Abstract
Aims/Introduction
Sodium–glucose cotransporter 2 inhibitors (SGLT2i) have shown beneficial effects on cardiometabolic risk factors (hemoglobin A1c, body mass index, systolic blood pressure) in patients with type 2 diabetes mellitus. We compared combined cardiometabolic effects of SGLT2i on hemoglobin A1c, body mass index and systolic blood pressure versus dipeptidyl peptidase‐4 inhibitors (DPP4i) in Japanese patients with type 2 diabetes mellitus.
Materials and Methods
This Japanese retrospective cohort study used the JMDC claims database. Patients newly treated with an SGLT2i (n = 18,936) or DPP4i (n = 55,484) were enrolled (January 2015–March 2020) and matched 1:1 using the propensity score. The primary end‐point was the proportion of patients achieving a composite outcome (i.e., simultaneous absolute/percent reduction in hemoglobin A1c ≥0.5%, body mass index ≥3% and systolic blood pressure ≥2 mmHg) 1 year after first SGLT2i or DPP4i prescription; Mantel–Haenszel common risk difference and its 95% confidence interval were estimated. Other end‐points included treatment persistence, with the associated hazard ratio calculated using the Cox proportional hazards model.
Results
After matching, patient characteristics were balanced (7,302 patients each). The proportion of patients achieving the composite outcome was significantly greater in patients receiving an SGLT2i than those receiving a DPP4i (31.0% [1,279/4,120] vs 12.9% [524/4,070], risk difference 18.6%, 95% confidence interval 16.3, 20.9, P < 0.001). Risk of treatment discontinuation was significantly lower in the SGLT2i group than in the DPP4i group (hazard ratio 0.85, 95% confidence interval 0.81, 0.90, P < 0.001).
Conclusions
In the present study, SGLT2i showed favorable cardiometabolic risk reduction and longer treatment persistence than DPP4i in Japanese patients with type 2 diabetes mellitus.
Keywords: Cardiometabolic risk factors, Diabetes mellitus, type 2, Sodium–glucose cotransporter 2 inhibitors
In this large retrospective cohort study using a Japanese administrative database, the proportion of patients with type 2 diabetes mellitus achieving a simultaneous improvement in hemoglobin A1c, body mass index and systolic blood pressure was significantly greater in patients newly treated with a sodium–glucose cotransporter 2 inhibitor than those newly treated with a dipeptidyl peptidase‐4 inhibitor. Furthermore, sodium–glucose cotransporter 2 inhibitor treatment showed better treatment persistence than dipeptidyl peptidase‐4 inhibitor treatment.

INTRODUCTION
Type 2 diabetes mellitus is associated with an increased risk of cardiovascular disease (CVD), which is the leading cause of death in patients with type 2 diabetes mellitus 1 , 2 , 3 , 4 . Given the close association between type 2 diabetes mellitus and CVD, the importance of a comprehensive treatment approach to address multiple cardiovascular risk reductions, including hypertension and dyslipidemia, has been highlighted 5 . Current Japanese treatment guidelines and the joint consensus statement from the Japanese Circulation Society and Japan Diabetes Society recommend intensive lifestyle and pharmacological interventions to improve glycemic control and reduce cardiovascular risks 6 , 7 . Therefore, antidiabetic drugs with cardiovascular benefits have been intensively studied and used in clinical practice for type 2 diabetes mellitus treatment.
Sodium–glucose cotransporter 2 inhibitors (SGLT2i), which were approved in Europe in 2012 8 , the USA in 2013 9 and Japan in 2014 10 , have shown various cardiometabolic effects, including significant reductions in hemoglobin A1c (HbA1c), bodyweight, blood pressure and lipid levels, in patients with type 2 diabetes mellitus 11 , 12 . Long‐term durability of improved glycemic control, and reductions in bodyweight and systolic blood pressure (SBP) have also been reported as major characteristics of SGLT2i compared with dipeptidyl peptidase‐4 inhibitors (DPP4i) and other glucose‐lowering drugs, suggesting their efficacy in preventing chronic diabetic vascular complications 13 , 14 . Furthermore, a recent Italian study reported that patients initiating dapagliflozin, an SGLT2i, had a greater probability of attaining simultaneous reduction in HbA1c, bodyweight and SBP than those initiating a DPP4i 15 . However, data on cardiometabolic effects of SGLT2i in real‐world clinical settings in Japan are limited and are of great interest. Given that improved persistence of antidiabetic drugs leads to better glycemic control 16 , assessment of patients' persistence to SGLT2i treatment in real‐world clinical practice is important.
We compared the effects of SGLT2i versus DPP4i on simultaneously improving HbA1c, body mass index (BMI) and SBP in Japanese patients with type 2 diabetes mellitus. Furthermore, we compared treatment persistence and change from baseline in cardiometabolic risk factors between new users of an SGLT2i and new users of a DPP4i.
MATERIALS AND METHODS
Data source
The present retrospective cohort study used the JMDC claims database (JMDC Inc., Tokyo, Japan), consisting of inpatients, outpatients and dispensing claims, and medical examination data of ~14 million cumulative individuals from health insurance associations (as of February 2022). The study was carried out in accordance with the protocol, ethical principles of the Declaration of Helsinki, and all relevant regulations and guidelines governing clinical study conduct. The protocol was approved by Medical Affairs Protocol Approval Committee (protocol number: 1941‐MA‐3372). No institutional ethics review nor informed consent were required, because retrospective de‐identified data were used.
Study population
Eligible patients were aged ≥18 years, had one or more prescription record for an SGLT2i or a DPP4i in the enrollment period (1 January 2015–31 March 2020; Figure S1; index treatment codes were reported previously 17 ) and had a diagnosis of type 2 diabetes mellitus (International Classification of Diseases, 10th Revision [ICD‐10]: E11.x) or unspecified diabetes mellitus (ICD‐10: E14x). Patients excluded had type 1 diabetes mellitus (ICD‐10: E10.x) diagnosis on or before the index date (date of first recorded receipt of an SGLT2i or a DPP4i on or after January 1, 2015); gestational diabetes (ICD‐10: O24.x) diagnosis in the pre‐index period (time between 1 year and 1 day before the index date) or on the index date; prescription records for an SGLT2i and/or a DPP4i, including those in a fixed‐dose combination of SGLT2i and DPP4i, during the pre‐index period or on the index date; or <1 year of record in the database on or before the index date.
