. J Eur Acad Dermatol Venereol. 2021;35(7):1449–1459. doi: 10.1111/jdv.17275. [DOI] [PubMed] [Google Scholar]
- 199.Kang E, Yoon HM, Lee BH. Neurofibromatosis type I: points to be considered by general pediatricians. Clin Exp Pediatr. 2021;64(4):149–156. doi: 10.3345/cep.2020.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dufresne H, Compain S, de Longcamp A, Morice-Picard F, Deladrière E, Bekel L, et al. Co-construction d’un programme d’éducation thérapeutique dédié à l’albinisme. Ann Dermatol Venereol. 2020;147(12):A272–A273. doi: 10.1016/j.annder.2020.09.389. [DOI] [Google Scholar]
- 201.Bronfenbrenner U. Ecology of Human Development – Experiments by Nature & Design. Reprint édition. Cambridge, Mass: Harvard University Press; 1979. 368 p.
- 202.Stangl AL, Earnshaw VA, Logie CH, van Brakel W, C Simbayi L, Barré I, Dovidio JF. The health stigma and discrimination framework: a global, crosscutting framework to inform research, intervention development, and policy on health-related stigmas. BMC Med. 2019;17(1):31. doi: 10.1186/s12916-019-1271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Legere D. Comment la génétique vient bousculer la famille et questionne le transgénérationnel: la place de la culpabilité dans un système familial. 47èmes Journées d’Étude de l’ALFPHV; 2021. Nantes.
- 204.Laurent K, Daviau L, Lebret C. Interculturalité/familles migrantes/transmissions en lien avec la déficience visuelle et les pathologies génétiques. 47èmes Journées d’Étude de l’ALFPHV; 2021. Nantes.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Evaluation grid for qualitative exploratory studies.
Data Availability Statement
Not applicable.