Outcome measures
The primary end‐point was the proportion of patients achieving a composite outcome (i.e., simultaneous achievement of the improvement criterion for HbA1c, BMI and SBP). Improvement criterion for each outcome was absolute reduction in HbA1c ≥0.5%, percent reduction in BMI ≥3% and absolute reduction in SBP ≥2 mmHg, relative to baseline values; criteria were based on previous studies and guidelines 15 , 18 , 19 , 20 , 21 , 22 . Secondary end‐points were treatment persistence from index date, assessed by estimating time to discontinuation of the index treatment and time to switch or add‐on of the comparator drug or a new antidiabetic drug; change from baseline in each cardiometabolic outcome (HbA1c, BMI, SBP, total cholesterol, triglyceride, low‐density lipoprotein cholesterol and high‐density lipoprotein cholesterol [HDL‐C]); and the proportion of patients achieving the improvement criterion for each outcome (HbA1c, BMI and SBP). Baseline cardiometabolic data were extracted within 180 days before the index date. Post‐index data, which could be extracted at different times for each outcome, were those extracted closest to 1 year (365 days), and between days 180 and 540 after the index date; if multiple measurements were available, a single measurement closest to 1 year after the index date was used. The primary and secondary end‐points were compared between treatment groups in the crude and matched populations, and by subgroup (HbA1c [<7.5%, ≥7.5%], BMI [<25 kg/m2, ≥25 kg/m2], SBP [<130 mmHg, ≥130 mmHg], presence of previous treatment [treatment naïve, non‐treatment naïve], duration of consecutive index treatment [<1 year, ≥1 year]). Furthermore, outcomes except for treatment persistence were analyzed using the “while‐on treatment strategy” and/or “treatment policy strategy”. The “while‐on treatment strategy” was defined as an approach where data from patients who discontinued treatment, switched or added on a comparator drug before having post‐index data available were excluded. The “treatment policy strategy” was defined as an approach where data from all patients, including those who discontinued treatment, switched or added on a comparator drug before having post‐index data available, were included.
Statistical analysis
All eligible patients were included. Patients newly treated with an SGLT2i or a DPP4i were matched 1:1 using the propensity score, estimated by multivariable logistic regression model. Missing values were not imputed before propensity score calculation. The propensity score was assessed using standardized mean difference, with standardized mean difference of >0.1 between treatment groups showing covariate imbalance. Baseline characteristics, including age, comorbidities, Charlson Comorbidity Index (CCI) 23 and adapted Diabetes Complication Severity Index (aDCSI) scores 24 , and concomitant medications, collected during the pre‐index period and on the index date, were summarized using descriptive statistics. Codes for CCI have been previously reported 17 , and codes for aDCSI and other relevant codes are shown in Tables S1–S4. Covariates in the logistic regression model were demographics (sex, age), smoking history, metabolic measurements (HbA1c, BMI, SBP) in the baseline period, CCI, aDCSI, coronary revascularization and relevant medication use in the baseline period.
The primary outcome was analyzed among matched patients (“while‐on treatment strategy”) as the primary analysis, and among matched patients (“treatment policy strategy”) and crude population (both strategies) as supplementary analyses. Patients with missing pre‐index and/or post‐index data were excluded from analysis. Mantel–Haenszel common risk difference and 95% confidence interval (CI) were estimated, and the Cochran–Mantel–Haenszel test was used for between‐group comparison; P < 0.05 was considered statistically significant. Time to treatment discontinuation and time to treatment switch or add‐on were estimated using Kaplan–Meier analyses. Cox proportional hazard models and log‐rank tests were carried out for between‐group comparison; P < 0.05 was considered statistically significant. Patients were considered as having discontinued the index treatment or switched or added on a treatment if the time between the previous prescription date plus days' supply of the drug and the current prescription date was >60 days, or if the patient switched and/or added on a comparator drug or another antidiabetic drug. For time to treatment discontinuation, patients who continued the index treatment were censored at the end of patient data or study period, whichever occurred first. For time to treatment switch or add‐on, patients were censored at the end of patient data, or study period, or at discontinuation of index treatment, whichever occurred first. Changes from baseline in cardiometabolic outcomes were also compared using t‐tests in the matched and/or crude populations (“while‐on treatment strategy”). Statistical analyses were carried out using SAS Release 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Study population
Of 74,420 eligible patients, 18,936 were newly treated with an SGLT2i (alone or in combination with anti‐diabetic drugs other than a DPP4i) and 55,484 were newly treated with a DPP4i (alone or in combination with antidiabetic drugs other than an SGLT2i), constituting the crude population (Figure 1). After matching, the SGLT2i and DPP4i groups included 7,302 patients each.
Figure 1.
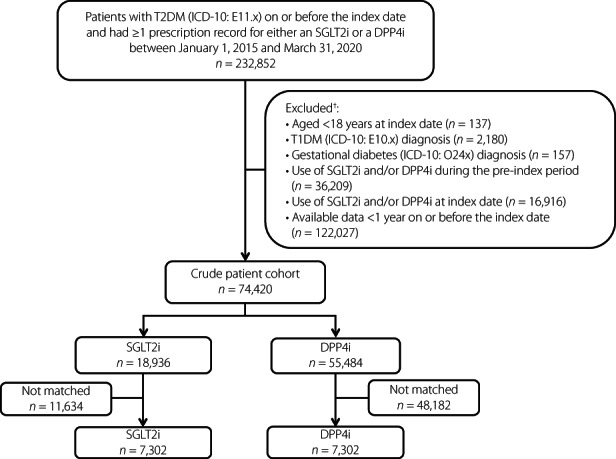
Patient disposition. †Not mutually exclusive. DPP4i, dipeptidyl peptidase‐4 inhibitors; ICD‐10, International Classification of Diseases, 10th Revision; SGLT2i, sodium–glucose cotransporter 2 inhibitors; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
Demographic and baseline clinical characteristics
Before matching, new users of an SGLT2i were younger and had lower HbA1c and HDL‐C, but higher BMI, compared with new users of a DPP4i (Table 1). Overall, CCI and aDCSI scores were similar between treatment groups. However, a greater proportion of SGLT2i‐treated patients had comorbid hypertension, nephropathy and dyslipidemia than DPP4i‐treated patients. Some concomitant drugs, including biguanides and glucagon‐like peptide‐1 receptor agonists, were prescribed more frequently in the SGLT2i group than in the DPP4i group. After matching, baseline characteristics were well balanced between groups, with standardized mean difference of ≤0.1 (Table 1). In the matched cohorts, ~80% patients were male; mean age was 51 years, mean HbA1c was 7.75%, mean SBP was ~133 mmHg, and mean BMI was 29.36 kg/m2 (SGLT2i group) and 29.50 kg/m2 (DPP4i group).
Table 1.
Baseline demographic and clinical characteristics
| Characteristic | Crude population | Matched patients | ||||
|---|---|---|---|---|---|---|
| SGLT2i (N = 18,936) | DPP4i (N = 55,484) | Standardized difference | SGLT2i (N = 7,302) | DPP4i (N = 7,302) | Standardized difference | |
| Male, n (%) | 13,804 (72.9) | 40,497 (73.0) | −0.002 | 5,891 (80.7) | 5,897 (80.8) | −0.002 |
| Age, n (years) | 18,936 | 55,484 | 7,302 | 7,302 | ||
| Mean, years (SD) | 50.8 (9.69) | 54.1 (9.75) | −0.342 | 51.4 (8.33) | 51.2 (8.57) | 0.022 |
| Smoking history (n) | 13,644 | 38,759 | 7,302 | 7,302 | ||
| Yes, n (%) † | 4,353 (31.9) | 13,808 (35.6) | −0.079 | 2,339 (32.0) | 2,353 (32.2) | −0.004 |
| HbA1c (n) | 7,763 | 22,085 | 7,302 | 7,302 | ||
| Mean, % (SD) | 7.74 (1.698) | 8.04 (1.803) | −0.170 | 7.75 (1.712) | 7.75 (1.500) | <0.001 |
| BMI (n) | 8,371 | 24,193 | 7,302 | 7,302 | ||
| Mean, kg/m2 (SD) | 29.68 (5.124) | 26.75 (4.703) | 0.595 | 29.36 (4.707) | 29.50 (5.257) | −0.028 |
| SBP (n) | 8,372 | 24,189 | 7,302 | 7,302 | ||
| Mean, mmHg (SD) | 132.5 (17.07) | 131.8 (17.95) | 0.038 | 132.5 (17.06) | 132.7 (17.36) | −0.014 |
| Total cholesterol (n) | 2,622 | 7,022 | 2,332 | 2,216 | ||
| Mean, mg/dL (SD) | 211.0 (39.01) | 214.9 (42.12) | −0.095 | 211.1 (39.09) | 210.5 (41.98) | 0.013 |
| Triglycerides (n) | 8,313 | 23,999 | 7,286 | 7,285 | ||
| Mean, mg/dL (SD) | 193.1 (158.74) | 185.3 (161.94) | 0.049 | 193.4 (155.49) | 188.6 (156.90) | 0.030 |
| LDL‐C (n) | 8,324 | 24,024 | 7,297 | 7,287 | ||
| Mean, mg/dL (SD) | 129.3 (34.23) | 131.2 (35.86) | −0.055 | 129.4 (34.19) | 129.2 (34.65) | 0.005 |
| HDL‐C (n) | 8,323 | 24,034 | 7,298 | 7,294 | ||
| Mean, mg/dL (SD) | 50.9 (12.74) | 52.8 (14.04) | −0.147 | 50.9 (12.78) | 51.0 (12.63) | −0.003 |
| Presence of comorbidity, yes, n (%) | ||||||
| Hypertension | 11,105 (58.6) | 29,238 (52.7) | 0.120 | 4,160 (57.0) | 4,124 (56.5) | 0.010 |
| Chronic kidney disease | 376 (2.0) | 1,294 (2.3) | −0.024 | 136 (1.9) | 146 (2.0) | −0.010 |
| Nephropathy | 2,923 (15.4) | 6,486 (11.7) | 0.110 | 999 (13.7) | 865 (11.8) | 0.055 |
| Peripheral neuropathy | 932 (4.9) | 2,453 (4.4) | 0.024 | 285 (3.9) | 286 (3.9) | −0.001 |
| Dyslipidemia | 12,447 (65.7) | 33,300 (60.0) | 0.119 | 4,735 (64.8) | 4,705 (64.4) | 0.009 |
| Hypoglycemia | 95 (0.5) | 202 (0.4) | 0.021 | 29 (0.4) | 19 (0.3) | 0.024 |
| CCI score (n) | 18,936 | 55,484 | 7,302 | 7,302 | ||
| Mean (SD) | 1.5 (1.41) | 1.4 (1.52) | 0.055 | 1.3 (1.30) | 1.3 (1.39) | 0.013 |
| aDCSI score (n) | 18,936 | 55,484 | 7,302 | 7,302 | ||
| Mean (SD) | 1.1 (1.77) | 1.0 (1.72) | 0.082 | 1.0 (1.61) | 1.0 (1.70) | 0.009 |
| Presence of coronary revascularization, n (%) | 205 (1.1) | 438 (0.8) | 0.031 | 72 (1.0) | 66 (0.9) | 0.008 |
| Concomitant drugs, n (%) | ||||||
| Sulfonylureas | 1,513 (8.0) | 4,222 (7.6) | 0.014 | 463 (6.3) | 454 (6.2) | 0.005 |
| Glinides | 485 (2.6) | 1,089 (2.0) | 0.040 | 141 (1.9) | 135 (1.8) | 0.006 |
| Biguanides | 5,802 (30.6) | 10,473 (18.9) | 0.276 | 1,875 (25.7) | 1,849 (25.3) | 0.008 |
| Thiazolidinediones | 1,138 (6.0) | 1,779 (3.2) | 0.135 | 344 (4.7) | 339 (4.6) | 0.003 |
| Alpha‐glucosidase inhibitors | 1,200 (6.3) | 3,419 (6.2) | 0.007 | 392 (5.4) | 391 (5.4) | 0.001 |
| GLP‐1 receptor agonists | 973 (5.1) | 333 (0.6) | 0.305 | 153 (2.1) | 94 (1.3) | 0.063 |
| Insulins | 2,284 (12.1) | 4,819 (8.7) | 0.111 | 583 (8.0) | 528 (7.2) | 0.028 |
| Lipid‐lowering drugs | 7,392 (39.0) | 17,444 (31.4) | 0.160 | 2,705 (37.0) | 2,724 (37.3) | −0.005 |
| Antiplatelet drugs | 1,432 (7.6) | 3,995 (7.2) | 0.014 | 477 (6.5) | 459 (6.3) | 0.010 |
| Antihypertensives | 8,515 (45.0) | 21,079 (38.0) | 0.142 | 3,103 (42.5) | 3,137 (43.0) | −0.009 |
Percentage was calculated using “smoking history” as the denominator.
aDCSI, adapted Diabetes Complication Severity Index; BMI, body mass index; CCI, Charlson Comorbidity Index; DPP4i, dipeptidyl peptidase‐4 inhibitors; GLP‐1, glucagon‐like peptide‐1; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; SD, standard deviation; SGLT2i, sodium–glucose cotransporter 2 inhibitors.
Achievement of improvement criteria for HbA1c, BMI and SBP
Among matched patients (“while‐on treatment strategy”), a significantly greater proportion of new users of an SGLT2i achieved a composite outcome 1 year after the index date compared with new users of a DPP4i (31.0% vs 12.9%, risk difference 18.6%, 95% CI 16.3, 20.9, P < 0.001; Table 2). The proportion of patients achieving the improvement criterion for each outcome was also significantly greater in the SGLT2i group compared with the DPP4i group. Similar results were observed among matched patients (“treatment policy strategy”; Table 2). In the crude population (both strategies), the proportion achieving the HbA1c improvement criterion was greater in the DPP4i group than in the SGLT2i group (Table S5).
Table 2.
Proportion of patients who achieved a composite outcome (HbA1c, BMI and SBP) 1 year after the index date (matched patients) † , ‡
| Achieved, % (n/n) | SGLT2i (N = 7,302) | DPP4i (N = 7,302) | RD (95% CI) | P‐value |
|---|---|---|---|---|
| While‐on treatment strategy § | ||||
| Composite outcome ¶ | 31.0 (1,279/4,120) | 12.9 (524/4,070) | 18.6 (16.3, 20.9) | <0.001 |
| HbA1c | 78.3 (3,227/4,122) | 74.7 (3,045/4,075) | 4.7 (2.3, 7.2) | <0.001 |
| BMI | 54.5 (2,280/4,184) | 21.9 (903/4,122) | 32.4 (29.8, 35.0) | <0.001 |
| SBP | 59.0 (2,469/4,184) | 51.0 (2,102/4,122) | 8.6 (5.8, 11.4) | <0.001 |
| Treatment policy strategy †† | ||||
| Composite outcome ¶ | 27.4 (1,683/6,144) | 14.8 (902/6,087) | 12.5 (10.9, 14.0) | <0.001 |
| HbA1c | 75.2 (4,620/6,147) | 72.6 (4,427/6,094) | 2.4 (0.7, 4.1) | 0.0048 |
| BMI | 49.1 (3,067/6,243) | 27.4 (1,691/6,178) | 21.4 (19.6, 23.2) | <0.001 |
| SBP | 57.1 (3,567/6,244) | 51.4 (3,176/6,178) | 6.0 (4.1, 7.8) | <0.001 |
Patients with missing pre‐index and/or post‐index data were excluded from the analysis. Post‐index data were those extracted closest to 1 year (365 days), and between days 180 and 540 after the index date; data for each outcome could be extracted at different times, and if multiple measurements were available, a single measurement closest to 1 year after the index date was used.
Improvement criterion for each outcome was absolute reduction in HbA1c of ≥0.5%, SBP of ≥2 mmHg and percent reduction in BMI of ≥3%, relative from the baseline values.
“While‐on treatment strategy” was defined as an approach where data from patients who discontinued treatment, switched or added on a comparator drug before having post‐index data available were excluded.
Patients who achieved the composite outcome were those who simultaneously achieved the improvement criteria for all outcomes (HbA1c, BMI and SBP).
“Treatment policy strategy” was defined as an approach where data from all patients, including those who discontinued treatment, switched or added on a comparator drug before having post‐index data available, were included.
BMI, body mass index; CI, confidence interval; DPP4i, dipeptidyl peptidase‐4 inhibitors; HbA1c, hemoglobin A1c; RD, risk difference; SBP, systolic blood pressure; SGLT2i, sodium–glucose cotransporter 2 inhibitors.
Treatment persistence
Among matched patients, the SGLT2i group was significantly less likely to discontinue index treatment compared with the DPP4i group (hazard ratio 0.85, 95% CI 0.81, 0.90, P < 0.001; Figure 2a). The SGLT2i group was also significantly less likely to switch or add on a treatment (hazard ratio 0.80, 95% CI 0.75, 0.86, P < 0.001; Figure 2b). Among the crude population, treatment discontinuation was also less likely in the SGLT2i group versus the DPP4i group; however, the probability of switching or adding on a treatment was similar between groups (Figure S2).
Figure 2.
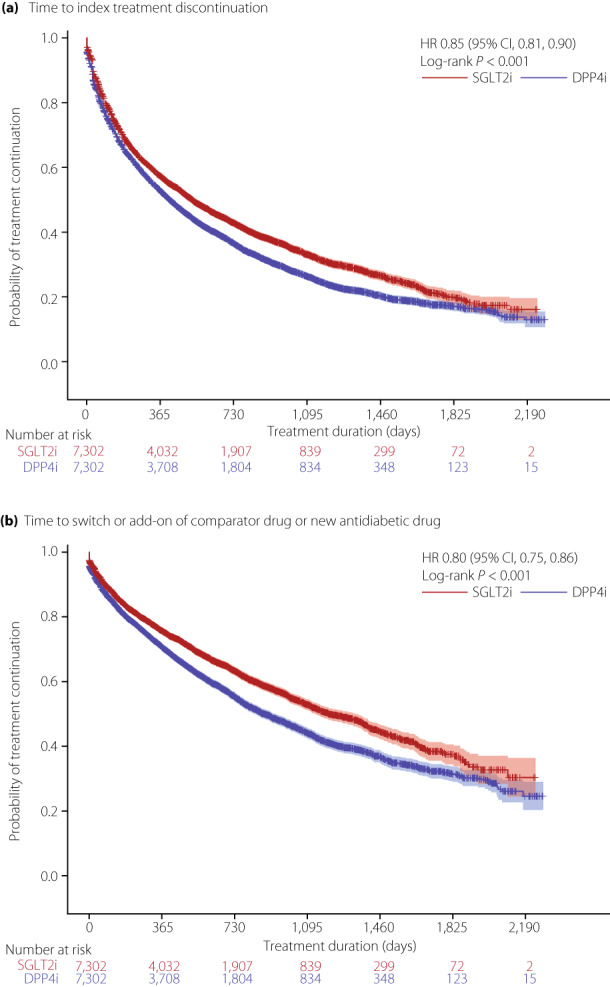
Kaplan–Meier curves for (a) time to treatment discontinuation of index treatment and (b) time to switch or add‐on of comparator drug or new antidiabetic drug (matched patients). For analysis of time to treatment discontinuation, patients who continued index treatment until the end of patient data or the study period (whichever occurred first) were censored. For analysis of time to treatment switch or add‐on, patients who discontinued index treatment, or continued index treatment until the end of patient data or the study period (whichever occurred first) were censored. CI, confidence interval; DPP4i, dipeptidyl peptidase‐4 inhibitors; HR, hazard ratio; SGLT2i, sodium–glucose cotransporter 2 inhibitors.
Change from baseline in cardiometabolic outcomes
Among matched patients (“while‐on treatment strategy”), compared with the DPP4i group, the SGLT2i group had a significantly greater reduction from baseline in each individual outcome (HbA1c, BMI and SBP), and a significantly greater increase from baseline in HDL‐C, 1 year after the index date (Table 3). Conversely, a reduction from baseline in total cholesterol was significantly greater in the DPP4i group than in the SGLT2i group. No significant difference was observed between treatment groups for the remaining outcomes (Table 3). Among the crude population, reductions from baseline in BMI and SBP, and an increase in HDL‐C, were significantly greater in the SGLT2i group than in the DPP4i group (Table S6); reduction in low‐density lipoprotein cholesterol was significantly greater in the DPP4i group than in the SGLT2i group.
Table 3.
Change from baseline in cardiometabolic outcomes (matched patients, while‐on treatment strategy † )
| SGLT2i | DPP4i | P‐value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Post‐index treatment | Change from baseline ‡ | Baseline | Post‐index treatment | Change from baseline ‡ | ||
| HbA1c (n) | 7,302 | 4,122 | 4,122 | 7,302 | 4,075 | 4,075 | |
| Mean, % (SD) | 7.75 (1.712) | 6.71 (0.841) | −0.89 (1.355) | 7.75 (1.500) | 6.85 (0.986) | −0.75 (1.381) | <0.001 |
| BMI (n) | 7,302 | 4,184 | 4,184 | 7,302 | 4,122 | 4,122 | |
| Mean, kg/m2 (SD) | 29.36 (4.707) | 28.35 (4.561) | −1.12 (1.353) | 29.50 (5.257) | 28.93 (5.009) | −0.22 (1.378) | <0.001 |
| −3.74 (4.429) § | −0.65 (4.527) § | <0.001 | |||||
| SBP (n) | 7,302 | 4,184 | 4,184 | 7,302 | 4,122 | 4,122 | |
| Mean, mmHg (SD) | 132.5 (17.06) | 127.0 (14.57) | −5.2 (15.06) | 132.7 (17.36) | 129.7 (15.00) | −2.8 (15.17) | <0.001 |
| Total cholesterol (n) | 2,332 | 1,428 | 1,212 | 2,216 | 1,413 | 1,178 | |
| Mean, mg/dL (SD) | 211.1 (39.09) | 199.2 (36.28) | −8.7 (35.13) | 210.5 (41.98) | 192.6 (33.79) | −13.8 (36.56) | 0.0381 |
| Triglycerides (n) | 7,286 | 4,175 | 4,170 | 7,285 | 4,115 | 4,108 | |
| Mean, mg/dL (SD) | 193.4 (155.49) | 160.2 (125.91) | −29.9 (132.31) | 188.6 (156.90) | 154.0 (110.12) | −29.2 (128.81) | 0.7217 |
| LDL‐C (n) | 7,297 | 4,176 | 4,174 | 7,287 | 4,112 | 4,105 | |
| Mean, mg/dL (SD) | 129.4 (34.19) | 118.6 (29.43) | −8.6 (31.39) | 129.2 (34.65) | 117.1 (29.47) | −10.1 (31.52) | 0.4637 |
| HDL‐C (n) | 7,298 | 4,177 | 4,176 | 7,294 | 4,115 | 4,111 | |
| Mean, mg/dL (SD) | 50.9 (12.78) | 54.9 (13.56) | 4.0 (7.68) | 51.0 (12.63) | 51.9 (12.77) | 0.5 (7.36) | <0.001 |
“While‐on treatment strategy” was defined as an approach where data from patients who discontinued treatment, switched or added on a comparator drug before having post‐index data available were excluded.
Absolute change from baseline.
Percent change from baseline.
BMI, body mass index; DPP4i, dipeptidyl peptidase‐4 inhibitors; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; SD, standard deviation; SGLT2i, sodium–glucose cotransporter 2 inhibitors.
Subgroup analyses
Achievement of improvement criteria for HbA1c, BMI and SBP
Among matched patients (both strategies), the proportion of patients achieving a composite outcome 1 year after the index date was significantly greater in the SGLT2i group than in the DPP4i group for all subgroups, except for the subgroup who had continued index treatment for <1 year (“treatment policy strategy”; Table 4). The proportion of matched patients (“while‐on treatment strategy”) achieving the improvement criterion for HbA1c 1 year after the index date was significantly greater in SGLT2i‐treated patients than in DPP4i‐treated patients for all subgroups, except for the subgroups with baseline HbA1c <7.5%, BMI <25 kg/m2, SBP <130 mmHg and non–treatment‐naïve patients (Table S7). For all subgroups, a significantly greater proportion of SGLT2i‐treated patients achieved the improvement criterion for BMI and SBP than DPP4i‐treated patients.
Table 4.
Proportion of patients by subgroup who achieved a composite outcome (HbA1c, BMI and SBP) 1 year after the index date (matched patients) † , ‡
| Achieved, % (n/n) | SGLT2i (N = 7,302) | DPP4i (N = 7,302) | RD (95% CI) | P‐value |
|---|---|---|---|---|
| While‐on treatment strategy § | ||||
| Baseline HbA1c | ||||
| <7.5% | 29.6 (720/2,433) | 12.5 (295/2,367) | 16.8 (13.0, 20.6) | <0.001 |
| ≥7.5% | 33.1 (559/1,687) | 13.4 (229/1,703) | 23.2 (17.8, 28.5) | <0.001 |
| Baseline BMI | ||||
| <25 kg/m2 | 27.5 (170/619) | 11.5 (97/847) | 20.3 (12.2, 28.5) | <0.001 |
| ≥25 kg/m2 | 31.7 (1,109/3,501) | 13.2 (427/3,223) | 18.4 (15.7, 21.2) | <0.001 |
| Baseline SBP | ||||
| <130 mmHg | 22.5 (431/1,919) | 8.8 (166/1,884) | 16.5 (12.1, 20.9) | <0.001 |
| ≥130 mmHg | 38.5 (848/2,201) | 16.4 (358/2,186) | 25.0 (20.2, 29.7) | <0.001 |
| Presence of previous treatment | ||||
| Treatment naïve | 28.7 (870/3,034) | 10.9 (324/2,978) | 17.0 (14.0, 19.9) | <0.001 |
| Non‐treatment naïve | 37.7 (409/1,086) | 18.3 (200/1,092) | 22.6 (14.3, 30.9) | <0.001 |
| Duration of index treatment | ||||
| <1 year | 27.1 (152/561) | 9.6 (77/798) | 12.1 (1.4, 22.8) | 0.0325 |
| ≥1 year | 31.7 (1,127/3,559) | 13.7 (447/3,272) | 17.6 (14.7, 20.4) | <0.001 |
| Treatment policy strategy ¶ | ||||
| Baseline HbA1c | ||||
| <7.5% | 25.4 (889/3,497) | 13.4 (442/3,304) | 11.2 (8.5, 13.8) | <0.001 |
| ≥7.5% | 30.0 (794/2,647) | 16.5 (460/2,783) | 14.2 (10.8, 17.7) | <0.001 |
| Baseline BMI | ||||
| <25 kg/m2 | 23.8 (233/977) | 11.5 (134/1,166) | 13.8 (8.9, 18.8) | <0.001 |
| ≥25 kg/m2 | 28.1 (1,450/5,167) | 15.6 (768/4,921) | 12.2 (10.4, 14.0) | <0.001 |
| Baseline SBP | ||||
| <130 mmHg | 19.8 (567/2,863) | 9.3 (262/2,809) | 11.3 (8.5, 14.2) | <0.001 |
| ≥130 mmHg | 34.0 (1,116/3,281) | 19.5 (640/3,278) | 15.7 (12.6, 18.9) | <0.001 |
| Presence of previous treatment | ||||
| Treatment naïve | 25.6 (1,074/4,198) | 13.0 (543/4,166) | 11.8 (9.7, 13.9) | <0.001 |
| Non‐treatment naïve | 31.3 (609/1,946) | 18.7 (359/1,921) | 14.4 (9.9, 19.0) | <0.001 |
| Duration of index treatment | ||||
| <1 year | 21.6 (554/2,565) | 16.2 (453/2,800) | 2.2 (−1.2, 5.6) | 0.2040 |
| ≥1 year | 31.5 (1,129/3,579) | 13.7 (449/3,287) | 17.5 (14.7, 20.3) | <0.001 |
Patients with missing pre‐index and/or post‐index data were excluded from the analysis. Post‐index data were those extracted closest to 1 year (365 days), and between days 180 and 540 after the index date; data for each outcome could be extracted at different times, and if multiple measurements were available, a single measurement closest to 1 year after the index date was used.
Improvement criterion for each outcome was absolute reduction in HbA1c of ≥0.5%, SBP of ≥2 mmHg and percent reduction in BMI of ≥3%, relative from the baseline values.
“While‐on treatment strategy” was defined as an approach where data from patients who discontinued treatment, switched or added on a comparator drug before having post‐index data available were excluded.
“Treatment policy strategy” was defined as an approach where data from all patients, including those who discontinued treatment, switched or added on a comparator drug before having post‐index data available, were included.
BMI, body mass index; CI, confidence interval; DPP4i, dipeptidyl peptidase‐4 inhibitors; HbA1c, hemoglobin A1c; RD, risk difference; SBP, systolic blood pressure; SGLT2i, sodium‐glucose cotransporter 2 inhibitors.
Change from baseline in cardiometabolic outcomes
Changes in cardiometabolic outcomes were analyzed among matched patients (“while‐on treatment strategy”). In both HbA1c subgroups, SGLT2i‐treated patients had significantly greater changes from baseline in BMI, SBP and HDL‐C compared with DPP4i‐treated patients (Table S8). A significantly greater reduction in HbA1c was observed in SGLT2i‐treated patients compared with DPP4i‐treated patients, but only in the baseline HbA1c ≥7.5% subgroup.
In both BMI subgroups, significantly greater changes from baseline in HbA1c, BMI, SBP and HDL‐C were observed in SGLT2i‐treated patients than in DPP4i‐treated patients (Table S9).
In the baseline SBP <130 mmHg subgroup, significantly greater changes in BMI and HDL‐C only were observed for SGLT2i‐treated patients versus DPP4i‐treated patients (Table S10). In the baseline SBP ≥130 mmHg subgroup, significantly greater changes in HbA1c, BMI, SBP and HDL‐C were observed in SGLT2i‐treated patients (Table S10).
Among treatment‐naïve patients, changes from baseline in HbA1c, BMI, SBP and HDL‐C were significantly greater for SGLT2i‐treated patients than for DPP4i‐treated patients, and for non–treatment‐naïve patients, those treated with an SGLT2i had significantly greater changes from baseline in HbA1c, BMI and HDL‐C (Table S11).
Among patients who received index treatment for <1 year or ≥1 year, SGLT2i‐treated patients had significantly greater changes from baseline in HbA1c, BMI, SBP and HDL‐C compared with DPP4i‐treated patients (Table S12).
DISCUSSION
To our knowledge, this is the first retrospective cohort study using a large administrative database to report the cardiometabolic effects of SGLT2i versus DPP4i in patients with type 2 diabetes mellitus in clinical practice in Japan. In the present study, a significantly greater proportion of new users of an SGLT2i achieved simultaneous improvement in HbA1c, BMI and SBP 1 year after the index date than new users of a DPP4i. A significantly greater change from baseline was observed for most cardiometabolic outcomes in new users of an SGLT2i than in new users of a DPP4i. Furthermore, SGLT2i was associated with a higher rate of treatment continuation and longer treatment persistence than DPP4i. These results suggest that SGLT2i treatment can provide better management of cardiometabolic risks compared with DPP4i in Japanese patients with type 2 diabetes mellitus.
Improvements in blood pressure, excessive bodyweight and lipid abnormalities are important for type 2 diabetes mellitus management to prevent and manage diabetes‐related complications, including CVD 5 , 6 . Two previous Japanese studies reported no significant differences in improvements in glycemic and coronary risk factors between SGLT2i and DPP4i treatments 25 , 26 . However, a recent Italian multicenter, retrospective, real‐world study reported that the proportion of patients achieving simultaneous reduction of HbA1c ≥0.5%, bodyweight ≥2 kg and SBP ≥2 mmHg was greater in patients newly starting dapagliflozin than those newly starting a DPP4i (17.6% vs 11.7%, relative risk 1.50, 95% CI 1.21, 1.86, P < 0.001) 15 . Furthermore, in the present study, a greater proportion of new users of an SGLT2i achieved simultaneous reduction of HbA1c ≥0.5%, BMI ≥3% and SBP ≥2 mmHg than new users of a DPP4i, with similar results observed for most subgroups. Therefore, SGLT2i treatment might enable comprehensive and simultaneous management of cardiometabolic risks and prevent diabetes‐related complications in Japanese patients with type 2 diabetes mellitus. Given that the current type 2 diabetes mellitus treatment guideline recommends to also focus on managing CVD risk factors, these results support the clinical significance of using SGLT2i. In fact, in a previous study 17 , SGLT2i were associated with a significant reduction in CVD events in Japanese patients with type 2 diabetes mellitus, including those without a CVD history. Therefore, SGLT2i might show cardioprotective effects in patients without CVD when initiated early and should not be excluded as a first‐line treatment option for type 2 diabetes mellitus.
In the present study, improvements in HbA1c, BMI, SBP and HDL‐C were significantly greater in new users of an SGLT2i than new users of a DPP4i. A prospective, open‐label, randomized study of Japanese patients with type 2 diabetes mellitus showed that improvements in BMI and HDL‐C were significantly greater in patients receiving dapagliflozin than in those receiving sitagliptin (P < 0.001); there was no significant difference between treatment groups for HbA1c and SBP 27 . Similarly, in another prospective, open‐label, randomized study of Japanese patients with type 2 diabetes mellitus, compared with patients treated with sitagliptin, those treated with ipragliflozin had significant improvements in bodyweight (P < 0.0001) and HDL‐C (P = 0.0033), but not in HbA1c and SBP 26 . These conflicting results might be explained by higher baseline HbA1c levels in the present study compared with previous studies. Additionally, higher mean BMIs (~29 kg/m2) in new users of SGLT2i and DPP4i in the present study might also have contributed. Subgroup analysis of the present study showed a significant improvement in HbA1c in new users of an SGLT2i versus a DPP4i among patients with baseline HbA1c ≥7.5% or BMI ≥25 kg/m2, but not among those with baseline HbA1c <7.5% or BMI <25 kg/m2. These findings suggest that patient baseline characteristics might contribute to the effectiveness of SGLT2i on individual metabolic outcomes; in particular, the effects of SGLT2i on HbA1c might be comparable with DPP4i in specific patient groups.
Better persistence can improve glycemic control and lower overall health care costs 16 , 28 , 29 . In the consensus statement recently released by the Japan Diabetes Society, treatment persistence and adherence were included as factors to consider when choosing treatment 30 . The present study showed that new users of an SGLT2i were less likely to discontinue treatment, had a longer time to treatment discontinuation and had a longer time to switch or add on a treatment than new users of a DPP4i. However, a previous Japanese real‐world database study of patients with type 2 diabetes mellitus reported that DPP4i has the highest 12‐month treatment persistence rate among all antidiabetic drugs in untreated and previously treated patients; the median time to discontinuation was also longer in patients treated with a DPP4i compared with those treated with an SGLT2i 31 . Immediately after the first SGLT2i approval in Japan in 2014, a special report on proper use of SGLT2i was released by the Japanese diabetes experts 32 . Recommendations included an immediate discontinuation of SGLT2i in patients who develop skin complications, which was based on reports that the incidence of skin complications is high in patients treated with SGLT2i 33 . Therefore, physicians might have been extremely cautious about adverse events when SGLT2i first became available, potentially leading to the shorter time to discontinuation of SGLT2i treatment. The benefits of SGLT2i treatment became clearer after 2015, when findings from major clinical trials, including the Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients trial 34 , were published, which might have then led to a reduction in discontinuation of SGLT2i treatment. Furthermore, in Japan, prescriptions for all newly approved drugs were previously restricted for 2 weeks for the first year after approval. Given that many SGLT2i were approved in 2014 and 2015, many SGLT2i prescriptions might have been restricted between 2014 and 2015; this might have also affected the treatment persistence of SGLT2i. Therefore, given that the patient selection period was between January 2011 and December 2015 for the previous study, and January 2015 and March 2020 for the present study, results from the present study may better reflect the effects of SGLT2i on treatment persistence. The present results suggest that patients are more likely to continue SGLT2i treatment than DPP4i treatment and maintain an optimal metabolic control, which could contribute to the long‐term prevention of CVD events in patients with type 2 diabetes mellitus.
To our knowledge, this is the first Japanese real‐world study that reports simultaneous improvement in cardiometabolic effects in new users of an SGLT2i versus new users of a DPP4i. The study used longitudinal data of a large sample size of >7,000 matched patients in each treatment group collected from a nationwide administrative database; patients can be tracked across multiple health facilities and followed up, even when they change clinics or hospitals. In the present study, various cardiometabolic outcomes were evaluated. Furthermore, the study showed that treatment persistence, an important factor to consider when choosing treatment, was longer with SGLT2i treatment than with DPP4i treatment. Therefore, results from this study can be used as a guide for choosing type 2 diabetes mellitus treatments.
The present study had some limitations. Potential unmeasured confounding factors that might affect treatment selection and outcomes are not accounted for in the analyses. Patient's laboratory test results were limited to only those carried out as part of a regular annual health checkup, and actual usage of prescribed drugs could not be determined from the drug claims. Post‐index data were extracted between a large window of 180 and 540 days after the index date, and data of each outcome might have been extracted at different times. After propensity score matching, the resemblance of the matched DPP4i‐treated patients to the actual Japanese type 2 diabetes mellitus population newly treated with a DPP4i might have been reduced. Nevertheless, the study results are generalizable, because consistent results were also observed in the crude population, except for HbA1c. Furthermore, the study did not assess any safety outcomes of SGLT2i compared with DPP4i.
In conclusion, the present large real‐world study showed that a simultaneous improvement in HbA1c, BMI and SBP was significantly greater in patients with type 2 diabetes mellitus newly treated with an SGLT2i than those newly treated with a DPP4i in Japan. Furthermore, SGLT2i was associated with a lower risk of treatment discontinuation, suggesting better treatment persistence than DPP4i. The results might further indicate the cardiometabolic risk benefits of SGLT2i compared with DPP4i and support the current treatment guidelines for type 2 diabetes mellitus.
DISCLOSURE
AK received funding as an adviser for Sunstar Group. SS, YK, KA and MR are employees of Astellas Pharma Inc. TK is an employee of Clinical Study Support, Inc, who received funding for this study and manuscript writing from Astellas Pharma Inc., and received payment to their institution from Pfizer Inc. and Viatris Inc.
Approval of the research protocol: Medical Affairs Japan Protocol Approval Committee (protocol number: 1941‐MA‐3372).
Informed consent: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Supporting information
Table S1 | Adapted Diabetes Complication Severity Index codes.
Table S2 | International Classification of Diseases, 10th Revision codes (2013 version) for relevant medical history.
Table S3 | Drug codes.
Table S4 | Procedure codes.
Table S5 | Proportion of patients achieving improvement criteria for hemoglobin A1c, body mass index and systolic blood pressure 1 year after the index date (crude population).
Table S6 | Change from baseline in cardiometabolic outcomes (crude population, while‐on treatment strategy).
Table S7 | Proportion of patients by subgroup who achieved the improvement criteria for hemoglobin A1c, body mass index and systolic blood pressure 1 year after the index date (matched patients, while‐on treatment strategy).
Table S8 | Change from baseline in cardiometabolic outcomes by hemoglobin A1c subgroups (matched patients, while‐on treatment strategy).
Table S9 | Change from baseline in cardiometabolic outcomes by body mass index subgroups (matched patients, while‐on treatment strategy).
Table S10 | Change from baseline in cardiometabolic outcomes by systolic blood pressure subgroups (matched patients, while‐on treatment strategy).
Table S11 | Change from baseline in cardiometabolic outcomes by presence of previous treatment subgroups (matched patients, while‐on treatment strategy).
Table S12 | Change from baseline in cardiometabolic outcomes by duration of index treatment (matched patients, while‐on treatment strategy).
Figure S1 | Study design.
Figure S2 | Kaplan–Meier curves for (a) time to treatment discontinuation and (b) time to switch to or add‐on of new anti‐diabetic drugs (crude population).
ACKNOWLEDGMENTS
This study was funded by Astellas Pharma Inc., who was involved in the study design, data collection and analysis, and manuscript preparation. Statistical analysis was carried out by Clinical Study Support, Inc, and medical writing assistance was provided by Envision Pharma Group, funded by Astellas Pharma Inc.
DATA AVAILABILITY STATEMENT
Researchers may request access to anonymized participant level data, trial level data and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see: https://clinicalstudydatarequest.com/Study‐Sponsors/Study‐Sponsors‐Astellas.aspx.
REFERENCES
- 1. Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 1999; 100: 1134–1146. [DOI] [PubMed] [Google Scholar]
- 2. Wilson PW. Diabetes mellitus and coronary heart disease. Am J Kidney Dis 1998; 32: S89–S100. [DOI] [PubMed] [Google Scholar]
- 3. Gæde P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348: 383–393. [DOI] [PubMed] [Google Scholar]
- 4. Einarson TR, Acs A, Ludwig C, et al. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol 2018; 17: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Diabetes Association Professional Practice Committee . 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2022 . Diabetes Care 2021; 45: S144–S174. [DOI] [PubMed] [Google Scholar]
- 6. Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int 2020; 11: 165–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Araki E, Tanaka A, Inagaki N, et al; Directors of the JCS and JDS. Diagnosis, prevention, and treatment of cardiovascular diseases in people with type 2 diabetes and prediabetes – a consensus statement jointly from the Japanese Circulation Society and the Japan Diabetes Society. Circ J 2020; 85: 82–125. [DOI] [PubMed] [Google Scholar]
- 8. Giugliano D, Esposito K. Class effect for SGLT‐2 inhibitors: a tale of 9 drugs. Cardiovasc Diabetol 2019; 18: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rieg T, Vallon V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia 2018; 61: 2079–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haas B, Eckstein N, Pfeifer V, et al. Efficacy, safety and regulatory status of SGLT2 inhibitors: focus on canagliflozin. Nutr Diabetes 2014; 4: e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heerspink HJ, Perkins BA, Fitchett DH, et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016; 134: 752–772. [DOI] [PubMed] [Google Scholar]
- 12. Kashiwagi A, Sakatani T, Nakamura I, et al. Improved cardiometabolic risk factors in Japanese patients with type 2 diabetes treated with ipragliflozin: a pooled analysis of six randomized, placebo‐controlled trials. Endocr J 2018; 65: 693–705. [DOI] [PubMed] [Google Scholar]
- 13. Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52‐week randomized trial. Diabetes Care 2013; 36: 2508–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leiter LA, Yoon K‐H, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double‐blind, phase 3 study. Diabetes Care 2015; 38: 355–364. [DOI] [PubMed] [Google Scholar]
- 15. Morieri ML, Consoli A, Sesti G, et al. Comparative effectiveness of dapagliflozin vs DPP‐4 inhibitors on a composite endpoint of HbA1c, body weight and blood pressure reduction in the real world. Diabetes Metab Res Rev 2021; 37: e3353. [DOI] [PubMed] [Google Scholar]
- 16. Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Ther 2011; 33: 74–109. [DOI] [PubMed] [Google Scholar]
- 17. Kashiwagi A, Shoji S, Onozawa S, et al. Reduction in cardiovascular disease events in patients with type 2 diabetes mellitus treated with a sodium‐glucose cotransporter 2 inhibitor versus a dipeptidyl peptidase‐4 inhibitor: a real‐world retrospective administrative database analysis in Japan. J Diabetes Investig 2022; 13: 1175–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muramoto A, Matsushita M, Kato A, et al. Three percent weight reduction is the minimum requirement to improve health hazards in obese and overweight people in Japan. Obes Res Clin Pract 2014; 8: e466–e475. [DOI] [PubMed] [Google Scholar]
- 19. National Institute for Health and Care Excellence . Type 2 diabetes in adults: management. Available from: https://www.ncbi.nlm.nih.gov/books/NBK338142 Accessed September 30, 2022. [PubMed]
- 20. Ueshima H. “Health Japan 21” and the development of cardiovascular disease countermeasures. Article in Japanese. Nihon Junkankibyou Yobou Gakkaishi 2001; 36: 135–139. [Google Scholar]
- 21. Matsuoka H. Hypertension treatment guidelines 2009 (JSH 2009). Article in Japanese. Ningen Dock 2009; 24: 837–843. [Google Scholar]
- 22. Miyazaki S. Obesity clinical practice guidelines 2016. Article in Japanese. Nippon Naika Gakkai Zasshi 2018; 107: 262–268. [Google Scholar]
- 23. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 24. Chang H‐Y, Weiner JP, Richards TM, et al. Validating the adapted Diabetes Complications Severity Index in claims data. Am J Manag Care 2012; 18: 721–726. [PubMed] [Google Scholar]
- 25. Kitazawa M, Katagiri T, Suzuki H, et al. A 52‐week randomized controlled trial of ipragliflozin or sitagliptin in type 2 diabetes combined with metformin: the N‐ISM study. Diabetes Obes Metab 2021; 23: 811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsurutani Y, Nakai K, Inoue K, et al. Comparative study of the effects of ipragliflozin and sitagliptin on multiple metabolic variables in Japanese patients with type 2 diabetes: a multicentre, randomized, prospective, open‐label, active‐controlled study. Diabetes Obes Metab 2018; 20: 2675–2679. [DOI] [PubMed] [Google Scholar]
- 27. Fuchigami A, Shigiyama F, Kitazawa T, et al. Efficacy of dapagliflozin versus sitagliptin on cardiometabolic risk factors in Japanese patients with type 2 diabetes: a prospective, randomized study (DIVERSITY‐CVR). Cardiovasc Diabetol 2020; 19: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kennedy‐Martin T, Boye KS, Peng X. Cost of medication adherence and persistence in type 2 diabetes mellitus: a literature review. Patient Prefer Adherence 2017; 11: 1103–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guerci B, Chanan N, Kaur S, et al. Lack of treatment persistence and treatment nonadherence as barriers to glycaemic control in patients with type 2 diabetes. Diabetes Ther 2019; 10: 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bouchi R, Kondo T, Ohta Y, et al; The Committee of Establishing Consensus Statements. A proposed algorithm for pharmacotherapy in people with type 2 diabetes. Article in Japanese. J Jpn Diabetes Soc 2022; 65: 419–434. [Google Scholar]
- 31. Nishimura R, Kato H, Kisanuki K, et al. Treatment patterns, persistence and adherence rates in patients with type 2 diabetes mellitus in Japan: a claims‐based cohort study. BMJ Open 2019; 9: e025806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Committee on the Proper Use of SGLT2 Inhibitors . Recommendations on the proper use of SGLT2 inhibitors. J Diabetes Investig 2020; 11: 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakamura I, Maegawa H, Tobe K, et al. Safety and effectiveness of ipragliflozin for type 2 diabetes in Japan: 12‐month interim results of the STELLA‐LONG TERM post‐marketing surveillance study. Adv Ther 2019; 36: 923–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Adapted Diabetes Complication Severity Index codes.
Table S2 | International Classification of Diseases, 10th Revision codes (2013 version) for relevant medical history.
Table S3 | Drug codes.
Table S4 | Procedure codes.
Table S5 | Proportion of patients achieving improvement criteria for hemoglobin A1c, body mass index and systolic blood pressure 1 year after the index date (crude population).
Table S6 | Change from baseline in cardiometabolic outcomes (crude population, while‐on treatment strategy).
Table S7 | Proportion of patients by subgroup who achieved the improvement criteria for hemoglobin A1c, body mass index and systolic blood pressure 1 year after the index date (matched patients, while‐on treatment strategy).
Table S8 | Change from baseline in cardiometabolic outcomes by hemoglobin A1c subgroups (matched patients, while‐on treatment strategy).
Table S9 | Change from baseline in cardiometabolic outcomes by body mass index subgroups (matched patients, while‐on treatment strategy).
Table S10 | Change from baseline in cardiometabolic outcomes by systolic blood pressure subgroups (matched patients, while‐on treatment strategy).
Table S11 | Change from baseline in cardiometabolic outcomes by presence of previous treatment subgroups (matched patients, while‐on treatment strategy).
Table S12 | Change from baseline in cardiometabolic outcomes by duration of index treatment (matched patients, while‐on treatment strategy).
Figure S1 | Study design.
Figure S2 | Kaplan–Meier curves for (a) time to treatment discontinuation and (b) time to switch to or add‐on of new anti‐diabetic drugs (crude population).
Data Availability Statement
Researchers may request access to anonymized participant level data, trial level data and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see: https://clinicalstudydatarequest.com/Study‐Sponsors/Study‐Sponsors‐Astellas.aspx.


